Abstract
Background
Therapeutic drug monitoring of mycophenolic acid (MPA) is usually performed with a limited sampling strategy (LSS), which relies on a limited number of blood samples and subsequent extrapolation of the global exposure to MPA. LSS is usually performed successfully with mycophenolate mofetil (MMF), but data on enteric-coated mycophenolate sodium (EC-MPS) are scarce. Here, we evaluated the feasibility of 6-h LSS therapeutic drug monitoring with EC-MPS compared with MMF monitoring among kidney transplant recipients.
Methods
Sixty-two patients who received EC-MPS during the first 6 months of transplantation were compared with a matched group of 64 MMF-treated kidney transplant recipients. The area under the curve (AUC) was computed by LSS using multiple concentration time points (0, 1, 2, 3 and 6 h post-dose) and a trapezoidal rule. Patients had MPA therapeutic drug monitoring performed on two occasions, one within 2 weeks and the second after 3–4 months of transplantation.
Results
EC-MPS monitoring and MMF therapeutic drug monitoring were not interpretable in 34.5% (n = 40/116) and 1.8% (n = 2/112) of patients, respectively {relative risk [RR] 19.3 [95% confidence interval (CI) 4.8–78.0]; P < 0.0001}. The main cause of abnormal EC-MPS therapeutic drug monitoring was delayed absorption of both the previous evening and the morning dose, resulting in MPA plasma levels before the next morning dose being higher than MPA plasma levels measured at 1, 2 and 3 h after taking EC-MPS. Cyclosporin in association with MMF significantly increased the risk of low AUC values (<30 mg h/L) in comparison with tacrolimus [55% (n = 11/20) and 10% (n = 9/88), respectively; RR 5.4 (95% CI 2.6–11.2); P < 0.0001].
Conclusions
The risk of therapeutic drug monitoring failure with EC-MPS is >30% during the first 6 months of renal transplantation. Delayed pharmacokinetics was the main reason. In contrast, the risk of therapeutic drug monitoring failure was substantially lower with MMF.
Keywords: suppression of area under curves, enteric coating, kidney transplantation, mycophenolate mofetil, pharmacokinetics
Introduction
Mycophenolic acid (MPA), the active compound of mycophenolate mofetil (MMF) and enteric-coated mycophenolate sodium (EC-MPS), is the most widely used antiproliferative agent in kidney transplantation [1, 2]. The main side effects of MPA are gastrointestinal disturbances, haematologic disorders (e.g. anaemia and leucopenia) and infections [3–5]. These adverse events are reported more frequently with high MPA exposure (>60 mg h/L of plasma). In contrast, the relative risk (RR) of acute rejection significantly increases with low MPA exposure (<30 mg h/L) [3, 4, 6–8]. Accordingly, an MPA exposure between 30 and 60 mg h/L is considered optimal [9].
Like some other immunosuppressive drugs, MPA exhibits a narrow therapeutic index and large inter- and intra-individual variability [10, 11]. Therefore, an expert panel has recommended MPA therapeutic drug monitoring for kidney transplant recipients who receive dual immunosuppressive therapy, reduced-dosage calcineurin inhibitor (CNI) therapy, CNI switch or withdrawal and in recipients with high immunologic risk [9]. The full area under the plasma concentration–time curve (AUC 0–12 h) is the gold standard method to assess MPA exposure, but it is time-consuming and expensive [4–6, 9, 12]. In routine clinical practice, limited sampling strategies (LSSs) from multiple concentration time points are perhaps a more practical method to evaluate the MPA AUC in the hours following drug intake. LSSs are translated into AUCs by computing the results by trapezoidal rule, multiple linear regressions or Bayesian estimation [9]. While both MPA formulations, MMF and EC-MPS, have shown equivalent efficacy in kidney transplant recipients [13–15], EC-MPS exhibits delayed and prolonged intestinal absorption. Therefore, LSS may not be accurate to evaluate MPA AUCs. Indeed, LSS is frequently performed with MMF [9, 16] but remains rarely reported with EC-MPS [8, 17–19]. Here, we retrospectively reviewed the performance of a trapezoidal rule-based LSS in kidney transplant recipients receiving either EC-MPS or MMF in combination with CNI and steroids.
Materials and methods
Study design
We started performing MPA AUC in our centre in September 2005. We retrospectively reviewed the charts of all kidney transplant recipients (N = 434) who had transplants performed at our centre between September 2005 and May 2011. We excluded 15 patients who received a combined graft (kidney and pancreas or liver), 5 patients who were already transplanted with another solid organ transplant and 23 paediatric recipients. Among the remaining 391 patients, 72 received EC-MPS from the transplantation, but 2 patients had no MPA AUC during the first 6 months and 8 patients had incomplete blood sampling. A total of 62 evaluable patients were therefore included in analyses. For comparison, sex- and age-matched MMF-treated kidney transplant recipients with an MPA AUC performed during the first 6 months were selected as a comparison group (N = 64). By centre protocol, most MPA AUCs were performed during the first 10 days, with the objective of avoiding MPA underexposure (EC-MPS, N = 52/116; MMF, N = 57/112; ‘early therapeutic drug monitoring’), or after (almost always during months 2–3), mainly to avoid MPA overexposure (EC-MPS, N = 64/116; MMF, N= 55/112; ‘late therapeutic drug monitoring’).
Monitoring methods
The MPA AUC level was computed on the basis of five sampling time points (before and 1, 2, 3 and 6 h after medication intake). As previously reported, MPA plasma levels were measured at 1, 2 and 3 h (C1, C2 and C3) to evaluate peak concentration (Cmax) [8, 10, 20]. A later time, 6 h (C6), was selected to assess enterohepatic MPA cycling. AUC values were computed by the trapezoidal rule developed in our centre in 2005 according to Hale et al.'s publication in kidney transplant recipients [21, 22]. Internal validation consistently demonstrated that trapezoidal rule was equivalent to several published equations derived from linear regression and was validated by full 12-h AUC measurements that were performed in kidney transplant recipients (n = 8, unpublished data). MPA plasma concentrations were measured by high-performance liquid chromatography–ultraviolet light as recommended [23]. According to their kinetic profile, curves were classified as interpretable or not. Curve qualification criteria required that peak MPA plasma concentrations (Cmax) be higher than those at both C6 and C0. In other words, Cmax had to occur at C1, C2 or C3. Indeed, the occurrence of Cmax at C0 or C6 strongly suggests that the 6-h sampling period does not capture adequate MPA exposure.
Patient demographics
The analysis included 62 and 64 kidney transplant recipients who were treated with EC-MPS and MMF, respectively. Demographics are summarized in Table 1. Within the EC-MPS group, cyclosporin was the most frequently used CNI (n = 59/62), whereas it was tacrolimus (n = 50/64) among MMF patients. The systematic association of EC-MPS with cyclosporin in the context of a clinical trial explained this difference in CNI distribution between the two patient populations.
Table 1.
Patient characteristics
| MMF (N = 64) | EC-MPS (N = 62) | P-value | |
|---|---|---|---|
| Recipient age (mean ± SD; years) | 54 ± 13 | 52 ± 14 | 0.38 |
| Donor age (mean ± SD; years) | 45 ± 14 | 44 ± 14 | 0.77 |
| BMI (mean ± SD; kg/m2) | 26 ± 5 | 25 ± 5 | 0.55 |
| Proportion of male (%, n) | 69% (44) | 73% (45) | 0.70 |
| Proportion of first graft (%, n) | 94% (60) | 100% (62) | 0.12 |
| PRA max >0% | 11% (7) | 5% (3) | 0.32 |
| AUCs, early period (%, n) | 51% (57) | 45% (52) | 0.43 |
| AUCs, late period (%, n) | 49% (55) | 55% (64) | |
| Treatment by cyclosporin (%, n) | 19% (12) | 95% (59) | <0.0001 |
| MPA dose, early period (mean ± SD; mg/kg) | 28 ± 6 | 20 ± 4 | NA |
| MPA dose, late period (mean ± SD; mg/kg) | 28 ± 7 | 20 ± 5 | NA |
| Creatinine, early period (mean ± SD, mg/dL)a | 3.3 ± 3.0 | 3.2 ± 2.2 | 0.35 |
| Creatinine, late period (mean ± SD, mg/dL)b | 1.4 ± 1.2 | 1.4 ± 0.7 | 0.08 |
Cyclosporin was more frequently prescribed in the EC-MPS group in comparison to the MMF group (mainly treated with tacrolimus) (see text).
BMI, body mass index; PRA, panel reactive antibody; NA, not applicable.
aMean on day of MPA assay: 5.7 ± 1.8 (n = 57, MMF) and 4.8 ± 1.7 (n = 52, EC-MPS).
bMean on day of MPA assay: 83.7 ± 42.9 (n = 55, MMF) and 74.4 ± 46.2 (n = 64, EC-MPS).
Data collection
For each patient, the following data were collected: age, sex, body weight at transplant, graft characteristics (date of transplant, donor age, graft order, current panel reactive antibody), the date the AUC was calculated, characteristics of concomitant immunosuppressive drugs, classical biological parameters (e.g. creatinine) and comorbidities (e.g. diabetes, oesophagitis and digestive ulcus). For EC-MPS-treated kidney transplant recipients, we also focussed on factors that may influence MPA exposure, such as oesophagitis and/or gastritis (n = 23), chronic viral hepatitis (n = 4), anti-HIV therapy (n = 0) and other co-medications taken at the time of therapeutic drug monitoring and possibly interfering with MPA absorption/metabolism {i.e. prophylactic use of drugs against herpetic virus [i.e. aciclovir, valaciclovir, ganciclovir or valganciclovir; n = 46], proton pump inhibitor [PPI] use [n = 95] and antibiotics [i.e. penicillins (n = 6), quinolone (n = 2)]}. No patients were taking metronidazole or ryfamicin.
Statistical analyses
Continuous data are expressed as mean (SD) or median [interquartile range (IQR)] as appropriate. The D'Agostino and Pearson omnibus normality test was used to determine normality. Unpaired non-parametric data were compared by the Mann–Whitney test. For some data, contingency tables were established and χ2 was determined. Correlations were obtained by linear regression analysis and the non-parametric Spearman rank correlation coefficient. P-values <0.05 were considered statistically significant. Statistical analyses were performed using Prism 5.0a (GraphPad Software, La Jolla, CA, USA).
Results
Adequacy of therapeutic drug monitoring
A mean of 1.87 and 1.75 AUC was obtained for EC-MPS- and MMF-treated kidney transplant recipients, respectively. EC-MPS monitoring and MMF therapeutic drug monitoring were not interpretable in 34.5% (n = 40/116) and 1.8% (n = 2/112) of assays, respectively {RR 19.3 [95% confidence interval (CI) 4.8–78.0]; P < 0.0001}. Within the MMF-treated group, the rate of therapeutic drug monitoring failure was low whether patients were taking cyclosporin A (n = 12) or tacrolimus (n = 42) (4.8 versus 1.1%, respectively; P = 0.34). For EC-MPS, the proportion of inadequate therapeutic drug monitoring was similar whether they were performed early or late [n = 18/52 (35%) versus 22/64 (34%); P = 1.00]. We failed to identify any risk factor for an abnormal kinetic profile among EC-MPS patients (i.e. oesophagitis and/or gastritis, diabetes, early versus late post-transplant period, PPI use, cyclosporin or antiherpetic drugs and obesity) (Table 2).
Table 2.
Risk factors for therapeutic drug monitoring failure with EC-MPS
| Proportion of therapeutic drug monitoring failures, % (n/N) |
|||
|---|---|---|---|
| Absent | Present | P-value | |
| Oesophagitis/gastritis | 31 (12/39) | 39 (9/23) | 0.58 |
| Diabetes | 35 (17/48) | 29 (4/14) | 0.76 |
| Early period | 33 (4/12) | 34 (17/50) | 1.00 |
| PPI use | 33 (4/12) | 34 (17/50) | 1.00 |
| Antiviral drugs (herpes virus) intake | 38 (6/16) | 32 (15/46) | 0.76 |
| BMI ≥30 mg/kg | 32 (17/53) | 44 (4/9) | 0.47 |
PPI, proton pump inhibitors.
MPA pharmacokinetics
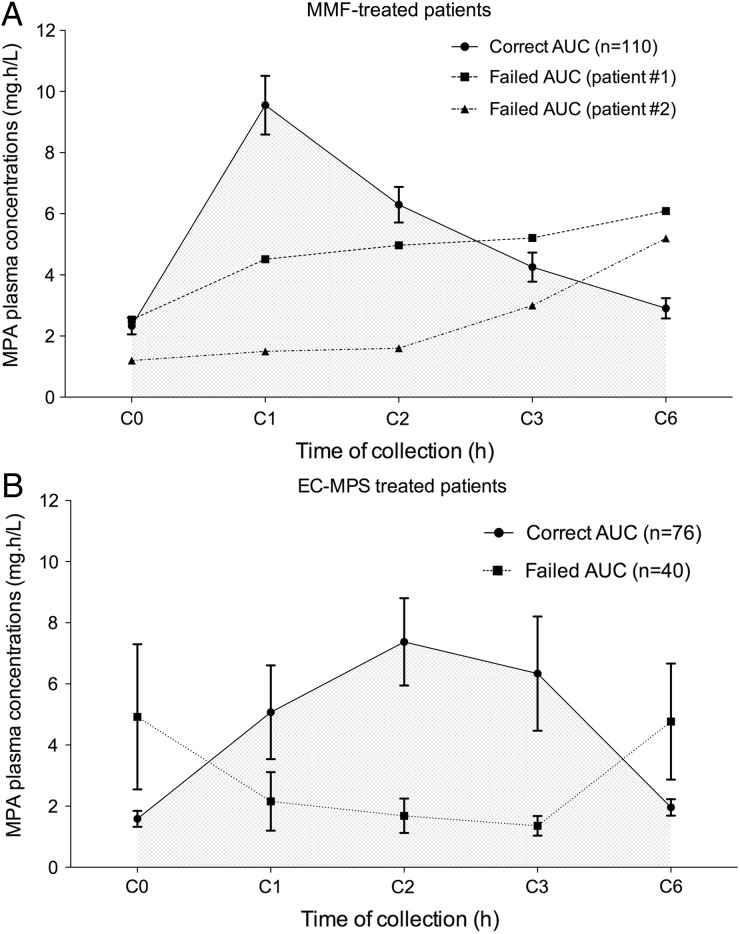
MPA plasma concentrations were plotted according to time for EC-MPS (n = 116) and MMF (n = 112) (Figure 1). In the case of a valid AUC, EC-MPS (n = 76) showed delayed peak MPA plasma concentrations compared with MMF (n = 110). Peak MPA plasma concentrations were shown after 1 h in 74% (81/110) of MMF and 32% (24/76) of EC-MPS assays (P < 0.0001). Peak MPA plasma concentrations were shown most often at 2 h with EC-MPS (41%, n = 31/76). Cmax values were similar (10.5 ± 4.8 and 12.5 ± 9.0 mg/L for MMF and EC-MPS, respectively; P = 0.23). The two abnormal MMF AUCs showed lower peak concentrations and higher C6, suggesting delayed absorption of the morning dose (Figure 1A). In comparison with valid EC-MPS AUCs (n = 74), abnormal EC-MPS AUCs (n = 40) exhibited higher C0 (4.9 ± 7.4 versus 1.6 ± 1.1 mg/L; P = 0.04) and delayed peak MPA plasma levels (Figure 1B). As previously described, cyclosporin was associated with lower exposure to MPA when compared with tacrolimus [cyclosporin + MMF: 32.3 ± 13.8 mg/h/L (n = 20) versus Tac + MMF: 48.4 ± 18.8 mg/h/L (n = 88); P < 0.0001; cyclosporin + EC-MPS: 35.3 ± 15.2 mg/h/L (n = 73) versus Tac + EC-MPS: 45.9 ± 1.0 mg h/L (n = 3)]. Accordingly, cyclosporin in association with MMF increased the risk of low AUC values (<30 mg/h/L) by nearly 50% in comparison with tacrolimus [55% (n = 11/20) and 10% (n = 9/88), respectively; RR 5.4 (95% CI 2.6–11.2); P < 0.0001].
FIGURE 1:
(A) MPA AUC after one dose of MMF. Normal AUC values, solid line (n = 110); abnormal AUC values, dashed line (n = 2). Vertical lines represent 95% CI. (B) MPA AUC after one dose of EC-MPS. Normal AUC values, solid line (n = 76); abnormal AUC values, dashed line (n = 40). Vertical lines represent 95% CI. MPA plasma levels, hours after intake.
Discussion
The main finding of our study is the poor performance of the 6-h LSS for therapeutic drug monitoring of EC-MPS. Indeed, about one-third of therapeutic drug monitoring failed with EC-MPS, whereas it was uncommon with MMF. It seems that by virtue of its pharmacokinetic properties, the extended-release EC-MPS formulation leads to an unusually delayed absorption in a significant subset of patients. As a result, absorption of the previous evening dose is still ongoing when the patient undergoes the MPA AUC, and the intake of the morning dose also results in a similar late absorption. Cattaneo et al. [10] reported that Cmax could occur as late as 16 h after EC-MPS intake. In our study, the pharmacokinetic profile of MPA (i.e. plasma concentration–time curves) was similar to that reported in the literature for both formulations of MPA [20]. de Winter et al. [24] have also reported the weak performance of LSS with EC-MPS, but their pharmacokinetic analysis was limited to 3 h. We noted high pharmacokinetic variabilities of EC-MPS, reflected in wide CIs in plasma MPA concentrations, which could contribute to therapeutic drug monitoring failure [10, 20, 24].
We investigated whether factors known to influence the absorption of MPA could help to identify patients at risk of EC-MPS LSS failure. The presence of diabetes, obesity, PPI use, antiherpetic drug intake and the timing of LSS (i.e. early versus late) did not predict EC-MPS therapeutic drug monitoring failure (although this study is too small to rule out small effects of these factors on therapeutic drug monitoring failure). Other study limitations include the retrospective design, the low acute graft rejection rate and the fact that MMF or EC-MPS dose modification in response to therapeutic drug monitoring could have reduced the reporting of side effects. Most patients on MMF were taking tacrolimus. In contrast, most patients on EC-MPS were taking cyclosporin. This distribution bias could be a limitation because cyclosporin exhibits well-documented interactions with MPA, whereas tacrolimus does not. However, the virtual absence of MMF therapeutic drug monitoring failure among patients taking cyclosporin strongly suggests that EC-MPS therapeutic drug monitoring failure is an intrinsic characteristic of EC-MPS, irrespective of the associated CNI. Finally, an additional limitation is the absence of full 12-h AUC values for MMF and EC-MPS in order to compare the ability of the LSS to predict exposure in the two groups. However, we believe that prolongation of the LSS is futile, as the need for a longer collection time could be impractical from a clinical point of view.
In conclusion, EC-MPS therapeutic drug monitoring by a 6-h LSS is associated with a high failure rate because of its delayed absorption. These patients may require the performance of a full 12-h AUC to capture MPA exposure efficiently [9]. For patients who require MPA therapeutic drug monitoring, MMF is currently the most practical therapeutic option and EC-MPS might be best reserved for use in those kidney transplant recipients who do not require therapeutic drug monitoring.
Conflict of interest statement
We declare that the results presented in this paper have not been published previously in whole or part, except in abstract format.
References
- 1.Knight SR, Russell NK, Barcena L, et al. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation 2009; 87: 785–794 [DOI] [PubMed] [Google Scholar]
- 2.Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007; 357: 2562–2575 [DOI] [PubMed] [Google Scholar]
- 3.van Gelder T, Hilbrands LB, Vanrenterghem Y, et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation 1999; 68: 261–266 [DOI] [PubMed] [Google Scholar]
- 4.Le Meur Y, Buchler M, Thierry A, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant 2007; 7: 2496–2503 [DOI] [PubMed] [Google Scholar]
- 5.Gaston RS, Kaplan B, Shah T, et al. Fixed- or controlled-dose mycophenolate mofetil with standard- or reduced-dose calcineurin inhibitors: the Opticept trial. Am J Transplant 2009; 9: 1607–1619 [DOI] [PubMed] [Google Scholar]
- 6.van Gelder T, Silva HT, de Fijter JW, et al. Comparing mycophenolate mofetil regimens for de novo renal transplant recipients: the fixed-dose concentration-controlled trial. Transplantation 2008; 86: 1043–1051 [DOI] [PubMed] [Google Scholar]
- 7.Kuypers DR, de Jonge H, Naesens M, et al. Current target ranges of mycophenolic acid exposure and drug-related adverse events: a 5-year, open-label, prospective, clinical follow-up study in renal allograft recipients. Clin Ther 2008; 30: 673–683 [DOI] [PubMed] [Google Scholar]
- 8.Sommerer C, Muller-Krebs S, Schaier M, et al. Pharmacokinetic and pharmacodynamic analysis of enteric-coated mycophenolate sodium: limited sampling strategies and clinical outcome in renal transplant patients. Br J Clin Pharmacol 2010; 69: 346–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuypers DR, Le Meur Y, Cantarovich M, et al. Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation. Clin J Am Soc Nephrol 2010; 5: 341–358 [DOI] [PubMed] [Google Scholar]
- 10.Cattaneo D, Cortinovis M, Baldelli S, et al. Pharmacokinetics of mycophenolate sodium and comparison with the mofetil formulation in stable kidney transplant recipients. Clin J Am Soc Nephrol 2007; 2: 1147–1155 [DOI] [PubMed] [Google Scholar]
- 11.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet 2007; 46: 13–58 [DOI] [PubMed] [Google Scholar]
- 12.Knight SR, Morris PJ. Does the evidence support the use of mycophenolate mofetil therapeutic drug monitoring in clinical practice? A systematic review. Transplantation 2008; 85: 1675–1685 [DOI] [PubMed] [Google Scholar]
- 13.Budde K, Curtis J, Knoll G, et al. Enteric-coated mycophenolate sodium can be safely administered in maintenance renal transplant patients: results of a 1-year study. Am J Transplant 2004; 4: 237–243 [DOI] [PubMed] [Google Scholar]
- 14.Johnston A, He X, Holt DW. Bioequivalence of enteric-coated mycophenolate sodium and mycophenolate mofetil: a meta-analysis of three studies in stable renal transplant recipients. Transplantation 2006; 82: 1413–1418 [DOI] [PubMed] [Google Scholar]
- 15.Salvadori M, Holzer H, de Mattos A, et al. Enteric-coated mycophenolate sodium is therapeutically equivalent to mycophenolate mofetil in de novo renal transplant patients. Am J Transplant 2004; 4: 231–236 [DOI] [PubMed] [Google Scholar]
- 16.Pawinski T, Hale M, Korecka M, et al. Limited sampling strategy for the estimation of mycophenolic acid area under the curve in adult renal transplant patients treated with concomitant tacrolimus. Clin Chem 2002; 48: 1497–1504 [PubMed] [Google Scholar]
- 17.Capone D, Tarantino G, Kadilli I, et al. Evaluation of mycophenolic acid systemic exposure by limited sampling strategy in kidney transplant recipients receiving enteric-coated mycophenolate sodium (EC-MPS) and cyclosporine. Nephrol Dial Transplant 2011; 26: 3019–3025 [DOI] [PubMed] [Google Scholar]
- 18.de Winter BC, van Gelder T, Mathot RA, et al. Limited sampling strategies drawn within 3 hours postdose poorly predict mycophenolic acid area-under-the-curve after enteric-coated mycophenolate sodium. Ther Drug Monit 2009; 31: 585–591 [DOI] [PubMed] [Google Scholar]
- 19.Fleming DH, Mathew BS, Prasanna S, et al. A possible simplification for the estimation of area under the curve (AUC0–12) of enteric-coated mycophenolate sodium in renal transplant patients receiving tacrolimus. Ther Drug Monit 2011; 33: 165–170 [DOI] [PubMed] [Google Scholar]
- 20.Budde K, Glander P, Kramer BK, et al. Conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in maintenance renal transplant recipients receiving tacrolimus: clinical, pharmacokinetic, and pharmacodynamic outcomes. Transplantation 2007; 83: 417–424 [DOI] [PubMed] [Google Scholar]
- 21.Hale MD, Nicholls AJ, Bullingham RE, et al. The pharmacokinetic-pharmacodynamic relationship for mycophenolate mofetil in renal transplantation. Clin Pharmacol Ther 1998; 64: 672–683 [DOI] [PubMed] [Google Scholar]
- 22.Chaabane A, Aouam K, Ben Fredj N, et al. Limited sampling strategy of mycophenolic acid in adult kidney transplant recipients: influence of the post-transplant period and the pharmacokinetic profile. J Clin Pharmacol 2013; 53: 925–933 [DOI] [PubMed] [Google Scholar]
- 23.Martiny D, Macours P, Cotton F, et al. Reliability of mycophenolic acid monitoring by an enzyme multiplied immunoassay technique. Clin Lab 2010; 56: 345–353 [PubMed] [Google Scholar]
- 24.de Winter BC, van Gelder T, Glander P, et al. Population pharmacokinetics of mycophenolic acid: a comparison between enteric-coated mycophenolate sodium and mycophenolate mofetil in renal transplant recipients. Clin Pharmacokinet 2008; 47: 827–838 [DOI] [PubMed] [Google Scholar]