Abstract
Articular cartilage undergoes matrix degradation and loss of mechanical properties when stimulated with proinflammatory cytokines such as interleukin-1 (IL-1). Aggrecanases and matrix metalloproteinases (MMPs) are thought to be principal downstream effectors of cytokine-induced matrix catabolism, and aggrecanase- or MMP-selective inhibitors reduce or block matrix destruction in several model systems. The objective of this study was to use metalloproteinase inhibitors to perturb IL-1-induced matrix catabolism in bovine cartilage explants and examine their effects on changes in tissue compression and shear properties. Explanted tissue was stimulated with IL-1 for up to 24 days in the absence or presence of inhibitors which were aggrecanase-selective, MMP-selective, or non-selective. Analysis of conditioned media and explant digests revealed that aggrecanase-mediated aggrecanolysis was delayed to varying extents with all inhibitor treatments, but that aggrecan release persisted. Collagen degradation was abrogated by MMP- and non-selective inhibitors and reduced by the aggrecanase inhibitor. The inhibitors delayed but did not reduce loss of the equilibrium compression modulus, whereas the loss of dynamic compression and shear moduli was delayed and reduced. The data suggest that non-metalloproteinase mechanisms participate in IL-1-induced matrix degradation and loss of tissue material properties.
Keywords: Cartilage, Degradation, Mechanical properties, Interleukin-1, Aggrecanase, MMP
Introduction
Articular cartilage provides a low-friction surface for joint motion, and disease or damage to the tissue causes chronic pain and loss of joint function. The dense, highly hydrated extracellular matrix (ECM) of articular cartilage is composed primarily of water, type II collagen, and aggrecan. The aggrecan core protein bears a large number of the sulfated glycosaminoglycans (sGAG) such as chondroitin sulfate and keratan sulfate. Aggregation of aggrecan monomers on hyaluronan chains entangled in the collagen network results in a high matrix fixed charge density and generates an osmotic swelling pressure that resists compression during joint loading (Eisenberg and Grodzinsky, 1985). Aggrecan has also been shown to contribute to the shear properties of the tissue (Jin and Grodzinsky, 2001). Progressive tissue degeneration in vivo is marked by release of aggrecan from the cartilage ECM and loss of compression and shear properties.
Proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin 1 alpha and beta (IL-1α,β), stimulate chondrocytes to degrade cartilage aggrecan and collagen through the production of activated aggrecanases (such ADAMTS-4 and -5) and collagenases (such as MMP-13), repectively. Many cartilage explant studies have shown that aggrecanolysis preceeds collagenolysis (Billinghurst, et al., 1997; Kuroki, et al., 2005; Pratta, et al., 2003b; Zwerina, et al., 2004), and it has been suggested (Pratta, et al., 2003b) that aggrecan protects the collagen network from proteolytic attack.
Proinflammatory cytokines also shut down synthesis of matrix molecules, exacerbating their disruption of homeostatic ECM remodeling. The physiologic importance of these findings is that IL-1 can be detected in degenerative cartilage (Towle, et al., 1997), and is present in synovial fluid at concentrations ranging from tens of picograms to nanograms per milliliter (Fontana, et al., 1982; Hopkins, et al., 1988). IL-1, therefore, has been implicated in the progression of arthritis, and in vitro stimulation of explanted cartilage with IL-1 has demonstrated utility as a model of the catabolic events leading to cartilage resorption.
Matrix remodeling in articular cartilage is mediated in part by the MMPs. Several MMPs, including the collagenases MMP-1, -8, and -13, MMP-3 (stromelysin-1), the gelatinases MMP-2 and -9, and membrane type MMP-14 and -17, are expressed in articular c artilage. Active MMPs readily degrade type II collagen and aggrecan (Beekman, et al., 1998; Koshy, et al., 2002), and exogenous MMPs were shown to modulate the composition and material properties of bovine cartilage explants (Bonassar, et al., 1996). The primary substrate for MMPs on the aggrecan core protein is within the interglobular domain (IGD) at the VIPEN360—361FFG bond (the equivalent substrate in bovine aggrecan is VDIPES360—361FFG) (Flannery, et al., 1992). Importantly, MMP-mediated aggrecan catabolism appears to operate independent of aggrecanases and is thought to be a quantitatively minor mechanism of aggrecan degradation in injured or osteoarthritic cartilage (Fosang, et al., 1996; Little, et al., 1999; Sandy, et al., 1992; Sandy and Verscharen, 2001).
Chondrocytes express aggrecanases of the ADAMTS family of enzymes (including ADAMTS-1, -4, -5, -8, -9, and -15). Proinflammatory stimuli may upregulate transcription of aggrecanase genes (Bau, et al., 2002; Koshy, et al., 2002), and there is mounting evidence for substantial post-translational processing of the enzymes that alter aggrecanase activity and specificity (Pratta, et al., 2003a). In human chondrosarcoma cells and bovine cartilage explants, MMP-17 (MT4-MMP) appears to be responsible for C-terminal truncation of ADAMTS-4, a process which converts the enzyme from one which can cleave only the sGAG-rich region to one which can also cleave the interglobular domain (IGD) (Gao, et al., 2004; Patwari, et al., 2005). Aggrecanase activity within the IGD is marked by scission of the NITEGE392—393ARGSVI bond (Sandy, et al., 1991). ADAMTS-4 and -5 (aggrecanase-1 and -2, respectively) appear to mediate the bulk of destructive sGAG release from osteoarthritic human cartilage explants (Arner, et al., 1999), and ADAMTS-5 is primarily responsible for destructive aggrecanolysis in the mouse (Stanton, et al., 2005).
MMPs, aggrecanases, and their post-translational activation mechanisms are obvious targets for clinical intervention in arthritis, and many natural and synthetic inhibitors have been investigated for potential therapeutic use (Chan, et al., 2005; Close, 2001). Indeed, a broad spectrum metalloproteinase inhibitor was found to reduce aggrecan depletion and loss of material properties in IL-1-stimulated cartilage explants (Bonassar, et al., 1997). Inhibitors of glycophosphatidyl inositol-anchor formation, including mannosamine and glucosamine, interfere with MMP-17-mediated activation of ADAMTS-4 and reduce IL-1-induced sGAG release and loss of material properties in cartilage explants (Patwari, et al., 2000). Synthetic aggrecanase inhibitors can delay sGAG and collagen release from IL-1-stimulated nasal cartilage, and preservation of aggrecan using an aggrecanase inhibitor protected the collagen network from proteolytic attack (Pratta, et al., 2003b). Collectively, these studies suggest that inhibitors of aggrecanases specifically or metalloproteinases generally (ADAMTSs and MMPs) can attenuate cell-mediated aggrecan catabolism and loss of tissue function associated with arthritic disease.
While several reports have shown that metalloproteinase inhibitors can abrogate IL-1-induced cartilage degradation, non-metalloproteinase pathways can also be quantitatively important. For example, Sugimoto et al. demonstrated that a broad spectrum inhibitor of MMPs and aggrecanases perturbed, but did not block, loss of aggrecan from IL-1-stimulated cartilage explants and the authors concluded that IL-1 was stimulating hyaluronidase activity (Sugimoto, et al., 2004). In other work, it was also concluded that depolymerization of hyaluronic acid may contribute to extrusion of aggrecan from diseased or injured tissue (Sztrolovics, et al., 2002). The effects of aggrecan depletion by metalloproteinase-independent pathways on changes on the material properties of cartilage, however, have not been characterized. Studies coupling analysis of molecular level changes in extracellular matrix with tissue level changes in matrix mechanical property are useful for evaluating the therapeutic potential of metalloproteinase inhibitors and permit investigation of the relationships between matrix composition, structure, and function. The objective of the current study was to examine the time–course of ECM catabolism and loss of mechanical properties in IL-1-stimulated articular cartilage explants treated with selective or non-selective metalloproteinase inhibitors. These studies show that inhibition of MMPs and/or aggrecanases does not effectively block IL-1-induced ECM destruction and support the idea that other enzymes, such as hyaluronidase, participate in aggrecan degradation and loss of tissue function.
Results
Selective and non-selective (NS) metalloproteinase inhibitors were used to perturb the catabolic cascade and progressive loss of tissue function in a well-established bovine cartilage explant model. Inhibitor selectivities, determined by recombinant enzyme-fluorescent substrate assays and ELISA, are summarized in Table 1 as concentrations of half-m aximal inhibition (IC50). The MMP-selective inhibitor effectively blocked (IC50<50nM) the collagenases MMP-8 and MMP-13, the gelatinase MMP-2, MMP-3, and the membrane-type MMPs-14 and -17, but it had weaker activity (IC50>1200nM) against MMP-1, MMP-7, and ADAMTS-4. The aggrecanase-selective inhibitor was ineffective (IC50>5600nM) against most MMPs, partially effective (IC50~710nM) against MMP-14 and highly inhibitory (IC50~8nM) against ADAMTS-4. The non-selective metalloproteinase inhibitor was highly inhibitory (IC50<7.5nM) to MMPs-2,3,8,9,13,14, and 17 and ADAMTS-4 and partially effective (IC50>260nM) against MMPs-1 and 7.
Table. 1. Inhibitor IC50s.
Inhibitors demonstrate differential selectivity for MMPs and aggrecanases. Inhibitor selectivities, indicated by concentrations of half maximal inhibition (IC50, in nM), were determined by recombinant enzyme-fluorescent substrate assay (MMPs) and ELISA (ADAMTS-4).
| Aggrecanase Inhibitor RO-3310769 |
MMP Inhibitor RO-1136222 |
Non-Selective Inhibitor RO-4002855 |
|
|---|---|---|---|
| ADAMTS-4 | 8 | 7200 | 1.5 |
| MMP-1 | 6500 | 1800 | 260 |
| MMP-8 | 5600 | 4 | 1.7 |
| MMP-13 | 5720 | 0.61 | 2.4 |
| MMP-2 | NT | 0.22 | 0.28 |
| MMP-9 | NT | NT | 7.5 |
| MMP-3 | NT | 0.52 | 3.4 |
| MMP-7 | 16000 | 1200 | 2400 |
| MMP-14 | 710 | 0.32 | 0.87 |
| MMP-17 | >10,000 | 50 | 0.6 |
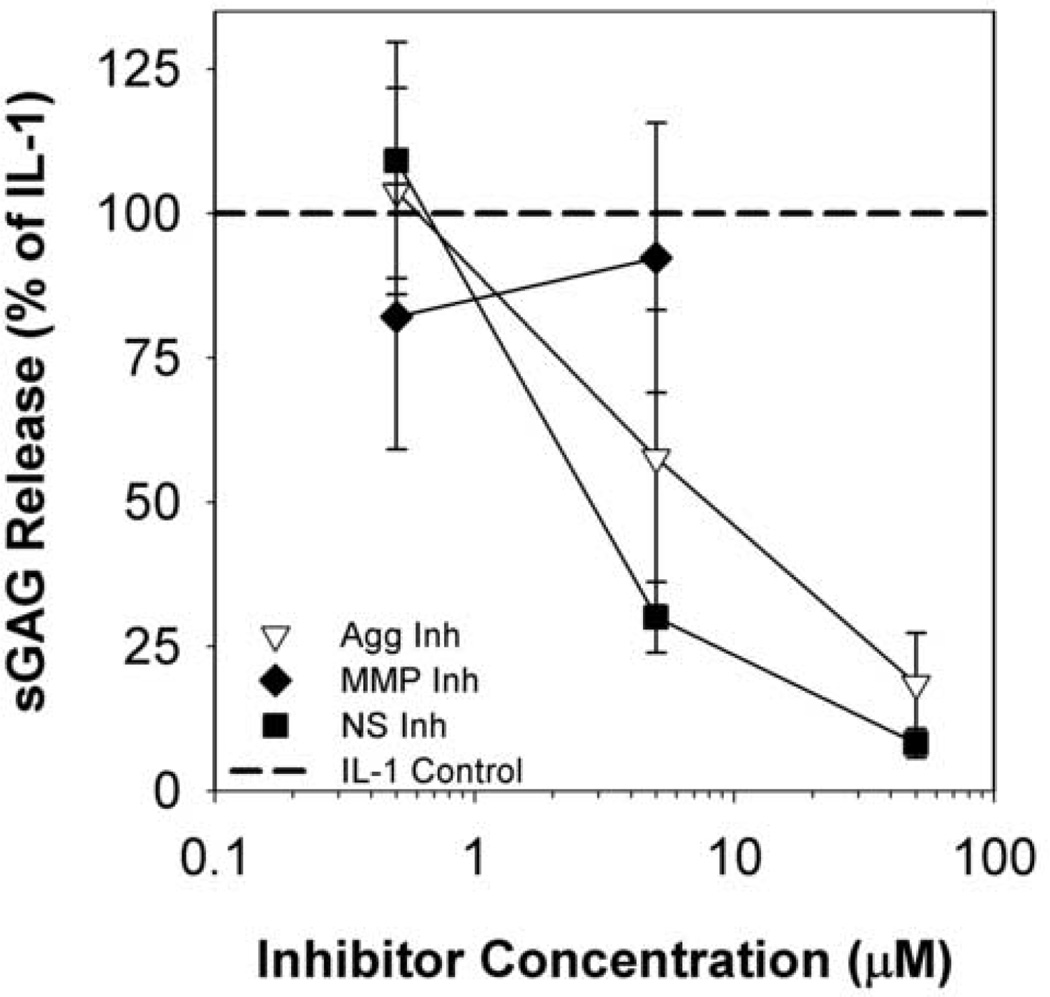
As predicted from the selectivity assay IC50 data, the inhibitors exhibited different effects on IL-1-induced aggrecan release (as indicated by sGAG release to media) from explanted bovine cartilage (Fig. 1). The aggrecanase-selective and non-selective compounds demonstrated dose-dependent inhibition of IL-1-induced aggrecan release in the 0.5–50µM range (Fig. 1), and this was not due to cytotoxic effects as shown by Live/Dead staining (data not shown). The MMP-selective inhibitor had no effect on sGAG release over 8 days at 0.5–5µM and was cytotoxic at 50µM. Based on these results, we carried out kinetic release studies from cartilage explants for 24 days at a concentration of 5µM for the MMP- and non-selective inhibitors and a concentration of 20µM for the aggrecanase-selective inhibitor. The aggrecanase inhibitor was used at a four-fold higher concentration than the non-selective inhibitor to account for differences in inhibitor potency against ADAMTS-4 (in substrate assays) and IL-1-induced sGAG release (in explant studies).
Fig 1. Dose Response sGAG release.
Metalloproteinase inhibitors demonstrate dose-dependent reduction in IL-1-stimulated sGAG release. Articular cartilage explants were stimulated with 20ng/mL rhIL-1α and treated with 0.05, 5, or 50µM inhibitor for 8 days. Cumulative sGAG release was measured by the DMMB-dye binding assay. Data are mean +/− SEM, n = 5. Cytotoxicity was observed at 50µM for the MMP-selective inhibitor.
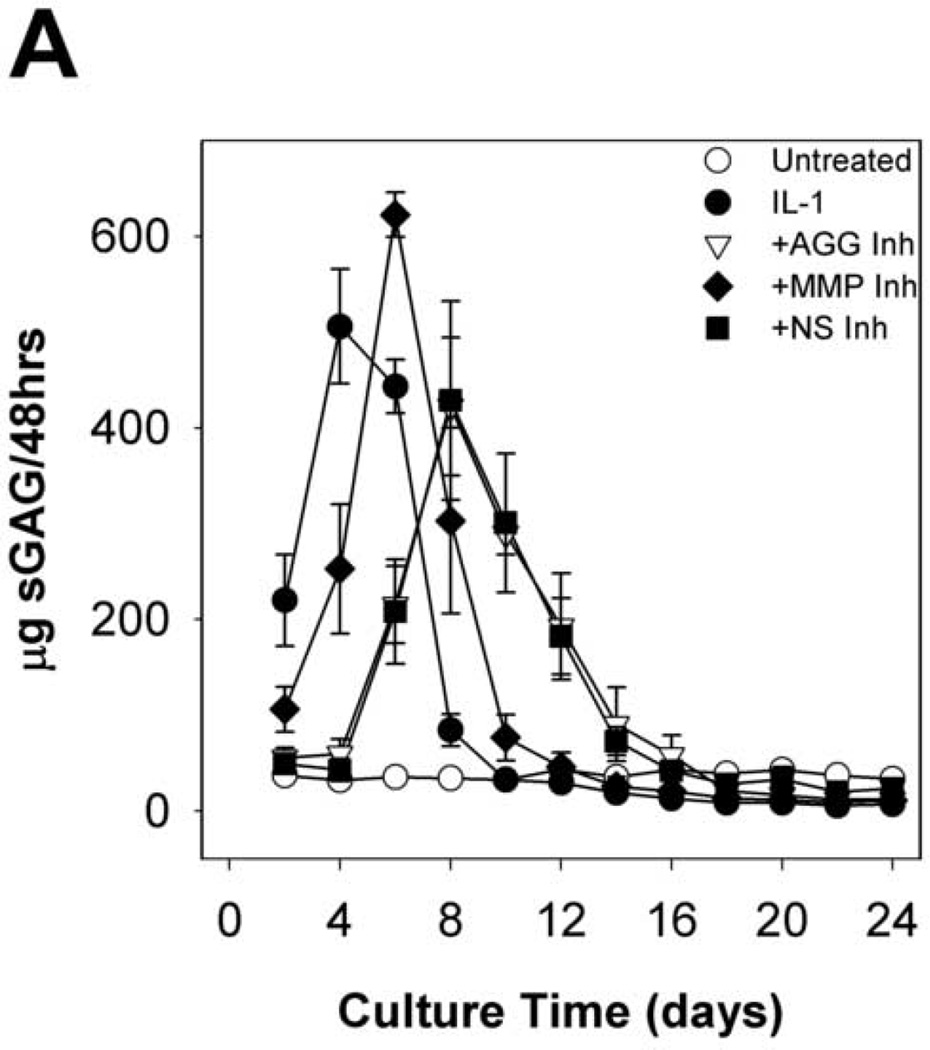
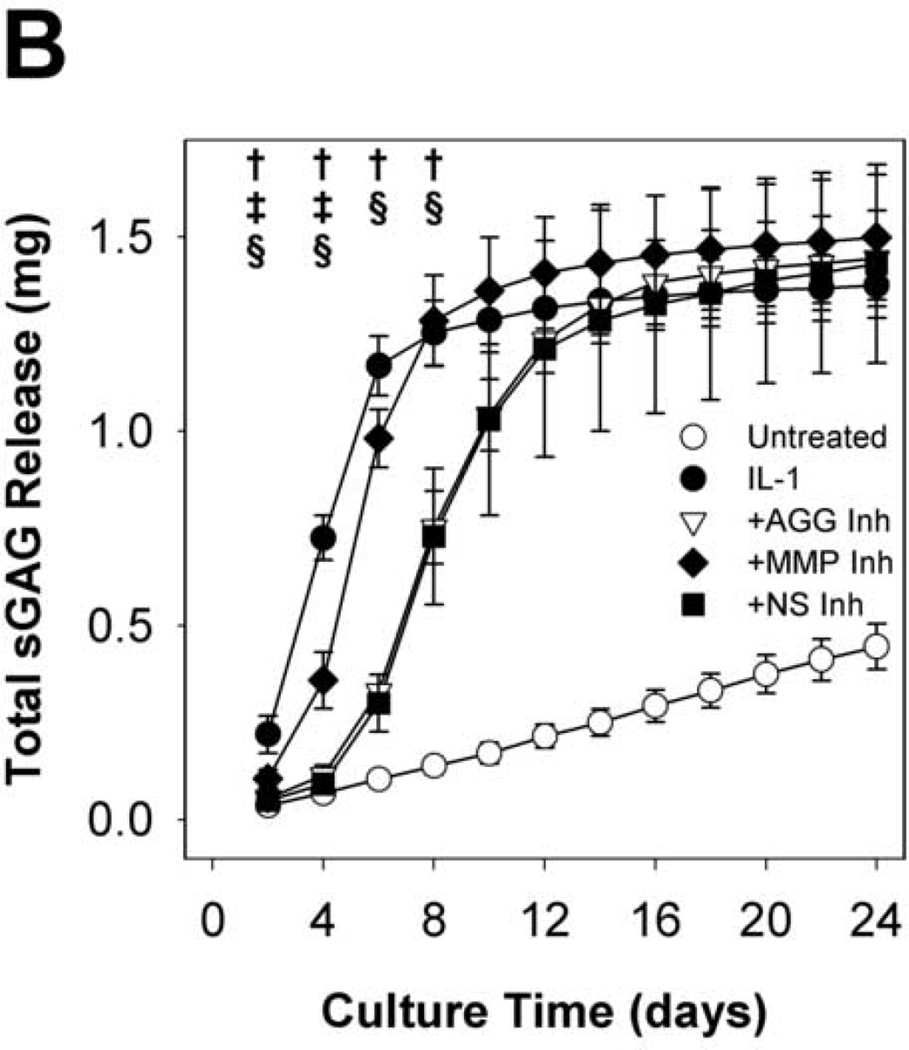
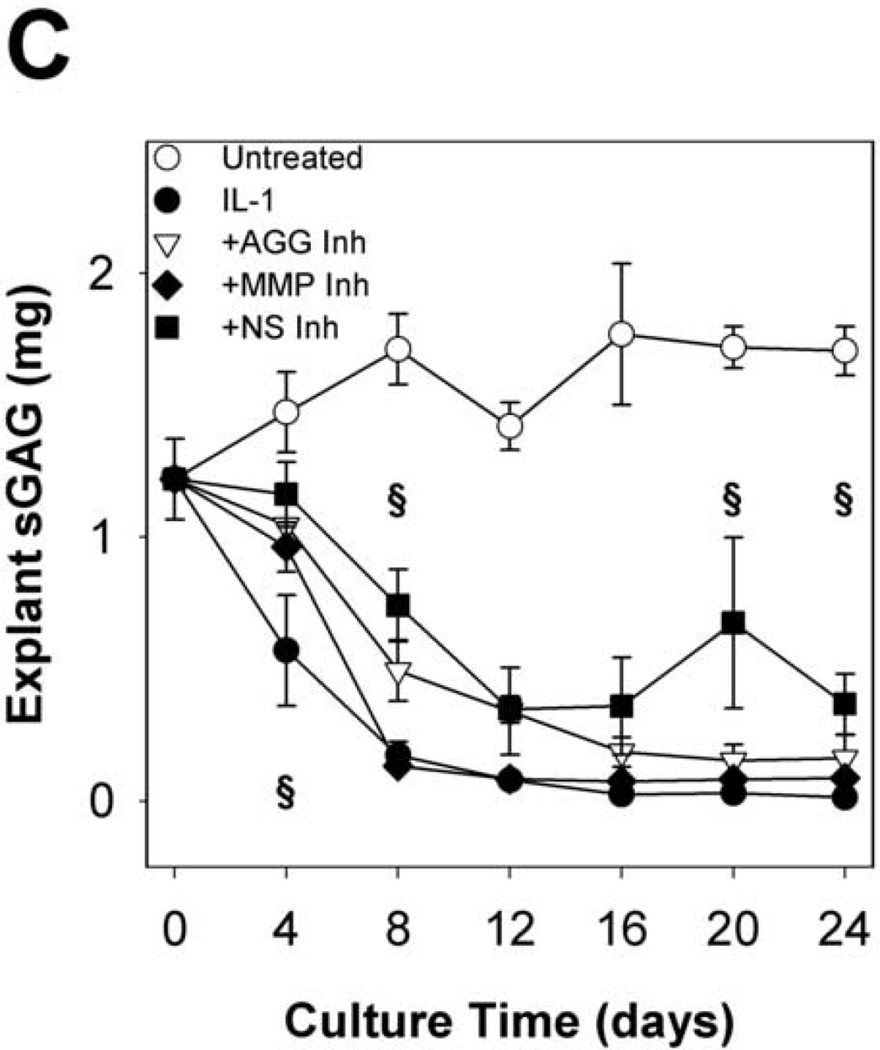
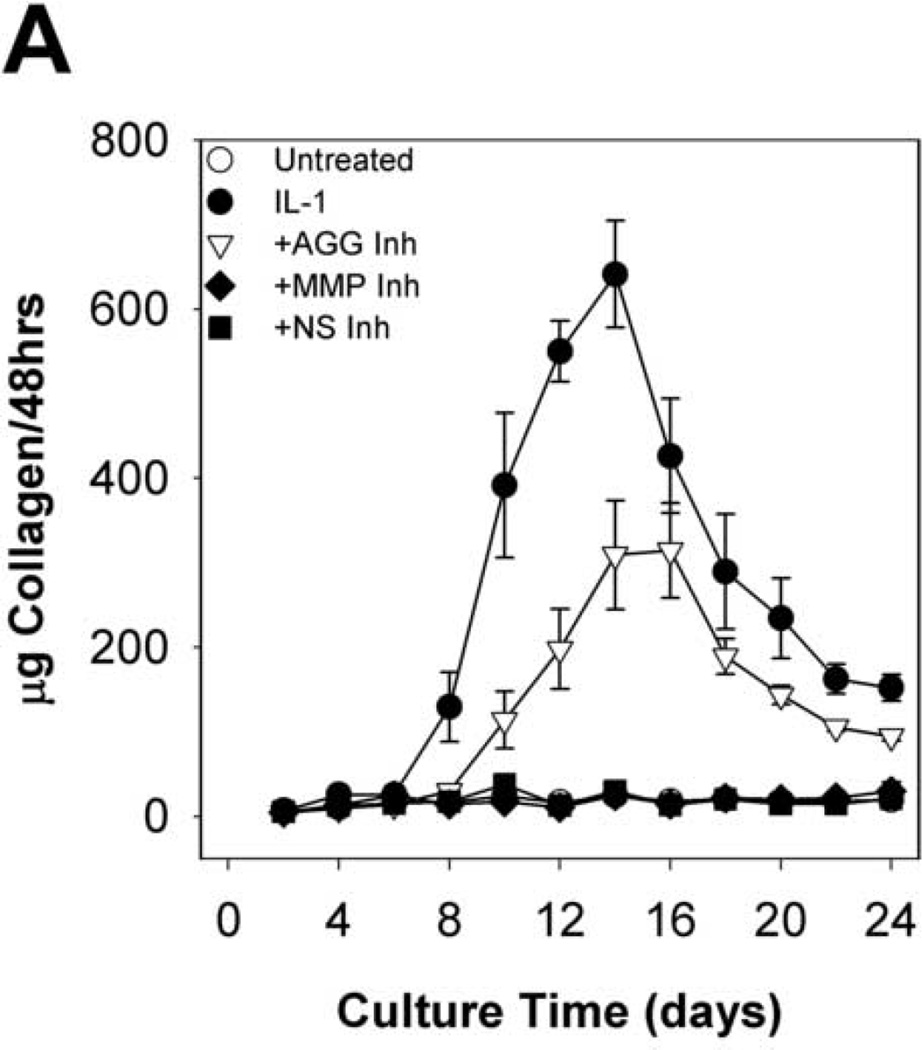
Time courses of sGAG release to the media (per 48h in Fig. 2A cumulative in Fig. 2B) and explant sGAG content (Fig. 2C) were used to assess aggrecan degradation. As expected, IL-1α treatment caused rapid (peak rates in days 2–4) and extensive aggrecan release with nearly complete depletion (~93%) by day 8. The MMP-selective inhibitor delayed the peak rate of IL-1-induced release (from days 2–4 to days 4–6) but did not affect the extent of depletion (~93%) at day 8. The aggrecanase inhibitor caused longer delays (from days 2–4 to days 6–8), but it had no significant effect on the extent of depletion after 24 days (Fig.2C). The non-selective inhibitor caused a delay which was almost identical to the aggrecanase inhibitor, and it reduced the extent of depletion by about 25% relative to IL-1.
Fig 2. sGAG release and explant sGAG.
Metalloproteinase inhibitors delay, but do not block, IL-1-stimulated proteoglycan degradation. sGAG content of conditioned media (A, B) and explant digests (C) was measured by the DMMB dye-binding assay. Data are mean +/− SEM, n = 5. In B and C, †, ‡, § = p<0.05 vs. IL-1 for AGG Inh, MMP Inh, and NS Inh, respectively.
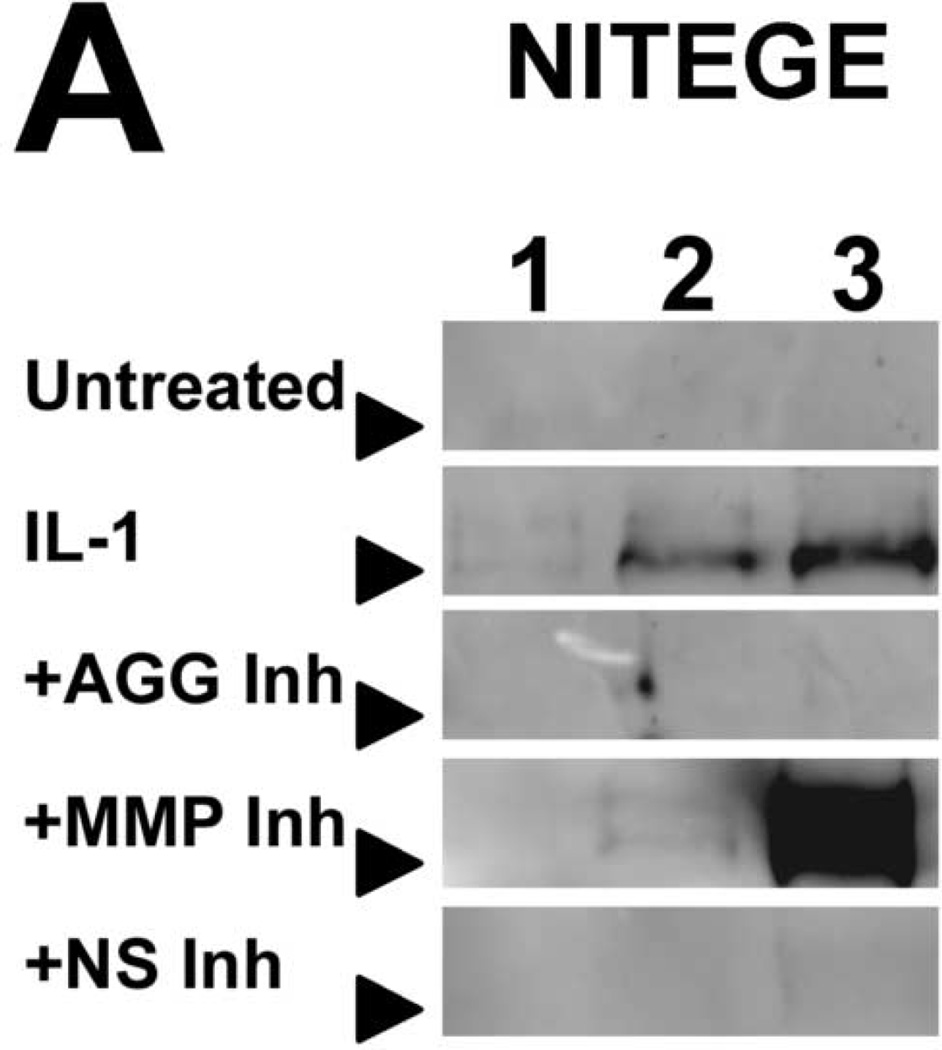
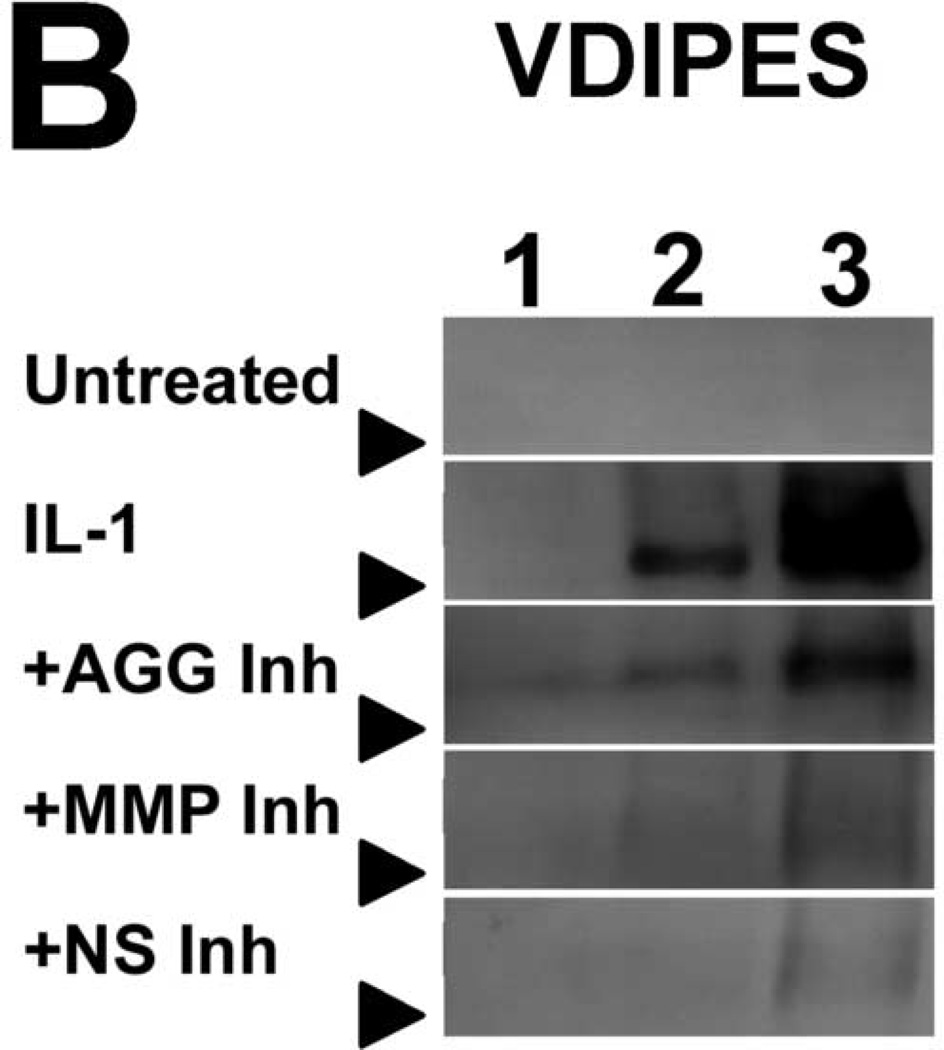
To characterize IL-1-induced aggrecan degradation in the presence of selective- and non-selective metalloproteinase inhibitors, conditioned media were immunoblotted for fragments of aggrecan core protein (Fig. 3). Conditioned media of IL-1-stimulated tissue, but not of untreated tissue, contained aggrecan species migrating at 50kDa and 65–70kDa corresponding to the G1-VDIPES and G1-NITEGE fragments, respectively. Treatment with an aggrecanase- or non-selective inhibitor abrogated release of the NITEGE-positive fragment through day 12, and an MMP-selective inhibitor delayed release of this fragment 2–4 days (Fig. 3). In contrast, release of the G1-VDIPES fragment was blocked by the MMP- and non-selective inhibitors. The aggrecanase-selective inhibitor (20µM) also reduced release of the MMP-generated fragment, but much less potently than the MMP- and non-selective inhibitors.
Fig 3. Immunoblots of conditioned media.
Selective and non-selective metalloproteinase inhibitors perturb release of aggrecan fragments from IL-1-stimulated articular cartilage. Aggrecan cleavage in conditioned media was detected by immunoblot for the NITEGE (A) and VDIPES (B) neoepitopes. Lanes 1, 2, and 3 contain media pooled from days 2 and 4, 6 and 8, and 10 and 12, respectively. Arrows indicate migration of 64kDa (A) or 50kDa (B) markers.
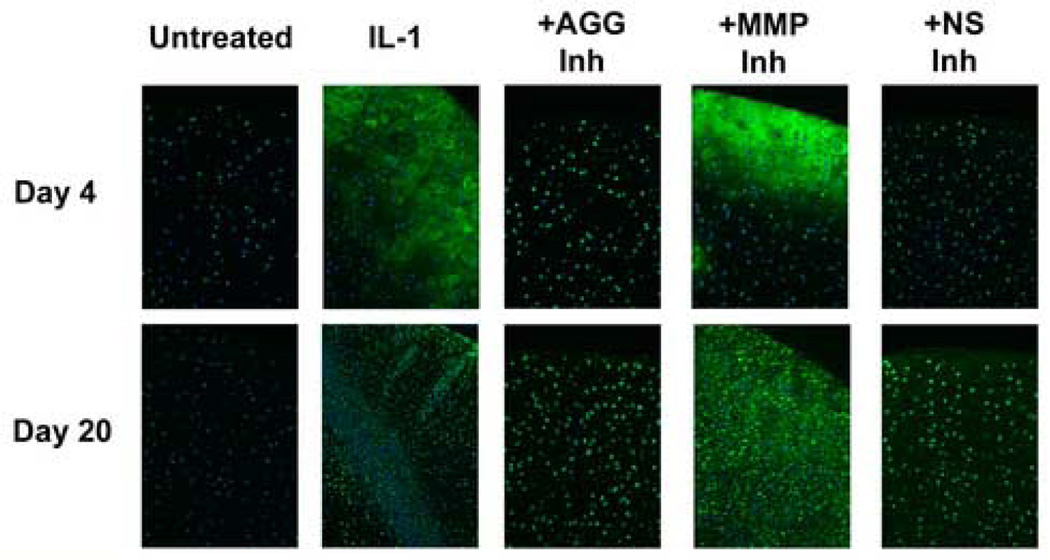
IL-1-induced aggrecan catabolism was further characterized by immunofluorescent detection of aggrecan cleavage fragments within the explants (Fig. 4). The NITEGE fragment was localized in IL-1-stimulated tissue at days 4 and (to a lesser extent) 20. Treatment with an aggrecanase- or non-selective inhibitor yielded weaker interterritorial staining with intense intra- or peri-cellular staining. The MMP-selective inhibitor did not substantially reduce or alter the spatial distribution of NITEGE staining, and appears to have inhibited release of the fragment at later times.
Fig 4. Immunostaining of tissue sections.
Selective and non-selective metalloproteinase inhibitors perturb aggrecanase-mediated aggrecanolysis in IL-1-stimulated articular cartilage. Aggrecan cleavage fragments were localized by immunofluorescent detection of the NITEGE neoepitope. NITEGE-positive regions are green, and cell nuclei are blue. Non-immune IgG-treated negative controls showed no background staining. Original magnification = 10×.
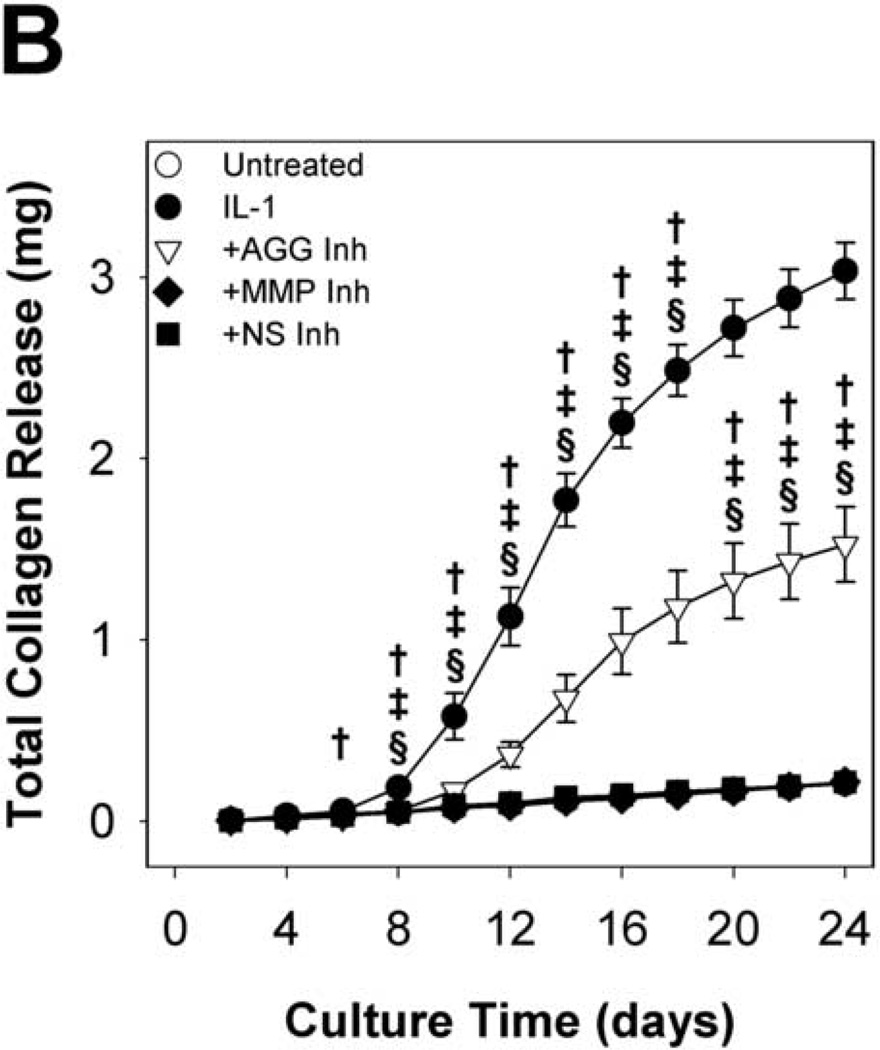
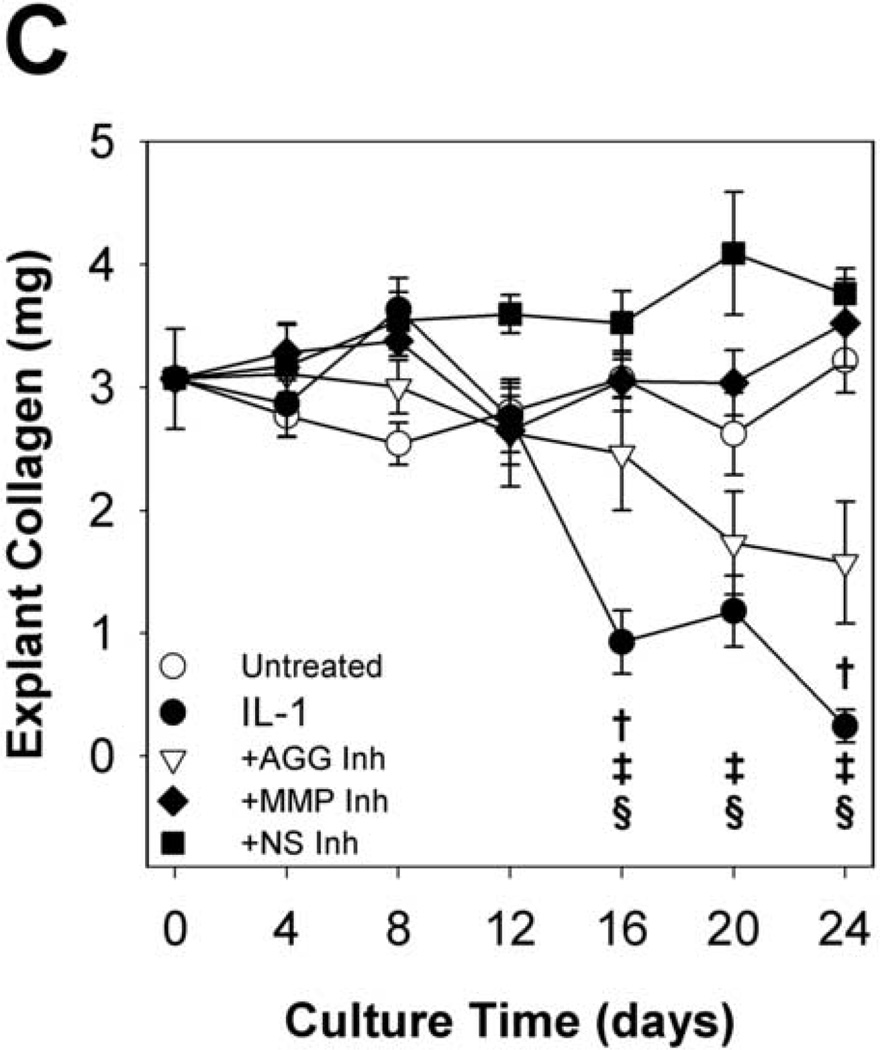
Hydroxyproline release to the conditioned media (Fig. 5A&B) and explant hydroxyproline content (Fig. 5C) were measured to examine collagen degradation. As observed in previous studies of IL-1-stimulated cartilage, IL-1-induced collagen release began after nearly complete depletion of aggrecan. Cell-mediated collagen destruction approached completion by day 24, with approximately 8% of t = 0 collagen content remaining. The MMP-selective and non-selective metalloproteinase inhibitors completely blocked collagen release from the tissue throughout the 24 days, indicating a central role for MMPs in degradation of the collagen network. Interestingly, the aggrecanase-selective inhibitor delayed and reduced hydroxyproline release to the media and loss from the explant by 50%. These results are consistent with a previously proposed model in which aggrecan protects the collagen network from proteolytic attack in bovine nasal cartilage (Pratta, et al., 2003b).
Fig 5. Collagen release and explant collagen.
MMP inhibitors block, and an aggrecanase inhibitor reduces, IL-1-induced collagen degradation. Hydroxyproline content of conditioned media (A, B) and explant digests (C) was measured by the chloramine-T/pDAB reaction; collagen content was calculated assuming a collagen:hydroxyproline mass ratio of 8:1. Data are mean +/− SEM, n = 5. In B and C, †, ‡, § = p<0.05 vs. IL-1 for AGG Inh, MMP Inh, and NS Inh, respectively.
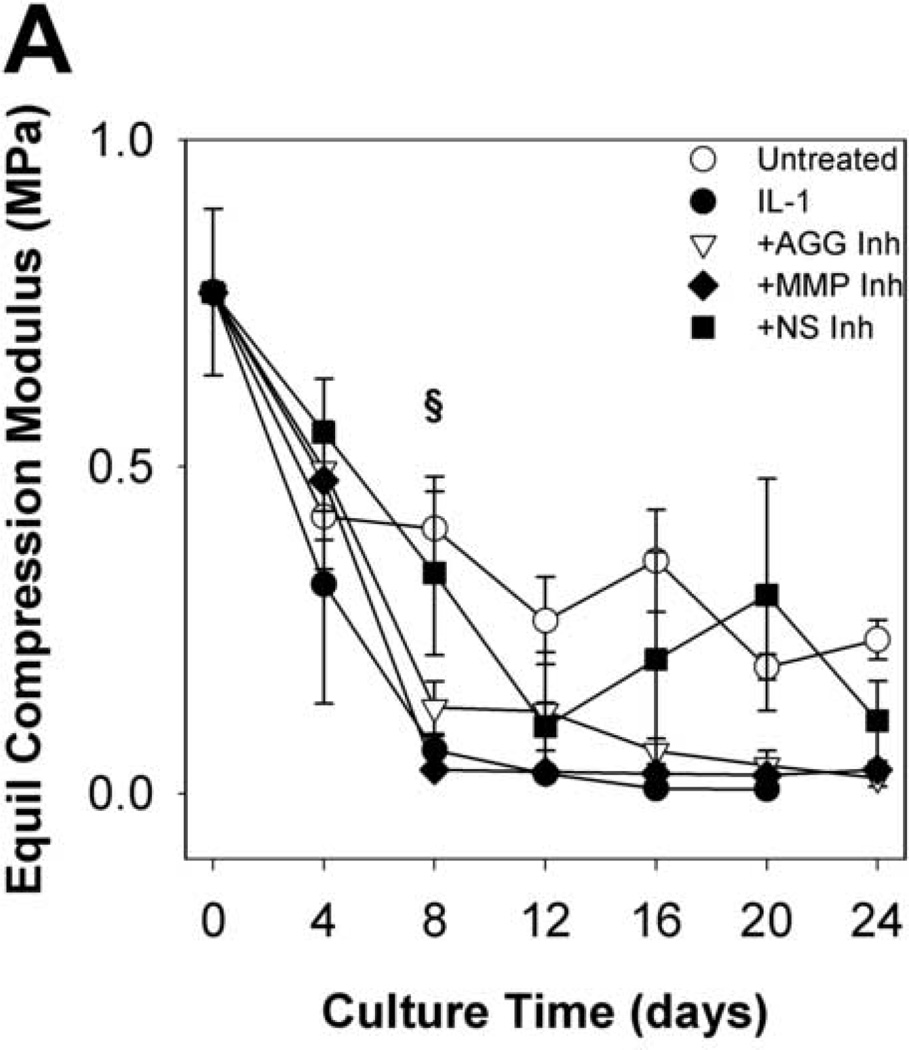
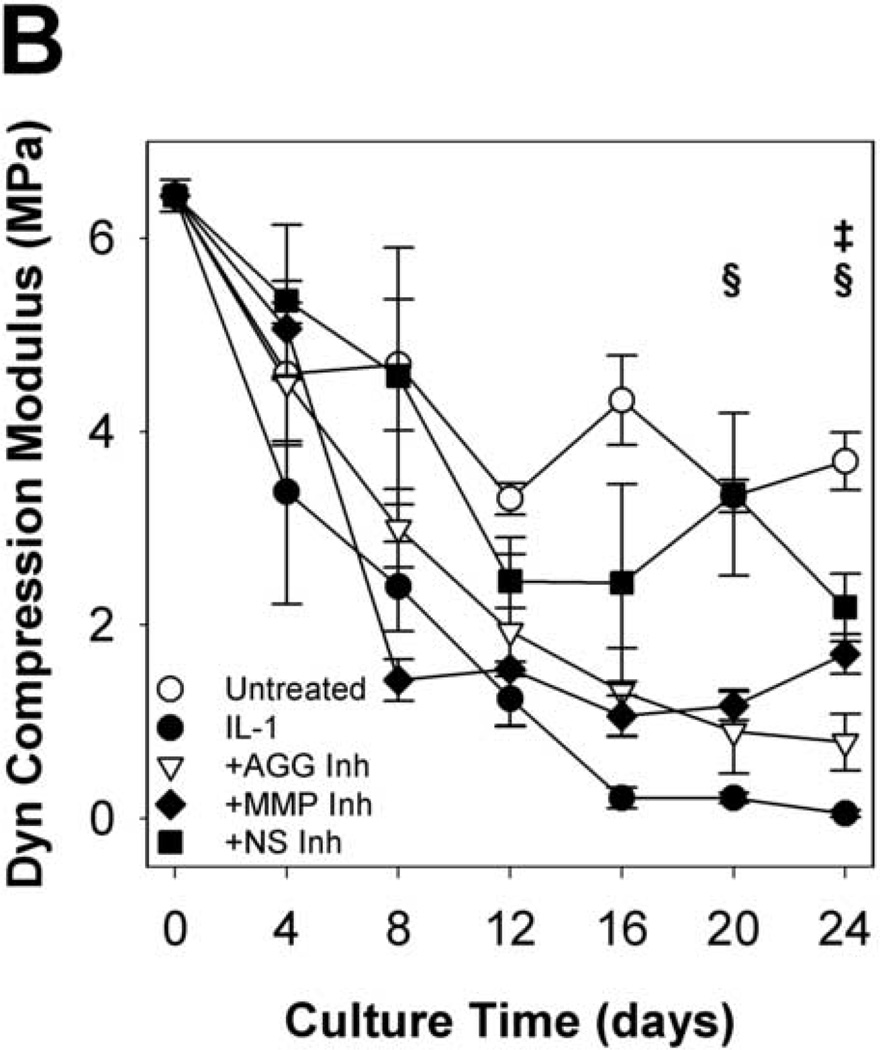
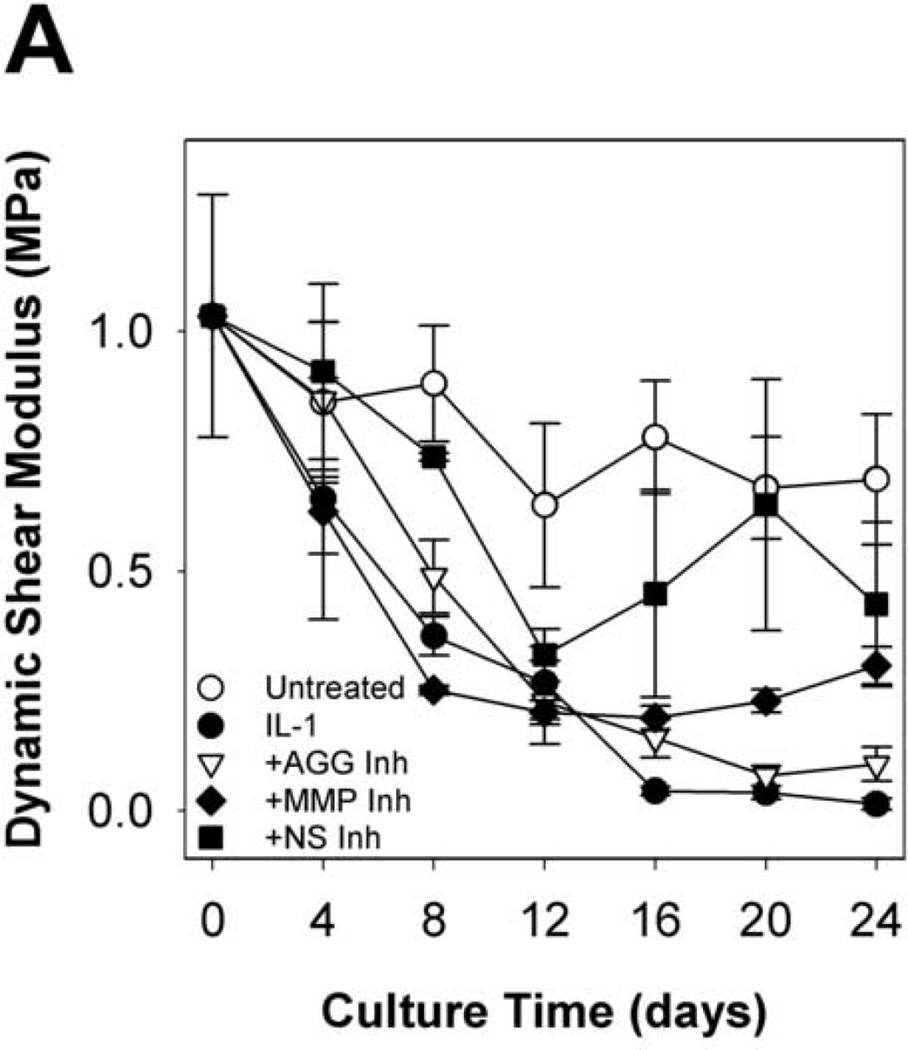
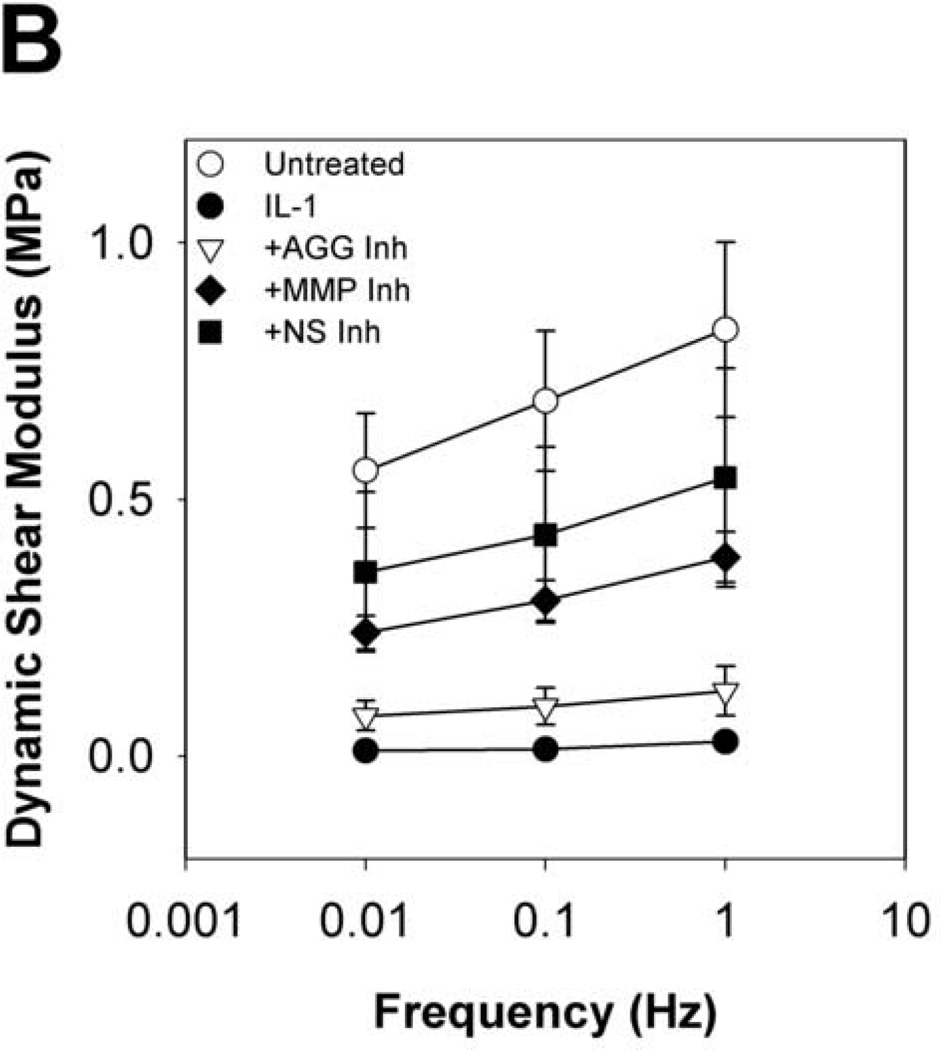
To evaluate the functional implications of perturbing metalloproteinase activity in IL-1-stimulated articular cartilage, explants were subjected to compression and shear testing. IL-1-stimulated tissue underwent substantial loss (91% reduction from t = 0 explants) of the equilibrium compression modulus by day 8 (Fig.6A), with kinetics similar to proteoglycan depletion. By day 16, the dynamic compression (Fig. 6B) and shear (Fig. 7A) moduli were dramatically reduced (by 97% and 96%, respectively, from t = 0 explants). Treatment with the MMP-selective inhibitor did not prevent loss of the equilibrium modulus, but reduced loss of the dynamic compression and shear moduli after day 8. The aggrecanase-selective inhibitor delayed, but did not block loss of equilibrium properties and reduced loss of the dynamic properties. The non-selective metalloproteinase inhibitor was able to delay and reduce, but did not block, loss of compression properties, and conferred the greatest protection of tissue function in IL-1-stimulated tissue (with 47%, 59%, and 62% of same-day untreated explants’ E, E*, and G*, respectively). The protective effects of inhibitor treatments on dynamic shear modulus were comparable across all frequencies tested (Fig. 7B). Similarly, protection of the dynamic compression modulus by each inhibitor was comparable for all frequencies tested (data not shown).
Fig 6. Equilibrium and Dynamic Compression Moduli.
Selective and non-selective metalloproteinase inhibitors reduce, but do not block, IL-1-stimulated loss of compression properties. Equilibrium (A) and dynamic (B) compression moduli were measured by stress relaxation and oscillatory loading (0.1Hz) tests, respectively. Equilibrium moduli were below detection by 24 days of IL-1 treatment. Data are mean +/− SEM, n = 5. ‡, § = p<0.05 vs. IL-1 for MMP Inh and NS Inh, respectively.
Fig 7. Dynamic Shear Modulus and frequency dependence.
Selective and non-selective metalloproteinase inhibitors reduce, but do not block, IL-1-stimulated loss of shear properties. Dynamic shear moduli as a function of culture time (A, at 0.1Hz) and frequency (B, at day 24) were measured by oscillatory torsion tests. Data are mean +/− SEM, n = 5.
Discussion
Selective proteinase inhibitors have demonstrated utility in the investigation of cartilage degeneration mechanisms and may have clinical use in the management of arthritis. The efficacy of these inhibitors is most often assessed by biochemical outcomes, and the studies presented here extend previous work that examines the functional consequences of targeted perturbations in cell-mediated degradation (Bonassar, et al., 1996; Bonassar, et al., 1997). In the present study, treatment of immature bovine cartilage with metalloproteinase inhibitors delayed or reduced IL-1-induced matrix degradation. These inhibitors were shown to potently and selectively target MMP or aggrecanase activity, or to broadly inhibit metalloproteinase activity in vitro. In control experiments (data not shown), explants treated with metalloproteinase inhibitors (but not exposed to IL-1) had DNA, aggrecan, and collagen contents and basal levels of aggrecan release similar to untreated controls, indicating that these inhibitors do not strongly influence cell proliferation or normal matrix metabolism. The compounds used to study protease activity in these experiments appear to inhibit their targets without disrupting physiologic processes, though it is possible that they have non-specific effects on cell metabolism. It is also noteworthy that the doses required to modify aggrecanolysis in explants were 2–3 orders of magnitude higher than the IC50 values measured in the metalloproteinase enzyme assays. This discrepancy in concentrations may be attributed to cellular metabolism of the compounds, transport limitations (e.g., partitioning) or other factors that influence inhibitor stability or delivery. In addition, the inhibitor selectivity assays were done with recombinant enzymes and artificial fluorescent substrates which may not model the natural process accurately. Nonetheless, the results of the explant studies support the specificity and relative activity of the inhibitors suggested by the selectivity assays. Complementary experiments with small interfering RNA, knockout animals, or dominant negative mutants perturbing protease activity may be required to more definitively establish the roles of individual enzymes.
Biochemical analysis of conditioned media and explant digests revealed that inhibition of MMPs and ADAMTSs delayed and/or reduced IL-1-induced aggrecan release, but did not prevent it. The aggrecanase- and non-selective inhibitors delayed IL-1-induced release of aggrecan to conditioned media and attenuated generation of the NITEGE neoepitope in immunoblots of conditioned media and in the tissue as shown by immunohistochemistry. These data are consistent with previous reports indicating that aggrecanases are the primary downstream effectors of the cell-mediated catabolic response to IL-1 (Arner, et al., 1998; Little, et al., 1999; Tortorella, et al., 2001). The less profound effects seen with the MMP-inhibitor may have been due to an inhibition of MMP-mediated activation of aggrecanases. A mechanism by which MMP-17 cleaves ADAMTS-4 at the cell surface appears to be active in IL-1-stimulated bovine cartilage explants (Patwari, et al., 2005), and inhibition of this mechanism with the MMP-selective inhibitor may have resulted in reduced levels of fully activated enzyme (Flannery, et al., 2002; Gao, et al., 2004). The MMP- and non-selective inhibitors prevented IL-1-induced generation of the VDIPES neoepitope, indicating that MMPs also degrade aggrecan in this model. Interestingly, stimulation with a lower dose (2ng/mL) of IL-1 permits a more robust reduction in proteoglycan release by the compounds used in this study (data not shown). More potent inhibitors of aggrecanase activity, such as tissue inhibitors of metalloproteinases (TIMP)-3, may also confer long-term protection of the cartilage ECM.
Our data suggest the existence of non-metalloproteinase-mediated pathways for aggrecan release in cartilage explants treated with IL-1. Chondrocyte-derived hyaluronidases may be responsible, although there is conflicting evidence for upregulated expression and activity of hyaluronidases in response to inflammatory cytokines (Chow and Knudson, 2005; Flannery, et al., 1998). Chondrocytes also express non-metalloproteinase enzymes such as cathepsin-B (Fosang, et al., 1992) and m-calpain (Oshita, et al., 2004) which have been shown to cleave the aggrecan core protein, and these enzymes may be upregulated in response to IL-1 treatment.
The delayed aggrecan release observed in tissue treated with aggrecanase-selective and non-selective inhibitors may be due to differential selectivity of these inhibitors for various aggrecanase activation states or enzymes. ADAMTS-4 undergoes post-translational processing to at least 4 activation states, each with distinct substrate specificity and matrix-binding properties that could influence inhibitor potency (Gao, et al., 2002; Kashiwagi, et al., 2004; Tortorella, et al., 2000). As a result, the aggrecanase inhibitors used in this study may efficiently block aggrecanase activity in the aggrecan IGD and yet permit less destructive activity (at sites in the CS-2 domain) characteristic of lower activation states. In addition, the selectivity of these inhibitors for different aggrecanases in the ADAMTS family of enzymes has not been fully characterized. The ELISA used to establish inhibitor selectivity for aggrecanases tested the inhibitor potency against recombinant ADAMTS-4 (and with similar potency against ADAMTS-5, data not shown), but ADAMTS-1, -8, -9, and -15 are also capable of generating the classical aggrecanase cleavage products. While the non-selective inhibitor is a more potent inhibitor of aggrecanases, a higher dose of the aggrecanase-selective inhibitor was used in these experiments to account for the difference in potency.
Treatment with the MMP-selective inhibitor delayed IL-1-induced release of the G1-NITEGE neoepitope by several days and inhibited release of the fragment. The specific role of MMPs in release of the proteoglycan aggregate is unclear, but may be related to destruction of the collagen network. Blockade of collagen degradation may be sufficient to preserve hyaluronan entanglement and thereby inhibit its diffusion (and diffusion of aggregable aggrecan fragments) from the ECM. Link protein, which stabilizes the interaction between aggrecan and hyaluronan, is also a substrate for MMPs including matrilysin and stromelysin-1 and -2 (Nguyen, et al., 1993). The compounds used in this study may, then, interfere with MMP-mediated cleavage of link protein and release of aggrecan.
Analysis of collagen content in explant digests and conditioned media confirmed the role of MMPs in collagenolysis. MMP-selective and non-selective metalloproteinase inhibitors completely blocked release of collagen to the media and depletion of collagen from the tissue. IL-1 upregulates expression of MMP-1, -3, and -13 in bovine cartilage (Flannery, et al., 1999), and IL-1-induces exhaustive collagen degradation over a month or less of treatment (Nixon, et al., 1991). Interestingly, the aggrecanase-selective inhibitor also conferred some protection of the collagen network. Using a different small-molecule aggrecanase inhibitor, Pratta et al observed a similar result and hypothesized that aggrecan molecules can prevent MMPs from reaching their substrates on collagen fibers, perhaps by steric exclusion (Pratta, et al., 2003b). Treatment of IL-1-stimulated cartilage with the aggrecanase-selective inhibitor reduced cumulative collagen release by 50% through day 24 of the experiment, and delayed but did not prevent aggrecan release over the same period. Generation of the G1-NITEGE fragment, however, was reduced in this group, indicating that alternative paths of aggrecan processing had occurred to release the aggrecan. Several enzymes (e.g., m-calpain) truncate aggrecan at C-terminal sites in the sGAG-rich region and leave an intact IGD, yielding a “trimmed” aggrecan that could contribute to partial protection of the collagen network.
Mechanical testing in compression and shear revealed that IL-1-induced reductions in explant material properties are attenuated by inhibition of metalloproteinase activity. Compression and shear moduli are indicators of tissue mechanical function and depend on the abundance and integrity of ECM constituents (Rieppo, et al., 2003; Setton, et al., 1999; Zhu, et al., 1993). Whereas IL-1-stimulated tissue retains compression properties approximately 0–4% of the initial (t = 0) values by day 24, treatment with the non-selective metalloproteinase inhibitor was effective at preserving 15% and 42% of the initial equilibrium and dynamic compression moduli, respectively. These data indicate that MMPs and aggrecanases mediate part of the IL-1-induced loss of cartilage compression properties, and further suggest that other enzyme systems or mechanisms of ECM catabolism may participate. The MMP-selective inhibitor attenuated IL-1-induced loss of the dynamic compression modulus, but the aggrecanase-selective inhibitor did not confer significant protection of either compression property by day 24. These data are consistent with the ideas that equilibrium behavior of cartilage is governed by the abundance of aggrecan and the dynamic loading behavior is influenced by the integrity of both aggrecan aggregates and the collagen network (Laasanen, et al., 2003). In preventing degradation of the collagen network, the MMP-selective inhibitor partly preserves the tissue’s response to dynamic loading. The aggrecanase-selective inhibitor fails to sufficiently protect the aggrecan or collagen and does not prevent loss of equilibrium or dynamic properties. Indeed, destruction of the collagen network, rather than the aggrecan aggregate, is considered the “point of no return” in cartilage degeneration. Of note, the equilibrium and dynamic compression moduli of untreated controls fell to 31% and 54%, respectively, of initial values after 24 days of in vitro culture. These changes are attributed in part to collagen network damage at the cut surfaces sustained during explant preparation.
Compounds that inhibit MMP activity protected dynamic material properties of IL-1-stimulated tissue, whereas an aggrecanase inhibitor did not. The MMP-selective and non-selective metalloproteinase inhibitors reduced the loss of the dynamic compression modulus over 24 days of IL-1 stimulation and treatment with the non-selective inhibitor also reduced the loss of the dynamic shear modulus. The aggrecanase-selective inhibitor delayed (by ~4 days) and reduced loss of the dynamic compression modulus, but did not substantially alter loss of the equilibrium compression or dynamic shear moduli. These data are consistent with previously published findings that the dynamic material properties of cartilage depend upon collagen content (Appleyard, et al., 2003). Recent work in our lab, however, also demonstrated significant correlations between aggrecan contents and the dynamic shear and compression properties in IL-1-stimulated cartilage (Palmer, et al., 2005), and the improved retention of explant aggrecan content by a non-selective metalloproteinase inhibitor observed here may contribute to protection of dynamic material properties. Trends in the dynamic shear properties generally followed those observed in the dynamic compression moduli and revealed similar sensitivities of these properties to IL-1-induced degradation.
The results of this study indicate that selective or broad inhibition of metalloproteinase activity perturbs cell-mediated ECM degradation induced by IL-1-stimulation of bovine cartilage explants. Disruption of the metalloproteinases was insufficient to prevent exhaustive depletion of aggrecan aggregates in this model system and may allow alternative pathways of aggrecan processing to proceed. In addition, these studies demonstrate that non-metalloproteinase mechanisms of aggrecan depletion can mediate IL-1-induced loss of tissue mechanical properties. Identification of these pathways may reveal new therapeutic requirements for clinical management of cartilage degradation.
Materials & Methods
Inhibitor Selectivity Assays
The potencies and selectivities of the inhibitors for various MMPs were determined using recombinant mouse or human MMPs (R&D Systems, Minneapolis, MN) and the fluorogenic peptide substrate MCA-Pro-Leu-Gly-Leu-DAP(DNP)-Ala-Arg-NH2 (Bachem, Heidelberg, Germany). Recombinant enzymes were activated with 1mM aminophenylmercuric acetate (Sigma), and reacted with the substrate in the presence of an aggrecanase-selective inhibitor (RO3310769, Roche-Palo Alto, Palo Alto, CA), a MMP-selective inhibitor (RO1136222, Roche-Palo Alto), or a non-selective metalloproteinase inhibitor (RO4002855, Roche-Palo Alto). The inhibitors were tested at concentrations ranging from 0–200µM. Reaction rates were measured by detection of the cleaved substrate’s fluorescent signal (excitation λ = 334nm, emission λ = 390nm) over 20min at 37°C. The results are reported as IC50, the inhibitor concentration causing 50% of the maximal reduction in the rate of substrate cleavage.
The potencies and selectivities of the inhibitors for ADAMTS-4 were determined via an enzyme linked immunosorbent assay (ELISA). Recombinant human ADAMTS-4 was produced in Sf9 cells and purified by column chromatography as previously described (Roughley, et al., 2003). Aggrecan substrate was isolated from young adult bovine nasal cartilage by extraction in 4M guanidine hydrochloride and subsequent fractionation by CsCl density gradient centrifugation. Microplates were treated with 660ng aggrecan/well overnight at 4°C, and blocked with 1% BSA for 1h at room temperature. Recombinant ADAMTS-4 in reaction buffer containing 50mM Tris-HCl, 1% glycerol, 10mM CaCl2, pH 7.5 was added with varying concentrations of inhibitors.
The digestions were carried out at room temperature for 2.5h. Reaction products were incubated with anti-NITEGE primary antibody diluted 1:3000 over 2h at room temperature. The extent of digestion was then measured by reaction of an alkaline phosphatase-conjugated secondary antibody with the chemiluminescent substrate disodium 3-(4-methoxyspiro{1,2-dioxetane-3,2'-(5'-chloro)tricyclo[3.3.1.13,7]decan}-4-yl)phenyl phosphate (CSPD, Applied Biosystems, Bedford, MA). The results are reported as IC50, the inhibitor concentration causing 50% of the maximal reduction in abundance of NITEGE-positive aggrecan fragments.
Tissue Culture
Articular cartilage was harvested aseptically from the femoral condyles and femoropatellar grooves of 1–2 week old calves (Research 87, Marlborough, MA) using a 4mm diameter biopsy punch. Full thickness explants were then cut to 2mm thick on a custom sizing block., The explants were cultured in 5% CO2, 95% humidity and 37°C for 72 hrs in the presence of serum-free media consisting of high glucose DMEM (Invitrogen, Carlsbad, CA), 10mM non-essential amino acids (Invitrogen), 50ug/mL gentamicin (Invitrogen, Carlsbad, CA), and 50µg/mL ascorbic acid (Sigma, St. Louis, MO). In a dose-response study, explants were stimulated with 20ng/mL recombinant human IL-1α (Peprotech, Rocky Hill, NJ) and treated with 0.5, 5, or 50µM aggrecanase-, MMP-, or non-selective inhibitors. Stock solutions of the compounds were prepared with dimethylsulfoxide (DMSO), and final concentrations of DMSO in the culture media did not exceed 0.2%. At doses below 5%, DMSO has been shown to have no effects on cartilage explant proteoglycan synthesis or viability (Smith, et al., 2000).
Explants were cultured for up to 24 days with or without 20ng/mL rhIL-1α. Some explants were additionally treated with 20µM of the aggrecanase-selective inhibitor, 5µM of the MMP-selective inhibitor or 5µM of the non-selective inhibitor. The four-fold higher dose of aggrecanase-selective inhibitor was chosen, based on the dose-response study and aggrecanase inhibition assay data, to yield similar inhibition of IL-1-induced sGAG release as the non-selective inhibitor. Media were collected and replenished every two days, and conditioned media were stored at −20°C. Inhibitors were added to the media with each media change. Explants harvested at days 0, 4, 8, 12, 16, 20 and 24 days of culture (n = 5/treatment/time point) were stored frozen until mechanical testing in Dulbecco’s phosphate buffered saline (DPBS) with a proteinase inhibitor cocktail (Calbiochem, San Diego, CA) including ethylenediaminetetraacetic acid (EDTA), 4-(2-aminoethyl)benzenesulfonylfluoride, leupeptin, and aprotinin. In a second study, explants harvested at days 0, 4, 8, and 20 of culture (n = 4/treatment/time point) were immediately fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned to 4µm for immunostaining. The kinetics of sGAG release to the media were similar between these two studies (data not shown).
Biochemistry
Following mechanical testing, explants were lyophilized, weighed dry, and digested overnight in 0.0125 mg/mg tissue proteinase K (Invitrogen) at 60°C. Explant digests and conditioned media were spectrophotometrically assayed for sGAG content by the dimethylmethylene blue (DMMB) dye-binding method (Farndale, et al., 1986) and for hydroxyproline content by the chloramine-T/para-dimethylaminobenzadehyde reaction (Woessner, 1961). Collagen content was calculated from the hydroxyproline content assuming a collagen:hydroxyproline mass ratio of 8:1 (Reddy and Enwemeka, 1996).
Immunodetection
Tissue sections were deparaffinized using a Leica autostainer and loaded into Sequenza immunostaining racks (ThermoShandon, Waltham, MA). Sections were deglycosylated with 0.1U/mL chondroitinase ABC (Sigma) for 1hr at 37°C and blocked in 2% normal goat serum, 0.1% gelatin, 1% bovine serum albumin, and 0.05% Tween-20 for 1h at room temperature. Primary antibody or species-matched non-immune IgG diluted to 10µg/mL was then applied to sections for 1hr at room temperature. A goat anti-rabbit-Alexa Fluor 488 secondary antibody (Molecular Probes) was used for detection and cell nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI, Molecular Probes). Images were captured through individual fluorescein isothiocyanate (FITC) and DAPI filters using a Ziess Axiovert 200 epifluorescent microscope with a CCD camera. All images were captured with the same exposure time, and the FITC and DAPI images were combined using image analysis software (Carl Zeiss MicroImaging, Thornwood, NY).
To assess MMP and aggrecanase activity, conditioned media from the second experiment were analyzed by immunoblot with antibodies to the VDIPEN360 neoepitope (JSCVDI) or the NITEGE392 neoepitope (JSCNIT). Antibodies raised against the VDIPEN neoepitope have been previously shown to detect the bovine VDIPES sequence (Little, et al., 2002). Pooled media samples from days 2 and 4, 6 and 8, or 10 and 12 of the experiment were treated with ice cold ethanol/5mM sodium acetate to precipitate proteoglycans, and precipitates were deglycosylated by sequential digestion with protease-free chondroitinase ABC (Sigma) and Keratanases I (Sigma) and II (Associates of Cape Cod, East Falmouth, MA) as previously described (Sandy and Verscharen, 2001). Equal quantities of sGAG (5 µg) were loaded into 4–12% gradient Tris-glycine gels (Invitrogen). Following electrophoresis and transfer to nitrocellulose, aggrecan fragments were identified with primary antibodies at 1µg/mL. Blots were developed using a secondary antibody conjugated with alkaline phosphatase followed by exposure to the fluorescent substrate ECF (Amersham, Piscataway, NJ). Bands were visualized using a Fuji FLA3000 Phospho-imager.
Mechanical Testing
Explants were thawed to room temperature in DPBS with protease inhibitors and weighed wet before testing in torsional shear on a CVO120 rheometer (Bohlin, East Brunswick, NJ) and in unconfined compression on an ELF3200 uniaxial loading frame (Enduratec, Minnetonka, MN). After application of a 10% compressive strain and relaxation for 12min, each explant was first tested in oscillatory torsion with 0.5% shear strain applied at 0.01–10Hz to yield a frequency-dependent dynamic shear modulus G*. Following a 12min reequilibration to the free-swelling state, each explant was then tested in compression through 4 steps of stress relaxation (5% each, 10min./step) to determine the equilibrium modulus E and through oscillatory compression about a 10% offset of ±1.5% strain at 0.001–1Hz.to determine the frequency-dependent dynamic compression modulus E*.
Statistics
Differences between treatment groups at a given day were evaluated by one-way analysis of variance and Dunnett’s post-hoc test using the IL-1 treated group as a control and significance at p<0.05.
Acknowledgements
This work was supported by an Arthritis Foundation Arthritis Investigator grant, by the ERC program of the NSF under award number EEC-9731643 (Georgia Tech/Emory Center (GTEC) for the Engineering of Living Tissues), by a graduate fellowship from the Cellular and Tissue Engineering Training Grant Program under NIH award number 5 T32 GM008433-13 (CGW), a graduate fellowship under NSF IGERT award number 0221600 (AWP), and by Roche-Palo Alto.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appleyard RC, Burkhardt D, Ghosh P, Read R, Cake M, Swain MV, Murrell GA. Topographical analysis of the structural, biochemical and dynamic biomechanical properties of cartilage in an ovine model of osteoarthritis. Osteoarthritis Cartilage. 2003;11:65–77. doi: 10.1053/joca.2002.0867. [DOI] [PubMed] [Google Scholar]
- Arner EC, Hughes CE, Decicco CP, Caterson B, Tortorella MD. Cytokine-induced cartilage proteoglycan degradation is mediated by aggrecanase. Osteoarthritis Cartilage. 1998;6:214–228. doi: 10.1053/joca.1998.0114. [DOI] [PubMed] [Google Scholar]
- Arner EC, Pratta MA, Decicco CP, Xue CB, Newton RC, Trzaskos JM, Magolda RL, Tortorella MD. Aggrecanase. A target for the design of inhibitors of cartilage degradation. Ann N Y Acad Sci. 1999;878:92–107. doi: 10.1111/j.1749-6632.1999.tb07676.x. [DOI] [PubMed] [Google Scholar]
- Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46:2648–2657. doi: 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]
- Beekman B, Verzijl N, de Roos JA, TeKoppele JM. Matrix degradation by chondrocytes cultured in alginate: IL-1 beta induces proteoglycan degradation and proMMP synthesis but does not result in collagen degradation. Osteoarthritis Cartilage. 1998;6:330–340. doi: 10.1053/joca.1998.0132. [DOI] [PubMed] [Google Scholar]
- Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, Van Wart H, Poole AR. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonassar LJ, Stinn JL, Paguio CG, Frank EH, Moore VL, Lark MW, Sandy JD, Hollander AP, Poole AR, Grodzinsky AJ. Activation and inhibition of endogenous matrix metalloproteinases in articular cartilage: effects on composition and biophysical properties. Arch Biochem Biophys. 1996;333:359–367. doi: 10.1006/abbi.1996.0402. [DOI] [PubMed] [Google Scholar]
- Bonassar LJ, Sandy JD, Lark MW, Plaas AH, Frank EH, Grodzinsky AJ. Inhibition of cartilage degradation and changes in physical properties induced by IL-1beta and retinoic acid using matrix metalloproteinase inhibitors. Arch Biochem Biophys. 1997;344:404–412. doi: 10.1006/abbi.1997.0205. [DOI] [PubMed] [Google Scholar]
- Chan PS, Caron JP, Orth MW. Effect of glucosamine and chondroitin sulfate on regulation of gene expression of proteolytic enzymes and their inhibitors in interleukin-1- challenged bovine articular cartilage explants. Am J Vet Res. 2005;66:1870–1876. doi: 10.2460/ajvr.2005.66.1870. [DOI] [PubMed] [Google Scholar]
- Chow G, Knudson W. Characterization of promoter elements of the human HYAL-2 gene. J Biol Chem. 2005;280:26904–26912. doi: 10.1074/jbc.M413845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close DR. Matrix metalloproteinase inhibitors in rheumatic diseases. Ann Rheum Dis. 2001;60(Suppl 3):iii62–iii67. doi: 10.1136/ard.60.90003.iii62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg SR, Grodzinsky AJ. Swelling of articular c artilage and other connective tissues: electromechanochemical forces. J Orthop Res. 1985;3:148–159. doi: 10.1002/jor.1100030204. [DOI] [PubMed] [Google Scholar]
- Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Flannery CR, Lark MW, Sandy JD. Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan. Evidence for proteolysis at this site in vivo in human articular cartilage. J Biol Chem. 1992;267:1008–1014. [PubMed] [Google Scholar]
- Flannery CR, Little CB, Hughes CE, Caterson B. Expression and activity of articular cartilage hyaluronidases. Biochem Biophys Res Commun. 1998;251:824–829. doi: 10.1006/bbrc.1998.9561. [DOI] [PubMed] [Google Scholar]
- Flannery CR, Little CB, Caterson B, Hughes CE. Effects of culture conditions and exposure to catabolic stimulators (IL-1 and retinoic acid) on the expression of matrix metalloproteinases (MMPs) and disintegrin metalloproteinases (ADAMs) by articular cartilage chondrocytes. Matrix Biol. 1999;18:225–237. doi: 10.1016/s0945-053x(99)00024-4. [DOI] [PubMed] [Google Scholar]
- Flannery CR, Zeng W, Corcoran C, Collins-Racie LA, Chockalingam PS, Hebert T, Mackie SA, McDonagh T, Crawford TK, Tomkinson KN, LaVallie ER, Morris EA. Autocatalytic cleavage of ADAMTS-4 (Aggrecanase-1) reveals multiple glycosaminoglycan-binding sites. J Biol Chem. 2002;277:42775–42780. doi: 10.1074/jbc.M205309200. [DOI] [PubMed] [Google Scholar]
- Fontana A, Hengartner H, Weber E, Fehr K, Grob PJ, Cohen G. Interleukin 1 activity in the synovial fluid of patients with rheumatoid arthritis. Rheumatol Int. 1982;2:49–53. doi: 10.1007/BF00541245. [DOI] [PubMed] [Google Scholar]
- Fosang AJ, Neame PJ, Last K, Hardingham TE, Murphy G, Hamilton JA. The interglobular domain of cartilage aggrecan is cleaved by PUMP, gelatinases, and cathepsin B. J Biol Chem. 1992;267:19470–19474. [PubMed] [Google Scholar]
- Fosang AJ, Last K, Maciewicz RA. Aggrecan is degraded by matrix metalloproteinases in human arthritis. Evidence that matrix metalloproteinase and aggrecanase activities can be independent. J Clin Invest. 1996;98:2292–2299. doi: 10.1172/JCI119040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Westling J, Thompson VP, Howell TD, Gottschall PE, Sandy JD. Activation of the proteolytic activity of ADAMTS4 (aggrecanase-1) by C-terminal truncation. J Biol Chem. 2002;277:11034–11041. doi: 10.1074/jbc.M107443200. [DOI] [PubMed] [Google Scholar]
- Gao G, Plaas A, Thompson VP, Jin S, Zuo F, Sandy JD. ADAMTS4 (aggrecanase-1) activation on the cell surface involves C-terminal cleavage by glycosylphosphatidyl inositol-anchored membrane type 4-matrix metalloproteinase and binding of the activated proteinase to chondroitin sulfate and heparan sulfate on syndecan-1. J Biol Chem. 2004;279:10042–10051. doi: 10.1074/jbc.M312100200. [DOI] [PubMed] [Google Scholar]
- Hopkins SJ, Humphreys M, Jayson MI. Cytokines in synovial fluid. I. The presence of biologically active and immunoreactive IL-1. Clin Exp Immunol. 1988;72:422–427. [PMC free article] [PubMed] [Google Scholar]
- Jin M, Grodzinsky A. Effect of electrostatic interactions between glycosaminoglycans on the shear stiffness of cartilage: A molecular model and experiments. MACROMOLECULES. 2001;34:8330–8339. [Google Scholar]
- Kashiwagi M, Enghild JJ, Gendron C, Hughes C, Caterson B, Itoh Y, Nagase H. Altered proteolytic activities of ADAMTS-4 expressed by C-terminal processing. J Biol Chem. 2004;279:10109–10119. doi: 10.1074/jbc.M312123200. [DOI] [PubMed] [Google Scholar]
- Koshy PJ, Lundy CJ, Rowan AD, Porter S, Edwards DR, Hogan A, Clark IM, Cawston TE. The modulation of matrix metalloproteinase and ADAM gene expression in human chondrocytes by interleukin-1 and oncostatin M: a time-course study using real-time quantitative reverse transcription-polymerase chain reaction. Arthritis Rheum. 2002;46:961–967. doi: 10.1002/art.10212. [DOI] [PubMed] [Google Scholar]
- Kozaci LD, Buttle DJ, Hollander AP. Degradation of type II collagen, but not proteoglycan, correlates with matrix metalloproteinase activity in cartilage explant cultures. Arthritis Rheum. 1997;40:164–174. doi: 10.1002/art.1780400121. [DOI] [PubMed] [Google Scholar]
- Kuroki K, Stoker AM, Cook JL. Effects of proinflammatory cytokines on canine articular chondrocytes in a three-dimensional culture. Am J Vet Res. 2005;66:1187–1196. doi: 10.2460/ajvr.2005.66.1187. [DOI] [PubMed] [Google Scholar]
- Laasanen MS, Toyras J, Korhonen RK, Rieppo J, Saarakkala S, Nieminen MT, Hirvonen J, Jurvelin JS. Biomechanical properties of knee articular cartilage. Biorheology. 2003;40:133–140. [PubMed] [Google Scholar]
- Little CB, Flannery CR, Hughes CE, Mort JS, Roughley PJ, Dent C, Caterson B. Aggrecanase versus matrix metalloproteinases in the catabolism of the interglobular domain of aggrecan in vitro. Biochem J. 1999;344(Pt 1):61–68. [PMC free article] [PubMed] [Google Scholar]
- Little CB, Hughes CE, Curtis CL, Janusz MJ, Bohne R, Wang-Weigand S, Taiwo YO, Mitchell PG, Otterness IG, Flannery CR, Caterson B. Matrix metalloproteinases are involved in C-terminal and interglobular domain processing of cartilage aggrecan in late stage cartilage degradation. Matrix Biol. 2002;21:271–288. doi: 10.1016/s0945-053x(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Nguyen Q, Murphy G, Hughes CE, Mort JS, Roughley PJ. Matrix metalloproteinases cleave at two distinct sites on human cartilage link protein. Biochem J. 1993;295(Pt 2):595–598. doi: 10.1042/bj2950595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon JS, Bottomley KM, Broadhurst MJ, Brown PA, Johnson WH, Lawton G, Marley J, Sedgwick AD, Wilkinson SE. Potent collagenase inhibitors prevent interleukin-1-induced cartilage degradation in vitro. Int J Tissue React. 1991;13:237–241. [PubMed] [Google Scholar]
- Oshita H, Sandy JD, Suzuki K, Akaike A, Bai Y, Sasaki T, Shimizu K. Mature bovine articular cartilage contains abundant aggrecan that is C-terminally truncated at Ala719–Ala720, a site which is readily cleaved by m-calpain. Biochem J. 2004;382:253–259. doi: 10.1042/BJ20040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AW, Wilson CG, Zuo F, Eugui E, Levenston ME. Altered Composition-Function Relationships During IL-1-Induced Cartilage Degradation. Journal of Orthopaedic Research submitted. 2005 [Google Scholar]
- Patwari P, Kurz B, Sandy JD, Grodzinsky AJ. Mannosamine inhibits aggrecanase-mediated changes in the physical properties and biochemical composition of articular cartilage. Arch Biochem Biophys. 2000;374:79–85. doi: 10.1006/abbi.1999.1538. [DOI] [PubMed] [Google Scholar]
- Patwari P, Gao G, Lee JH, Grodzinsky AJ, Sandy JD. Analysis of ADAMTS4 and MT4-MMP indicates that both are involved in aggrecanolysis in interleukin-1-treated bovine cartilage. Osteoarthritis Cartilage. 2005;13:269–277. doi: 10.1016/j.joca.2004.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratta MA, Scherle PA, Yang G, Liu RQ, Newton RC. Induction of aggrecanase 1 (ADAM-TS4) by interleukin-1 occurs through activation of constitutively produced protein. Arthritis Rheum. 2003a;48:119–133. doi: 10.1002/art.10726. [DOI] [PubMed] [Google Scholar]
- Pratta MA, Yao W, Decicco C, Tortorella MD, Liu RQ, Copeland RA, Magolda R, Newton RC, Trzaskos JM, Arner EC. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem. 2003b;278:45539–45545. doi: 10.1074/jbc.M303737200. [DOI] [PubMed] [Google Scholar]
- Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–229. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- Rieppo J, Toyras J, Nieminen MT, Kovanen V, Hyttinen MM, Korhonen RK, Jurvelin JS, Helminen HJ. Structure-function relationships in enzymatically modified articular cartilage. Cells Tissues Organs. 2003;175:121–132. doi: 10.1159/000074628. [DOI] [PubMed] [Google Scholar]
- Roughley PJ, Barnett J, Zuo F, Mort JS. Variations in aggrecan structure modulate its susceptibility to aggrecanases. Biochem J. 2003;375:183–189. doi: 10.1042/BJ20030609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy JD, Neame PJ, Boynton RE, Flannery CR. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991;266:8683–8685. [PubMed] [Google Scholar]
- Sandy JD, Flannery CR, Neame PJ, Lohmander LS. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J Clin Invest. 1992;89:1512–1516. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy JD, Verscharen C. Analysis of aggrecan in human knee cartilage and synovial fluid indicates that aggrecanase (ADAMTS) activity is responsible for the catabolic turnover and loss of whole aggrecan whereas other protease activity is required for C-terminal processing in vivo. Biochem J. 2001;358:615–626. doi: 10.1042/0264-6021:3580615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setton LA, Elliott DM, Mow VC. Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthritis Cartilage. 1999;7:2–14. doi: 10.1053/joca.1998.0170. [DOI] [PubMed] [Google Scholar]
- Smith CL, MacDonald MH, Tesch AM, Willits NH. In vitro evaluation of the effect of dimethyl sulfoxide on equine articular cartilage matrix metabolism. Vet Surg. 2000;29:347–357. doi: 10.1053/jvet.2000.5607. [DOI] [PubMed] [Google Scholar]
- Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, Fourie AM, Fosang AJ. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Iizawa T, Harada H, Yamada K, Katsumata M, Takahashi M. Cartilage degradation independent of MMP/aggrecanases. Osteoarthritis Cartilage. 2004;12:1006–1014. doi: 10.1016/j.joca.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Sztrolovics R, Recklies AD, Roughley PJ, Mort JS. Hyaluronate degradation as an alternative mechanism for proteoglycan release from cartilage during interleukin-1beta-stimulated catabolism. Biochem J. 2002;362:473–479. doi: 10.1042/0264-6021:3620473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorella M, Pratta M, Liu RQ, Abbaszade I, Ross H, Burn T, Arner E. The thrombospondin motif of aggrecanase-1 (ADAMTS-4) is critical for aggrecan substrate recognition and cleavage. J Biol Chem. 2000;275:25791–25797. doi: 10.1074/jbc.M001065200. [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Malfait AM, Deccico C, Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9:539–552. doi: 10.1053/joca.2001.0427. [DOI] [PubMed] [Google Scholar]
- Towle CA, Hung HH, Bonassar LJ, Treadwell BV, Mangham DC. Detection of interleukin-1 in the cartilage of patients with osteoarthritis: a possible autocrine/paracrine role in pathogenesis. Osteoarthritis Cartilage. 1997;5:293–300. doi: 10.1016/s1063-4584(97)80008-8. [DOI] [PubMed] [Google Scholar]
- Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small portions of this imino acid. Archives of Biochemical Biophysics. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Zhu W, Mow VC, Koob TJ, Eyre DR. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J Orthop Res. 1993;11:771–781. doi: 10.1002/jor.1100110602. [DOI] [PubMed] [Google Scholar]
- Zwerina J, Hayer S, Tohidast-Akrad M, Bergmeister H, Redlich K, Feige U, Dunstan C, Kollias G, Steiner G, Smolen J, Schett G. Single and combined inhibition of tumor necrosis factor, interleukin-1, and RANKL pathways in tumor necrosis factor-induced arthritis: effects on synovial inflammation, bone erosion, and cartilage destruction. Arthritis Rheum. 2004;50:277–290. doi: 10.1002/art.11487. [DOI] [PubMed] [Google Scholar]