Abstract
Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis is observed in various conditions, including depression and obesity, which are also often related. Glucocorticoid (GC) resistance and desensitization of peripheral GC receptors (GRs) are often the case in HPA dysregulation seen in depression, and GC plays a critical role in regulation of inflammation. Given the growing evidence that inflammation is a central feature of some depression cases and obesity, we aimed to investigate the immune-regulatory role of GC-GR in relation to depressive mood and obesity in 35 healthy men and women. Depressive mood and level of obesity were assessed, using Beck Depression Inventory (BDI-Ia) and body mass index (BMI), respectively. We measured plasma cortisol levels via enzyme-linked immunosorbent assay and lipopolysaccharide-stimulated intracellular tumor necrosis factor (TNF) production by monocytes, using flow cytometry. Cortisol sensitivity was determined by the difference in monocytic TNF production between the conditions of 1 and 0 μM cortisol incubation (“cortisol-mediated inflammation regulation, CoMIR”). GR vs. mineralocorticoid receptor (MR) antagonism for CoMIR was examined by using mifepristone and spironolactone. A series of multiple regression analyses were performed to investigate independent contribution of depressive mood vs. obesity after controlling for age, gender, systolic blood pressure (SBP), and plasma cortisol in predicting CoMIR. CoMIR was explained by somatic subcomponents of depressive mood (BDI-S: β= −0.499, p= 0.001), or BMI (β= −0.466, p< 0.01) in separate models. The effects of BMI disappeared when BDI-S was controlled for in the model, while BDI-S remained a significant independent predictor for CoMIR (β= −0.369, p< 0.05). However, BMI remained the only independent predictor when BDI-T or BDI-C were controlled for in the model. Mediation analyses also revealed that the relationship between BMI and CoMIR was mediated by BDI-S. The exploratory findings of the relative GR vs. MR roles in CoMIR, using GR and MR blockers, indicated that CoMIR in our cellular model was predominantly mediated by GRs at the higher cortisol dose (1 μM). There was initial indication that greater obesity and somatic depressive symptoms were associated with smaller efficacy of the blockers, which warrants further investigation. Our findings, although in a preclinical sample, signify the shared pathophysiology of immune dysregulation in depression and obesity and warrant further mechanistic investigation.
Keywords: cortisol, glucocorticoid sensitivity, monocytes, obesity, somatic depression, TNF
Introduction
The link between depression and inflammation has been shown via numerous reports of increased levels of various inflammatory markers among depressed patients (Dowlati et al., 2010), including elevated circulating cytokines interleukin (IL)-6 (Alesci et al., 2005), C-reactive protein (Ford and Erlinger, 2004), and tumor necrosis factor (TNF). Increased inflammatory cytokines appear to induce clinically significant depressive symptoms, as seen in longitudinal studies that report elevated CRP and IL-6 levels with future depressive symptom development (Valkanova et al., 2013) and in interferon treatment studies among hepatitis (Udina et al., 2012) and melanoma patients (Musselman et al., 2001). The depression-inflammation association appears to be bi-directional, as studies also report a temporal relationship of depression to future inflammatory cytokine levels (Copeland et al., 2012). Notably, the inflammation-related depression is evident only in a subset of depressed patients (Glassman and Miller, 2007; Kiecolt-Glaser et al., 2015; Raison and Miller, 2011).
Although pathogenesis of the depression-inflammation link remains to be further elucidated, a likely primary pathway is the hypothalamic-pituitary-adrenal (HPA) axis through immunomodulatory actions of glucocorticoids (GCs) (Popova et al., 2011; Varga et al., 2014). Increased levels of cortisol, corticotropin releasing hormone, and the size and activity of pituitary and adrenal glands are often, although not always, found in major depression (Pace and Miller, 2009; Stetler and Miller, 2011; Zunszain et al., 2011). Elevation of cortisol levels in depression is indicative of HPA dysregulation, as many patients with depression exhibit impaired negative feedback of cortisol production by dexamethasone administration (Zunszain et al., 2011), likely attributed to down-regulation or desensitization of glucocorticoid receptors (GRs) (Carvalho and Pariante, 2008; Mokhtari et al., 2013). Phosphorylation of GRs in peripheral blood mononuclear cells (PBMCs) differs between depressed patients and healthy individuals such that GRs of depressed individuals exhibit decreased transcriptional activity and nuclear translocation (Simic et al., 2013). In addition, impaired or reduced GR function co-exists with increased circulating and upregulation of inflammatory markers in patients with depression (Carvalho et al., 2014; Nikkheslat et al., 2015). In examining development of depression, a large longitudinal study also found that decreased GC response by monocytes and T-cells of soldiers before deployment predicted development of greater depressive symptoms after returning (van Zuiden et al., 2015). Given the well-documented immunomodulatory role of GCs, GR functions in inflammatory cytokine regulation may be of clinical significance in mood disorders and other conditions that present with HPA dysregulation and hypercortisolism such as chronic stress (Rohleder et al., 2010).
GC sensitivity is also impaired in obesity, supported by similar data to depression such as blunted dexamethasone suppression in obese children (Longui et al., 2003) or decreased GC feedback sensitivity in obese men (Mattsson et al., 2009). Glucocorticoid elevation is associated with higher abdominal obesity, impaired glucose tolerance, and blood lipid levels (Bose et al., 2009; Schäfer et al., 2013; Wallerius et al., 2003). Chronic exposure to cortisol is associated with accumulation of visceral fat, though systemic cortisol elevation is not required for obesity to occur (Chapman et al., 2013b). Furthermore, increased expression of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD-1), which converts inert cortisone to active cortisol, facilitates weight gain in mice models of diet-induced obesity (Kershaw et al., 2005; Lee et al., 2014; Liu et al., 2008). Thus, impaired GR function is likely a shared mechanism for elevated inflammation in depression and obesity. Elevated11β-HSD-1 activity is hypothesized to influence inflammation in obesity (Chapman et al., 2013a), potentially through decreased feedback inhibition that would normally suppress inflammatory processes. BMI was associated with lower GC sensitivity assessed by lipopolysaccharide (LPS)-stimulated TNF release with dexamethasone treatment in response to a laboratory psychological stressor (Wirtz et al., 2008). Therefore, impaired responsivity of GRs to GC may be the critical factor that drives chronic low-grade inflammation in obesity (McInnis et al., 2014).
Co-morbidity of depression and obesity is found in a high percent of affected individuals, and bi-directional temporal predictability between depression and obesity is evident (Carey et al., 2014; Luppino et al., 2010; Onyike et al., 2003). Furthermore, obesity is related to sustained depressive symptoms even among the individuals without clinical depression (Heo et al., 2006; van der Merwe, 2007). In spite of the accumulating evidence for the association between GC/GR-mediated inflammation regulation and depression or obesity separately, there are limited existing data to clarify cellular mechanisms that underlie the co-condition of obesity and subclinical depressive mood. Thus, we hypothesized that regulation of inflammation mediated by GC/GR is a pathophysiology that underlies the link between depressive symptoms and obesity even among individuals without clinical depression. We investigated the level of cortisol-mediated suppression of TNF production among healthy individuals with a wide range of depressive mood and obesity states. A previously established ex vivo model of LPS-stimulated intracellular expression of TNF by peripheral blood monocytes (Dimitrov et al., 2013; Hong et al., 2014) was adapted to examine responsivity of monocytes to cortisol in inhibition of TNF production. By using a whole blood set-up and cortisol doses representing physiological levels, we aimed to create an ex vivo cellular system that closely resembled an in vivo environment. In addition, given the cortisol action on both GRs and mineralocorticoid receptors (MRs), pharmacological antagonists were used to differentiate cortisol effects on each receptor type in the context of TNF production, which has not been examined in previous studies.
2. Materials and Methods
2.1. Participants
35 otherwise healthy participants with normal to mildly elevated blood pressure (BP) from an ongoing parent prehypertension study at University of California San Diego (UCSD) participated in this investigation. All participants gave written informed consent and were compensated for time and travel. The protocol for recruitment and human subject treatment was approved by the UCSD Institutional Review Board.
Initial screening of participants via telephone interviews followed by face-to-face confirmation determined the absence of several exclusion criteria: diabetes, current or recent history (past 6 months) of smoking or substance abuse, history of cardiovascular disease (e.g. symptomatic coronary or cerebral vascular disease, arrhythmia, myocardial infarction, cardiomyopathy, heart failure), history of bronchospastic pulmonary disease, inflammatory disorder or health conditions affecting immune function (e.g. recent vaccinations within 10 days of the study visit, active and current infections/illness, use of immunomodulatory medication, uncontrolled thyroid disease), psychosis, clinical depression, and clinical hypertension indicated by current intake of antihypertensive medication or laboratory-assessed BP > 145/90 mmHg, with an exception of one participant (152/76 mmHg) whose BP values fluctuated greatly.
2.2. Procedures
Average basal BPs and heart rates (HR) were calculated from two sets of three consecutive measurements at five–minute intervals, using a Dinamap Compact BP® monitor (Critikon, Tempa, FL). Each BP and HR measurement took less than a minute on average, and the two sets were separated by 40 to 60 minutes. To assess levels of obesity, standard anthropometrics (i.e., height, weight, waist and hip circumference) were collected via conventional tape ruler and scale. Subsequently, Body Mass Index (BMI) was calculated by the formula: BMI = weight in kg/(height in m)2. Depressive mood was measured via the Beck Depression Inventory (BDI-Ia), a comprehensive and clinically robust self-report 21-item questionnaire (Beck et al., 1996). Each question was scored from 0 to 3, summed to a BDI total score (BDI-T) that was also subcategorized into cognitive (BDI-C) and somatic (BDI-S) depressive mood scores.
Blood samples were obtained between 8am and 10am for all participants after 12 hours of fasting via an antecubital vein and collected in vacutainers (BD, Franklin Lakes, NJ), containing either heparin or ethylenediaminetetraacetic acid (EDTA) as an anticoagulant. Cellular assay was performed on whole blood aliquots from heparin vacutainer within one hour of collection, and EDTA-treated blood was kept on ice until plasma was separated by centrifugation and aliquoted for storage at −80°C for measuring plasma free cortisol level using enzyme-linked immunosorbent assay (ELISA).
2.3. Ex Vivo Cortisol and Antagonist Treatments of Blood Cells
In order to assess functional response of monocytes to cortisol, varying concentrations of exogenous cortisol (catalog # H0888, Sigma-Aldrich) were applied to LPS-stimulated whole blood cells along with combinations of mifepristone (catalog # M8046, Sigma-Aldrich) and spironolactone (catalog # S3378, Sigma-Aldrich), which are highly specific GR and MR blockers, respectively. The blockers were added 15 minutes prior to addition of cortisol and LPS, which were added concurrently. Effective cortisol concentrations used were 1 μM (36 μg/dl, high-dose, approximating stress levels), 0.2 μM (7.2 μg/dl, moderate-dose, approximating morning/daytime levels), and 0.1 μM (3.6 μg/dl, low-dose, approximating nighttime levels), while antagonist concentrations were 10 μM. Our series of pre-trial titration experiments showed that the dose-dependent nature of inhibition in LPS-stimulated TNF production by monocytes disappears past 1 μM of cortisol (high-dose), such that no further inhibition occurred with higher concentrations of cortisol. To ensure saturation and complete block of either GR or MR on blood monocytes, antagonists were diluted to a concentration of at least one order of magnitude greater than cortisol. Peripheral blood cells were treated with either PBS (control) or cortisol, and cells from a subset of participants (n= 25 of 35) were also treated with PBS, mifepristone, spironolactone, or a combination of both mifepristone and spironolactone. The GR and MR blocker experiments were performed as an exploratory investigation to primarily examine the relative contribution or potentially synergistic or antagonistic effects of GRs vs. MRs in mediating the inhibitory effects of cortisol on LPS-stimulated TNF expression by monocytes.
2.4. LPS-Stimulated Intracellular Monocyte TNF Production via Flow Cytometry
LPS-stimulated production of intracellular TNF was assessed in order to investigate monocytes’ functional responses to cortisol treatments as an indicator of their sensitivity to cortisol, using flow cytometry. Lipopolysaccharide (LPS; 200 pg/ml) (E. coli 0111:B4, catalog # L4391, Sigma-Aldrich, St. Louis, MO) was applied to whole blood via incubation for 3.5 hours at 37°C with 5% CO2. Brefeldin A (10 μg/mL, Sigma-Aldrich) was added during the last 3 hours of incubation to stop cytokine exocytosis. The 200 pg/ml LPS dose was pre-determined from a series of dose-response experiments as sufficient within a physiological range to induce inflammatory response in human blood monocytes ex vivo (Dimitrov et al., 2013; Hong et al., 2014).
Resulting intracellular monocyte TNF production was measured via multiparametric flow cytometry using fluorochrome-conjugated antibodies. Flow cytometry technology characterizes cells individually within a sample based on their fluorescence and light scattering properties. First, leukocytes were isolated via erythrocyte lysis with ammonium chloride solution, centrifugation (5-minute cycles of 500 x g), and wash with PBS containing 0.1 % azide and 0.5 % albumin. Staining of surface markers for monocyte identification proceeded via 15-minute incubation with previously titrated, appropriate concentrations of CD14/APC (Biolegend, San Diego, CA) and HLA-DR/PE (BD Biosciences, San Jose, CA). Cell fixation and permeabilization (Cytofix/Cytoperm Kit, BD Biosciences) preceded intracellular staining (30-minute incubation) with TNF/FITC (Biolegend). A dual-laser FACSCalibur (BD Biosciences) flow cytometer collected at minimum 10,000 gated monocytes per treatment condition, and the data were analyzed using FlowJo software (Tree Star, Inc, Ashland, OR). Monocytes were distinguished from granulocytes and lymphocytes based on forward and side scatter characteristics as well as fluorescence indicating CD14+/dimHLA-DR+ phenotype. Of these monocytes, presence (CD14+/dimHLA-DR+ TNF+) or lack of TNF production (CD14+/dimHLA-DR+ TNF−) was identified by intracellular detection of TNF, which allowed calculation of TNF-producing monocytes as a percentage of total monocytes (“% TNF+ monocytes”).
2.5. Cortisol-Mediation and Antagonist Efficacy in Regulation of Inflammatory Responses
Cortisol “sensitivity” in inflammation suppression at all concentrations of ex vivo cortisol stimulation (0.1, 0.2 and 1μM) was calculated by the difference in % TNF-producing monocytes between inhibited (by cortisol) and control (media control) conditions as Δ % mono TNFcort<sup>x</sup>= PBS – Cortx and termed cortisol-mediated inflammation regulation (CoMIR). The effects of the GR and MR blockers were presented as Δ %mono TNFcort<sup>x</sup> = blocker – Cortx The CoMIR and blocker effects at cortisol dose of 1μM were analyzed as the indicator of sensitivity to cortisol in inflammation regulation, and those at the doses of 0.1 and 0.2 μM were examined and presented primarily to demonstrate the dose response and the assay reliability.
2.6. Plasma Cortisol Measurement
Plasma samples were assayed for concentrations of free cortisol with ELISA kit (Parameter™, R&D Systems, Minneapolis, MN). Manufacturer’s protocols were followed. All samples were assayed using a single plate, and intra-assay coefficient of variability was 3.5%.
2.7. Statistical Analysis
Analyses were performed using SPSS Statistical Software (v22.0) and Microsoft Excel. Descriptive data are presented as means ± SD. Normality of the data was evaluated, using the Shapiro-Wilk test, skewness, and kurtosis. Natural-log transformation of the data was performed for plasma cortisol and total, cognitive, and somatic BDI scores, which initially presented large skewness and kurtosis (> +/− 1). Firstly, bivariate correlations were calculated, using Pearson’s test to examine simple associations among CoMIR, BMI, and depressive mood (BDI). Then, multiple regression analyses were performed, controlling for potential covariates to further determine the independent effect of depressive mood or obesity on cortisol sensitivity: regression models were constructed with basic demographic characteristics and BP (age, gender, systolic BP) as the first step of predictors, plasma cortisol as the second step, and each BDI score and/or BMI as the third step to determine their independent contributions to CoMIR. Age, gender, and SBP were controlled for their previously-shown associations with depressive mood, inflammation, or cortisol sensitivity. Plasma levels of cortisol were included in the model, as our ex vivo cellular measurements were performed in whole blood which included baseline levels of endogenous cortisol, which could have influenced CoMIR. Additionally, a series of univariate regression analyses were performed to formally investigate the mediating effects of BDI scores on the association between BMI and CoMIR based on the methods by Baron and Kenny (Baron and Kenny, 1986). The results were considered statistically significant at p< 0.05, and all tests were two-tailed.
3. Results
3.1. Participant Characteristics and Plasma Cortisol Levels
Participants (n=35) were young to middle-aged adults, with nearly 50% being Caucasian and 60% being men, and overweight or obese individuals on average (Table 1). When compared by BMI-based categories of less than 25 kg/m2 (normal), between 25 kg/m2 and 30 kg/m2 (overweight), and above 30 kg/m2 (obese), over 50% of participants were obese. Depressive mood was under the threshold for clinical evaluation (i.e., BDI-T score ≥ 17) for most subjects, as a current diagnosis of major depression was an exclusion criterion. Average BDI-T score was 5.4 ± 6.5, while cognitive/affective and somatic subscores were 2.9 ± 4.6 and 2.5 ± 2.3, respectively. Average BP of the group was within a normotensive (<125/80 mmHg at study visit) to pre-hypertensive (≥125/80 mmHg & <145/90 mmHg) range. Two out of 14 women reported oral contraceptive use. The average level of plasma cortisol of the study participants was 8.73 ± 5.65 μg/dL (approximately 0.2 μM).
Table 1.
Demographic characteristics.
| Participants (N) | 35 |
|---|---|
| Sex (% Male, Female) | 60, 40 |
| Age (years) | 38 (12), 20 – 59 |
| Race (% White, African American, Asian, Other) | 46, 26, 17, 11 |
| BMI (kg/m2) | 31 (6.8), 18.8 – 45.2 |
| BMI category (% Normal, Overweight, Obese) | 23, 23, 54 |
| BDI-T (score) | 5.4 (6.5), 0 – 26 |
| BDI-C (score) | 2.9 (4.6), 0 – 17 |
| BDI-S (score) | 2.5 (2.3), 0 – 10 |
| Systolic Blood Pressure (mmHg) | 123.9 (14.0), 98 – 152 |
| Diastolic Blood Pressure (mmHg) | 72.8 (8.2), 57 – 89.2 |
Values reported are in format of sample mean (standard deviation), and range where applicable. Percentages are rounded to integers.
3.2. LPS-stimulated TNF Production and CoMIR
3.2.1. Monocyte TNF production with and without cortisol treatment
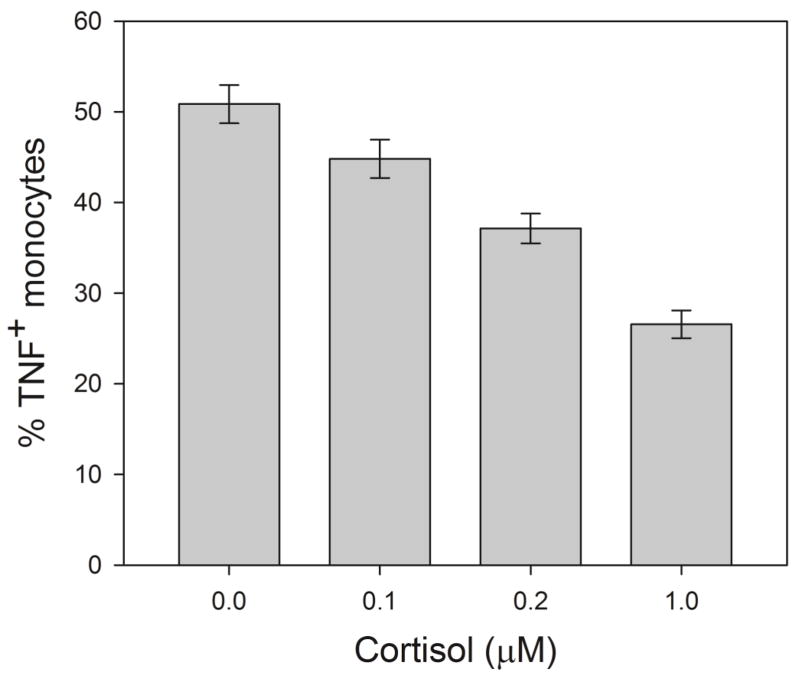
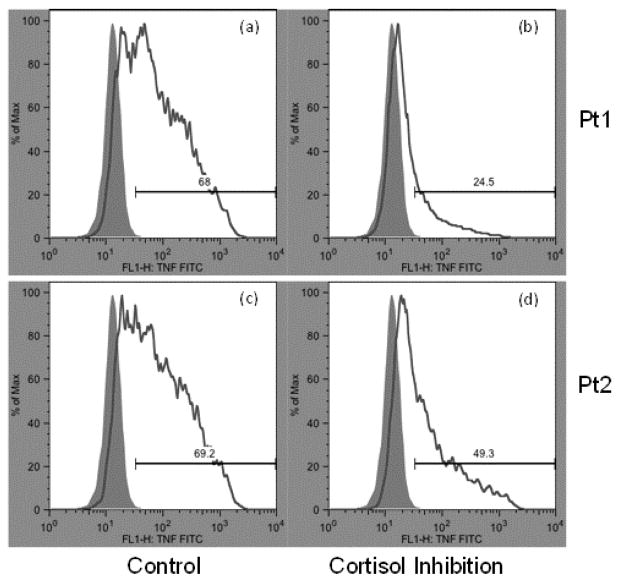
On average, 50.8% (SD of 12.5%, range of 26.1 to 80.1%) of monocytes produced TNF when stimulated with LPS without cortisol treatment. Repeated measures ANOVA with pairwise comparisons revealed that cortisol treatment of all doses significantly suppressed intracellular TNF production by monocytes (p’s between 0.01 and 0.001; Figure 2). On average, monocyte TNF expression was suppressed by cortisol in a reliable dose-response fashion, in which the mean % TNF+ monocytes decreased by about 50% from baseline (media control) to 26.3 ± 9.1 % at 1 μM cortisol treatment (Figure 2), resulting in Δ %mono TNF of 24.1 ± 7.5 %. Cortisol doses of 0.2 and 0.1 μM resulted in 37.1 ± 7.7 % and 44.8 ± 12.5 % of monocytes that produced TNF. The levels of cortisol-mediated inhibition differed among the individuals regardless of the baseline TNF production levels without cortisol treatment (PBS control). For example, Figure 1 depicts that despite both participants 1 and 2, having exhibited about 70% of total monocytes producing TNF upon LPS stimulation at baseline (similar immunological response by monocytes), 24.5% vs. 49.4% of monocytes produced TNF when treated with 1μM cortisol, indicating differing levels of inhibitory effects of cortisol between the two individuals.
Figure 2. Human monocyte LSP-stimulated TNF production from ex vivo treatment with three doses of cortisol.
A dose-dependent relationship is shown between cortisol treatment and intracellular monocytic TNF production. Mean values are reported with standard error bars. Repeated measures ANOVA results indicated the differences between all groups were significant at p< 0.05.
Figure 1. Examples of flow cytometry outputs measuring % TNF+ monocytes from human blood after ex vivo stimulation with LPS with and without cortisol treatment (1 μM).
Monocyte frequency on the Y axes is shown against fluorescence intensity of FITC-conjugated intracellular TNF antibody on the X axes. Solid gray (purple in the web version) areas indicate unstimulated control samples (without LPS), while white areas under the line indicate LPS-stimulated samples. Baseline fluorescence for TNF/FITC was determined and used to gate and differentiate between TNF+ and TNF− monocytes. The difference in percentage of TNF+ monocytes between LPS and control samples is calculated and termed % TNF+ monocytes. This value for each participant is shown above each bar (e.g. 68% for histogram (a) and 24.5% for histogram (b)). Histograms (a) and (b) are from one participant (Pt1) that show treatment without and with cortisol, respectively. Histograms (c) and (d) are from another participant (Pt2). The histograms (b) vs. (d) showcase the differing degree of cortisol-induced inhibition of TNF production indicative of impaired cortisol-mediated inflammation regulation (CoMIR) for Pt2.
3.2.2. CoMIR negatively correlates with BMI and depressive mood
Without cortisol treatment, baseline LPS-stimulated % TNF+ monocytes was negatively correlated with BDI-S at a marginal level (r= −0.31, p= 0.07), indicating an association between greater somatic depressive symptoms and lower basal immunological/inflammatory responses to LPS. No significant associations were found between the baseline % TNF production and the cognitive/affective or total depressive mood scores. Baseline % TNF also negatively correlated with diastolic BP (r= −0.449, p= 0.007) and mean arterial pressure (r= −0.357, p= 0.035). CoMIR (at 1 μM cortisol) was correlated with BDI-S and BDI-T scores (Table 2, Figure 3).
Table 2.
Correlation matrix (Pearson’s r) among BMI, BDI scores, and cortisol-mediated monocytic TNF production with and without GR and MR blockers.
| BMI | BDI-C | BDI-S | BDI-T | Δ %mono TNF (CoMIR)
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Cort only | Mifepris. | Spirono. | |||||||
| BDI-C | r | .020 | |||||||
| p | .910 | ||||||||
|
| |||||||||
| BDI-S | r | .427* | .559** | ||||||
| p | .010 | .000 | |||||||
|
| |||||||||
| BDI-T | r | .308 | .860** | .848** | |||||
| p | .072 | .000 | .000 | ||||||
|
| |||||||||
| Δ %mono TNF (CoMIR) | Cort only | r | −.396* | −.163 | −.461** | −.414* | |||
| p | .018 | .348 | .005 | .013 | |||||
|
| |||||||||
| Mifepris. | r | −.241 | .452* | .178 | .376# | .417# | |||
| p | .280 | .035 | .429 | .084 | .053 | ||||
|
| |||||||||
| Spirono. | r | −.298 | .145 | .128 | .136 | −.301 | .256 | ||
| p | .178 | .520 | .571 | .547 | .173 | .263 | |||
|
| |||||||||
| Combo | r | −.587** | .331 | −.432# | −.142 | .604** | .771** | .358 | |
| p | .007 | .154 | .057 | .550 | .005 | .000 | .121 | ||
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
Correlation is at the 0.10 level (2-tailed).
Body mass index (BMI); Beck Depression Inventory (BDI: C, cognitive/affective; S, somatic; T, total scores). CoMIR, cortisol-mediated inflammation regulation; Cort only, 1μM cortisol with no blockers; Mifepris, cortisol with mifepristone; Spirono, cortisol with spironolactone; Combo, both antagonists; %mono TNF, % TNF-expressing monocytes.
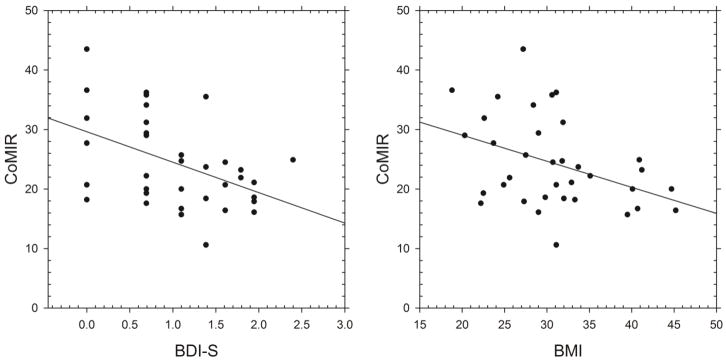
Figure 3. Scatter plots of CoMIR with BMI and BDI-S.
Scatter plots show that cortisol-mediated inflammation regulation (CoMIR) values, calculated by Δ %mono TNF, were negatively associated with obesity, indicated by body mass index (BMI) (r= −.396, p= .018), and depressive mood, assessed by Beck Depression Inventory-Somatic subscale (BDI-S) (r= −.461, p= .005). These associations indicate that individuals with greater depressive mood or obesity are more likely to exhibit smaller CoMIR values which indicate decreased responsivity to GC in suppressing inflammation.
Multivariate regression models, controlling for covariates (Step 1: age, gender, SBP; Step 2: plasma cortisol; Step 3: BMI or BDI), revealed that BMI was significantly predictive of CoMIR (β= −0.466, p= 0.004) and explained an additional 19.8% of the total variance after controlling for demographic variables, SBP, and plasma cortisol levels. Meanwhile, BDI-S was independently associated with CoMIR (β= −0.499, p= 0.001) and explained an additional 24.4% of the total variance after controlling for the covariates. Since a greater CoMIR value indicates greater cortisol-mediated inhibition, a negative β value from our regression models indicates that greater BDI-S scores were predictive of smaller CoMIR, thus impaired suppressive effects of cortisol or GR/MR responsivity to cortisol in suppressing inflammatory responses. BDI-T or BDI-C scores were not significantly associated with CoMIR after controlling for the covariates. When both BMI and each BDI score were entered into the model, BDI-S remained as a significant predictor of CoMIR, but BMI did not (Table 3). However, BMI remained as a significant predictor of CoMIR when BDI-T or BDI-C was controlled for in the model. Age also remained as a significant predictor of CoMIR in the final models.
Table 3.
Multiple regression results with model summary for each steps and coefficient statistics for all predictors in the final step for the outcome variable of CoMIR.
| Models | Predictors | β-coefficientstd | t | R2 | R2adj | ΔR2 | ΔF |
|---|---|---|---|---|---|---|---|
| BDI-T | Age* | −.319 | −2.161 | ||||
| Gender | −.079 | −.550 | |||||
| SBP | −.207 | −1.379 | .200 | .122 | .200 | 2.581 | |
| Plasma Cort | −.168 | −1.163 | .219 | .115 | .019 | .725 | |
| BDI-T# | −.269 | −1.792 | .373 | .265 | .154 | 7.122* | |
| BMI* | −.364 | −2.357 | .477 | .364 | .104 | 5.556* | |
| BDI-S | Age* | −.341 | −2.445 | ||||
| Gender | −.101 | −.736 | |||||
| SBP | −.231 | −1.596 | .200 | .122 | .200 | 2.581 | |
| Plasma Cort | −.161 | −1.176 | .219 | .115 | .019 | .725 | |
| BDI-S* | −.369 | −2.466 | .463 | .370 | .244 | 13.162*** | |
| BMI# | −.286 | −1.842 | .521 | .418 | .058 | 3.395 | |
| BDI-C | Age* | −.352 | −2.254 | ||||
| Gender | −.077 | −.515 | |||||
| SBP | −.166 | −1.054 | .200 | .122 | .200 | 2.581 | |
| Plasma Cort | −.205 | −1.354 | .219 | .115 | .019 | .725 | |
| BDI-C | −.068 | −.454 | .230 | .097 | .011 | .418 | |
| BMI** | −.460 | −3.039 | .421 | .297 | .191 | 9.236** |
Each model for the BDI scores included age, gender, and systolic blood pressure as the 1st step and plasma cortisol as the 2nd step. Then, BDI scores and BMI were entered as the 3rd step. Significance was determined at
p ≤ 0.05,
p ≤ 0.01,
p ≤ 0.001 for the predictors and the F change for each step.
notes p< .10.
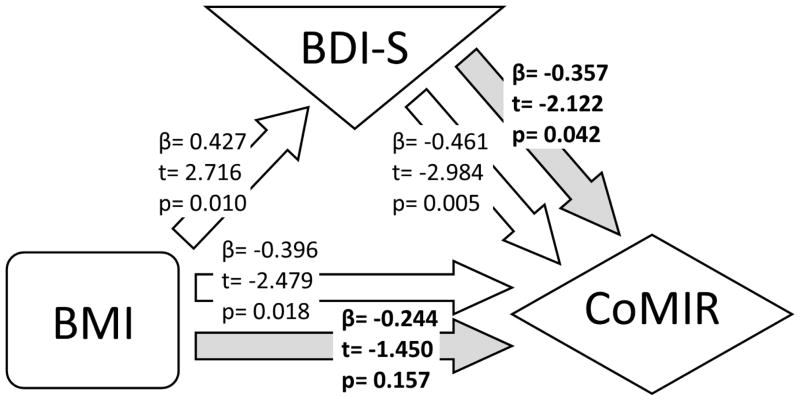
We further investigated whether BDI-S mediated the relationship between BMI and CoMIR by testing a series of univariate regression models followed by a model including both BDI-S and BMI, predicting CoMIR, based on the mediation analysis method by Baron and Kenny (Baron and Kenny, 1986). As shown in Figure 4, mediation analyses confirmed the results from multivariate regression models that BDI-S was a mediator in the BMI-CoMIR association.
Figure 4. Mediation analysis among BMI, BDI-S, and CoMIR.
Relationship between BMI, BDI-S, and CoMIR are displayed with results from a series of regression analyses, which are represented by arrows pointing from predictors to outcome variables. Bolded results are from regression model with both BDI-S and BMI as independent variables.
3.3. Pharmacologic Receptor Antagonism
3.3.1. GR vs. MR antagonism in cortisol inhibition of TNF production
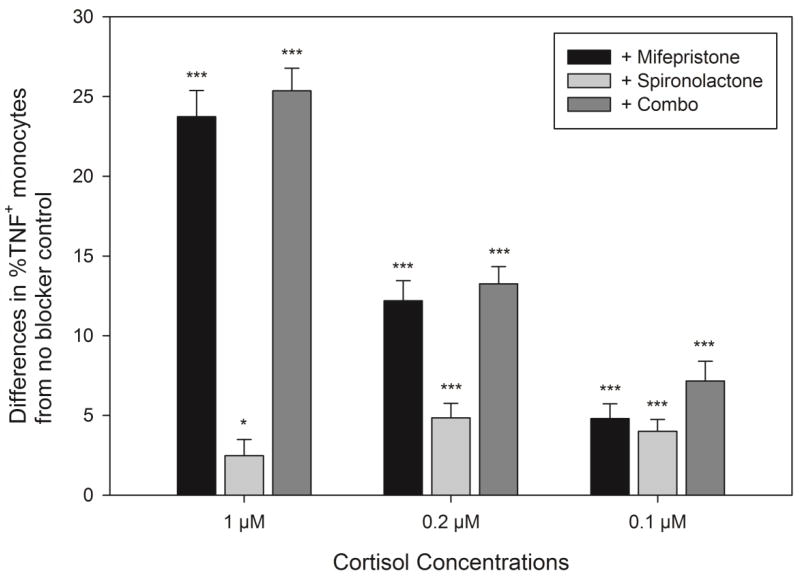
Repeated measures ANOVA with paired comparisons revealed that %TNF-producing monocytes in the mifepristone (GR blockade), spironolactone (MR blockade), and a combination of both blocker conditions were significantly greater than the cortisol-only condition (p’s between < 0.01 and 0.001), indicating significant antagonistic effects of the blockers (Figure 5). At the same time, the difference in %TNF-producing monocytes between control and MR blocker conditions was significantly smaller compared to the same difference between control and GR blocker or combination blocker conditions, indicating that the blocker effects of spironolactone were limited. The GR, MR, and the combination blockers resulted in increases in %TNF-producing monocytes from 25.9% with 1 μM cortisol treatment to 49.5%, 28.9%, and 51.3%, respectively. With differing cortisol doses, the relative antagonistic efficacy of mifepristone and spironolactone also differed. GR blockade reversed TNF-producing monocytes by 12.2 ± 5.8% at 0.2 μM and 4.8 ± 4.2% at 0.1 μM cortisol, whereas an MR blocker led to the recovery of 4.9 ± 4.3% and 4.0 ± 3.8% monocytes at 0.2 μM and 0.1 μM cortisol, respectively, indicating that the blocker effects of MR blocker appeared to be greater at lower cortisol doses. The combination of both blockers reversed the % monocytes by 13.2 ± 5.1% at 0.2 μM and 7.2 ± 5.5% at 0.1 μM cortisol.
Figure 5. Recovery of %TNF-producing monocytes by antagonists of GR (Mifepristone) and of MR (Spironolactone) from ex vivo treatment with three doses of cortisol.
Recovery is calculated as the difference in %TNF+ monocytes between cortisol only (without the blockers) and cortisol with antagonist(s) treatments. Significance was denoted with *p< 0.05 and ***p< 0.001 for the difference between each blocker condition and cortisol only treatment in %TNF-producing monocytes from pairwise comparisons with Bonferroni correction.
3.3.2. BMI and BDI associations with responses to cortisol and antagonists
Bivariate correlation analyses showed that GR blocker effects [Δ %mono TNFcort<sup>x</sup>= blocker – Cortx] were positively associated with BDI-C scores and also with BDI-T scores at a marginal level (Table 2). The effects of combined blockers were strongly and negatively associated with BMI and also negatively with BDI-S scores at a marginal level, indicating that greater obesity and somatic depressive symptoms were associated with smaller efficacy of the blockers. Multiple regression analysis showed that BMI was a significant predictor of the combined antagonist effects on CoMIR (β= −0.540, p= 0.036) even after controlling for demographic factors, SBP, plasma cortisol, and BDI-T. BMI was predictive of the combined antagonist effects on CoMIR at a marginal level when BDI-S or BDI-C with other covariates were controlled for in the model (β= −0.479, p= 0.051; β= −0.496, p= 0.056, respectively).
4. Discussion
In this investigation we showed that the immunomodulatory action of cortisol in suppression of LPS-stimulated monocytic production of TNF was significantly diminished in relation to depressive mood and obesity after controlling for covariates. Furthermore, obesity-related declines in cortisol-mediated regulation of inflammatory responses (“CoMIR”) were mediated by somatic depressive symptoms. On the contrary, in the absence of cortisol treatment monocytes of those with greater depressive mood produced less inflammatory cytokine TNF when exposed to LPS ex vivo, indicating that baseline inflammatory immune responses to an antigenic challenge was diminished among the participants who reported greater depressive mood. Thus, using an ex vivo cellular system, we uncovered that both functional immune responses and cortisol-mediated immune regulatory actions were impaired in individuals who reported elevated depressive mood or who were obese. Some cases of obesity-related chronic low-grade inflammation may be attributed to elevated somatic depressive symptoms and dysregulation of CoMIR of immune cells. These findings do not imply a causal relationship between depressive mood and obesity-related impairment in cortisol-mediated regulation of inflammatory processes, but they indicate CoMIR as a shared mechanism that underlies elevated inflammation in obesity and a subset of depression.
Our results are in agreement with previous studies that reported associations between depressive symptoms and GC insensitivity in functional assessment of immune cells. For example, impaired suppression of T-cell proliferation by dexamethasone was prospectively associated with greater depressive symptoms in deployed military personnel (van Zuiden et al., 2012) and multiple sclerosis patients (Fischer et al., 2012). However, the findings of TNF production in relation to GC sensitivity are limited; to our knowledge, no other reports are currently available in which intracellular monocytic TNF was measured under cortisol treatment to directly investigate cellular responses in a well-defined monocytic population. In addition, using cortisol at levels that resemble physiological values and a whole blood set-up, our ex vivo model highly mimicked the in vivo environment, whereas many previous studies used cortisol doses that were far beyond a physiological range or isolated cell subpopulations. Furthermore, we report that even healthy individuals without a clinical depression diagnosis, reporting mildly increased depressive symptoms, exhibit cortisol resistance in immune/inflammation modulation.
Of note, in comparison to lower TNF production by monocytes upon LPS stimulation in relation to greater depressive mood in our study, levels of various plasma inflammatory cytokines such as IL-6, transforming growth factor-1, and interferon-gamma were shown to be elevated in depression (Kim et al., 2007). Also, LPS-stimulated monocytes from depressed patients produced greater amounts of IL-6 and IL-1β but less prostaglandin E2, highlighting diverse regulatory pathways of inflammatory monocyte reactivity that may be impaired in depression (Lisi et al., 2013). These discrepancies in findings between these previous and our studies are likely due to differences in the immunological outcomes and methods of cellular investigations. Many previous reports of positive associations between baseline or stimulated inflammatory cytokine levels and clinical depression focused on plasma or mixed cell culture supernatant measurements of extracellular cytokines. However, we examined intracellular TNF expression by a carefully-defined cell population of monocytes based on phenotypic marker expression by flow cytometry, which is independent of differences in the composition of cell subpopulations. Findings of elevated circulating inflammatory cytokine levels in relation to depression or obesity indicate constant and systemic low-grade inflammation, while our findings further highlight that a key monocytic function in mounting immune responses to an endotoxin is impaired, coupled by reduced inflammation regulation via cortisol/GR. It is also imperative to note that only a subset of depressed patients present elevated inflammation (Kiecolt-Glaser et al., 2015; Raison and Miller, 2011; Raison et al., 2013), and the pathogenesis of this inflammation-related depression remains unclear. Thus, focused investigations to elucidate the mechanisms underlying the relationship between depression and inflammation and to scrutinize co-morbid conditions, including obesity, are in great need.
Findings of cellular signaling mechanisms underlying HPA and GR dysregulation in the context of inflammation reflect a complex picture of a bidirectional association that may be a perpetual loop. Peripheral blood monocytes of individuals who reported chronic stress exhibited reduced expression of response elements for GC but increased response elements for NFκ-B, a key pro-inflammatory transcription factor (Hayden and Ghosh, 2014). Conversely, effects of varying cytokines on the HPA axis were found at multiple levels, from GC secretion to GR translocation and post-translational modifications (Zunszain et al., 2011). For example, proinflammatory cytokine IL-1 can activate mitogen-activated protein kinase (MAPK) kinase, regulating GR phosphorylation via activation of c-Jun amino-terminal kinase or p38 MAPKs (Quan et al., 2003). Furthermore, deletion of the IL-1 receptor in mice preserved GC sensitivity compared to wildtype mice when both were exposed to social disruption stressors, indicating that IL-1 may be necessary to the development of GC resistance (Engler et al., 2008). TNF can also further promote inflammation via activation of inhibitor κ-B kinase β (IKKβ), which phosphorylates Iκ-B to translocate NFκ-B (Zhang et al., 2014). Phosphorylation of GR prevents nuclear translocation of NFκ-B, and NFκ-B prevents GR-DNA binding (Hayden and Ghosh, 2014; Pace and Miller, 2009). These findings strengthen the postulation that one pathway by which depression leads to inflammation dysregulation is insensitivity of the GRs on immune cells to GC, leading to dysregulation of cellular inflammatory activities, resulting in further GC insensitivity.
In this investigation we explored the receptor subtypes through which cortisol exerted its anti-inflammatory effects on peripheral blood monocytes. The involvement of MRs in inflammation regulation through cortisol was shown in this study and by others (Sauer et al., 1996). MR-mediated low-dose GC inhibition of macrophages attenuated immune activation (Lim et al., 2007), and expression of MRs on macrophages was suppressed by LPS antigen challenge (Barish et al., 2005). But, the existing evidence remains limited in the role of MRs in inflammation regulation relative to that of GRs. GR and MR share 94% in their DNA binding domains and could be expected to perform similar immunosuppressive functions in regulating expression of proinflammatory cytokines such as IL-6 and TNF (Zen et al., 2011). We found that blocking MR receptors on monocytes, using spironolactone, resulted in similar levels of blocker effects upon low-dose cortisol treatments but in limited levels of blocker effects at a higher level of cortisol dose (1μM, mimicking a level under stress) in comparison to the effects of a GR blocker. While MR and GR share 57% ligand binding homology (Arriza et al., 1987), the affinity for cortisol is greater for MR than for GR, resulting in greater cortisol-MR binding at lower levels of cortisol. The level of GR binding increases as cortisol levels rise above the resting levels (Zunszain et al., 2011). Thus, the immunomodulatory effects of HPA dysregulation in the context of depression or chronic stress are likely mediated by GRs more so than MRs. Our hypothesis, that the association between HPA dysregulation in depression and/or obesity and impaired CoMIR is GR-driven primarily, needs further verification in a larger sample-sized study, as our results of blocker efficacy in relation to BMI or BDI scores were not entirely conclusive. Our results provide initial insight into inflammation regulatory pathways mediated by GRs vs. MRs for targeted therapeutics.
We report that older age was consistently related to CoMIR in addition to depressive symptoms or BMI, indicating the impact of senescence on HPA regulation of inflammation. Previous studies show that immunosenescence has been associated with changes similarly seen in chronic stress or GC treatment such as diminished responses of lymphocytes from older individuals to ex vivo GC treatments (Bauer, 2005). Overall dysregulation of HPA axis is also seen in the elderly, where dexamethasone induced suppression of ACTH and GC production is reduced (Hatzinger et al., 2011). Although found insignificant in this study, gender may be related to CoMIR. Data in the literature are variable on gender and GC sensitivity; IL-6 and TNF production was higher, but cortisol sensitivity was lower in men (Wirtz et al., 2004). Oral contraceptive use was associated with greater GC sensitivity in response to a laboratory stressor in women (Rohleder et al., 2003), which could not be further explored in our study. In addition, as hypertension has been observed in hypercortisolemia (Nishikawa et al., 2013), BP is also a documented correlate with HPA dysregulation. Thus, certain demographic basic health characteristics should be considered in deciphering the depression-obesity-GC resistance.
A number of considerations exist in interpretations of our data and promising future directions. In spite of significant associations among depression, obesity, and CoMIR of moderate effect sizes, a limited sample size is a primary limitation of our study, especially for interpretations of our findings of GR vs. MR blocker effects in CoMIR in relation to depression and obesity. In addition, replication and expansion of our results in a larger sample would allow controlling for a more extensive list of covariates (e.g., socioeconomic status, physical activity levels, sleep pattern, etc.) and enhance the generalizability of our findings. Potential cortisol rhythm dysregulation among individuals experiencing greater depressive mood may have contributed to the lack of significant associations between plasma cortisol levels and depressive mood. This could have also been due to inter-subject variations in circadian rhythms of cortisol in our group of participants, whose plasma cortisol levels were sampled at a single timepoint (i.e., 8 – 10 AM). A cellular investigation at multiple timepoints of the day would provide further insight on this topic. From an assay and analytical perspective, a future study may further investigate individual’s cortisol-mediated TNF regulation pattern by using a wider range and a greater number of cortisol doses. The ability to obtain either IC50 or regression coefficients from a model of a good-fit, using analytical methods such as linear mixed modeling, may provide more comprehensive insight into cellular responses to cortisol in inflammation regulation. Lastly, individual differences for intermediates of the GR and MR signaling pathways likely also contributed to a greater variance in the proposed models of association; thus, molecular and additional intracellular protein investigations deserve further consideration in future studies. While molecular pathways of inflammation dysregulation in the intersection of obesity, depression, and cortisol insensitivity remain to be clarified, our findings indicate the significance of depressive mood in cortisol sensitivity in the context of inflammation regulation and its potential implications for the depression-obesity comorbidity.
Highlights.
We measured human monocyte’s inflammatory response and its suppression by cortisol.
We examined the link between obesity, depressive mood, and cortisol mediated regulation of inflammation in monocytes.
Depressive mood and obesity are both negatively associated with cortisol mediated suppression of inflammation.
The relationship between obesity and regulation of inflammation is mediated by depressive mood.
Acknowledgments
Supported by the research grants R01HL090975, American Recovery and Reinvestment Act (ARRA) HL090975-S, University of California San Diego (UCSD) Academic Senate Grant to SH, and UL1RR031980 for the UCSD Clinical and Translational Science Award from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, Listwak SJ, Ayala AR, Licinio J, Gold HK, Kling MA, Chrousos GP, Gold PW. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J Clin Endocrinol Metab. 2005;90:2522–30. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268–75. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- Barish GD, Downes M, Alaynick WA, Yu RT, Ocampo CB, Bookout AL, Mangelsdorf DJ, Evans RM. A Nuclear Receptor Atlas: macrophage activation. Mol Endocrinol. 2005;19:2466–77. doi: 10.1210/me.2004-0529. [DOI] [PubMed] [Google Scholar]
- Baron R, Kenny D. The moderator-mediator variable distinction in social psychological research. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bauer ME. Stress, glucocorticoids and ageing of the immune system. Stress. 2005;8:69–83. doi: 10.1080/10253890500100240. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bose M, Oliván B, Laferrère B. Stress and obesity: the role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Curr Opin Endocrinol Diabetes Obes. 2009;16:340–6. doi: 10.1097/MED.0b013e32832fa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey M, Small H, Yoong SL, Boyes A, Bisquera A, Sanson-Fisher R. Prevalence of comorbid depression and obesity in general practice: a cross-sectional survey. Br J Gen Pract. 2014;64:e122–7. doi: 10.3399/bjgp14X677482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho LA, Bergink V, Sumaski L, Wijkhuijs J, Hoogendijk WJ, Birkenhager TK, Drexhage HA. Inflammatory activation is associated with a reduced glucocorticoid receptor alpha/beta expression ratio in monocytes of inpatients with melancholic major depressive disorder. Transl Psychiatry. 2014;4:e344. doi: 10.1038/tp.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho LA, Pariante CM. In vitro modulation of the glucocorticoid receptor by antidepressants. Stress. 2008;11:411–24. doi: 10.1080/10253890701850759. [DOI] [PubMed] [Google Scholar]
- Chapman K, Coutinho AE, Zhang Z, Kipari T, Savill JS, Seckl JR. Changing glucocorticoid action: 11β-hydroxysteroid dehydrogenase type 1 in acute and chronic inflammation. J Steroid Biochem Mol Biol. 2013a;137:82–92. doi: 10.1016/j.jsbmb.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K, Holmes M, Seckl J. 11β-hydroxysteroid dehydrogenases: intracellular gatekeepers of tissue glucocorticoid action. Physiol Rev. 2013b;93:1139–206. doi: 10.1152/physrev.00020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. Cumulative depression episodes predict later C-reactive protein levels: A prospective analysis. Biol Psychiatry. 2012;71:15–21. doi: 10.1016/j.biopsych.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S, Shaikh F, Pruitt C, Green M, Wilson K, Beg N, Hong S. Differential TNF production by monocyte subsets under physical stress: blunted mobilization of proinflammatory monocytes in prehypertensive individuals. Brain Behav Immun. 2013;27:101–8. doi: 10.1016/j.bbi.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Engler H, Bailey MT, Engler A, Stiner-Jones LM, Quan N, Sheridan JF. Interleukin-1 receptor type 1-deficient mice fail to develop social stress-associated glucocorticoid resistance in the spleen. Psychoneuroendocrinology. 2008;33:108–17. doi: 10.1016/j.psyneuen.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Otte C, Krieger T, Nicholls RA, Krüger S, Ziegler KJ, Schulz KH, Heesen C, Gold SM. Decreased hydrocortisone sensitivity of T cell function in multiple sclerosis-associated major depression. Psychoneuroendocrinology. 2012;37:1712–8. doi: 10.1016/j.psyneuen.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2004;164:1010–4. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Miller GE. Where there is depression, there is inflammation . sometimes! Biol Psychiatry. 2007;62:280–1. doi: 10.1016/j.biopsych.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Hatzinger M, Brand S, Herzig N, Holsboer-Trachsler E. In healthy young and elderly adults, hypothalamic-pituitary-adrenocortical axis reactivity (HPA AR) varies with increasing pharmacological challenge and with age, but not with gender. J Psychiatr Res. 2011;45:1373–80. doi: 10.1016/j.jpsychires.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Regulation of NF-κB by TNF family cytokines. Semin Immunol. 2014;26:253–66. doi: 10.1016/j.smim.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo M, Pietrobelli A, Fontaine KR, Sirey JA, Faith MS. Depressive mood and obesity in US adults: comparison and moderation by sex, age, and race. Int J Obes. 2006;30:513–519. doi: 10.1038/sj.ijo.0803122. [DOI] [PubMed] [Google Scholar]
- Hong S, Dimitrov S, Pruitt C, Shaikh F, Beg N. Benefit of physical fitness against inflammation in obesity: role of beta adrenergic receptors. Brain Behav Immun. 2014;39:113–20. doi: 10.1016/j.bbi.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw EE, Morton NM, Dhillon H, Ramage L, Seckl JR, Flier JS. Adipocyte-specific glucocorticoid inactivation protects against diet-induced obesity. Diabetes. 2005;54:1023–31. doi: 10.2337/diabetes.54.4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: Depression Fans the Flames and Feasts on the Heat. Am J Psychiatry. 2015;172:1075–1091. doi: 10.1176/appi.ajp.2015.15020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Na KS, Shin KH, Jung HY, Choi SH, Kim JB. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1044–53. doi: 10.1016/j.pnpbp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta. 2014;1842:473–81. doi: 10.1016/j.bbadis.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HY, Müller N, Herold MJ, van den Brandt J, Reichardt HM. Glucocorticoids exert opposing effects on macrophage function dependent on their concentration. Immunology. 2007;122:47–53. doi: 10.1111/j.1365-2567.2007.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisi L, Camardese G, Treglia M, Tringali G, Carrozza C, Janiri L, Dello Russo C, Navarra P. Monocytes from depressed patients display an altered pattern of response to endotoxin challenge. PLoS One. 2013;8:e52585. doi: 10.1371/journal.pone.0052585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Nakagawa Y, Wang Y, Liu L, Du H, Wang W, Ren X, Lutfy K, Friedman TC. Reduction of hepatic glucocorticoid receptor and hexose-6-phosphate dehydrogenase expression ameliorates diet-induced obesity and insulin resistance in mice. J Mol Endocrinol. 2008;41:53–64. doi: 10.1677/JME-08-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longui CA, Giusti MMCG, Calliari LEP, Katiki T, Kochi C, Monte O. Partial glucocorticoid resistance in obese children detected by very low dose dexamethasone suppression test. J Pediatr Endocrinol Metab. 2003;16:1277–82. doi: 10.1515/jpem.2003.16.9.1277. [DOI] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH, Zitman FG. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–9. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- Mattsson C, Reynolds RM, Simonyte K, Olsson T, Walker BR. Combined receptor antagonist stimulation of the hypothalamic-pituitary-adrenal axis test identifies impaired negative feedback sensitivity to cortisol in obese men. J Clin Endocrinol Metab. 2009;94:1347–52. doi: 10.1210/jc.2008-2054. [DOI] [PubMed] [Google Scholar]
- McInnis CM, Thoma MV, Gianferante D, Hanlin L, Chen X, Breines JG, Hong S, Rohleder N. Measures of adiposity predict interleukin-6 responses to repeated psychosocial stress. Brain Behav Immun. 2014;42:33–40. doi: 10.1016/j.bbi.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhtari M, Arfken C, Boutros N. The DEX/CRH test for major depression: a potentially useful diagnostic test. Psychiatry Res. 2013;208:131–9. doi: 10.1016/j.psychres.2012.09.032. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–6. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- Nikkheslat N, Zunszain PA, Horowitz MA, Barbosa IG, Parker JA, Myint AM, Schwarz MJ, Tylee AT, Carvalho LA, Pariante CM. Insufficient glucocorticoid signaling and elevated inflammation in coronary heart disease patients with comorbid depression. Brain Behav Immun. 2015;48:8–18. doi: 10.1016/j.bbi.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Omura M, Saito J, Matsuzawa Y. The possibility of resistant hypertension during the treatment of hypertensive patients. Hypertens Res. 2013;36:924–9. doi: 10.1038/hr.2013.107. [DOI] [PubMed] [Google Scholar]
- Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158:1139–47. doi: 10.1093/aje/kwg275. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci. 2009;1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova A, Kzhyshkowska J, Nurgazieva D, Goerdt S, Gratchev A. Pro- and anti-inflammatory control of M-CSF-mediated macrophage differentiation. Immunobiology. 2011;216:164–72. doi: 10.1016/j.imbio.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Lai W, Dhabhar F, Sheridan JF. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. J Neuroimmunol. 2003;137:51–8. doi: 10.1016/s0165-5728(03)00042-0. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13:467–75. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, Wolf JM, Piel M, Kirschbaum C. Impact of oral contraceptive use on glucocorticoid sensitivity of pro-inflammatory cytokine production after psychosocial stress. Psychoneuroendocrinology. 2003;28:261–73. doi: 10.1016/s0306-4530(02)00019-7. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Wolf JM, Wolf OT. Glucocorticoid sensitivity of cognitive and inflammatory processes in depression and posttraumatic stress disorder. Neurosci Biobehav Rev. 2010;35:104–14. doi: 10.1016/j.neubiorev.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Sauer J, Castren M, Hopfner U, Holsboer F, Stalla GK, Arzt E. Inhibition of lipopolysaccharide-induced monocyte interleukin-1 receptor antagonist synthesis by cortisol: involvement of the mineralocorticoid receptor. J Clin Endocrinol Metab. 1996;81:73–9. doi: 10.1210/jcem.81.1.8550797. [DOI] [PubMed] [Google Scholar]
- Schäfer HH, de Villiers JD, Sivukhina E, Lewis J, Wande D, Perembe B, Jirikowski G. Altered homeostasis of systemic glucocorticoids as related to obesity, glucose tolerance, and smoking. Horm Metab Res. 2013;45:245–51. doi: 10.1055/s-0032-1323741. [DOI] [PubMed] [Google Scholar]
- Simic I, Maric NP, Mitic M, Soldatovic I, Pavlovic Z, Mihaljevic M, Andric S, Radojcic MB, Adzic M. Phosphorylation of leukocyte glucocorticoid receptor in patients with current episode of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:281–5. doi: 10.1016/j.pnpbp.2012.10.021. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med. 2011;73:114–26. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Udina M, Castellví P, Moreno-España J, Navinés R, Valdés M, Forns X, Langohr K, Solà R, Vieta E, Martín-Santos R. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73:1128–38. doi: 10.4088/JCP.12r07694. [DOI] [PubMed] [Google Scholar]
- Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150:736–44. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- van der Merwe MT. Psychological correlates of obesity in women. Int J Obes (Lond) 2007;31(Suppl 2):S14–8. doi: 10.1038/sj.ijo.0803731. discussion S31–2. [DOI] [PubMed] [Google Scholar]
- van Zuiden M, Heijnen CJ, Maas M, Amarouchi K, Vermetten E, Geuze E, Kavelaars A. Glucocorticoid sensitivity of leukocytes predicts PTSD, depressive and fatigue symptoms after military deployment: A prospective study. Psychoneuroendocrinology. 2012;37:1822–36. doi: 10.1016/j.psyneuen.2012.03.018. [DOI] [PubMed] [Google Scholar]
- van Zuiden M, Kavelaars A, Vermetten E, Olff M, Geuze E, Heijnen C. Pre-deployment differences in glucocorticoid sensitivity of leukocytes in soldiers developing symptoms of PTSD, depression or fatigue persist after return from military deployment. Psychoneuroendocrinology. 2015;51:513–24. doi: 10.1016/j.psyneuen.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Varga G, Ehrchen J, Brockhausen A, Weinhage T, Nippe N, Belz M, Tsianakas A, Ross M, Bettenworth D, Spieker T, Wolf M, Lippe R, Tenbrock K, Leenen PJM, Roth J, Sunderkötter C. Immune suppression via glucocorticoid-stimulated monocytes: a novel mechanism to cope with inflammation. J Immunol. 2014;193:1090–9. doi: 10.4049/jimmunol.1300891. [DOI] [PubMed] [Google Scholar]
- Wallerius S, Rosmond R, Ljung T, Holm G, Björntorp P. Rise in morning saliva cortisol is associated with abdominal obesity in men: a preliminary report. J Endocrinol Invest. 2003;26:616–9. doi: 10.1007/BF03347017. [DOI] [PubMed] [Google Scholar]
- Wirtz PH, Ehlert U, Emini L, Suter T. Higher body mass index (BMI) is associated with reduced glucocorticoid inhibition of inflammatory cytokine production following acute psychosocial stress in men. Psychoneuroendocrinology. 2008;33:1102–10. doi: 10.1016/j.psyneuen.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Wirtz PH, von Känel R, Rohleder N, Fischer JE. Monocyte proinflammatory cytokine release is higher and glucocorticoid sensitivity is lower in middle aged men than in women independent of cardiovascular risk factors. Heart. 2004;90:853–8. doi: 10.1136/hrt.2002.003426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen M, Canova M, Campana C, Bettio S, Nalotto L, Rampudda M, Ramonda R, Iaccarino L, Doria A. The kaleidoscope of glucorticoid effects on immune system. Autoimmun Rev. 2011;10:305–10. doi: 10.1016/j.autrev.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Zhang J, Clark K, Lawrence T, Peggie MW, Cohen P. An unexpected twist to the activation of IKKβ: TAK1 primes IKKβ for activation by autophosphorylation. Biochem J. 2014;461:531–7. doi: 10.1042/BJ20140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:722–9. doi: 10.1016/j.pnpbp.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]