Abstract
The approval of a multitargeted receptor tyrosine kinase inhibitor, sorafenib, with activity against vascular endothelial growth factor receptor-2 and -3, Raf-1 and B-Raf, platelet-derived growth factor receptor-α and -β, and other kinases, has ushered in the era of molecular targeted agents in advanced hepatocellular carcinoma (HCC). Sunitinib malate is an oral, multitargeted inhibitor of vascular endothelial growth factor receptor-1, -2, and -3, platelet-derived growth factor receptor-α and -β, and other kinases implicated in tumor growth, angiogenesis, and metastasis. Sunitinib has been approved in metastatic renal cell carcinoma and gastrointestinal stromal tumor and is undergoing active clinical development in HCC. Early evidence of antitumor activity and a promising safety profile for this agent have emerged from single arm phase II trials in United States, European, and Asian patients with advanced HCC. Correlative studies of imaging and circulating biomarkers have provided insights into the potential mechanism of action of sunitinib. Additional phase II studies using either single agent or in combination with chemotherapeutic agents are ongoing, and a phase III trial comparing sunitinib and sorafenib in advanced HCC is actively accruing patients. Here, we review the current progress and future directions for the development of sunitinib in advanced HCC.
Keywords: sunitinib, hepatocellular carcinoma, angiogenesis, multitargeted receptor tyrosine kinase inhibitor, clinical trials
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide and is responsible for approximately 600,000 deaths per year.1 Despite many available treatment options for patients with early stage HCC, the mortality rate remains very high making HCC the third leading cause of cancer-related death worldwide.1 This high mortality rate reflects the poor prognosis for patients with advanced stage HCC, the pattern of presentation, and the poor outcomes associated with cirrhosis. Most patients present with advanced disease, only 30% of patients present with resectable disease, and up to 80% have underlying cirrhosis.2
Although many investigators have explored the use of systemic agents, including hormonal and chemotherapy in HCC, none of them has demonstrated improved overall survival (OS).3 The first and only approved drug that has shown to date improvement in survival in a phase III trial in patients with advanced HCC is sorafenib (Nexavar; Bayer HealthCare Pharmaceuticals, West Haven, CT, and Onyx Pharmaceuticals, Emeryville, CA). Sorafenib is a small molecule that inhibits tumor-cell proliferation and tumor angiogenesis by targeting the serine-threonine kinases, Raf-1 and B-Raf, and the receptor tyrosine kinases (RTK) of vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-2, and VEGFR-3, and platelet-derived growth factor receptor (PDGFR)-α and -β. Based on the strong rationale of mechanism of action, promising preclinical data against HCC, and early evidence of antitumor activity from the phase II study,4 the international, phase III, placebo-controlled Sorafenib HCC Assessment Randomized Protocol (SHARP) trial was subsequently conducted and demonstrated a longer OS time and time-to-tumor progression (TTP) compared with placebo.5 The median OS time was 10.7 months in the sorafenib group and 7.9 months in the placebo group (hazard ratio for the sorafenib group, 0.69; P < 0.001). The median TTP was 5.5 months in the sorafenib group and 2.8 months in the placebo group (P < 0.001). In another randomized phase III study conducted in Asia, sorafenib also demonstrated a longer OS in patients with advanced HCC.6 The OS was 6.5 months in the sorafenib group versus 4.2 months in the placebo group (hazard ratio for the sorafenib group, 0.68; P = 0.014). Based on these studies, sorafenib has become the standard of care for the patients with advanced HCC and the new standard for ongoing and future clinical trials.
The development of sorafenib in HCC has several important implications. First, it validates the use of molecularly targeted agents in HCC. Second, it sets a standard for future clinical trials in advanced disease. Third, it generates new promise that understanding the mechanism of action of this targeted agent could help develop the predictive biomarkers to identify patients more likely to respond and for assessing efficacy and toxicity for individual patients. Finally, it justifies the development of other agents with overlapping target inhibition, such as sunitinib, in advanced HCC.
RATIONALE FOR DEVELOPING SUNITINIB IN HCC
New vessel formation or angiogenesis is a hallmark of cancer.7 Tumor angiogenesis is a complex and a dynamic process involving factors essential for the development of new tumor blood vessels, tumor growth, and metastasis. HCCs express elevated levels of VEGF and VEGF receptors and have a high microvascular density.8–14 Several studies have correlated elevated VEGF with the biology of HCC and clinical outcomes in HCC. Elevated levels of VEGF have been correlated with more invasive disease, shorter survival, and worse outcomes after surgery and local therapy.15–19 Recently, molecular studies of clinical tissue have identified high-level genomic gains of the VEGF-A gene and corresponding increased gene expression in a subset of liver cancers.20 Taken together, these data suggest a role for dysregulated angiogenesis in HCC and provide the rationale for targeting angiogenesis as a potential therapeutic strategy in HCC.
Angiogenesis is also modulated by factors other than VEGF. PDGF pathway is a key mediator of angiogenesis recently implicated in the pathogenesis of HCC.7,21 PDGF conveys through its receptors (PDGFR-α and -β) survival and migratory signals to pericytes that provide support to vascular endothelial cells.22 Inhibiting PDGFRs have been shown to cause pericyte detachment from the endothelium, leaving endothelial cells more susceptible to VEGF inhibition.22 PDGFR-α is overexpressed in HCC,23 and PDGF overexpression has been linked to the increased metastatic potential of HCC.24 We recently reported that PDGFR-β, in addition to PDGFR-α, can be ectopically detected on the tumor endothelial cells in HCC.25
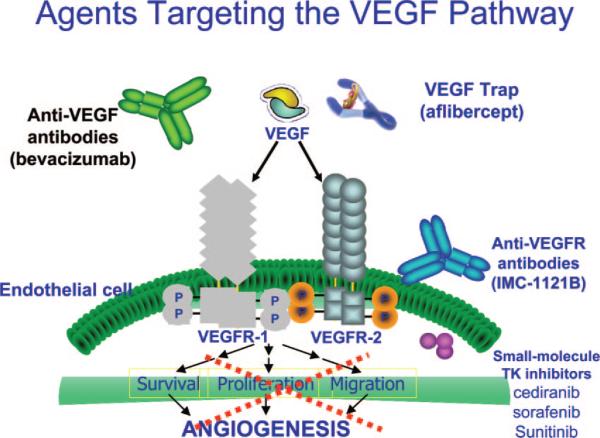
These insights into the molecular pathogenesis of HCC have led to the development of agents that target these important signaling pathways. At this stage, targeting angiogenesis through VEGF and its receptors has gained most experience in HCC. As illustrated in Figure 1, there are several classes of agents targeting this important axis. Each class has unique mechanism of action, target inhibition spectrum, and side-effect profile. Bevacizumab is a humanized monoclonal antibody against VEGF. Aflibercept/VEGF Trap has been developed by incorporating domains of both VEGFR-1 and VEGFR-2 fused to the constant region of human immunoglobulin G1, which acts as a soluble decoy receptor for VEGF with very high affinity for all isoforms of VEGF-A (<1 pM) and placental growth factor. IMC-1121B is a fully humanized monoclonal antibody of the immunoglobulin G, subclass 1 (IgG1) that specifically binds to the extracellular domain of VEGFR-2 with high affinity. This antibody potently blocks the binding of VEGF to VEGFR-2 and downstream signaling in the endothelial cells. Many small molecule RTK inhibitors target VEGFRs. Some of these are more selective (eg, cediranib), others have broad inhibitory spectra (sorafenib and sunitinib).26
FIGURE 1.
Different agents that target the VEGF pathway and their molecular mechanism of action.
Sunitinib is an oral, multitargeted RTK inhibitor with antitumor and antiangiogenic properties.27–29 Sunitinib has a partially overlapping but distinct spectrum of target inhibition compared with sorafenib (Table 1). Sunitinib inhibits multiple kinases including VEGFR-1, -2, and -3, PDGFR-α and -β, stem-cell factor receptor (KIT), FMS-like tyrosine kinase 3, colony-stimulating factor receptor type 1, and the glial cell line-derived neurotrophic factor receptor (RET). Some of these pathways have been implicated in tumor growth, angiogenesis, and metastasis.30–34 Compared with sorafenib, sunitinib has more potent anti-VEGFR activities in vitro. Sunitinib is approved for the treatment of advanced renal cell carcinoma (RCC) and imatinib-resistant or -intolerant gastrointestinal stromal tumor (GIST).35,36 Sunitinib is in clinical development for several solid tumor types, including lung, colorectal, and HCC.
TABLE 1.
| Kinase | Sunitinib IC50 (nM) | Sorafenib IC50 (nM) |
|---|---|---|
| VEGFR-1 | 2*† | 26 |
| VEGFR-2 | 9* | 90 |
| VEGFR-3 | 17*† | 20‡ |
| PDGFR-α | ND | ND |
| PDGFR-β | 8* | 57‡ |
| Raf-1 | ND | 6 |
| CSF-1R | 50-100 | ND |
| KIT | 1-10 | 68 |
| FLT3 | 250 | 58 |
| RET | 50§ | 47 |
| MET | 4000 | >10,000 |
| FGFR-1 | 830* | 580 |
K (nM) values reported.
Pfizer, unpublished data.
Values indicate IC50 for murine enzyme.
Value indicates inhibition of C634W mutant RET.
CSF-1R indicates colony-stimulating factor receptor type 1; FLT3, FMS-like tyrosine kinase-3; RET, glial-cell line-derived neurotrophic factor receptor, rearranged during transfection; MET, hepatocyte growth factor receptor; FGFR, fibroblast growth factor receptor; ND, not determined.
In preclinical studies, sunitinib inhibited endothelial cell proliferation and the formation of tube-like structures, which are important processes during angiogenesis.37 Furthermore, it inhibited VEGF- and PDGF-induced endothelial cell growth.32 These data indicated that sunitinib could potentially inhibit tumor angiogenesis. In addition, direct antitumor activity was demonstrated by the ability of sunitinib to inhibit the growth of solid tumor cell lines expressing some of its targets. In mouse xenograft models, sunitinib was able to induce regression, growth arrest, or considerable growth retardation of established xenografts from human tumor cell lines, including colon carcinoma, glioblastoma, lung carcinoma, and RCC.38 In vivo antiangiogenic activity of sunitinib in a model of human colon cancer (HT-29) was also assessed using dynamic contrast-enhanced magnetic resonance imaging, which showed that sunitinib reduced vascular permeability in the tumor rim within 24 hours of administration of a single dose.39 This early effect of sunitinib on tumor angiogenesis likely reflects potent inhibition of VEGFR activity, and may precede any effect of treatment on inhibition of tumor growth. Although there are no published data on the antitumor activity in HCC in preclinical models, in vivo antitumor efficacy against HCC xenografts has been observed (unpublished data, Pfizer).
Based on the strong rationale of mechanism of action, supporting preclinical data, and proven efficacy in metastatic RCC and in GIST, sunitinib is under clinical development in HCC.
EARLY CLINICAL EXPERIENCE IN HCC
The current clinical experience is based on 3 single arm open-label phase II studies. Our group performed one of these phase II studies of sunitinib in advanced HCC.25 Thirty-four patients with histologically confirmed advanced HCC received sunitinib at 37.5 mg/d for 4 weeks followed by 2 weeks rest/cycle. Of these, 33 patients (97%) had underlying Child-Pugh A cirrhosis. Thirteen patients (38%) had Cancer of the Liver Italian Program (CLIP) score 1, 12 patients (35%) had CLIP score 2, and 9 patients (27%) had CLIP score 3. Twenty-nine patients (85%) had Barcelona Clinic Liver Cancer stage C, and 5 patients (15%) had Barcelona Clinic Liver Cancer stage B. The majority of patients (n = 28, 82%) had no prior systemic treatments. The primary endpoint was progression-free survival (PFS). Sunitinib induced a partial response (PR) of 20 months duration in one patient (2.9%; 95% confidence interval [CI], 0.2%–14.9%) and achieved stable disease (SD) in 17 patients (50%; 95% CI 34.1%–65.9%). Three patients (8.8%) showed a greater than 50% decrease in alphafetoprotein (AFP). With a median follow-up of 8.1 months, the PFS of this cohort was 3.9 months (95% CI 2.6–6.9 months), the TTP was 4.1 months (95% CI 2.8–9.2 months), and the median OS was 9.8 months (95% CI 7.4 months–not available).
Preliminary antitumor activity of sunitinib has also been observed in another phase II study of European and Asian populations. Patients (N = 37) with unresectable HCC who had not received prior systemic treatment received sunitinib, 50 mg/d for 4 weeks followed 2 weeks rest per cycle. Sunitinib was associated with a disease control rate of 37.8%, with 13 patients experiencing SD for >3 months and 8 patients with SD for >6 months. One patient experienced a confirmed PR. Sunitinib was associated with a median TTP of 4.8 months and a median OS of 10.1 months. Hoda et al40 presented their early experience of 23 patients with unresectable or metastatic HCC who received sunitinib at 37.5 mg daily for 4 weeks on and 2 weeks off every cycle. Of the 17 evaluable patients, one had PR and additional 6 patients had SD. The efficacy data from the phase II trials are summarized in Table 2.
TABLE 2.
| Zhu et al (N = 34) | Faivre et al (N = 37) | Hoda et al (N = 17) | |
|---|---|---|---|
| Objective response rate, n (%) | 1 (3) | 1 (3) | 1 (6) |
| Disease control rate, n (%)* | 16 (52) | 14 (38) | 9 (53) |
| Overall survival (mo) | 9.8 | 10.1 | — |
| Time to progression (mo) | 4.1 | 4.8 | — |
| Progression-free survival (mo) | 3.9 | — | — |
Partial response or stable disease for >3 mo.
In these phase II studies, adverse events were reported as generally manageable. Major toxicities associated with sunitinib were myelo-suppression, elevation of transaminases, fatigue, asthenia, diarrhea, and nausea. The grade 3 to 4 events observed in these 2 studies are summarized in Table 3. Of note, only one study has completely published toxicity report, and the other 2 are still preliminary.25 In the Asian/European trial, grade 5 adverse events possibly attributable to sunitinib occurred in 4 patients: in 3 patients (two of whom were Child-Pugh class B) due to bleeding or hepatic encephalopathy during the first cycle; and in one patient due to renal failure during the seventh cycle. The last patient had experienced arterial hypertension, dyslipidemia, and a renal cyst with an expansive process since the first cycle of treatment; sunitinib doses were not reduced during the study period. In our study, 2 patients died during the first 4 weeks, likely due to rapid disease progression and hepatic failure. However, the possibility of any causal relationship with the investigational agent could not be ruled out. In the preliminary study reported by Hoda et al, grade 3 to 4 toxicities included fatigue (3), elevated aspartate aminotransferase or alanine aminotransferase (3), anorexia (2), nausea (1), vomiting (1), and diarrhea (1). In the phase II sunitinib trials, adverse events were managed by reducing or interrupting sunitinib dosing if and when necessary. From these initial experiences, it seems that sunitinib could be given safely in the majority of patients with close monitoring. The frequency of severe adverse events occurring in these studies suggests a potentially better tolerability of the lower dose of 37.5 mg given in a 4 weeks on and 2 weeks off schedule. Whether sunitinib could induce life-threatening hepatic toxicity with liver failure, as has been reported previously in ovarian cancer,41 should be carefully evaluated in larger HCC populations in the future.
TABLE 3.
| Grade 3-4 Toxicity | Zhu et al (N = 34) | Faivre et al (N = 37) | Hoda et al (N = 23) |
|---|---|---|---|
| Leukopenia | 6 (18) | 4 (11) | — |
| Neutropenia | 6 (18) | 9 (24) | |
| Lymphopenia | 6 (18) | — | — |
| AST | 6 (18) | — | 3 (13) |
| Thrombocytopenia | 4 (12) | 13 (35) | |
| Fatigue | 4 (12) | 8 (22) | 3 (13) |
| ALT | 3 (9) | — | 3 (13) |
| Nausea | 2 (6) | 2 (5) | 1 (4) |
| Anorexia | 2 (6) | — | 2 (9) |
| Total bilirubin | 2 (6) | — | |
| Hand-foot syndrome | 2 (6) | — | — |
| Hypertension | 2 (6) | — | — |
| Upper gastrointestianl bleeding | 2 (6) | 5 (14) | — |
| Anemia | 1 (3) | 7 (19) | — |
| Hypophosphatemia | 1 (3) | — | — |
| Vomiting | 1 (3) | — | 1 (4) |
| Stomatitis | 1 (3) | — | — |
| Rash | 1 (3) | — | — |
| Hyponatremia | 1 (3) | — | |
| Pulmonary embolism | 1 (3) | — | |
| Ataxia | 1 (3) | — | |
| Diarrhea | — | 1 (4) |
AST indicates asparatate aminotransferase (SGOT); ALT, alanine aminotransferase (SGPT).
Sunitinib is undergoing further phase II investigation in several other studies in HCC, both as a single agent and in combination regimens, ie, in combination with capecitabine (Table 4). Moreover, based on the initial clinical experience from the 2 phase II studies, a multinational, randomized, phase III trial to evaluate the efficacy and the safety of sunitinib compared with sorafenib in patients with advanced HCC is ongoing. A total of 1200 patients will be enrolled—600 to receive sunitinib at 37.5 mg daily on a continuous dosing schedule and 600 to receive sorafenib 400 mg twice daily. The trial will be open to patients with histologically confirmed, locally advanced, or metastatic HCC with Eastern Cooperative Oncology Group performance status 0 or 1 and Child-Pugh class A. The primary endpoint for the trial is OS, and a total of 929 events (deaths) will be required to detect a statistically significant difference between treatment arms. If no difference is observed by a 1-sided stratified log-rank test, a superiority or noninferiority hybrid test will be performed, as described by Freidlin et al.42
TABLE 4.
Ongoing Studies of Sunitinib in Hepatocellular Carcinoma
| Design | Country; Clinicaltrials.gov Identifier | Sunitinib Dosing Schedule |
|---|---|---|
| Phase II with continuous sunitinib treatment in patients with unresectable HCC | Switzerland; NCT00514228 | Once daily, starting dose 37.5 mg; continuous dosing |
| Phase II open-label study of sunitinib in patients with metastatic and/or surgically unresectable HCC | United States; NCT00495625 | Once at 37.5 mg daily; schedule 4/2* |
| A phase II study of sunitinib and capecitabine for the treatment of unresectable or metastatic HCC | United States; NCT00787787 | Sunitinib Capecitabine |
| A phase II study sunitinib and chemoembolization in patients with unresectable HCC | United States; NCT00524316 | Cycle 1: sunitinib once daily, days 1-7 (chemoembolization day 8); subsequent cycles: schedule 4/2* |
| A multinational, randomized, open-label, phase III study of sunitinib malate versus sorafenib in patients with advanced HCC | Multinational; NCT00699374 | Sunitinib: 37.5 mg daily continuously Sorafenib: 400 mg twice daily |
Schedule 4/2 = 4 wk on treatment followed by 2 wk off.
CORRELATIVE STUDIES WITH SUNITINIB IN ADVANCED HCC
Despite the demonstration of improved OS by sorafenib in advanced HCC, many questions remain unanswered: What are the molecular and clinical predictors of clinical benefits of sorafenib? What are the potential mechanisms of action of sorafenib that lead to clinical benefits? Is this due to the VEGFR blockage or inhibition of Raf/mitogen-activated protein kinase/extracellular signal-regulated protein kinase signaling pathway? Why do certain patients develop severe toxicities from sorafenib therapy? Why is the benefit from sorafenib seen only in some patients? How do we preselect these patients? What is the escape mechanism(s) that contribute to the resistance to sorafenib? Although we are developing other antiangiogenic and targeted agents in HCC, it is imperative that we continue our efforts to identify validated surrogate and predictive biomarkers that would be helpful to predict clinical efficacy, toxicity, and resistance to antiangiogenic therapy.
In an attempt to evaluate the mechanisms of action and to identify useful biomarkers, extensive correlative studies have been performed in the 2 phase II studies of sunitinib. Because we detected ectopic PDGFR-β expression in HCC endothelial cells, we hypothesized that sunitinib may induce similar antivascular and antipermeability effects in HCC as seen with cediranib (another RTK inhibitor with activity against VEGFR and PDGFR) in glioblastomas, consistent with vascular normalization.43 By comparing clinical outcome with dynamic contrast-enhanced magnetic resonance imaging parameters (eg, Ktrans at baseline and day 14 posttreatment) and circulating biomarkers involved in angiogenic and inflammatory pathways (at baseline, changes after 2 weeks of treatment, and changes at 6 time points during the first 3 cycles of treatment), we attempted to search for biomarkers that might be correlated with clinical efficacy.
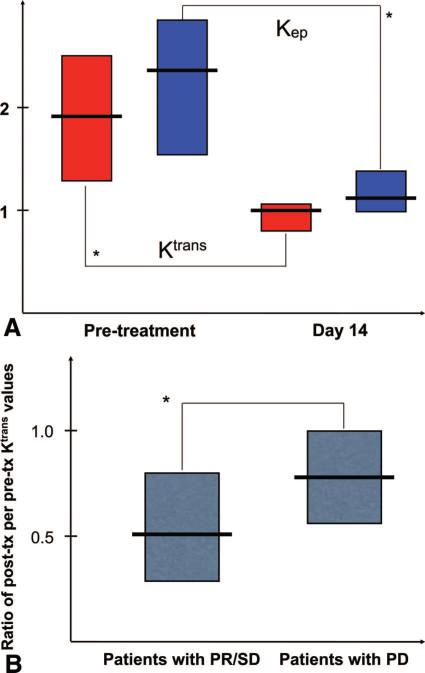
In patients with valid pre- and posttreatment MRI measurements, we found significant decreases in Ktrans and Kep to approximately half of pretreatment values (P < 0.0001; Fig. 2). Moreover, the extent of decrease in Ktrans in patients who experienced PR or SD (n = 17) was significantly greater (2-fold on average) compared with that in patients with PD or who died (n = 8) during the first 2 cycles of therapy (ie, by day 84; P < 0.05). Thus, the extent of the decrease in Ktrans was greater in patients with delayed progression, suggesting that control of vessel leakiness may be a determinant of HCC response to sunitinib.25
FIGURE 2.
Measurement of the effects of sunitinib using dynamic contrast-enhanced magnetic resonance imaging.25 A, Suninitib significantly decreased Ktrans (red boxes) and Kep (blue boxes) in advanced HCC patients (P < 0.0001, data shown as medians with 95% confidence intervals). B, Correlation between the extent of Ktrans decrease at day 14 in HCC patients with partial response or stable disease versus patients with progressive disease after sunitinib (P < 0.05). Reproduced with permission from Ref. 25.
Sunitinib treatment induced significant and sustained increases in the plasma VEGF, placental growth factor, and stromal-derived factor (SDF)-1α and decreases in the plasma soluble VEGFR (sVEGFR)-2 and sVEGFR-3 and circulating progenitor cells (Table 5). In addition, sunitinib tended to decrease plasma levels of VEGF-C and soluble c-KIT, but not other angiogenic and inflammatory biomarkers: plasma bFGF, sVEGFR-1, tumor necrosis factor-α, interleukin (IL)-1β, IL-6 and IL-8, or circulating endothelial cells. Data from the other phase II study are consistent with these findings: sunitinib at a higher dose induced significant elevations in plasma VEGF, and decreases in plasma sVEGFR-2, sVEGFR-3, VEGF-C and soluble c-KIT.44
TABLE 5.
Circulating Biomarker Kinetics After Sunitinib Treatment
| First Cycle |
Later Time Points | |||
|---|---|---|---|---|
| Plasma | 2wk | 4 wk | 6wk | |
| VEGF | Increased25,44 | Increased25,44 | NS44 | Increased44 |
| PlGF | Increased25 | Increased25* | N/A | Increased25 |
| sVEGFR2 | Decreased25,44 | Decreased44 | Decreased44 | Decreased25,44 |
| VEGF-C | NS25 | Decreased25,44* | Decreased44 | NS25 |
| sVEGFR3 | Decreased25,44 | Decreased25,44 | Decreased44 | NS25 |
| sol-c-KIT | Decreased25,44 | Decreased25,44 | Decreased44 | Decreased44 |
| SDF-1α | Increased25 | Increased25 | N/A | Increased25 |
| CPCs | Decreased25 | Decreased25 | N/A | NS25 |
Blood molecular and cellular biomarkers that change after sunitinib treatment compared with baseline in two translational clinical studies in the HCC patients.
Nonsignificant trend for change (P = 0.05-0.1).
PIGF indicates placental growth factor; CPC, circulating progenitor cell.
We also tested if these systemic changes in circulating proangiogenic and proinflammatory factors associate with PFS or OS in HCC patients, after stratifying them by their disease stage using the CLIP score. We found significantly higher baseline serum levels of AFP and plasma levels of the inflammatory cytokines IL-8, IL-6, SDF-1α and tumor necrosis factor-α in patients with rapid tumor progression and/or mortality after sunitinib (P < 0.05, Table 6). Moreover, patients with decreases in plasma IL-6 and soluble c-KIT after 14 days of sunitinib treatment had significantly improved PFS and OS (P < 0.05). In line with this finding, greater decreases in soluble c-KIT associated with decreased tumor “density” measured on computer tomography scans in the other phase II study.44 Finally, analysis performed in a time-dependent proportional hazards model showed that patients with more elevated AFP, IL-6, soluble c-KIT, SDF-1α, sVEGFR-1, and circulating progenitor cells at any time point during sunitinib treatment were associated with higher hazard of immediate progression or mortality (P < 0.05).25 Collectively, these circulating biomarker data suggest a critical role for the balance between angiogenic and inflammatory pathways in HCC response and resistance to sunitinib treatment. Successful modulation of these inflammatory markers might be critical for achieving treatment response with sunitinib and potentially other antiangiogenic agents. The findings of these hypothesis generating studies should be validated in large prospective trials. It will be particularly important to explore these biomarkers for other anti-VEGF RTK inhibitors, such as sorafenib, to better understand the significance of these findings for antiangiogenic therapy and improve the outcome of treatment in HCC.
TABLE 6.
Correlations Between Circulating Biomarkers and Outcome in HCC Patients After Sunitinib
| Biomarker | Pretreatment Measurement | Early Change (d 14) | Time-Dependent Change |
|---|---|---|---|
| AFP | Correlated25 | N/A | Correlated25 |
| VEGF | Correlated25 | NS25 | NS25 |
| IL-6 | Correlated25 | Correlated25 | Correlated25 |
| VEGF-C | Correlated44 | ||
| Soluble c-KIT | NS25 | Correlated25 | Correlated25 |
| IL-8 | Correlated25 | NS25 | NS25 |
| SDF-1α | Correlated25 | NS25 | Correlated25 |
| TNF-α | Correlated25 | NS25 | NS25 |
| sVEGFR1 | NS25 | NS25 | Correlated25 |
| CPCs | NS25 | N/A | Correlated25 |
Blood biomarkers significantly associated with outcome of sunitinib treatment in patients with advanced HCC in translational studies. Biomarkers were evaluated at baseline (d 1), early after sunitinib (d 14) and after subsequent cycles of treatment (d 3).
TNF indicates tumor necrosis factor; CPC, circulating progenitor cell.
In the European and Asian study, Faivre et al also examined the changes in imaging biomarkers after sunitinib treatment and their correlation with clinical outcome.44,46 They observed an induction of tumor necrosis measured by computer tomography scan. However, how to reliably quantify tumor necrosis and whether tumor necrosis is truly associated with improved clinical outcome remain to be defined.
The correlative studies from the above 2 studies are exploratory in nature and warrant further characterization and validation in prospective studies. However, they provide critical initial insights into the potential mechanism of action of sunitinib and the potential value of some of these imaging and circulating markers as predictive or prognostic markers in HCC.
CHALLENGES IN DEVELOPING SUNITINIB IN HCC
Several challenges remain for the ongoing and future development of sunitinib in HCC. What is the relative efficacy of sunitinib in comparison with sorafenib, the current standard of care? Do the efficacy data from the phase II studies justify the head-to-head comparison between these 2 agents in the ongoing phase III study? Will sunitinib have favorable safety profiles in a larger population? What is the risk for hepatic toxicity of this agent? What is the optimum dose and schedule for sunitinib in HCC? What imaging and circulating biomarkers should be chosen for validation in future prospective studies?
The efficacy data from the single arm phase II studies suggest some antitumor activity for this agent. Despite the low response rate, the TTP/PFS was around 4 months. However, compared with the experience of sunitinib in RCC, the objective response data in HCC seemed to be modest at best. In addition, due to the single arm nature of these studies and potential patient selection bias and inherent heterogeneity, it is hard to assess the relative efficacy in comparison with sorafenib. Ideally, one would like to see a direct comparison of these 2 agents in the same patient population in a randomized phase II study, and the results can be used to help the design of the phase III study. This will provide stronger rationale if a superiority design for OS improvement is realistic. Given the partially nonoverlapping target inhibition spectrum, it is reasonable to examine the efficacy of this agent in sorafenib refractory or intolerable population. Combining sunitinib with either other targeted agents or chemotherapy represent an alternative strategy, but these combinations will need to be examined carefully for tolerability and safety in specific phase I studies in HCC.
Tolerability and toxicity profiles will be the key considerations for developing this agent in HCC. Based on the experience to date, the toxicity encountered in HCC population seemed to be higher than the prior experience in RCC, GIST, and other solid tumor populations. Although 37.5 mg given in a 4 weeks on/2 weeks off schedule seemed to be more favorable compared with the standard 50 mg given in a 4 weeks on/two weeks off schedule; currently, there are no mature data using the 37.5 mg continuous dosing schedule for HCC population-the schedule selected for ongoing phase III sunitinib dosing schedule. It would be interesting to see the mature safety data of this study across different populations around the world. We need more prospective safety data on a larger patient population regarding the rare but potentially serious toxicity of sunitinib in HCC.
It would be critical to continue the efforts developing useful imaging and circulating biomarkers and validate the predictive value of some of these markers in HCC in ongoing and future sunitinib trials. It is conceivable that these efforts will eventually lead to the identification of the right patients who will benefit from sunitinib use and those who can be spared from serious toxicities.45
CONCLUSIONS
After the approval of sorafenib for advanced HCC, sunitinib represents the next agent, among a few, that reaches advanced clinical development in HCC. Single arm phase II studies with sunitinib have demonstrated preliminary antitumor activity. The toxicity profiles seemed to be manageable in most patients, particularly with the lower intermittent dose schedule (37.5 mg 4 weeks on/two weeks off). Despite the lack of head-to-head comparison of sunitinib and sorafenib in a randomized phase II study, a randomized phase III study comparing these 2 agents in advanced HCC is actively enrolling patients. This study will provide important information on the relative efficacy and safety profiles of sunitinib in comparison with sorafenib. Preliminary correlative studies have identified several promising imaging and circulating biomarker candidates that have shown significant associations with clinical outcome. Future efforts to develop and validate biomarkers of response and resistance hold promise for the optimization of treatment for individual HCC patients.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 3.Zhu AX. Systemic therapy of advanced hepatocellular carcinoma: how hopeful should we be? Oncologist. 2006;11:790–800. doi: 10.1634/theoncologist.11-7-790. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 8.Miura H, Miyazaki T, Kuroda M, et al. Increased expression of vascular endothelial growth factor in human hepatocellular carcinoma. J Hepatol. 1997;27:854–861. doi: 10.1016/s0168-8278(97)80323-6. [DOI] [PubMed] [Google Scholar]
- 9.Messerini L, Novelli L, Comin CE. Microvessel density and clinicopatho-logical characteristics in hepatitis C virus and hepatitis B virus related hepatocellular carcinoma. J Clin Pathol. 2004;57:867–871. doi: 10.1136/jcp.2003.015784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi R, Yano H, Nakashima Y, et al. Expression and localization of vascular endothelial growth factor receptors in human hepatocellular carcinoma and non-HCC tissues. Oncol Rep. 2000;7:725–729. doi: 10.3892/or.7.4.725. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi R, Yano H, Iemura A, et al. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology. 1998;28:68–77. doi: 10.1002/hep.510280111. [DOI] [PubMed] [Google Scholar]
- 12.Ng IO, Poon RT, Lee JM, et al. Microvessel density, vascular endothelial growth factor and its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am J Clin Pathol. 2001;116:838–845. doi: 10.1309/FXNL-QTN1-94FH-AB3A. [DOI] [PubMed] [Google Scholar]
- 13.Dhar DK, Ono T, Yamanoi A, et al. Serum endostatin predicts tumor vascularity in hepatocellular carcinoma. Cancer. 2002;95:2188–2195. doi: 10.1002/cncr.10972. [DOI] [PubMed] [Google Scholar]
- 14.Moon WS, Rhyu KH, Kang MJ, et al. Overexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma? Mod Pathol. 2003;16:552–557. doi: 10.1097/01.MP.0000071841.17900.69. [DOI] [PubMed] [Google Scholar]
- 15.Poon TC, Yip TT, Chan AT, et al. Comprehensive proteomic profiling identifies serum proteomic signatures for detection of hepatocellular carcinoma and its subtypes. Clin Chem. 2003;49:752–760. doi: 10.1373/49.5.752. [DOI] [PubMed] [Google Scholar]
- 16.Poon RT, Lau CP, Ho JW, et al. Tissue factor expression correlates with tumor angiogenesis and invasiveness in human hepatocellular carcinoma. Clin Cancer Res. 2003;9:5339–5345. [PubMed] [Google Scholar]
- 17.Poon RT, Lau CP, Cheung ST, et al. Quantitative correlation of serum levels and tumor expression of vascular endothelial growth factor in patients with hepatocellular carcinoma. Cancer Res. 2003;63:3121–3126. [PubMed] [Google Scholar]
- 18.Jeng KS, Sheen IS, Wang YC, et al. Is the vascular endothelial growth factor messenger RNA expression in resectable hepatocellular carcinoma of prognostic value after resection? World J Gastroenterol. 2004;10:676–681. doi: 10.3748/wjg.v10.i5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao Y, Li CP, Chau GY, et al. Prognostic significance of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin in patients with resectable hepatocellular carcinoma after surgery. Ann Surg Oncol. 2003;10:355–362. doi: 10.1245/aso.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Chiang DY, Villanueva A, Hoshida Y, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68:6779–6788. doi: 10.1158/0008-5472.CAN-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu AX. Development of sorafenib and other molecularly targeted agents in hepatocellular carcinoma. Cancer. 2008;112:250–259. doi: 10.1002/cncr.23175. [DOI] [PubMed] [Google Scholar]
- 22.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 23.Stock P, Monga D, Tan X, et al. Platelet-derived growth factor receptor-alpha: a novel therapeutic target in human hepatocellular cancer. Mol Cancer Ther. 2007;6:1932–1941. doi: 10.1158/1535-7163.MCT-06-0720. [DOI] [PubMed] [Google Scholar]
- 24.Zhang T, Sun HC, Xu Y, et al. Overexpression of platelet-derived growth factor receptor alpha in endothelial cells of hepatocellular carcinoma associated with high metastatic potential. Clin Cancer Res. 2005;11(24 Pt 1):8557–8563. doi: 10.1158/1078-0432.CCR-05-0944. [DOI] [PubMed] [Google Scholar]
- 25.Zhu AX, Sahani DV, Duda DG, et al. Efficacy, safety and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27:3027–3035. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3(1):24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 27.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 28.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 29.Faivre S, Demetri G, Sargent W, et al. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6:734–745. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 30.Abrams TJ, Lee LB, Murray LJ, et al. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2:471–478. [PubMed] [Google Scholar]
- 31.Kim DW, Jo YS, Jung HS, et al. An orally administered multitarget tyrosine kinase inhibitor, SU11248, is a novel potent inhibitor of thyroid oncogenic RET/papillary thyroid cancer kinases. J Clin Endocrinol Metab. 2006;91:4070–4076. doi: 10.1210/jc.2005-2845. [DOI] [PubMed] [Google Scholar]
- 32.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 33.Murray LJ, Abrams TJ, Long KR, et al. SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis. 2003;20:757–766. doi: 10.1023/b:clin.0000006873.65590.68. [DOI] [PubMed] [Google Scholar]
- 34.O'Farrell AM, Abrams TJ, Yuen HA, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597–3605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 35.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 36.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 37.Osusky KL, Hallahan DE, Fu A, et al. The receptor tyrosine kinase inhibitor SU11248 impedes endothelial cell migration, tubule formation, and blood vessel formation in vivo, but has little effect on existing tumor vessels. Angiogenesis. 2004;7:225–233. doi: 10.1007/s10456-004-3149-y. [DOI] [PubMed] [Google Scholar]
- 38.Potapova O, Laird AD, Nannini MA, et al. Contribution of individual targets to the antitumor efficacy of the multitargeted receptor tyrosine kinase inhibitor SU11248. Mol Cancer Ther. 2006;5:1280–1289. doi: 10.1158/1535-7163.MCT-03-0156. [DOI] [PubMed] [Google Scholar]
- 39.Marzola P, Degrassi A, Calderan L, et al. Early antiangiogenic activity of SU11248 evaluated in vivo by dynamic contrast-enhanced magnetic resonance imaging in an experimental model of colon carcinoma. Clin Cancer Res. 2005;11:5827–5832. doi: 10.1158/1078-0432.CCR-04-2655. [DOI] [PubMed] [Google Scholar]
- 40.Hoda D, Catherine C, Strosberg J, et al. Phase II study of sunitinib malate in adult pts (pts) with metastatic or surgically unresectable hepatocellular carcinoma (HCC) [abstract 267]. 2008 ASCO Gastrointestinal Cancers Symposium. 2008 [Google Scholar]
- 41.Taran A, Ignatov A, Smith B, et al. Acute hepatic failure following mono-therapy with sunitinib for ovarian cancer. Cancer Chemother Pharmacol. 2009;63:971–972. doi: 10.1007/s00280-008-0814-7. [DOI] [PubMed] [Google Scholar]
- 42.Freidlin B, Korn EL, George SL, et al. Randomized clinical trial design for assessing noninferiority when superiority is expected. J Clin Oncol. 2007;25:5019–5023. doi: 10.1200/JCO.2007.11.8711. [DOI] [PubMed] [Google Scholar]
- 43.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deprimo SE, Cheng A, Lanzalone S, et al. Circulating biomarkers of sunitinib in patients with unresectable hepatocellular carcinoma (HCC): analysis of correlations with outcome and tumor imaging parameters. J Clin Oncol. 2008:26. abstract 4593. [Google Scholar]
- 45.Jain RK, Duda DG, Willett CG, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6:327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faivre SJ, Raymond E, Douillard JY, et al. Assessment of safety and drug-induced tumor necrosis with sunitinib in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol. 2007;25(18S) abstract 3546. [Google Scholar]