Abstract
Levamisole is an antihelminthic agent widely used as an adulterant of illicit cocaine recently implicated as a cause of antineutrophil cytoplasmic antibody (ANCA)–associated microscopic polyangiitis in cocaine abusers. An isolated case of membranous nephropathy (MN) associated with levamisole exposure has also been reported. We report the first case, to our knowledge, of a patient with both microscopic polyangiitis manifest as a pauci-immune necrotizing and crescentic glomerulonephritis and concurrent MN in the setting of chronic cocaine abuse and presumed levamisole exposure, raising the hypothesis that levamisole was the causative agent in the development of this rare dual glomerulopathy.
Keywords: antineutrophil cytoplasmic antibody (ANCA), kidney biopsy, levamisole, membranous nephropathy (MN)
Introduction
Levamisole, which was first developed as an antihelminthic agent in the 1960s, is widely utilized to adulterate cocaine. It has been detected with increased frequency in the illicit cocaine supply in the USA over the last decade and is now estimated to be present in 70–80% of the cocaine entering the country [1, 2]. Prolonged exposure to this compound can induce a distinct clinical syndrome characterized by cutaneous vasculitis (polyangiitis) and/or agranulocytosis accompanied by an unusual constellation of serologic abnormalities including the presence of antiphospholipid antibodies, lupus anticoagulants and high titers of antineutrophil cytoplasmic antibodies (ANCAs) [3]. There have been an increasing number of case reports suggesting that levamisole-adulterated cocaine may lead to renal disease in the form of a pauci-immune complex type of necrotizing and crescentic glomerulonephritis (GN), alternately called microscopic polyangiitis [4, 5]. An isolated case of membranous nephropathy (MN) associated with levamisole exposure has been reported recently in abstract form [6]. Concomitant necrotizing and crescentic GN and MN is a rare finding in renal biopsies [7]. We present the first case, to our knowledge, of a patient with pauci-immune complex type necrotizing and crescentic GN and concurrent MN in the setting of chronic cocaine abuse with probable ingestion of levamisole.
Clinical history
A 34-year-old female with a history of chronic alcohol and cocaine abuse was referred for the management of acute renal failure. She had been hospitalized 3 weeks prior due to multiple ulcers involving both lower extremities, requiring ulcer debridement and skin grafting. She was homeless and regularly smoked ‘crack’ cocaine, but denied intravenous drug abuse. A review of her medical records showed anti-hepatitis C virus antibody positivity in the past, with subsequent spontaneous clearance and a recent negative polymerase chain reaction (PCR) test for viremia.
On admission, the patient was afebrile with a blood pressure (BP) of 116/78 mmHg and a heart rate of 93 beats per minute. On examination, she was noted to have extensive, painful, bilateral lower extremity wounds, which on the left leg were fairly clean, but on the lower right leg the skin was focally necrotic.
Laboratory testing showed a serum creatinine of 4.2 mg/dL, increased from a baseline of 0.70 mg/dL measured 2 weeks previously. Hemoglobin was 9.9 g/dL, hematocrit 28.5%, white blood cell count 5.6 × 10 × 3/μL, platelet count 69.000/μL, haptoglobin 252 mg/dL, serum albumin 2.3 mg/dL, serum total protein 5.8 g/dL and normal liver function test results. A protein-to-creatinine ratio measurement in the patient's spot urine sample was 2.4 mg/mg. Urinalysis revealed hematuria with dysmorphic red blood cells. A urine toxicology screen was positive for cocaine and barbiturates. Serologic tests were weakly positive for antinuclear antibody but negative for anti-double-stranded DNA, anti-glomerular basement membrane (anti-GBM) antibody, anti-HIV, anti-hepatitis B virus and anti-hepatitis C virus antibodies. Complement levels were normal. Cryoglobulins were not detected. Serologic studies were notable for the presence of circulating perinuclear anti-ANCAs (pANCAs); a test for the corresponding presence of circulating antibodies to myeloperoxidase (MPO) was markedly elevated (311.4 U, normal <1 U), while a test for circulating anti-PR3 antibodies was negative. A coagulation panel revealed a prolonged partial thromboplastin time (PTT) of 51 s (normal 26–36 s) and an increased dilute Russell viper venom time (DRVVT) of 1.9 (normal 0.0–1.1), raising suspicion of a lupus anticoagulant (LAC). Testing for anticardiolipin was not performed.
Kidney biopsy
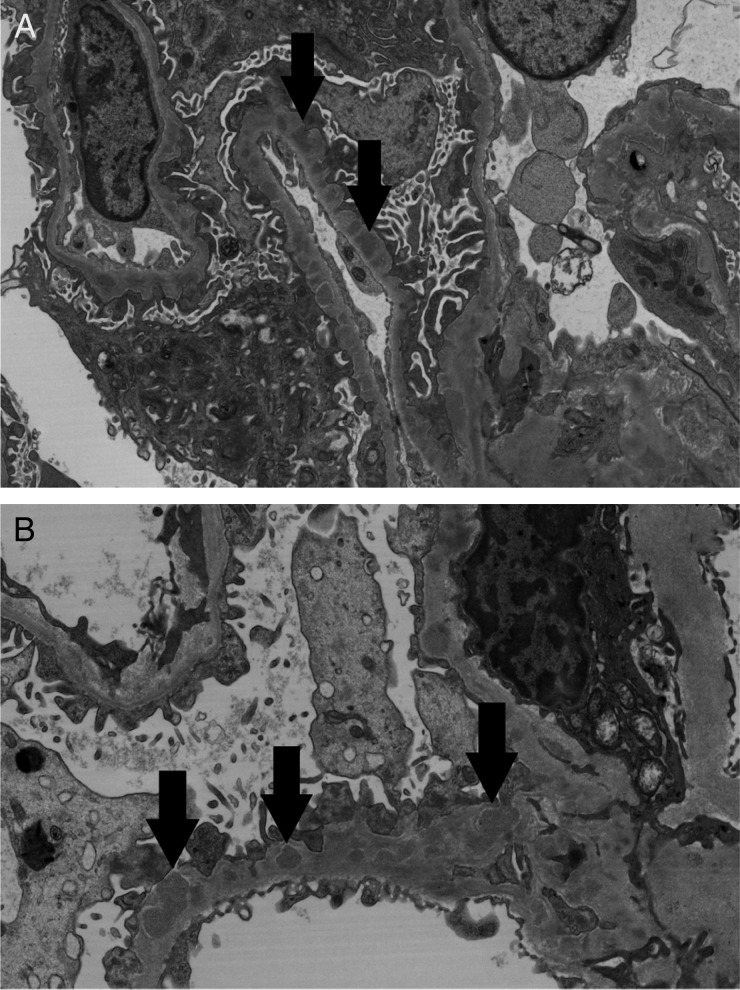
A renal biopsy showed ∼9 glomeruli per level section with necrotizing and crescentic GN involving up to 30–40% of the glomeruli (Figure 1A and B). Intact portions of glomeruli were without prominent inflammatory cell infiltration. Intact glomerular capillary loops were thickened and contained rarefactions, which were best seen on Jones' silver stain. The tubular parenchyma showed focal red blood cell casts, moderate interstitial inflammation and edema and mild tubulointerstitial fibrosis. Arterial and arteriolar vessels were without evidence of vasculitis or significant sclerosis. Immunofluorescence (IF) microscopy (Figure 1C) revealed finely granular staining for IgG kappa and lambda light chains of 2+ intensity (on a semiquantitative scale of 0 to 4+) predominantly in glomerular capillary walls, including a few portions of capillary walls where they were reflected over mesangial regions; there was similarly distributed staining of trace intensity for C3. There was no significant glomerular staining for IgA, IgM, C1q or albumin. An IF stain for the presence of phospholipase A2 receptor (PLA2R) antigen within the glomerular immune deposits was negative. Electron microscopy (Figure 2A and B) detected numerous immune-type electron-dense deposits in peripheral capillary walls in intramembranous and subepithelial locations. The deposits were somewhat irregularly distributed and a minority of capillary basement membranes were without such deposits. Rare, small, ill-defined electron densities consistent with either hyaline or possible deposits of immune complexes were present in mesangial regions. Based on these biopsy results, the distribution of the patient's skin lesions and positive serologies, a diagnosis of cocaine/levamisole-induced ANCA-associated systemic microscopic polyangiitis and concurrent MN was made.
Fig. 1.
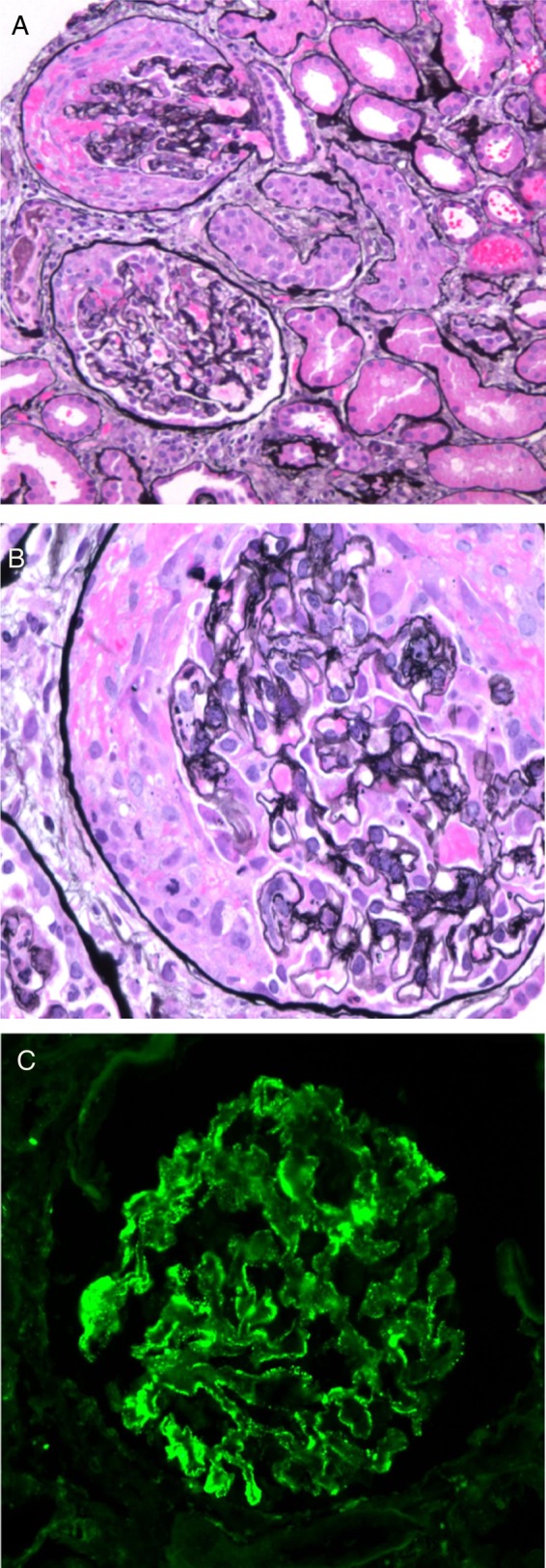
(A) Two glomeruli show segmental necrotizing lesions and are involved by cellular crescents compressing the glomerular tufts. A periglomerular inflammatory infiltrate is present (Jones silver stain; original magnification ×100). (B) A glomerulus involved by a circumferential cellular crescent (Jones silver stain; original magnification ×600). (C) IF staining revealed finely granular staining of 2+ intensity for IgG predominantly in glomerular capillary walls but also involving a few mesangial regions (original magnification ×200).
Fig. 2.
(A and B) Electron micrographs show numerous immune-type electron-dense deposits in peripheral capillary walls in intramembranous and subepithelial locations (arrows). There are ‘spikes’ of basement membrane matrix separating the deposits and incorporating them into the basement membranes [original magnification: (A) ×7960; (B) ×11900].
Clinical follow-up
The patient was started on pulse methylprednisolone (3 doses of 1 g each) with subsequent taper, along with plasmapheresis (7 total treatments) and rituximab infusions (2 doses of 1 g each). Due to the severity of the renal impairment, temporary hemodialysis treatment was required. Overall, she tolerated these therapies well and her kidney function improved considerably, with creatinine levels dropping from 5 to 1.6 mg/dL and cessation of her hemodialysis requirement. Her lower extremity wounds improved during this treatment course. The clinical course during hospitalization was complicated by spontaneous right colon rupture secondary to constipation from chronic opioid use, requiring right hemicolectomy. Two months after discharge, the patient was noted to have resumed cocaine use, and a urine toxicology screen at that time was positive for cocaine. She has been lost for further follow-up.
Discussion
Levamisole may enhance noradrenergic neurotransmission by partially metabolizing into an amphetamine-like compound or increasing endogenous opioids. The possible stimulant properties of levamisole led it to be used as a cocaine adulterant (cocaine cutting agent). An adulterant is a substance found within other substances (e.g. food, beverages), although not legally allowed. Levamisole reversibly and noncompetitively inhibits most isoforms of alkaline phosphatase (e.g. human liver, bone, kidney and spleen). It is thus used to reduce background alkaline phosphatase activity in biomedical assays such as in situ hybridization or western blot protocols where alkaline phosphatase is used for signal amplification. Levamisole is readily absorbed from the gastrointestinal tract and metabolized in the liver, excreted primarily through the kidneys.
Levamisole has been used therapeutically as an immunomodulatory agent and has been shown to promote neutrophil mobility and chemotaxis, enhance dendritic cell maturation, promote T cell proliferation and induce circulating autoantibodies [8–10]. These effects on innate and adaptive immune responses may explain its propensity to induce autoimmunity and vasculitis [4, 11, 12]. Toxicity eventually led to its withdrawal from human use in 1999 [5, 13]. In the last decade, levamisole toxicity has reemerged as a medical problem in the USA, with multiple reports linking the use of levamisole-adulterated cocaine to neutropenia, life-threatening agranulocytosis and vasculitis-like purpuric skin eruption of the face and extremities [3, 14–18].
There is growing evidence that prolonged use of levamisole-adulterated cocaine can lead to a drug-induced autoimmune disease (microscopic polyangiitis) complicated by a pauci-immune complex type GN [1, 4, 5, 19].
Our case is notable for the unusual concurrence of both pauci-immune complex necrotizing and crescentic GN and MN. In renal biopsies of MN, fibrinoid necrosis and crescent formation are rarely encountered [7]. When present, these changes suggest three diagnostic possibilities. First, is a combination of focal or diffuse lupus nephritis with crescent formation and MN (Class III or IV plus V lupus nephritis) in patients with systemic lupus erythematosus (SLE). In the absence of clinical evidence of SLE or pathologic findings highly suggestive of lupus MN, other possibilities include MN with superimposed anti-GBM disease or ANCA-associated necrotizing and crescentic GN. Concurrent anti-GBM disease and MN was first reported by Klassen et al. in 1974 [20], and since that time at least 25 cases have been described [21–23]. The negative serologic test and the absence of characteristic linear glomerular basement membrane staining for IgG excluded this possibility. Coexistent MN and ANCA-associated necrotizing and crescentic GN is a rare occurrence. Nasr et al. [7] reported the largest clinical experience to date (14 patients). There are also rare reports of concomitant MN and crescentic GN in which there is no evidence of anti-GBM disease, ANCA seropositivity or SLE. In these cases, crescent formation may represent an unusual morphologic expression of MN [24, 25]. In our case, the finding of high-titer pANCA in the presence of crescentic and necrotizing lesions suggested a pauci-immune, ANCA-associated GN superimposed upon preexisting MN. This is the first case, to our knowledge, where this dual glomerulopathy process occurred in the setting of chronic cocaine abuse and presumptive levamisole exposure.
It is possible to speculate that these two disease processes are pathogenetically linked. One possible scenario would be damage to the GBM occurring from membranous immune deposits, resulting in the release of antigens to the circulation that in turn leads to formation of autoantibodies, or vice versa. However, MN is a relatively common glomerular disease, but MN complicated by ANCA-associated disease is not [7], and so the clinical evidence to support such a scenario is limited. We know of no experimental animal models whereby MN-type lesions lead to a subsequent polyangiitis type of glomerular injury, and so are unable to draw from the experimental literature in support of such a scenario.
Interpretation of negative IF staining for PLAR2R in our case provides some evidence in favor of a secondary form of MN. The complex medical history of polysubstance abuse, the remote exposure to HCV and the presence of multiple possible sources of bacterial infection (extensive skin ulcers) make it difficult to establish a clear etiologic basis for the MN in this case, but the history of chronic cocaine abuse suggests the intriguing hypothesis of levamisole as a causative agent in the development of MN. To our knowledge, the only case of MN associated with levamisole-adulterated cocaine was described by Wang and Morfin [6] in a recent abstract. The kidney biopsy in this patient showed typical features of MN, but a necrotizing and crescentic GN was not present. Staining for IgG4 was negative, suggesting a secondary type of MN.
As in the previously published series, a lack of documentation of levamisole in patient samples is clearly a limitation in our case. The adulterant can be detected in serum and urine using gas chromatography and mass spectrometry, but its half-life is quite short (5.6 h) and these tests are not routinely performed in the clinical setting [26]. However, recent data show that exposure to levamisole has become almost ubiquitous among regular cocaine users within the USA, supporting our interpretation that this patient's renal disease is likely consequent to levamisole exposure [2–4, 11]. Furthermore, patients with vasculitis associated with levamisole-adulterated cocaine classically demonstrate unique serologic abnormalities characterized by unusually high titers of p-ANCA and, the majority of cases, show positivity for antiphospholipid antibodies as well as antinuclear and anti-double-stranded DNA antibodies [1, 19]. All of these, except for positive anti-double-stranded DNA antibodies, were present in our case. Clinically, besides the renal involvement, the dramatic cutaneous lesions presented by our patient are also a typical feature of this syndrome.
In summary, this case demonstrates a unique constellation of renal pathology findings in a patient who has been exposed to levamisole by virtue of her use of illicit cocaine. One type of renal toxicity, microscopic polyangiitis, is a well-known toxicity of this drug, but MN has been rarely associated with levamisole use. This combination of renal pathology changes in the setting of levamisole exposure is without precedent.
Conflicts of interest statement
None declared.
Acknowledgements
The results presented in this article have not been published previously.
References
- 1.Nolan A, Jen K. Pathologic manifestations of levamisole-adulterated cocaine exposure. Diagn Pathol 2015; 10: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchanan J, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA 2011; 305: 1657–1658 [DOI] [PubMed] [Google Scholar]
- 3.Roberts A, Chévez-Barrios P. Levamisole-induced vasculitis: a characteristic cutaneous vasculitis associated with levamisole-adulterated cocaine. Arch Pathol Lab Med 2015; 139: 1058–1061 [DOI] [PubMed] [Google Scholar]
- 4.McGrath M, Isakova T, Rennke H, et al. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated disease. Clin J Am Soc Nephrol 2011; 6: 2799–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson A, Tuot D, Jen K, et al. Pauci-immune glomerulonephritis in individuals with disease associated with levamisole-adulterated cocaine: a series of 4 cases. Medicine (Baltimore) 2014; 93: 290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Morfin J. Unusual case of membranous glomerulonephritis associated with levamisole [NFK abstract 383]. Am J Kidney Dis 2014; 63: A114 [Google Scholar]
- 7.Nasr S, Said S, Valeri A, et al. Membranous glomerulonephritis with ANCA-associated necrotizing and crescentic glomerulonephritis. Clin J Am Soc Nephrol 2009; 4: 299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Lin Y, Chiang B. Levamisole enhances immune response by affecting the activation and maturation of human monocyte-derived dendritic cells. Clin Exp Immunol 2008; 151: 174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levamisole. In Physician's Desk Reference. Montvale, NJ: Medical Economics, 1995, pp. 1670–1672 [Google Scholar]
- 10.Szeto C, Gillespie K, Mathieson P. Levamisole induces interleukin-18 and shifts type 1/type 2 cytokine balance. Immunology 2000; 100: 217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogan J, Markowitz G, Radhakrishnan J. Drug-induced glomerular disease: immune-mediated injury. Clin J Am Soc Nephrol 2015; 10: 1300–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laux-End R, Inaebnit D, Gerber H. Vasculitis associated with levamisole and circulating autoantibodies. Arch Dis Child 1996; 75: 355–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souied O, Baydoun H, Ghandour Z, et al. Levamisole-contaminated cocaine: an emergent cause of vasculitis and skin necrosis. Case Rep Med 2014; 2014: 434717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poon S, Baliog CJ, Sams R, et al. Syndrome of cocaine-levamisole-induced cutaneous vasculitis and immune-mediated leukopenia. Semin Arthritis Rheum 2011; 41: 434–444 [DOI] [PubMed] [Google Scholar]
- 15.Waller J, Feramisco J, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol 2010; 63: 530–535 [DOI] [PubMed] [Google Scholar]
- 16.Walsh N, Green P, Burlingame R, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole. J Cutan Pathol 2010; 37: 1212–1219 [DOI] [PubMed] [Google Scholar]
- 17.Zhu N, Legatt D, Turner A. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med 2009; 150: 287–289 [DOI] [PubMed] [Google Scholar]
- 18.Chung C, Tumeh P, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol 2011; 65: 722–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulati S, Donato A. Lupus anticoagulant and ANCA associated thrombotic vasculopathy due to cocaine contaminated with levamisole: a case report and review of the literature. J Thromb Thrombolysis 2012; 34: 7–10 [DOI] [PubMed] [Google Scholar]
- 20.Klassen J, Elwood C, Grossberg A, et al. Evolution of membranous nephropathy into anti-glomerular-basement-membrane glomerulonephritis. N Engl J Med 1974; 290: 1340–1344 [DOI] [PubMed] [Google Scholar]
- 21.Basford A, Lewis J, Dwyer J, et al. Membranous nephropathy with crescents. J Am Soc Nephrol 2011; 22: 1804–1808 [DOI] [PubMed] [Google Scholar]
- 22.Ghassan B, Bruce A, Jian L, et al. Rituximab for the treatment of refractory simultaneous anti-glomerular basement membrane (anti-GBM) and membranous nephropathy. Clin Kidney J 2014; 7: 53–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jurcić V, Vizjak A, Rigler A, et al. Goodpasture's syndrome with concomitant immune complex mixed membranous and proliferative glomerulonephritis. Clin Nephrol 2014; 81: 216–223 [DOI] [PubMed] [Google Scholar]
- 24.Tse W, Howie A, Adu D, et al. Association of vasculitic glomerulonephritis with membranous nephropathy: a report of 10 cases. Nephrol Dial Transplant 1997; 12: 1017–1027 [DOI] [PubMed] [Google Scholar]
- 25.Arrizabalaga P, Sans Boix A, Torras Rabassa A, et al. Monoclonal antibody analysis of crescentic membranous glomerulonephropathy. Am J Nephrol 1998; 18: 77–82 [DOI] [PubMed] [Google Scholar]
- 26.Kouassi E. Novel assay and pharmacokinetics of levamisole and p-hydroxylevamisole in human plasma and urine. Biopharm Drug Dispos 1986; 7: 71–89 [DOI] [PubMed] [Google Scholar]