Abstract
Classical fear conditioning creates an association between an aversive stimulus and a neutral stimulus. Although the requisite neural circuitry is well understood in mature organisms, the development of these circuits is less well studied. The current experiments examine the ontogeny of fear conditioning and relate it to neuronal activation assessed through immediate early gene (IEG) expression in the amygdala, hippocampus, perirhinal cortex, and hypothalamus of periweanling rats. Rat pups were fear conditioned, or not, during the 3rd or 4th weeks of life. Neuronal activation was assessed by quantifying expression of FBJ osteosarcoma oncogene (FOS) using immunohistochemistry (IHC) in Experiment 1. Fos and early growth response gene-1 (EGR1) expression was assessed using qRT-PCR in Experiment 2. Behavioral data confirm that both auditory and contextual fear continue to emerge between PD 17 and 24. The IEG expression data are highly consistent with these behavioral results. IHC results demonstrate significantly more FOS protein expression in the basal amygdala of fear conditioned PD 23 subjects compared to control subjects, but no significant difference at PD 17. qRT-PCR results suggest specific activation of the amygdala only in older subjects during auditory fear expression. A similar effect of age and conditioning status was also observed in the perirhinal cortex during both contextual and auditory fear expression. Overall, the development of fear conditioning occurring between the 3rd and 4th weeks of life appears to be at least partly attributable to changes in activation of the amygdala and perirhinal cortex during fear conditioning or expression.
Keywords: anxiety, development, ontogeny, hippocampus, fos, egr1
Classical fear conditioning involves the formation of an association between an aversive, unconditioned stimulus (US; e.g. footshock) and a neutral, conditioned stimulus (CS; e.g. tone). It is a widely used as a tool for researching anxiety and fear-related disorders, in part because the neural circuitry underlying fear conditioning is well understood in adult organisms (Phillips & LeDoux, 1992; Campeau & Davis, 1995; Fendt & Fanselow, 1999; Maren, 2008; Blair, Schafe, Bauer, Rodrigues, & LeDoux, 2001; LeDoux, 1998). In adult animals, it is now clearly established that fear conditioning relies on a well-defined circuit centered on the amygdala. The basal and lateral nuclei of the amygdala (also referred to as the basolateral complex; BLA) are considered the input region of the amygdala, while the central nucleus of the amygdala is considered the main output region (Campeau & Davis, 1995; Kim & Fanselow, 1992; Kim & Jung, 2006; LeDoux, 2007; Maren & Fanselow, 1995; Maren, 2001; Phillips & LeDoux, 1992; Schettino & Otto, 2001). However, despite the fact that anxiety disorders emerges relatively early in development, the ontogeny of these circuits is less well understood.
Although the association of simple conditioned stimuli and painful unconditioned stimuli appears to only require the simple circuit outlined above, more complex stimuli require additional circuitry (Phillips & LeDoux, 1992; Rudy, Huff, & Matus-Amat, 2004; Fendt & Fanselow, 1999; Maren, 2001; Raineki et al., 2010; Gross & Canteras, 2012). For example, the dorsal hippocampus is a region of the fear circuit thought to be responsible for the formation of contextual representations necessary for contextual fear conditioning (Sacchetti, Lorenzini, Baldi, Tassoni, & Bucherelli, 1999; Fanselow, 2000; Maren, 2001; Matus-Amat, Higgins, Barrientos, & Rudy, 2004). However, in contrast to the ventral hippocampus, which has dense reciprocal connections, the dorsal portion of the hippocampus has only sparse connections with the amygdala (Majak & Pitkanen, 2003; Pikkarainen, Rönkkö, Savander, Insausti, & Pitkänen, 1999; Pitkänen, Pikkarainen, Nurminen, & Ylinen, 2000; van Groen & Wyss, 1990). Importantly, dorsal and ventral hippocampus are not highly inter-connected (Moser & Moser, 1998), leading to the conclusion that other regions may be necessary for the integration of contextual and aversive information. One possibility is the perirhinal cortex, which along with the entorhinal and postrhinal cortex, is known to reciprocally connect with both the BLA and the dorsal hippocampus and is involved in utilizing highly processed sensory information (Rosen et al., 1992; Campeau & Davis, 1995; Maren, 2001; Stoop & Pralong, 2000).
Elucidating the functional emergence of the fear circuit pathway over the course of development is an ongoing process. The traditional view is that fear conditioning to an explicit cue (i.e. tone or visual cue) and context dissociate during development (Rudy, 1993; Pugh & Rudy, 1996; Stanton, 2000; Raineki et al., 2010; Schiffino, Murawski, Rosen, & Stanton, 2011; Jablonski, Schiffino, & Stanton, 2012). Auditory fear develops by postnatal day (PD) 15–17 as relevant sensory systems come online, while fear of a context (contextual fear) does not fully emerge until PD 23 or 24. Given the role of the hippocampus in contextual, but not auditory, fear conditioning, this pattern has been interpreted as suggesting that the hippocampus may be relatively late to mature (See Raineki et al., 2010).
However, other data are not consistent with this interpretation. Recent research in our lab suggests that auditory fear continues to emerge with contextual fear between PD 17 and 24 (Burman, Erickson, Deal, & Jacobson, 2014). This is consistent with studies suggesting PD 17 rats require greater training than PD 24 subjects to achieve similar levels of freezing (Kim & Richardson, 2007, 2008; Rudy & Moreledge 1994). In addition, the first 3 weeks life is a time of significant growth and change in the basal, lateral, and accessory basal nuclei of the rat amygdala (Chareyron, Lavenex, & Lavenex, 2012). Furthermore, although electrophysiological phenomena, such as long-term potentiation (LTP), can be shown to emerge in the amygdala with the emergence of learned aversions around PD 12 (Thompson, Sullivan, & Wilson, 2008), there appears to be continuing changes in the electrophysiological properties in the BLA at least until PD 28 (Ehrlich, Ryan, & Rainnie, 2012) and in the cortical and thalamic pathways to the lateral nucleus of the amygdala through adulthood in mice (Pan, Ito, & Morozov, 2009). Thus, the amygdala appears to slowly develop over a fairly broad timeframe.
Similarly, in the hippocampus, evidence suggests that hippocampal LTP is present as early as the first week of postnatal development and reaches adult levels by the second week of development in the CA1 region of the hippocampus and the third week in the dentate gyrus (Durand, Kovalchuk, & Konnerth, 1996; Bekenstein & Lothman, 1991). Additionally, contextual learning may emerge earlier than suggested by the “traditional view” (Brasser & Spear, 1998; Brasser & Spear, 2004; Esmorís-Arranz, Méndez, & Spear, 2008; Foster & Burman, 2010). Thus, it seems likely that several structures, and the connectivity between them, are continuing to emerge during the periweaning time period, making a strict dissociation between auditory and contextual fear conditioning somewhat unlikely.
The current experiments test the hypothesis that fear conditioning in general will continue to emerge during the 3rd and 4th weeks of life and that this emergence will correspond with changes in amygdala activation, likely driven by development of cortical inputs. To accomplish this, we measured levels of immediate early gene (IEG) expression following fear conditioning or fear expression (Campeau et al., 1991; Hoffman, Smith, & Verbalis, 1993; Herrera & Robertson, 1996; Chaudhuri, 1997; Tischmeyer & Grimm, 1999; Malkani & Rosen, 2000). IEGs, including FOS and EGR-1, are a group of genes with rapid and transient expression that are often associated with neuroplasticity and are required for variety of behavioral paradigms including spatial learning tasks (Vann, Brown, Erichsen, & Aggleton, 2000; Teather, Packard, Smith, Ellis-Behnke, & Bazan, 2005) and fear conditioning (Rosen, Fanselow, Young, Sitcoske, & Maren, 1998; Huff et al., 2006; Schreiber, Asok, Jablonski, Rosen, & Stanton, 2014; Botterill et al., 2014; Bailey, Kim, Sun, Thompson, & Helmstetter, 1999; Schafe & LeDoux, 2000; Maren, Ferrario, Corcoran, Desmond, & Frey, 2003). Together these studies demonstrate continuing development between PD 17 and PD 24 in critical regions of the fear circuit.
Methods
Subjects
138 offspring (71 females and 67 males) from 35 litters were used for Experiment 1: Immunohistochemistry and behavioral analysis. 82 offspring (37 female and 45 male) from 17 litters were used for Experiment 2: behavioral and qPCR analysis. All subjects were delivered by timed-pregnant Sprague-Dawley rats (Charles River) arriving on either GD 12 or 18 and were checked daily for birth. All subjects were born on GD 21 or 22. For scheduling reasons, GD 22 was considered the day of birth (postnatal day 0) for all litters. Litters were housed in 43 cm × 44 cm × 20 cm transparent PET cages (Innovive, San Diego CA) in the University of New England rat vivarium. All experiments were approved by the IACUC and conducted in accordance with relevant guidelines. On postnatal day (PD) 3, litters were culled to 10 pups per litter (5 male and 5 female) whenever possible. All subjects lived with littermates and mother prior to weaning, which occurred on PD 21. Post-weaning subjects were housed with their same sex littermates (approximately 5 per cage). All subjects were maintained on a 12:12 hour light/dark cycle and had free access to food and water at all times in the vivarium.
Apparatus
Four Startfear chambers (Harvard Apparatus/Panlab model #58722) were used for fear conditioning in two separate configurations (see Burman et al., 2014). Contexts differed in shape, color, floor texture and cleaning solution (smell). A lack of movement below a fixed threshold, as assessed through changes in relative weight positioning across the floor was defined as freezing (Burman et al., 2014).
Behavioral testing
Rats were randomly assigned to a condition. No more than one same-sex littermate was assigned to any given experimental condition. Experiments 1A and 1B compared fear conditioned with unpaired control groups, in which the tone and shocks were presented in pseudo-random order and did not overlap, and tone-alone groups that did not receive any shock to avoid both auditory and contextual fear conditioning, but otherwise maintain similar levels of stress and stimulation (Figure 1). Experiment 1C compared fear conditioned animals and tone-alone control subjects. Experiments 2A, B and C, compared fear conditioned, tone-alone and completely undisturbed (home-cage) control subjects. Home-cage control subjects were generally taken from the same litters as, and sacrificed alongside, the fear conditioned and tone-alone groups. Due to the similar age and treatment, in order to conserve animals, 4 PD 17 and 4 PD 24 home-cage control subjects were used for analysis of both context fear and tone fear tests. These rats were littermates with subjects in only one of the experiments, but were otherwise treated identically. The experiment began on either PD 17 or PD 24. The same experimenter performed the behavioral protocol between 9:00 a.m. and 11:00 a.m. each day. On each day of the procedure, the home cage was brought into the lab and stored in a holding room. Rats were weighed, labeled with permanent marker on the tail and placed into individual transport cages measuring 24 cm × 18 cm × 13 cm and made of clear plastic. Ambient sound was muted and rats were transported to the testing room and placed into individual Startfear conditioning chambers. After each test, rats were returned to their home cages.
Figure 1. Timeline of Experiments.
Experiments 1A & 1B involve Conditioning on Day 1, Context Test on Day 2 and Tone Test on Day 3. Experiment 1C involves fear conditioning followed 1 hour later by tissue harvesting for immunohistochemistry. Experiment 2A, 2B and 2C involves tissue harvesting ½ hour after conditioning, or one of the test sessions for qPCR
Training
Fear Acquisition
On PD 17 or 23/24, animals in the fear conditioned group were pre-exposed to the experimental chamber for 5 minutes. This was followed by 10 conditioning trials in which a 10-s 4-Khz 67 (Experiment 2) or 70 (Experiment 1) dB tone co-terminated with an aversive stimulus. The US was a 2-s 1.0-mA footshock, except for Experiment 1A which utilized a 0.3-mA shock. Trials were separated by randomly chosen intervals (mean = 2.5 min, range = 1.5–3.5 min). Subjects in the “unpaired” group were subjected to the same stimuli, but presented in a pseudo-random order with presentation of any individual stimuli separated by an average ITI of 1.75 min. This ought to prevent auditory fear conditioning, but would still produce contextual fear conditioning. Subjects in the “tone-alone” group experienced the same procedure, but did not receive any footshocks, a control group used when no fearful learning is desired. Although each brain structure will express c-FOS and EGR-1 with a different time course, in an attempt to maximize RNA and protein expression, 30 (qPCR; Experiment 2) or 60 min (immunohistochemistry; Experiment 1C) following training, animals were anesthetized and brain tissue was harvested (Cullinan et al., 1995; Xiu et al., 2014).
Context Testing
Approximately 24 hours after training, rats being tested for contextual fear were returned to the original conditioning context chamber for 5 minutes during which freezing behavior was recorded. Following the 5-minute context fear test, rats were returned to their home cage for 30 minutes prior to tissue collection for qPCR..
Novel Context and Tone Testing
For the behavioral experiments, this phase took place 24 hours after contextual testing. For the qPCR experiments, contextual testing was omitted and this phase took place 24 hours after conditioning. Rats being tested for fear to the tone were placed into a novel conditioning chamber (in the opposite contextual configuration) for 5 minutes during which freezing was record. This was followed by 10 exposures to the 10-s 4-Khz tone, separated by 20-s intervals over a 5-minute period. Freezing was also recorded during the tone exposure period. Rats were returned to their home cage after test. 30 minutes later, subjects were euthanized and tissue was collected for qPCR.
Context testing was conducted before tone testing for our behavioral experiments (Experiment 1A, 1B), as the contextual extinction that occurs during the contextual fear test session facilitates assessment of auditory-cue induced freezing on the subsequent day (Burman et al., 2014; unpublished observations) and allows us to assess both behaviors in a single subjects. In contrast, for Experiment 2 (PCR), we wanted to be able to compare the patterns of neurological activation produced by contextual and auditory cue recall, without interference from the other, and thus subjected rats to either one or the other on day 2, but not both.
Immunohistochemistry
The expression of protein was observed following fear acquisition on PD 17 or 23. 60 min following completion of the fear acquisition session (~90 minutes after the first tone-shock pairing), subjects were anesthetized and perfused transcardially using physiological saline followed by a 4% paraformaldehyde (PFA) solution. Brains were removed and submerged in 4% PFA for 60 min and then placed in a 30% sucrose solution overnight at 4° C. Brains were sliced using a freezing microtome at a thickness of 40 μm with every 5th section kept, collecting a total of 24 slices per brain. In order to visualize the FOS protein, slices were stained using immunoperoxidase-IHC. Slices were rinsed on a rotating table for 10 min in a 0.3% hydrogen peroxide, 10% methanol solution followed by a 60 min incubation in a 3% normal goat serum blocking solution. Following this rinse, slices were incubated in rabbit anti-FOS primary antibody (Santa Cruz Biotechnology; sc-7202) diluted in 1% normal goat serum and 3% triton X rotating overnight at 4° C. Due to clear differences in efficacy between different lots of the primary antibody, concentrations of either 1:1000 or 1:250 were used depending on the antibody lot, with care taken to ensure that this produced similar levels of staining (Average FOS-like immunoreactive cells per mm^3 using lot 1=254.33 and lot 2=271.22; T-test: p=.630) and both antibody lots were equally distributed across experimental conditions and ages. The following day, slices were rinsed on a rotating table three times in a 1% normal goat serum blocking solution for 10 min per rinse. The secondary antibody (biotinylated goat anti-rabbit; Vector Laboratories; BA-1000) was applied at a concentration of 1:500 and left to rotate for 60 min and then rinsed with three 10 min rinses in phosphate buffered saline (PBS). Slices were then incubated in ABC solution (Vector Laboratories; Vectastain ABC Standard Kit) for 60 min followed by three 10 min washes in phosphate buffer (PB). Finally, slices were rinsed in DAB (0.07% w/v DAB, 0.067% w/v urea hydrogen peroxide, 0.01% nickel ammonium sulfate, 0.016 % cobalt chloride) until an even light brown coloring was apparent on the slices (about 5–8 min) followed by three 10-min PB rinses. Slices were mounted onto slides immediately following the final PB rinse and allowed to dry overnight at room temperature prior to coverslipping. A control test performed to confirm antibody specificity showed no difference from background staining levels in the absence of the primary antibody.
Imaging/Stereology
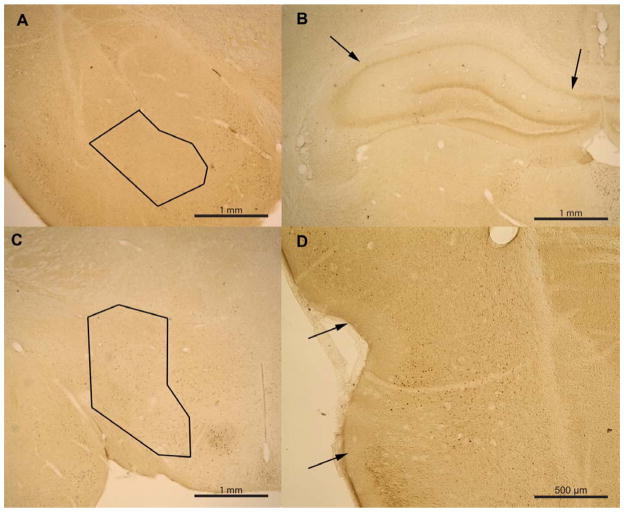
Data were collected using a commercially available computerized stereology system (Stereologer, Stereology Resource Center, Tampa-St. Petersburg, FL) by an experimenter blinded to treatment group. Four reference spaces (regions of interest) were anatomically identified for quantification of the total number of positively stained cells and total region volume: basal amygdala, dorsal hippocampus CA1, perirhinal cortex, and lateral hypothalamus (see Table 1 for n values). Sample size per region varied because not all regions were quantifiable in all brains due to tissue folding and tearing. Regions were determined using multiple atlases (Sherwood & Timiras, 1970; Paxinos & Watson, 1998), previous literature (Burwell, Witter, & Amaral, 1995; Burwell & Amaral, 1998), as well as using claustral and hippocampal morphology. We defined the rostral and caudal borders of the perirhinal cortex to be around 3.0 mm and 1.8 mm anterior to the interaural line respectively (Sherwood & Timiras, 1970). The rostral and caudal borders of the basal nucleus of the amygdala were defined to be around 4.4 mm and 2.9 mm anterior to the interaural line respectively. The rostral and caudal borders of the lateral hypothalamus were defined to be around 5.6 mm and 2.6 mm anterior to the interaural line respectively. The rostral and caudal borders of the dorsal CA1 region of the hippocampus were defined to be around 3.5 mm and 1.2 mm anterior to the interaural line respectively. Sample outlines of regions of interest can be seen in figure 2.
Table 1.
Number of samples analyzed via IHC per region in each group.
| Fear Conditioned | Tone-Alone | |
|---|---|---|
| PD 17 | Amyg: n=5; Hippo: n=5; Hypo: n=4; Per: n=5 | Amyg: n=5; Hippo: n=4; Hypo: n=5; Per: n=4 |
| PD 23 | Amyg: n=6; Hippo: n=6; Hypo: n=6; Per: n=5 | Amyg: n=6; Hippo: n=6; Hypo: n=6; Per: n=6 |
Figure 2. Sample of region outlines used when quantifying FOS-like immunoreactivity in the basal amygdala.
(A), CA1/CA2 region of the hippocampus (B), lateral hypothalamus (C), and perirhinal cortex (D).
Due to the relatively small number of FOS-like immunoreactive cells, total cell counts for each reference space region were calculated using the Rare Event Protocol (Mouton, 2011), an unbiased variation for rare events of Gundersen’s fractionator method (1986). With the Rare Event Protocol, the total number of rare events (cells) is quantified in an unbiased manner by counting all immunoreactive cells within a known fraction of the reference volume. In brief, a known section sampling fraction, ssf (1/5th) of sections were sampled from the entire number of section through the entire reference volume. On each sampled section the reference area was outlined at low magnification (5x) and the software automatically quantified total region volume using the Cavalieri point-counting method (Gundersen, Jensen, Kiêu, & Nielsen, 1999). At a higher magnification (60x), the user scanned within each outlined reference area to identify dark brown immunoreactive cells circular or oval in shape. After the total number of immunoreactive cells counted within sampled sections were determined, the software calculated the total number (total N) of immunoreactive cells using the formula: Total N = ΣQ− • F1 where, ΣQ− = total # cells with tops present with the sampled sections and F1 = reciprocal of the ssf = 1/number of sampled sections/ total number of sections through entire reference space.
Note that total N is quantified using the Rare Event Protocol with 1 for both the area sampling fraction (asf) and the thickness sampling fraction (tsf) since all immunoreactive cells are counted within each sampled section.
Tissue collection and RNA isolation for qPCR
Rats were retrieved from their home cage and sacrificed 30 minutes after completion of the training session (fear acquisition), context fear test or tone fear test (Campeau et al., 1991). Animals in the home cage control group remained in their home cages until they were sacrificed on PD 17 or 24 (fear acquisition) or PD 18 or 25 (context fear test and tone fear test) at the same time as subjects in the fear conditioned and tone-alone groups. Rats were anesthetized with an overdose of pentobarbital and decapitated within 5 minutes of injection and prior to death. Brains were frozen immediately in liquid nitrogen upon removal and stored at −80°C.
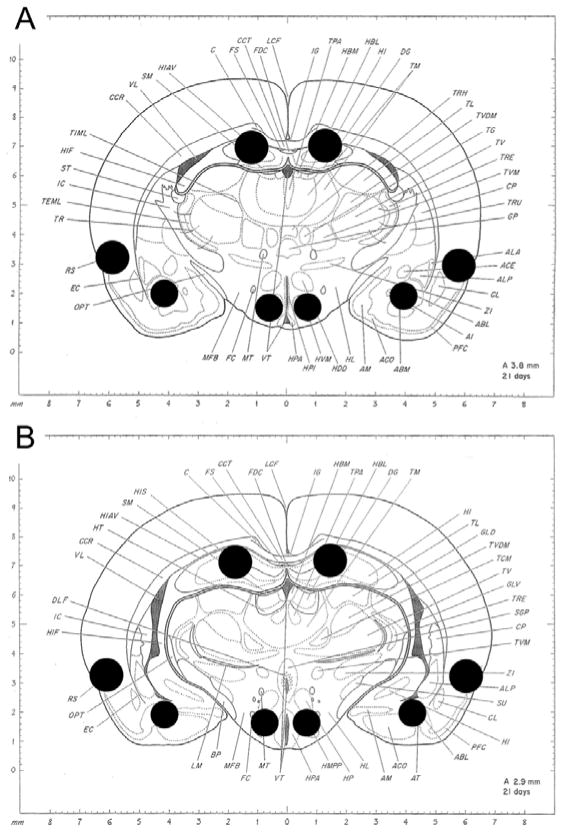
To collect tissue from specific regions, a coronal section approximately 1mm thick was taken from each brain (rostral side approximately 3.8mm from the interaural line; see figure 3). A small portion of the amygdala, perirhinal cortex, hippocampus and hypothalamus were excised bilaterally using a 1mm (basolateral amygdala and ventromedial hypothalamus) or 1.25mm (perirhinal cortex and CA1/2 of hippocampus) diameter tissue punch. In the amygdala, the punch area was centered on the basolateral amygdala, but may have contained neighboring regions of the basomedial amygdala and cortical nucleus. The targeted region of the hypothalamus was the ventromedial hypothalamus, but likely included tissue from the periventricular nucleus, premamillary nucleus and lateral hypothalamus. In the hippocampus, the targeted region was the dorsal CA1/CA2 region, but likely included portions of the dentate gyrus and fornix. The targeted region of the perirhinal cortex included Area 35 and Area 36 surrounding the rhinal sulcus, but may have included tissue from surrounding cortical areas such as the dorsal entorhinal cortex. Excised tissue was placed in a 1.5 mL microcentrifuge tube containing 140 μL of RNA lysis buffer (Promega, Madison WI). Tissue was homogenized and lysed. The samples were collected and total RNA was isolated using the Promega SV Total RNA Isolation System. RNA quantity was analyzed using the NanoDrop 2000c spectrophotometer (Thermo Scientific, Waltham MA). RNA was stored at −80°C until needed.
Figure 3. Locations of tissue samples taken for qRT-PCR.
A) Approximate rostral limit of tissue samples collected. B) Approximate caudal limit of tissue samples collected. From: A Stereotaxic Atlas of the Developing Rat Brain. Pages 107 & 113 by N Sherwood & P Timiras, P 1970, Berkeley: University of California Press. Copyright 1970 N. Sherwood. Reprinted with Permission.
Reverse transcription
Total RNA was synthesized into cDNA using Q-Script cDNA synthesis kit from VWR Scientific (VWR, Radnor PA) according to manufacturer’s instructions. To control for varying levels of total RNA, RNA input to the cDNA synthesis reaction was standardized to the sample containing the lowest quantity of RNA determined using NanoDrop spectrophotometry. Total reaction volume was 20 μL for all samples. Samples were incubated at 25°C for 5 minutes, followed by 30 minute incubation at 42°C for the reverse transcription reaction and 5-minute incubation at 85°C for inactivation of reverse transcriptase.
qRT-PCR
Real-time qPCR was completed using the Bio-Rad CFX90. A common mastermix was created and used for all samples in each replication. Samples were run in triplicate and a non-template control was included in every run. Primers were commercially available RT2 qPCR Primer Assays from Qiagen. Genes of interest were two immediate early genes (IEGs), FBJ osteosarcoma oncogene (Fos; Qiagen cat. no. PPR55248C, accession no. NM_022197.2) and early growth response gene (Egr1; Qiagen cat. no. PPR44272B, accession no. NM_012551.2; also known as Zif-268 or Ngf1). Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) was used as a reference gene (Qiagen cat. no. PPR06557B, accession no. NM_017008.4) for all samples.
qRT-PCR reactions began with incubation for 10 minutes at 95°C, followed by 40 cycles of 1 minute at 60°C for annealing/extension followed by 15 seconds at 95°C for denaturation. After completion of the 40 amplification cycles, amplicons were run through a melt curve analysis consisting of 10 second steps from 65–95°C to ensure that a single product was amplified within each reaction. Results were analyzed using the delta-delta CT method (Livak & Schmittgen, 2001). To perform these calculations, first data were averaged from the triplicate replications. Clearly aberrant single replications (>2.5 SD from others) were excluded. All data were then normalized to reference gene Gapdh by calculating difference scores between Ct values for the gene of interest and Gapdh. Gapdh served to control for quantity of RNA analyzed as well as quality. Next, the normalized home cage samples were averaged to create a calibrator value for each age and brain region. Home cage calibrator values were then used to normalize data from the fear conditioned and tone-alone samples, by once again calculating difference scores between the target data point and the calibrator values. It is important to note that all samples for each replication of an experiment were run simultaneously using a common master-mix. This includes the common home-cage animals, which underwent PCR separately for each experiment. Data were removed as outliers if the value for both Fos and Egr1 was more than 2.5 standard deviations outside the mean. Using this analysis, out of the 120 brain/region combinations for each experiment, 5 individual data points were removed in the fear acquisition analysis, 6 data points were removed in the context fear test analysis and 4 data points were removed from the tone fear test analysis, though no more than 1 data point was removed from a single group (i.e. PD 24 tone-alone amygdala, etc.) in each analysis. One sample from the fear acquisition study (PD 17 home cage hypothalamus) could not be analyzed due to highly inconsistent amplification and was excluded.
Data Analysis and Statistics
Data were analyzed using SPSS 20 (IBM, Armonk NY) on Windows XP or JMP 11 (SAS) for Macintosh. We found no effects of sex in our initial analyses, similar to previous findings (Burman et al. 2014; Foster & Burman 2010), and therefore all further analyses are combined across sex. Behavioral data (percent time spent freezing) was analyzed using a 3 (condition; fear conditioned, unpaired, tone alone) × 2 (age; PD 17 or 24) × 4 (test; habituation, context test, novel context, tone test; within subjects) mixed model MANOVA. A tone-difference score was calculated by subtracting percent freezing during the novel context from percent freezing during the tone, to determine the amount of freezing specifically attributable to the auditory cue and analyzed using a 3 (condition) × 2 (age) ANOVA. Follow-up tests examined the context fear and tone fear tests using 2 (age: PD 17 or 24) × 2 (condition: tone-alone or fear conditioned) ANOVAS with additional one-way ANOVAS when appropriate. FOS protein expression was analyzed using 2 (age: PD 17 vs. 23) × 2 (condition: fear conditioned vs. tone-alone) MANOVAs performed for each region analyzed (amygdala, hippocampus, perirhinal cortex, and hypothalamus) as well as Tukey’s post-hoc tests when appropriate. Fos and Egr1 mRNA levels in home cage, tone-alone and fear conditioned subjects after the fear acquisition training, context fear test or tone fear test was analyzed separately for each region of interest using 2(age) × 3 (condition) ANOVAs with Tukey’s post-hoc tests (see Table 2 for n values for each region per group). Specific effects of either age or condition on IEG expression was examined using one-way ANOVAs within each brain region to investigate the hypothesis that the neural circuitry underlying both auditory and contextual fear undergoes developmental changes during this period. For simplicity, due to the large number of comparisons, results from the one-way ANOVAs and subsequent post-hoc tests are represented in the figures, but not all of them are reported in the text. Alpha level was set at 0.05 for all statistical tests.
Table 2.
Number of samples analyzed via PCR per region in each group.
| Fear Acquisition | Fear Condition | Tone-Alone | Home Cage |
|---|---|---|---|
| PD 17 | Amyg: n=5; Hippo: n=5; Hypo: n=5; Per: n=5 | Amyg: n=5; Hippo: n=5; Hypo: n=5; Per: n=5 | Amyg: n=5; Hippo: n=5; Hypo: n=4; Per: n=4 |
| PD 24 | Amyg: n=5; Hippo: n=4; Hypo: n=5; Per: n=5 | Amyg: n=5; Hippo: n=4; Hypo: n=5; Per: n=5 | Amyg: n=4; Hippo: n=4; Hypo: n=5; Per: n=5 |
| Context Fear | |||
| PD 17 | Amyg: n=5; Hippo: n=5; Hypo: n=5; Per: n=5 | Amyg: n=5; Hippo: n=5; Hypo: n=4; Per: n=4 | Amyg: n=5; Hippo: n=4; Hypo: n=5; Per: n=5 |
| PD 24 | Amyg: n=5; Hippo: n=5; Hypo: n=5; Per: n=5 | Amyg: n=5; Hippo: n=5; Hypo: n=5; Per: n=5 | Amyg: n=4; Hippo: n=4; Hypo: n=4; Per: n=4 |
| Tone Fear test | |||
| PD 17 | Amyg: n=5; Hippo: n=5; Hypo: n=5; Per: n=5 | Amyg: n=5; Hippo: n=5; Hypo: n=5; Per: n=5 | Amyg: n=5; Hippo: n=5; Hypo: n=4; Per: n=5 |
| PD 24 | Amyg: n=4; Hippo: n=5; Hypo: n=4; Per: n=5 | Amyg: n=5; Hippo: n=5; Hypo: n=5; Per: n=4 | Amyg: n=5; Hippo: n=4; Hypo: n=5; Per: n=5 |
Results
Experiment 1: Fear conditioning and c-Fos IHC
Experiment 1A: Fear Conditioning with a 0.3 mA Shock
Three rats were excluded as statistical outliers from Experiment 1A (2 unpaired PD 23, 1 unpaired PD 17) for differing by more than 2.5 standard deviations from the mean (2 were above, 1 was below).
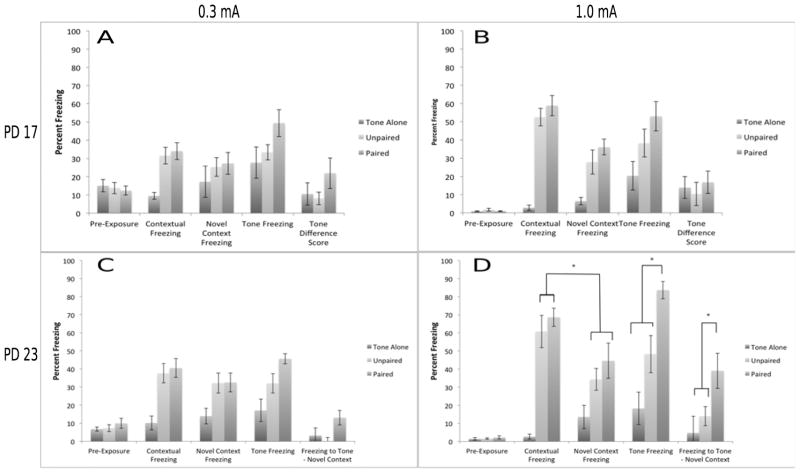
The data from this experiment is consistent with previous studies demonstrating that contextual conditioning develops between PD 17 and 23 (Figure 4). In contrast, freezing to the auditory cue failed to differentiate between the paired and control groups at both ages. A 2 (age: PD 17 vs 23) × 3 (Training Condition: paired, unpaired and tone alone) × 4 (Test Session: habituation, context freezing, novel context freezing, auditory cue freezing) omnibus mixed-model MANOVA revealed a main effect of condition F(2,55) = 15.86, p<.01, a main effect of test session F(3,53) = 72.12, p<.01, but not a main effect of age F(1,55) = 0.05, p>.10. The two-way interaction between test and age reached significance (p<.01), while the other interactions merely demonstrated a trend towards significance (ps = .07 and .08). Follow-up tests examining the paired groups across ages confirm that contextual freezing differs between PD 23 and 17 (p<.01), whereas auditory freezing does not (p>.10). Further analyses are separated by age.
Figure 4. Experiments 1A & 1B. Significant enhancement in both contextual and auditory fear conditioning between PD 17 and PD 23 subjects.
Fear conditioning at either PD 17 (A,C) or 23 (B,D) in Tone Alone, Unpaired and Paired groups, using a mild (0.3 mA; A,B) or strong (1.0 mA; C,D) unconditioned stimulus. Older subjects show greater contextual freezing, contextual discrimination and auditory freezing using two measures compared to younger animals. * = significant at p<.05
Context Freezing
We expected that both paired and unpaired conditioning would yield greater freezing to the context relative to the tone-alone groups and that this would be especially prevalent at PD 23 (e.g. Burman et al., 2014). At PD 17, this pattern was confirmed by an ANOVA comparing freezing to the conditioning context as a function of training condition F (2,31) = 7.65, p<.01 and Tukey’s HSD tests (ps <.05). However, freezing was not specific to the conditioning context, as a 3 (Training Condition) × 2 (Conditioning Context vs Novel Context) MANOVA failed to detect a significant difference, p>.10, precluding further analyses. At PD 23, the one-way ANOVA was also significant, F (2,28) = 45.67, p<.01, as were the Tukey’s HSD tests (ps <.05). Unlike in the younger animals, this freezing did appear to be specific to the conditioning context as the 3 × 2 ANOVA detected a significant interaction (p<.01). Indeed, post-hoc Tukey’s HSD tests confirm that freezing to the conditioning context is significantly greater than freezing to the novel context following both paired and unpaired training (ps<.05).
Tone Freezing
When measuring freezing to the auditory cue, we expected the paired groups to show greater freezing to the tone than either the unpaired or tone alone groups, but only at PD 23 (Burman et al., 2014). Indeed, at PD 17 a one-way ANOVA examining the effects of condition on freezing to the auditory cue failed to find a significant effect, F (2,31) = 1.84, p>.10. Furthermore, a 3 (condition) × 2 (novel context vs. tone freezing) mixed model ANOVA failed to produce a significant interaction (p>.10), suggesting that the tone failed to elicit greater freezing than the background context. Together these findings suggest that non-associative processes can account for any freezing observed in these subjects. Surprisingly, in the older subjects, the one-way ANOVA examining the effects of condition on freezing to the auditory cue also failed to find a significant effect F (2,28) = 2.19, p>.10 as did the follow-up analyses. Similarly, a 3 (condition) × 2 (age) ANOVA on tone difference scores fails to find any significant effects. These highly unexpected results suggest that non-associative processes can account for any freezing observed to the tone in these subjects. One possible explanation is that a 0.3 mA shock was simply too weak to observe meaningful conditioning to an explicit cue in this experiment.
Experiment 1B: Fear Conditioning with a 1.0 mA Shock
In contrast to conditioning with a weak shock, the results from this experiment suggest that both contextual and auditory conditioning continue to emerge between PD 17 and 23, similar to our previous results (Burman et al., 2014). A 2 (age) × 3 (Training Condition) × 4 (Test Session) mixed-model MANOVA revealed a main effect of condition F(2,47) = 51.88, p<.01, a main effect of test session F(3,45) = 110.27, p<.01 and a main effect of age F(1,47) = 9.51, p<.01. All interactions were statistically significant (ps<.01). Follow-up tests found significant differences between paired subjects at PD 17 and 23 in both freezing to the context and the auditory cue (ps<.01), confirming that both tasks are continuing to develop during this period.
Context Freezing
One-way ANOVAs examined freezing to the conditioning context as a function of training condition. For PD 17 subjects, the ANOVA F(2,28) = 13.27, p<.01 and Tukey’s HSD tests (ps <.05) confirmed that conditioning produced greater freezing to the context than the tone alone. However, as with the weaker shock, freezing was not specific to the conditioning context, as a 3 (Training Condition) × 2 (Conditioning Context vs Novel Context) ANOVA failed to detect a significant interaction, p>.10, precluding further analyses. For PD 23 subjects, a similar pattern was found in the ANOVA, F(2,23) = 47.30, p<.01 and Tukey’s HSD tests (ps <.05). However, this freezing did appear to be specific to the conditioning context, as the 3 × 2 ANOVA detected a significant interaction, p=.01 and a planned contrast between context test and novel context freezing yielded a significant difference (p<.01)..
Tone Freezing
We expected that freezing to the auditory cue would be greater in the subjects receiving paired conditioning as opposed to the control groups, especially in the older subjects. A one-way ANOVA examining freezing to the auditory cue as a function of training condition in PD 17 subjects was significant F (2,28) = 8.38, p<.01. However, the Tukey’s HSD tests offered only partial support for specific freezing due to paired conditioning. Freezing in the paired group was significantly higher than the tone-alone group (p<.05), but not in the unpaired group (p>.10). If conditioning were successful, paired subjects, but not control subjects, would show greater freezing to the tone than the novel context. Indeed, a 3 (condition) × 2 (novel context vs. tone freezing) mixed model ANOVA produced a significant interaction F(2, 26) = 4.16, p<.05. However, posthoc tests show that freezing to the tone does not significantly differ from novel context freezing in any training condition. As with the weaker shock level, these findings suggest that non-associative processes may account for the freezing observed in the PD 17 subjects.
For PD 23 subjects, an ANOVA examining freezing to the auditory cue as a function of training condition found a significant effect F(2,23) = 16.44, p<.01. Tukey’s HSD tests found all groups significantly differed from one another. Again, we expected that paired subjects, but not control subjects, would show greater freezing to the tone than the novel context. Indeed, a 3 (condition) × 2 (novel context vs. tone freezing) mixed model ANOVA produced a significant interaction, p<.05. In this case, Tukey’s HSD post-hoc tests confirm that only the paired group shows significantly greater freezing to the tone compared to the novel context.
A 3 (condition) × 2 (age) ANOVA on tone difference scores finds significant effects of age F(1,47) = 7.61, p<.01 and condition F(2,47) = 7.74, p<.01, but no interaction. However, follow-up tests demonstrate that the PD 23 fear conditioned group differences from all others (p<.05), which do not differ from each other. As a whole, we can conclude that it is only with the older animals and the stronger shock level that we see specific associative freezing to the tone.
Experiment 1C: FOS Immunohistochemistry
FOS protein expression was examined one hour after fear acquisition with a 1.0 mA shock. FOS-like immunoreactivity was quantified in the basal amygdala, dorsal hippocampus CA-1, perirhinal cortex, and lateral hypothalamus. To address the hypothesis that immediate-early gene expression will differ between older, but not younger, conditioned and control subjects, a series of 2 (age: PD 17, PD 23) × 2 (condition: fear conditioned, tone-alone) ANOVAs were used (Figure 5).
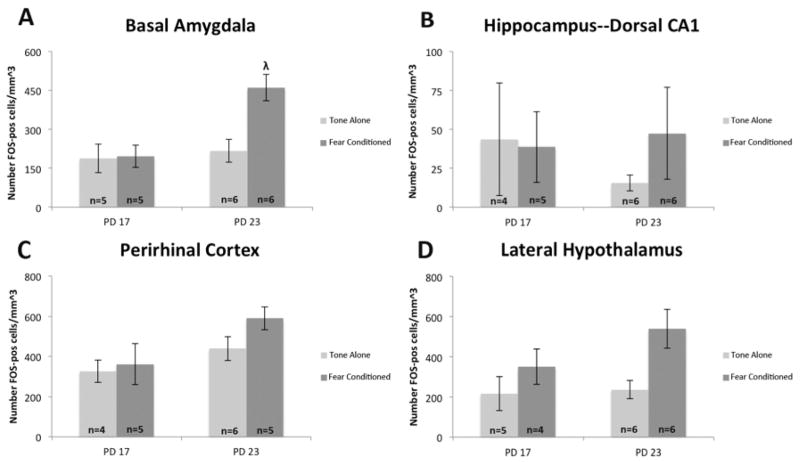
Figure 5. Significant increase in basal amygdala FOS-like immunoreactive cells in fear conditioned PD 23 subjects compared to control.
FOS-like immunoreactive cell expression in fear conditioned and tone-alone control subjects in the basal amygdala, dorsal hippocampus CA1, perirhinal cortex, and lateral hypothalamus. The number of FOS-like positive cells per mm^3 of the basal amygdala was significantly higher after fear conditioning in PD 23 subjects though not PD 17 using a strong US. The lateral hypothalamus showed significantly higher FOS-like positive cell expression after fear conditioning regardless of age. n=22; Different from tone-alone: λ = p<.05
Consistent with our hypothesis, data from the basal amygdala showed a clear increase in the expression of FOS-like immunoreactive cells after fear conditioning in older subjects, but not younger ones (Fig. 4A). Analysis showed a main effect of age F(1,18)=9.219, p<.05 and condition F(1,18)=6.760, p<.05 and an interaction between the two F(1,18)=5.924, p<.05 on the number of FOS-like cells estimated in the basal amygdala. A Tukey’s post-hoc test demonstrated that fear conditioned PD 23 subjects had significantly higher FOS-like cell expression than the control subjects whereas there was no difference between the FOS-like cell levels for either PD 17 condition. These data suggest that fear conditioning at PD 23 in rats, but not at PD 17, produces an increase in the production of FOS protein in the basal amygdala.
Data collected from the perirhinal cortex demonstrated a numerically similar pattern as seen in the basal amygdala, showing a greater increase following fear conditioning in the expression of FOS-like cells in the older subjects when compared to the younger ones (Fig. 4B). However, analysis showed an effect of age F(1,16)=5.536, p<.05, but not condition F(1,16)=1.649, p>.10 and no interaction F(1,16)=0.634, p>.10. A Tukey’s post-hoc test showed that there were significantly more FOS-like cells estimated in the older subjects compared to the younger ones. These data suggest that during this developmental period, age, but not fear conditioning, is a factor in c-FOS production.
Results from the dorsal CA1 region of the hippocampus showed low levels of FOS-like cell expression in all conditions while also trending towards a pattern similar to the basal amygdala (Fig. 4C). However, analysis of this region showed no effects of age F(1,17)=0.150, p>.05 or condition F(1,17)=0.299, p>.05 and no interaction F(1,17)=0.559, p>.05 on the expression of FOS-like immunoreactive cells. Due to the high variance, interpretation of these results is difficult. We suspect that we are sampling a heterogeneous region that bridges the dorsal and intermediate regions (Burman, Starr, & Gewirtz, 2006).
Data collected from the lateral hypothalamus showed increased FOS-like cell expression at both age groups after fear conditioning (Fig. 4D). Analysis of the lateral hypothalamus showed a main effect of condition F(1,17)=7.142, p<.05, but not age F(1,17)=1.632, p>.05 and no interaction F(1,17)=1.086, p>.05, on the estimated number of FOS-like cells. These data suggest that fear conditioning increases activity in the lateral hypothalamus regardless of age during this developmental period, and confirms that shock can activate FOS expression at PD 17.
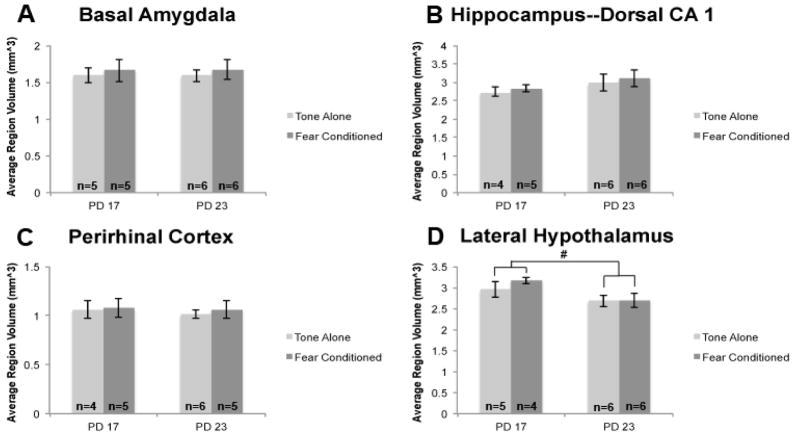
Volume data collected via unbiased stereology was also collected and analyzed (Figure 6). Significant volume change was only observed in the lateral hypothalamus between PD 17 to 23 (F(1,17)=5.457, p<.05), which was, somewhat surprisingly, a decrease. Basal amygdala, dorsal hippocampus CA1, and perirhinal cortex did not change between PD 17 and 23 F(1,18)=0.0005, p>.10; F(1,17)=2.534, p>.10; F(1,16)=0.1862, p>.10 respectively). As expected, condition had no effect at any age and there were no interactions in the 4 regions quantified.
Figure 6. Lateral hypothalamus volume decreases significantly between PD 17 to 23 subjects.
Total region volume (mm^3) was estimated for the basal amygdala, dorsal hippocampus CA1, perirhinal cortex, and lateral hypothalamus via unbiased stereology. Volume was found to decrease significantly in the lateral hypothalamus between PD 17 and 23. No other region volumes changed significantly during this time period. n=22
Experiment 2: Fear Conditioning and qPCR
Experiment 2A: qPCR following Fear Acquisition
Behavioral Data
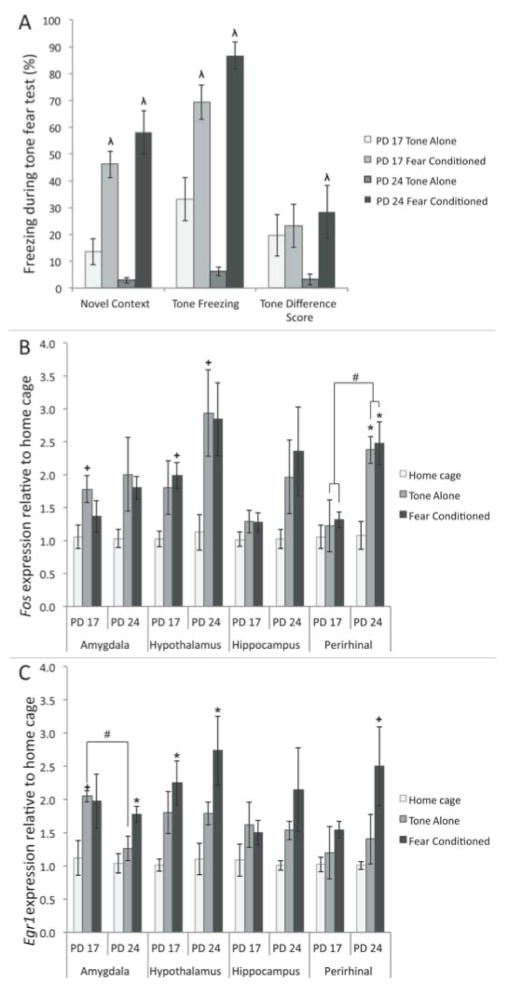
Behavioral data from the conditioning session was collected and analyzed during the acquisition of the CS, reported in Figure 7. CS acquisition freezing data were analyzed using a 2 (age: PD 17, PD 24) × 2 (condition: tone-alone, fear conditioned) ANOVA (Fig 6A). These data show an expected main effect of condition F(1,16)=11.717, p<.05, and a significant interaction F(1,16)=16.167, p<.05. Fear conditioned older subjects were found to freeze more than older tone-alone subjects, while there was no difference in the younger subjects. Additionally, fear conditioned subjects froze more than tone-alone subjects, though this was driven mainly by the large difference between the older subjects. Younger subjects appear to freeze equally in the tone-alone and fear conditioning groups, suggesting that the novelty and tone were anxiogenic at this age.
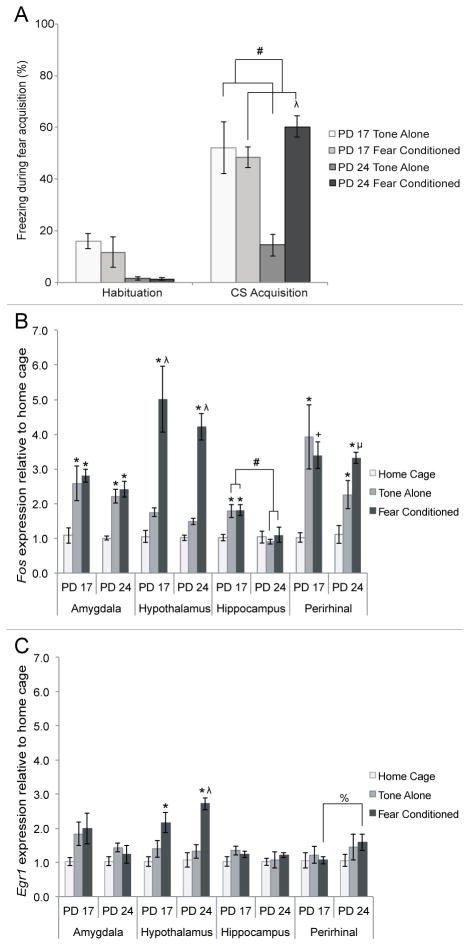
Figure 7. Behavioral and IEG mRNA concentration at PD 17 or PD 24 following fear conditioning (fear conditioned), exposure to tone alone (tone-alone) or no conditioning (home cage).
A) Amount of freezing behavior measured during habituation and while subjects were exposed to the tone during fear acquisition. Older, not younger, subjects exposed to both the tone and shock exhibited higher amounts of freezing compared to those exposed to just the tone. Additionally, tone-alone subjects were found to freeze less than fear conditioned ones. B) Fos mRNA levels in the amygdala, hypothalamus, hippocampus and perirhinal cortex following fear acquisition. One-way ANOVAs for age and condition showed significant differences due to condition in all four regions, and a significant difference due to age in the hippocampus. C) Egr1 levels in the amygdala, hypothalamus, hippocampus and perirhinal cortex following fear acquisition. One-way ANOVAs for age and condition revealed a significant difference due to condition in the hypothalamus of fear conditioned subjects only. Different from home cage: * = p<.05, + = p<.10; Different from tone-alone: λ = p<.05, μ = p<.10; All other differences: # = p<.05, % = p<.10
IEG Expression
To investigate our immediate-early gene-expression hypothesis, we used a series of 2 (age: PD 17, PD 24) × 3 (condition: home cage, tone-alone, fear conditioned) ANOVAs on both relative Fos and Egr1 mRNA levels following fear conditioning (Figure 7). Fos expression in perirhinal cortex appears to most closely predict freezing levels. This is confirmed by analysis. In both the amygdala and perirhinal cortex, there was a main effect of condition on Fos concentration, F(2,23)=18.023 and F(2,23)=13.117 respectively, ps<.05. There were no effects of age and no interactions between the two factors. Further analysis using Tukey’s post-hoc tests revealed that in both of these structures relative Fos concentration is elevated in both the fear conditioned and tone-alone conditions compared to the home-cage condition. The fear conditioned and tone-alone subjects do not differ from each other. The one exception is that PD 24 fear conditioned subjects trended towards a difference from tone-alone subjects in the perirhinal cortex. This suggests that the handling and novelty components of the task, rather than tone-shock pairings, are enough to induce Fos expression in both of these structures. Neither age nor conditioning status significantly affected Egr1 concentration in the amygdala or perirhinal cortex.
In the hypothalamus, there was a significant main effect of condition on both Fos and Egr1 levels F(2,23)=37.420 and F(2,22)=19.879 respectively, ps<.05. Tukey’s post-hoc tests show that in this region, relative Fos and Egr1 concentration was greater in the fear conditioned subjects than either the tone-alone or home-cage groups (Figure 7), suggesting exposure to footshock specifically activates IEG expression. Age did not significantly affect Fos or Egr1 levels in the hypothalamus.
In the hippocampus, there was a significant main effect of condition on Fos expression F(2,21)=3.850, p<.05, as well as a main effect of age, F(1,21)=16.315, p<.05, and a significant interaction, F(2,21)=4.554, p<.05. Counter to our hypothesis, one-way ANOVAs showed that PD 17 tone-alone and fear conditioned subjects had greater Fos levels than their PD 24 counterparts (ps>.05; Figure 7A). Neither age nor condition significantly affected Egr1 concentration in the hippocampus.
Experiment 2B: qPCR following Context Freezing
Behavioral Data
Subjects in this study were returned to the original conditioning chamber 24 hours after fear conditioning for a five minute test of contextual freezing. The hypothesis that PD 24 fear conditioned subjects would display the greatest amount of contextual fear was tested using a 2 (age: PD 17, PD 24) × 2 (condition: tone-alone, fear conditioned) ANOVA on freezing to the context. This analysis revealed significant main effects of age, F(1,16)=17.281, p<.05, and condition, F(1,16)=235.793, p<.05, as well as a significant interaction of age and condition, F(1,16)=25.605, p<.05, as seen in Figure 8. To analyze the interaction, one-way ANOVAs were used to further examine the effects of age on contextual freezing between the fear conditioned and tone-alone groups. Consistent with our hypothesis, there was a significant main effect of age, F(1,8)=22.115, p<.05, on contextual freezing in the fear conditioned group, with PD 24 subjects freezing more than PD 17 subjects. As expected, there was no effect of age on contextual freezing in the tone-alone condition.
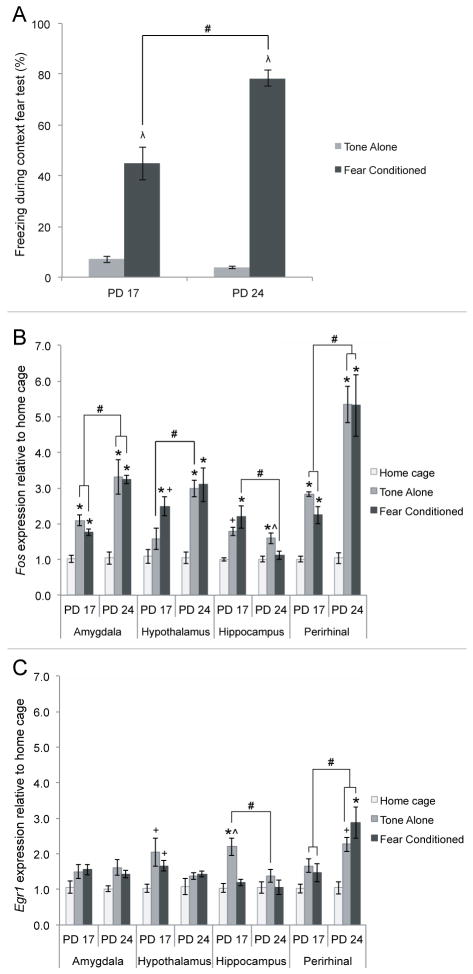
Figure 8. Behavioral and IEG mRNA concentration results following the context fear test.
A) Behavioral findings from the context fear test show that PD 24 fear conditioned subjects froze significantly more in the context than PD 17 fear conditioned subjects. B) Fos mRNA levels in the amygdala, hypothalamus, hippocampus and perirhinal cortex following the context fear test in PD 17 and PD 24 subjects. One-way ANOVAs for condition and age reveal effects of context exposure on Fos levels in all four regions, while age differences were found in all regions except the hypothalamus. C) Egr1 mRNA concentration in the amygdala, hypothalamus, hippocampus and perirhinal cortex following the context fear test. One-way ANOVAs for condition and age reveal significant effects of condition in the PD 17 hypothalamus and hippocampus, and the PD 24 perirhinal cortex. Effects of age were found in the tone-alone condition in the hippocampus and both tone-alone and fear conditioned groups in the perirhinal cortex. Different from home cage: * = p<.05, + = p<.10; Different from tone-alone: λ = p<.05; Different from fear conditioned: ^ = p<.05; All other differences: # = p<.05
IEG Expression
To assess the effects of age and condition on IEG mRNA levels in the amygdala, hypothalamus, hippocampus and perirhinal cortex following a context fear test, we used a series of 2(age) × 3 (condition) ANOVAs for both Fos and Egr1 concentrations in each region of interest. Here, Egr1 levels in the perirhinal cortex appear to most closely predict freezing levels. The analysis shows that in the amygdala and perirhinal cortex, there were main effects of age F(1,23)=22.363 and F(1,22)=24.627, ps<.05 and condition F(2,23)=29.210 and F(2,22)=26.092, ps<.05, as well as significant age × condition interactions F(2,23)=5.329 and F(2,22)=6.122, ps<0.5 for relative Fos concentration. The age × condition interactions were explored with one-way ANOVAS and post-hoc tests as seen in Figure 8B. These demonstrate that in both the amygdala and perirhinal cortex, fear conditioned and tone-alone groups had significantly greater relative Fos levels than the home cage condition, but did not differ from each other. Moreover, PD 24 subjects in these conditions had greater relative Fos expression than PD 17 subjects, suggesting that the amygdala and perirhinal cortex are both developing during this period.
There was also a main effect of condition on relative Egr1 expression in the amygdala, F(2,23)=5.656, p<.05, and perirhinal cortex, F(2,21)=9.410, p<.05. However, post-hoc tests on the effect of condition on Egr1 concentration showed that only the tone-alone condition trended towards significance from home cage controls in the amygdala. This should be treated with caution, as this effect was lost when examining each age separately. In the perirhinal cortex, post-hoc tests showed that the main effect of condition was primarily driven by the PD 24 fear conditioned group. There was no significant difference between conditions in Egr1 expression in PD 17 subjects. In contrast, at PD 24 the relative Egr1 concentration was significantly increased only in the fear conditioned group, while the tone-alone condition merely trended towards a significant difference from home cage controls. Additionally, there was also a main effect of age on Egr1 in the perirhinal cortex, F(2,21)=11.009, p<.05, with PD 24 subjects showing greater relative Egr1 expression than PD 17 subjects.
In the hippocampus, there were significant main effects of condition, F(2,22)=8.830, p<.05, and age, F(1,22)=8.660, p<.05, on Fos concentration following the context fear test, as well as a significant interaction between age and condition, F(2,22)=5.568, p<.05. To evaluate the interaction, follow-up one-way ANOVAs revealed that PD 17 fear conditioned subjects had greater Fos levels than PD 24 fear conditioned subjects (Figure 8B). There were also significant main effects of condition, F(2,24)=10.847, p<.05, and age, F(1,24)=4.921, p<.05, on Egr1 concentration in the hippocampus following the context fear test, but no significant interaction (Figure 8C). Tukey’s post-hoc testing showed that, similar to PD 24 Fos levels, the tone-alone condition had a greater Egr1 concentration than both the fear conditioned and home cage groups at PD 17. The effect of age revealed that PD 17 subjects overall had elevated Egr1 levels in the hippocampus compared to PD 24 subjects, though this age effect was driven almost entirely by the increase in PD 17 tone-alone Egr1 levels.
Finally, in the hypothalamus, there were main effects of condition, F(2,22)=17.939, p<.05, and age, F(1,22)=7.369, p<.05, on Fos concentration, as well as a main effect of condition on Egr1 concentration, F(2,21)=5.658, p<.05, and no interaction between age and condition for either IEG mRNA. For Fos concentration, Tukey’s post-hoc tests showed that subjects in the fear conditioned and tone-alone groups had significantly elevated Fos compared to home cage subjects but did not differ from each other at PD 24, while at PD 17 relative Fos expression was significantly increased only in the fear conditioned subjects. Additionally, PD 24 subjects had significantly greater Fos levels than PD 17 subjects in the tone-alone condition. Post-hoc analysis of the effect of condition on Egr1 concentration revealed that both the fear conditioned and tone-alone groups trended towards significantly more Egr1 compared to the home cage condition at PD 17, but not at PD 24.
Experiment 2C: qPCR on Auditory Freezing
Behavioral Data
Subjects in our third study were placed in a novel conditioning chamber 24 hours after the initial fear conditioning and re-exposed to the 70 dB tone to test auditory fear conditioning. The hypothesis that PD 24 fear conditioned subjects would show greater fear than PD 17 subjects was tested with a 2 (age) × 2 (condition (fear conditioning vs. tone alone) ANOVA for novel context freezing, tone freezing, and tone difference score, calculated from novel context and tone freezing values. There were significant main effects of condition on novel context freezing, F(1,16)=68.16, p<.05, and tone freezing, F(1,16)=100.458, p<.05, as well as a trend towards significance for tone difference score, F(1,16)=3.701, p=.072. Given the small sample size, this lack of power should not be surprising. There was also a significant condition × age interaction for novel context freezing, F(1,16)=4.564, p<.05, and tone freezing, F(1,16)=14.49, p<.05. As seen in Figure 9A, each age was then analyzed separately for effects of condition on novel context freezing, tone freezing and tone-difference score. This revealed a significant effect of condition on novel context freezing and tone-freezing at both ages (ps<.05), suggesting that subjects at both ages show some enhanced freezing as a result of the shock. The effect of condition on tone-difference score was significant only at PD 24, p<.05.
Figure 9. Behavioral and IEG expression results following the tone fear test in PD 17 and PD 24 subjects.
A) Behavioral results from the tone fear test show that tone freezing differs significantly by age and condition. Tone freezing differs significantly between PD 17-PD 24 tone-alone subjects and trends towards significance (p<.07) between PD 17-PD 24 fear conditioned subjects. B) Fos expression in the amygdala, hypothalamus, hippocampus and perirhinal cortex following the tone fear test. One-way ANOVAs for age and condition reveal a significant effect of age in the PD 24 hypothalamus and perirhinal cortex, and a significant effect of age in the perirhinal cortex. C) Egr1 expression in the amygdala, hypothalamus, hippocampus and perirhinal cortex following the tone fear test. One-way ANOVAs for age and condition reveal a significant effect of age in the amygdala of tone-alone subjects, as well as significant effects of condition in the hypothalamus at both ages and the amygdala and perirhinal cortex at PD 24. Different from home cage: * = p<.05, + = p<.10; Different from tone-alone: λ = p<.05; All other differences: # = p<.05
IEG Expression
We tested the hypothesis that IEG mRNA concentration in the auditory fear conditioning circuit would shift between PD 17–24 using a 2 (age) × 3 (condition) ANOVAs for Fos and Egr1 concentrations in each region of interest (Figure 9). Indeed, egr1 levels in the amygdala appear to closely match levels of freezing. In the amygdala, there was a main effect of condition on Fos levels, F(2,23)=4.395, p<.05. Tukey’s post-hoc testing revealed that the tone-alone condition trended towards a greater relative Fos concentration than the home cage condition, while the fear conditioned subjects did not differ from home cage or tone-alone (Figure 9B). This suggests that Fos mRNA in the amygdala is not activated by the presentation of a fear-inducing tone any more than by a neutral tone. There was also a main effect of condition on Egr1 levels in the amygdala, F(2,23)=6.125, p<.05. Interestingly, in the amygdala only the PD 24 fear conditioned subjects displayed significantly greater Egr1 concentration than the home-cage subjects, consistent with the observed developmental changes in freezing expression. Tukey’s post-hoc tests also showed significantly more Egr1 in PD 17 tone-alone subjects compared to the PD 24 tone-alone subjects, consistent with the observed freezing.
In the hypothalamus and perirhinal cortex, there were main effects of condition on both Fos F(2,22)=6.391 and F(2,23)=6.158, p<.05 and Egr1 levels F(2,22)=10.958 and F(2,23)=4.951, p<.05, although Tukey’s post-hoc tests showed that these effects differed between the two genes. For Fos, both the fear conditioned and tone-alone groups in the perirhinal cortex had greater Fos than the home cage subjects at PD 24, but not at PD 17. However, for Egr1, there was a trend towards a greater Egr1 concentration in the fear-conditioned subjects compared the home-cage controls, but only at PD 24, and not the tone-alone groups,. There was no significant change in Egr1 levels across groups at PD 17. Additionally, there was a main effect of age on Fos concentration in the perirhinal cortex, F(1,23)=13.102, p<.05, where PD 24 tone-alone and fear conditioned subjects had a significantly greater relative Egr1 concentration than PD 17 tone-alone and fear conditioned subjects (Figure 9C). There were no other significant effects of age in any region and no significant effects of condition or interactions in the hippocampus.
Discussion
This paper confirms that there are dramatic changes in the ability of rats to acquire conditioned fear between PD 17 and 24 and begins to shed light on the underlying neurological development that underlies the emergence of fear conditioning. First, fear conditioning to both the context and auditory cue continues to develop between PD 17 and 24, consistent with our previously published findings (Burman et al., 2014). Second, alterations in immediate early gene expression in various limbic system regions in response to fear conditioning or expression, most notably in the amygdala and perirhinal cortex, are consistent with these behavioral changes. Therefore, the current data contribute to our understanding that changes in the amygdala and associated circuitry underlie the emergence of classical fear conditioning.
The current findings that rats at both PD 17 and PD 24 demonstrate enhanced freezing when re-exposed to the conditioning context, but older subjects exhibit greater contextual freezing than the younger subjects, is consistent with the majority of published work in this area (e.g. Rudy, 1993; Raineki et al., 2010; Stanton 2000). The older subjects also show greater contextual discrimination than the younger subjects, consistent with other work on generalization during development (i.e. Brown & Stanton, 2008). What is perhaps less commonly reported is that that older subjects also displayed enhanced auditory cue-induced freezing relative to the pre-weaning subjects, although this is a routine finding in our hands (Burman et al., 2014). Other published work does also suggest that PD 17 subjects condition less well than older counterparts, as they require additional trials to reach similar levels of freezing (Kim & Richardson, 2007, 2008) or show significantly reduced freezing (Rudy & Morledge, 1994). Although there are many possible reasons for these differences, we suspect that pre- and neonatal husbandry factors may play a role. We use timed pregnant rats. Stress during shipping, combined with strain differences, may alter the developmental time course slightly. The current data also show that other parameters, such as shock level, may be critical. Nevertheless, these experiments clearly demonstrate that the emergence of fear conditioning in general may depend upon changes in amygdala and cortical circuitry. This is likely true regardless of the specific age at which the behavioral emergence occurs.
In the current paper, we have chosen to assess auditory fear conditioning by examining both the percentage of time spent freezing during the tone and by constructing a difference score between freezing to the tone and the novel context (Jacobs, Cushman, & Fanselow, 2010). Although the difference score would appear to be the preferred measure, as it attempts to account for any baseline freezing levels, it remains somewhat unclear which is the best method to accurately assess auditory conditioning levels. It is worth noting that Jacobs et al. (2010) demonstrated that manipulations that might be expected to reduce auditory conditioning levels do indeed affect difference scores, while leaving other freezing measures unaffected.
For the IEG expression studies, we chose to use tone alone, rather than unpaired, control groups. Critically, unpaired subjects show substantial contextual learning, which depends upon the amygdala. Thus, the “unpaired” subjects are not a “no learning” control. The fear conditioning they acquire would be expected to cause amygdala, hypothalamus and hippocampus activation. To achieve a control group that has similar stress and procedural variable, but no fear learning, these experiments used a “tone alone” group as the critical comparison group. This group would be expected to have low levels of stimulus-induced amygdala, hypothalamus and hippocampus activation.
We observe high levels of novel context freezing, especially in Experiment 2. This suggests that the additional context test in Experiment 1 may help extinguish contextual fear, a finding also suggested by the importance of the order of testing observed in our previous work (Burman et al., 2011; unpublished observations). It is also important to note that we observe very little difference in freezing to the novel context and conditioning context at PD 17, whereas we do see significant differences after weaning. Thus, the high levels of novel context freezing are also likely caused by an age-dependent relative inability to distinguish between conditioning contexts.
That the emergence of fear conditioning depends upon the amygdala is not surprising. Here we observe that only PD 23, and not PD 17, subjects show enhanced FOS protein expression during fear conditioning, consistent with our behavioral results. Although our qPCR results show that Fos mRNA levels do not predict conditioning, Egr1 levels in the amygdala are a strong predictor of freezing during the auditory fear test. Together, these findings suggest that novelty or stress may be sufficient to prepare the subject for learning by activating transcription of Fos, but only exposure to the fear conditioning procedure itself regulates FOS activity in the amygdala at the translational level during learning. Moreover, the signaling mechanisms underlying the acquisition and expression of auditory fear may be different, at least at these ages.
Egr1 mRNA concentration is often increased in the amygdala following both fear conditioning and expression compared to controls (Rosen et al., 1998; Asok, Schreiber, Jablonski, Rosen, & Stanton, 2013; Schreiber et al., 2014; Malkani & Rosen, 2000; Hall, Thomas, & Everitt, 2001), an effect not seen during fear acquisition in the current study. However, given that our subjects appear to be the youngest subjects yet examined for mRNA expression in auditory and contextual fear conditioning, (Asok et al., 2013; Hall et al., 2001; Malkani & Rosen, 2000; Rosen et al., 1998; Schreiber et al., 2014), we believe that most differences can be attributed to developmental stage. Indeed, Schreiber et al. (2014) found an increased Egr1 expression in the amygdala during fear acquisition at PD 31 using a modified protocol called “the context pre-exposure facilitation effect”. However, similar to the current data, they showed no change in Egr1 expression following standard fear conditioning when comparing shock-exposed and context-exposed subjects.
FOS protein expression results seen in the current experiments are also similar to previous literature demonstrating an increase in FOS protein expression in the central and lateral amygdala following fear acquisition (Holahan & White, 2004) and the ventral region of the dorsal nucleus of the lateral amygdala following aversive conditioning (Radwanska, Nikolaev, Knapska, & Kaczmarek, 2002), as well as others examining fear conditioning in the adult rat (Beck & Fibiger, 1995; Radwanska, Nikolaev, Knapska, & Kaczmarek, 2002; Fujisaki, Hashimoto, Iyo, & Chiba, 2004). Wiedenmayer and Barr (2001) observed changes in FOS expression in multiple nuclei that included the lateral and medial amygdala, though no change in the basal amygdala, between PD 7, 14, and 21 when measuring the immobility of young rats in response to adult male rat exposure. Interestingly, the lateral amygdala showed increased FOS at PD 14 and the medial showed increased FOS expression at PD 21. These findings suggest a pattern of FOS expression specific to age in response to a fear expression.
That the amygdala undergoes critical development during this period is well established in the literature (Chareyron et al., 2012; Verwer, Van Vulpen, & Van Uum, 1996; Bouwmeester, Smits, & Van Ree, 2002; Bouwmeester, Wolterink, & Van Ree, 2002). For example, Chareyron, Lavenex, and Lavenex (2012) conducted a stereology study examining the number of neurons and size of the amygdala over development. They found that while the number of neurons in the amygdala did not change after birth, volume increased during the first 3 weeks of development. Following the 3rd week however, and consistent with our findings, there was no change in volume of the lateral, basal, and accessory basal nuclei of the amygdala. Astrocyte and oligodendrocyte numbers in the basal, lateral, central, medial, and accessory basal nuclei continued to change at least through the first 7 months of postnatal development. Functional changes also occur during this period. Recent work suggests that GABA-ergic transmission, despite the earlier presence of receptors, is continuing to emerge until at least PD 28 in mice (Bosch & Ehrlich, 2015). Chen, Shemyakin, and Wiedenmayer (2006) found that PD14 rat pups froze more than PD18 subjects when exposed to an unfamiliar adult male rat and demonstrated that this was due to changes in the medial and lateral amygdala nuclei and the processing of olfactory information by the amygdala. Our data support the conclusion that the activation of the amygdala during fear conditioning is also continuing to change during the 3rd or 4th weeks of life, at least in the basal nucleus of the amygdala.
The perirhinal cortex also appears to be undergoing significant changes in its response to fear expression as a function of age. Differential expression patterns of Fos and Egr1 mRNA during fear acquisition and recall observed in the older subjects, consistent with the changes in behavior. This demonstrates that this region is undergoing development related to fear conditioning during this period. Changes in cortical layer morphology have also been observed over a similar time period (Furtak, Moyer, & Brown, 2007). The role of the perirhinal cortex in fear conditioning is complex, although it has been implicated using IEG expression studies (Beck & Fibiger, 1995; Albrechet-Souza, Borelli, Almada, & Brandão, 2011; VanElzakker, Fevurly, Breindel, & Spencer, 2008; Schettino & Otto, 2001). Through the use of lesion studies, it is has been suggested that the role of the perirhinal cortex in fear conditioning is critical for contextual memory, but not cued fear conditioning (VanElzakker et al., 2008; Bucci, Phillips, & Burwell, 2000; Albrechet-Souza et al., 2011). Conversely, other studies that do suggest the perirhinal cortex is involved in conditioned fear to auditory and visual cues (Rosen et al., 1992; Campeau & Davis, 1995; Corodimas & LeDoux, 1995; Suzuki, 1996). Supporting this literature, the specific Fos and Egr1 increases that we see in the older conditioned subjects compared to home-cage controls indicates that there the perirhinal cortex is likely a critical site of development for contextual and auditory cue fear retrieval during this period.
A surprising aspect of our study is that we do not see greater developmental changes in IEG expression in the hippocampus, due to the hypothesized role of the hippocampus in the fear circuit and specifically contextual fear (Phillips & LeDoux, 1992; Rudy, Barrientos, & O’Reilly, 2002; Sanders, Wiltgen, & Fanselow, 2003; Fendt & Fanselow, 1999). We found no significant differences in the expression of FOS protein in the dorsal CA1 region of the hippocampus following fear acquisition. In addition, younger subjects exposed to both the tone-alone and fear conditioning procedures showed more Fos mRNA following fear acquisition and contextual fear testing than older subjects. With one exception (PD 24 tone-alone subjects following contextual fear testing), the PD 24 subjects did not show increased IEG expression in the dorsal CA1 region of the hippocampus. This is contrary to previous published observations of an increased involvement of the hippocampus in fear conditioning in rats. Raineki et al. (2010) found increased FOS protein expression in the CA1, CA3, and dentate gyrus (DG) in PD24, but not PD 21, rats following fear conditioning. It should be noted that odor was used as the explicit cue by Raineki et al. compared to the tone used in the current experiments. It is possible that this procedural difference elicited a greater increase in FOS production in these hippocampal regions given the early onset of the olfactory circuit in the rats (Moriceau, Roth, Okotoghaide, & Sullivan, 2004). In contrast, similar to the current data, Beck and Fibiger (1995) did not see a change in dorsal CA1 FOS protein expression following contextual fear testing. The specific task, gene measured and age of the subject are also likely important factors. Egr1 levels in CA1 of the dorsal hippocampus are enhanced following fear conditioning at PD 31 (Schreiber et al., 2014) and contextual fear expression in adults (Hall et al., 2001). Similarly, Asok et al. (2013) found that Egr1 expression in the CA1 region of the dorsal hippocampus may have been driven by exposure to a particular context, rather than contextual fear conditioning.
A predicted outcome of the current study was the increased IEG expression due to fear conditioning at both ages on in the lateral hypothalamus, a region involved in endocrine activity and an output region of the fear circuit via projections from the central nucleus of the amygdala (Maren, 2001; Fendt & Fanselow, 1999; LeDoux, Iwata, Cicchetti, & Reis, 1988). IEG expression at both the transcriptional and translational levels was increased specifically in the fear conditioned subjects regardless of age following fear acquisition. Auditory fear expression was also accompanied by an increase in Egr1 at both ages specifically to the tone following tone fear testing. The increased expression observed is supported by Beck and Fibiger (1995) who saw an increase in FOS protein expression in the lateral hypothalamus following contextual fear testing. This is consistent with a role of the hypothalamus in the autonomic (but not behavioral) response to fear (LeDoux et al., 1988). Our findings suggest that the activity of the lateral hypothalamus in the fear circuit is established prior to PD17.
It is important to note that despite the functional development occurring during this period, the volumes of our regions of interest did not significantly increase with age. This lack of change in region volume is similar to that reported elsewhere in the literature. Chareyron et al. (2012) found that multiple nuclei of the amygdala, including the lateral, basal, and accessory basal, increase in size until the 3rd week of life and then maintains a consistent volume until around 7 months of age before continuing to increase in volume. Similarly, in the perirhinal cortex, Furtak, Moyer, and Brown (2007) found rapid increases in neuron populations in multiple layers of perirhinal cortex through PD 10–12 followed by a relatively stagnant periweaning period and a decrease in layer 5 neurons between PD 17 and 21. The decrease in volume observed in the lateral hypothalamus was surprising to us, although Schwarz, Sholar, and Bilbo (2012) found that the paraventricular nucleus of the hypothalamus also decreases in size from PD 4 to PD 30. Schwarz et al. also report a decrease in the CA1 of the hippocampus during that same time period. However, it should be noted that the present study looked specifically at dorsal CA1 whereas Schwarz et al. measured the entire CA1. Importantly, this relatively static period in volumetric development makes it easier to compare across ages in our qPCR experiments, which rely on the use of fixed volume tissue punches.
Overall Conclusion
This paper tested the hypothesis that critical development for the emergence of fear conditioning is occurring in the amygdala or the cortico-amygdala circuitry between PD 17 and 24. We find evidence using IEG expression to support this hypothesis in the basal nucleus of the amygdala and the perirhinal cortex. Thus, while confirming that the transition from infancy to early childhood is a critical period in the emergence of fear, these data also suggest that attention should continue to be paid to the amygdala and essential fear circuit, which appear to contribute to a gradual change in the mechanisms of fear conditioning during the peri-weaning period.
Acknowledgments
We would like to acknowledge the following people for their contributions to these projects: Peter Mouton for assistance with the stereology, Dan Brazeau for aiding us in qRT-PCR techniques, Ian Meng for help in immunohistochemistry procedures, Linda Biagini for help in using the stereology program and microscope, and Miles Hughes for collecting and staining tissue. Stereology and Histology were conducted with the help of UNE’s COBRE Histology and Imaging Core, which is funded by the NIGMS (grant number P20GM103643). Funding for these projects was in part by the National Institute of Mental Health of the National Institute of Health under Award Number R15MH093950 to MAB.
References
- Albrechet-Souza L, Borelli KG, Almada RC, Brandão ML. Midazolam reduces the selective activation of the rhinal cortex by contextual fear stimuli. Behavioural Brain Research. 2011;216(2):631–8. doi: 10.1016/j.bbr.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Asok A, Schreiber WB, Jablonski SA, Rosen JB, Stanton ME. Egr-1 increases in the prefrontal cortex following training in the context preexposure facilitation effect (CPFE) paradigm. Neurobiology of Learning and Memory. 2013;106:145–53. doi: 10.1016/j.nlm.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DJ, Kim JJ, Sun W, Thompson RF, Helmstetter FJ. Acquisition of fear conditioning in rats requires the synthesis of mRNA in the amygdala. Behavioral Neuroscience. 1999;113(2):276–82. doi: 10.1037//0735-7044.113.2.276. [DOI] [PubMed] [Google Scholar]
- Beck CH, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. The Journal of Neuroscience. 1995;15(1):709–20. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekenstein JW, Lothman EW. An in vivo study of the ontogeny of long-term potentiation (LTP) in the CA1 region and in the dentate gyrus of the rat hippocampal formation. Brain Research. Developmental Brain Research. 1991;63(1–2):245–51. doi: 10.1016/0165-3806(91)90084-v. [DOI] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learning & Memory. 2001;8(5):229–42. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Bosch D, Ehrlich I. Postnatal maturation of GABAergic modulation of sensory inputs onto lateral amygdala principal neurons. The Journal of Physiology. 2015 doi: 10.1113/JP270645. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botterill JJ, Fournier NM, Guskjolen AJ, Lussier AL, Marks WN, Kalynchuk LE. Amygdala kindling disrupts trace and delay fear conditioning with parallel changes in Fos protein expression throughout the limbic brain. Neuroscience. 2014;265:158–71. doi: 10.1016/j.neuroscience.2014.01.040. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H, Smits K, Van Ree JM. Neonatal development of projections to the basolateral amygdala from prefrontal and thalamic structures in rat. The Journal of Comparative Neurology. 2002;450(3):241–55. doi: 10.1002/cne.10321. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H, Wolterink G, van Ree JM. Neonatal development of projections from the basolateral amygdala to prefrontal, striatal, and thalamic structures in the rat. The Journal of Comparative Neurology. 2002;442(3):239–49. doi: 10.1002/cne.10084. [DOI] [PubMed] [Google Scholar]
- Brasser SM, Spear NE. A sensory-enhanced context facilitates learning and multiple measures of unconditioned stimulus processing in the preweanling rat. Behavioral Neuroscience. 1998;112(1):126–40. doi: 10.1037//0735-7044.112.1.126. [DOI] [PubMed] [Google Scholar]
- Brasser SM, Spear NE. Contextual conditioning in infants, but not older animals, is facilitated by CS conditioning. Neurobiology of Learning and Memory. 2004;81(1):46–59. doi: 10.1016/s1074-7427(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Brown KL, Stanton ME. Cross-modal transfer of the conditioned eyeblink response during interstimulus interval discrimination training in young rats. Developmental Psychobiology. 2008;50(7):647–664. doi: 10.1002/dev.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, Phillips RG, Burwell RD. Contributions of postrhinal and perirhinal cortex to contextual information processing. Behavioral Neuroscience. 2000;114(5):882–94. doi: 10.1037//0735-7044.114.5.882. [DOI] [PubMed] [Google Scholar]
- Burman MA, Erickson KJ, Deal AL, Jacobson RE. Contextual and auditory fear conditioning continue to emerge during the periweaning period in rats. PloS One. 2014;9(6):e100807. doi: 10.1371/journal.pone.0100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman MA, Starr MJ, Gewirtz JC. Dissociable effects of hippocampus lesions on expression of fear and trace fear conditioning memories in rats. Hippocampus. 2006;16(2):103–13. doi: 10.1002/hipo.20137. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. The Journal of Comparative Neurology. 1998;391(3):293–321. doi: 10.1002/(sici)1096-9861(19980216)391:3<293::aid-cne2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Witter MP, Amaral DG. Perirhinal and postrhinal cortices of the rat: a review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus. 1995;5(5):390–408. doi: 10.1002/hipo.450050503. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. The Journal of Neuroscience. 1995;15(3):2312–27. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Hayward MD, Hope BT, Rosen JB, Nestler EJ, Davis M. Induction of the c-fos proto-oncogene in rat amygdala during unconditioned and conditioned fear. Brain Research. 1991;565(2):349–52. doi: 10.1016/0006-8993(91)91669-r. [DOI] [PubMed] [Google Scholar]
- Chareyron LJ, Lavenex PB, Lavenex P. Postnatal development of the amygdala: a stereological study in rats. The Journal of Comparative Neurology. 2012;520(16):3745–63. doi: 10.1002/cne.23132. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A. Neural activity mapping with inducible transcription factors. Neuroreport. 1997;8(16):v–ix. [PubMed] [Google Scholar]
- Chen SWC, Shemyakin A, Wiedenmayer CP. The role of the amygdala and olfaction in unconditioned fear in developing rats. The Journal of Neuroscience. 2006;26(1):233–40. doi: 10.1523/JNEUROSCI.2890-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corodimas KP, LeDoux JE. Disruptive effects of posttraining perirhinal cortex lesions on conditioned fear: contributions of contextual cues. Behavioral Neuroscience. 1995;109(4):613–9. doi: 10.1037//0735-7044.109.4.613. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64(2):477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381(6577):71–5. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Ehrlich DE, Ryan SJ, Rainnie DG. Postnatal development of electrophysiological properties of principal neurons in the rat basolateral amygdala. The Journal of Physiology. 2012;590(19):4819–38. doi: 10.1113/jphysiol.2012.237453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmorís-Arranz FJ, Méndez C, Spear NE. Contextual fear conditioning differs for infant, adolescent, and adult rats. Behavioural Processes. 2008;78(3):340–50. doi: 10.1016/j.beproc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behavioural Brain Research. 2000;110(1–2):73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neuroscience and Biobehavioral Reviews. 1999;23(5):743–60. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Foster JA, Burman MA. Evidence for hippocampus-dependent contextual learning at postnatal day 17 in the rat. Learning & Memory. 2010;17(5):259–66. doi: 10.1101/lm.1755810. [DOI] [PubMed] [Google Scholar]
- Fujisaki M, Hashimoto K, Iyo M, Chiba T. Role of the amygdalo-hippocampal transition area in the fear expression: evaluation by behavior and immediate early gene expression. Neuroscience. 2004;124(1):247–60. doi: 10.1016/j.neuroscience.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Moyer JR, Brown TH. Morphology and ontogeny of rat perirhinal cortical neurons. The Journal of Comparative Neurology. 2007;505(5):493–510. doi: 10.1002/cne.21516. [DOI] [PubMed] [Google Scholar]
- Gross CT, Canteras NS. The many paths to fear. Nature Reviews. Neuroscience. 2012;13(9):651–8. doi: 10.1038/nrn3301. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. Journal of Microscopy. 1986;143(Pt 1):3–45. [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kiêu K, Nielsen J. The efficiency of systematic sampling in stereology--reconsidered. Journal of Microscopy. 1999;193(Pt 3):199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. The Journal of Neuroscience. 2001;21(6):2186–93. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera DG, Robertson HA. Activation of c-fos in the brain. Progress in Neurobiology. 1996;50(2–3):83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Frontiers in Neuroendocrinology. 1993;14(3):173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- Holahan MR, White NM. Amygdala c-Fos induction corresponds to unconditioned and conditioned aversive stimuli but not to freezing. Behavioural Brain Research. 2004;152(1):109–20. doi: 10.1016/j.bbr.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, Rudy JW. Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. The Journal of Neuroscience. 2006;26(5):1616–23. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski SA, Schiffino FL, Stanton ME. Role of age, post-training consolidation, and conjunctive associations in the ontogeny of the context preexposure facilitation effect. Developmental Psychobiology. 2012;54(7):714–22. doi: 10.1002/dev.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs NS, Cushman JD, Fanselow MS. The accurate measurement of fear memory in Pavlovian conditioning: resolving the baseline issue. Journal of neuroscience methods. 2010;190(2):235–239. doi: 10.1016/j.jneumeth.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Richardson R. A developmental dissociation of context and GABA effects on extinguished fear in rats. Behavioral Neuroscience. 2007;121(1):131–9. doi: 10.1037/0735-7044.121.1.131. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. The effect of temporary amygdala inactivation on extinction and reextinction of fear in the developing rat: unlearning as a potential mechanism for extinction early in development. The Journal of Neuroscience. 2008;28(6):1282–90. doi: 10.1523/JNEUROSCI.4736-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neuroscience and Biobehavioral Reviews. 2006;30(2):188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. Fear and the brain: where have we been, and where are we going? Biological Psychiatry. 1998;44(12):1229–1238. doi: 10.1016/S0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Current Biology. 2007;17(20):R868–74. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. The Journal of Neuroscience. 1988;8(7):2517–29. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Majak K, Pitkanen A. Activation of the amygdalo-entorhinal pathway in fear-conditioning in rat. European Journal of Neuroscience. 2003;18(6):1652–1659. doi: 10.1046/j.1460-9568.2003.02854.x. [DOI] [PubMed] [Google Scholar]
- Malkani S, Rosen JB. Specific induction of early growth response gene 1 in the lateral nucleus of the amygdala following contextual fear conditioning in rats. Neuroscience. 2000;97(4):693–702. doi: 10.1016/s0306-4522(00)00058-0. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. The European Journal of Neuroscience. 2008;28(8):1661–6. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. The Journal of Neuroscience. 1995;15(11):7548–64. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Ferrario CR, Corcoran KA, Desmond TJ, Frey KA. Protein synthesis in the amygdala, but not the auditory thalamus, is required for consolidation of Pavlovian fear conditioning in rats. The European Journal of Neuroscience. 2003;18(11):3080–8. doi: 10.1111/j.1460-9568.2003.03063.x. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. The Journal of Neuroscience. 2004;24(10):2431–9. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Okotoghaide T, Sullivan RM. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. International Journal of Developmental Neuroscience. 2004;22(5–6):415–22. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8(6):608–19. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Mouton P. Unbiased Stereology: A Concise Guide. Baltimore: The Johns Hopkins University Press; 2011. [Google Scholar]
- Pan BX, Ito W, Morozov A. Divergence between thalamic and cortical inputs to lateral amygdala during juvenile-adult transition in mice. Biological Psychiatry. 2009;66(10):964–71. doi: 10.1016/j.biopsych.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego: Academic Press; 1998. [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106(2):274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M, Rönkkö S, Savander V, Insausti R, Pitkänen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. The Journal of Comparative Neurology. 1999;403(2):229–60. [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal Connections between the Amygdala and the Hippocampal Formation, Perirhinal Cortex, and Postrhinal Cortex in Rat: A Review. Annals of the New York Academy of Sciences. 2000;911(1):369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Rudy JW. A developmental analysis of contextual fear conditioning. Developmental Psychobiology. 1996;29(2):87–100. doi: 10.1002/(SICI)1098-2302(199603)29:2<87::AID-DEV1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Radwanska K, Nikolaev E, Knapska E, Kaczmarek L. Differential response of two subdivisions of lateral amygdala to aversive conditioning as revealed by c-Fos and P-ERK mapping. Neuroreport. 2002;13(17):2241–6. doi: 10.1097/01.wnr.0000045006.52875.93. [DOI] [PubMed] [Google Scholar]
- Raineki C, Holman PJ, Debiec J, Bugg M, Beasley A, Sullivan RM. Functional emergence of the hippocampus in context fear learning in infant rats. Hippocampus. 2010;20(9):1037–46. doi: 10.1002/hipo.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB, Fanselow MS, Young SL, Sitcoske M, Maren S. Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning. Brain Research. 1998;796(1–2):132–42. doi: 10.1016/s0006-8993(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Hitchcock JM, Miserendino MJ, Falls WA, Campeau S, Davis M. Lesions of the perirhinal cortex but not of the frontal, medial prefrontal, visual, or insular cortex block fear-potentiated startle using a visual conditioned stimulus. The Journal of Neuroscience. 1992;12(12):4624–33. doi: 10.1523/JNEUROSCI.12-12-04624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behavioral Neuroscience. 1993;107(5):887–91. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O’Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behavioral Neuroscience. 2002;116(4):530–8. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neuroscience and Biobehavioral Reviews. 2004;28(7):675–85. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behavioral Neuroscience. 1994;108(2):227–34. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Lorenzini CA, Baldi E, Tassoni G, Bucherelli C. Auditory thalamus, dorsal hippocampus, basolateral amygdala, and perirhinal cortex role in the consolidation of conditioned freezing to context and to acoustic conditioned stimulus in the rat. The Journal of Neuroscience. 1999;19(21):9570–8. doi: 10.1523/JNEUROSCI.19-21-09570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. European Journal of Pharmacology. 2003;463(1–3):217–23. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. The Journal of Neuroscience. 2000;20(18):RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettino LF, Otto T. Patterns of Fos expression in the amygdala and ventral perirhinal cortex induced by training in an olfactory fear conditioning paradigm. Behavioral Neuroscience. 2001;115(6):1257–72. doi: 10.1037//0735-7044.115.6.1257. [DOI] [PubMed] [Google Scholar]
- Schiffino FL, Murawski NJ, Rosen JB, Stanton ME. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiology of Learning and Memory. 2011;95(2):190–8. doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber WB, Asok A, Jablonski SA, Rosen JB, Stanton ME. Egr-1 mRNA expression patterns in the prefrontal cortex, hippocampus, and amygdala during variants of contextual fear conditioning in adolescent rats. Brain Research. 2014;1576:63–72. doi: 10.1016/j.brainres.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. Journal of Neurochemistry. 2012;120(6):948–63. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood N, Timiras P. A Stereotaxic Atlas of the Developing Rat Brain. Berkeley: University of California Press; 1970. [Google Scholar]
- Stanton ME. Multiple memory systems, development and conditioning. Behavioural Brain Research. 2000;110(1–2):25–37. doi: 10.1016/s0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- Stoop R, Pralong E. Functional connections and epileptic spread between hippocampus, entorhinal cortex and amygdala in a modified horizontal slice preparation of the rat brain. The European Journal of Neuroscience. 2000;12(10):3651–63. doi: 10.1046/j.1460-9568.2000.00253.x. [DOI] [PubMed] [Google Scholar]
- Suzuki WA. The anatomy, physiology and functions of the perirhinal cortex. Current Opinion in Neurobiology. 1996;6(2):179–86. doi: 10.1016/s0959-4388(96)80071-7. [DOI] [PubMed] [Google Scholar]
- Teather LA, Packard MG, Smith DE, Ellis-Behnke RG, Bazan NG. Differential induction of c-Jun and Fos-like proteins in rat hippocampus and dorsal striatum after training in two water maze tasks. Neurobiology of Learning and Memory. 2005;84(2):75–84. doi: 10.1016/j.nlm.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Thompson JV, Sullivan RM, Wilson DA. Developmental emergence of fear learning corresponds with changes in amygdala synaptic plasticity. Brain Research. 2008;1200:58–65. doi: 10.1016/j.brainres.2008.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischmeyer W, Grimm R. Activation of immediate early genes and memory formation. Cellular and Molecular Life Sciences. 1999;55(4):564–74. doi: 10.1007/s000180050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. The Journal of Comparative Neurology. 1990;302(3):515–28. doi: 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- VanElzakker M, Fevurly RD, Breindel T, Spencer RL. Environmental novelty is associated with a selective increase in Fos expression in the output elements of the hippocampal formation and the perirhinal cortex. Learning & Memory. 2008;15(12):899–908. doi: 10.1101/lm.1196508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Brown MW, Erichsen JT, Aggleton JP. Fos imaging reveals differential patterns of hippocampal and parahippocampal subfield activation in rats in response to different spatial memory tests. The Journal of Neuroscience. 2000;20(7):2711–8. doi: 10.1523/JNEUROSCI.20-07-02711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer RW, Van Vulpen EH, Van Uum JF. Postnatal development of amygdaloid projections to the prefrontal cortex in the rat studied with retrograde and anterograde tracers. The Journal of Comparative Neurology. 1996;376(1):75–96. doi: 10.1002/(SICI)1096-9861(19961202)376:1<75::AID-CNE5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Barr GA. Developmental changes in c-fos expression to an age-specific social stressor in infant rats. Behavioural Brain Research. 2001;126(1–2):147–57. doi: 10.1016/s0166-4328(01)00260-1. [DOI] [PubMed] [Google Scholar]
- Xiu J, Zhang Q, Zhou T, Zhou TT, Chen Y, Hu H. Visualizing an emotional valence map in the limbic forebrain by TAI-FISH. Nature neuroscience. 2014;17(11):1552–1559. doi: 10.1038/nn.3813. [DOI] [PubMed] [Google Scholar]