Abstract
Background
The extent and the progression of vascular calcification (VC) are independent predictors of cardiovascular risk in the haemodialysis population. Vitamin K is essential for the activation of matrix gla protein (MGP), a powerful inhibitor of tissue calcification. Functional vitamin K deficiency may contribute to the high VC burden in haemodialysis patients. In addition, haemodialysis patients are frequently treated with vitamin K antagonists, mainly to prevent stroke in atrial fibrillation, potentially compounding the cardiovascular risk in these already vulnerable patients. New oral anticoagulants (NOACs) are valuable alternatives to vitamin K antagonists in the general population, but their use in dialysis has been encumbered by substantial renal clearance. However, a recent pharmacokinetic study provided information on how to use rivaroxaban in haemodialysis patients.
Methods
We conduct a randomized, prospective, multicentre, open-label interventional clinical trial that will include 117 chronic haemodialysis patients with non-valvular atrial fibrillation, treated with or candidates for treatment with vitamin K antagonists. Patients will be randomized to a vitamin K antagonist titrated weekly to an international normalized ratio between 2 and 3, a daily dose of rivaroxaban of 10 mg, or a daily dose of rivaroxaban 10 mg with a thrice weekly supplement of 2000 µg vitamin K2. Cardiac computed tomography, pulse wave velocity (PWV) measurements and MGP sampling will be performed at baseline, 6 months, 12 months and 18 months. Primary endpoints include progression of coronary artery and thoracic aorta calcification and changes in PWV. Secondary endpoints are progression of aortic and mitral valve calcification, all-cause mortality, major adverse cardiovascular events, stroke and bleeding. The ClinicalTrials.gov database was searched to retrieve related trials.
Results
Seven trials, three of which are performed in the haemodialysis population, evaluate whether pharmacological doses of vitamin K1 or K2 retard progression of VC. Five studies compare the effect of warfarin and NOACs on progression of VC, the present study being the only conducted in the dialysis population.
Conclusion
Vitamin K deficiency may be a modifiable cardiovascular risk factor in the haemodialysis population. Conversely, vitamin K antagonists may aggravate VC burden in haemodialysis patients. Several ongoing trials may provide an answer to these questions in the near future.
Keywords: haemodialysis, rivaroxaban, vascular calcifications, vitamin K, vitamin K antagonists
Background and objectives
Patients on chronic haemodialysis suffer from extensive vascular calcifications (VC). The presence of VC is directly related to increased cardiovascular morbidity and mortality in the dialysis population and progression of VC provides independent incremental prognostic information [1].
Matrix Gla protein (MGP) is a powerful vascular wall-based inhibitor of VC, that requires vitamin K-dependent post-translational modification to be fully active [2]. Haemodialysis patients exhibit reduced vitamin K intake [3, 4], and uraemia interferes with vitamin K recycling [5]. Levels of dephosphorylated uncarboxylated MGP (dp-uc-MGP), the inactive form of MGP, increase with chronic kidney disease stage [6]. Haemodialysis patients thus represent a population with a very high overall vitamin K deficiency and vitamin K supplementation may be an attractive novel approach to attenuate progression of VC in haemodialysis patients. The optimal dose and form of vitamin K to achieve this goal is unknown. The group of K vitamins comprises phylloquinone (vitamin K1) and several menaquinones (vitamin K2). Menaquinones have side chains of varying length and are termed MK-n, in which n denotes the number of unsaturated isoprenoid residues. Vitamin K1 predominantly accumulates in the liver where it catalyses the carboxylation of the coagulation factors, whereas vitamin K2 is more readily transported to the extrahepatic tissues and is involved in the carboxylation of MGP and osteocalcin. Within the extrahepatic tissues, vitamin K1 may be converted to vitamin K2, mainly MK-4 [2]. Two dose-finding studies in haemodialysis patients have shown an almost linear dose–response of dp-uc-MGP to the long-chain menaquinone MK-7, with doses ranging between 45 µg/day and 1080 µg trice weekly [4, 7]. A plateau phase was not obtained and even the highest dose of MK-7 did not normalize dp-uc-MGP, suggesting that the optimal dose of MK-7 supplementation in dialysis patients may be even higher. There is no documented toxicity for phylloquinone or menaquinones and the WHO has set no upper tolerance level for vitamin K intake [2]. An animal study reported no toxicity associated with synthetic MK-7 administered in a single oral dose up to 2000 mg/kg or for 90 days of oral administration of 10 mg/kg per day [8]. A theoretical consideration could be that excessive vitamin K results in an increased thrombosis risk. However, vitamin K-dependent proteins have a limited number of Glu residues capable of gamma-carboxylation per molecule, beyond which there can be no further gamma-carboxylation. Vitamin K supplementation had no adverse effects on thrombin generation [9]. Further, the anticoagulant proteins S and C are activated in parallel with the coagulation factors. No side events were recorded in a Japanese trial of 2185 postmenopausal osteoporotic women receiving 45 mg menatetrenone for 3 years [10]. Vitamin K intake was not associated with stroke risk in a prospective cohort of 35 476 healthy subjects [11]. Another potential problem relates to the vitamin K-dependent carboxylation of osteocalcin, also termed Bone Gla protein [12]. Besides its known role in bone health, osteocalcin may affect glucose homeostasis. So far, no clinical data suggest a potential adverse effect of vitamin K intake or supplements on diabetes control [12], but strict vigilance remains warranted.
Vitamin K antagonists are frequently used in chronic haemodialysis patients, mainly for the prevention of stroke and systemic embolism in atrial fibrillation, a condition that is highly prevalent in this population [13]. Vitamin K antagonists are increasingly implicated in the development of VC [14–16], precisely because they block not only the gamma-carboxylation of coagulation factors, but also prevent the activation of MGP. The use of anticoagulants alternative to warfarin has been problematic in the dialysis population for lack of data, given their substantial renal clearance. Rivaroxaban is a factor Xa inhibitor, shown to be effective and safe in a large trial on the prevention of stroke and systemic embolism in non-valvular atrial fibrillation [17]. Rivaroxaban is partially eliminated via the kidneys, resulting in a decreased clearance in subjects with renal failure and its use is officially contraindicated in patients with a creatinine clearance <15 mL/min. A recent pharmacokinetic study in patients on chronic haemodialysis demonstrated that a daily dose of 10 mg rivaroxaban in dialysis patients provides an anticoagulant effect that is comparable to that of 20 mg in healthy volunteers [18]. Dialysis had no significant effect on the plasma levels of rivaroxaban [18].
The present study targets dialysis patients with non-valvular atrial fibrillation that are candidates for treatment with vitamin K antagonists according to the most recent guidelines [19]. It addresses the question of whether replacement of the vitamin K antagonist by rivaroxaban is able to slow progression of VC. The second research question is whether addition of vitamin K2 to rivaroxaban can further beneficially affect the progression of VC. Two non-invasive methods are used to evaluate the impact of the interventions on the progression of VC: i.e. coronary artery calcification (CAC) and pulse wave velocity (PWV) measurements. Although controversial as treatment target, the detection of CAC is a frequently used surrogate marker and is predictive of future cardiovascular events in haemodialysis patients [20]. Stiffening of the central elastic-type arteries is an independent predictor of cardiovascular morbidity and mortality in haemodialysis patients [21].
Trial design
This study is a randomized, prospective, multicentre and open-label interventional clinical trial. The study is performed in accordance with the ethical standards of the responsible institutional committee on human experimentation and with the Helsinki Declaration of 1975 (and as revised in 1983). The study is registered on ClinicalTrials.gov (Identifier NCT02610933).
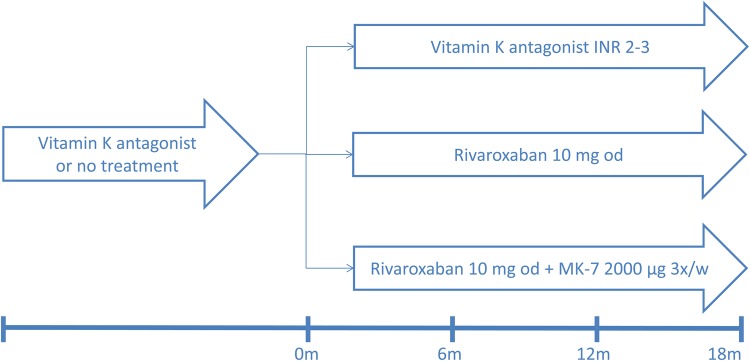
The study has a three-arm parallel group design with a 1:1:1 allocation ratio. Patients will be screened in order to randomize 117 patients (Figure 1). The total expected trial duration is 3 years. Each patient will have a follow-up of at least 18 months.
Fig. 1.
Trial design.
Participants
The study population are adults on chronic haemodialysis with non-valvular atrial fibrillation, who have a CHA2DS2-VASc Score of ≥2 and therefore are candidates for anticoagulation [19] or already receive vitamin K antagonists, and are able to provide informed consent.
Exclusion criteria are:
known intestinal malabsorption or inability to take oral medication
inability to stop co-medication that causes major interactions with rivaroxaban (e.g. ketoconazole, itraconazole, voriconazole, posaconazole, ritonavir, rifampicin, phenytoin, carbamazepine, phenobarbital or St John's wort)
investigator's assessment that the subject's life expectancy is <1 year
prosthetic mechanical heart valve
contraindication for anticoagulation
liver dysfunction Child-Pugh grade B–C
pregnancy, breastfeeding, inadequate contraception
incompliance with medication and scheduled investigations
Interventions and measurements
Patients in the first treatment arm are started on a vitamin K antagonist or continue their vitamin K antagonist, with dose adjustments to achieve an international normalized ratio (INR) of 2–3 on the basis of weekly INR measurements. Time within the therapeutic range is recorded. Patients in the second treatment arm receive a daily dose of 10 mg rivaroxaban. Patients in the third treatment arm receive a daily dose of 10 mg rivaroxaban and a thrice weekly dose of 2000 µg MK-7.
Clinical data, biochemical data, imaging and PWV data are collected at baseline, 6 months, 12 months and 18 months. Subjective tolerability is evaluated by questioning the patients about any adverse events or by spontaneous reporting of adverse events by the patients. Objective tolerability is evaluated by monitoring vital signs and routine clinical laboratory tests.
Analysis of dp-uc-MGP
Venous blood samples are taken at the start of dialysis at the dialyser inlet line. EDTA plasma is obtained by 15 min of centrifugation at Rotina 38R and stored at −80°C in 3 aliquots of 1 mL until dry ice shipment for analysis. Circulating dp-uc-MGP is quantified using a previously described dual antibody enzyme-linked immunosorbent assay [22].
Imaging
For calcification assessment, an unenhanced electrocardiographically gated computed tomography of the heart and thoracic aorta is performed at 120 kV on a Revolution (GE Healthcare), Aquilion One (Toshiba) or Somatom Definition Flash (Siemens) scanner. To limit artefacts owing to high heart rates (>70 bpm), beta-blockade (bisoprolol 2.5–5 mg) is administered orally 60 min before scanning when necessary. Calcium scores are calculated on 2.5 mm slices using Smartscore v.4.0 (GE Healthcare) by the Agatston method [23], by the volume method [24] and by the mass score. All foci within the arteries with attenuation >130 Hounsfield units (Hu) and a minimum area of 1 mm2 are considered significant and will be counted into the total score. An Agatston score for each calcific lesion is calculated by multiplying the density factor (1 = 130–199 Hu, 2 = 200–299 Hu, 3 = 300–399 Hu and 4 = ≥400 Hu) by the area. A total Agatston score is obtained by adding up the scores of all individual lesions in all slice levels. The total volume score is obtained by adding the volumes of all >130 Hu lesions. The mass score calculates the mass (mg) of calcified plaque above the 130 Hu threshold. One experienced investigator reviews all scans for consistency of interpretation. For each subject, all imaging procedures are done on the same equipment using the same parameters at each session to permit valid image comparisons. Scans start in the upper thorax above the aortic arch, advancing caudally to the level of the diaphragm to include the coronary arteries, the aortic and mitral valve, and the ascending and descending thoracic aorta.
Pulse wave velocity
Haemodynamic measurements are performed in the supine position by trained research nurses during a haemodialysis session. PWV is obtained by sequential recording of electrocardiogram-gated carotid and femoral artery pressure waves using applanation tonometry (SphygmoCor version 7, AtCor Medical, Sydney, Australia), as described previously [25]. The path length is calculated as 0.8 times the direct distance between the carotid and femoral recording site. PWV is calculated as the path length divided by transit time (m/s). A mean of three measurements is made. Based on a combination of practical and theoretical issues, PWV is measured during the first hour of the mid-week dialysis session [26].
Endpoints
Primary endpoints
Progression of CAC (absolute and relative change versus baseline).
Progression of thoracic aortic calcification (absolute and relative change versus baseline).
Absolute and relative change in PWV.
Secondary endpoints
Progression of aortic valve calcification (absolute and relative change versus baseline).
Progression of mitral valve calcification (absolute and relative change versus baseline).
Mortality from any cause.
Myocardial infarction, acute coronary syndrome, symptom-driven revascularization, hospitalization for heart failure and death from cardiovascular cause.
Stroke, defined as sudden onset of focal neurological deficit consistent with the territory of a major cerebral artery and categorized as ischaemic, haemorrhagic or unspecified. Haemorrhagic transformation of ischaemic stroke is not deemed to be haemorrhagic stroke. Systemic embolism, defined as an acute vascular occlusion of a limb or organ documented by imaging, surgery or autopsy.
Major bleeding, defined as a requirement for transfusion of two or more units of blood or a decrease in haemoglobin of 2 g/dL or more. Life-threatening bleeding, defined as fatal bleeding, symptomatic intracranial bleeding, a decrease in haemoglobin of 5 g/dL or more, or a requirement for transfusion of four or more units of blood, inotropic agents or surgery. All other bleedings are regarded as minor.
Subgroup analysis
Prevalent users of vitamin K antagonists versus patients with incident indication for vitamin K antagonists.
Sample size calculation
Assuming an average CAC score of 800 at baseline and accounting for an anticipated annual CAC progression of 60% in the vitamin K antagonist arm, 30% in the rivaroxaban arm and 0% in the rivaroxaban + MK-7 arm [27–30], power calculations revealed that 27 patients in each arm are required to provide 80% statistical power at the α = 0.05 significance level. Based on an estimated drop-out rate of 30% during 18 months of follow-up, 39 patients per arm are included in the trial.
Randomization and blinding
Patients are assigned to groups through computer-generated randomization. The patients, site investigators, and members of the data analysis committee are aware of the treatment assignments. Due to the requirement for frequent INR measurements to adjust the dose of warfarin, an adequate complete blinding would be challenging. Therefore, the study is performed open-labelled. To exclude information bias, the primary endpoints are objectively measured by investigators blinded to the treatment allocation.
Statistical methods
Since the distribution of calcification scores and PWV measurements as well as their progression are usually skewed, median (P10-P90) is used as measure of central tendency and variation. Baseline and end of study characteristics are compared between the study arms according to Fisher's exact test for categorical variables and the Kruskal–Wallis test for continuous variables. To account for the correlation between multiple observations from the same subject, differences between treatment groups in calcification progression and changes in PWV are analysed by evaluating the treatment-by-time interaction in a mixed-effects model. P-values are calculated using the Satterthwaite approximation of the denominator degrees of freedom for the F-statistics. An additional adjustment for differences in patient characteristics occurring at baseline is done if necessary. To meet model requirements, PWV values are ln-transformed and calcification scores are transformed as to ln(CAC+1). All probability values are two-tailed and P-values ≤0.05 are considered as indicating statistical significance. Analyses are conducted using SAS (release 9.4, Cary, NC, USA). All statistical analyses are based on the intention-to-treat principle.
Review of other ongoing trials
Population studies on dietary vitamin K consumption reported a correlation between low menaquinone (vitamin K2) intake and VC or cardiovascular endpoints in some [31–33] but not all [11, 34] studies. Dietary intake of phylloquinone (vitamin K1) was not associated with vascular health in any of the studies [11, 31, 32, 34, 35]. In a study of 387 haemodialysis patients, deficiency of the vitamin K2 species MK-4 and MK-7 correlated with the presence of VC [36]. A few interventional trials with vitamin K on intermediate cardiovascular endpoints have yielded inconsistent results. In 388 older men and women with normal renal function, 500 µg daily phylloquinone did not reduce CAC progression at the end of 3 years of follow-up [37]. However, in a subgroup analysis of participants who were ≥85% adherent to treatment, there was less CAC progression in the phylloquinone group than in the controls [37]. In healthy postmenopausal women, a daily supplement of 1000 µg phylloquinone and vitamin D preserved the elastic properties of the vessel wall, as compared with placebo or vitamin D alone [38]. On the other hand, CAC progression was not affected by 500 µg daily phylloquinone supplementation for 3 years in 374 older adults without coronary heart disease [39]. Three years of supplementation with 180 µg daily MK-7 improved arterial stiffness in a trial of 244 healthy postmenopausal women [40]. In contrast, CAC progression was not significantly affected by a 270-day course of 90 µg MK-7 in 42 patients with chronic kidney disease stage 3–5 not on dialysis [41]. We searched for ongoing trials of the effect of vitamin K supplementation on the progression of VC entering ‘vitamin K’ and ‘vascular calcification’ as search terms in the ClinicalTrials.gov search engine. Seven studies were retrieved (Table 1), three of which are conducted in the haemodialysis population. All use pharmacological doses of either vitamin K1 (three studies) or vitamin K2 (four studies).
Table 1.
Characteristics of ongoing trials evaluating the effect of Vitamin K supplements on vascular calcification
| ClinicalTrials.gov Identifier |
Study design | Country | Population | Type of vitamin K | Duration (months) | Primary endpoint |
|---|---|---|---|---|---|---|
| NCT01742273a | International, multicentre, prospective, controlled, open label, randomized, interventional clinical trial | Europe | 348 prevalent haemodialysis patients | Vitamin K1 (phylloquinone) 5 mg thrice weekly | 18 | Progression of thoracic aorta and coronary calcification |
| NCT01528800b | Prospective, placebo controlled, double-blinded, randomized, interventional clinical trial | Canada | 80 incident haemodialysis patients | Vitamin K1 (phytonadione) 10 mg thrice weekly | 12 | Progression of CAC, cardiovascular events |
| NCT00785109c | Prospective, placebo-controlled, open label, randomized, interventional clinical trial | Germany | 200 patients >50 years with echocardiographically verified aortic valve calcification | Vitamin K1 2 mg daily | 18 | Progression of aortic valve calcification |
| NCT01002157d | Prospective, placebo-controlled, double-blinded, randomized, interventional clinical trial | Netherlands | 180 patients with established CAC | Vitamin K2 (MK-7) dose not mentioned | 24 | Progression of coronary calcification |
| NCT01922804e | Prospective, placebo-controlled, double-blinded, randomized, interventional clinical trial | Denmark | 150 postmenopausal women with osteopaenia | Vitamin K2 (MK-7) 375 µg daily | 36 | Change in bone mineral density, insulin sensitivity, arterial stiffness |
| NCT02404519f | Prospective, placebo-controlled, double-blinded, randomized, interventional clinical trial | Netherlands | 240 men and women between 40 and 70 years with functional Vitamin K deficiency | Vitamin K2 (MK-7) 180 µg daily | 12 | Change in arterial stiffness |
| Our studyg | Multicentre, prospective, controlled, open label, randomized, interventional clinical trial | Belgium | 117 prevalent haemodialysis patients with AF | Vitamin K2 (MK-7) 2000 µg thrice weekly | 18 | Progression of thoracic aorta and CAC, progression of arterial stiffness |
AF, atrial fibrillation.
aVitamin K1 to Slow Progression of Vascular Calcification in Haemodialysis Patients: VitaVask Study.
bInhibit Progression of Coronary Artery Calcification With Vitamin K in HemoDialysis Patients: iPACKHD Study.
cVitamin K Containing Nutritional Supplement for Activation of Matrix-Gla-proteins (MGP) and Inhibition of Aortic Valve Calcification Process.
dThe Effects of Vitamin K2 Supplementation on the Progression of Coronary Artery Calcification: VitaK-CAC Study.
eInvestigations of the Effect of MK-7 on Bone and Glucose Metabolism and Arterial Calcification.
fIntervention Study on the Effect of Vitamin K2 (Menaquinone-7) Supplementation on the Vascular Stiffness in Subjects With Poor Vitamin K-status.
gThe effect of replacement of vitamin K antagonist by rivaroxaban with or without vitamin K2 supplementation on vascular calcifications in chronic haemodialysis patients: a randomized controlled trial.
New oral anticoagulants (NOAC) compare favourably with vitamin K antagonists for the prevention of stroke and systemic embolism, with a beneficial effect on total mortality in a recent meta-analysis [42]. However, none of the individual trials was designed to specifically answer the question whether warfarin accelerates progression of VC. We searched for ongoing trials comparing NOACs with vitamin K antagonists in terms of their effects on vascular calcification. The following search terms were entered in random combinations in the ClinicalTrials.gov search engine: ‘rivaroxaban’, ‘apixaban’, ‘dabigatran’, ‘novel oral anticoagulant’, ‘vascular calcification’, ‘vitamin K antagonist’, ‘warfarin’. Five studies were retrieved, using rivaroxaban (four studies) or apixaban (one study) as the comparator for warfarin (Table 2). The present study is the first to test the hypothesis in haemodialysis patients, using information obtained in a recent pharmacokinetic study of rivaroxaban in this population [18].
Table 2.
Characteristics of ongoing trials evaluating the effect of Vitamin K antagonists (VKA) versus novel oral anticoagulants (NOAC) on vascular calcification
| ClinicalTrials.gov Identifier |
Study design | Country | Population | NOAC (versus VKA) | Duration (months) | Primary endpoint |
|---|---|---|---|---|---|---|
| NCT02066662a | Single centre, prospective, controlled, open label, randomized, interventional clinical trial | Germany | 253 patients with AF or PE and eGFR >15 mL/min/1.73 m2 | Rivaroxaban 15/20 mg | 12 | Progression of coronary and aortic valve calcification (Agatston score) |
| NCT02161965b | Prospective, controlled, open label, randomized, interventional clinical trial | France | 150 patients with AF, PE or venous thrombosis and eGFR > 30 mL/min/1.73 m2 | Rivaroxaban 15/20 mg | 12 | Progression of coronary calcification and arterial stiffness |
| NCT02090075c | Prospective, controlled, open label, randomized, interventional clinical trial | USA | 66 patients with AF and eGFR >50 mL/min/1.73 m2 | Apixaban 5 mg | 12 | Progression of coronary calcification |
| NCT02376010d | Prospective, controlled, open label, randomized, interventional clinical trial | USA | 110 patients with AF and eGFR >50 mL/min/1.73 m2 | Rivaroxaban 15/20 mg | 12 | Progression of coronary calcification |
| Our studye | Multicentre, prospective, controlled, open label, randomized, interventional clinical trial | Belgium | 117 patients with AF on haemodialysis | Rivaroxaban 10 mg | 18 | Progression of aortic and coronary calcification and arterial stiffness |
AF, atrial fibrillation; PE, pulmonary embolism; eGFR, estimated glomerular filtration rate.
aInfluence of Rivaroxaban Compared to Vitamin K Antagonist Treatment Upon Development of Cardiovascular Calcification in Patients With Atrial Fibrillation and/ or Pulmonary Embolism (IRIVASC- Trial).
bThe VICTORIA Study (Vascular CalcIfiCation and sTiffness Induced by ORal antIcoAgulation) Comparison Anti-vitamin K Versus Anti-Xa.
cApixaban Versus Warfarin in the Evaluation of Progression of Atherosclerotic Calcification and Vulnerable Plaque.
dRivaroxaban Versus Warfarin in the Evaluation of Progression of Coronary Calcium.
eThe effect of replacement of vitamin K antagonist by rivaroxaban with or without vitamin K2 supplementation on vascular calcifications in chronic haemodialysis patients: a randomized controlled trial.
Conclusion
Over the past decade, a large body of evidence has accumulated establishing that vitamin K is essential for vascular health. Vitamin K supplementation may be a simple means to prevent progression of VC in haemodialysis patients, a population characterized by severe functional vitamin K deficiency. Conversely, Vitamin K antagonists have garnered attention as they may potentially aggravate progression of VC in dialysis patients. The scientific community is now poised to conduct randomized controlled trials to supersede the evidence from basic science and observational studies and yield therapies that may improve the dire prognosis of dialysis patients.
Conflict of interest statement
The results presented in this paper have not been published previously in whole or part.
References
- 1.Bellasi A, Raggi P. Vascular imaging in chronic kidney disease. Curr Opin Nephrol Hypertens 2012; 21: 382–388 [DOI] [PubMed] [Google Scholar]
- 2.Brandenburg VM, Schurgers LJ, Kaesler N, et al. Prevention of vasculopathy by vitamin K supplementation: can we turn fiction into fact? Atherosclerosis 2015; 240: 10–16 [DOI] [PubMed] [Google Scholar]
- 3.Cranenburg ECM, Schurgers LJ, Uiterwijk HH, et al. Vitamin K intake and status are low in hemodialysis patients. Kidney Int 2012; 82: 605–610 [DOI] [PubMed] [Google Scholar]
- 4.Caluwé R, Vandecasteele S, Van Vlem B, et al. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant 2013; 29: 1385–1390 [DOI] [PubMed] [Google Scholar]
- 5.Kaesler N, Magdeleyns E, Herfs M, et al. Impaired vitamin K recycling in uremia is rescued by vitamin K supplementation. Kidney Int 2014; 86: 286–293 [DOI] [PubMed] [Google Scholar]
- 6.Schurgers LJ, Barreto DV, Barreto FC, et al. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol 2010; 5: 568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westenfeld R, Krueger T, Schlieper G, et al. Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: a randomized trial. Am J Kidney Dis 2012; 59: 186–195 [DOI] [PubMed] [Google Scholar]
- 8.Pucaj K, Rasmussen H, Møller M, et al. Safety and toxicological evaluation of a synthetic vitamin K2, menaquinone-7. Toxicol Mech Methods 2011; 21: 520–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theuwissen E, Cranenburg EC, Knapen MH, et al. Low-dose menaquinone-7 supplementation improved extra-hepatic vitamin K status, but had no effect on thrombin generation in healthy subjects. Br J Nutr 2012; 108: 1652–1657 [DOI] [PubMed] [Google Scholar]
- 10.Inoue T, Fujita T, Kishimoto H, et al. Randomized controlled study on the prevention of osteoporotic fractures (OF study): a phase IV clinical study of 15-mg menatetrenone capsules. J Bone Miner Metab 2009; 27: 66–75 [DOI] [PubMed] [Google Scholar]
- 11.Vissers LET, Dalmeijer GW, Boer JMA, et al. Intake of dietary phylloquinone and menaquinones and risk of stroke. J Am Heart Assoc 2013; 2: e000455–e000455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gundberg CM, Lian JB, Booth SL. Vitamin K-dependent carboxylation of osteocalcin: friend or foe? Adv Nutr 2012; 3: 149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkelmayer WC, Patrick AR, Liu J, et al. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol 2011; 22: 349–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y-T, Tang Z-Y. Research progress of warfarin-associated vascular calcification and its possible therapy. J Cardiovasc Pharmacol 2014; 63: 76–82 [DOI] [PubMed] [Google Scholar]
- 15.Krüger T, Floege J. Vitamin K antagonists: beyond bleeding. Semin Dial 2014; 27: 37–41 [DOI] [PubMed] [Google Scholar]
- 16.Fusaro M, Tripepi G, Noale M, et al. Prevalence of vertebral fractures, vascular calcifications, and mortality in warfarin treated hemodialysis patients. Curr Vasc Pharmacol 2015; 13: 248–258 [DOI] [PubMed] [Google Scholar]
- 17.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891 [DOI] [PubMed] [Google Scholar]
- 18.De Vriese AS, Caluwé R, Bailleul E, et al. Dose-finding study of rivaroxaban in hemodialysis patients. Am J Kidney Dis 2015; 66: 91–98 [DOI] [PubMed] [Google Scholar]
- 19.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64: e1–e76 [DOI] [PubMed] [Google Scholar]
- 20.Langou RA, Huang EK, Kelley MJ, et al. Predictive accuracy of coronary artery calcification and abnormal exercise test for coronary artery disease in asymptomatic men. Circulation 1980; 62: 1196–1203 [DOI] [PubMed] [Google Scholar]
- 21.Verbeke F, Van Biesen W, Honkanen E, et al. Prognostic value of aortic stiffness and calcification for cardiovascular events and mortality in dialysis patients: outcome of the Calcification Outcome in Renal Disease (CORD) Study. Clin J Am Soc Nephrol 2011; 6: 153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cranenburg E, Koos R, Schurgers LJ, et al. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost 2010; 104: 811–822 [DOI] [PubMed] [Google Scholar]
- 23.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990; 15: 827–832 [DOI] [PubMed] [Google Scholar]
- 24.Callister TQ, Cooil B, Raya SP, et al. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology 1998; 208: 807–814 [DOI] [PubMed] [Google Scholar]
- 25.Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012; 30: 445–448 [DOI] [PubMed] [Google Scholar]
- 26.Di Iorio B, Nazzaro P, Cucciniello E, et al. Influence of haemodialysis on variability of pulse wave velocity in chronic haemodialysis patients. Nephrol Dial Transplant 2010; 25: 1579–1583 [DOI] [PubMed] [Google Scholar]
- 27.Chertow GM, Burke SK, Raggi P, Treat to Goal Working Group. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 2002; 62: 245–252 [DOI] [PubMed] [Google Scholar]
- 28.Barreto DV, Barreto F de C, de Carvalho AB, et al. Phosphate binder impact on bone remodeling and coronary calcification—results from the BRiC study. Nephron Clin Pract 2008; 110: c273–c283 [DOI] [PubMed] [Google Scholar]
- 29.Qunibi W, Moustafa M, Muenz LR, et al. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis 2008; 51: 952–965 [DOI] [PubMed] [Google Scholar]
- 30.Raggi P, Chertow GM, Torres PU, et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant 2011; 26: 1327–1339 [DOI] [PubMed] [Google Scholar]
- 31.Geleijnse JM, Vermeer C, Grobbee DE, et al. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr 2004; 134: 3100–3105 [DOI] [PubMed] [Google Scholar]
- 32.Beulens JWJ, Bots ML, Atsma F, et al. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis 2009; 203: 489–493 [DOI] [PubMed] [Google Scholar]
- 33.Gast GCM, de Roos NM, Sluijs I, et al. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis 2009; 19: 504–510 [DOI] [PubMed] [Google Scholar]
- 34.Maas AHEM, van der Schouw YT, Beijerinck D, et al. Vitamin K intake and calcifications in breast arteries. Maturitas 2007; 56: 273–279 [DOI] [PubMed] [Google Scholar]
- 35.Villines TC, Hatzigeorgiou C, Feuerstein IM, et al. Vitamin K1 intake and coronary calcification. Coron Artery Dis 2005; 16: 199. [DOI] [PubMed] [Google Scholar]
- 36.Fusaro M, Noale M, Viola V, et al. Vitamin K, vertebral fractures, vascular calcifications, and mortality: VItamin K Italian (VIKI) dialysis study. J Bone Miner Res 2012; 27: 2271–2278 [DOI] [PubMed] [Google Scholar]
- 37.Shea MK, O'Donnell CJ, Hoffmann U, et al. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr 2009; 89: 1799–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braam LAJLM, Hoeks APG, Brouns F, et al. Beneficial effects of vitamins D and K on the elastic properties of the vessel wall in postmenopausal women: a follow-up study. Thromb Haemost 2004; 91: 373–380 [DOI] [PubMed] [Google Scholar]
- 39.Shea MK, O'Donnell CJ, Vermeer C, et al. Circulating uncarboxylated matrix gla protein is associated with vitamin K nutritional status, but not coronary artery calcium, in older adults. J Nutr 2011; 141: 1529–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knapen MHJ, Braam LAJLM, Drummen NE, et al. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. Thromb Haemost 2015; 113: 1135–1144 [DOI] [PubMed] [Google Scholar]
- 41.Kurnatowska I, Grzelak P, Masajtis-Zagajewska A. Effect of vitamin K2 on progression of atherosclerosis and vascular calcification in non-dialyzed patients with chronic kidney disease stage 3-5. Pol Arch Med Wewn 2015; 125: 631–640 [DOI] [PubMed] [Google Scholar]
- 42.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383: 955–962 [DOI] [PubMed] [Google Scholar]