Abstract
PURPOSE
To explore the effects of zoledronic acid on the healing process in osteoporotic patients following spinal fusion in a randomized, placebo controlled, and triple-blinded study.
METHODS
Seventy-nine osteoporotic patients with single-level degenerative spondylolisthesis were randomly assigned to receive either zoledronic acid infusion (zoledronic acid group) or saline infusion (controls) after spinal fusion. Functional radiography and CT scans were used to evaluate fusion status. Bone formation was graded into 3 categories: Grade A (bridging bone bonding with adjacent vertebral bodies), Grade B (bridging bone bonding with either superior or inferior vertebral body), or Grade C (incomplete bony bridging). A solid fusion was defined as less than 5 degree of angular motion with Grade A or B bone formation. Adjacent vertebral compression fractures (VCF) were assessed on MRI at 12 months after surgery. Serum level of carboxy terminal cross-linked telopeptide of type I collagen (β-CTX) and amino-terminal propeptide of type I procollagen (PINP) was measured. Bone mineral density (BMD) was measured by DXA. Oswestry Disability Index (ODI) was used to assess the clinical outcomes.
RESULTS
Grade A or B bridging bone was more frequently observed in zoledronic acid group at 3, 6, and 9 months post-operation compared to the control group (p < 0.05). At 12-months postoperation, bridging bone and solid fusion were not significantly different between groups. No patients in zoledronic acid group showed aVCF, whereas 6 patients (17%) in the control group did (p < 0.05). Both β-CTX and PINP were suppressed in zoledronic acid group. BMD at femoral neck decreased rapidly and did not return to the preoperative level in the controls at 3 (−1.4%), 6 (−2.5%), and 12 (−0.8%) months after surgery. Zoledronic acid prevented this immobilization-induced bone loss and increased BMD. ODI showed the improved clinical outcomes compared with controls at 9 and 12 months post-surgery.
CONCLUSION
Treatment with zoledronic acid in osteoporotic patients with spinal fusion shortens the time to fusion, improves the fusion rate, prevents subsequent aVCFs, and improves clinical outcomes.
Keywords: bisphosphonates, zoledronic acid, spinal fusion, posterior lumbar inter-body fusion
INTRODUCTION
Spinal fusion with instrumentation is one of the most common orthopedic procedures for the treatment for spinal instability, trauma, and deformity. As the population ages, disability associated with spinal pathology and spinal surgery is rapidly increasing and there is a concomitant increase in prevalence of osteoporosis which is a detrimental factor for spinal fusion and instrumentation. Osteoporosis-related bone fragility is a primary reason for implant fixation failure, spinal fusion failure, and vertebral compression fractures (VCFs) above or below the fusion sites [1]. In order to minimize fusion-associated sequelae, expandable pedicle screws, bone cement augmented techniques [2], and bone morphogenetic proteins [3] were utilized. However, these methods don’t prevent all the problems associated with bone fusion. Osteoporosis is a complex systemic metabolic bone disease characterized by an imbalance between osteoblastic and osteoclastic activities, resulting in progressive bone fragility [4]. We therefore hypothesized that intervention with an anti-osteoporosis agent would improve outcomes in spinal fusion and instrumentation.
Bisphosphonates, used as first-line therapies for osteoporosis, work by inhibiting osteoclastic function and subsequently inducing osteoclast apoptosis [5]. They have been shown to reduce the bone turnover rate, increase bone mineral density (BMD) and prevent VCFs [6]. Successful spinal fusion and instrumentation requires optimal coordination between bone formation and resorption. Bisphosphonates through extensive inhibition of bone resorption may lead to decreased bone formation as a result of uncoupling of the balance between osteoclastic and osteoblastic activities [7]. However, studies in animal models [11–13] and in humans [14] on the role of alendronate in spinal fusion yielded inconsistent results. Furthermore, zoledronic acid, a third-generation nitrogen-containing bisphosphonate, differs from previous bisphosphonates in that bone resorption is inhibited, which leads to an increase in secondary mineralisation and in refilling of remodeling space or existing resorptive pits [8, 21]. Furthermore, in a rabbit spinal fusion model, zoledronic acid had been shown to increased fusion mass size and bone mineral content [9]. These results suggest that zoledronic acid may provide some benefits for spinal fusions. To our knowledge, clinical investigation using zoledronic acid for spinal fusion and instrumentation has not been reported.
The objectives of this prospectively randomized, placebo controlled, and triple-blind trial were to evaluate the effect of zoledronic acid on spinal fusion and clinical efficacy in osteoporotic patients following single-level posterior lumbar inter-body fusion (PLIF).
MATERIALS AND METHODS
Study Design
In this prospectively randomized study, participants, investigators and outcome assessors were unaware of the group assignments. A 1-year follow-up period was planned. Enrollment commenced in October 2011 and concluded at the end of March 2012, and the follow-up period ended in March 2013. The institute review board and human research ethics committee at our university approved this study. All participants provided written informed consent.
The inclusion criteria was a diagnosis of single-level degenerative spondylolisthesis with symptoms of low-back pain and/or leg pain for at least 3 months, which was not be adequately controlled by nonoperative treatments including bed rest, bracing, non-steroidal anti-inflammatory drugs, and physical therapy. Another inclusion criterion was the diagnosis of osteoporosis, defined as a bone mineral density (BMD) at lumbar or femoral neck with 2.5 standard deviations or more below the mean peak bone mass measured by dual-energy X-ray absorptiometry (DXA). Exclusion criteria were evidence or suspicion of neoplasm, infection, acute vertebral fractures, history of lumbar surgery, history of a anti-osteoporosis medication, severe spinal deformities such as degenerative scoliosis, uncorrected bleeding diatheses, renal insufficiency (Creatinine Clearance Rate < 35ml/min), and other metabolic disorders.
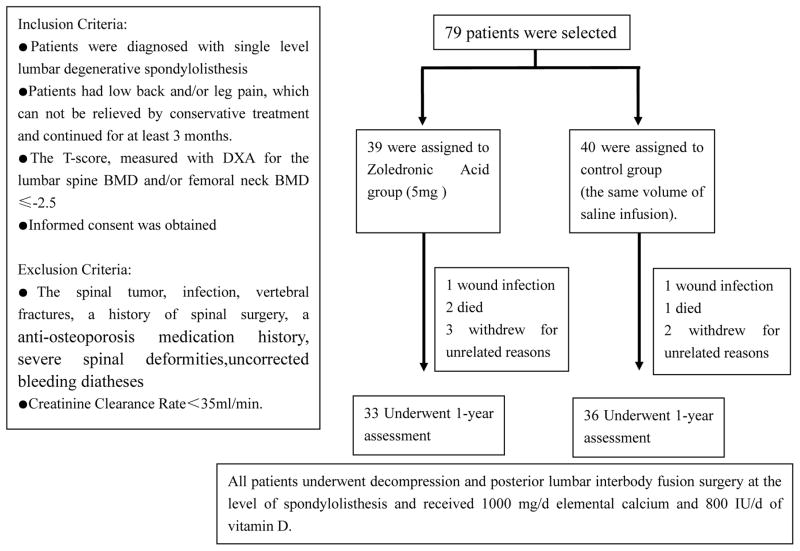
A total of 79 subjects (65 women and 14 men) in our hospital were recruited for this study and randomly assigned to receive either zoledronic acid infusion (5mg) (zoledronic acid group), or placebo as the same volume of saline (control group), after single-level PLIF. All Patients received daily 1000 mg elemental calcium and 800 IU vitamin D.
Ten patients were lost to follow-up because of death, deep wound infection, or withdrawal from participation. In the zoledronic acid group, one died of traffic accident and one of stroke. One in control group died of pneumonia. A total of 69 patients completed the 1-year follow-up (33 in the zoledronic acid group, 36 in the control group) (Figure 1). No significant differences between groups were detected in age, gender, BMD, Oswestry disability index (ODI) score, or the level and grade of degenerative spondylolisthesis (Table 1).
Figure 1.
Enrollment and Follow-up.
Table 1.
Baseline characteristics of the both groups (mean ± standard deviation).
| Group | N(M:F) | Age (y) | BMD (g/cm2) | ODI Score | Surgery Level | Grade | |||
|---|---|---|---|---|---|---|---|---|---|
| L1–4 | Femur neck | L4/5 | L5/S1 | I | II | ||||
| Control | 36(7:29) | 63±7 | 0.698± 0.004 | 0.483± 0.005 | 21.9±2.6 | 25 | 11 | 32 | 4 |
| Zoledronic Acid | 33(6:27) | 65±8 | 0.709± 0.003 | 0.485± 0.005 | 20.8±2.6 | 24 | 9 | 30 | 3 |
N, number of patient; M, male; F, female; y, years; BMD, bone mineral density; ODI, Oswestry disability index.
Surgical Procedure and Postoperative Care
Patients were placed on an appropriate surgical frame in a prone position under general anesthesia. Through a routine posterior approach, the spinous processes were exposed and the paraspinal muscles were retracted to the tips of the transverse processes. Pedicle screws were inserted through the pedicles of the vertebrae that were proximal and distal to the vertebral space afflicted by spondylolisthesis. Laminectomy was performed for almost all of the caudal two-thirds of the spinous process and laminae including both inferior facets of the spondylolisthesis level, to decompress the nerve roots and enlarge epidural space. Rods were applied to pedicle screws. Expanding pincers were used to recover the spine alignment and enlarge the intervertebral space under fluoroscopy. All the disc materials were removed to ensure proper end-plate preparation. The bone graft materials, a mixture of local bone harvested from the lamina and allograft bone, were packed as tightly as possible into the disc space. Compressing pincers were used and the screws were tightened to apply the compressive load to the bone graft materials. All surgeries were performed by the same surgical team.
Wound drains were removed when the output was less than 30 ml/24hr. Bed rest was advised for the first month after surgery. Zoledronic acid (5mg) or the same amount of saline was infused at 3 days after surgery.
Radiographic Evaluation
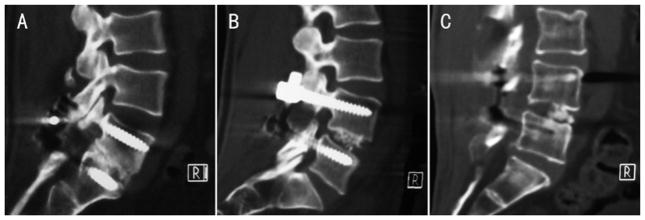
Posterior-anterior radiography was taken to observe the integrity and the location of the screws and rods at 3, 6, 9, and 12 months postoperation. The functional radiography and CT scans at 3, 6, 9, and 12 months after surgery were evaluated for fusion status. Flexion radiography was obtained with the patient in a standing position trying to bend forward as far as possible, and an extension one was obtained with the patient maximally arching the back. Coronal and sagittal multiplanar CT reconstruction was obtained through the spinal fusion level by 4-mm contiguous slices. Bone formation was graded into the following 3 categories: Grade A as bridging bone bonding with both adjacent vertebral bodies, Grade B as bridging bone bonding with either superior or inferior vertebral body, or Grade C as incomplete bony bridging (Fig. 2). A solid fusion was defined as less than 5 degree of angular motion on flexion-extension radiographs at the fusion level and the presence of Grade A or B bone formation [14]. Subsequent VCF was assessed on MR imaging, including T1-weighted, T2- weighted, and STIR images, at 12 months postoperation.
Figure 2.
Representative sagittal reconstructed computed tomography (CT) images demonstrate a 3-category grading system to evaluate bone formation. Grade A: bridging bone bonding with adjacent vertebral bodies; Grade B: bridging bone bonding with one vertebral body; and Grade C: incomplete bony bridging.
Bone metabolic marker measurements and BMD assessment
Bone resorption was assessed by measuring the serum level of carboxy terminal cross-linked telopeptide of type I collagen (β-CTX). The level of amino-terminal propeptide of type I procollagen (PINP) was measured in serum to evaluate bone formation. To minimize diurnal variation of bone metabolic markers, the samples were obtained in the morning.
Due to instrumentation at the lumbar site, BMD was measured only at the femoral neck by DXA at 3, 6, and 12 months after surgery.
Clinical Evaluation
Clinical outcome was evaluated using ODI score derived from the Oswestry Low Back Pain Questionnaire used by clinicians and researchers to measure degree of disability and estimate quality of life in a person with low back pain. This self-completed questionnaire contains ten topics and each topic category is followed by 6 statements with the first statement being zero indicating the least amount of disability, and the last statement scored 5 indicating most severe disability [19]. The data were recorded before surgery, 3, 6, 9, and 12 months after surgery. All adverse events were reported, too.
Statistical Analyses
The results are presented as mean ± standard deviation (SD). The data distribution was determined. Non-parametric tests were used to compare the difference between zoledronic acid and control groups. In multiple comparison procedure, Bonferroni adjustment was used. Significance of difference in frequency between groups was evaluated with 2-tail Fisher’s exact test. The SPSS 16.0 software package (SPSS Inc., Chicago, IL, USA) was used for statistical analyses.
RESULTS
Table 1 shows demographic characteristics of the patients before surgery. There was no significant difference in the patients’ background, BMD, ODI score, or the level and grade of degenerative spondylolisthesis.
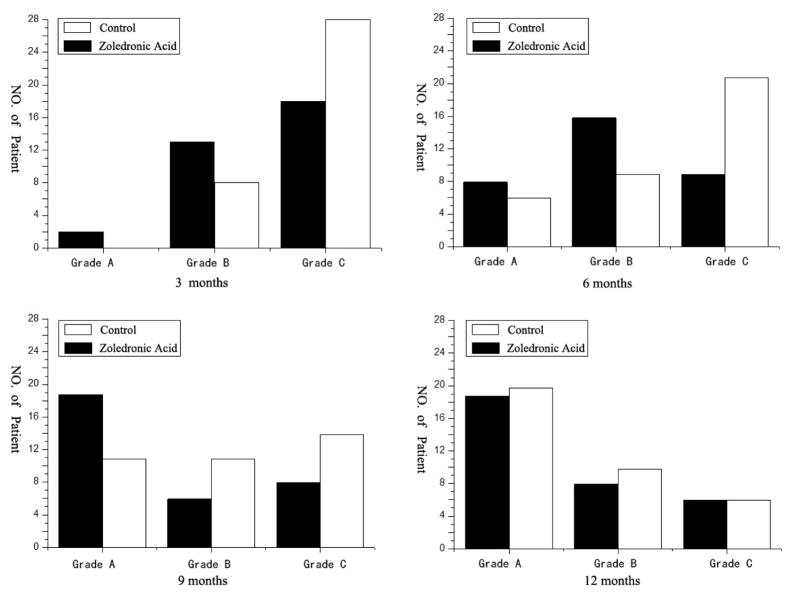
There were no implant fixation failures detected during the follow-up. Grade A or B bone formation assessed by CT was more frequently observed in the zoledronic acid group at 3, 6, and 9 months postoperatively compared to the control group (p < 0.05) (Fig. 3). However, there was no significant difference in bone formation status between the two groups at 12 months post-operation. At 12 months post surgery, there were 19 patients in Grade A bone formation (58%), 8 patients in Grade B (24%), 6 patients in Grade C (18%) in the zoledronic acid group, and 20 patients in Grade A bone formation (56%), 10 patients in Grade B (28%), 6 patients in Grade C (17%) in the control group. At the 1-year observation, 27 of 33 patients (82%) in the zoledronic acid group and 30 of 36 patients (83%) in the control group had solid fusion (p > 0.05). No subsequent VCFs occurred in the zoledronic acid group, while 6 patients (17%) in the control group developed VCFs (p < 0.05). In addition, 4 of the 6 patients who developed subsequent VCFs showed no solid fusion.
Figure 3.
Grade A or B bone formation is significantly more frequently observed in the zoledronic acid group at 3, 6, and 9 months post surgery (p<0.05). There was no significant difference in bone formation status between two groups at 12 months postoperation.
Table 2 shows the time course of serum bone metabolic markers β-CTX and PINP in both groups. In the control group, the serum level of β-CTX and PINP fluctuated around the preoperative baseline. However, the serum level of β-CTX in the zoledronic acid group was below the preoperative baseline at all postoperative periods. The level decreased rapidly by 91.8% on the 10th day after operation and did not return to the preoperative baseline even at the end of the study, with −76% at 3 months, −73% at 6 months, −69% at 9 months, and −66% at 12 months after surgery. The serum level of PINP as a marker of bone formation in the zoledronic acid group also decreased by −11% on the 10th day after operation, plummeted to a nadir at 3 months postoperation by −61%, and gradually increased but remained below the preoperative baseline, by −49% at 9 months and −43% at 12 months after surgery. There was significant difference in bone resorption and formation between the groups at all postoperative periods (p < 0.05).
Table 2.
Bone metabolic markers and BMD at femur neck (mean ± standard deviation)
| β–CTX (μg/ml) | PINP (μg/ml) | Femur neck BMD (g/cm2) | |
|---|---|---|---|
| Controls (n=36) | |||
| Pre-surgery | 0.42±0.15 | 40±15 | 0.483±0.005 |
| 10 days post-surgery | 0.43±0.14 | 38±15 | |
| 3 months post-surgery | 0.42±0.15 | 41±15 | 0.476±0.003# |
| 6 months post-surgery | 0.45±0.14 | 43±15 | 0.478±0.005# |
| 9 months post-surgery | 0.44±0.14 | 42±15 | |
| 12 months post-surgery | 0.45±0.15 | 41±15 | 0.479±0.004# |
| Zoledronic Acid (n=33) | |||
| Pre-operation | 0.414±0.212 | 41±16 | 0.485±0.005 |
| 10 days post-surgery | 0.034±0.008* | 36±14* | |
| 3 months post-surgery | 0.101±0.010* | 16±7* | 0.491±0.004* |
| 6 months post-surgery | 0.112±0.009* | 19±8* | 0.497±0.005* |
| 9 months post-surgery | 0.127±0.011* | 21±9* | |
| 12 months post-surgery | 0.139±0.010* | 23±9* | 0.501±0.004* |
β-CTX: Carboxy terminal cross-linked telopeptide of type I collagen; PINP: Amino-terminal propeptide of type I procollagen; BMD: bone mineral density.
p<0.05 vs. pre-surgery and vs. controls; and
p<0.05 vs. pre-surgery.
Table 2 shows the change in BMD at the femoral neck. In the zoledronic acid group, the BMD was elevated by 1.2% at 3 months after surgery, 2.5% at 6 months after surgery, and 3.3% at 12 months after surgery. In the control group; however, the BMD decreased below the preoperative baseline at all postoperative periods, with −1.4% at 3 months, −2.5% at 6 months, −0.8% at 12 months after surgery. There was significant difference in the percentage change of the BMD between the groups at all postoperative periods (p < 0.05).
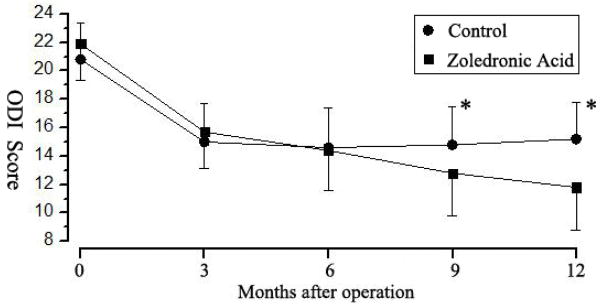
The mean ODI score in the zoledronic acid group consistently decreased until 12 months after surgery (p < 0.01) (Fig. 4). In the control group, the mean ODI score decreased until 3 months post-operation and reached a plateau thereafter. The difference in ODI scores between the groups was statistically significant at 9 months and 12 months after surgery (p < 0.05).
Figure 4.
The mean Oswestry disability index (ODI) score in the zoledronic acid group is consistently decreased until 12 months after surgery (p <0.01 vs preoperative baseline). In the control group, the mean ODI score decreased until 3 months postoperation, but thereafter reached a plateau. The difference in ODI scores between the groups was statistically significant at 9 months and 12 months after surgery. * p < 0.05.
In the zoledronic acid group, there were 3 (9%) patients with spine fusion failure, without any VCF detected on MRI. In the control group, there were 5 (14%) patients with spine fusion failure, and 6 (17%) with VCFs. These patients had poor clinical outcomes, with less than 20% improvement in ODI score.
DISCUSSION
Our investigation is the first randomized controlled clinical trial to examine the effect of zoledronic acid on the post-surgical formation of spine fusion. In our study, use of zoledronic acid shortened the duration of time to fusion, prevented the subsequent VCFs, and improved the clinical outcome after instrumented lumbar inter-vertebral fusion in osteoporotic patients.
The biology of spinal fusion and instrumentation is a complex process that requires de novo bone formation and remodeling by the appropriate coordination between bone formation and resorption. Our rationale for the study was that therapies for osteoporosis targeting this complex process might improve outcomes in spinal fusion. Nitrogen-containing bisphosphonates, such as alendronate, risedronate, ibandronate, and zoledronic acid, were currently used as first-line agent for prevention and treatment for osteoporosis [10]. Bisphosphonates increase BMD, decrease the levels of biomarkers of bone resorption, and reduce the risk of osteoporotic fractures, which results from both their affinity for bone mineral and their inhibitory effect on osteoclastic cell function [6]. In animal studies, the effect of bisphosphonates on spine fusion and instrumentation are mixed. To our knowledge, the first report on the role of bisphosphonates in spine fusion in animals was published in 2003 [11]. In that study, pigs underwent anterior intervertebral lumbar arthrodesis and alendronate was given orally to the animals. Histomorphometric analysis showed that a relatively low dose and short-term alendronate treatment did not impair the formation of new bone, but increases bone ingrowth into the central hole of the porous tantalum ring and the pores of the porous tantalum in the porcine model. Xue et al [12] investigated the effects of alendronate on bone–pedicle screw interface fixation using a pig posterolateral lumbar fusion model with the CD Horizon pedicle screw system. They demonstrated that alendronate enlarged the bone–screw contact surface, but did not improve the biomechanical strength of the bone/implant interface. Huang et al. [13] used histomorphometric and radiographic assessments to determine whether alendronate enhanced fusion rate in a rat posterolateral lumbar fusion model and demonstrated that alendronate increased the size and density of the fusion mass but decreased the chance of a solid fusion. Because of the different biologic and biomechanical environment between animal and human, Nagahama et al. conducted a human trial of 36 patients with regard to the effects of alendronate on spine fusion [14]. In that study, alendronate shortened the duration of fusion, improved the fusion rate, prevented the subsequent VCFs and improved the clinical outcome.
Zoledronic acid shows significant differences in binding affinity to bone mineral and the degree to which it reduces osteoclastic activity by inhibition of farnesyl pyrophosphate synthase compared to other bisphosphonates Recker et al. [8] used histomorphologic analysis and micro-computed tomography (micro CT) to examine bone specimens from postmenopausal women with osteoporosis treated by intravenous zoledronic acid at 5 mg yearly for 3 consecutive years. The bone specimens showed a reduction in activity of bone remodeling, accompanied by increased volume of trabeculae and a much higher mineralization rate. The findings suggest that injection of zoledronic acid once a year had little effect on osteoblasts. Ramen spectroscopy further revealed an increased mineral-to-matrix ratio after zoledronic acid treatment. The mineralized crystals in newly formed bone were orderly arranged, which was suggestive that zoledronic acid promoted bone matrix formation [15]. Bransford et al [16] used manual palpation and radiographic assessment to determine whether zoledronic acid enhanced fusion rate in a rabbit intertransverse fusion model. They demonstrated that zoledronic acid increased the size and bone mineral content of the fusion mass and led to an increased fusion rate and recommended that bisphosphonate treatment should be maintained to enhance successful spinal fusion. Yalcin et al. [17] demonstrated the positive role of zoledronic acid in posterolateral lumbar fusion in rabbits, too. These studies in animals on the role of zoledronic acid in spinal fusion have yielded consistent results. Tu et al. [20] retrospectively analyzed 64 patients with both degenerative lumbar spondylolisthesis and osteoporosis who underwent lumbar interbody fusion surgery with regard to the effects of zoledronic acid on spine fusion. Zoledronic acid lowered the incidence of final subsequent VCFs, pedicle screw loosening, cage subsidence, and improved the clinical outcomes. In a hip fracture study with a very large sample size [22], protection against hip fracture-associated osteoporosis did not occur when zoledronic acid was given within 6 weeks of fracture. This was likely due to the distribution of zoledronic acid to the fracture site as opposed to systemic distribution. We plan to continue to follow up the subjects of the current study because the protected effect on the hip BMD in the current study may translate into hip fracture reduction in the future. Future study is warranted to investigate the optimal timing of administration of medication for consistently enhanced fusion by one year and beyond.
Our radiographic results showed that the numbers of patients achieving grade A or grade B bone graft fusion and solid fusion were much higher in the zoledronic acid group than in the control group in most of the follow ups except 12-month post surgery. Treatment with zoledronic acid facilitated spine fusion and shortened the time to fusion. This study confirmed that BMD at femur neck decreases rapidly because of the immobilization after surgery and did not return to the preoperative level even at the end of the trial. Zoledronic acid was effective in preventing the bone loss and result in increased BMD at the femur neck. Because of spinal instrumentation, the post-operative BMD at the lumbar vertebrae could not be measured, but extensive studies indicate a good correlation between BMD at the femur neck and that at lumbar vertebrae. An improved BMD could effectively prevent subsequent VCFs. There was no subsequent VCF in the zoledronic acid group, while 6 cases (17%) in the controls showed VCFs. There was statistically significant difference in clinical outcomes between the two groups, and the poor clinical results were associated with placebo fusion and subsequent VCFs. Biochemical analyses of the bone metabolic markers demonstrated that zoledronic acid rapidly inhibited bone resorption from the early phase of the bone fusion process and bone formation was also suppressed. Bone formation might be down-regulated by a consequence of osteoclast inhibition through coupling processing [18]. Our study confirms a similar study [23] that residronate did not decrease spine fusion. Anabolic agent such as PTH showed robust effects to reduce pedicle screw loosening in spinal surgery [23]. In our study, no pedicle screw loosening was identified in either group.
Apart from the strength of our randomized placebo controlled study, this study has its limitations. The sample size of this prospective study is relatively small. In addition, the follow-up time over 12 months is relatively short, though the study already showed effect on vertebral fracture in the placebo group. Finally, the definition of a solid fusion by assessing instability between the flexion and extension positions could potentially underestimate fusion status, because pedicle screw fixation provides immediate stability to the operative segments and might lead to overestimation of fusion status, but we applied the same evaluation to both groups.
Conclusions
The current study evaluated the efficacy of zoledronic acid infusion for spine fusion after instrumented lumbar intervertebral fusion in patients with osteoporosis. Bone metabolic marker analyses showed suppression of both bone formation and bone resorption by zoledronic acid. Compared to placebo, zoledronic acid treatment prevented the subsequent VCFs, shortened the time to fusion, improved the clinical outcome, and prevented immobilization induced bone loss in the hip.
Acknowledgments
This research was supported by “the Fundamental Research Funds for the Central Universities” (2012QNZT148), Science and Technology Plan of Hunan Province (2012WK3039) and National Institutes of Health (P01 CA093900)
Footnotes
CONFLICT OF INTERSET
Fei Chen, Zhehao Dai, Yijun Kang, Guohua Lv, Evan Keller, and Yebin Jiang declare that they have no conflict of interest.
The ethics committee of Central South University approved the study.
References
- 1.Etebar S, Cahill DW. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J Neurosurg. 1999;90(2 Suppl):163–169. doi: 10.3171/spi.1999.90.2.0163. [DOI] [PubMed] [Google Scholar]
- 2.Ponnusamy KE, Iyer S, Gupta G, Khanna AJ. Instrumentation of the osteoporotic spine: biomechanical and clinical considerations. Spine J. 2011 Jan;11(1):54–63. doi: 10.1016/j.spinee.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Guppy K, Paxton L, Harris J, Alvarez J, Bernbeck J. Does Bone Morphogenic Protein (BMP) Change the Operative Nonunion Rates in Spine Fusions? Spine (Phila Pa 1976) 2014 Oct;39(22):1831–1839. doi: 10.1097/BRS.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 4.Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995 Feb 2;332(5):305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 5.Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014 Jul;142:155–170. doi: 10.1016/j.jsbmb.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai Zhehao, Dai Ruchun, Xiao Yi, et al. Progress in anti-osteoporosis drug treatment. Chin J Osteoporos. 2010;16(11):894–906. [Google Scholar]
- 7.Schimmer RC, Bauss F. Effect of daily and intermittent use of ibandronate on bone mass and bone turnover in postmenopausal osteoporosis: a review of three phase II studies. Clin Ther. 2003 Jan;25(1):19–34. doi: 10.1016/s0149-2918(03)90005-1. [DOI] [PubMed] [Google Scholar]
- 8.Recker RR, Delmas PD, Halse J, et al. Effects of intravenous zoledronic acid once yearly on bone remodeling and bone structure. J Bone Miner Res. 2008;23(1):6–16. doi: 10.1359/jbmr.070906. [DOI] [PubMed] [Google Scholar]
- 9.Bransford R, Goergens E, Briody J, et al. Effect of zoledronic acid in an L6–L7 rabbit spine fusion model. Eur Spine J. 2007;16(4):557–562. doi: 10.1007/s00586-006-0212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008 Jun;19(6):733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 11.Zou X, Xue Q, Li H, Bünger M, Lind M, Bünge C. Effect of alendronate on bone ingrowth into porous tantalum and carbon fiber interbody devices: an experimental study on spinal fusion in pigs. Acta Orthop Scand. 2003 Oct;74(5):596–603. doi: 10.1080/00016470310018027. [DOI] [PubMed] [Google Scholar]
- 12.Xue Q, Li H, Zou X, et al. Alendronate treatment improves bone-pedicle screw interface fixation in posterior lateral spine fusion: an experimental study in a porcine model. Int Orthop. 2010;34(3):447–451. doi: 10.1007/s00264-009-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang RC, Khan SN, Sandhu HS, et al. Alendronate inhibits spine fusion in a rat model. Spine. 2005;30(22):2516–2522. doi: 10.1097/01.brs.0000186470.28070.7b. [DOI] [PubMed] [Google Scholar]
- 14.Nagahama K, Kanayama M, Togawa D, Hashimoto T, Minami A. Does alendronate disturb the healing process of posterior lumbar interbody fusion? A prospective randomized trial. J Neurosurg Spine. 2011 Apr;14(4):500–507. doi: 10.3171/2010.11.SPINE10245. [DOI] [PubMed] [Google Scholar]
- 15.Gamsjaeger S, Buchinger B, Zwettler E, et al. Bone material properties in actively bone-forming trabeculae in postmenopausal women with osteoporosis after three years of treatment with once-yearly zoledronic acid. J Bone Miner Res. 2011;26(1):12–18. doi: 10.1002/jbmr.180. [DOI] [PubMed] [Google Scholar]
- 16.Bransford R, Goergens E, Briody J, et al. Effect of zoledronic acid in an L6–L7 rabbit spine fusion model. Eur Spine J. 2007;16(4):557–562. doi: 10.1007/s00586-006-0212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yalçin N, Öztürk A, Ozkan Y. The effects of zoledronic acid and hyperbaric oxygen on posterior lumbar fusion in a rabbit model. J Bone Joint Surg Br. 2011;93(6):793–800. doi: 10.1302/0301-620X.93B6.24257. [DOI] [PubMed] [Google Scholar]
- 18.Tamma R, Zallone A. Osteoblast and osteoclast crosstalks: from OAF to Ephrin. Inflamm Allergy Drug Targets. 2012;11(3):196–200. doi: 10.2174/187152812800392670. [DOI] [PubMed] [Google Scholar]
- 19.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940–2952. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 20.Tu CW, Huang KF, Hsu HT, et al. Zoledronic acid infusion for lumbar interbody fusion in osteoporosis. J Surg Res. 2014;192(1):112–116. doi: 10.1016/j.jss.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 21.Seeman E, Martin TJ. Co-administration of Antiresorptive and Anabolic Agents: A Missed Opportunity. J Bone Miner Res. 2015;30(5):753–764. doi: 10.1002/jbmr.2496. [DOI] [PubMed] [Google Scholar]
- 22.Boonen S, Orwoll E, Magaziner J, et al. Once-yearly zoledronic acid in older men compared with women with recent hip fracture. J Am Geriatr Soc. 2011;59(11):2084–2090. doi: 10.1111/j.1532-5415.2011.03666.x. [DOI] [PubMed] [Google Scholar]
- 23.Ohtori S, Inoue G, Orita S, et al. Comparison of teriparatide and bisphosphonate treatment to reduce pedicle screw loosening after lumbar spinal fusion surgery in postmenopausal women with osteoporosis from a bone quality perspective. Spine. 2013;38(8):E487–492. doi: 10.1097/BRS.0b013e31828826dd. [DOI] [PubMed] [Google Scholar]