Abstract
Advanced melanoma has been traditionally unresponsive to standard chemotherapy agents and used to have a dismal prognosis. Genetically targeted small-molecule inhibitors of the oncogenic BRAF V600 mutation or a downstream signaling partner (MEK mitogen-activated protein kinase) are effective treatment options for the 40–50% of melanomas that harbor mutations in BRAF. Selective BRAF and MEK inhibitors induce frequent and dramatic objective responses and markedly improve survival compared with cytotoxic chemotherapy. In the past decade after discovery of this mutation, drugs such as vemurafenib and dabrafenib have been approved by the US Food and Drug Administration (FDA) and the European Medicines Agency for the treatment of V600-mutated melanomas. While the initial trials did not signal any renal toxicities with the BRAF inhibitors, recent case reports, case series and FDA adverse reporting systems have uncovered significant nephrotoxicities with these agents. In this article, we systematically review the nephrotoxicities of these agents. Based on recently published data, it appears that there are lower rates of kidney disease and cutaneous lesions seen with dabrafenib compared with vemurafenib. The pathology reported in the few kidney biopsies done so far are suggestive of tubulo interstitial damage with an acute and chronic component. Electrolyte disorders such as hypokalemia, hyponatremia and hypophosphatemia have been reported as well. Routine monitoring of serum creatinine and electrolytes and calculation of glomerular filtration rate prior to the first administration when treating with dabrafenib and vemurafenib are essential.
Keywords: BRAF inhibitors, dabrafenib, onconephrology, vemurafenib
Introduction
Mutations in v-Raf murine sarcoma viral oncogene homolog B (BRAF) were first discovered in 2002 [1]. Since that time, this discovery has helped uncover mutations in many cancers. The discovery of mutations in BRAF, part of the mitogen-activated protein kinase (MAPK) signaling pathway, is creating a new era of targeted therapy for patients with malignant melanoma, colorectal cancer and non-small cell lung cancer [2–10]. In addition, mutations in BRAF have been described in multiple myeloma, hairy cell leukemia and thyroid cancer as well [11].
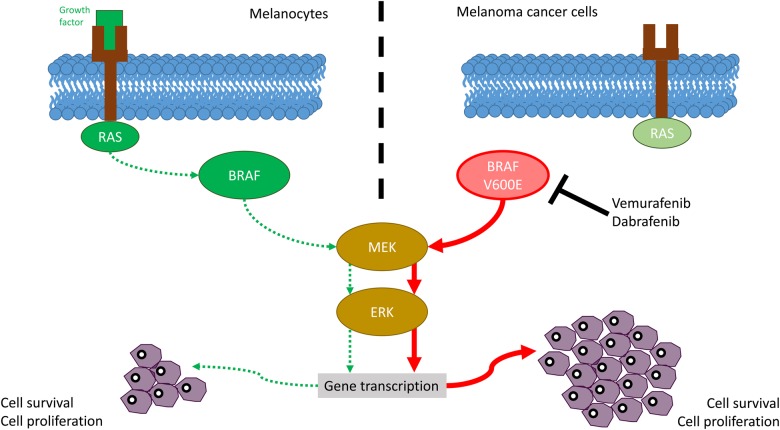
The MAPK/extracellular regulated protein kinase (ERK) pathway is a chain of proteins that helps communicate a signal from cell receptors to the cell's DNA. The pathway includes many proteins, including BRAF and MEK (mitogen-activated protein kinases). They act as ‘on’ and ‘off’ switches for the signal. Particular mutations may change resultant proteins, locking them in either the ‘on’ or ‘off’ positions, contributing to cancer development and progression. One such mutation is the above-mentioned BRAF V600 mutation. Mutated BRAF results in persistently elevated ERK phosphorylation and target gene transcription. In addition, it is resistant to negative feedback signals that attempt to counterbalance the ERK activation [11]. Figure 1 depicts the key components of the MAPK/ERK pathway. The most common mutation encountered is the V600E mutation that results in an amino acid substitution from valine (V) to glutamic acid (E). It occurs in 40–50% of malignant melanomas. Advanced melanoma has been traditionally unresponsive to standard chemotherapy agents and used to have a dismal prognosis. After the advent of BRAF inhibitors, marked improvements in outcomes from historic norms have been observed. Genetically targeted small-molecule inhibitors of the oncogenic BRAF V600 mutation or a downstream signaling partner (MEK—mitogen activating protein kinase) are effective treatment options for the 40–50% of melanomas that harbor mutations in BRAF. Selective BRAF and MEK inhibitors induce frequent and dramatic objective responses and markedly improve survival compared with cytotoxic chemotherapy. In the past decade after discovery of this mutation, drugs such as vemurafenib [7] and dabrafenib [8] were approved by the US Food and Drug Administration (FDA) and the European Medicines Agency for the treatment of BRAF V600-mutated melanomas.
Fig. 1.
Mechanism of action of BRAF inhibitors.
Vemurafenib, the first selective BRAF inhibitor, was evaluated in clinical trials. In a Phase III trial [7] comparing vemurafenib with dacarbazine, patients in the vemurafenib group had a decreased risk of progression [hazard ratio (HR) 0.26, P<0.001] and death (HR 0.37, P<0.001), leading to regulatory approval of vemurafenib. Extended follow-up confirmed the benefit of vemurafenib, with improved median overall survival (13.6 versus 9.7 months; P<0.001) and median progression-free survival (6.9 versus 1.6 months; P<0.001).
Dabrafenib, another BRAF inhibitor, was subsequently developed and showed similar activity to vemurafenib. A Phase III clinical trial then compared dabrafenib with chemotherapy; this study demonstrated a 53% objective response rate (ORR) for the dabrafenib group and improved progression-free survival (HR 0.3, P<0.001) [8].
BRAF inhibitor monotherapy is generally well tolerated, although arthralgias, skin rash and cutaneous squamous cell carcinomas occur frequently. Although both agents are similar, vemurafenib causes more phototoxic effects and likely more cutaneous squamous cell carcinomas, whereas fevers occur more often with dabrafenib. A recent review [12] of BRAF inhibitor–related toxicities included QTc prolongation, diarrhea, fatigue, increased liver function tests, hyperglycemia, ophthalmologic complications, pyrexia and skin toxicities. This review did not report on the nephrotoxicities noted with these agents.
Knowledge of renal toxicities of BRAF inhibitors is extremely important. When we reviewed ClinicalTrials.gov [13, 14] for trials pertaining to these agents, numerous studies are recruiting patients for alternate cancers and disease states such as thyroid cancer, colon cancer, ameloblastoma, Erdheim–Chester disease and gliomas. Given the wide use of these agents in other cancers, it is important that data on nephrotoxicities are available to clinicians for review.
Methods
To better understand recent published contributions related to vemurafenib- and dabrafenib-induced renal toxicities, a Medline search of indexed manuscripts was conducted. The search term ‘renal failure’ and subheadings ‘vemurafenib’ and ‘dabrafenib’ were employed. In addition, search terms with all electrolyte disorders such as ‘hypokalemia, hypocalcemia, hyponatremia, hypophosphatemia’ were also searched. A search with the subheadings ‘acute kidney injury’ and ‘nephrotoxicities’ was also utilized. A total of five citations were found. One article was not relevant. Abstracts from scientific meetings were searched as well. One additional study was found. In addition, references of the four citations that were found revealed two additional articles. Primary data from the initial studies of both agents were also reviewed to look for data on nephrotoxicities and dosing in patients with chronic kidney disease (CKD) and end-stage kidney disease (ESKD).
Results
BRAF inhibitors–associated renal toxicity
Vemurafenib
During the initial Phase III trial, 51 patients developed edema, but there were no reports of proteinuria. There was no reported incidence of acute kidney injury (AKI) [7, 15]. In 2013, in a letter to the editor, Uthurriague et al. [16] were first to report AKI with this drug [16]. They reviewed 16 patients on this agent for >8 months. A total of 15 patients experienced significant decline in glomerular filtration rate (GFR) in 1 month, with a mean reduction of 29 mL/min, and this decline persisted for 3 months. For patients who had a maximum 8-month follow-up, there was a persistent decline at 8 months. In addition, a total of five patients had a persistent non-nephrotic range proteinuria of 500 mg/24 h. No kidney biopsies were performed in that series of patients (Table 1).
Table 1.
Summary of cases of AKI reported with vemurafenib
| Publication | Total number of patients | Time of onset of renal dysfunction after starting vemurafenib | Cancer | Mean age (years) | Males/females | Pathology reported | Outcomes |
|---|---|---|---|---|---|---|---|
| Uthurriague et al. [16] | 15 | After 1 month | Melanoma | Not reported | 10/6 | None | Decrease in renal function persisted for 3 months. In the patients with the longest follow-up (8 months), CKD persisted |
| Regnier-Rosencher et al. [17] | 4 | After 1–2 weeks | Melanoma | 76 | 4/0 | None | See Table 2 |
| Launay-Vacher et al. [18] | 8 | 50% after 1–2 months; 50% after 1–2 weeks |
Melanoma | 66 | 6/2 | ATN (one biopsy) | See Table 2 |
ATN, acute tubular necrosis.
Following that, Regnier-Rosencher et al. reported four cases of AKI with skin eruptions [17]. Four patients presented with a generalized Grade 3 erythematous eruption and hyperkeratosis pilaris 5–14 days after the introduction of vemurafenib (Tables 1 and 2). These symptoms were associated with AKI in all patients and transitory hypereosinophilia in two patients. Vemurafenib was stopped in three patients and the dose was reduced in the fourth, leading to a gradual improvement of skin lesions and renal function. Vemurafenib, when reintroduced at a lower dose, resulted in deterioration of renal function without reappearance of the rash. Similar to the first report, no kidney biopsies were performed (Tables 1 and 2).
Table 2.
Twelve detailed cases of AKI reported with vemurafenib
| Publication | Patient no. | Age/sex | Baseline GFR (mL/min/1.73 m2) and CKD stage | Clinical presentation | Renal outcome | Cancer outcome | Kidney biopsy performed |
|---|---|---|---|---|---|---|---|
| Regnier-Rosencher et al. [17] | 1 | 81/Male | 87 (CKD Stage 2) | 1–2 weeks following treatment, presented with AKI, microscopic hematuria, non-nephrotic range proteinuria and peripheral eosinophilia | Drug was stopped and AKI resolved. Reintroduction caused relapse of AKI | Complete response | No |
| Regnier-Rosencher et al. [17] | 2 | 86/Male | 50 (CKD Stage 3A) | 1–2 weeks after treatment, developed grade 3 skin eruption and AKI, hematuria, and non-nephrotic range proteinuria | Dose of drug was reduced to 75% of original dose and topical steroids given. Renal function improved but remained above baseline | Partial response | No |
| Regnier-Rosencher et al. [17] | 3 | 54/Male | 73 (CKD Stage 2) | 1 week after treatment, developed skin rash and AKI and non-nephrotic range proteinuria and peripheral eosinophilia | Drug was stopped, leading to improvement of skin and renal condition. Reintroduction 1 month later at a lower dose led to skin rash and AKI | Complete response | No |
| Regnier-Rosencher et al. [17] | 4 | 82/Male | 70 (CKD Stage 2) | 1–2 weeks after treatment, developed rash and AKI | Drug was stopped and that led to gradual improvement of rash and renal function | Complete response | No |
| Launay-Vacher et al. [18] | 5 | 71/Female | 57 (CKD Stage 3A) | One more after treatment, had AKI and rash. No proteinuria | Drug was reduced by 50% and renal function stabilized at a new baseline | No data provided | No |
| Launay-Vacher et al. [18] | 6 | 68/Male | 66 (CKD Stage 2) | 1 week following treatment, AKI was noted | After stopping agent, a kidney biopsy was done showing ATN with arteriosclerosis lesions. Drug with 75% reduced dose was reintroduced, but creatinine continued to rise and therapy was stopped. Patient would have required dialysis but chose not to. Patient expired | Progression of disease | Yes—ATN |
| Launay-Vacher et al. [18] | 7 | 80/Female | 50 (CKD Stage 3A) | After 2 months on treatment, developed AKI | Drug was maintained for 4 months and renal function gradually returned to baseline | No data provided | No |
| Launay-Vacher et al. [18] | 8 | 77/Male | 62 (CKD Stage 2) | 1 week after starting treatment, developed AKI with non-nephrotic range proteinuria and skin rash | Drug was maintained and renal function spontaneously improved and plateaued 3 weeks later | Progression of disease | No |
| Launay-Vacher et al. [18] | 9 | 63/Male | 88 (CKD Stage 2) | 1 week after starting treatment, developed AKI and non-nephrotic range proteinuria | Drug was continued at 75% of the dose and AKI did not improve. Eventually, drug was stopped and renal function improved. Reintroduction at 50% decreased dose still led to AKI, but then stabilized and remained stable on the drug | No data provided | No |
| Launay-Vacher et al. [18] | 10 | 41/Male | 83 (CKD Stage 2) | 2 weeks after treatment, developed AKI | Drug was stopped. When drug was reintroduced at 75% of the dose, renal function again worsened. Once stopped, renal function completely recovered | Disease progression | No |
| Launay-Vacher et al. [18] | 11 | 69/Male | 57 (CKD Stage 3A) | 1 month after treatment, developed AKI and photosensitivity | Drug was maintained and serum creatinine remained elevated. Once drug was changed to fotemustine, renal function rapidly improved | Disease progression | No |
| Launay-Vacher et al. [18] | 12 | 61/Male | 51 (CKD Stage 3A) | 1 month after treatment, developed AKI with hematuria and non-nephrotic range proteinuria | Drug was reduced to 75% dose and renal function eventually stabilized on the drug | Complete response | No |
AKI, acute kidney injury; CKD, chronic kidney disease; GFR, glomerular filtration rate; ATN, acute tubular necrosis.
Launay-Vacher et al. published a series of eight cases from France in 2014 [18]. All eight patients had a decrease in GFR ranging from 20 to 74%. One patient had a kidney biopsy that showed acute tubular necrosis (ATN). One patient would have required dialysis but died due to disease progression. Twenty-five percent of the patients recovered renal function on the drug, while 25% did not recover renal function despite stopping treatment. Thirty-five percent recovered renal function once the drug was stopped. Three patients had a severe photosensitivity reaction as noted in prior series from Regnier-Rosencher et al. [17] (Table 2). Tables 1 and 2 summarize the above three case series of 27 patients with metastatic melanoma in the literature. On observation, 10 of the 16 patients in the Uthurriague et al. series, all 4 patients in Regnier-Rosencher et al. and 6 of the 8 patients in Launay-Vacher et al. were male. Table 2 summarizes the clinical data of the 12 cases [17, 18] that have all substantial information in the reported series. At baseline, most patients had CKD Stage 2 or 3 before receiving vemurafenib. It appears that kidney toxicity is more common in older male patients with this mutation. A form of the toxicity appears more acutely within 1–2 weeks of drug initiation and another form within 1–2 months of starting vemurafenib.
Yorio et al. recently published a case of vemurafenib-induced Sweet's syndrome [19]. In the case, the patient developed AKI requiring hemodialysis. In addition, Denis et al. published a case of Fanconi's syndrome with severe hypokalemia following treatment with vemurafenib, which improved with interruption of therapy [20].
Muzet et al. [21] presented their data as an oral abstract from a single center at the recent Cancer and the Kidney International Network (C-KIN) meeting in Brussels, Belgium, in April 2015. They conducted a retrospective study of 74 patients with metastatic melanoma treated with vemurafenib. The patients were divided into two groups—with and without AKI. Kidney biopsies were performed in three patients. The mean duration of treatment was 10 months and 58 patients (78%) developed AKI. This occurred mainly in the first 3 months of treatment. The median time of onset was 2 months. When compared with the non-AKI group, there was no difference in baseline blood pressure, diabetes and cardiovascular disease. Similar to the above-summarized observations, cases of AKI were noted more often in men than in women. Kidney biopsies showed tubular toxicity and interstitial fibrosis. One biopsy showed focal, non-inflammatory and discrete lesions of interstitial fibrosis 7 months after the first observation of AKI. The two others performed <3 months after AKI showed marked specific chronic tubular and interstitial lesions and acute and focal lesions of epithelial damage compatible with ATN. Changes were chronic in two of the three cases [21].
A recent study by Jhaveri et al. [22] reviewed the FDA Adverse Event Reporting System's (FAERS) quarterly legacy data file from the third quarter of 2011 to the second quarter of 2014 for vemurafenib. Vemurafenib-related renal adverse event data were extracted from the database through formation of a query using FAERS-assigned unique case identifiers. Search terms utilized were ‘renal insufficiency, elevated creatinine, renal failure, renal injury, proteinuria, renal impairment, blood creatinine increase, renal failure acute, low phosphorus, hypophosphatemia, hypercreatinemia, hyponatremia, hypokalemia, renal damage’. A total of 132 cases of AKI were reported secondary to vemurafenib to the FAERS in the time frame reviewed. Eighty-five patients were men and 47 women (P = 0.00094). The average age of the men was 65 years and 59 years for the women (P = 0.0392). The cases were reported from around the world with France, the USA and Germany having most of the cases. Fourteen cases of electrolyte disorders were reported (hypokalemia six cases and hyponatremia eight cases). The most common indication was for treatment of malignant melanoma.
Dabrafenib
In our review using the above-mentioned search queries, there are no published reports of renal failure with dabrafenib. This might be partly due to limited experience. However, the European summary of product characteristics of the drug reports that renal failure has been identified in <1% of patients treated with dabrafenib [23]. Observed cases were generally associated with fever and dehydration and responded well to dose interruption and general supportive measures. Hypophosphatemia has been reported in 7% of patients included in the clinical trials, more than half of them (4% of total) of Grade 3 in severity (National Cancer Institute Common Toxicity Crietria). Granulomatous nephritis has also been reported per the summary product report. In the trial by Flaherty et al. [24], when used in combination with trametinib (MEK inhibitor), there were reports of hyponatremia, hypophosphatemia, increased serum creatinine and hypokalemia. There were lesser incidences of hypomagnesemia, hypocalcemia and hypercalcemia. While there were few cases of renal injury reported in the dabrafenib-only arm, the renal injury cases increased as increasing doses of trametinib were added. Roberts et al. [25] compared vemurafenib to dabrafenib + trametinib to show a better survival in the combined therapy. There were more reported events of increased creatinine in the vemurafenib arm (11%) compared with the dabrafenib+ trametinib arm (4%). No renal events were noted in a more recent study by Long et al. that compared dabrafenib solo therapy to dabrafenib +trametinib [26].
In a recent review by Jhaveri et al. [22] of the FAERS's quarterly legacy data file from the second quarter of 2013 to the second quarter of 2014 for dabrafenib using similar search terms as above, AKI was detected. A total of 13 cases of AKI with dabrafenib were reported to the FAERS in the time frame stated above. Twelve patients were men. The average ages of men and women were 55 and 75 years, respectively (P = 0.0022). Eight cases of electrolyte disorders were reported (hypokalemia two cases and hyponatremia six cases). Contrary to prior publications, no cases of hypophosphatemia were found [22].
Mechanism of injury
In an elegant murine study, BRAF was shown to be expressed and localized in developing and mature glomerular podocytes [27]. However, clinically it appears that BRAF inhibition targets the tubulo interstitial compartment. Based on the literature review and four biopsies reported so far, the common findings are tubular and interstitial damage with an acute and chronic component. As noted in Table 1, the three published case series suggest one form of injury that appears immediately after drug initiation (1–2 weeks) and another form of kidney injury that is more subacute in nature that appears within 1–2 months. The former injury is likely a drug reaction leading to photosensitivity and allergic interstitial nephritis. The latter injury is likely acute tubular toxicity. This drug either is tubular toxic or can lead to an allergic interstitial nephritis. Given tubular toxicity, it is not unusual to see Fanconi's syndrome in some cases and isolated electrolyte disorders in others. In addition, the proteinuria is in the subnephrotic range, suggesting a tubular variant of proteinuria. Table 3 summarizes the toxicities commonly noted with vemurafenib and dabrafenib.
Table 3.
BRAF inhibitor–related toxicity summary
| Allergic interstitial disease |
| Acute tubular necrosis |
| Proximal tubular damage (Fanconi's syndrome) |
| Hypophosphatemia |
| Hyponatremia |
| Hypokalemia |
| Subnephrotic-range proteinuria |
| Acute/subacute decrease in GFR by 20–40% |
| Urinalysis may have hematuria, proteinuria and white blood cells |
| Urinary sediment may show granular casts or white blood cell casts |
Based on the above data, it appears that there are lower rates of kidney disease and cutaneous lesions seen with dabrafenib compared with vemurafenib. It is possible that this is due to higher potency against BRAF V600E, whereas vemurafenib may be relatively equipotent. Given more cutaneous lesions in vemurafenib, one might see an increased incidence of allergic interstitial nephritis with vemurafenib. It is also possible that vemurafenib has off-target effects on the kidney more than the dabrafenib.
Use of BRAF agents in CKD and ESKD
Vemurafenib is highly protein bound, metabolized primarily in the liver and excreted via feces (94%) with minimal excretion via urine (1%), so it can probably be used at its usual dosage in patients with renal impairment [15, 28]. Although, no dose adjustment is recommended in patients with mild to moderate renal impairment, one has to exercise caution in patients with severe renal impairment (GFR <30 mL/min), as data regarding its use are limited, involving just one patient in clinical trials [29].
In a study published by Iddawela et al. [30], this drug was used in a patient with ESKD on peritoneal dialysis. The patient tolerated the drug but developed asymptomatic QTc prolongation, which was managed with dose reduction. Although QTc prolongation is a common adverse effect seen with this drug, the probability of this being a drug reaction in this patient was deemed low, as he had a history of a prolonged QT preceding drug use [23]. Regardless, given the high prevalence of electrolyte disturbances, structural heart disease and changes in fluid shifts in dialysis patients, we recommend close monitoring of electrolytes and baseline and serial EKGs in dialysis patients receiving this drug.
Dabrafenib, like vemurafenib, is also highly protein bound with a large volume of distribution. However, renal elimination of the drug is higher than that of vemurafenib (71% fecal excretion and 23% urinary excretion). This drug can also be used in mild–moderate renal impairment, with no data being available for use in severe renal impairment [31, 32].
C-KIN recommendations
We recommend routine monitoring of serum creatinine and electrolytes during treatment with dabrafenib and vemurafenib, with calculation of the GFR prior to the first administration. Given that the incidence of renal injury is in the initiation phase of treatment, we suggest monitoring monthly serum creatinine, serum eosinophils and electrolytes such as potassium, phosphorus, calcium, magnesium and sodium. In addition, a urinalysis is essential to monitor for proteinuria. If there is no renal toxicity in the first 2 months, then monitoring can be increased to every 3 months.
When possible, a kidney biopsy should be performed to illustrate the type of pathology that is leading to renal dysfunction. For example, evidence of tubular toxicity should lead to drug interruption with potential re-introduction when renal function has normalized. In cases of AKI, a trial of drug cessation can be attempted with a gradual reintroduction at a lower dose. In addition, worsening renal dysfunction risk has to be discussed in the context of treatment for a malignant cancer with the patient. Electrolyte disorders should be managed aggressively to avoid admissions via intravenous or oral supplementations when appropriate.
Conclusions
Vemurafenib and dabrafenib are drugs that showed efficacy in metastatic melanoma in patients carrying a specific mutation (V600) on the BRAF enzyme. Renal toxicity seen with vemurafenib shows a male predominance. Dabrafenib-related renal toxicity is rare, but more data and time are needed before concluding that these drugs are not nephrotoxic. Oncologists, dermatologists and nephrologists need to be aware of the nephrotoxicities of these agents.
Authors’ contribution
R.W. and K.D.J. wrote the initial draft of the manuscript. V.L.-V. and G.D. edited the manuscript. V.L.-V. is current President of C-KIN. G.D. is on the executive committee of C-KIN.
Conflict of interest statement
This work was part of a C-KIN working group on BRAF inhibitor nephrotoxicities. V.L.-V. and G.D. have received unrestricted educational grants and honoraria from Roche for last 3 years.
References
- 1.Davis H, Bignell GR, Cox C, et al. Mutations in BRAF gene in human cancer. Nature 2002; 417: 949–954 [DOI] [PubMed] [Google Scholar]
- 2.Andrulis M, Lehners N, Capper D, et al. Targeting the BRAF V600E mutation in multiple myeloma. Cancer Discov 2013; 3: 862–869 [DOI] [PubMed] [Google Scholar]
- 3.Bonello L, Voena C, Ladetto M, et al. BRAF gene is not mutated in plasma cell leukemia and multiple myeloma. Leukemia 2003; 17: 2238–2240 [DOI] [PubMed] [Google Scholar]
- 4.Chapman MA, Lawrence MS, Keats JJ, et al. Initial genome sequencing and analysis of multiple myeloma. Nature 2011; 471: 467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura ET, Nikiforova MN, Zhu Z, et al. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 2003; 63: 1454–1457 [PubMed] [Google Scholar]
- 6.Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med 2011; 364: 2305–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364: 2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 2012; 379: 1893–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010; 363: 809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012; 380: 358–365 [DOI] [PubMed] [Google Scholar]
- 11.Hall R, Kudchadkar R. BRAF mutations: signaling, epidemiology and clinical experience in multiple malignancies. Cancer Control 2014; 21: 221–230 [DOI] [PubMed] [Google Scholar]
- 12.Welsh S, Corrie P. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol 2015; 7: 122–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dabrafenib in Treating Patients With Solid Tumors and Kidney or Liver Dysfunction. https://clinicaltrials.gov/ct2/show/NCT01907802?term=dabrafenib&rank=9. (20 April 2015, date last accessed)
- 14. Vemurafenib. Open Studies. https://clinicaltrials.gov/ct2/results?term=vemurafenib&recr=Open&pg=3. (20 April 2015, date last accessed)
- 15.US Food and Drug Administration. Zolboraf Highlights of prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202429s000lbl.pdf Revised August 2011
- 16.Uthurruague C, Thellier S, Ribes D, et al. Vemurafenib significantly decreases glomerular filtration rate. J Eur Acad Dermatol Venerol 2014; 28: 978–979 [DOI] [PubMed] [Google Scholar]
- 17.Regnier-Rosencher E, Lazareth H, Gressier L, et al. Acute kidney injury in patients with severe rash on vemurafenib treatment for metastatic melanomas. Br J Dermatol 2013; 169: 934–938 [DOI] [PubMed] [Google Scholar]
- 18.Launay-Vacher V, Zimner-Rapuch S, Poulalhon N, et al. Acute renal failure associated with the new BRAF inhibitor vemurafenib: a case series of 8 patients. Cancer 2014; 120: 2158–2163 [DOI] [PubMed] [Google Scholar]
- 19.Yorio JT, Mays SR, Ciurea AM, et al. Case of vemurafenib-induced Sweet's syndrome. J Dermatol 2014; 41: 817–820 [DOI] [PubMed] [Google Scholar]
- 20.Denis D, Franck N, Fichel F, et al. Fanconi syndrome induced by vemurafenib: a new renal adverse event. JAMA Dermatol 2015; 151: 453–454 [DOI] [PubMed] [Google Scholar]
- 21.Teuma C, Muzet CP, Pelletier S, et al. New insights in renal toxicity of BRAF inhibitor vemurafenib in patients with metastatic melanoma [abstract]. Cancer and Kidney International Network (CKIN), Annual Meeting, Brussels, Belgium, 2015 [Google Scholar]
- 22.Jhaveri KD, Sakhiya V, Fishbane S. BRAF inhibitor related nephrotoxicity. JAMA Oncol 2015; 1: 1133–1134 [DOI] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration. Tefinlar Highlights of prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/202806s000lbl.pdf Last revised May 2013
- 24.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012; 367: 1694–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015; 372: 30–39 [DOI] [PubMed] [Google Scholar]
- 26.Long G, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015; 386: 44–51 [DOI] [PubMed] [Google Scholar]
- 27.Chaib H, Hoskins BE, Ashraf S, et al. Identification of BRAF as a new interactor of PLCepsilon1, the protein mutated in nephrotic syndrome type 3. Am J Physiol Renal Physiol 2008; 294: F93–F99 [DOI] [PubMed] [Google Scholar]
- 28.Heakal Y, Kester M, Savage S. Vemurafenib (PLX4032): an orally available inhibitor of mutated BRAF for the treatment of metastatic melanoma. Ann Pharmacother 2011; 45: 1399–1405 [DOI] [PubMed] [Google Scholar]
- 29.Flaherty KT, Yasothan U, Kirkpatrick P. Vemurafenib. Nat Rev Drug Discov 2011; 10: 811–812 [DOI] [PubMed] [Google Scholar]
- 30.Iddawela M, Cook S, George L, et al. Safety and efficacy of vemurafenib in end stage renal failure. MBC Cancer 2103; 13: 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ouellet D, Gibiansky E, Leonowens C, et al. Population pharmacokinetics of dabrafenib, a BRAF inhibitor: effect of dose, time, covariates, and relationship with its metabolites. J Clin Pharmacol 2014; 54: 696–706 [DOI] [PubMed] [Google Scholar]
- 32.Naranjo CA, Busto U, Sellers EM. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239–224 [DOI] [PubMed] [Google Scholar]