Abstract
Polydipsia and polyuria are common symptoms in patients with diabetes insipidus (DI), which can be due to inadequate vasopressin production (cranial DI) or vasopressin insensitivity (nephrogenic DI). Clinical diagnosis of the subtypes of DI can be tricky. We present a 44-year-old man with a strong family history of DI who had been diagnosed with autosomal dominant nephrogenic DI from infancy. At the age of 40, he had progressed to end-stage renal failure. When he experienced unresolving severe polyuria after renal transplant, further investigations revealed that he was misdiagnosed and that he had a novel mutation causing autosomal dominant cranial DI.
Keywords: chronic renal failure, congenital cranial diabetes insipidus, genetic testing, hereditary polyuric states, kidney transplantation
Background
Diabetes insipidus (DI) is a condition characterized by a failure to concentrate urine appropriately and the excretion of large volumes (>3 L/day) of dilute urine (<300 mOsmol/kg) [1]. The four main forms of clinical DI are cranial (CDI), nephrogenic (NDI), gestational DI and primary polydipsia. CDI has a prevalence of 1 in 25 000 and is due to defective vasopressin secretion. Insensitivity to vasopressin causes NDI, whose prevalence is unknown. Most cases of DI are acquired, but some cases are genetic, which have helped to clarify the cellular mechanisms underlying DI. Approximately 90% of inherited NDI is X-linked recessive, 10% autosomal recessive and the remaining cases autosomal dominant [2]. In contrast, the majority of inherited CDI is by an autosomal dominant transmission [3]. Differentiating between these two types of DI can be difficult, even with a family history. However, it is important to establish a diagnosis early, since undiagnosed patients, especially those with NDI, are at risk of developing lethal electrolyte disturbances and irreversible renal tract damage [4, 5].
Case report
The patient was a 44-year-old male with a background of presumed autosomal dominant NDI and end-stage renal failure, who presented with sudden onset and prolonged polyuria following a cadaveric renal transplant.
As an infant, he had presented to another hospital with vomiting, polyuria and failure to thrive. He was diagnosed clinically with dominantly inherited NDI, because of his similarly affected brother, sister and father. He developed and grew normally, despite ongoing polydipsia and polyuria (10–15 L/day). Routine renal ultrasonography at the age of 6 revealed bilateral hydronephrosis and a grossly distended bladder. He was diagnosed with bladder outflow obstruction and treated initially with a cystomyotomy. Despite this, the hydronephrosis progressed, which prompted further investigation to exclude an anatomical cause. He was found to have mild spina bifida occulta, but this was not thought to explain his bladder problem. No obstructive cause was found and his renal function began to deteriorate from the age of 14, resulting in end-stage renal failure by the age of 40 when he had come to the attention of our nephrology service. He underwent a brief period of haemodialysis, during which he continued to pass urine, though less than previously, before receiving a successful cadaveric renal transplant.
Polyuria immediately after a renal transplant is not unusual and is often exaggerated by the need and tendency to maintain a positive fluid balance postoperatively. However, following his renal transplant, he remained significantly polyuric (and more than when on dialysis), despite a reduction in his intravenous fluids. This prompted a reconsideration of the original diagnosis of NDI.
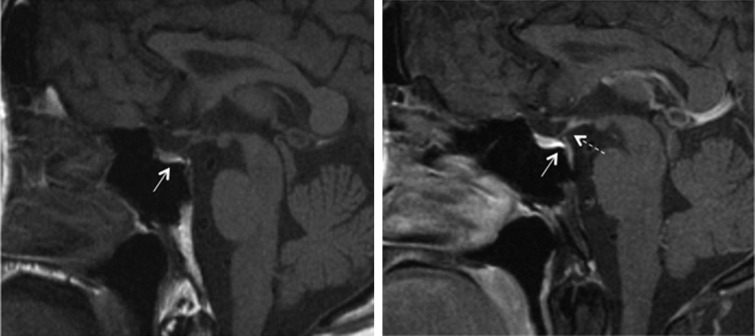
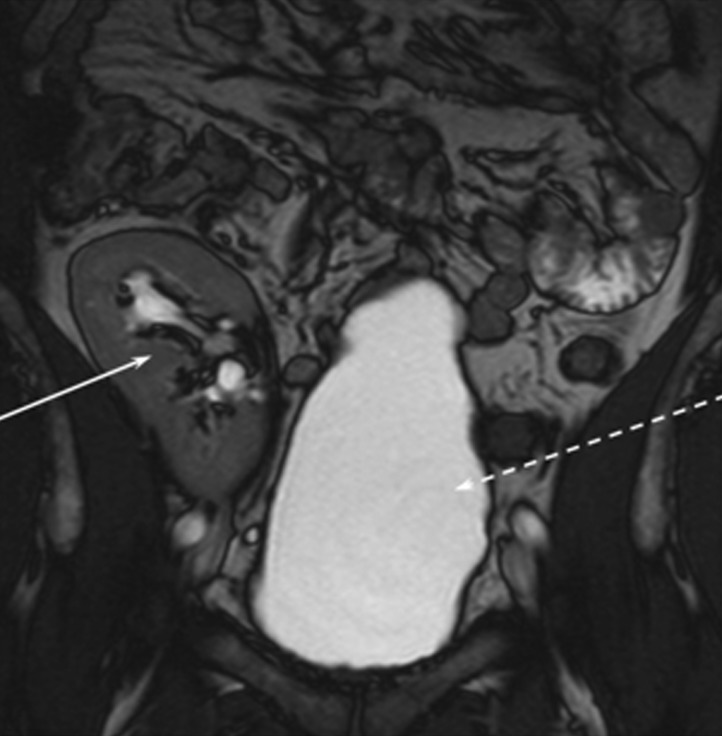
There was a clear family history of dominantly inherited DI (Figure 1), but the data supporting the original diagnosis of NDI were no longer available. Therefore, affected members of the family with polyuria were traced and invited to undergo further investigation. Renal tract ultrasonography of the patient's sister (II:5) and her three sons (III:1, III:2, III:3) showed significantly increased bladder capacity, but adequate bladder emptying. Blood and urine tests showed no impairment of renal function or proteinuria. T1 magnetic resonance imaging (MRI) scans of the patient (Figure 2) and his affected sister revealed reduced signal intensity of the posterior lobe of the pituitary gland, which is consistent with CDI or NDI. There was also some bladder distension after his renal transplant (Figure 3). A therapeutic trial of low-dose desmopressin (DDAVP; 0.1 mg orally) was performed. His weight, plasma sodium, plasma osmolarity, urinary sodium and urinary osmolarity were measured at the start of the test (0 h), shortly before DDAVP was administered. Fluid intake was not restricted. The various parameters were measured again at 2 and 4 h post-dose. The test resulted in urinary osmolality rising from 155 mOsmol/kg at 0 h to 400 mOsmol/kg at 4 h post-dose (normal average range 300–900 mOsmol/kg). Urinary sodium levels also increased from 35 mmol/L at 0 h to 78 mmol/L at 4 h. His weight, plasma osmolarity and plasma sodium levels remained stable during the test. Similar responses to oral DDAVP were found in the other family members (Table 1).
Fig. 1.
Pedigree of the index patient and his family. The arrow indicates the index patient (II:3) and the number in parentheses refers to the age of diagnosis.
Fig. 2.
T1-weighted sagittal pituitary MRI of the patient. The images are pre-gadolinium (left) and post-gadolinium (right). Arrows indicate relative hypointensity of the posterior pituitary. Dashed arrow indicates the thin inferior portion of the pituitary stalk.
Fig. 3.
Coronal MRI of the patient. This image shows the transplant kidney (arrow) and a distended bladder (dashed arrow).
Table 1.
Blood results of the affected individuals during the desmopressin stimulation test
| Laboratory parameters | II:3 |
II:5 |
III:5 |
III:6 |
III:7 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 2 h | 4 h | 0 h | 2 h | 4 h | 0 h | 2 h | 4 h | 0 h | 2 h | 4 h | 0 h | 2 h | 4 h | |
| P-osm (mOsm/kg) | 284 | 281 | 280 | 287 | 268 | 273 | 292 | 293 | 293 | 287 | 286 | 294 | 287 | 288 | 293 |
| P-Na (mmol/L) | 138 | 136 | 135 | 140 | 132 | 132 | 143 | 144 | 141 | 142 | 142 | 141 | 138 | 142 | 143 |
| U-osm (mOsmol/kg) | 155 | 260 | 400 | 95 | 232 | 361 | 160 | 250 | 400 | 110 | 250 | 448 | 125 | 327 | 401 |
| U-Na (mmol/kg) | 35 | 65 | 78 | 18 | 53 | 49 | 18 | 30 | 66 | 22 | 40 | 46 | 12 | 17 | 50 |
| Weight (kg) | 90 | 90 | 90 | 60 | 60 | 60 | 79 | 79 | 79 | 50 | 50 | 50 | 50 | 50 | 50 |
Low-dose desmopressin (0.1 mg orally) was given at 0 h. No restriction of fluids.
P-osm, plasma osmolarity; P-Na, plasma sodium; U-osm, urine osmolarity; U-Na, urine sodium.
Genotyping with flanking microsatellite markers showed no evidence of genetic linkage with either the arginine vasopressin receptor (AVPR2) or aquaporin-2 (AQP2) genes, mutations in which are known causes of NDI. Linkage analysis using the flanking microsatellite markers D20S842 and D20S181 showed co-segregation between the arginine vasopressin (AVP) gene and affected status (Figure 3). Sequencing all the exons and exon–intron boundaries of AVP identified a novel c.225>G point mutation in the AVP gene of only those affected.
Unlike NDI, the treatment for CDI is oral or nasal DDAVP; however, the patient declined treatment and said he had always been able to cope with his polyuria. He remains well with a functioning renal transplant. The affected relatives of the index patient had also remained well despite refusing desmopressin therapy.
Discussion
Diagnostic difficulties in diabetes insipidus
DI is diagnosed in individuals with a total urine volume of >50 mL/kg/day and with a low osmolality (<300 mOsmol/kg) under ad libitum fluid intake conditions [6]. The patient was initially misdiagnosed with NDI, which demonstrates the diagnostic difficulty with DI. Traditionally, a fluid deprivation test and response to DDAVP are used. Sequential measurements of urine osmolarity are taken following fluid deprivation (usually ≤7 h) and after the administration of DDAVP. However, interpretation of the results can often be problematic, because similar results can be seen in partial forms of DI. The test performed by our group seemed to suggest that there was a positive response to the desmopressin stimulation test (>50% increase in urine osmolality), which can be seen in CDI. However, the diagnosis of CDI was only confirmed in conjunction with genotyping and pituitary studies. The hypertonic saline infusion (5% saline given at 0.05 mL/kg/min for 2 h [7]) with AVP assay test can be useful when fluid deprivation causes urine concentration before plasma osmolarity and plasma sodium rise above the normal range. This test should be administered and monitored carefully for signs of thrombophlebitis at the site of infusion and dehydration. Plasma vasopressin concentration is measured with a vasopressin assay. In CDI, plasma vasopressin levels remain low or undetectable or may increase slightly; in NDI, there is usually a further significant rise in levels following osmotic vasopressin stimulation.
MRI of the pituitary gland is another useful diagnostic tool. The normally hyperintense signal seen in the posterior pituitary with T1-weighted MRI tends to be diminished or absent in CDI, because of a loss of vasopressin [8]. However, this is not always observed, since a lack of the posterior pituitary ‘bright spot’ is also characteristic of NDI (attributed to the chronic stimulation of vasopressin release and its high turnover) as well as in ∼20% of normal subjects (more common in the elderly and may explain some age-related reduced urinary concentrating ability) [9]. The main value of MRI is in distinguishing partial forms of CDI and NDI from primary polydipsia, in which vasopressin release is suppressed and the ‘bright spot’ is preserved or even heightened.
Infants with NDI tend to present with signs and symptoms of failure to thrive and fluid depletion, sometimes with life-threatening hypernatraemia, soon after birth, whereas those with CDI tend to show signs of thirst and polyuria much later in early childhood [2, 10]. This difference is attributed to the gradual loss of pituitary vasopressin in inherited CDI, as was the pattern observed in our patient, his siblings and father (who recalled drinking rainwater from puddles as a child).
Long-standing severe polyuria with a high voiding load in DI can lead to bladder distension and high pressure with reflux, causing hydronephrosis and functional obstruction, especially if double micturition (voiding) is not taught and practised [11–13]. Voluntary retention of urine due to social embarrassment may also be an important contributory factor in children and young adults. Functional obstruction can result in urinary stasis and recurrent urinary tract infections with sepsis, adding to the loss of renal function [6]. Our patient seems to be the first reported example of CDI progressing to renal failure. He was not hypertensive and never underwent a renal biopsy, making the loss of renal function difficult to explain. We postulate that the cause was due to chronic outflow tract obstruction with reflux, exacerbated by subclinical recurrent urinary tract infections. So far, the other affected family members with CDI, despite large bladder volumes, have preserved renal function.
Linkage analysis and pathogenesis
Linkage analysis using the flanking microsatellite markers D20S842 and D20S181 showed co-segregation between AVP and the disease. The logarithm of odds (LOD) score is a statistical test performed to determine the likelihood of genetic linkage, and a positive score supports the presence of genetic linkage. A maximum LOD score of 1.8 was obtained for D20S181 (9Kb distal to AVP). The novel c.225>G point mutation in affected individuals translates to a substitution of cysteine by tryptophan at position 75 in the neurophysin II domain of the AVP gene. Familial CDI has been associated with ∼70 mutations [14]. These mutations change the primary structure of the preprohormone such that there is impaired folding, dimerization and disulphide bonding of the vasopressin precursor. These mutant hormone precursors may be cytotoxic and cause progressive degeneration of the AVP-producing magnocellular neurons in the posterior pituitary. This is known as the misfolding-neurotoxicity hypothesis [15]. The variable age of onset and severity of symptoms in affected individuals could be due to the variable cytotoxic accumulation of misfolded protein, which is likely to depend on the degree of impaired protein folding [3].
Conclusion
Although DI can be difficult to diagnose clinically, measurement of endogenous vasopressin levels is usually the easiest way to differentiate CDI (low or undetectable levels) from NDI (high levels). A hypertonic saline infusion as a challenge test for vasopressin stimulation can be used in cases of diagnostic uncertainty. However, this depends on an available and validated vasopressin assay and appropriate blood sample collection with rapid separation of plasma by centrifugation and freezing for storage. Obtaining a careful family history is also very important, as is a properly conducted water deprivation and vasopressin stimulation test, when indicated. Finally, patients with persistent polyuria following a renal transplant should be monitored closely and investigated further. Early diagnosis and treatment, along with regular kidney and bladder surveillance, in patients with DI may impede progression to end-stage renal failure.
Conflict of interest statement
None declared.
Acknowledgements
We would like to thank Claire Willoughby, Fayha Ahmed, Nikhil Johri, Ana Teixeira, William White, James Rosenberg, Jessica Mozersky and Daniel Bichet for their help and advice.
References
- 1.Robertson GL. Diabetes insipidus. Endocrinol Metab Clin North Am 1995; 24: 549–572 [PubMed] [Google Scholar]
- 2.Bichet DG. Nephrogenic diabetes insipidus. Adv Chronic Kidney Dis 2006; 13: 96–104 [DOI] [PubMed] [Google Scholar]
- 3.Christensen JH, Rittig S. Familial neurohypophyseal diabetes insipidus—an update. Semin. Nephrol 2006; 26: 209–223 [DOI] [PubMed] [Google Scholar]
- 4.Zender HO, Ruedin P, Moser F, et al. Traumatic rupture of the urinary tract in a patient presenting nephrogenic diabetes insipidus associated with hydronephrosis and chronic renal failure: case report and review of the literature. Clin Nephrol 1992; 38: 196–202 [PubMed] [Google Scholar]
- 5.Linshaw MA, Stapleton FB, Knapp J. Growth failure: nephrogenic diabetes insipidus. J Kans Med Soc 1977; 78: 345–347 [PubMed] [Google Scholar]
- 6.Babey M, Kopp P, Robertson GL. Familial forms of diabetes insipidus: clinical and molecular characteristics. Nat Rev Endocrinol 2011; 7: 701–714 [DOI] [PubMed] [Google Scholar]
- 7.Bockenhauer D, Bichet DG. Pathophysiology, diagnosis and management of nephrogenic diabetes insipidus. Nat Rev Nephrol 2015; 11: 576–588 [DOI] [PubMed] [Google Scholar]
- 8.Kurokawa H, Fujisawa I, Nakano Y, et al. Posterior lobe of the pituitary gland: correlation between signal intensity on T1-weighted MR images and vasopressin concentration. Radiology 1998; 207: 79–83 [DOI] [PubMed] [Google Scholar]
- 9.Terano T, Seya A, Tamura Y, et al. Characteristics of the pituitary gland in elderly subjects from magnetic resonance images: relationship to pituitary hormone secretion. Clin Endocrinol (Oxf) 1996; 45: 273–279 [DOI] [PubMed] [Google Scholar]
- 10.van Lieburg AF, Knoers NV, Monnens LA. Clinical presentation and follow-up of 30 patients with congenital nephrogenic diabetes insipidus. J Am Soc Nephrol 1999; 10: 1958–1964 [DOI] [PubMed] [Google Scholar]
- 11.Nakada T, Miyauchi T, Sumiya H, et al. Nonobstructive urinary tract dilatation in nephrogenic diabetes insipidus. Int Urol Nephrol 1990; 22: 419–427 [DOI] [PubMed] [Google Scholar]
- 12.Uribarri J, Kaskas M. Hereditary nephrogenic diabetes insipidus and bilateral nonobstructive hydronephrosis. Nephron 1993; 65: 346–349 [DOI] [PubMed] [Google Scholar]
- 13.Lindenthal V, Mainberger A, Morris-Rosendahl DJ, et al. Dilatative uropathy as a manifestation of neurohypophyseal diabetes insipidus due to a novel mutation in the arginine vasopressin-neurophysin-II gene. Klin Padiatr 2013; 225: 407–412 [DOI] [PubMed] [Google Scholar]
- 14.Leroy C, Karrouz W, Douillard C, et al. Diabetes insipidus. Ann Endocrinol (Paris) 2013; 74: 496–507 [DOI] [PubMed] [Google Scholar]
- 15.Rittig S, Robertson GL, Siggaard C, et al. Identification of 13 new mutations in the vasopressin-neurophysin II gene in 17 kindreds with familial autosomal dominant neurohypophyseal diabetes insipidus. Am J Hum Genet 1996; 58: 107–117 [PMC free article] [PubMed] [Google Scholar]