Abstract
BK virus (BKV) is a non-enveloped DNA virus of the polyomaviridae family that causes an interstitial nephritis in immunosuppressed patients. BKV nephropathy is now a leading cause of chronic kidney disease and early allograft failure following kidney transplantation. It is also known to cause renal disease with a progressive decline in kidney function in non-renal solid organ transplant (NRSOT) recipients, although the disease may not be recognized nor its impact appreciated in this patient population. In this report, we review the existing literature to highlight our current understanding of its incidence in NRSOT populations, the approaches to diagnosis and the potential treatment options.
Keywords: BK polyoma virus, immunosuppression complication, interstitial nephritis, non-renal solid organ transplantation
Introduction
BK virus (BKV) is a non-enveloped DNA virus, a member of the polyomaviridae family. BKV and JC virus (JCV) were the first human polyoma viruses isolated from immunosuppressed patients [1, 2]. Since then as many as 13 polyoma viruses have been discovered [3] and more are likely in the future, as novel molecular screening techniques are used in identification [4]. BKV takes its name from the initials of the first patient in whom it was isolated [1]. BKV causes an interstitial nephritis in kidney transplant patients, but has also been reported to cause renal disease in non-renal solid organ transplant (NRSOT) patients and bone marrow transplant recipients. The significance of BKV infection in NRSOT is poorly understood, although kidney disease from BKV may not be always recognized. In this review, we summarize the known epidemiology of BKV infection and discuss the pathophysiology and presentation of BKV nephropathy (BKVN) in kidney transplant recipients and the diagnosis and management of BKV infection in NRSOT patients. We illustrate this review with a clinical case that highlights the presentation and treatment of BKVN in a lung transplant recipient.
Epidemiology
Primary BKV infection is mainly asymptomatic or results in a mild respiratory illness [5]. The natural route of transmission is not established. Seroprevalence studies indicate a high exposure rate to BKV during childhood, with antibodies being detected in >50% of children by the age of 3 and >90% by the age of 10 [6, 7]. Due to the presence of viral DNA in tonsillar tissue, transmission is thought to occur via a respiratory route [5, 8]. There is also evidence for other possible routes of transmission such as fecal–oral, urino-oral and transplacental transmission, and via blood transfusion [9].
There are four BKV genotypes, designated I, II, III and IV [10], that are well recognized, and V and VI are also proposed to exist [11]. After primary infection, BKV enters a latency phase and tends to persist indefinitely. Autopsy studies have detected BKV mainly in kidney parenchyma, renal pelvis, ureter and urinary bladder of immunocompetent individuals [12]. Asymptomatic shedding of BKV particles into the urine has been reported in 5–10% of immunocompetent adults at any given time [9, 13]. Reactivation of BKV replication is observed in states of relative or absolute immunodeficiency such as transplantation [14], pregnancy [15, 16], diabetes [17], cancer [17], HIV infection [18] and systemic lupus erythematosus [19]. Unchecked BKV replication can then lead to BKVN and other organ disease. It remains unclear if there is any correlation between genotype and the likelihood of clinical disease [20].
For the purposes of this review, BKV infection is defined as any evidence of exposure to BKV. Positive BKV serology and/or low-level BKV DNA in the urine probably indicates an asymptomatic latent phase of infection. BKV reactivation is defined as evidence of viral multiplication noted by one of the following: BKV virions in target tissues on electron microscopy, BKV-specific structural proteins in target tissues by immunohistochemistry, BKV mRNA expression of late genes in body fluids or affected tissues, BKV DNA detection in non-latency sites (plasma and CSF) or increasing BKV DNA copies in urine [14]. BKVN and other forms of BKV-related pathology indicate clinical disease from BKV replication.
Pathophysiology
Typically, BKV remains latent for the life of the host [21]. Under some circumstances, when immunity is lowered, the dormant viruses begin to replicate in the epithelial cells of the kidney, ureter and bladder [22]. Electron microscopic studies in kidney transplant patients have demonstrated BK virions entering the renal tubular cell in smooth vesicles, aggregating and then using tubulovesicular networks to gain access to paranuclear areas and the nucleus [23]. The nucleus subsequently becomes markedly enlarged from the accumulation of daughter viral particles and then the nuclear membrane ruptures. Cytoplasmic swelling together with generalized disruption of the intracellular organelles then leads to cell death [23]. Lysis of the infected cells results in massive shedding of the virions into the tubular lumen and into the intercellular spaces causing cell-to-cell spread [23, 24].
Today, after kidney transplantation, 30–60% of transplant recipients develop BK viruria, 10–20% develop BK viremia and 5–10% develop BKVN [25–32]. Although rarely tested for at that time, it is thought that BK reactivation in kidney transplant recipients occurred very infrequently in the 1970s–80s. The increased prevalence in recent times is attributed, in part, to more potent calcineurin inhibitor (CNI)-based immunosuppressive regimens. It is important to acknowledge that better understanding of BKV infection has led to screening protocols in kidney transplantation that has resulted in the increased recognition of asymptomatic BKV infections. The true incidence of BKV reactivation after NRSOT is unknown, but is considerably lower than that in the kidney transplant population [33].
Diagnosis
BKV infection is manifest by BK viruria, BK viremia and BKVN. BK viruria precedes BK viremia by a median of 4 weeks and BKVN by a median of 12 weeks [28, 34]. One of the earlier methods to detect BKV infection was by the detection of ‘decoy’ cells in the urine. Decoy cells originate from infected renal tubular cells with nuclei altered by BKV inclusions. They can be observed on urine cytology using Papanicolaou stains (Pap) or on phase contrast microscopy. The urine Pap smear, though sensitive for the diagnosis of BKVN, has a positive predictive value (PPV) of only 29% [28]. Measurement of BK viral load by polymerase chain reaction (PCR) in the urine is another method used to monitor BKV infection [35]. Low levels of viruria may reflect asymptomatic shedding during the latent phase and increasing viral load is indicative of active BKV replication. The negative predictive value (NPV) of BKV DNA in urine for BKVN is close to 100%, but has a PPV of only ∼40–67% [36, 37]. Many variables, such as micturition intervals, fluctuations in urine content and the method of sample processing and shipment, can contribute to interassay variations. BK viral copy number may also vary depending on whether or not supernatants, cell pellets or resuspended urine are used for DNA preparation [38].
Other tests for BK viruria include the measurement of BKV mRNA in urine and the detection of cast-like three-dimensional BKV aggregates (Haufen) in urine by electron microscopy [39]. The amplification of viral VP1 mRNA in urine may be a better test for BK viruria as it represents active BKV replication [40]. Using a cutoff value of 6.5 × 105 BKV VP1 mRNA copies per nanogram total RNA, authors in one study reported a 94% sensitivity and specificity for BKVN. In a more recent study, the group further validated their original findings in a larger cohort of patients. They reported that urinary BKV VP1 mRNA expression continued to accurately diagnose BKVN. Furthermore, elevated levels of mRNA for granzyme B and proteinase inhibitor-9 in urine predicted those who developed graft dysfunction in the 12 months post-BKVN diagnosis [41]. This shows promise as a non-invasive test that can predict BKVN, but will need to be replicated. Using electron microscopy to detect BK virions (Haufen) in the urine is also reported to accurately correlate with BKVN [39], with a PPV of 97% and an NPV of 100% for BKVN [39]. This experience is also limited and requires validation.
BK viremia can be measured quantitatively by PCR in plasma. In one study, a BKV PCR value of 5000 copies/mL had a sensitivity of 100%, for BKVN, but yielded a false-positive diagnosis in 15.2%. A higher threshold of 1 × 105 copies/mL reduced the false-positive diagnosis to 6.1%, but the sensitivity decreased to 70% [36]. In another study, the sensitivity of any BK viremia for BKVN was found to be 100%, with a specificity of 88% and PPV of 82% [29]. In a prospective analysis of BKV replication and BKVN in renal transplant recipients, patients with BKVN had a viral load >7700 copies/mL, with a mean viral load of 28 000 copies/mL [28]. These observations are complicated by occasional reports of BKVN with viremia as low as 1000 copies/mL [37]. It is clear that BK viremia is seen in all patients with BKVN, but a threshold value that can accurately diagnose BKVN without the need for a biopsy does not exist. PCR assays can detect a wide range of viremia and this can vary from laboratory to laboratory. It is important to be aware of the limits of detection and the thresholds for quantitation in individual laboratories when interpreting PCR results.
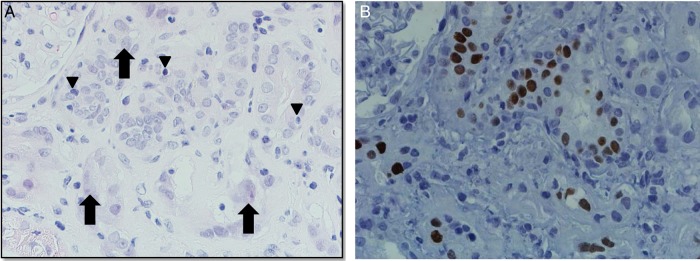
The definitive diagnosis of BKVN still requires a kidney biopsy where intranuclear polyomavirus inclusion bodies in tubular epithelial and/or glomerular parietal cells can be identified (Figure 1A). Inclusion bodies are basophilic structures seen on light microscopy [42–44]. The cytopathic changes are often associated with epithelial cell necrosis resulting in denudation of tubular basement membranes and acute tubular injury. The associated inflammatory response is variable and may be rich in lymphocytes, plasma cells and/or polymorphonuclear leukocytes [38]. These findings are not pathognomonic and most centers probe biopsy specimens with antibodies against T antigen, a polyoma protein present among BKV, JCV and Simian Virus 40 (SV40) species [38]. The histopathological changes and the T Ag positivity on immunohistochemistry can be patchy, multifocal and in the medulla and/or cortex (Figure 1B). It is possible to miss BKVN with a single biopsy core and two cores of kidney tissue, with at least one containing medullary parenchyma, should preferably be evaluated.
Fig. 1.
Polyomavirus nephropathy in a lung transplant recipient. (A) Hematoxylin & Eosin stain. Interstitial inflammation with mononuclear, lymphocyte and plasma cells, and areas of tubulitis (arrowheads): mononuclear and lymphocyte inflammatory cells damaging epithelium of the renal tubules. Viral cytopathic changes including nuclear atypia, vesicular changes, and finely granular and coarsely clumped inclusions (black arrow) are shown. (B) Positive (tan-brown) SV-40 large T antigen (polyomavirus) immunohistochemical stain in nuclei of tubular epithelial cells.
Three histologic patterns (A, B and C) of injury have been described [44]. In early disease (Pattern A), the cytopathic changes are present with little to no inflammation or tubular atrophy. Pattern B consists of viral cytopathic changes with varying degrees of inflammation, tubular atrophy and fibrosis. In late BKVN (Pattern C), cytopathic changes often are less apparent as a result of a tubular atrophy, interstitial fibrosis and chronic inflammatory infiltrate. The degree of damage corresponds to the degree of allograft dysfunction and correlates with allograft outcome.
BKV reactivation following kidney transplant should be considered in the differential diagnosis of acute kidney injury (AKI) or chronic kidney disease (CKD). Other common causes of AKI in kidney transplant recipients are CNI nephrotoxicity and allograft rejection. A kidney biopsy is necessary to definitively differentiate these, although the specific clinical scenario can raise suspicion for BKVN. For example, a rising creatinine within the first few months following intensification of immunosuppression but with therapeutic CNI levels increases the pretest probability of BKVN. On the other hand, in a highly sensitized transplant recipient with AKI in the setting of low-CNI levels suspicion for acute rejection should be high. A rising creatinine with elevated CNI levels especially months and years after transplantation is more likely to be secondary to CNI nephrotoxicity and should be responsive to drug dose reduction.
One of the strategies to treat BKVN is reduction in immunosuppression (discussed in the Treatment section). After control of BKV is achieved, an increase in creatinine should prompt an evaluation for acute rejection. With aggressive BKV surveillance protocols, early changes in creatinine can lead to evaluations for BKVN by renal biopsy. In patients who have had stable graft function for several years, surveillance for BKV replication are generally not as frequent as in the initial post-transplant period since BKVN is less likely to occur late when there has not been any intensification in the immunosuppression regimen.
BKV incidence and/or prevalence in NRSOT
BK viremia and viruria in liver transplant recipients
In a prospective, cross-sectional study of 59 consecutive pediatric liver transplant recipients, random blood and urine samples were obtained to determine the prevalence of BKV infection. Nine patients (15.3%) had viruria, although eight had low-level viruria (median 610 copies/mL, range 18–3.9 × 105 copies/mL) and only one had high-level viruria (2.2 × 109 copies/mL). One patient had low-level viremia (98 copies/mL) and no viruria [45]. In all cases, the viruria resolved spontaneously on follow-up and no impairment in renal function was evident. In another prospective prevalence study at a mean of 2187 days after transplant (range 20–5671 days), 100 consecutive pediatric liver transplant recipients had urine screened for BKV. A plasma analysis by PCR was done if >100 000 copies/mL of BKV were detected in urine. BK viruria was found in 15 patients (median copies 25 930 copies/mL; range 200–300 000 copies/mL) and none had viremia [46]. Concurrent determination of estimated glomerular filtration rate (eGFR) showed no difference between the median eGFR in patients with and without BK viruria. No long-term follow of renal function or BK viremia was reported. In a prospective longitudinal study, 62 adult liver transplant recipients were tested for BKV infection with urine and plasma PCR at specific time points after transplantation. BK viruria was detected in 21% with median urine BK viral load 7.58 × 106 (range 9.8 × 102–1.4 × 1013) copies/mL. BK viremia was detected in 18% (11 patients) with a median plasma BK viral load 2.01 × 105 (range 3.4 × 102–2.9 × 1014) copies/mL [47]. Five of the nine with viremia who were also tested for viruria were positive. In 10 of 11 cases, BK viremia was detected in the first 3 months after transplant. Renal function was similar in those with BK viremia (1.2 mg/dL) compared with those without (1.1 mg/dL). Three patients with persistent viremia displayed AKI. The first patient had high viral load (2.9 × 1013 copies/mL) and developed AKI concomitant with an acute cellular rejection of the liver allograft, with renal function improving coincident with the treatment of rejection. This patient died a month later with multiorgan failure and a request for autopsy was declined. The other two patients also developed renal failure, and in both these cases, the attending physicians attributed it to CNI toxicity. CNI doses were decreased and renal function improved. A patient with persistent viremia showed higher mean BK viral loads (5 × 1012 versus 292 775 copies/mL) [47]. In 121 patients who enrolled in a CMV prevention study comparing valganciclovir with ganciclovir, blood was also drawn for BKV and JCV surveillance at 2, 6 and 10 weeks, and 3, 4, 4.5, 6, 8 and 12 months after transplant. BK viremia was detected in five (4.1%) transplant patients. No renal dysfunction was noted in any of the patients with BK viremia a month before and a month after detection of BKV, but no longer term follow-up is available [48]. In another single-center study, urine was prospectively monitored for BKV at the time of transplant and then 3, 6 and 9 months later. If urine was positive, then plasma was tested. Three of 25 patients had BK viruria, but no viremia was detected in any patient. There was no association between BK viruria and renal dysfunction [33]. In five other studies, 147 liver transplant recipients were assessed for BK infection at various times after transplant. A total of 28 of 147 had viruria and of 114 tested, none had viremia [49–53]. Based on the above studies, it appears that BK viruria can be detected in up to 25% of liver transplant recipients [49], but the incidence of BK viremia is low although 18% of patients serially tested at multiple time points were positive for BK viremia at one point or another [47]. Two patients with AKI and BK viremia responded to reduction in CNI dose, which would be expected with either drug nephrotoxicity or with BKVN. Since no patient with high-level BK viremia or AKI had a renal biopsy, it remains possible that some patients developed BKVN.
BK viremia and viruria in heart transplant recipients
In a prospective study of 28 consecutive heart transplant recipients [54], urine and plasma samples collected at Week 1 and Months 1, 3, 6, 9 and 12 after transplant were tested by PCR. BK viruria was found in 12 patients (42.8%), 5 of whom also had viremia (17.8%) and 1 had transient viremia with no viruria. Median plasma BK viral load was 3.5 × 104 (range 5.8 × 103–8.6 × 104) copies/mL and median urine BK viral load was 3.7 × 1010 (range 2.2 × 103–2.2 × 1011) copies/mL. Median time to BK viremia was 30 days after transplant. One patient had transient BK viremia without viruria at 1 week after transplant and two others were transiently positive at 1 week and 1 month. Persistent BK viremia was noted from Months 1–3 in one patient and between Months 6 and 18 in another. Both developed new renal impairment. In the first case, renal dysfunction was attributed to BKVN, but BKV was only demonstrated by biopsy in the urinary bladder and in the second, the authors speculate that CNI toxicity could have contributed to renal failure. Patients with BKV infection (BK viruria and/or viremia) were reported to be more likely to have a higher median creatinine value compared with those without, although the relevance of this statistic is unclear. In a cross-sectional analysis of 111 cardiac transplantation patients, 14 patients had evidence of BK viruria and none had evidence of BK viremia. The mean serum creatinine value did not differ significantly between the patients with and without BK viruria [55]. In 45 heart transplant patients enrolled in a study comparing valganciclovir with ganciclovir in the prevention of CMV infection, blood was drawn for BKV and JCV surveillance at 2, 6 and 10 weeks, and 3, 4, 4.5, 6, 8 and 12 months after transplant. BK viremia was detected in three heart (6.7%) transplant patients, all after treatment for rejection. No renal dysfunction was noted in any of the patients with BK viremia a month before and a month after detection of BKV, but no longer term follow-up is available [48]. In another single-center study, urine was prospectively monitored for BKV at the time of transplant and then 3, 6 and 9 months later. If urine was positive, then plasma was tested. One of the seven patients had BK viruria, but no viremia was detected in any of the patients. There was no association between BK viruria and renal dysfunction [33].
BK viremia and viruria in lung transplant recipients
In a prospective lung transplant study, 50 recipients were tested for BKV in urine and plasma. Thirty-two percent had BKV in at least one urine specimen (mean viral load 5.0 × 1010 copies/mL). All blood samples were negative for BKV and there was no difference in the calculated creatinine clearance between those with BK viruria and those without [56]. In a later study from the same center, 87 adult lung and 3 heart lung transplant recipients were enrolled prospectively to provide urine samples for BKV. Forty-two percent of patients were positive for BKV. Two patients in the study developed end-stage renal disease (ESRD): 1 of the 38 with BK viruria and 1 of the 52 who did not. Unfortunately, in both these patients BK viremia was not evaluated nor was a kidney biopsy done to confirm BKV as cause for ESRD. Longitudinal analysis of renal function revealed no association between BK viruria and renal function [57]. In another single-center study, urine was prospectively monitored for BKV at the time of transplant and then 3, 6 and 9 months later. If urine was positive, then plasma was tested. Five of 28 patients had BK viruria, but no viremia was detected in any of the patients. There was no association between BK viruria and renal dysfunction [33]. Twenty-three lung transplant recipients and a heart lung recipient at a single center with evidence of renal dysfunction (creatinine >1.8 or ≥30% decline in GFR from highest GFR or GFR at 1 month after transplant) had blood and urine checked for BKV. Patients were enrolled at a median time of 3.5 years after transplant. Of these 24 patients (16.7%), 4 had viruria and none had viremia. Mean estimated creatinine clearance was similar in patients with or without BK viruria [53]. No patient had a renal biopsy to determine the cause of renal dysfunction.
To summarize, the studies discussed above indicate that BK viruria is sometimes detectable by routine screening after NRSOT. In pediatric liver transplant recipients, its incidence can range up to 15.3% [45, 46], in adult liver transplant recipients from 12.5 to 52% [33, 47, 49–52], heart transplant recipients from 2.9 to 42.8% [33, 53–55] and lung recipients from 11.7 to 42% [33, 53, 56, 57]. The incidence of BK viremia is lower and ranges between 0–18% in adult liver recipients [33, 47–52], 0–17.8% in heart recipients [33, 48, 53–55] and because of limited studies, unknown in lung transplant recipients [33, 53, 56]. There were far too few studies to determine the median time to viremia after NRSOT, although almost all patients [47, 48, 54] had viruria at the time of detection of viremia [54]. The median time to develop viruria in the heart transplant population was 220 days, but no information is available for liver or lung recipients [47]. Based on data from the above studies, the association between BK viruria and renal dysfunction is poor and there is insufficient information to determine if any patient with BK viremia had renal dysfunction.
BKVN in NRSOT
Although apparently rare, BKVN has been reported in lung [58, 59], heart [60, 61] and pancreas [62] transplant recipients. In these nine cases, patients were being evaluated for CKD without a clear cause. All patients were detected to have BK viremia and all patients had BK viruria. In a review of these cases of BKVN, the median plasma BK viral load was reported to be 5.2 log10 copies/mL and urine viral load was >7 log10 copies/mL [59]. Five of six were treated with immunosuppression reduction and cidofovir and the other with immunosuppression reduction and leflunomide. The treatment regimen in the other three patients was not reported. Of these nine cases, two cleared the virus from plasma, but follow-up information on BKV was not reported in the others. Five patients progressed to ESRD needing dialysis, one patient had stabilization of renal function and the renal outcome for three others was not reported [59]. Case reports of BKVN with poor outcomes reflect the ascertainment bias that comes with biopsies done in patients with progressive CKD and the publication bias that comes from positive biopsy evidence of BKVN, and does not necessarily indicate poorer outcomes of BKVN [59] in NRSOT who develop BKVN compared with those that do not. Furthermore, because of the small numbers reported it is difficult to truly compare the difference in outcomes between BKVN [61, 62] in NRSOT with those in the kidney transplant population. It is important to note that there is an especially high incidence of CKD and ESRD in some types of NRSOT, which has been attributed to CNI nephrotoxicity. In one large database analysis of chronic renal failure after a NRSOT, the incidence of CKD ranged from 1.7 to 9.6% at 12 months after transplant and 4.2 to 14.2% at 36 months after transplantation [63].
We illustrate the clinical presentation and treatment of a lung transplant patient at our center who developed BK viremia with CKD from BKVN.
A 63-year-old Caucasian male with severe chronic obstructive pulmonary disease and long-standing hypertension underwent bilateral lung transplantation. Patient's pre-transplant creatinine ranged from 0.7 to 0.9 mg/dL with an eGFR of 100.4–90.6 mL/min/1.73 m2 (Chronic Kidney Disease Epidemiology Collaboration). He was induced with anti-thymocyte globulin and maintained on tacrolimus, azathioprine and prednisone. His tacrolimus levels were kept between 6 and 9 ng/mL. He did not have any allograft rejection episodes and did not require intensification of his immunosuppression. His initial serum creatinine ranged from 0.7 to 1.0 mg/dL (eGFR 100.4–79.7 mL/min/1.73m2) and 2 years following transplant it increased to range between 1.4 to 1.8 mg/dL (eGFR 54.1–40.4 mL/min/1.73m2). Over the next 2 years, it slowly increased to 2.4–2.8 mg/dL (eGFR 26.9–22.3 mL/min/1.73 m2). This prompted testing for BKV in plasma with a quantitative PCR assay, which was positive at 87 900 copies/mL. His azathioprine was stopped and he began treatment with leflunomide at 20 mg daily. Two weeks later, the dose was increased to 40 mg to attain a teriflunomide level of >40 µg/mL. Despite this therapy, his creatinine increased to 3.0–3.4 mg/dL (eGFR 22.3–17.7 mL/min/1.73 m2). Patient was noted to have an unremarkable urinalysis with a random urine–protein creatinine ratio of 0.4. A kidney biopsy was performed that showed patchy, mononuclear and lymphoplasmacytic tubulointerstitial inflammation, with rare admixed neutrophils and eosinophils on hematoxylin and eosin staining (Figure 1A). There was also significant global glomerulosclerosis, moderate to severe interstitial fibrosis and tubular atrophy (IFTA). Immunohistochemical stain for polyomavirus (SV-40) was positive in renal tubular epithelial cell nuclei (Figure 1B). Standard immunofluorescence and electron microscopy studies were non-contributory. A diagnosis of BKVN with possible chronic CNI-related nephrotoxicity was made. The dose of leflunomide was increased to 60 mg daily and ciprofloxacin at 250 mg daily was added. Ciprofloxacin was continued for a total of 8 weeks. The patient's tacrolimus dose was reduced to achieve therapeutic targets between 4 and 6 ng/mL, but his prednisone was continued at 5 mg/day. His BKV counts began to fall and about a year later, his BKV counts reached a nadir of 1500 copies/mL and renal function has stabilized with creatinine measuring between 2.9 and 3.0 mg/dL (eGFR 21.4–20.5 mL/min/1.73 m2).
Recommendations for diagnosis of BKV infection in NRSOT
When should BKV infection be considered? The risk factors for BK viremia and BKVN in kidney transplant recipients are thought to include intense maintenance immunosuppression, prior episodes of allograft rejection, induction therapy with anti-thymocyte globulin, older age and male gender with immunosuppressive therapy thought to account for most of this risk [64, 65]. However, immunosuppression alone cannot account for the high incidence of BKV reactivation in transplant recipients since NRSOT recipients have a much lower incidence of BKV reactivation. It is likely that immunosuppression occurs in the setting of an adverse environment or a second hit. Mice infected with polyoma virus will not develop viral nephropathy unless exposed to either an ischemic or chemical injury to the kidney that probably creates a permissive environment for viral replication [66]. In humans, such an injury could occur at the time of renal transplant secondary to ischemia–reperfusion injury, drug toxicity, ureteral stent placement or donor–recipient HLA mismatch or the development of allograft rejection [55].
Since there is a high incidence of BK viremia and BKVN in the kidney transplant population, most kidney transplant centers are serially testing their recipients for BKV. Plasma and/or urine BKV screening is based on the paradigm that all patients with BKVN first have evidence of preceding BK viremia or BK viruria. The incidence of BK viremia appears to be very low in NRSOT patients and it is likely that BKVN is much less common in NRSOT patients compared with kidney transplant recipients, although with the paucity of publications on renal biopsy findings in NRSOT patients, it is difficult to be certain. Many NRSOT patients are presumed to have tacrolimus nephrotoxicity when they develop CKD, but this should be a diagnosis of exclusion.
We recommend that BKV infection be considered in all NRSOT patients with AKI or CKD. We believe that plasma BKV by PCR should be tested in those recipients who have renal dysfunction. Though there is a lack of data on the correlation of viruria, viremia and nephropathy in NRSOT, extrapolating from data in the kidney transplant population, a negative plasma BKV screen can be used to exclude BKVN in NRSOT. In the case of BK viremia and CKD or AKI, a renal biopsy with specific evaluation for BKVN should be considered. While early in BKVN the biopsy findings demonstrate an acute interstitial nephritis with tubular cytopathic changes, in late disease the disease may be missed if not specifically considered and biopsy samples tested for T Ag staining (Figure 1B). Biopsy findings in advanced CNI nephrotoxicity and late BKVN may be nonspecific and include IFTA with or without a modest chronic inflammatory infiltrate and if T Ag staining is not done, it may be difficult to differentiate. In those with no biopsy evidence of BKVN, it is important to closely monitor BKV plasma levels. If renal function is stable, continued monitoring by PCR is reasonable. Increasing BKV viremia increases the risk for BKVN and then further immunosuppression reduction or anti-BKV therapy should be considered. In addition, a repeat renal biopsy may be necessary when there is further deterioration in renal function.
The available literature does not support the need for routine surveillance for BKV infection after NRSOT. Yet, it is clear that BKVN in NRSOT can lead to irreversible renal dysfunction. Until we have data to the contrary, it is our opinion that patients who develop BK viremia are at risk for BKVN and once detected, regular monitoring of BKV and renal function is necessary. Additionally, any patient with unexplained AKI following a solid organ transplant should be tested for BKV replication and if necessary, the cause of the AKI further evaluated with appropriate studies, which may include a renal biopsy.
Treatment
The goal in treating BKV replication is to eliminate the virus while preserving the maximal amount of renal function. Treatment options for BKV infections come from studies in the kidney and bone marrow transplant population. In this section, we review the management options for BKV in kidney transplant recipients and make recommendations for BKV in NRSOT.
As BK infection involves reactivation of a latent virus in the setting of a suppressed immune system, the most widely accepted intervention is to reduce immunosuppression. The prevention of BKVN by monitoring BK viral load and appropriately decreasing immunosuppression for BKV replication appears to improve graft survival in non-randomized trials in the kidney transplant population [34, 67]. In one study, urine and serum samples were monitored weekly for 16 weeks, and at Months 5, 6, 9 and 12 after kidney transplant. BK viruria was taken as evidence of active viral infection and the presence of sustained viruria prompted review of patient's immunosuppression and appropriate adjustment in accordance with their protocol. Progression to BK viremia led to discontinuation of the anti-proliferative component (azathioprine and mycophenolate mofetil) of immunosuppressive regimen. If viremia failed to clear 3–4 weeks later, the CNI was tapered to minimum acceptable levels (cyclosporine 12-h trough levels of 100–200 ng/mL and tacrolimus levels of 3–5 ng/mL). Using this protocol, 35% were detected to have viruria and 11.5% viremia by 1 year. After reduction in immunosuppression, viremia resolved in 95%, without increased acute rejection, allograft dysfunction or graft loss [34]. At 5-year follow-up (available on 97% of patients), graft survival was 84% [67].
Despite reduction in immunosuppression, renal allograft losses have been reported and this has led to the use of antiviral agents in addition to reduction in immunosuppression [43, 68]. Quinolones that act via inhibiting the DNA gyrase have in vitro and in vivo activity against BKV [69–71]. Based on this evidence, a 1-month fluoroquinolone course after renal transplantation was associated with significantly lower rates of BK viremia at 1 year compared with those with no fluoroquinolones [72]. A more recent randomized control trial using a 3-month course of levofloxacin initiated early following renal transplantation did not prevent BK viruria [73]. Another multicenter double-blinded placebo-controlled trial also confirmed that a 30-day course of levofloxacin did not improve BK viral load reduction [74]. These data clearly indicate that quinolones have no role in the treatment of BK reactivation after kidney transplantation.
The active metabolite of leflunomide, A77 1726, has also been shown to have substantial antiviral activity in vitro and in animals [75]. In 17 kidney transplant recipients with biopsy-proven BKVN, leflunomide therapy along with discontinuation of prednisone and mycophenolate, and reduced tacrolimus dosing (target trough 4–6 ng/mL), demonstrated clearance of viremia or progressive reductions in the viral load in blood and urine (P < 0.001) [76]. Leflunomide treatment consisted of a loading dose of 100 mg per day for 5 days and maintenance doses of 20–60 mg per day, with a target blood level of 50–100 µg/mL [76]. However, in a Phase 2, randomized, open-label, parallel-group, 6-month study in renal transplant patients, FK778 (derived from an active metabolite of leflunomide) was compared with the current standard of care (reduction in immunosuppression) for the treatment of newly diagnosed or untreated BKN, confirmed by renal biopsy [77]. Despite a greater decrease in plasma BK viral load, treatment with FK778 was associated with more rejection, and less favorable renal function and safety profile than standard of care. The authors concluded that reduction in immunosuppression with careful monitoring is paramount importance in the prevention of progressive renal dysfunction and graft loss in renal transplant patients with newly diagnosed BKVN [77].
There has also been interest in the use of mammalian target of rapamycin inhibitor, sirolimus, for the treatment of BKV after kidney transplants. Though there are no randomized trials that have shown the superiority of a regimen using sirolimus in the treatment of BK infections, a retrospective analysis has shown that patients taking a sirolimus-containing immunosuppressive regimen appear to have a lower incidence of BK infection [78].
Cidofovir, a cytosine analog and viral DNA polymerase inhibitor, inhibits BKV replication [79]. In a cohort of 21 recipients with BKVN, no graft loss was reported in eight kidney transplant recipients who received treatment with cidofovir but a 70% graft loss in the 13 who did not [80]. Low-dose cidofovir at dosages ranging from 0.25 to 1 mg/kg every 1–3 weeks has been successfully employed in an uncontrolled fashion for the treatment of BKVN in adult and pediatric renal recipients [35, 81]. However, it is primarily excreted by the kidneys [82] and is nephrotoxic [83]. The nephrotoxicity and the lack of randomized studies have led to reluctance to adopt it widely.
Brincidofovir (CMX001) is an investigational orally administered, ether-lipid ester conjugated prodrug of cidofovir. The nephrotoxic effect of cidofovir is apparently abrogated by lipid conjugation [84]. In a pediatric kidney transplant recipient with BKVN, treatment with reduction in immunosuppression, leflunomide and ciprofloxacin, failed to prevent allograft dysfunction. With FDA approval, the patients were started on a 36-week course with the investigational drug. This lead to an improvement in creatinine, though BKV DNA loads remained low [85]. Further studies are necessary to better understand the role of brincidofovir in the treatment of BKVN.
Intravenous immunoglobulin (IVIg) is believed to contain antibodies that can bind and neutralize BKV. Analysis of commercially available IVIg preparations from different suppliers revealed that co-incubation of BKV with clinically relevant concentrations of IVIg derived from healthy and hepatitis B vaccinated subjects caused >90% inhibition of viral DNA yield after 7 days in culture [86]. Immunosuppression reduction together with IVIg at a dose of 2 g/kg over 2–5 days was used in eight kidney transplant recipients diagnosed with BKVN [87]. After a mean follow-up of 15 months, only one patient returned to dialysis but four were unable to clear the virus [87]. In another series of 12 cases of BKVN treated with IVIg, there was no robust protective effect on maintaining renal function, although 10 of them had also received cidofovir with IVIg [88]. Despite these data, clinicians continue to consider IVIG as a treatment option.
For the management of BKV infection in NRSOT, we suggest that those with BK viremia and normal renal function should be closely monitoring for increasing viremia and/or the development of AKI. In those with viremia and AKI or CKD, a kidney biopsy is necessary to confirm BKVN. With BKVN, the mainstay of therapy is the reduction in immunosuppression, although this may increase the risk of rejection and that risk in a life-sustaining allograft such as the heart, lung or liver must be weighed against the risk of progressive CKD. If immunosuppression can be reduced, we recommend reducing or discontinuing mycophenolate mofetil (MMF) and beginning leflunomide therapy targeting teriflunomide levels >40 µg/mL in serum, while monitoring BK PCR levels on a 2–4 weekly basis. Decreasing viral loads with or without improving renal function indicates a response to treatment. If there is increasing viremia or worsening renal function, additional reduction in immunosuppression such as reducing the tacrolimus dose to achieve a lower-drug level and the introduction of other agents including low-dose cidofovir and IVIg should be considered.
Conclusions
The prevalence of BKV infection after NRSOT is not clearly established and the clinical significance of BK viruria remains unclear. Data that have been reviewed in this study indicate that BK viruria in NRSOT is as prevalent as in the renal transplant population. Viremia on the other hand is not as common and the factors that underlie this difference are unclear. The available literature does not support the need for routine surveillance for BKV infection after NRSOT. Yet, it is clear that BKVN in NRSOT can lead to irreversible renal dysfunction. The risk factors for this are poorly understood.
BKVN should be considered in the differential diagnosis of AKI and CKD after NRSOT in any patient with BK viremia, especially if associated with minimal proteinuria and bland urine on urinalysis, features suggestive of a chronic tubulointerstitial disease. It is important to note that a large fraction of CKD and ESRD after NRSOT is attributed to CNI nephrotoxicity, which also presents features of a chronic tubulointerstitial disease. If BK viremia is present, the definitive diagnosis of BKVN can be made by kidney biopsy and the microscopic examination should include an immunohistochemical stain for SV40 T antigen.
The treatment of BK viremia and BKVN involves reduction in immunosuppression, although this must be balanced against the risk of rejection in the allograft. In situations when immunosuppression can be reduced, a stepwise approach begins with discontinuing the antimetabolite (MMF or azathioprine). Serial monitoring of plasma BKV PCR and renal function should guide further therapy. Additional agents to consider include leflunomide, cidofovir, sirolimus or IVIg. Consultation with transplant nephrologists who regularly manage BKV infections after kidney transplant may be helpful.
Conflict of interest statement
The authors declare that the results presented in this paper have not been published previously in whole or in part.
References
- 1.Gardner SD, Field AM, Coleman DV, et al. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet 1971; 1: 1253–1257 [DOI] [PubMed] [Google Scholar]
- 2.Padgett BL, Walker DL, ZuRhein GM, et al. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet 1971; 1: 1257–1260 [DOI] [PubMed] [Google Scholar]
- 3.DeCaprio JA, Garcea RL. A cornucopia of human polyomaviruses. Nat Rev Microbiol 2013; 11: 264–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nickeleit V, Singh HK. Polyomaviruses and disease: is there more to know than viremia and viruria? Curr Opin Organ Transplant 2015; 20: 348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goudsmit J, Wertheim-van Dillen P, van Strien A, et al. The role of BK virus in acute respiratory tract disease and the presence of BKV DNA in tonsils. J Med Virol 1982; 10: 91–99 [DOI] [PubMed] [Google Scholar]
- 6.Shah KV, Daniel RW, Warszawski RM. High prevalence of antibodies to BK virus, an SV40-related papovavirus, in residents of Maryland. J Infect Dis 1973; 128: 784–787 [DOI] [PubMed] [Google Scholar]
- 7.Knowles WA, Pipkin P, Andrews N, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol 2003; 71: 115–123 [DOI] [PubMed] [Google Scholar]
- 8.Knowles WA. Discovery and epidemiology of the human polyomaviruses BK virus (BKV) and JC virus (JCV). Adv Exp Med Biol 2006; 577: 19–45 [DOI] [PubMed] [Google Scholar]
- 9.Jiang M, Abend JR, Johnson SF, et al. The role of polyomaviruses in human disease. Virology 2009; 384: 266–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin L, Gibson PE, Booth JC, et al. Genomic typing of BK virus in clinical specimens by direct sequencing of polymerase chain reaction products. J Med Virol 1993; 41: 11–17 [DOI] [PubMed] [Google Scholar]
- 11.Sharma PM, Gupta G, Vats A, et al. Phylogenetic analysis of polyomavirus BK sequences. J Virol 2006; 80: 8869–8879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boldorini R, Veggiani C, Barco D, et al. Kidney and urinary tract polyomavirus infection and distribution: molecular biology investigation of 10 consecutive autopsies. Arch Pathol Lab Med 2005; 129: 69–73 [DOI] [PubMed] [Google Scholar]
- 13.Behzad-Behbahani A, Klapper PE, Vallely PJ, et al. Detection of BK virus and JC virus DNA in urine samples from immunocompromised (HIV-infected) and immunocompetent (HIV-non-infected) patients using polymerase chain reaction and microplate hybridisation. J Clin Virol 2004; 29: 224–229 [DOI] [PubMed] [Google Scholar]
- 14.Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis 2003; 3: 611–623 [DOI] [PubMed] [Google Scholar]
- 15.Chang D, Wang M, Ou WC, et al. Genotypes of human polyomaviruses in urine samples of pregnant women in Taiwan. J Med Virol 1996; 48: 95–101 [DOI] [PubMed] [Google Scholar]
- 16.Coleman DV, Gardner SD, Mulholland C, et al. Human polyomavirus in pregnancy. A model for the study of defence mechanisms to virus reactivation. Clin Exp Immunol 1983; 53: 289–296 [PMC free article] [PubMed] [Google Scholar]
- 17.Kahan AV, Coleman DV, Koss LG. Activation of human polyomavirus infection-detection by cytologic technics. Am J Clin Pathol 1980; 74: 326–332 [DOI] [PubMed] [Google Scholar]
- 18.Sundsfjord A, Flaegstad T, Flo R, et al. BK and JC viruses in human immunodeficiency virus type 1-infected persons: prevalence, excretion, viremia, and viral regulatory regions. J Infect Dis 1994; 169: 485–490 [DOI] [PubMed] [Google Scholar]
- 19.Sundsfjord A, Osei A, Rosenqvist H, et al. BK and JC viruses in patients with systemic lupus erythematosus: prevalent and persistent BK viruria, sequence stability of the viral regulatory regions, and nondetectable viremia. J Infect Dis 1999; 180: 1–9 [DOI] [PubMed] [Google Scholar]
- 20.Jin L, Gibson PE, Knowles WA, et al. BK virus antigenic variants: sequence analysis within the capsid VP1 epitope. J Med Virol 1993; 39: 50–56 [DOI] [PubMed] [Google Scholar]
- 21.Heritage J, Chesters PM, McCance DJ. The persistence of papovavirus BK DNA sequences in normal human renal tissue. J Med Virol 1981; 8: 143–150 [DOI] [PubMed] [Google Scholar]
- 22.Shinohara T, Matsuda M, Cheng SH, et al. BK virus infection of the human urinary tract. J Med Virol 1993; 41: 301–305 [DOI] [PubMed] [Google Scholar]
- 23.Drachenberg CB, Papadimitriou JC, Wali R, et al. BK polyoma virus allograft nephropathy: ultrastructural features from viral cell entry to lysis. Am J Transplant 2003; 3: 1383–1392 [DOI] [PubMed] [Google Scholar]
- 24.Nickeleit V, Hirsch HH, Zeiler M, et al. BK-virus nephropathy in renal transplants-tubular necrosis, MHC-class II expression and rejection in a puzzling game. Nephrol Dial Transplant 2000; 15: 324–332 [DOI] [PubMed] [Google Scholar]
- 25.Mackenzie EF, Poulding JM, Harrison PR, et al. Human polyoma virus (HPV)—a significant pathogen in renal transplantation. Proc Eur Dial Transplant Assoc 1978; 15: 352–360 [PubMed] [Google Scholar]
- 26.Gardner SD, MacKenzie EF, Smith C, et al. Prospective study of the human polyomaviruses BK and JC and cytomegalovirus in renal transplant recipients. J Clin Pathol 1984; 37: 578–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogan TF, Borden EC, McBain JA, et al. Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann Intern Med 1980; 92: 373–378 [DOI] [PubMed] [Google Scholar]
- 28.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med 2002; 347: 488–496 [DOI] [PubMed] [Google Scholar]
- 29.Nickeleit V, Klimkait T, Binet IF, et al. Testing for polyomavirus type BK DNA in plasma to identify renal-allograft recipients with viral nephropathy. N Engl J Med 2000; 342: 1309–1315 [DOI] [PubMed] [Google Scholar]
- 30.Ramos E, Drachenberg CB, Portocarrero M, et al. BK virus nephropathy diagnosis and treatment: experience at the University of Maryland Renal Transplant Program. Clin Transpl 2002; 143–153 [PubMed] [Google Scholar]
- 31.Ramos E, Drachenberg CB, Papadimitriou JC, et al. Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J Am Soc Nephrol 2002; 13: 2145–2151 [DOI] [PubMed] [Google Scholar]
- 32.Buehrig CK, Lager DJ, Stegall MD, et al. Influence of surveillance renal allograft biopsy on diagnosis and prognosis of polyomavirus-associated nephropathy. Kidney Int 2003; 64: 665–673 [DOI] [PubMed] [Google Scholar]
- 33.Doucette KE, Pang XL, Jackson K, et al. Prospective monitoring of BK polyomavirus infection early posttransplantation in nonrenal solid organ transplant recipients. Transplantation 2008; 85: 1733–1736 [DOI] [PubMed] [Google Scholar]
- 34.Brennan DC, Agha I, Bohl DL, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant 2005; 5: 582–594 [DOI] [PubMed] [Google Scholar]
- 35.Vats A, Shapiro R, Singh Randhawa P, et al. Quantitative viral load monitoring and cidofovir therapy for the management of BK virus-associated nephropathy in children and adults. Transplantation 2003; 75: 105–112 [DOI] [PubMed] [Google Scholar]
- 36.Randhawa P, Ho A, Shapiro R, et al. Correlates of quantitative measurement of BK polyomavirus (BKV) DNA with clinical course of BKV infection in renal transplant patients. J Clin Microbiol 2004; 42: 1176–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hariharan S. BK virus nephritis after renal transplantation. Kidney Int 2006; 69: 655–662 [DOI] [PubMed] [Google Scholar]
- 38.Hirsch HH, Brennan DC, Drachenberg CB, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation 2005; 79: 1277–1286 [DOI] [PubMed] [Google Scholar]
- 39.Singh HK, Andreoni KA, Madden V, et al. Presence of urinary Haufen accurately predicts polyomavirus nephropathy. J Am Soc Nephrol 2009; 20: 416–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding R, Medeiros M, Dadhania D, et al. Noninvasive diagnosis of BK virus nephritis by measurement of messenger RNA for BK virus VP1 in urine. Transplantation 2002; 74: 987–994 [DOI] [PubMed] [Google Scholar]
- 41.Dadhania D, Snopkowski C, Ding R, et al. Validation of noninvasive diagnosis of BK virus nephropathy and identification of prognostic biomarkers. Transplantation 2010; 90: 189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nickeleit V, Hirsch HH, Binet IF, et al. Polyomavirus infection of renal allograft recipients: from latent infection to manifest disease. J Am Soc Nephrol 1999; 10: 1080–1089 [DOI] [PubMed] [Google Scholar]
- 43.Randhawa PS, Finkelstein S, Scantlebury V, et al. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation 1999; 67: 103–109 [DOI] [PubMed] [Google Scholar]
- 44.Drachenberg CB, Papadimitriou JC, Hirsch HH, et al. Histological patterns of polyomavirus nephropathy: correlation with graft outcome and viral load. Am J Transplant 2004; 4: 2082–2092 [DOI] [PubMed] [Google Scholar]
- 45.Amir A, Shapiro R, Shulman LM, et al. BK virus infection and its effect on renal function in pediatric liver-transplant recipients: a cross-sectional, longitudinal, prospective study. Transplantation 2011; 92: 943–946 [DOI] [PubMed] [Google Scholar]
- 46.Brinkert F, Briem-Richter A, Ilchmann C, et al. Prevalence of polyomavirus viruria (JC virus/BK virus) in children following liver transplantation. Pediatr Transplant 2010; 14: 105–108 [DOI] [PubMed] [Google Scholar]
- 47.Loeches B, Valerio M, Perez M, et al. BK virus in liver transplant recipients: a prospective study. Transplant Proc 2009; 41: 1033–1037 [DOI] [PubMed] [Google Scholar]
- 48.Razonable RR, Brown RA, Humar A, et al. A longitudinal molecular surveillance study of human polyomavirus viremia in heart, kidney, liver, and pancreas transplant patients. J Infect Dis 2005; 192: 1349–1354 [DOI] [PubMed] [Google Scholar]
- 49.Salama M, Boudville N, Speers D, et al. Decline in native kidney function in liver transplant recipients is not associated with BK virus infection. Liver Transpl 2008; 14: 1787–1792 [DOI] [PubMed] [Google Scholar]
- 50.Randhawa P, Uhrmacher J, Pasculle W, et al. A comparative study of BK and JC virus infections in organ transplant recipients. J Med Virol 2005; 77: 238–243 [DOI] [PubMed] [Google Scholar]
- 51.Splendiani G, Cipriani S, Condo S, et al. Polyoma virus BK and renal dysfunction in a transplanted population. Transplant Proc 2004; 36: 713–715 [DOI] [PubMed] [Google Scholar]
- 52.Kusne S, Vilchez RA, Zanwar P, et al. Polyomavirus JC urinary shedding in kidney and liver transplant recipients associated with reduced creatinine clearance. J Infect Dis 2012; 206: 875–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barton TD, Blumberg EA, Doyle A, et al. A prospective cross-sectional study of BK virus infection in non-renal solid organ transplant recipients with chronic renal dysfunction. Transpl Infect Dis 2006; 8: 102–107 [DOI] [PubMed] [Google Scholar]
- 54.Loeches B, Valerio M, Palomo J, et al. BK virus in heart transplant recipients: a prospective study. J Heart Lung Transplant 2011; 30: 109–111 [DOI] [PubMed] [Google Scholar]
- 55.Pendse SS, Vadivel N, Ramos E, et al. BK viral reactivation in cardiac transplant patients: evidence for a double-hit hypothesis. J Heart Lung Transplant 2006; 25: 814–819 [DOI] [PubMed] [Google Scholar]
- 56.Thomas LD, Vilchez RA, White ZS, et al. A prospective longitudinal study of polyomavirus shedding in lung-transplant recipients. J Infect Dis 2007; 195: 442–449 [DOI] [PubMed] [Google Scholar]
- 57.Thomas LD, Milstone AP, Vilchez RA, et al. Polyomavirus infection and its impact on renal function and long-term outcomes after lung transplantation. Transplantation 2009; 88: 360–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwarz A, Mengel M, Haller H, et al. Polyoma virus nephropathy in native kidneys after lung transplantation. Am J Transplant 2005; 5: 2582–2585 [DOI] [PubMed] [Google Scholar]
- 59.Egli A, Helmersen DS, Taub K, et al. Renal failure five years after lung transplantation due to polyomavirus BK-associated nephropathy. Am J Transplant 2010; 10: 2324–2330 [DOI] [PubMed] [Google Scholar]
- 60.Menahem SA, McDougall KM, Thomson NM, et al. Native kidney BK nephropathy post cardiac transplantation. Transplantation 2005; 79: 259–260 [DOI] [PubMed] [Google Scholar]
- 61.Schmid H, Burg M, Kretzler M, et al. BK virus associated nephropathy in native kidneys of a heart allograft recipient. Am J Transplant 2005; 5: 1562–1568 [DOI] [PubMed] [Google Scholar]
- 62.Haririan A, Ramos ER, Drachenberg CB, et al. Polyomavirus nephropathy in native kidneys of a solitary pancreas transplant recipient. Transplantation 2002; 73: 1350–1353 [DOI] [PubMed] [Google Scholar]
- 63.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003; 349: 931–940 [DOI] [PubMed] [Google Scholar]
- 64.Hirsch HH, Babel N, Comoli P, et al. European perspective on human polyomavirus infection, replication and disease in solid organ transplantation. Clin Microbiol Infect 2014; 20(Suppl 7): 74–88 [DOI] [PubMed] [Google Scholar]
- 65.Sood P, Senanayake S, Sujeet K, et al. Management and outcome of BK viremia in renal transplant recipients: a prospective single-center study. Transplantation 2012; 94: 814–821 [DOI] [PubMed] [Google Scholar]
- 66.Atencio IA, Shadan FF, Zhou XJ, et al. Adult mouse kidneys become permissive to acute polyomavirus infection and reactivate persistent infections in response to cellular damage and regeneration. J Virol 1993; 67: 1424–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hardinger KL, Koch MJ, Bohl DJ, et al. BK-virus and the impact of pre-emptive immunosuppression reduction: 5-year results. Am J Transplant 2010; 10: 407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vasudev B, Hariharan S, Hussain SA, et al. BK virus nephritis: risk factors, timing, and outcome in renal transplant recipients. Kidney Int 2005; 68: 1834–1839 [DOI] [PubMed] [Google Scholar]
- 69.Leung AY, Chan MT, Yuen KY, et al. Ciprofloxacin decreased polyoma BK virus load in patients who underwent allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2005; 40: 528–537 [DOI] [PubMed] [Google Scholar]
- 70.Randhawa PS. Anti-BK virus activity of ciprofloxacin and related antibiotics. Clin Infect Dis 2005; 41: 1366–1367; author reply 7 [DOI] [PubMed] [Google Scholar]
- 71.Portolani M, Pietrosemoli P, Cermelli C, et al. Suppression of BK virus replication and cytopathic effect by inhibitors of prokaryotic DNA gyrase. Antiviral Res 1988; 9: 205–218 [DOI] [PubMed] [Google Scholar]
- 72.Gabardi S, Waikar SS, Martin S, et al. Evaluation of fluoroquinolones for the prevention of BK viremia after renal transplantation. Clin J Am Soc Nephrol 2010; 5: 1298–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Knoll GA, Humar A, Fergusson D, et al. Levofloxacin for BK virus prophylaxis following kidney transplantation: a randomized clinical trial. JAMA 2014; 312: 2106–2114 [DOI] [PubMed] [Google Scholar]
- 74.Lee BT, Gabardi S, Grafals M, et al. Efficacy of levofloxacin in the treatment of BK viremia: a multicenter, double-blinded, randomized, placebo-controlled trial. Clin J Am Soc Nephrol 2014; 9: 583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Waldman WJ, Knight DA, Blinder L, et al. Inhibition of cytomegalovirus in vitro and in vivo by the experimental immunosuppressive agent leflunomide. Intervirology 1999; 42: 412–418 [DOI] [PubMed] [Google Scholar]
- 76.Williams JW, Javaid B, Kadambi PV, et al. Leflunomide for polyomavirus type BK nephropathy. N Engl J Med 2005; 352: 1157–1158 [DOI] [PubMed] [Google Scholar]
- 77.Guasch A, Roy-Chaudhury P, Woodle ES, et al. Assessment of efficacy and safety of FK778 in comparison with standard care in renal transplant recipients with untreated BK nephropathy. Transplantation 2010; 90: 891–897 [DOI] [PubMed] [Google Scholar]
- 78.Tohme FA, Kalil RS, Thomas CP. Conversion to a sirolimus-based regimen is associated with lower incidence of BK viremia in low-risk kidney transplant recipients. Transpl Infect Dis 2015; 17: 66–72 [DOI] [PubMed] [Google Scholar]
- 79.Andrei G, Snoeck R, Vandeputte M, et al. Activities of various compounds against murine and primate polyomaviruses. Antimicrob Agents Chemother 1997; 41: 587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuypers DR, Vandooren AK, Lerut E, et al. Adjuvant low-dose cidofovir therapy for BK polyomavirus interstitial nephritis in renal transplant recipients. Am J Transplant 2005; 5: 1997–2004 [DOI] [PubMed] [Google Scholar]
- 81.Kuten SA, Patel SJ, Knight RJ, et al. Observations on the use of cidofovir for BK virus infection in renal transplantation. Transpl Infect Dis 2014; 16: 975–983 [DOI] [PubMed] [Google Scholar]
- 82.Brody SR, Humphreys MH, Gambertoglio JG, et al. Pharmacokinetics of cidofovir in renal insufficiency and in continuous ambulatory peritoneal dialysis or high-flux hemodialysis. Clin Pharmacol Ther 1999; 65: 21–28 [DOI] [PubMed] [Google Scholar]
- 83.Ortiz A, Justo P, Sanz A, et al. Tubular cell apoptosis and cidofovir-induced acute renal failure. Antivir Ther 2005; 10: 185–190 [PubMed] [Google Scholar]
- 84.Lanier R, Trost L, Tippin T, et al. Development of CMX001 for the treatment of Poxvirus infections. Viruses 2010; 2: 2740–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reisman L, Habib S, McClure GB, et al. Treatment of BK virus-associated nephropathy with CMX001 after kidney transplantation in a young child. Pediatr Transplant 2014; 18: E227–E231 [DOI] [PubMed] [Google Scholar]
- 86.Randhawa PS, Schonder K, Shapiro R, et al. Polyomavirus BK neutralizing activity in human immunoglobulin preparations. Transplantation 2010; 89: 1462–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sener A, House AA, Jevnikar AM, et al. Intravenous immunoglobulin as a treatment for BK virus associated nephropathy: one-year follow-up of renal allograft recipients. Transplantation 2006; 81: 117–120 [DOI] [PubMed] [Google Scholar]
- 88.Wadei HM, Rule AD, Lewin M, et al. Kidney transplant function and histological clearance of virus following diagnosis of polyomavirus-associated nephropathy (PVAN). Am J Transplant 2006; 6: 1025–1032 [DOI] [PubMed] [Google Scholar]