Abstract
This is a report of a patient with minimal change disease (MCD) onset after bevacizumab administration. A 72-year-old man with inoperable Grade 3 astrocytoma was treated with a combination of temozolomide and the vascular endothelial growth factor monoclonal antibody bevacizumab. After two biweekly treatments, he developed nephrotic syndrome. Despite cessation of bevacizumab, his renal function deteriorated and a renal biopsy disclosed MCD. Thereafter, he was started on high-dose oral prednisone and renal function immediately improved. Within weeks, the nephrotic syndrome resolved. Although rare, biologic agents can cause various glomerulopathies that can have important therapeutic implications. MCD should be considered in patients who develop nephrotic syndrome while exposed to antiangiogenic agents.
Keywords: angiogenesis, glomerulonephritis, nephrotic syndrome, nephrotoxicity, proteinuria
Introduction
Bevacizumab (Avastin; Genentech, South San Francisco, CA, USA) is a humanized monoclonal antibody to the vascular endothelial growth factor (VEGF). It is currently indicated for non-small cell lung cancer, renal cell carcinoma, breast cancer and ovarian cancer [1]. It was recently approved for recurrent high-grade gliomas, particularly glioblastoma multiforme [1–6]. The safety profile is well established and there have been several studies confirming its efficacy as well as tolerability in elderly patients with advanced malignancies [7].
Bevacizumab inhibits angiogenesis by direct binding of VEGF and disruption of VEGF receptor signaling. VEGF receptors are expressed predominantly on vascular endothelial cells. Other sites with increased expression are glomerular podocytes where VEGF receptors cover the glomerular basement membrane [8, 9]. Pathological VEGF signaling has been implicated in proteinuria associated with pre-eclampsia, diabetic nephropathy and treatment with mammalian target of rapamycin (m-TOR) inhibitors such as sirolimus and everolimus [10, 11].
Proteinuria, hypertension and thrombotic microangiopathy (TMA) have been increasingly reported as adverse effects from the administration of bevacizumab and other antiangiogenesis inhibitors [5, 12]. Acute kidney injury (AKI) due to TMA from this class of agents has been well described [13]. This lesion is associated with severe renal failure and is usually treated by withdrawal of the VEGF inhibitor rather than plasmapheresis [14]. The transient nature of proteinuria and self-limiting nature of avastin-induced disease practically means that proteinuria resolves only by withholding the offending agent. Biopsies are often only obtained for rare cases of glomerular disease resulting in ongoing proteinuria. New expert opinions are being formed as to when biopsies in anti-VEGF renal disease should be obtained [15]. Minimal change disease (MCD) in association with bevacizumab use has not been described de novo in adults with systemic anti-VEGF therapy. Previous case reports by and Pérez-Valdivia et al. [16] and Soto et al. [17] reported relapses of MCD after intravitreal administrations of bevacizumab, and both were treated with systemic steroids. Another case report from Japan noted persistent proteinuria with long-term use of systemic bevacizumab and described a complex glomerulopathy with finding of double contours without evidence of antibody staining, which attenuated with losartan use. In this case, however, the renal biopsy pattern was not consistent with MCD as seen here [18].
Case report
A 72-year-old Caucasian man diagnosed with a World Health Organization (WHO) Grade 3 anaplastic astrocytoma presented to Cedars Sinai Medical Center with AKI and 3+ proteinuria on urinalysis 4 weeks after the initiation of bevacizumab. He was initially diagnosed with glioblastoma multiforme in January 2012, after presenting with a generalized tonic–clonic seizure. He received radiotherapy and chemotherapy with dose-dense temozolomide in April 2012, with a good response.
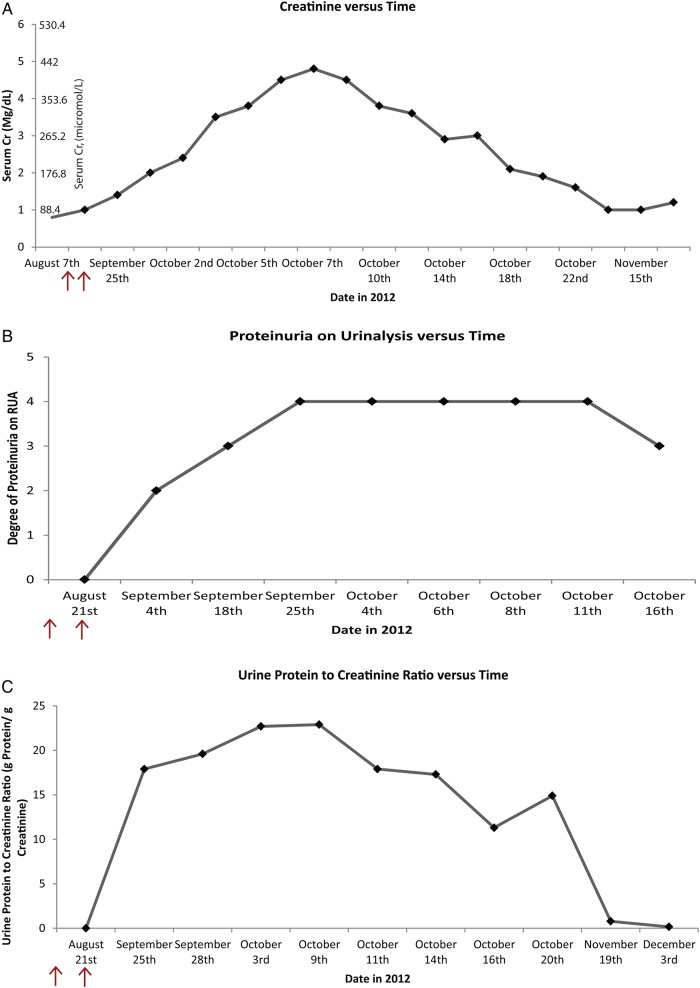
On 7 August 2012, the patient began a combination of temozolomide and bevacizumab, with scheduled bevacizumab every 2 weeks. Prior to initiating therapy he was noted to have type 2 diabetes mellitus without evidence of either diabetic retinopathy or high-grade proteinuria (he had microalbuminuria). Four weeks later, before his third treatment, he was found to have 3+ proteinuria on urinalysis and a mildly elevated serum creatinine of 88.4 μmol/L [1 mg/dL; baseline 70.72 μmol/L (0.8 mg/dL)]. Bevacizimab was held. The urine protein-to-creatinine ratio was markedly elevated (17.89 g of protein of creatinine). Two weeks later, he returned to his neuro-oncologist with 4+ proteinuria, hypertension, anasarca, severe hypoalbuminemia with serum albumin 231.84 μmol/L (1.6 g/dL) and worsening AKI with a serum creatinine of 123.76 μmol/L (1.4 mg/dL). Repeat urine protein-to-creatinine ratio was 19.51 g/g of creatinine (Figure 1 ).
Fig. 1.
Trends of serum creatinine, urinalysis proteinuria/albuminuria and urine protein-to-creatinine ratio. (A) Trend of serum creatinine versus time (serum creatinine in mg/dL). (B) Trend of urinalysis proteinuria versus time (1+ to 4+ proteinuria). (C) Trend of urine protein-to-creatinine ratio (g protein/g creatinine). Arrows: temporal representation of two avastin administrations timed 7 and 21 August 2012.
He was admitted for further renal evaluation. During that admission he developed progressive nonoliguric AKI with a peak creatinine of 424.32 μmol/L (4.8 mg/dL). Obstructive uropathy, acute interstitial nephritis (AIN) and acute tubular necrosis (ATN) were considered unlikely causes of AKI. Glomerulonephritis was suspected given the patient's high-grade proteinuria. Bevacizumab was thought to be the etiology of proteinuria and AKI, although the clinical presentation did not conform to the classic pattern of bevacizumab-induced TMA. A computed tomography (CT)–guided renal biopsy was performed to investigate the etiology of nephrotic syndrome and AKI.
The kidney biopsy included portions of cortex with 17 glomeruli, 4 of which were completely sclerosed. Importantly, there was no evidence of focal and segmental sclerosis (FSGS) on light microscopy, and renal pathology confirmed that the findings did not suggest FSGS. Most of the remaining glomeruli were structurally normal with patent capillary lumina and single contoured capillary walls. Few fresh fibrin thrombi were in some capillary lumina of three glomeruli. Cells of many proximal tubules were irregular and flattened and some lacked brush border staining. There was mild tubular atrophy with interstitial fibrosis. Other light microscopic changes were not evident. Immunofluorescence was negative in all glomeruli in that portion of the specimen [3].
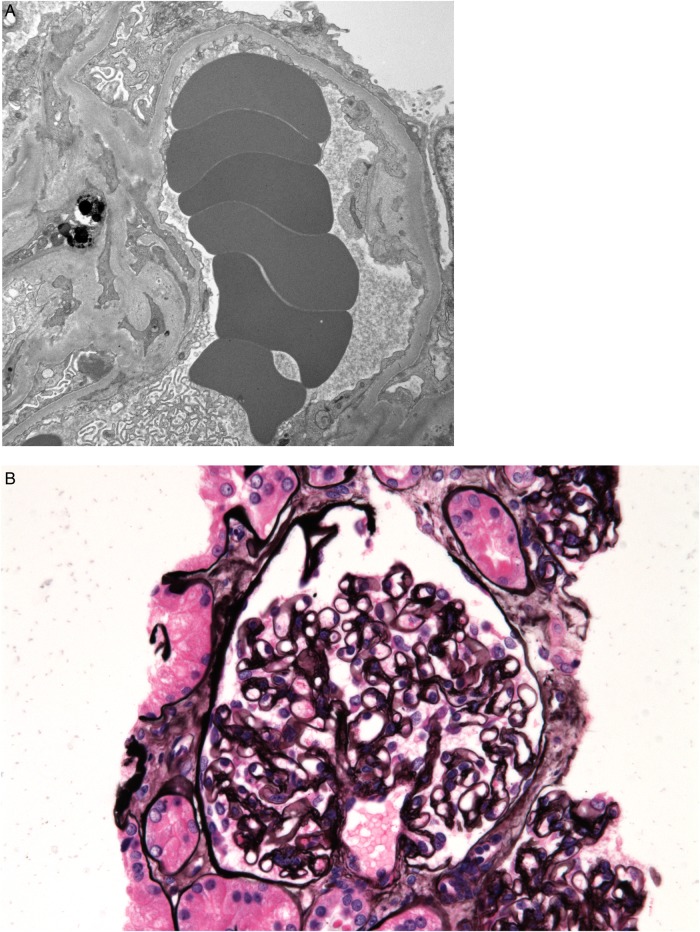
Electron microscopy disclosed complete effacement of podocyte foot processes, lack of electron dense deposits and no evidence of luminal or mural fibrin in the capillaries (Figure 2).
Fig. 2.
Biopsy specimens showing bevacizumab-induced MCD and fibrin thrombi. (A) Complete podocyte foot process effacement seen on electron microscopy. (B) Normal glomerulus (periodic acid-Schiff stain).
AKI and proteinuria persisted for >2 weeks after the withdrawal of bevacizumab. Deterioration of renal function resulted in worsening volume overload and uremia. Renal replacement therapy was considered. After the biopsy results, 1 mg/kg oral prednisone was started to treat the MCD.
Within 4 days after starting high-dose steroids, the patient's serum creatinine improved and renal replacement therapy was not needed. Proteinuria improved more gradually and decreased from 19 to 11 g in <1 week. It decreased further to <1 g after 6 weeks of steroid and angiotensin-converting enzyme inhibitor therapy, and subsequently resolved after nearly 2 months of therapy for MCD (Figure 1).
Discussion
To our knowledge, this is the first reported case of renal failure and de novo MCD associated temporally with systemic bevacizumab use. Previous cases noted above (Pérez-Valdivia et al. [16] and Soto et al. [17]) showed a relapse of MCD after intravitreal bevacizumab administration and Haruhara et al. [18] showed an atypical light microscopic pattern of double contouring without evidence of immune complex deposition. Renal failure, hypertension and transient proteinuria occur in up to 18% of patients receiving bevacizimab, although sustained proteinuria and renal failure are both rare features of bevacizimab nephrotoxicity [19]. It is important to recognize MCD as an adverse effect of therapy with bevacizimab since this patient did not respond to cessation of therapy alone and required high-dose steroid therapy for restoration of renal functions.
There have been other glomerulopathies associated with treatments of cancer with anti-VEGF and anti-epidermal growth factor (EGF) biologic agents. Bevacizimab has been associated with cryoglobulinemic glomerulonephritis, collapsing glomerulonephritis, membranoproliferative glomerulonephritis (MPGN) and the more commonly reported lesions of TMA [19]. Trastuzumab, which is a related antiendothelial growth factor receptor monoclonal antibody, has been linked to one case of MCD [19]. Sorafenib, axitinib, gefitinib, imatinib, sunitinib and small molecule tyrosine kinase inhibitors, which target signaling proteins downstream of the VEGF receptor, have been associated with focal segmental glomerulosclerosis (FSGS), ischemic glomerulonephritis, ATN and AIN (see Table 1) [15–30]. Sorafenib has recently been linked with MCD as well [31]. In the case of Haruhara et al. [19] mentioned previously, a complex glomerulopathy was noted that responded to renin–angiotensin systemic blockade with losartan.
Table 1.
Cases reports of glomerulopathies and kidney injury due to VEGF and EGF receptor inhibition
| Drug | Pathology | Citation |
|---|---|---|
| Anti-VEGF monoclonal antibodies (IV, intravitreal) adapted from Izzedine et al. [19] and updated | ||
| Bevacizumab-IV | MCD | Our case (Hanna et al.) reported 2015 |
| Bevacizumab-IV | Glomerulopathy with double contouring, nonimmune | Haruhara et al. [18] |
| Bevacizumab-intravitreal | MCD | Soto et al. [17] |
| Bevacizumab-intravitreal | MCD | Perez-Valdivia et al. [16] |
| Bevacizumab-IV | Collapsing glomerulonephritis | Johnson et al. [24] (compiled in Izzedine et al.) |
| Bevacizumab-IV | MPGN | Miller et al. [27] (compiled in Izzedine et al.) |
| Bevacizumab-IV | Thrombotic microangiopathy | Many reviews including Eremina et al. [21] |
| VEGF-trap/Aflibercept-route unknown | Crescentic glomerulonephritis | From unpublished data per Izzedine et al. |
| Anti-EGF monoclonal antibodies adapted from Izzedine et al. [19] | ||
| Trastuzumab-IV | MCD | Vidal [29] (compiled in Izzedine et al.) |
| Trastuzumab-IV | Focal and segmental glomerulosclerosis | Vidal [29] (compiled in Izzedine et al.) |
| Trastuzumab-IV | Fibrillary glomerulonephritis | Vidal [29] (compiled in Izzedine et al.) |
| Tyrosine kinase inhibitors (PO) downstream from VEGF and EGF signaling pathways adapted from Izzedine et al. [19] | ||
| Axitinib-PO | Collapsing glomerulonephritis | From unpublished data per Izzedine et al. |
| Gefitinib-PO | Minimal change nephropathy | Kumasuka et al. [26] (compiled in Izzedine et al.) |
| Gefitinib-PO | ATN | Wan and Yao [30] (compiled in Izzedine et al.) |
| Imatinib-PO | Thrombotic microangiopathy | Al aly et al. [20] (compiled in Izzedine et al.) |
| Imatinib-PO | ATN | Pou et al. [28] (compiled in Izzedine et al.) |
| Imatinib-PO | ATN | Kitiyakara and Atichartakarn [25] (compiled in Izzedine et al.) |
| Sorafenib-PO | AIN | From unpublished data per Izzedine et al. |
| Sunitinib-PO | Ischemic glomerulonephritis | From unpublished data per Izzedine et al. |
There are many reported glomerulopathies that have been reported chronologically and were first compiled by Izzedine et al. [19] in an article published in the American Journal of Kidney Disease [15–30]. A table was compiled to show all the reported cases of glomerulopathies noted in cases of anti-EGF and anti-VEGF therapies and expanded to include the new cases mentioned above [16–18]. The table's focus is on expanding the new case reports of glomerular disease specific to bevacizumab, while other references to diseases due to anti-EGF therapies and tyrosine kinase inhibitors are adapted from Izzedine et al. [19] (see Table 1).
It is striking that while these angiogenesis inhibitors act on the same pathway, they can cause renal failure by various pathophysiologic mechanisms. These adverse events are rare, but compounded together, they form a growing understanding of how these drugs affect renal molecular biology and physiology.
It is important to record the clinical course we observed in this patient, as this is the first de novo case of MCD linked to systemic bevacizimab. Usually, the proteinuria linked to this agent is transient and may resolve with withdrawal of the agent. The underlying pathologic diagnosis may be important in cases of persistent proteinuria.
Although rare, drug-induced glomerulopathies can be progressive and lead to end-stage renal disease unless diagnosed quickly and appropriately treated. In this patient, proteinuria and renal failure progressed despite stopping the offending agent and only improved after initiation of high-dose steroids. This suggests a role for high-dose steroids in patients with MCD associated with bevacizumab use. This diagnosis and associated treatment should be considered in patients with bevacizumab-associated nephrotic syndrome. Other case reports described above mention differing pathological patterns found on biopsy after development of glomerular proteinuria and AKI in conjunction with bevacizumab and other anti-VEGF and anti-EGF therapies [16–19]. In one of these cases, angiotensin receptor blockade resulted in attenuation of proteinuria due to bevacizumab therapy. The lesion in this case was associated with a double contour nonimmune glomerulopathy pattern on renal biopsy. Thus, it is important to distinguish that steroid therapy has only been shown to be efficacious in the literature with MCD pattern glomerular injury after anti-VEGF therapy with systemic or intravitreal bevacizumab [16–18].
Authors’ contribution
E.L. contributed to research on the glomerular disorders previously reported with bevacizumab use. J.W. contributed to the section on the pathophysiology of VEGF blockade. S.B. edited the document and contributed to the discussion section indispensably. A.H.C. is the pathologist who reviewed this case and contributed to the slide captions and the case report segment featuring the tissue pathology interpretation, as well as extensively editing the text.
Conflict of interest statement
None declared.
Acknowledgement
The results presented in this article have not been published previously in whole or part, except in abstract form.
References
- 1.Rossari JR, Metzger-Filho O, Paesmans M, et al. Bevacizumab and breast cancer: a meta-analysis of first-line phase III studies and a critical reappraisal of available evidence. J Oncol 2012; 2012: 417673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harshman LC, Xie W, Bjarnason GA, et al. Conditional survival of patients with metastatic renal-cell carcinoma treated with VEGF-targeted therapy: a population-based study. Lancet Oncol 2012; 13: 927–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyake TM, Sood AK, Coleman RL. Contemporary use of bevacizumab in ovarian cancer. Expert Opin Biol Ther 2013; 13: 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mrugala MM, Crew LK, Fink JR, et al. Carboplatin and bevacizumab for recurrent malignant glioma. Oncol Lett 2012; 4: 1082–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soria JC, Mauguen A, Reck M, et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol 2013; 24: 20–30 [DOI] [PubMed] [Google Scholar]
- 6.Sweet JA, Feinberg ML, Sherman JH. The role of avastin in the management of recurrent glioblastoma. Neurosurg Clin N Am 2012; 23: 331–341 [DOI] [PubMed] [Google Scholar]
- 7.Rosati G, Avallone A, Aprile G, et al. XELOX and bevacizumab followed by single-agent bevacizumab as maintenance therapy as first-line treatment in elderly patients with advanced colorectal cancer: the Boxe study. Cancer Chemother Pharmacol 2013; 71: 257–264 [DOI] [PubMed] [Google Scholar]
- 8.Henao DE, Cadavid AP, Saleem MA. Exogenous vascular endothelial growth factor supplementation can restore the podocyte barrier-forming capacity disrupted by sera of preeclamptic women. J Obstet Gynaecol Res 2013; 39: 46–52 [DOI] [PubMed] [Google Scholar]
- 9.Jin J, Sison K, Li C, et al. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell 2012; 151: 384–399 [DOI] [PubMed] [Google Scholar]
- 10.Kirsch AH, Riegelbauer V, Tagwerker A, et al. The mTOR-inhibitor rapamycin mediates proteinuria in nephrotoxic serum nephritis by activating the innate immune response. Am J Physiol Renal Physiol 2012; 303: F569–F575 [DOI] [PubMed] [Google Scholar]
- 11.Mima A, Kitada M, Geraldes P, et al. Glomerular VEGF resistance induced by PKCdelta/SHP-1 activation and contribution to diabetic nephropathy. FASEB J 2012; 26: 2963–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans T. Utility of hypertension as a surrogate marker for efficacy of antiangiogenic therapy in NSCLC. Anticancer Res 2012; 32: 4629–4638 [PubMed] [Google Scholar]
- 13.Bollee G, Patey N, Cazajous G, et al. Thrombotic microangiopathy secondary to VEGF pathway inhibition by sunitinib. Nephrol Dial Transplant 2009; 24: 682–685 [DOI] [PubMed] [Google Scholar]
- 14.Hayman SR, Leung N, Grande JP, et al. VEGF inhibition, hypertension, and renal toxicity. Curr Oncol Rep 2012; 14: 285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izzedine H. Anti-VEGF cancer therapy in nephrology practice. Int J Nephrol 2014; 2014: 143426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez-Valdivia MA, Lopez-Mendoza M, Toro-Prieto FJ, et al. Relapse of minimal change disease nephrotic syndrome after administering intravitreal bevacizumab. Nefrologia 2014; 34: 421–422 [DOI] [PubMed] [Google Scholar]
- 17.Sato T, Kawasaki Y, Waragai T, et al. Relapse of minimal change nephrotic syndrome after intravitreal bevacizumab. Pediatr Int 2013; 55: e46–e48 [DOI] [PubMed] [Google Scholar]
- 18.Haruhara K, Tsuboi N, Nakao M, et al. A case of glomerulopathy associated with the vascular endothelial growth factor inhibitor bevacizumab. Nihon Jinzo Gakkai Shi 2014; 56: 600–605 [PubMed] [Google Scholar]
- 19.Izzedine H, Rixe O, Billemont B, et al. Angiogenesis inhibitor therapies: focus on kidney toxicity and hypertension. Am J Kidney Dis 2007; 50: 203–218 [DOI] [PubMed] [Google Scholar]
- 20.Al Aly Z, Philoctete Ashley JM, Gellens ME, et al. Thrombotic thrombocytopenic purpura in a patient treated with imatinib mesylate: true association or mere coincidence? Am J Kidney Dis 2005; 45: 762–768 [DOI] [PubMed] [Google Scholar]
- 21.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 2008; 358: 1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izzedine H, Brocheriou I, Deray G, et al. Thrombotic microangiopathy and anti-VEGF agents. Nephrol Dial Transplant 2007; 22: 1481–1482 [DOI] [PubMed] [Google Scholar]
- 23.Izzedine H, Sene D, Hadoux J, et al. Thrombotic microangiopathy related to anti-VEGF agents: intensive versus conservative treatment? Ann Oncol 2011; 22: 487–490 [DOI] [PubMed] [Google Scholar]
- 24.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004; 22: 2184–2191 [DOI] [PubMed] [Google Scholar]
- 25.Kitiyakara C, Atichartakarn V. Renal failure associated with a specific inhibitor of BCR-ABL tyrosine kinase, STI 571. Nephrol Dial Transplant 2002; 17: 685–687 [DOI] [PubMed] [Google Scholar]
- 26.Kumasaka R, Nakamura N, Shirato K, et al. Side effects of therapy: case 1. Nephrotic syndrome associated with gefitinib therapy. J Clin Oncol 2004; 22: 2504–2505 [DOI] [PubMed] [Google Scholar]
- 27.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 2005; 23: 792–799 [DOI] [PubMed] [Google Scholar]
- 28.Pou M, Saval N, Vera M, et al. Acute renal failure secondary to imatinib mesylate treatment in chronic myeloid leukemia. Leuk Lymphoma 2003; 44: 1239–1241 [DOI] [PubMed] [Google Scholar]
- 29.2006. Vidal. Herceptin. http://www.vidal.fr. (12 September 2012, date last accessed)
- 30.Wan HL, Yao NS. Acute renal failure associated with gefitinib therapy. Lung 2006; 184: 249–250 [DOI] [PubMed] [Google Scholar]
- 31.Overkleeft EN, Goldschmeding R, van Reekum F, et al. Nephrotic syndrome caused by the angiogenesis inhibitor sorafenib. Ann Oncol 2010; 21: 184–185 [DOI] [PubMed] [Google Scholar]