Abstract
This review provides a guide to researchers who wish to establish a biobank. It also gives practical advice to investigators seeking access to samples of healthy or diseased human hearts. We begin with a brief history of the Sydney Heart Bank (SHB) from when it began in 1989, including the pivotal role played by the late Victor Chang. We discuss our standard operating procedures for tissue collection which include cryopreservation and the quality assurance needed to maintain the long-term molecular and cellular integrity of the samples. The SHB now contains about 16,000 heart samples derived from over 450 patients who underwent isotopic heart transplant procedures and from over 100 healthy organ donors. These enable us to provide samples from a wide range of categories of heart failure. So far, we have delivered heart samples to more than 50 laboratories over two decades, and we answer their most frequently asked questions. Other SHB services include the development of tissue microarrays (TMA). These enable end users to perform preliminary examinations of the expression and localisation of target molecules in diseased or aging donor hearts, all in a single section of the TMA. Finally, the processes involved in managing tissue requests from external users and logistics considerations for the shipment of human tissue are discussed in detail.
Electronic supplementary material
The online version of this article (doi:10.1007/s12551-015-0182-6) contains supplementary material, which is available to authorized users.
Keywords: BioBanking, Human heart tissue, Sydney Heart Bank, Heart failure, Healthy donor tissue
Background to the Sydney heart bank
In early 1989, collaboration began between the corresponding author and the late Dr Victor Chang (Companion of the Order of Australia, 1986). Dr. Chang provided access to failing hearts from his National Heart Transplant Program at St Vincent’s Hospital, Darlinghurst (established 1984), and Dr dos Remedios initiated a research program at the University of Sydney. The latter has developed into what is now recognised as the Sydney Heart Bank (SHB).
Today, the SHB comprises an Executive (Cris dos Remedios, Director; Sean Lal, Curator; Amy Li, Deputy Director) which manages the routine functions of the Bank, and the Board of the SHB (David Allen – Chair, Paul D Allen, Paul Bannon, Tim Cartmill, Roger Cooke, Alan Farnsworth, Anne Keogh) which oversees final decisions on operations.
Governance of the SHB
Once it became clear that the SHB was more than a mere collection of high-quality human heart samples, it began attracting an increasing number of requests for heart tissue. About 8 years ago, increased demand was reflected in the increasing scientific output that in turn accelerated the rate of new requests. In 2014-2015, the Executive consulted with the management of other tissue banks within the University of Sydney (see the related review by Byrne et al. in this issue of Biophysical Reviews) to see if their operations and governance were applicable to the SHB. As a result, the Executive realised that it needed to develop governance structures of the SHB Board. The Board now consists of one non-voting representative of the Executive and seven independent cardiac scientists, cardiologists and cardiac surgeons. Their collective task is to evaluate the merits of applications for every new project, both from external applicants as well as from within the SHB researchers.
The decisions of the Board can be to accept the proposal as submitted, reject the application but seek clarification, or reject the application. Final decisions are based on: (1) whether there were ethical issues such as a current HREC approval, and whether the research was being conducted on a for-profit basis contrary to patients and organ donors informed consent conditions; (2) practical matters such as whether there was sufficient material in the SHB to achieve the goals of the application, and whether requested amounts appeared to conflict with the applicant’s aims and methods; (3) whether there was a real or perceived conflict with other approved projects; and, most importantly, (4) whether the project as described has scientific merit.
In determining the best practices for the SHB, we examined the recommendations of the International Society for Biological and Environmental Repositories (ISBER). With the exception of their policy on cost recovery, the SHB complies with the principal recommendations of the ISBER.
This review is intended as a background and guide to researchers considering setting up a heart tissue biobank as well as those who require access to the Sydney Heart Bank (SHB). We will cover the following major frequently asked queries by both researchers and reviewers:
What and how many samples are stored in the SHB?
How are the samples protected from degradation?
Do the SHB donor hearts cover a wide range of ages?
What types of failing hearts are in the SHB?
How can tissue be requested from the SHB?
What does the SHB contain?
For a successful heart tissue bank, tissue samples must be obtained ethically, they must be of high quality, and they must collected in large numbers from all parts of the heart and from a wide range of categories of heart failure. Most SHB samples come from end-stage failing hearts from patients who underwent isotopic heart transplantation. However, we also collect samples from patients receiving coronary artery bypass grafts, replacement valves, repairs of ventricular or atrial septal defects, or from patients with hypertrophic cardiomyopathy who underwent surgical cardiomyectomy.
The SHB works closely with cardiothoracic surgeons to minimise the ischemic time between collection of the tissue and preservation of the samples by snap-freezing them in liquid nitrogen. In the great majority of instances, we find there are no appreciable molecular (e.g. RNA or protein) changes in these samples. Heart transplantation invariably requires at least two adjacent theatres at St Vincent’s Hospital, a circumstance which dictates that the procedure be scheduled in the middle of the night. The resulting loss of sleep for the SHB team on transplantation nights should not be underestimated!
How are the samples protected from degradation?
By 2000, the SHB had collected and stored samples from about 180 end-stage failing hearts, and about 40 healthy donor hearts. Today, the Bank has more than doubled (>400 failing and >120 donor hearts), and as our collaborators have become more confident about the absence of degradation in the samples, the rate of requests for tissue has increased and the rate of joint publications accelerated (see below: Publications that employ tissue from the Sydney Heart Bank).
Given the intrinsic variability of humans genes, lifestyles, diets, medications, these numbers are sufficient to establish a statistical basis for detecting molecular (DNA, RNA and protein) differences in failing hearts.
DNA is substantially more stable than RNA, and particularly since the ribonuclease inhibitor RNasin was not invented until much later. In all but a handful of hearts, our collaborators and ourselves were able to demonstrate that there was little or no mRNA breakdown in samples collected with the protocol described below.
It is a common misconception that the SHB donor hearts come from post mortem bodies. Some forms of RNA (particularly mRNA) are rapidly hydrolysed. The SHB has demonstrated that its tissue samples from patients in end-stage heart failure and from non-failing donor hearts show no signs of nucleotide degradation (Trahair et al. 1993; Fermin et al. 2008; Kong et al. 2010; Lu et al. 2010; Cordell et al. 2013; Koopmann et al. 2014).
Proteins tend to be more stable, but some proteins such as the titin (originally known as connectin) are extremely sensitive to hydrolysis and are significantly degraded under anoxic conditions. Titin, with its molecular weight of about 3.9 MDa, is by far the largest and most easily cleaved protein. Collaborators such as Wolfgang Linke were reluctant to examine our samples for titin, but when he discovered our donor samples showed no sign of proteolysis, we published several nice papers (Kruger et al 2009; Hamdani et al 2013; Koetter et al. 2013).
As the SHB reputation for high-quality failing and non-failing heart samples grew, it was imperative that the high degree of preservation of human heart samples achieved by collection within 30–40 min was not spoiled by degradation during transportation. Samples are shipped in nitrogen vapour dewars which maintain –194 °C for up to 3 weeks in transit. Our experience with FedEx has been almost faultless. In well over 100 round trips, all but 2 arrived at their destination frozen in nitrogen vapour. In both those instances, the dewar box must have been transported on its side or inverted. We solved this problem by using a dewar box that falls over when placed upside-down or rolls when placed on its side. The round trip from Sydney to virtually anywhere in the world costs about A$600.
SHB donor hearts from a wide range of ages
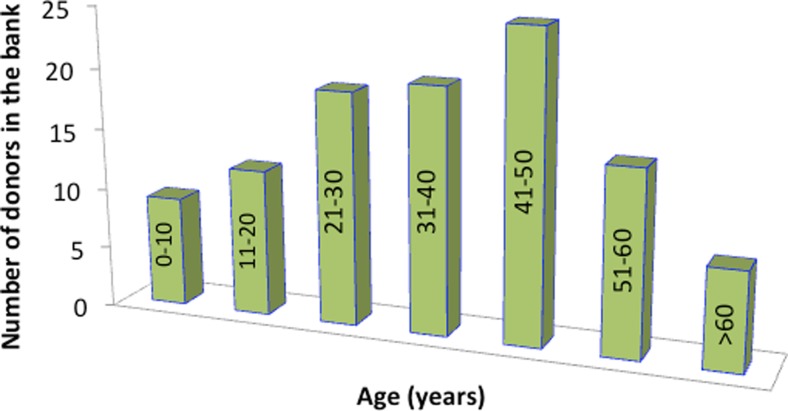
In the first year of operation, Dr Victor Chang provided access to about 30 failing hearts, but only one donor heart was available for research. Today, over 120 donor hearts have been collected which were considered by the transplant team to be unsuitable for isotopic transplantation. The age distribution of these hearts is illustrated in Fig. 1. Acquisition of healthy heart samples against which failing hearts were compared rapidly became a major obstacle since they were normally required for heart transplant patients.
Fig. 1.
The number of healthy donor hearts available in the SHB and their age (in the columns) distribution. Total number of donor hearts is 121. These hearts were surplus to the needs of the St Vincent’s Hospital Heart transplant Program
All hearts, whether failing or donor in origin, were considered potential donor sources of aortic and pulmonary semilunar allograft valves, but these had to be removed under sterile conditions before the rest of the heart could be available for research. These allograft valves, being avascular, could have been harvested the next morning, far too late to useful to the SHB. Helmi Albrecht, who was in charge of collecting the valves for the Sydney Heart Valve Clinic, did not want her precious valves to wait, so without fail she would arrive in good time to collect the valves immediately after removal from the patient or donor heart. She usually completed her job within minutes and promptly handed the remaining specimen to the SHB team.
Donors over 50 years of age were considered too old to be valve donors, so we could process them as soon as they were handed over by the transplant coordinators. All donor hearts, including those from within St Vincent’s Hospital, were perfused with ice-cold cardioplegic solution which effectively inhibited myocardial contraction, while the chilled solution effectively arrested molecular degradation. As a consequence, we were able to show that donor transport times from about 20 min (donors recovered in house) but no more than 4 h (by road or by private jet aircraft used to transport the surgical and transplant coordinator to and from the donor hospital) resulted in no detectable molecular degradation.
In the case of donors over the age of 50 years, the valves were considered unsuitable for transplantation, so we had access to them as soon as they arrived in Sydney.
Failing hearts in the SHB?
The SHB now contains hearts from over 150 IDCM patients. Subcategories of IDCM include: 10 patients with peripartum cardiomyopathy (a baffling category of DCM patients who rapidly progress into severe heart failure at around the time of parturition); 11 with alcohol-induced cardiomyopathy; and 15 with virus-induced dilated cardiomyopathy. The SHB has 92 hearts from patients with ischaemic heart disease including several with diabetes. We have 16 hearts with hypertrophic cardiomyopathy (HCM). Many of the HCM patients’ hearts have been sequenced and the gene mutations identified.
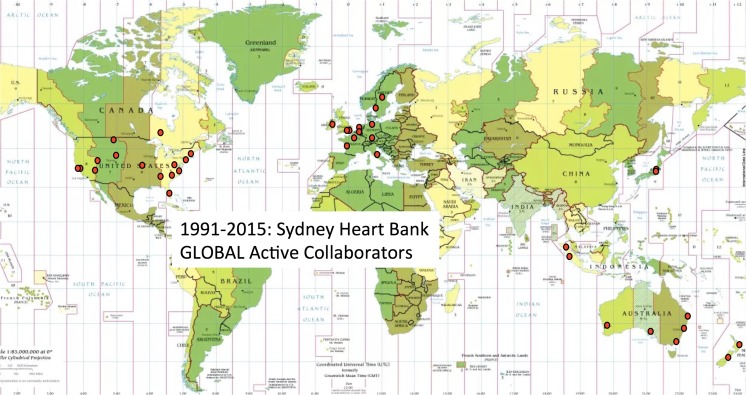
In addition to these main categories, we have a large number from patients with rare conditions such as adriamycin-induced cardiomyopathy (12 hearts), restrictive cardiomyopathy (9), Eisenmenger’s syndrome (8), valvular disease (5), sarcoid cardiomyopathy (4), myocarditis (4) pulmonary hypertension (3) rheumatic cardiomyopathy (3), Marfan syndrome (2), congestive cardiomyopathy (2), Tetralogy of Fallot (1), single ventricle (1), Chaga’s disease (1), Becker’s syndrome (1), and autoimmune diseases (Sjogren's syndrome, and systemic lupus erythematosus). The locations of the collaborating laboratories involved in the examination these hearts is illustrated in Fig. 2.
Fig. 2.
The red dots indicate the cities in which the Sydney Heart Bank has more than 50 global collaborating laboratories. Often, more than one collaborating laboratory is represented in each city (e.g. Baltimore, Boston, Rochester in the USA, Amsterdam, London, Oxford, Dublin and Stockholm in Europe, Tokyo and Singapore in Asia, and in Sydney, Melbourne, Brisbane, Canberra, Adelaide and Perth in Australia and in Auckland and Dunedin in New Zealand
Storage and transport of heart samples
All heart samples in Sydney are stored in either liquid nitrogen (–196 °C) or nitrogen vapour (–192 °C), and we advise recipients to maintain this temperature. On rare occasions, we received complaints that our samples were showing signs of degradation. This was a serious concern since we had not observed this over a period of 26 years. We recently discovered that, if the heart samples are allowed to warm to –80 °C for periods of 6 months, we detected signs of protein degradation on SDS PAGE gels. Storage for even a couple of weeks produces subtle changes. We therefore require that investigators maintained all samples at –192 °C or lower and that they are shipped by FedEx.
Provided the correct documents accompany shipments, transportation of human heart samples between countries has encountered essentially no obstacles as they enter national Customs and Immigration jurisdictions. Problems were encountered when empty dewars were returned to Australia, but we now have developed documentation with the Australian Quarantine & Inspection Service (AQIS) to circumvent this problem.
The process for requesting SHB tissue
Applicants are requested to submit the following documents:
A research proposal (two pages) that: (1) lists all investigators involved in the experiments; (2) provides a brief background; (3) states clear aims or objectives for the research; (4) specifies the methods to be used; (5) presents well-argued reasons for requesting the types of tissues, the number of samples requested (preferably based on a power calculation), and specifies the amounts (mg) tissue required for technical repeats; (6) specifies the patient clinical data required for the successful completion of the project; and (7) includes a request for age-matched donor samples.
A copy of a current HREC approval for this project (not simply a blanket approval for heart research). All recipients of tissue from the SHB are required to hold current approvals from their local Human Research Ethics Committee (HREC). The University of Sydney requires all new project applications for tissue from the SHB to hold current HREC approval at the applicant’s own institution. Regulations on the use of human tissue can vary. Usually, each project is submitted to the applicant’s institution for approval, but some institutions do not require approval, arguing that if approval has been granted by the institution that collected the samples, no further ethics approval is required. In that case, please provide a letter confirming this position.
A two-page CV summarizing the applicant’s track record relevant to this application.
For further information see the SHB website: http://sydney.edu.au/medicine/anatomy/research/labs/mru/sydney-heart-bank/index.php
The SHB Executive will then assess the application for completeness and apply to the University of Sydney HREC Office to have the team leader included in the SHB HREC approval (this step takes only a few days). We then assemble all the documents, examine the SHB tissue holding to check that the requested tissue is available, and present the submission to the SHB Board (see below) who will make its final decision (usually within 2 weeks).
The Office of General Counsel of the University will then draw up a Material Transfer Agreement between the University of Sydney and the receiving institution, and once that is in place, we will send the tissue using the applicant’s FedEx account.
Patient and donor consent for research
All heart transplant patients as well as healthy donors sign an informed consent specifically for research. They are provided with a patient information sheet that provides a broad description of the research that will be undertaken. This document specifically excludes the use of patient tissues for commercial development. This is means that patient consent excludes projects which are specifically designed to provide a profit to the recipient laboratory.
Sample De-identification
Human Research Ethics Committee (HREC) approvals for all failing and non-failing hearts specifically require that the tissue samples from patients and donors must be de-identified. Thus, sample labels must avoid the use of initials, date of birth, or transplant date. Each heart is given a four- or five-digit code that enables the SHB to track the use of all samples, Recipients of tissue are asked to include these codes in relevant research publications so that all laboratories receiving tissue from the same patients or donor hearts can be cross-correlated. Recipients are prohibited from transferring tissue to researchers outside those proscribed by the Material Transfer Agreement.
Detailed sample information
Victor Chang was the first person to be granted approval by the Australian Government to perform isotropic heart transplantation with a limit of about 100 hearts per year. This number was limited due to the very high costs involved. Today, heart transplantation is done in the capital cities of nearly all states, but the number of transplantations per year has not significantly increased, thus reducing the number of hearts available in Sydney.
Currently, the SHB currently contains about 16,000 individual cryovials of tissue from about more than 400 failing human hearts and about 120 non-failing donor hearts. Strictly speaking, these are not control hearts, although the aggregate of the donor hearts may resemble a statistical control population. The great majority of these come from explanted hearts from the St Vincent’s Hospital Darlinghurst Heart and Lung Transplantation Unit.
Approximately 100 samples are collected from each donor or failing heart and are frozen in the transplant theatres. These include: (1) transmural sections of the left ventricle (LV) collected and annotated to keep track of the elapsed time (within a few minutes) from aortic cross-clamp of the coronary arteries, and their location in the left ventricle (anterior, middle and posterior free wall, and some LV samples are divided into endocardial and epicardial regions); all LV samples are collected within 20 min of cross-clamp; (2) sections across the interventricular septum consisting mostly of the LV but must include a proportion of the right ventricle; (3) transmural sections of the right ventricle; (4) papillary muscles from the LV and RV; (5) right atrial samples including pieces from the crista terminalis and trabeculae pectinatae; (6) left atrial samples; and (7) blood vessels including the left and right coronary arteries and their branches and we have some pieces from the ascending aorta. Note: the collection of heart samples is generally completed within 40 min of cross-clamp.
The SHB has exchanged heart samples from other human heart tissue resources. We recently commenced banking samples from hypertrophic cardiomyopathy patients who underwent LV septal cardiomyectomy. This procedure excises gram quantities of tissue from the left side of the interventricular septum to remove obstruction to its outflow tract. We have also received myectomy samples from sources such as the Mayo Clinic in Rochester Minnesota, Imperial College in London, VU Medical Centre in Amsterdam, and the Cleveland Clinic in Ohio.
Patient clinical data and medications history
The SHB Executive recognises the importance of accurate and relevant clinical data for the failing heart samples. We endeavour to record this information at the time of the transplantation procedure. Where patients have been retained by St. Vincent’s Hospital (a period of 5–10 years), we are able to progressively amend patient data including: (1) NYHA classification; (2) LV ejection fraction; (3) echo data for LV end diastolic diameter (LVEDD) and LV end systolic diameter (LVESD) and fractional shortening; (4) the coronary angiogram data that estimate the degree of coronary occlusion in patients with ischaemic heart failure; (5) pathology reports on failing hearts (where available); (6) an extensive history of HF patient medications; and (7) the diagnosis provided by the cardiologists’ reports.
There will be instances where other clinical information may be requested by researchers. Within the time limit for retention of patient records (currently 5 years), this information can be provided by the cardiologists at the Heart Transplant Unit at St Vincent's Hospital.
Research output from the SHB and its collaborators
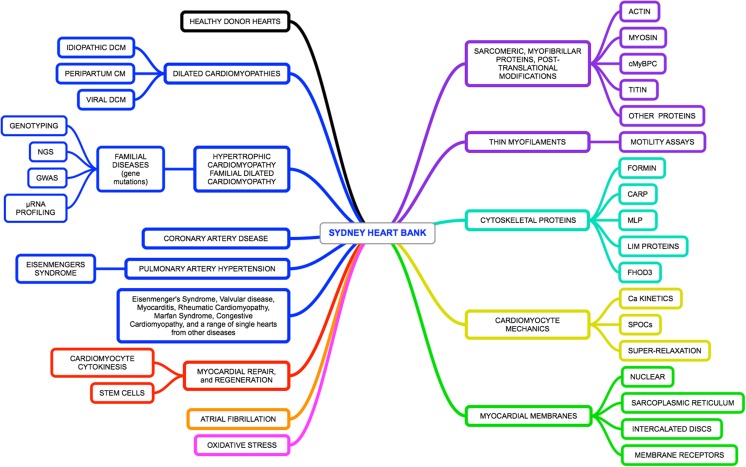
The scope and magnitude of tissue samples held by the Sydney Heart Bank is probably is one of the largest in existence. Since 1991, well over 50 research laboratories have received SHB tissue resulting in nearly 90 publications, usually in the highly ranked cardiac research journals. These papers cover a wide range of aspects of human heart failure as well as changes in healthy hearts as a function of age. The research fields covered by these papers are summarised in Fig. 3. Frequently, samples are used to study sarcomeric or cytoskeletal proteins either in isolation or in concert with functional experiments, such a muscle fibre contraction, myofibril contraction or myofilament motility assays. Other projects focus more on the type of human heart failure (mainly dilated and hypertrophic cardiomyopathies) and the processes of both failure and regeneration. Thus, while collaborations cover a wide range of investigations into failing human hearts, failure is invariably assessed against non-failing samples. The availability of a large number of non-failing hearts corresponded to a sharp increase in both the number of papers accepted for publication and an increase in citation rates.
Fig. 3.
The connections between key words in research papers produced by the SHB and its collaborators. On the left, we cover a wide range of forms of heart failure (blue boxes), myocardial repair and regenerations (red) the impact of atrial fibrillations (orange), and oxidative stress (lilac). These are compared to a wide range of healthy donor heart (black). On the right, we list research topics we focus on keywords arising from all >80 SHB publications (listed in the Supplementary reference) over the past two decades. The topics include changes in and modifications of sarcomeric protein (purple), cytoskeletal proteins (blue-green), and defects in myocardial membranes (green)
Tissue microarrays
Tissue microarrays have been described in more detail elsewhere (Li et al. 2013). In essence, these consist of 0.5-mm-diameter “cores” cut from formaldehyde-fixed and paraffin-embedded tissues, mainly from LV. Duplicate cores from 50–100 hearts are placed into a pre-drilled paraffin block, annealed and then sectioned. These TMAs provide an efficient method of rapidly screening failing and non-failing hearts for specific proteins. A limitation is the antibody must work for immunohistochemistry and then be capable of quantified by western blots. We recommend using Human Protein Atlas antibodies since these are designed to work on human tissue samples (Li et al. 2013) using both methods. The SHB will construct custom TMAs for projects. In fact, for many projects, we recommend a TMA-based preliminary study before any tissue is despatched. Examples of TMA technology have been published in Li et al. (2013).
Does snap-freezing affect sample analyses?
Snap-freezing of heart samples is done in pre-labelled 1.8-mL cryovials. The amount of tissue placed in each vial is important because freezing causes expansion of the tissue volume and can result in compression effects which distort cellular architecture and cell viability.
Samples snap-frozen in liquid nitrogen are routinely used to investigate the identity and quantification of proteins by SDS PAGE, mass spectrometry and other analytical procedures. We recommend that samples be ground to a very fine powder under liquid nitrogen before rapid thawing in extraction buffer.
We know that many of the non-cardiomyocyte cells (e.g. fibroblasts, leukocytes) in frozen LV tissue can survive rapid thawing from liquid nitrogen temperatures. Cardiomyocytes can be maintained in culture for 2–3 weeks after thawing (Ge et al. 2015).
Cardiomyocytes can be glycerol-treated to permeabilised and remove cellular membranes and contractile tension measurements can be made from single, isolated failing cardiomyocytes (Hoskins et al. 2010; Bayliss et al. 2013) compared to donor cells (Fig. 3). Other contractile activity such as auto-oscillations (Wolfe et al. 2011; Kagemoto et al. 2015), super-relaxation measurements (McNamara et al. 2015) and single filament motility assays are possible (Bayliss et al. 2013) (Fig. 3).
Measuring research outcomes
It is expected that laboratories which received tissue for research will acknowledge this in publications, conference proceedings, seminars and lectures and other communications. Reports should be in high-quality journals, and identification of heart samples in these publications should retain their original SHB coding. By doing so, other recipients of tissue from the same patients or donor hearts will be able to appreciate the connection between published data and their results.
Why Use animal models when human heart tissue is available
There are times in the progress of research when the only way forward to understanding the molecular mechanisms of human heart disease is to use animal models. On the other hand, the track record of the applicability of animal-derived data is less than outstanding. An editorial in Nature Medicine (2013, 19: 379) reported serious concerns that animals are poor models of human disease, and more recently another editorial (Sawinski & Maltzman 2015) raised concerns over the need to closely match murine controls when translating murine results to human transplant recipients. This growing awareness of the limitations and over-reliance on animal models of human disease is reflected in the shift by drug companies away from testing with animals (Perel et al. 2007).
The capacity of animal models to predict drug toxicity or efficacy in humans can be as low as 37–50 % suggesting it is no better than toss of a coin (van der Worp et al. 2010). As a result, clinicians and scientists in the UK joined forces in a bid to revise current practices on testing the safety of pharmaceuticals for human use. They published an open letter stating that “our reliance on animals to establish (drug) safety may result in the exposure of clinical volunteers and patients to treatments that are at best ineffective and at worst dangerous” (Archibald et al. 2011).
More than 90 % of newly synthesised drugs fail in clinical trials due to a lack of safety or efficacy in patients (Arrowsmith 2011). In the period from 2007 to 2010, the number of drugs entering phase I clinical trials fell by 47 %, those entering phase II saw a decline of 53 %, and for phase III trials, the number reduced by 55 % (Arrowsmith 2011). The pharmaceutical industry is well aware of the risks involved in developing drugs which pass the animal trial phases only to find they do not achieve the expected outcomes in humans. The odds can be as small as 1–2 out of 10,000 newly synthesised substances will eventually be marketed (Hughes et al. 2011). Much of this failure is due to significant structural and functional differences between animals and man. Even non-human primates, despite their relatively close evolutionary proximity to humans, are not necessarily faithful models of human disease (Duerr et al. 2012).
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 185 kb)
Acknowledgments
The SHB Executive is extremely grateful to Medical Advances Without Animals (MAWA) who provided major funding for the new nitrogen vapour tissue storage facility. The SHB is also grateful to the Discipline of Anatomy & Histology and the Department of Physiology for providing matching funding that matched the MAWA grant. A special mention must be made to recognise the contributions of the many PhD students who collected heart samples from the St Vincent’s Hospital Heart Transplant Program in the middle of the night over 26 years. Even though these students benefit from the award of their degrees and publications on human heart failure, their enthusiasm and many sleepless nights are very gratefully acknowledged. Currently, the SHB does not remunerate any person for contributing to the collection and of tissues. The considerable costs associated with collection and maintenance of the SHB are absorbed by the SHB. Recipients of tissue are asked to cover these costs, even those who pay the transportation costs of dewars to and from Sydney. Under these circumstances, it is not unusual for members of the SHB to be included either as authors of publications that arise, or by acknowledging their contribution in the appropriate section of these papers.
Compliance with Ethical Standards
ᅟ
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects performed by the authors.
Footnotes
Sean Lal and Amy Li are equal first Authors.
Electronic supplementary material
The online version of this article (doi:10.1007/s12551-015-0182-6) contains supplementary material, which is available to authorized users.
References
- Archibald K, Coleman R, Foster C, Signatories O. Open letter to UK Prime Minister David Cameron and Health Secretary Andrew Lansley on safety of medicines. Lancet. 2011;377:1915–1915. doi: 10.1016/S0140-6736(11)60802-7. [DOI] [PubMed] [Google Scholar]
- Arrowsmith J. TRIAL WATCH Phase III and submission failures: 2007-2010. Nat Rev Drug Discov. 2011;10:1. doi: 10.1038/nrd3347. [DOI] [PubMed] [Google Scholar]
- Bayliss CR, et al. Myofibrillar Ca2+-sensitivity is uncoupled from troponin I phosphorylation in hypertrophic obstructive cardiomyopathy due to abnormal troponin T. Cardiovasc Res. 2013;97:500–508. doi: 10.1093/cvr/cvs322. [DOI] [PubMed] [Google Scholar]
- Cordell HJ, et al. Genome-wide association study of multiple congenital heart disease phenotypes identifies a susceptibility locus for atrial septal defect at chromosome 4p16. Nat Genet. 2013;45:822–824. doi: 10.1038/ng.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr A, et al. Extended Follow-up Confirms Early Vaccine-Enhanced Risk of HIV Acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study) J Infect Dis. 2012;206:258–266. doi: 10.1093/infdis/jis342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermin DR, et al. Sex and age dimorphism of myocardial expression in nonischemic human heart failure. Circ Cardiovasc Genet. 2008;1:117–125. doi: 10.1161/CIRCGENETICS.108.802652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Lal S, Le TYL, dos Remedios CG, Chong JJH. Cardiac stem cells: Translation to human studies. Biophys Rev. 2015;7:127–139. doi: 10.1007/s12551-014-0148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdani N, et al. Crucial role for Ca2+/calmodulin dependent protein kinase-II in regulating diastolic stress of normal and failing hearts via titin phosphorylation. Circ Res. 2013;112:664–674. doi: 10.1161/CIRCRESAHA.111.300105. [DOI] [PubMed] [Google Scholar]
- Hoskins AC, et al. Normal passive viscoelasticity but abnormal myofibrillar force generation in human hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2010;49:737–745. doi: 10.1016/j.yjmcc.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JP, Rees S, Kalindjian SB, Philpott KL. Principles of early drug discovery. Br J Pharmacol. 2011;162:1239–1249. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagemoto T, Li A, dos Remedios CG, Ishiwata S. Spontaneous oscillatory contraction (SPOC) in cardiomyocytes. Biophys Rev. 2015;7:15–24. doi: 10.1007/s12551-015-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koetter S, et al. Differential changes in titin domain phosphorylation increase myofilament stiffness in failing human hearts. Cardiovasc Res. 2013;99:648–656. doi: 10.1093/cvr/cvt144. [DOI] [PubMed] [Google Scholar]
- Kong SW, et al. Heart failure associated changes in RNA splicing of sarcomere genes. Circ Cardiovasc Genet. 2010;3:138–146. doi: 10.1161/CIRCGENETICS.109.904698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmann T, et al. Genome-wide identification of expression quantitative trait loci (eQTLs) in human heart. PLoS ONE. 2014;9:e97380. doi: 10.1371/journal.pone.0097380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger M et al (2009) Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res 104:87–94 [DOI] [PubMed]
- Li A, Estigoy C, Raftery M, Cameron D, Odeberg J, Ponten F, Lal S, dos Remedios CG (2013) Heart research advances using database search engines, Human Protein Atlas and the Sydney Heart Bank. Heart Lung Circ 22:819–826 [DOI] [PubMed]
- Lu Z, et al. Oxidative stress regulates left ventricular PDE5 expression in the failing heart. Circulation. 2010;121:1474–1483. doi: 10.1161/CIRCULATIONAHA.109.906818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara JW, Li A, dos Remedios CG, Cooke R. The role of super-relaxed myosin in skeletal and cardiac muscle. Biophys Rev. 2015;7:5–14. doi: 10.1007/s12551-014-0151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perel P, et al. Comparison of treatment effects between animal experiments and clinical trials: systematic review. Br Med J. 2007;334:197–200. doi: 10.1136/bmj.39048.407928.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawinski D, Maltzman JS. Do mice need an order of fries to be relevant for transplant studies? Am J Transplant. 2015;15:2283–2284. doi: 10.1111/ajt.13348. [DOI] [PubMed] [Google Scholar]
- Trahair T, et al. Myosin light chain gene expression associated with diseased states of the human heart. J Mol Cell Cardiol. 1993;26:577–585. doi: 10.1006/jmcc.1993.1067. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, et al. (2010) Can animal models of disease reliably inform human studies? PLoS Med 7 [DOI] [PMC free article] [PubMed]
- Wolfe JE, et al. SPontaneous Oscillatory Contraction (SPOC): Auto-oscillations observed in striated muscle at partial activation. Biophys Rev. 2011;3:53–62. doi: 10.1007/s12551-011-0046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 185 kb)