Abstract
Background
Anti-glomerular basement membrane (anti-GBM) disease classically presents with aggressive necrotizing and crescentic glomerulonephritis, often with pulmonary hemorrhage. The pathologic hallmark is linear staining of GBMs for deposited immunoglobulin G (IgG), usually accompanied by serum autoantibodies to the collagen IV alpha-3 constituents of GBMs.
Methods
Renal pathology files were searched for cases with linear anti-GBM to identify cases with atypical or indolent course. Histopathology, laboratory studies, treatment and outcome of those cases was reviewed in detail.
Results
Five anti-GBM cases with atypical clinicopathologic features were identified (accounting for ∼8% of anti-GBM cases in our laboratory). Kidney biopsies showed minimal glomerular changes by light microscopy; one patient had monoclonal IgG deposits in an allograft (likely recurrent). Three patients did not have detectable serum anti-GBM by conventional assays. Three patients had indolent clinical courses after immunosuppressive treatment. One patient, untreated after presenting with brief mild hematuria, re-presented after a short interval with necrotizing and crescentic glomerulonephritis.
Conclusions
Thorough clinicopathologic characterization and close follow-up of patients with findings of atypical anti-GBM on renal biopsy are needed. Review of the literature reveals only rare well-documented atypical anti-GBM cases to date, only one of which progressed to end-stage kidney disease.
Keywords: anti-glomerular basement membrane disease, crescentic glomerulonephritis, Goodpasture's
Introduction
Anti-glomerular basement membrane (anti-GBM) disease encompasses tissue injury caused by autoantibodies to constituents of the GBM, most commonly the non-collagenous domain (NC1) of the alpha-3 subunit of type IV collagen [1–4]. The hallmark of this disease is continuous linear deposition of immunoglobulin [usually immunoglobulin G (IgG)] along GBMs, demonstrated by immunofluorescence microscopy [1–4]. Tissue injury typically manifests as diffuse necrotizing and crescentic glomerulonephritis [1–3]. Accompanying pulmonary hemorrhage occurs in ∼50% of patients when the antibody also reacts to this protein in pulmonary basement membranes (termed Goodpasture's syndrome). Rarely, patients present with pulmonary hemorrhage without glomerulonephritis [1, 2, 4]. Anti-GBM antibodies can be demonstrated in serum with conventional enzyme-linked immunosorbent assay (ELISA) in 87–90% of patients [1]. Given the intensity of the renal injury, most patients present with markedly elevated serum creatinine, hematuria, and active urine sediment with or without pulmonary hemorrhage. These features may be preceded by malaise and a viral-like prodrome.
We have observed patients with anti-GBM disease, as characterized by strong linear IgG immunofluorescence staining, with atypical histopathologic findings and/or clinical course, including several with subacute presentation. We highlight the variability of histopathology, clinical presentation, laboratory findings and outcome of patients with atypical anti-GBM disease.
Materials and methods
This study was approved by the Institutional Review Board of Oregon Health & Science University. The computerized pathology files (1999–2014) were searched for renal biopsies showing moderate to strong linear glomerular capillary wall IgG staining. One case from a prior time period was added from the author's file (D.C.H.). Patients with diabetes or heavy proteinuria were excluded as they have been associated with nonspecific linear IgG immunofluorescence. Cases with typical anti-GBM disease (acute clinical nephritis, with necrotizing and crescentic glomerulonephritis) were not further studied. Cases had been submitted for diagnostic review and were processed using standard methods for renal biopsy; available slides and images were reviewed. Clinical and laboratory data were obtained by chart review or from the referring nephrologists.
Results
A search of the Pathology files at Oregon Health & Science University yielded 47 cases of anti-GBM disease, ∼1% of the native biopsies submitted. Of those, four biopsies (8%) had atypical histopathologic or clinical features, and are described below, along with an additional case from one of the author's files.
Case 1
Clinical history
A 68-year-old Caucasian male underwent a renal allograft biopsy to evaluate gross hematuria and rising creatinine from 0.7 to 1.2 mg/dL, 3 months following transplantation (Table 1).
Table 1.
Clinical features of patients with atypical anti-GBM at index biopsy
| Patient |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Age (years) | 67a | 23 | 60b | 64 | 31 |
| Sex | Male | Male | Female | Male | Male |
| Smoking history | Former, quit 45 years agoa | Smoker | Never smoker | Unknown | Unknown |
| Serum creatinine (mg/dL) | 1.2 | 1.6 | 0.73 | 7.1 | ‘Normal’ |
| Hematuria | Yes, gross | Yes, 100–200 RBCs | Yes, >50 RBCs, non-dysmorphic | n/a | Yes |
| Proteinuria | Urine Pr:Cr = 1.2 | Urine Pr:Cr = 3.25 | 0.4 g/day | n/a | n/a |
| Anti-GBM | IFA positive once, ELISA and other time points negative | Negative | Weak positive at 1.5 (upper limit of normal = 1) | Positive 112 (nl <20) |
Positive |
| ANCA | Negative | Negative | Negative | Negative | n/a |
| Complements | Normal | Normal | Normal | Mildly low C3 = 82 (nl 83–177) C4 = 7 (nl 12–36) |
n/a |
| Other | ANA negative Hematologic workup negative, including sPEP/uPEP, free light chains and peripheral blood flow cytometry |
ANA negative RF negative Hepatitis B, C negative HIV negative sPEP normal |
ANA negative ESR and CRP elevated; IgG subclasses normal |
IgG-lambda monoclonal spike (>3 g), ESR = 144, Hgb = 7.6 with normal WBC and platelet count | Pulmonary hemorrhage |
ANA, anti-nuclear antibody; ANCA, anti-neutrophil cytoplasmic antibody; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ELISA, enzyme-linked immunosorbent assay; Hgb, hemoglobin; HIV, human immunodeficiency virus; IFA, indirect immunofluorescence assay; n/a, not available; nl, normal range; Pr:Cr, protein to creatinine ratio; RBC, red blood cell; RF, rheumatoid factor; sPEP/uPEP, serum protein electrophoresis/urine protein electrophoresis; WBC, white cell count.
aProteinuria at age 18 years; also occupational exposure to wood smoke and sulfuric acid fumes.
bFirst presentation at age 44 years.
His past medical history included asymptomatic proteinuria at the age of 18 years discovered during a pre-employment assessment, not further evaluated. At the age of 61 years, he underwent right nephrectomy for renal cell carcinoma (clear cell type, 3.1 cm size, stage pT1a, NX). This was followed by recurrent urinary tract infections requiring prolonged antibiotic treatment, new onset hypertension and hematuria attributed at that time to a renal stone. His renal function progressively deteriorated over the following year, and he began hemodialysis. He had no family history of renal disease, hearing defects, hematuria or vision abnormalities.
He received a four antigen HLA mismatched kidney transplant from a standard criteria deceased donor after 52 months on dialysis. Graft function was excellent with creatinine of 0.7 mg/dL (basiliximab induction; standard tacrolimus, prednisone and mycophenolate mofetil immunosuppression). Persistent microscopic hematuria was detected on Postoperative Day (POD) 15, and, gross hematuria developed 1 week after ureteral stent removal. His urine contained dysmorphic red blood cells (RBCs) without casts; proteinuria (UPr:Cr) of 1.2 (up from 0.4), and serum creatinine rose to 1.2 mg/dL. Serologic studies including anti-GBM antibody were negative (Table 1). Imaging was unrevealing, and cystoscopy noted allograft origin of the hematuria. The recipient of contralateral kidney from the same donor had no hematuria. An allograft biopsy was performed at POD 83.
Pathologic findings
Histopathologic findings are detailed in Table 2 and illustrated in Figure 1. Unexpectedly, serial allograft biopsies studied by immunofluorescence showed ribbon-like linear staining of GBMs for IgG-lambda; further characterized as IgG3-lambda monotypic. Despite the linear staining and the presence of RBC casts, cellular crescents were not seen; only mild glomerulitis and focal mild endocapillary/mesangial hypercellularity were noted (Figure 1). There were no deposits seen by electron microscopy. Progressing interstitial fibrosis and tubular atrophy (IFTA) have been observed on multiple biopsies examined over a period of 12 months since transplantation, without evidence of rejection. The 1-year biopsy demonstrated a greater proportion of glomeruli involved by mild mesangial and endocapillary hypercellularity, along with two fibrocellular crescents.
Table 2.
Histopathologic findings in atypical anti-GBM patients
| Patient |
|||||
|---|---|---|---|---|---|
| 1 (First biopsy) | 2 | 3 | 4 | 5 | |
| Number of glomeruli | 31 | 24 | 12 | 15 | 7 |
| Number of global sclerosis (%) | 0 (0%) | 2 (8%) | 3 (25%) | 3 (20%) | 0 (0%) |
| Number of segmental sclerosis/adhesion (%) | 0 (0%) | 5 (21%) | 0 (0%) | 1 (7%) | 0 (0%) |
| Number of cellular crescents | 0 | 2 (minute) | 1a segmental | 0 | 0 |
| Number of fibrous crescents | 0 | 4 | 0 | 0 | 0 |
| Other glomerular findings | Minimal glomerulitis; focal mild mesangial and endocapillary hypercellularity | Mesangial sclerosis and proliferation; several ischemic | 1-Amorphous fibrinous/fibrous debris with inflammation | 1-Endocapillary proliferation 1-intracapillary fibrin 1 loop mesangial sclerosis |
Mild mesangial sclerosis |
| IFTA (%) | 10 | 20 | 10 | 50 | 20 |
| Interstitial inflammation | None | Patchy with eosinophils | <5% | Acute interstitial nephritis (neutrophils, mononuclear cells) | None |
| Arteriosclerosis | Mild | Mild | Mild | Severe | Mild |
| RBC casts | Many, with ATN | Rare | Many | Rare | Absent |
| Immunofluorescence | 1 (Second biopsy) | 2 | 3 | 4 | 5 |
| IgG | 2–3+ Linear loop IgG3 1–2+ linear IgG1,2,4-negative |
3+ Linear loop | 2+ Linear loop with mesangial granular accentuation IgG1 1-2+ linear IgG2,3,4-negative |
2–3+ Linear loop | 3+ Linear loop |
| Kappa | Negative | Not done | 1+ as IgG | 2–3+ Linear loop | Not done |
| Lambda | 1–2+ linear loop | Not done | Trace as IgG | 2–3+ Linear loop | Not done |
| IgM | Negative | Not reviewed | Trace linear loop | Negative | Negative |
| IgA | Negative | Not reviewed | 2+ Mesangial | Negative | Negative |
| C3 | Negative | Not reviewed | 1+ Mesangial | Negative | 2+ Spotty but diffuse capillary wall |
| C1q | Negative | Not done | Negative | Negative | Not done |
| Fibrinogen | Negative | Not done | Negative | Not done | Negative |
| Electron microscopy | 1 (First and third biopsies, native nephrectomy) | 2 | 3 | 4 | 5 |
| Descriptive findings | No deposits. Normal GBM thickness and texture. Local podocyte effacement in first biopsy only | No deposits. GBMs thick (600 nm), and mesangial sclerosis | Few granular deposits in segmental mesangial zones; no organized deposits | No deposits. Some GBMs thickened | n/a |
ATN, acute tubular necrosis; GBM, glomerular basement membrane.
aNot present on initial 16 levels; seen on additional levels.
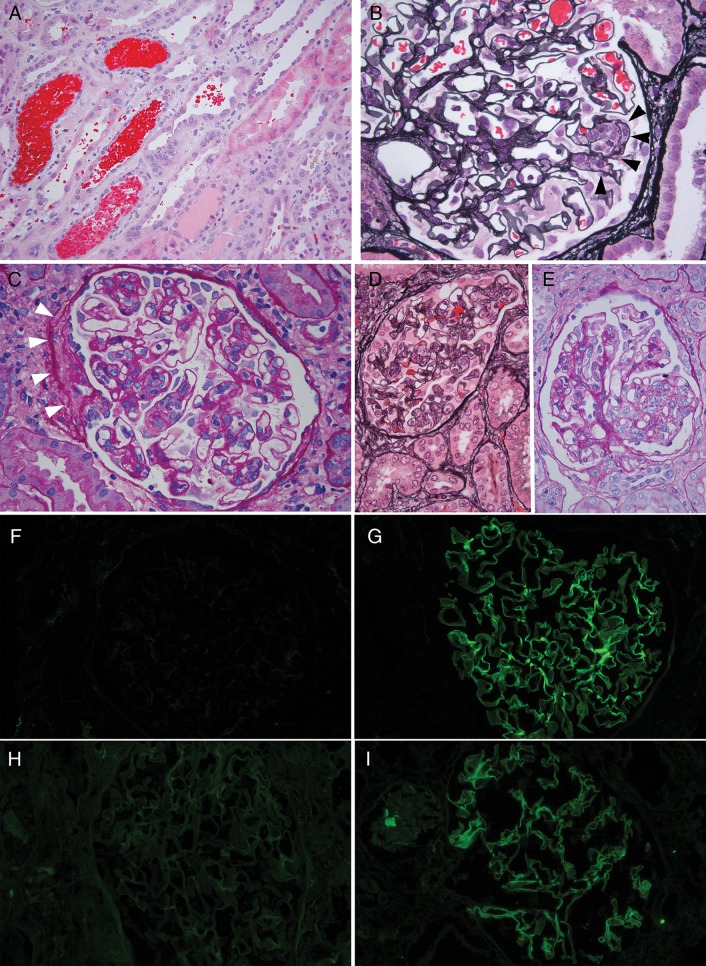
Fig. 1.
Pathology of allograft biopsies and native nephrectomy, Patient 1. (A) Biopsy Post-transplant Day 96 showing numerous RBC casts and tubular injury. (B) Biopsy Post-transplant Day 96, glomerulus with segmental endocapillary hypercellularity (black arrowheads). (C) Biopsy Post-transplant Day 342, glomerulus with segmental mesangial and endocapillary hypercellularity. White arrowheads highlight segmental fibrosis, consistent with fibrocellular crescent. There were also prominent RBC casts in tubules (not shown). (D) Native nephrectomy specimen showing glomerulus with endocapillary proliferation and reactive appearing extracapillary cells. (E) Native nephrectomy specimen with segmental mesangial proliferation. (F-I) Immunofluorescence microscopy. Each of the tested biopsies showed ultrathin linear glomerular capillary loop staining for IgG3 and lambda. IgG1 and kappa were negative. IgG2 and IgG4 were also negative (not shown). (F) IgG1 immunofluorescence, Day 342. (G) IgG3 immunofluorescence, Day 342. (H) Kappa light-chain immunofluorescence, Day 160. (I) Lambda light-chain immunofluorescence, Day 160. Hematoxylin & eosin (H&E) stain panel A; Jones silver stain panels B and D; Periodic acid Schiff (PAS) stain panel.
In addition, slides from the native nephrectomy specimen from 7 years earlier were obtained from the referring center for retrospective review (Figure 1). The findings included numerous RBC casts and mild segmental proliferation and glomerulitis, without crescents. There were no electron-dense or fibrillary deposits and no basement membrane changes of Alport's syndrome on retrospective electron microscopic analysis of the native nephrectomy.
Diagnosis and subsequent course
The findings were interpreted as a form of monoclonal anti-GBM disease in the allograft. Furthermore, findings in the native nephrectomy specimen were concerning for the same process. Additional anti-GBM studies were pursued; serum from POD 100 was reported as positive by indirect immunofluorescence antibody testing [immunofluorescence assay (IFA)], but was negative by qualitative multi-analyte fluorescence detection. Sera from later time points, however, were repeatedly negative for anti-GBM by ELISA or IFA tests. Given the apparent monotypia of the deposits, further hematologic studies included serum and urine protein electrophoresis, evaluation of peripheral blood for free light chains and flow cytometric analysis of lymphocytes, all of which were negative for a clonal lymphoproliferative process. The patient was treated with plasmapheresis, intravenous immunoglobulin, steroids and a single dose of rituximab. Thereafter, he resumed his baseline triple immunosuppressive therapy, and despite increasing IFTA, maintains graft function (creatinine 1.0 mg/dL) with 2+ proteinuria and >50 RBCs on spot urinalysis, 56 months post-transplant.
Case 2
Clinical history
A 23-year-old man was referred to Nephrology with hematuria, proteinuria (3.25 UPr:Cr, Table 1) and elevated creatinine (1.6 mg/dL). Past history included absent/small right kidney apparently congenital, and intermittent hematuria for at least 8 years, with negative cystoscopy. Additional laboratory studies were negative (Table 1), including repeated anti-GBM studies.
Pathologic findings
A kidney biopsy demonstrated glomerular mesangial sclerosis and hypercellularity. Small segmental cellular crescents were apparent in two glomeruli, while nine others showed evidence of fibrous crescents, and/or tuft adhesions (Table 2 and Figure 2). There were also moderate IFTA, focal mixed (lymphoplasmacytic with eosinophils) interstitial inflammation and mild arteriosclerosis.
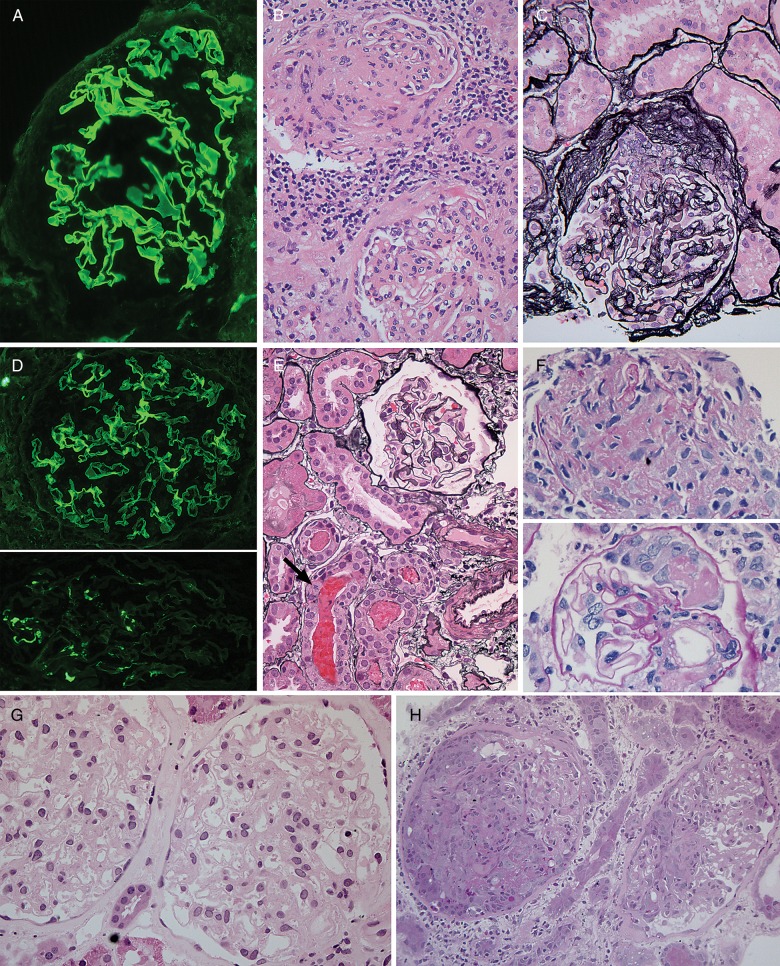
Fig. 2.
Histopathologic findings in atypical anti-GBM disease. (A-C) Patient 2. (A) IgG immunofluorescence staining shows strong, linear staining of GBMs. (B) H&E stain showing segmental glomerular scar (top), interstitial inflammation (middle) and glomerulus with mesangial expansion and tuft adhesions (bottom). (C) Jones silver stain shows fibrocellular crescent. (D-F) Patient 3. (D) IgG immunofluorescence staining shows strong, linear staining of GBMs in top panel. Bottom, IgA with granular mesangial staining. (E) The majority of glomeruli, as shown here with Jones silver stain, were histologically normal. RBC casts were seen, at bottom. Arrow denotes tubular epithelial cell with mitotic figure. (F) The only two abnormal glomerular foci, not present on initial levels, including fibrin-inflammatory focus (top panel), and small segmental crescent with fibrin (bottom panel), PAS stain. (G-H) Patient 5. (G) First biopsy with histologically normal glomeruli (H&E) despite strong linear IgG immunofluorescence staining (not shown). (H) Biopsy 3 months later with necrotizing and crescentic glomerulonephritis (PAS stain). Original magnifications: 200× B–C, H; 400×: A, D–G.
Immunofluorescence studies at the referring laboratory showed bright thin linear IgG staining along GBMs. Electron microscopy showed mesangial sclerosis and GBM thickening of ∼600 nm. A few loops exhibited basement membrane duplication with cellular interposition, but no immune-type or fibrillary electron-dense deposits.
Diagnosis and subsequent course
This biopsy was interpreted as anti-GBM disease with mesangial and GBM sclerosis and focal segmental glomerular scarring. The longstanding history of hematuria and the presence of fibrocellular crescents were consistent with a subacute course.
The patient was treated with a regimen of plasmapheresis (seven treatments, each one plasma volume), cyclophosphamide (175 mg oral/day for 3 months) and intravenous methylprednisolone (1 g × 3 days, with a 6-month oral taper). His proteinuria gradually decreased during the course of therapy, reaching UPr:Cr of 0.4 by the end of the cyclophosphamide course, and hematuria completely resolved. Creatinine had peaked at 1.8 mg/dL, improved with therapy, reaching a nadir of 1.3 mg/dL, and was sustained at 1.4–1.5 mg/dL at 2 years follow-up without hematuria, with angiotensin receptor blocker treatment.
Case 3
Clinical history
A 60-year-old woman presented with fever of unknown origin, with temperatures to 103°F followed by drenching night sweats. Her only other symptoms were dysphagia and a 20-lb weight loss. Physical exam showed minimal lymphadenopathy, and the patient had no joint, skin or lung complaints. Her creatinine was normal (0.73 mg/dL, Table 1), while urinalysis showed >50 RBCs (4+ blood) but no dysmorphic RBCs. Proteinuria on order of 400 mg/day, and urine eosinophils were noted. A single blood culture was negative. An anti-GBM study was weakly positive at 1.2–1.5 (normal <1).
Sixteen years earlier, the patient had experienced a similar febrile illness with hematuria, renal insufficiency, reportedly negative serologic studies and no proteinuria. At that time, she improved with prednisone and methotrexate therapy, with interim urinalysis negative for protein and blood.
Pathologic findings
A kidney biopsy at the time of contemporary re-presentation demonstrated histologically normal glomeruli, without mesangial proliferation or sclerosis (Table 2). One in a series of additional levels demonstrated a small segmental crescent and extracapillary fibrin in one glomerulus and another poorly defined inflammatory focus likely representing glomerular tuft necrosis. RBC casts were seen in cortical and medullary tubules (Figure 2). Otherwise, there was mild IFTA, accompanied by a minor mononuclear infiltrate.
Immunofluorescence studies showed both 2+ linear capillary loop staining (IgG, IgG1, kappa>lambda) and 2+ granular mesangial staining (IgA, C3, kappa, lambda, Table 2 and Figure 2). Electron microscopy confirmed the presence of mesangial electron-dense deposits, with essentially normal GBMs, podocytes and endothelium, without granular or fibrillary deposits in other locations.
Interestingly, two renal biopsies performed during the first illness, 16 years prior, reportedly demonstrated an unusual sequence of findings: early crescents with fibrin thrombi and mesangial electron-dense deposits (no anti-GBM staining or IgA deposits per report), followed 6 months later, after treatment with prednisone and methotrexate, by 2+ linear IgG staining, but no crescents.
Diagnosis and subsequent course
Based on the recent biopsy, the patient had IgA nephropathy, as well as anti-GBM disease given the low-positive anti-GBM serology, and linear IgG staining, yet glomerular injury was sparse. Despite being offered Cytoxan and prednisone, the patient accepted only prednisone and methotrexate (as 16 years prior). Her hematuria completely resolved in 6 weeks, and her energy level improved. At her most recent follow-up, 34 months after re-presentation, the creatinine remains normal at 0.8 mg/dL, with stable low-level proteinuria (800 mg/day), and no hematuria.
Case 4
Clinical history
A 64-year-old man presented with a 6-month history of weight loss, increasing tremor, weakness and recent onset of hypertension. Laboratory studies showed a positive anti-GBM antibody (112, normal <20), negative anti-neutrophil cytoplasmic antibody (ANCA) and an IgG-lambda monoclonal spike (>3 g), and anemia. His serum creatinine at presentation was 7.1 mg/dL. Bone marrow biopsy was negative, but imaging demonstrated a T9 vertebral lesion consistent with plasmacytoma.
Pathologic findings
Glomeruli in this biopsy exhibited variable mild mesangial expansion and hypercellularity; many showed ischemic wrinkling and tuft retraction (Table 2). A single glomerulus demonstrated segmental endocapillary hypercellularity, and another had intracapillary fibrin in one loop. One glomerulus had a small area of tuft adhesion, possibly associated with a fibrous crescent. Tubules were ectatic with flattened epithelial cells, consistent with acute tubular injury, and there was interstitial edema with a mixed interstitial inflammatory cell infiltrate, including neutrophils. Interstitial fibrosis, tubular atrophy and arteriosclerosis were extensive.
Immunofluorescence demonstrated a linear GBM pattern of staining for IgG and both light chains with 2–3+ intensity; other stains were negative (Table 2). Ultrastructural evaluation showed only wrinkling and thickening of GBMs in a few capillary loops. Neither granular nor fibrillary deposits were present.
Diagnosis and subsequent course
The immunofluorescence and serologic studies indicated anti-GBM disease, but the dominant histologic finding was acute interstitial nephritis, with only minimal glomerular injury. The patient was treated aggressively with Cytoxan, steroids and five daily courses of plasmapheresis, and serum anti-GBM decreased to 2 within 2 weeks. He was then treated with thalidomide for plasmacytoma. Adequate renal function returned after 10 months of dialysis. His serum creatinine was 1.6 mg/dL, 7 years after biopsy, shortly before his death; multiple myeloma was in remission, but a retroperitoneal biopsy showed well-differentiated neuroendocrine tumor.
Case 5
Clinical history
In 1983, the patient was a 31-year-old man who presented with blood-tinged sputum and was found to have microscopic hematuria, and a normal serum creatinine. A kidney biopsy was performed, and anti-GBM antibody was documented shortly thereafter.
Pathologic findings
The biopsy was normal except for focal mesangial sclerosis in a sample of seven glomeruli (Table 2 and Figure 2), but immunofluorescence staining showed 3+ uniform linear capillary loop staining for IgG and 2+ linear staining for C3. Other immunostains were negative (IgA, IgM, C4, fibrinogen). Tissue was not available for electron microscopy.
Diagnosis and subsequent course
The biopsy diagnosis was consistent with ‘Goodpasture's’ disease with minimal renal involvement. Both hemoptysis and hematuria ceased spontaneously shortly after the biopsy, and the patient was followed closely without treatment.
Abruptly, 3 months later he had an active urine sediment, and his creatinine and blood urea nitrogen were found to be 3.4 and 22 mg/dL, respectively. A second biopsy at that time contained cellular crescents in 15 of 22 glomeruli, most associated with fibrinoid tuft necrosis (Figure 2). Mixed inflammation was seen. No tissue was submitted for immunofluorescence or electron microscopy.
Despite 4 weeks of plasmapheresis, anti-GBM antibody titers remained positive and serum creatinine continued to rise, until he required hemodialysis. He received a deceased donor renal transplant 3 years later with OKT3 prophylaxis and was maintained on an immunosuppressive regimen of azathioprine and prednisone. He experienced excellent allograft function for more than 20 years, after which time he was no longer followed at our institution.
Discussion
Strong linear IgG staining along GBMs is diagnostic of anti-GBM disease and is typically accompanied by necrotizing and crescentic glomerulonephritis, along with measureable serum anti-GBM antibodies [1–4]. Up to half of affected patients have renal involvement alone; most of the rest also have pulmonary hemorrhage (Goodpasture's syndrome), and a small minority (∼5%) have pulmonary limited disease [1, 2, 4–8]. We present five patients with atypical manifestations of anti-GBM disease. All have had one or more instances of documented linear glomerular capillary wall Immunofluorescence (IF) staining but otherwise atypical clinicopathologic features, including several with a relatively indolent disease course. We considered the full differential diagnosis of quasi-linear IgG staining, including pitfalls and mimics (see Table 3), before attributing findings to atypical anti-GBM disease, a diagnosis of exclusion. A small number of atypical anti-GBM cases have been previously reported over the past five decades, reviewed in Table 4.
Table 3.
Differential diagnosis of ‘linear’ capillary loop IgG staining on renal biopsy
| Disease entity | Diagnostic features |
|---|---|
| Nonspecific staining | Weak linear staining in diabetic glomerulopathy, smoking associated glomerulosclerosis (idiopathic nodular glomerulosclerosis) or in patients with heavy proteinuria. Such were cases excluded from present study. |
| Fibrillary glomerulonephritis | Often ‘smudgy’ thicker IgG and light-chain staining along capillary loops. Electron microscopy demonstrates characteristic fibrillary deposits [9, 10]. |
| Membranous nephropathy | Texture of IgG staining is granular. Electron microscopy demonstrates closely spaced, discrete subepithelial deposits. |
| Monoclonal immunoglobulin deposition disease (light and heavy chains) | Light microscopy usually nodular mesangial sclerosis. Strong capillary loop, mesangial and TBM staining for monoclonal heavy and/or monoclonal light chain. Electron microscopy may show fine granular GBM (endothelial facing) and TBM (interstitial facing) deposits. |
| Proliferative glomerulonephritis with monoclonal immunoglobulins | Most commonly granular deposition of monoclonal IgG, rarely semilinear. Electron-dense deposits, usually granular [11]. |
| Anti-GBM disease | Favored diagnosis in reported cases (with atypical clinicopathologic presentation). Typical pathologic features include crescentic glomerulonephritis, strong thin linear staining along capillary loops with IgG, C3 and light chains. No electron-dense deposits by electron microscopy [1, 3]. |
| Anti-GBM disease post-transplant, in Alport's syndrome patients | Renal biopsy pathology similar to typical anti-GBM disease, rare even in patients with Alport's syndrome. |
GBM, glomerular basement membrane; TBM, tubular basement membrane.
Table 4.
Anti-GBM disease with indolent clinical course and/or atypical glomeruli histopathology: literature review
| Study (year) | Age (years)/sex | Renal histopathology | Immunofluorescence | Serology | Comments |
|---|---|---|---|---|---|
| Wilson and Dixon, Case 39 (1973) [12] | 14/M | n/a | IgG linear | Anti-GBM positive (IFA) | Incidental renal biopsy during splenectomy ‘hypersplenism and optic vasculitis’. Treated with steroids; normal renal function at 1 year. |
| Nilssen, Case 1 (1986) [13] | 19/M | ‘Focal glomerulonephritis’ ‘Mild changes in 2/14’ |
IgG linear C3 granular (IgG linear in lung) |
Anti-GBM positive (IFA) | First presentation with slight hemoptysis; biopsy 5 months later with normal creatinine (104–122 μmol/L). Treated with steroid, cyclophosphamide, then plasmapheresis for increasing creatinine and pulmonary hemorrhage. ESRD ∼20 months after presentation. |
| Nilssen, Case 2 (1986) [13] | 58/F | ‘Minimal changes’ | IgG linear, strong C3 granular (IgG linear lung with normal chest X-ray) |
Anti-GBM strongly positive | Hematuria, proteinuria and mildly elevated creatinine (163 μmol/L).Treated with steroid and cyclophosphamide, then steroid and azathioprine. Renal function stable. |
| Nilssen, Case 4 (1986) [13] | 74/F | Membranoproliferative pattern | IgG, IgM, C3 linear both kidneys | Anti-GBM positive (IFA) | Slight hematuria and elevated creatinine (157 μmol/L). Incidental biopsy in conjunction with angiomyolipoma resection. Serum anti-GBM resolves without therapy. Treated with steroid and briefly cyclophosphamide. Creatinine 133 μmol/L at 18 months. |
| Knoll (1993) [14] | 23/F | Crescents in 4/22; Sclerotic 1/22; 50% with mesangial proliferation; 25% normal glomeruli; No interstitial fibrosis |
IgG 3+ linear C3 focal weak |
Anti-GBM negative (IFA) ANCA negative |
Hematuria and proteinuria; normal renal function (Cr = 0.8 mg/dL), transient URI symptoms, no pulmonary hemorrhage. Biopsied 1 year after initial presentation. Treated with steroid, 10 days of plasmapheresis, then azathioprine. Renal function stable at 1 year. |
| Olaru et al. (2013) [15] | n/a | No crescents | IgG linear | Commercial anti-GBM negative; IgG4 restricted antibodies to NC1 hexamers present | Mild proteinuria, microscopic hematuria, elevated but stable serum creatinine. |
| Coley (2015) [16] | 53/M | Mild endocapillary proliferative and exudative GN; multifocal GBM breaks; focal RBC casts; no crescents | IgG1-kappa intense linear, C3 weak sparse granular |
Commercial anti-GBM negative; indirect IF negative ANCA negative; sPEP, uPEP, serum free light chain normal |
Decreased kidney function with upper respiratory tract infection (Cr = 3 mg/dL). Microscopic hematuria. Biopsy 9 years later with Cr = 2.15 mg/dL and hematuria showed similar findings with increased scarring. |
Cr, serum creatinine; IFA, indirect immunofluorescence assay.
The pathogenesis of anti-GBM disease involves development of autoantibodies to structural collagen IV elements of the glomerular (and alveolar) basement membranes. Most of these antibodies are directed against the non-collagenous domain of the alpha-3 subunit, reacting with epitopes (17–31 = EA, 127–141 = EB) that are normally hidden by intrachain methionine cross-links [2, 6, 17]; however, antibodies reactive with other collagen IV epitopes have been identified rarely [15]. Environmental exposure to cigarette smoke or inhaled toxins is hypothesized to play a role in exposure of cryptic collagen epitopes in the alveolar capillary basement membranes and may correlate with pulmonary involvement [2, 8]. Further, genetic predisposition and T-cell help are increasingly appreciated, as anti-GBM disease is positively associated with the HLA DR15 and DR4 alleles [2, 4, 6, 18].
Patients with anti-GBM disease and predominant/exclusive pulmonary involvement are rare but have been characterized in the literature, yet the pathogenesis of this presentation is not understood [7]. Some of these patients may have clinically occult kidney disease that remains uncharacterized if a renal biopsy is not performed. It has been suggested that pulmonary disease may precede renal manifestations in these patients [2, 6, 8], and that early aggressive treatment may logically attenuate renal involvement. In our series, Patient 5 presented with a histologically normal renal biopsy and linear IgG staining at first, followed 3 months later by necrotizing and crescentic glomerulonephritis, again with linear IgG. Thus, linear IgG in a seemingly ‘mild’ clinicopathologic context could merely represent an early phase of aggressive anti-GBM disease, and such patients must be followed closely. Some studies have suggested that patients with both anti-GBM disease and positive ANCA have better prognosis and less severe histologic findings than those patients with anti-GBM alone [1]. Although all four tested patients in our series were negative for ANCA, serologic testing is not always positive in patients with ANCA-associated disease (anti-MPO and PR3 testing results were not available in this cohort) [19].
In contrast to Patient 5, most of the patients we report had a mild course, including two with additional kidney biopsies over time (Patients 1 and 3). Further, serum anti-GBM antibodies were difficult to detect using standard contemporary assays in Patients 1–3. This raises the possibility of antibodies reactive with non-conventional GBM epitopes, of non-typical immunoglobulin subclass, of low affinity or low concentration (low rate of synthesis and/or high rate of clearance) [8, 15–17, 20]. The previous generation of qualitative indirect IFAs, in which patient serum is incubated with urea-treated human or primate renal tissue, have been largely replaced by ELISAs developed with solubilized GBM, purified collagen IV-alpha-3 (sometimes bovine) or recombinant epitopes, which have not been standardized [2, 21–23]. While these assays have advantages in consistency and specificity, some are less likely to detect antibodies of non-conventional immunoglobulin (such as IgA), isotype or epitope reactivity [18, 22, 24]. For instance, Patient 1 had low-level IFA positivity at one time point, yet ELISA testing was repeatedly negative. Indeed, several groups have reported cases of anti-GBM disease with classic histology in which conventional assays for serum antibodies were negative [15, 18, 25–27]. Several patients with anti-GBM IgA antibodies have been reported, including one with monotypic IgA1-kappa antibodies directed at alpha-1,2 collagen IV subunits, and recurrent in the kidney transplant [27–29]. Ohlsson recently reported four young female patients with severe pulmonary disease, negative conventional anti-GBM assays and positive IgG4-anti-GBM assays, one of whom had a normal kidney biopsy [26]. Interestingly, Olaru characterized in detail non-nephritogenic antibodies, also of the IgG4 subclass, to intact alpha-3,4,5 hexamers [15]. Their index patient had a normal kidney biopsy with linear IgG staining, and clinical presentation of microscopic hematuria, proteinuria and mildly elevated creatinine [15]. As we were working with retrospective and/or historic clinical samples, we were unable to further explore these alternative anti-GBM assay methods.
Linear IgG deposits were monotypic in Patient 1 (IgG3-lambda), and Patient 3 had only IgG1-linear staining (though light chains did not appear monotypic), yet hematologic studies and 3–16 years clinical follow-up have not revealed lymphoproliferative disease. Precedents for monotypic immune complex deposition in patients without hematologic malignancy include proliferative glomerulonephritis with monoclonal immunoglobulin deposits [30], post-infectious monoclonal immunoglobulin deposition associated with parvovirus [31] and a case of recurrent post-transplant membranous nephropathy [32], all with predilection for IgG3-kappa. Case 1 (and 3) most closely resembles the recent case report by Coley et al., which had a mildly proliferative and exudative glomerulonephritis, IgG1-kappa monotypic linear staining and negative serum anti-GBM studies at the time of an upper respiratory tract ailment [16]. Recently, additional cases of monotypic anti-GBM staining were reported in a series of atypical anti-GBM cases in abstract form [33]. As described, we hypothesize that the same process affected the native and allograft kidneys in Patient 1, which further emphasizes the importance of detailed renal pathology review of the non-neoplastic kidney accompanying tumor resections [34, 35].
In summary, we report in detail the varied histopathologic changes in five patients with anti-GBM reactivity in kidney biopsy tissue and atypical clinicopathologic presentations. These cases illustrate a spectrum of renal outcomes. At one end, the linear IgG ‘alone’ phenotype may represent the earliest manifestation of disease destined to evolve into florid glomerulonephritis (Patient 5). Conversely, Patients 1–3 had a documented history of clinically mild renal disease over years. We hypothesize that the latter group of patients have less nephritogenic antibody (reacting with alternate epitopes, restricted isotype, low affinity or titer, and so on), and/or with host factors (T cell phenotype or activation for instance), resulting in less aggressive disease. As early treatment of anti-GBM disease is crucial to preserve renal function and avoid severe pulmonary sequelae, careful clinicopathologic evaluation, with consideration for extended anti-GBM testing, and close follow-up are necessary in managing these patients.
Conflict of interest statement
None declared.
Acknowledgements
The authors thank Drs Feroz Aziz, Anuja Mittalhenkle, Pei-Li Wang and Amy Hackett for help with clinical data and follow-up.
References
- 1.Jennette JC, Nickeleit V. Anti-glomerular basement membrane glomerulonephritis and Goodpasture syndrome. In: Jennette JC, Silva FG, Olson JL, et al. (eds). Heptinstall's Pathology of the Kidney. 7th edn Philadelphia, PA: Wolters Kluwer, 2015, pp. 657–684 [Google Scholar]
- 2.Cui Z, Zhao M-H. Advances in human antiglomerular basement membrane disese. Nat Rev Nephrol 2011; 7: 697–705 [DOI] [PubMed] [Google Scholar]
- 3.Fischer EG, Lager DJ. Anti-glomerular basement membrane glomerulonephritis: a morphologic study of 80 cases. Am J Clin Pathol 2006; 125: 445–450 [DOI] [PubMed] [Google Scholar]
- 4.Lahmer T, Heemann U. Anti-glomerular basement membrane antibody disease: a rare autoimmune disorder affecting the kidney and the lung. Autoimmun Rev 2012; 12: 169–173 [DOI] [PubMed] [Google Scholar]
- 5.Ang C, Savige J, Dawborn J, et al. Anti-glomerular basement membrane (GBM)-antibody-mediated disease with normal renal function. Nephrol Dial Transplant 1998; 13: 935–939 [DOI] [PubMed] [Google Scholar]
- 6.Dammacco F, Battaglia S, Gesualdo L, et al. Goodpasture's disease: a report of ten cases and a review of the literature. Autoimmun Rev 2013; 12: 1101–1108 [DOI] [PubMed] [Google Scholar]
- 7.Lazor R, Bigay-Gamé L, Cottin V, et al. Alveolar hemorrhage in anti-basement membrane antibody disease: a series of 28 cases. Medicine (Baltimore) 2007; 86: 181–193 [DOI] [PubMed] [Google Scholar]
- 8.Cui Z, Zhao MH, Singh AK, et al. Antiglomerular basement membrane disease with normal renal function. Kidney Int 2007; 72: 1403–1408 [DOI] [PubMed] [Google Scholar]
- 9.Sharma P, Kuperman M, Racusen L, et al. Fibrillary glomerulonephritis presenting as rapidly progressive glomerulonephritis. Am J Kidney Dis 2012; 60: 157–159 [DOI] [PubMed] [Google Scholar]
- 10.Nasr SH, Valeri AM, Cornell LD, et al. Fibrillary glomerulonephritis: a report of 66 cases from a single institution. Clin J Am Soc Nephrol 2011; 6: 775–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasr SH, Satoskar A, Markowitz GS, et al. Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol 2009; 20: 2055–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson CB, Dixon FJ. Anti-glomerular basement membrane antibody-induced glomerulonephritis. Kidney Int 1973; 3: 74–89 [DOI] [PubMed] [Google Scholar]
- 13.Nilssen DE, Talseth T, Brodwall EK. The many faces of Goodpasture's syndrome. Acta Med Scand 1986; 220: 489–491 [DOI] [PubMed] [Google Scholar]
- 14.Knoll G, Rabin E, Burns BF. Antiglomerular basement membrane antibody-mediated nephritis with normal pulmonary and renal function. A case report and review of the literature. Am J Nephrol 1993; 13: 494–496 [DOI] [PubMed] [Google Scholar]
- 15.Olaru F, Wang X-P, Wentian Luo W, et al. Proteolysis breaks tolerance toward intact alpha345(IV) collagen, eliciting novel anti–glomerular basement membrane autoantibodies specific for alpha345NC1 hexamers. J Immunol 2013; 190: 1424–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coley SM, Shirazian S, Radhakrishnan J, et al. Monoclonal IgG1κ anti-glomerular basement membrane disease: a case report. Am J Kidney Dis 2015; 65: 322–326 [DOI] [PubMed] [Google Scholar]
- 17.Yang R, Hellmark T, Zhao J, et al. Levels of epitope-specific autoantibodies correlate with renal damage in anti-GBM disease. Nephrol Dial Transplant 2009; 24: 1838–1844 [DOI] [PubMed] [Google Scholar]
- 18.Salama AD, Levy JB. Tolerance and autoimmunity in anti-GBM disease. J Am Soc Nephrol 2003; 14: 2988–2989 [DOI] [PubMed] [Google Scholar]
- 19.Jennette JC, Thomas DB. Pauci-immune and antineutrophil cytoplasmic autoantibody-mediated crescentic glomerulonephritis and vasculitis. In: Jennette JC, Silva FG, Olson JL, et al. (eds). Heptinstall's Pathology of the Kidney. 7th edn Philadelphia, PA: Wolters Kluwer, 2015, pp. 685–709 [Google Scholar]
- 20.Segelmark M, Hellmark T, Wieslander J. The prognostic significance in goodpasture's disease of specificity, titre and affinity of anti-glomerular-basement-membrane antibodies. Nephron Clin Pract 2003; 94: c59–c68 [DOI] [PubMed] [Google Scholar]
- 21.Sinico RA, Radice A, Corace C, et al. Anti-glomerular basement membrane antibodies in the diagnosis of Goodpasture syndrome: a comparison of different assays. Nephrol Dial Transplant 2006; 21: 397–401 [DOI] [PubMed] [Google Scholar]
- 22.Jia X-Y, Qu Z, Cui Z, et al. Circulating anti-glomerular basement membrane autoantibodies against α3(IV)NC1 undetectable by commercially available enzyme-linked immunosorbent assays. Nephrology (Carlton) 2012; 17: 160–166 [DOI] [PubMed] [Google Scholar]
- 23.Mahler M, Radice A, Sinico RA, et al. Performance evaluation of a novel chemiluminescence assay for detection of anti-GBM antibodies: an international multicenter study. Nephrol Dial Transplant 2012; 27: 243–252 [DOI] [PubMed] [Google Scholar]
- 24.Stolk M, Carl D, Massey HD. Antibody-negative Goodpasture's disease. NDT Plus 2010; 3: 253–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellman MA, Gerhardt TM, Rabe C, et al. Goodpasture's syndrome with massive pulmonary haemorrhage in the absence of circulating anti-GBM antibodies? Nephrol Dial Transplant 2006; 21: 526–529 [DOI] [PubMed] [Google Scholar]
- 26.Ohlsson S, Herlitz H, Lundberg S, et al. Circulating anti-glomerular basement membrane antibodies with predominance of subclass IgG4 and false-negative immunoassay test results in anti-glomerular basement membrane disease. Am J Kidney Dis 2014; 63: 289–293 [DOI] [PubMed] [Google Scholar]
- 27.Fervenza FC, Terreros D, Boutaud A, et al. Recurrent Goodpasture's disease due to a monoclonal IgA-kappa circulating antibody. Am J Kidney Dis 1999; 34: 549–555 [DOI] [PubMed] [Google Scholar]
- 28.Borza DB, Chedid MF, Colon S, et al. Recurrent Goodpasture's disease secondary to a monoclonal IgA1-kappa antibody autoreactive with the alpha1/alpha2 chains of type IV collagen. Am J Kidney Dis 2005; 45: 397–406 [DOI] [PubMed] [Google Scholar]
- 29.Ho J, Gibson IW, Zacharias J, et al. Antigenic heterogeneity of IgA anti-GBM disease: new renal targets of IgA autoantibodies. Am J Kidney Dis 2008; 52: 761–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasr SH, Markowitz GS, Stokes MB, et al. Proliferative glomerulonephritis with monoclonal IgG deposits: a distinct entity mimicking immune-complex glomerulonephritis. Kidney Int 2004; 65: 85–96 [DOI] [PubMed] [Google Scholar]
- 31.Fujita E, Shimizu A, Kaneko T, et al. Proliferative glomerulonephritis with monoclonal immunoglobulin G3κ deposits in association with parvovirus B19 infection. Hum Pathol 2012; 43: 2326–2333 [DOI] [PubMed] [Google Scholar]
- 32.Debiec H, Hanoy M, Francois A, et al. Recurrent membranous nephropathy in an allograft caused by IgG3κ targeting the PLA2 receptor. J Am Soc Nephrol 2012; 23: 1949–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornell L, Collins A, Schraith D, et al. Atypical anti-glomerular basement membrane disease: a novel form of glomerulonephritis. Abstract. World Congress of Nephrology, Cape Town, South Africa, March 15, 2015.
- 34.Bijol V, Mendez GP, Hurwitz S, et al. Evaluation of the nonneoplastic pathology in tumor nephrectomy specimens: predicting the risk of progressive renal failure. Am J Surg Pathol 2006; 30: 575–584 [DOI] [PubMed] [Google Scholar]
- 35.Henriksen KJ, Meehan SM, Chang A. Non-neoplastic renal diseases are often unrecognized in adult tumor nephrectomy specimens: a review of 246 cases. Am J Surg Pathol 2007; 31: 1703–1708 [DOI] [PubMed] [Google Scholar]