Abstract
Aims
Catalase catalyzes the degradation of H2O2. Acinetobacter species have four predicted catalase genes, katA, katE, katG, and katX. The aims of the present study seek to determine which catalase(s) plays a predominant role in determining the resistance to H2O2, and to assess the role of catalase in Acinetobacter virulence.
Main Methods
Mutants of A. baumannii and A. nosocomialis with deficiencies in katA, katE, katG, and katX were tested for sensitivity to H2O2, either by halo assays or by liquid culture assays. Respiratory burst of neutrophils, in response to A. nosocomialis, was assessed by chemiluminescence to examine the effects of catalase on the production of reactive oxygen species (ROS)1 in neutrophils. Bacterial virulence was assessed using a Galleria mellonella larva infection model.
Key findings
The capacities of A. baumannii and A. nosocomialis to degrade H2O2 are largely dependent on katE. The resistance of both A. baumannii and A. nosocomialis to H2O2 is primarily determined by the katG gene, although katE also plays a minor role in H2O2 resistance. Bacteria lacking both the katG and katE genes exhibit the highest sensitivity to H2O2. While A. nosocomialis bacteria with katE and/or katG were able to decrease ROS production by neutrophils, these cells also induced a more robust respiratory burst in neutrophils than did cells deficient in both katE and katG. We also found that A. nosocomialis deficient in both katE and katG was more virulent than the wildtype A. nosocomialis strain.
Significance
Our findings suggest that inhibition of Acinetobacter catalase may help to overcome the resistance of Acinetobacter species to microbicidal H2O2 and facilitate bacterial disinfection.
Keywords: Acinetobacter, catalase, KatE, KatG, hydrogen peroxide, reactive oxygen species, neutrophils
INTRODUCTION
Acinetobacter is a catalase-positive, Gram-negative, and non-fermentative short rod bacterial genus known to cause nosocomial infections. The Acinetobacter calcocaceticus-baumannii (ACB) species complex comprises four species: A. baumannii, A. pittii, A. nosocomialis, and clinically unimportant A. calcoaceticus. A. baumannii is a well-established opportunistic pathogen that is becoming an increasingly important bacterial species for hospital-acquired infections. It has been estimated that A. baumannii accounts for more than 10% of all hospital-acquired infections in the United States and has a >50% mortality rate in patients with sepsis and pneumonia [16]. A. baumannii is often resistant to antibiotics and primarily affects people with a compromised immune system, particularly patients in the intensive care units (ICUs) after major surgical operations [3, 12]. While less well characterized, A. pittii and A. nosocomialis, which are also referred to as Acinetobacter genomic species 3 and 13TU, respectively, are highly similar to A. baumannii and are also frequently the source of human infections. A comprehensive analysis of the Acinetobacter isolates collected between 1995 and 2003 from 31 hospitals throughout the United States identified A. baumannii as the most prevalent Acinetobacter species, accounting for 63% of all isolates, followed by A. nosocomialis (21%) and A. pittii (8%) [42]. A similar study on the ACB species complex clinical isolates collected from six hospitals in Singapore indicated that A. baumannii constitutes 79% while A. pittii and A. nosocomialis constitute 9% and 12%, respectively, of the clinical isolates [19].
Acinetobacter species can cause life-threatening infections, particularly in immune compromised patients. A. baumannii is a common cause of hospital-acquired skin and soft-tissue infections, bacteremia, secondary meningitis, urinary tract infections, and nosocomial pneumonia, particularly late-onset ventilator-associated pneumonia, due to its ability to colonize indwelling medical devices [2, 6, 10, 26, 31]. It is also a common cause of periodontitis, endocarditis, intra-abdominal abscess, wound and surgical site infections [3, 12]. Multi-drug resistant A. baumannii strains have also become major bacterial species responsible for battle wound-associated infections in the United States military personnel injured in Iraq and Afghanistan [4, 6, 8]. Acinetobacter infections are notoriously difficult to treat because of their abilities to acquire resistance to a wide array of antibiotics. A. baumannii strains resistant to broad-spectrum cephalosporins, beta-lactam agents, aminoglycosides, and quinolones have been isolated [24, 36]. Resistance to carbapenems is also on the rise, raising serious concerns about the rapid decrease in clinically available antibiotics to treat these infections. It has been reported that hospitalized patients infected with A. baumannii have a mortality of about 30% [32]. For these reasons, strict guidelines have been developed to eliminate the transmission of multidrug-resistant A. baumannii in healthcare settings [1, 34]. In particular, emphases have been placed on infection prevention measures, including proper hand hygiene by healthcare providers, environmental and equipment cleaning, and disinfection.
Hydrogen peroxide is a powerful disinfectant with strong bactericidal activity. Vaporized H2O2 has been used to control outbreaks of multidrug-resistant A. baumannii infections in healthcare facilities [7, 33]. Hydrogen peroxide also plays an important role in the containment of bacterial infections by the immune system. Phagocytosis of bacterial particles by phagocytes, including neutrophils and macrophages, triggers a signal transduction pathway, leading to the assembly of NADPH oxidase complexes at the phagosomal membrane and the production of superoxide [17]. Superoxide is then converted by superoxide dismutase to H2O2, which is subsequently converted to a highly bactericidal substance hypochlorous acid by myeloperoxidase. This process is often referred to as the respiratory burst. Highlighting the critical role of the respiratory burst in the containment and clearance of infectious bacterial and fungal pathogens, defects in the respiratory burst process are identified as the underlying cause of chronic granulomatous disease [9]. Since Acinetobacter species contain multiple genes encoding for catalases, enzymes that degrade H2O2, we hypothesize that strains with mutations in the distinct catalases will exhibit differential sensitivity to H2O2. We further postulate that catalases of Acinetobacter species may attenuate the production of reactive oxygen species (ROS) by phagocytic cells of the innate immune system, thus, conferring resistance to respiratory burst-mediated killing by neutrophils, monocytes, and macrophages. In the present study, we assessed the sensitivities of various Acinetobacter strains lacking distinct catalases to H2O2 and examined the effects of catalase deficiency on neutrophil respiratory burst. We assessed the virulence of catalase-deficient A. nosocomialis strains using a Galleria mellonella larva infection model. Our studies demonstrate the pivotal role of the katG gene product in Acinetobacter resistance to H2O2. Our studies also show that although deletion of both katE and katG in Acinetobacter compromises their ability to attenuate ROS production in neutrophils, these catalase-deficient mutant actually exhibit great virulence in G. mellonella larvae, likely due to their attenuated induction of respiratory burst in phagocytes.
MATERIALS AND METHODS
Bacterial strains
The bacterial strains used in this study are listed in Table I. The A. baumannii strains were obtained from the University of Washington Transposon Mutant Collection. The parental strain, AB5075, a highly virulent strain of multi-drug resistant A. baumannii originally isolated from patients in the United States military health care system, has been previously described [11, 16]. The A. baumannii mutants lacking different catalase genes were generated by single insertion of the T26 transposons into the genome of AB5075 in the Manoil laboratory at the University of Washington [11]. The A. nosocomialis M2 strain has been described previously [5, 13]. Although it was initially regarded as an A. baumannii strain, the M2 strain has been re-categorized as an A. nosocomialis strain after determining the genomic sequence [5]. A. nosocomialis M2 mutants with deficiencies in various catalase genes were generated as previously described [5], using the primers listed in Table II. The authenticity of all genetic deletion mutants were confirmed by PCR and sequencing. All bacterial strains were grown at 37°C in tryptic soy broth medium (Becton, Dickinson and Company, Franklin Lakes, NJ).
Table I.
Bacterial strains used in this study
| Strain | Species | Strain nomenclature | Genotype | Means of disruption |
Catalase test |
|---|---|---|---|---|---|
| AB5075-UW | A. baumannii | WT | + | ||
| AB06621 | A. baumannii | tnab1_kr130916p04q128 | katA mutant::T26 | T26 | + |
| AB06618 | A. baumannii | tnab1_kr121204p05q190 | katA mutant::T26 | T26 | + |
| AB06423 | A. baumannii | tnab1_kr130917p12q154 | katE mutant::T26 | T26 | − |
| AB06425 | A. baumannii | tnab1_kr121213p04q158 | katE mutant::T26 | T26 | − |
| AB09109 | A. baumannii | tnab1_kr121212p02q109 | katG mutant::T26 | T26 | + |
| AB09110 | A. baumannii | tnab1_kr121127p08q183 | katG mutant::T26 | T26 | + |
| AB5352 | A. baumannii | tnab1_kr121204p05q181 | katX mutant::T26 | T26 | + |
| AB5354 | A. baumannii | tnab1_kr121203p08q171 | katX mutant::T26 | T26 | + |
| AB5355 | A. baumannii | tnab1_kr130913p10q131 | katX mutant::T26 | T26 | + |
| M2 | A. nosocomialis | WT | + | ||
| M2ΔkatE | A. nosocomialis | ΔkatE::FRT | Flp-FRT | − | |
| M2ΔkatG | A. nosocomialis | ΔkatG::FRT | Flp-FRT | + | |
| M2ΔkatX | A. nosocomialis | ΔkatX::FRT | Flp-FRT | + | |
| M2ΔkatEkatG | A. nosocomialis | ΔkatE::FRT ΔkatG::FRT | Flp-FRT | − | |
| M2ΔkatEkatX | A. nosocomialis | ΔkatE::FRT ΔkatX::FRT | Flp-FRT | − | |
| M2ΔkatGkatX | A. nosocomialis | ΔkatG::FRT ΔkatX::FRT | Flp-FRT | + | |
Table II.
Primers used for the disruption of the A. nosocomialis catalase genes
| Primer | Sequence | Function |
|---|---|---|
| M2katE_Fwd | ATGATGATCTTTTACGCAGTCT | To clone M2 katE and 1-kb flanking DNA into pGEM-T Easy |
| M2katE_Rev | GCATATTAATCACTATAAAGGGAC | |
| M2katErecomb_Fwd | TTTTCAACTTCAACCCAAGTTTAACTTTCAAAAC ATAGGTAATGGACATGATTCCGGGGATCCGTCG ACC |
Recombineering primers to delete M2 katE and replace with Tn903-sacB |
| M2katErecomb_Rev | GAAATCTTCAACTTTCAGGGCTTTTTTATTTAAG CCGGTACGTGTGCGGCTGTAGGCTGGAGCTGCT TCG |
|
| M2katG_Fwd | CGGGATTATGGTAGAGGTA | To clone M2 katG and 1-kb flanking DNA into pGEM-T Easy |
| M2katG_Rev | GCATATTAATCACTATAAAGGGAC | |
| M2katGrecomb_Fwd | GGTTCCATTAAGAGCTCAAATAAATATTACCCA GAGAGAATATAATCATGATTCCGGGGATCCGTC GACC |
Recombineering primers to delete M2 katG and replace with Tn903-sacB |
| M2katGrecomb_Rev | GAACTGGCTTTTTATTATTTCGACTTAAATTAAG CTAAGTCAAAACGGTCTGTAGGCTGGAGCTGCT TCG |
|
| M2katX_Fwd | ATCTATTAATATCACTTCTCCAG | To clone M2 katX and 1-kb flanking DNA into pGEM-T easy |
| M2katX_Rev | ATCAATGTTAGCTTTTTATTATCT | |
| M2katXrecomb_Fwd | ACATATTATTTTAATTCTAAAGTGGACTTTGCA ATAAGGAGCAATGAATGATTCCGGGGATCCGTC GACC |
Recombineering primers to delete M2 katX and replace with Tn903-sacB |
| M2katXrecomb_Rev | TTTATATGTAAAGTATGACGAACTCACTTTTAA GGCTCTTGTCGAGGGGCTGTAGGCTGGAGCTGC TTCG |
|
Chemicals and reagents
Tryptic soy broth powder was purchased from BD (Becton, Dickenson & Co., Sparks, MD, USA). Hydrogen peroxide and luminol were purchased from Sigma-Aldrich (St Louis, MO). FITC-conjugated rat monoclonal antibody against mouse Ly-6G and APC-conjugated rat monoclonal antibody against mouse CD11b were purchased from Biolegend (San Diego, CA).
Assessment of H2O2 sensitivity
For halo assays, bacteria were grown overnight at 37°C with shaking at 300 rpm. The bacteria were sub-cultured by diluting in fresh medium at 1:10 and allowing to grow for 5 h. The cultures were then diluted with fresh tryptic soy broth medium to an optical density (OD) of 0.1 measured at 600 nm. Eighty microliters of the diluted culture was added to 4 ml of heat-melted 0.7% soft agar maintained at 42°C, vortexed thoroughly, and overlaid onto solidified tryptic soy broth with 1.5% agar (10 ml) in petri dishes. The top agar was allowed to solidify for 30 min. Sterilized dry Whatman 3MM filter discs of 8-mm diameter were placed on the soft agar (which contained bacteria), and 10 µl of 2% H2O2 was spotted onto the discs. The petri dishes were incubated at 37°C in humidified environment overnight. The diameters of the halos were measured with a digital caliper (General Tools, Secaucus, NJ).
To measure the sensitivity of the bacterial strains to H2O2 in liquid culture, bacterial cultures were diluted in fresh medium to a final OD600 of 0.01. Hydrogen peroxide was then added to the cell suspensions to different concentrations ranging from 0–0.02% (equivalent to 0–8.5 mM). The mixtures were then added to 96 well plates in triplicate and placed in a SpectraMax M2 spectrophotometer (Molecular Devices, Sunnyvale CA). Bacteria were cultured at 37°C with constant shaking. The optical density of the culture in the wells were measured automatically every 30 min for 16 h.
Assessment of catalase gene expression
A. nosocomialis strain M2 was grown in LB, with shaking at 180 rpm, and cell density determined by measuring optical density at 600 nm. When cultures were in exponential phase, and then in early stationary phase, cells were split into two aliquots, one of which was challenged with 30 mM H2O2 for 10 min. Cells were then pelleted at 3,220×g for 10 min at 4°C, the medium removed and cells resuspended in TRIzol (Life Technologies, Carlsbad, CA). RNA was isolated and catalase expression determined as previously described [14]. Briefly, expression of catalase was determined by quantitative reverse transcription (RT)-PCR (qRT-PCR) using a one-step QuantiTect SYBR green RT-PCR kit (Qiagen, Valencia, CA), with the primers described in Table III. Five biological replicates and three technical replicates were performed for each condition tested. Threshold cycle (CT ) values were normalized to the value for the endogenous control gyrA, and relative quantitation was calculated from the median CT value using ΔΔCT . Statistical significance was determined using a Student’s two-tailed t test. A fold change in catalase expression greater than 2-fold and with a p value of <0.05 was assessed as significant.
Table III.
Primers used for the assessment of katE and katG expression
| Primer | Sequence | Function |
|---|---|---|
| M2 katE_qRTPCR F1 | AGATGGCGATAATTTAAATGCAGTGATG | Quantitative PCR for katE gene expression in A. nosocomialis |
| M2 katE_qRTPCR R1 | CGGTACGTGTGCGGCTATTTGTT | |
| M2 katG_qRTPCR F1 | TTGGTGGTTTACGTGTACTGGGCA | Quantitative PCR for katG gene expression in A. nosocomialis |
| M2 katG_qRTPCR R1 | CCGTCGGCTTGAGCATAAACCTC | |
| M2 gyrA_qRTPCR F1 | TGATTTCTGATGGTGGTACGCTTGTT | Quantitative PCR for gyrA gene expression in A. nosocomialis |
| M2 gyrA_qRTPCR R1 | ATACAACTTCTTCGCTATCAGTTTCAGTCGT | |
| 16S_RT_F | CTTCGGACCTTGCGCTAATA | Quantitative PCR for 16S rRNA expression in A. baumannii |
| 16S_RT_R | ATCCTCTCAGACCCGCTACA | |
| katG_RT_F | GGCGATGAAAAAGAATGGTTA | Quantitative PCR for katG expression in A. baumannii |
| katG_RT_R | ATTTCTTCATCATCCATTGCC | |
| katE_RT_F | AACTTTGACTTCGATTTGCTGGA | Quantitative PCR for katE expression in A. baumannii |
| katE_RT_R | TGTATGAAAATAGTCGGGCTTGT | |
To assess katE and katG expression in different growth phases in A. baumannii, the wildtype (WT) strain, AB5075, was grown in LB broth overnight at 37°C with shaking at 250 rpm. Subsequently, the cultures were diluted 1:100 (v/v) in fresh medium to 3-ml final volumes, and incubated at 37°C with shaking at 250 rpm. At an optical density of ~0.6 at 600 nm (exponential growth phase) and again at an A600~1.4 (early stationary phase) 1.5 ml of cells were removed for RNA extraction. RNA extractions were carried out using the RNeasy kit (Qiagen, Mississauga, ON, Canada) according to the manufacturer’s instructions. RNA samples (1 µg per sample) were then digested with DNase (Qiagen, Mississauga, ON, Canada) to remove genomic DNA. cDNA was synthesized using SuperScript® VILO™ cDNA synthesis kit (Life Technologies) along with a no reverse transcriptase control to determine each sample was free of genomic DNA. Levels of katG and katE cDNAs were assessed by qPCR using SYBR® Select Master Mix (Life Technologies) using primers presented in Table III. The 16s ribosomal RNA was used as an internal control for normalization. Fold of changes of gene expression was calculated from the median CT value using ΔΔCT .
Catalase activity testing
To categorize the catalase status of the bacteria, a small amount of A. baumannii bacteria was collected from an isolated colony after overnight growth 37°C, using a sterile inoculating loop, and placed on a microscope glass slide. A drop of 2% H2O2 was placed onto the bacteria. Bubble formation was examined under a microscope. The formation of bubbles indicative of oxygen generation is an indication of catalase positivity.
To quantify catalase activity in stationary phase, single colonies from strains of interest were inoculated into 2 ml of LB (Becton, Dickenson & Co., Sparks, MD, USA) and grown overnight at 37°C with shaking at 200 rpm. The next day catalase activity was measured by mixing 1.94 ml of 50 mM potassium phosphate ( pH 7.4) (Becton, Dickenson & Co.) with 50 µl of H2O2 (Fisher Scientific, ON, Canada) and 5–10 µl of the overnight culture. A Clark oxygen electrode (Cole-Parmer, QC, Canada 05520–13) connected to a Gilson oxygraph, was then used to measure oxygen generation, as previously described [35]. The catalase units/mg of dry weight was calculated by using the velocity of oxygen production, OD600 and dilution factors of the cultures [15]. One unit of catalase activity was defined as the amount that decomposes 1 µmol H2O2 in 1min at 37°C. Measurements for each strain were carried out in biological triplicate.
To quantify catalase activity at differential phase of growth, overnight bacterial cultures of A. baumannii strains were diluted 1:100 (v/v) in fresh medium, and incubated at 37°C with shaking at 250 rpm. At an optical density of ~0.6 at 600 nm (exponential growth phase) and again at an A600~1.4 (early stationary phase) cells were removed for catalase activity determination.
Respiratory burst assays
Neutrophils were purified from the bone marrow of C3H/HeN mice by antibody-mediated depletion of red cells and other leukocytes using a Neutrophil Isolation Kit (Miltenyl Biotech, San Diego, CA). The purified neutrophils were stained with a FITC-conjugated rat monoclonal antibody against mouse Ly-6G, a neutrophil cell surface marker, and an APC-conjugated rat monoclonal antibody against mouse CD11b, a myeloid lineage marker. The percentage of neutrophils in the purified cells was determined by flow cytometry. To prepare for opsonized bacteria, A. nosocomialis strains were grown overnight at 37°C in tryptic soy broth, washed three times with phosphate-buffered saline (PBS), and pelleted by centrifugation. Then bacteria (8×106−2.4×108 CFU) were suspended in 80 µl mouse serum, and incubated at 37°C for 1 h. Following opsonization, 10 µl of the bacteria/serum mixture were aliquoted into the wells on a 96 well plate. As a control, the same volume of mouse serum was added into additional wells. Respiratory burst assays were initiated by mixing 105 neutrophils with opsonized bacteria in the wells containing 100 µM luminol. Respiratory burst activity, measured using chemiluminescence, were monitored by taking sequential images in an IVIS Spectrum imaging system (Caliper Life Sciences, Hopkinton, MA) over 90 min, essentially as described [43]. Respiratory burst activity was calculated as the cumulative chemiluminescence in a given period.
Galleria mellonella larva infection model
The wild type (WT) A. nosocomialis M2 and the M2ΔkatEΔkatG strains were grown overnight at 37°C, with shaking at 300 rpm. The cultures were then diluted 10-fold into fresh medium and grown for 3 h. Cells were collected by centrifugation, washed twice in PBS, and resuspended in PBS to a concentration of 2 × 107 CFU/ml. G. mellonella larvae (Vanderhorst Wholesale, Saint Marys, OH) were used within 7 days of shipment from the vendor. Larvae were kept in the dark at room temperature before infection. Larvae weighing 200 to 300 mg were used in the survival assays as described previously [16]. Briefly, 5 µl of the bacteria suspension containing 1 × 105 CFU of bacteria was injected into the last left proleg of the larvae using a 10-µl glass syringe (Hamilton, Reno, NV) fitted with a 30-G needle (Novo Nordisk, Princeton, NJ). Each experiment included control groups of non-injected larvae or larvae injected with 5 µl sterile PBS. Injected larvae were incubated at 37°C in humidified environment, and death was assessed everyday over 8 days. Larvae were considered dead if they did not respond to physical stimuli. Experiments were repeated four times using 10–20 larvae per experimental group. Differences in survival between worms infected with the parental WT M2 strain and the M2 ΔkatEΔkatG double mutant of A. nosocomialis were determined by Kaplan-Meier analysis with log-rank test, using SigmaPlot software (Systat Software Inc, San Jose, CA).
RESULTS AND DISCUSSION
Catalase activity in the catalase gene-deficient A. baumannii mutants
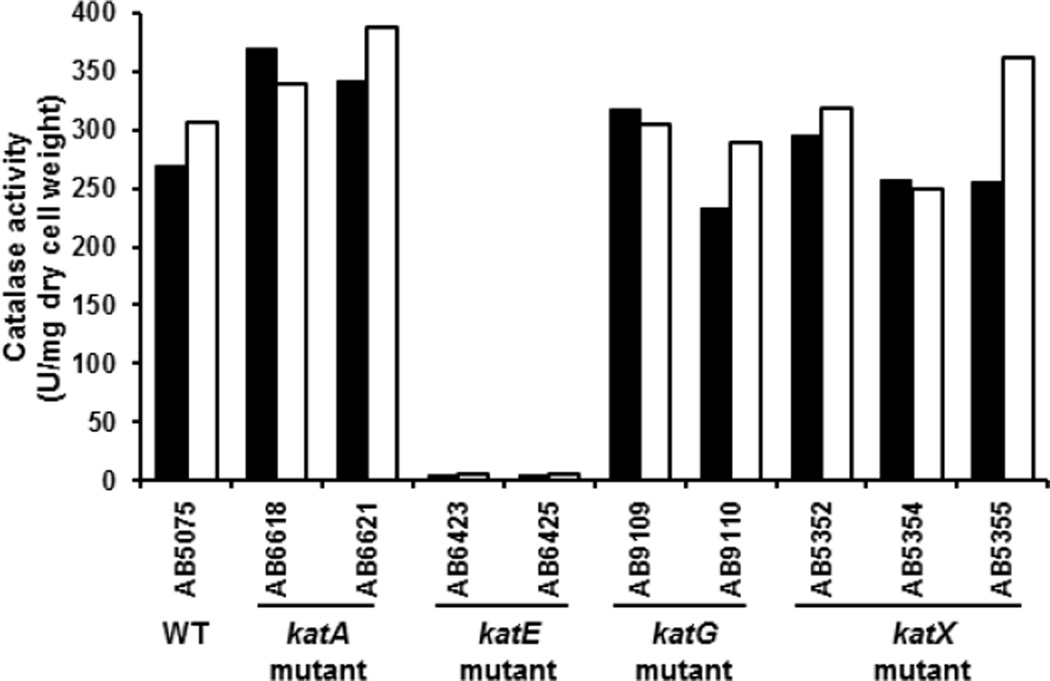
A.baumannii has four predicted catalase genes katA (Ab locus: ABUW_2504), katE (Ab locus: ABUW_2436), katG (Ab locus: ABUW_3469), and katX (Ab locus: ABUW_2059). The katA gene is referred to as “catalase” in the A. baumannii mutant library database (http://www.gs.washington.edu/labs/manoil/baumannii.htm), while katX is referred to as “catalase domain-containing”. katA encodes a small-subunit mono-functional catalase; katE encodes a large-subunit mono-functional catalase; and katG encodes a catalase-peroxidase whereas katX encodes a small protein with a catalase-domain. The parental WT strain, AB5075, has been described previously [16], and its genome was recently sequenced [11]. Strains with transposon insertions in each individual catalase gene were obtained from the Manoil Laboratory at The University of Washington, and cultured to test catalase activity. Since catalase degrades H2O2 to generate O2, catalase-positive bacteria produce oxygen bubbles upon incubation with H2O2. The two A. baumannii strains (AB6423 and AB6425) with transposon insertions in katE exhibited a catalase-negative phenotype. In contrast, strains with transposon insertions in katG (AB9109 and AB9110), katA (AB6618 and AB6621), and katX (AB5352, 5354, and 5355) exhibited a catalase-positive phenotype that was indistinguishable from WT strain (AB5075) (Table I). This qualitative visual assay of catalase activity was confirmed by a quantitative determination in cells grown into stationary phase. These analyses indicated that strains with transposon insertions in the katE gene had virtually no catalase activity, while strains with transposon insertions in katA, katG, and katX, had catalase activities comparable to that of the parental WT strain (Fig. 1). These results are consistent with the idea that KatE is the primary enzyme responsible for H2O2 degradation in stationary phase A. baumannii.
Figure 1.
The catalase activity of the WT and various catalase mutant A. baumannii strains. A. baumannii cells were cultured overnight, and catalase activity toward H2O2 was determined by measuring production using a Clark oxygen electrode connected to a Gilson oxygraph. Values were normalized to the dry weight of bacteria. Results represent data from two independent experiments. Solid and open bars represent results obtained from the two separate experiments.
katG of A. baumannii is a determinant of resistance to H2O2
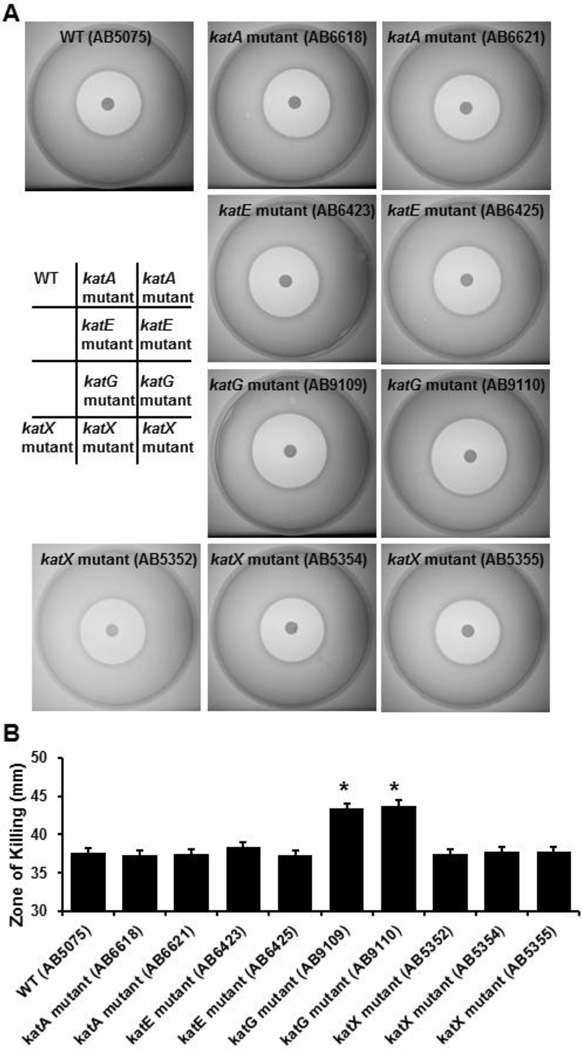
To assess the contributions of the different predicted catalases to the resistance of A. baumannii to H2O2, we performed halo assays. In these assays sensitivity of bacteria to H2O2 is indicated by the diameter of the zone of killing encircling the H2O2-impregnated filter disc (Fig. 2A). When all A. baumannii catalase mutants were tested, H2O2 produced a significantly greater zone of killing on the bacterial lawns of both katG mutants tested, as compared to the parental strain (Fig. 2B). This stands in contrast to the observation that loss of katG had no effect on total catalase activity in stationary phase (Fig. 1). Further, even though disruption of katE almost completely eliminated the total catalase activity in stationary phase A. baumannii, disruption of katE had no significant effect on the sensitivity of A. baumannii to H2O2. Similarly, loss of either katA or katX genes had no effect on the sensitivity of A. baumannii to H2O2. We also compared the sensitivities of the A. baumannii parental strain and the catalase mutants to the most common germicides used in both hospitals and laboratories. When the strains were tested against 30% acetic acid, 70% ethanol, bleach (6.15% sodium hypochlorite), and 1.75% iodine, no significant differences were detected (data not shown).
Figure 2.
The sensitivity of the different A. baumannii strains to H2O2. A baumannii strains were cultured overnight, and then grown for 5 hours in fresh medium. Bacteria were adjusted according to their optical density and seeded into soft agar on tryptic soy broth medium containing 1.5% agar. Hydrogen peroxide (2%, 10 µl) was spotted onto sterilized dry filter discs of 8-mm diameter. The plates were incubated at 37°C overnight. The halos were photographed and diameters were measured. A. Halo formation caused by H2O2 on lawn containing different A. baumannii strains. Representative images are shown. B. Diameters of the halos caused by H2O2 for distinct A. baumannii strains. Bars are means ± standard error from 5 independent experiments. *, p<0.05, compared to the WT strain (AB5075) (Student’s t-test).
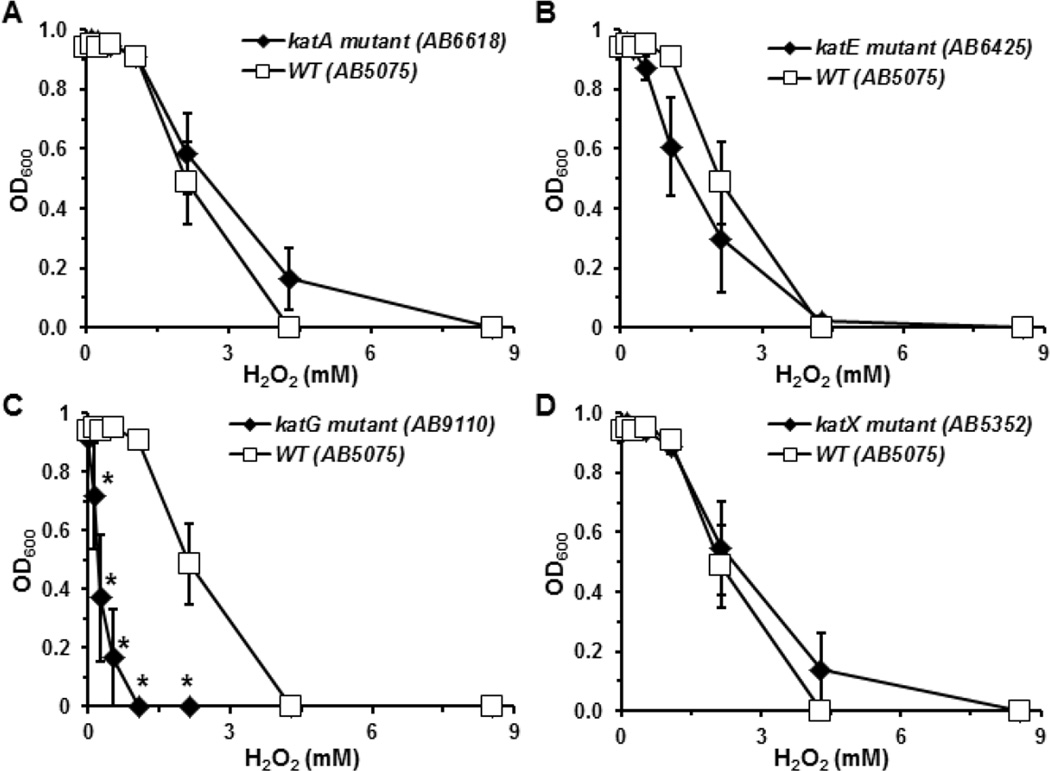
To assess whether similar H2O2 sensitivity profiles would be observed with cells grown in liquid culture, we grew the bacteria until they reached the exponential phase, then continued growth in the presence of H2O2 that ranged in concentration from 0 to 8.5 mM. A representative strain was chosen from each group for analysis. Consistent with the idea that KatG plays a dominant role in determining the resistance of A. baumannii, to H2O2, the katG mutant strain (AB9110) exhibited the greatest sensitivity to H2O2 amongst all A. baumannii strains tested (Fig. 3C). Conversely, the katA and katX mutant strains showed similar sensitivities to H2O2 as the parental strain (Fig. 3A, 3D), while the katE mutant strain (AB6423) of A. baumannii appeared only slightly more sensitive to H2O2 (Fig. 3B), despite the almost complete absence of measurable catalase activity in stationary phase cultures (Fig. 1). These results suggest that resistance to H2O2 in A. baumannii is not solely dependent on their ability to degrade H2O2.
Figure 3.
The inhibitory effects of H2O2 on the growth of different A. baumannii strains in liquid culture. A. baumannii strains were cultured overnight, and then grown for 5 hours in fresh medium. The cultures were diluted to a final optical density of 0.01 into new tryptic soy broth with increasing concentrations of H2O2. Bacteria were cultured at 37°C in a spectrophotometer with agitation for 16 h, and OD600 were measured every 30 min. Data presented were optical density of different strains reached after 12 h in medium containing the indicated concentrations of H2O2. Dose-dependent inhibition of katA, katE, katG, and katX mutants was presented in A, B, C, and D. Inhibition of growth of the parental strain by H2O2 is presented in comparison with the mutant strains. Note, all assays were done concurrently, so the same parental data is displayed on all four panels. Data were presented as means ± standard error of 5 independent experiments. *, p<0.05, compared to the WT strain (AB5075) at the same concentration of H2O2 (Student’s t-test).
KatG plays a predominant role in H2O2 resistance while KatE also contributes to H2O2 resistance
The Acinetobacter species A. nosocomialis and A. baumannii are closely related. In fact, A. nosocomialis was only categorized as a new species in 2011 [30]. Based on the genomic sequence, A. nosocomialis strain M2 is predicted to have three catalase genes, katE, katG, and katX. The KatE orthologues of A. baumannii and A. nosocomialis share 98% identity, while the KatG orthologues of the two species have 96% identity. The identity of the KatX orthologues of these two species are significantly lower (78%). A. nosocomialis M2 strains which lacked katE, katG or katX, as well as strains that lacked two catalase genes were tested for catalase activity using the slide-based oxygen bubble forming assay. Similar to what we observed with the A. baumannii strains, only the strains lacking the katE gene, either alone or in combination with another catalase gene, exhibited a catalase-negative phenotype (Table I). This result is consistent with katE being preferentially and highly expressed in stationary phase cells [22, 38].
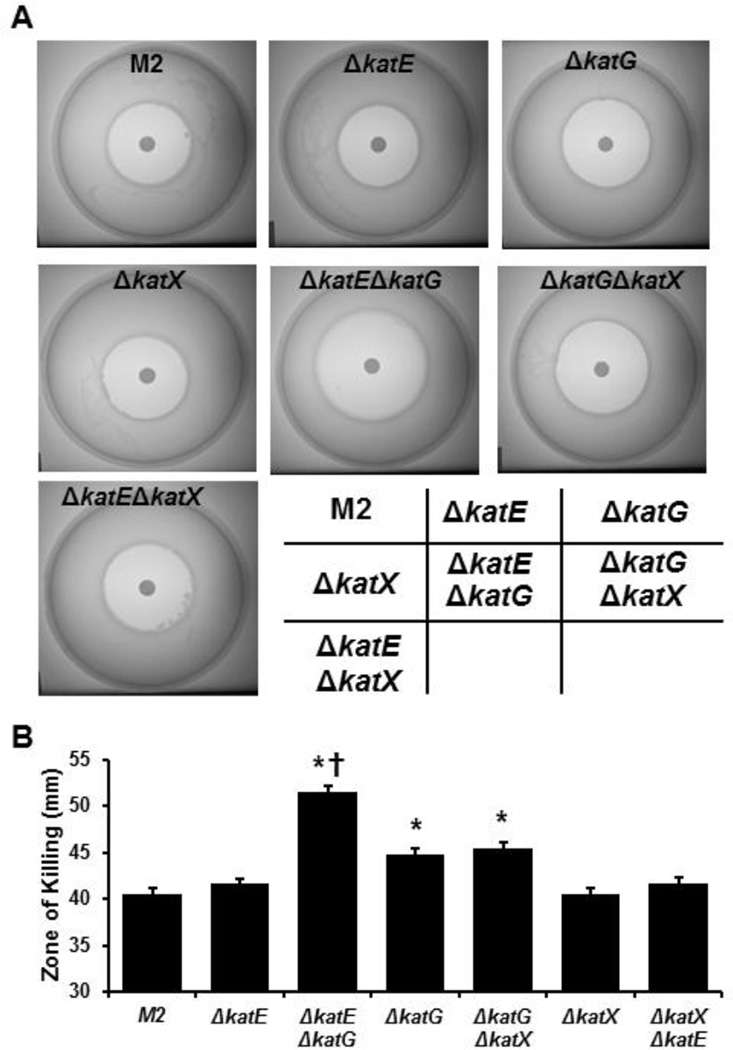
The sensitivities of the catalase-deficient A. nosocomialis strains to H2O2 were assessed using the halo assays (Fig. 4A), with results similar to those observed with A. baumannii strains. The zone of killing of the ΔkatG mutant, indicated by the halo, was significantly larger than that of the parental A. nosocomialis strain, while the zones of killing of the ΔkatE and ΔkatX strains were similar to that of the parental strain (Fig. 4B). Loss of both katG and katE resulted in an even greater zone of killing, whereas loss of katG and katX produced a similar level of killing as loss of katG alone. These results suggest that in A. nosocomialis there is a hierarchy of importance in the catalases with regard to protection against the killing by H2O2: KatG plays the predominant role, KatE plays a minor role, while KatX does not appear to be important.
Figure 4.
The sensitivity of WT M2 A. nosocomialis strain and its derivatives deficient in katE, katG, both katE and katG to H2O2. The A. nosocomialis strains were cultured overnight, and then grown for 5 hours in fresh medium. Bacteria were adjusted according to their optical densities and seeded into soft agar on tryptic soy broth medium containing 1.5% agar. Hydrogen peroxide (2%, 10 µl) was spotted onto sterilized dry filter discs of 8-mm diameter. The plates were incubated at 37°C overnight. The halos were photographed and diameters of the halos were measured using a digital caliper. A. Halo formation caused by H2O2 on lawn containing different A. nosocomialis strains. Representative images are shown. B. Diameters of the halos caused by H2O2 for distinct A. nosocomialis strains. Bars are means ± standard error from 5 independent experiments. *, p<0.05, compared to the parental M2 strain. †, p<0.05, compared to the ΔkatG strain. Student’s t-test was used for comparison.
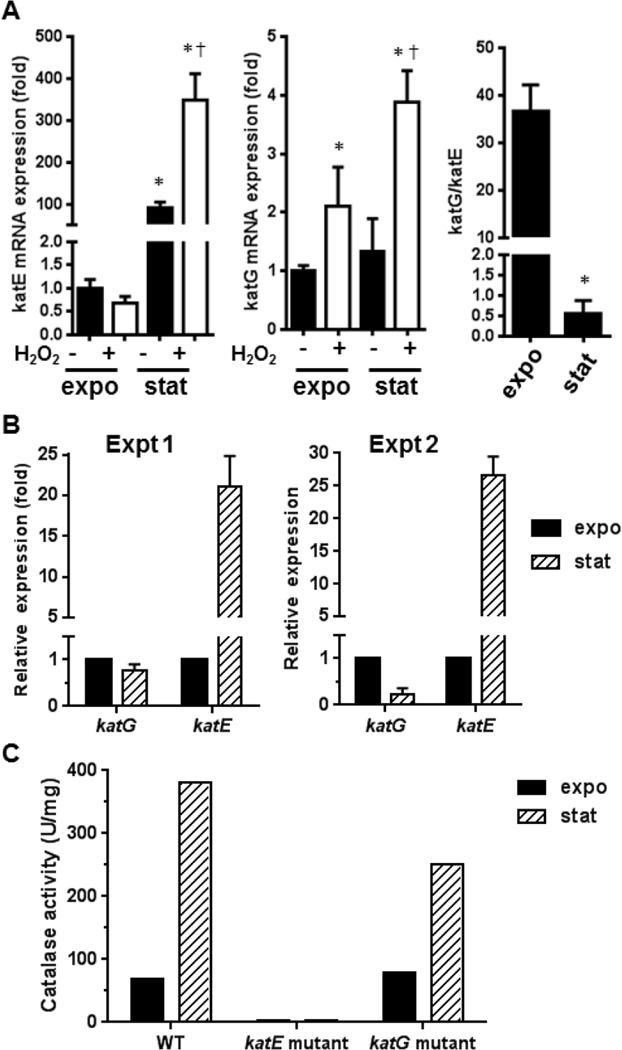
Previously, it has been shown that in E. coli katG expression is rapidly induced during the rapid exponential growth phase [22, 27]. Additionally, katG transcription is substantially enhanced in response to H2O2 through a transcriptional mechanism mediated by OxyR [27], a transcription factor sensitive to oxidative stress [39]. Conversely, katE is transcribed at the transition from exponential growth to stationary phase by RNA polymerase containing the alternative sigma subunit σs, the product of the rpoS gene [23, 28]. Thus, it is possible that upon inoculation into fresh medium containing H2O2, OxyR induces katG expression and KatG gradually becomes the enzyme that contributes to the majority of the catalase activity mediating the degradation of H2O2. In contrast, in stationary phase KatE contributes to most of the catalase activity (Fig. 1), but during exponential growth phase (likely the growth state of cells in both the halo assays and the culture growth assays), KatE contributes a smaller portion of the total catalase activity. To explore this possibility, either exponentially growing A. nosocomialis strain M2 or cells in stationary phase were treated with with H2O2 then expression of katE and katG quantified by qRT-PCR (Fig. 5A). The katE gene was preferentially expressed in the stationary phase. Stimulation of A. nosocomialis cells with H2O2 increased katE expression at the stationary phase, but had little effect during exponential growth (Fig. 5A, left graph). In contrast, basal katG expression levels were similar in both stationary and exponential growth phases. H2O2 stimulation enhanced katG expression in both phases (Fig. 5A, middle graph). The differential expression of the katE and katG genes at different phases is shown in the right hand graph of Figure 5A.
Figure 5.
The effects of growth phase and H2O2 on the expression of katE and katG in A. nosocomilis and A. baumannii A. Expression levels of katE and katG in exponential growth phase and stationary phase A. nosocomialis in the presence and absence of H2O2. A. nosocomialis M2 strain was grown to exponential growth phase (labeled as expo) or early stationary phase (labeled as stat), and then treated with 30 mM H2O2 for 10 min. The mRNA levels of katE and katG were assessed by qRT-PCR. Five biological replicates and three technical replicates were performed for each condition tested. The expression level at the exponential growth phase in the absence of H2O2 was set as 1. Comparison between treatment groups were made using Student’s two-tailed t test. *, p<0.05, comparing to exponential growth phase. †, p<0.05, comparing to cells that were not stimulated with H2O2. B. Expression levels of katE and katG in exponential growth phase and stationary phase A. baumannii. WT A. baumannii strain (AB5075) was grown to exponential growth phase (OD600=0.6) or early stationary phase (OD600=1.4). Cells were harvested to purify total RNA. The mRNA levels of katE and katG were assessed by qRT-PCR. The expression level at the exponential growth phase was set as 1. Data presented are from two independent experiments. C. Catalase activity of WT, the katE mutant, and the katG mutant at exponential growth phase and stationary phase A. baumannii. A. baumannii cells were grown to exponential growth phase or early stationary phase as in B, catalase activity in the cell lysate were determined as in Figure 1. Results from a representative experiment were shown.
Similarly, we also examined the expression patterns of katE and katG in A. baumannii during exponential growth and in stationary phase. As observed in A. nosocomilis, katE expression levels in the parental A. baumannii strain (AB5075) were dramatically increased in stationary phase, as compared to during exponential growth (Fig. 5B). In contrast, katG expression appeared to be greater during exponential growth as compared to stationary phase. Measurement of the enzymatic activity in exponential growth and stationary phases clearly indicates that catalase activity was substantially greater in stationary phase than in the exponential growth phase in both the parent and the katG mutant strains (Fig. 5C). It should be stressed that total catalase activity in the katG mutant strain were comparable to that in the parental A. baumannii strain during both exponential growth and in stationary phase, whereas catalase activity in the katE mutant strain were almost absent in both exponential growth and stationary phases. Thus, although katG expression appeared to be greater during exponential growth phase, the H2O2-sensitive phenotype cannot be explained by a decrease in total catalase activity in the exponential growth phase. Our results suggest that the H2O2-sensitive phenotype of the katG mutant is not likely due to a defect in cells’ catalase activity, as catalase activity in these katG mutant cells was comparable to those of parental cells. This is also consistent with the observation that the katE mutant was almost completely deficient in catalase activity but exhibited nearly normal H2O2 resistance (Fig. 5C).
A. nosocomialis deficient in both katE and katG elicit a weaker ROS production by neutrophils and display enhanced virulence in a G. mellonella larvae model
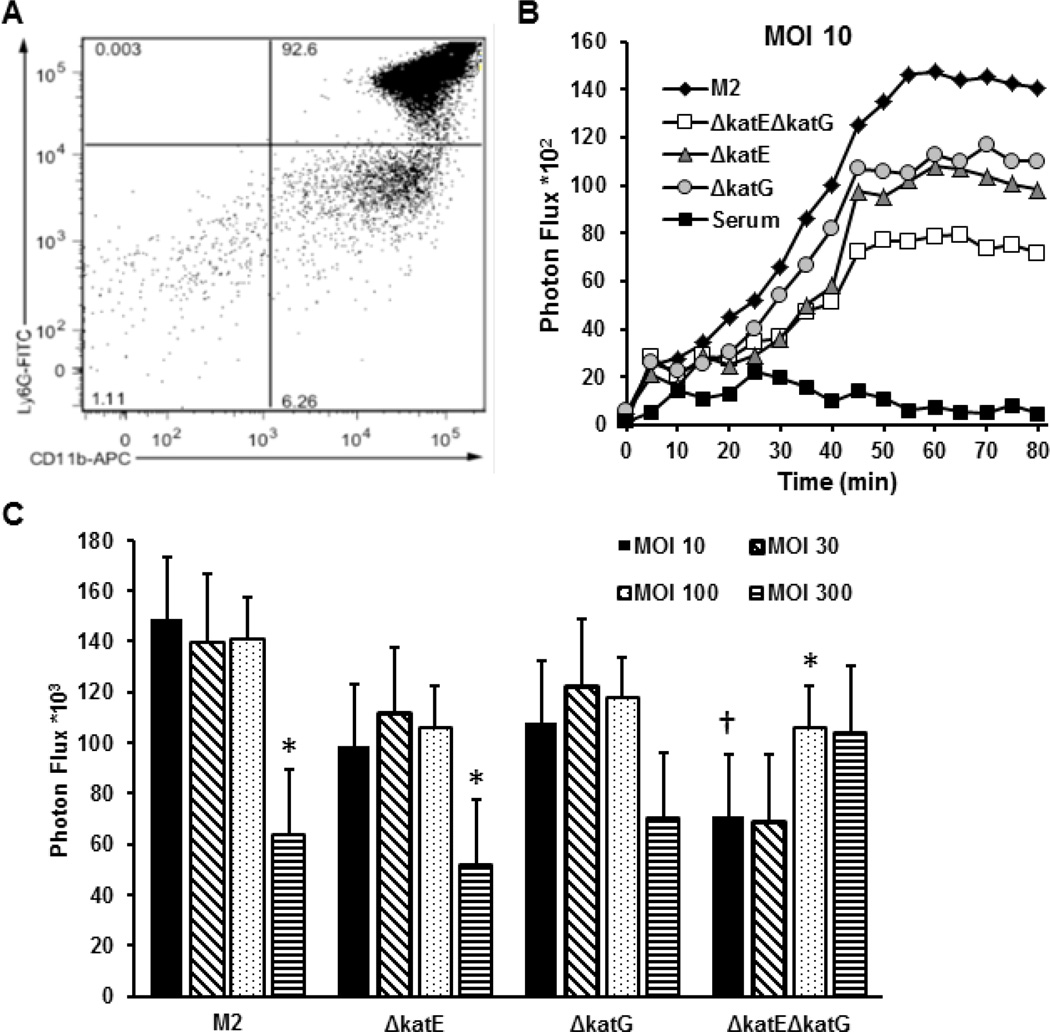
Phagocytes such as neutrophils, monocytes, and macrophages produce H2O2 and other ROS for bacterial killing through a process often referred to as respiratory burst [17, 29]. We hypothesized that strains deficient in catalase would exhibit a lower capacity to counteract the respiratory burst and so be more sensitive to killing by phagocytic cells. We tested this hypothesis using purified murine neutrophils isolated from mouse bone marrow. Flow cytometry analysis indicated that the cell preparations contained ~92% Ly-6G+CD11b+, ~6% Ly-6G-CD11b+, and ~2% Ly-6G‒CD11b‒ cells (Fig. 6A). As Ly-6G is only expressed in neutrophils of the CD11b+ myeloid class, we concluded that neutrophils constituted ~92% of the neutrophil preparations, with ~6% were likely monocytes (CD11b+). To assess the effect of bacterial catalase on ROS production in neutrophils in response to bacteria, we opsonized WT A. nosocomialis strain M2 and the ΔkatE, ΔkatG, and ΔkatEΔkatG mutant strains with normal murine serum. The opsonized bacteria were then incubated with purified murine neutrophils in the presence of luminol. The kinetics of ROS production by the neutrophils were documented by measuring the time course of the photon flux. As shown in Figure 5B, normal murine serum did not stimulate a production of ROS. Exposure of neutrophils to all four A. nosocomialis strains (M2, ΔkatE, ΔkatG, and ΔkatEΔkatG) induced a time-dependent increase in ROS production that reached its peak levels at 45 to 55 min, indicating an increase in respiratory burst activity in neutrophils after phagocytosis of the bacteria (Fig. 6B). Consistent with the notion that KatE and KatG in A. nosocomialis cells counteract production of ROS by neutrophils, at a high multiplicity of infection (MOI) of 300 the parent and the ΔkatE and ΔkatG mutant strains caused a reduced level of ROS production, but the ΔkatEΔkatG mutant caused an increase in ROS production. The fact that either KatE or KatG was sufficient to reduce peroxide production suggests overlapping function of the two catalases in the degradation of ROS. Paradoxically, ROS production induced by the ΔkatEΔkatG mutant cells at MOI of 10 or 30 was far lower than that induced by the parental A. nosocomialis cells, suggesting that the KatE and/or KatG protein(s) facilitate(s) the recognition of bacteria or activation of the respiratory burst by neutrophils (Fig. 6C). In other words, the catalases exhibit competing roles in destroying peroxide to confer resistance to respiratory burst-mediated bacterial killing while at the same time enhancing the respiratory burst of the innate immune cells.
Figure 6.
Neutrophil respiratory burst induced by the A. nosocomialis parent and mutant strains deficient in katE, katG, or both katE and katG. A. Scot plot of the flow cytometry data on a neutrophil preparation after labeling with FITC-Ly-6G and APC-CD11b. Murine neutrophils were purified from bone marrow of C3H/HeN mice using a neutrophil isolation kit. The purity of neutrophils was assessed by flow cytometry after staining with FITC-Ly-6G and APC-CD11b. B. Kinetics of the respiratory burst of neutrophils exposed to parent, ΔkatE, ΔkatG, and ΔkatEΔkatG A. nosocomialis at a MOI of 10, or exposed to the same volume of serum. Respiratory burst activity of neutrophils was measured by luminol chemiluminescence after incubating neutrophils with opsonized bacteria and luminol in an IVIS Spectrum system. C. Cumulative respiratory burst activity of neutrophils in 90 min after incubation with escalating amounts of parent, ΔkatE, ΔkatG, and ΔkatEΔkatG A. nosocomialis. Data are presented as means ± standard errors of the values from 3 independent experiments. *, p<0.05 compared to value at MOI of 10 of the same strain. †, p<0.05, compared to M2 strain at the MOI of 10 (Student’s t-test).
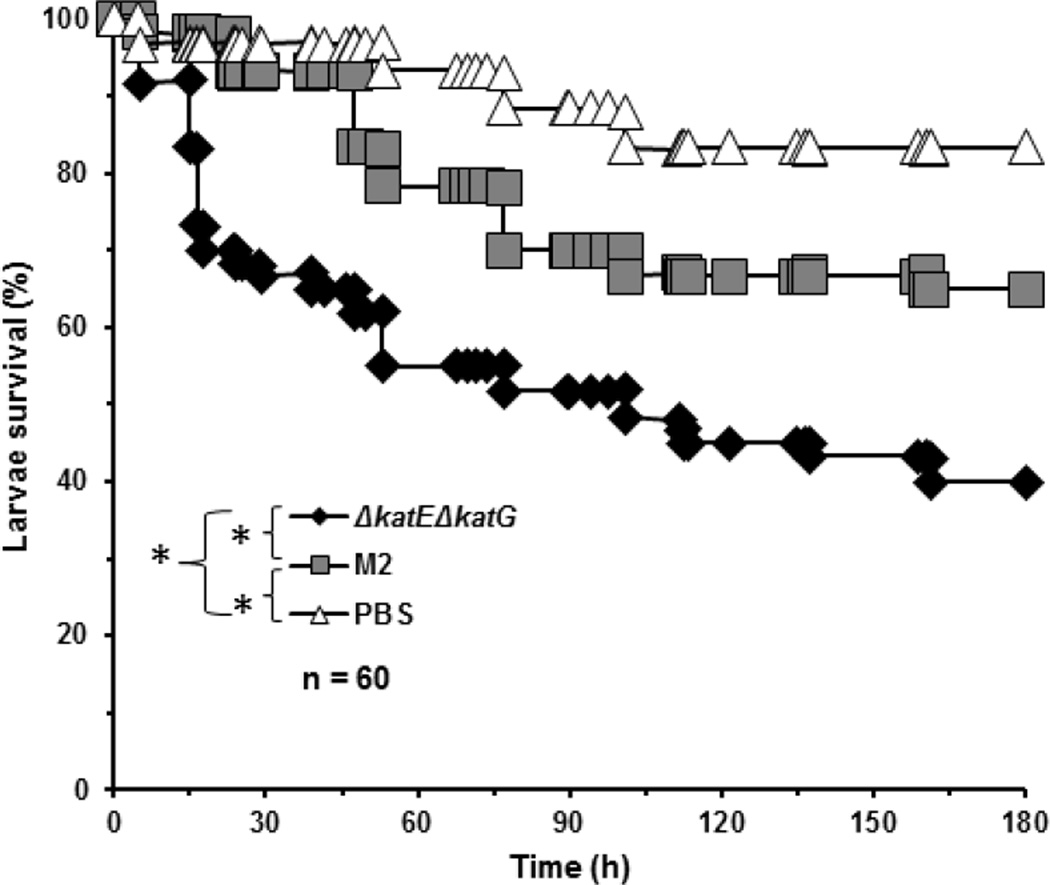
To assess whether loss of both katE and katG alters the virulence of Acinetobacter, we infected G. mellonella larvae with 1 × 105 CFU of either the A. nosocomialis parent or the ΔkatEΔkatG mutant strain, and assessed survival of the larvae every day for 8 days (Fig. 7). As expected, the parental A. nosocomialis strain induced greater mortality in G. mellonella larvae than did PBS. Surprisingly, ΔkatEΔkatG mutant cells induced a significantly higher mortality than did the parental A. nosocomialis cells. The decreased survival rate of larvae infected with the ΔkatEΔkatG mutant, relative to the parent, indicates that the lack of KatE and KatG actually made these bacteria more virulent. As increased virulence is often associated with increased bacterial resistance to the bactericidal mechanisms of the immune system, these findings suggest that KatE and/or KatG actually hinder the survival of A. nosocomialis during the interaction with the immune system, despite conferring bacterial resistance to H2O2 (Fig. 6).
Figure 7.
Survival curves of G. mellonella larvae infected with the A. nosocomialis parent or the ΔkatEΔkatG mutant strain. Overnight bacterial culture were inoculated into fresh medium and allow to grow for 3 h. Cells were collected by centrifugation, washed and resuspended in PBS to a concentration of 2× 107 CFU/ml. Each G. mellonella larva was injected with 1× 105 CFU of the A. nosocomialis parent or the ΔkatEΔkatG mutant strain (in 5 µl PBS), or injected with 5 µl PBS through the last left proleg. Injected larvae were incubated at 37°C in humidified environment. Survival was monitored for 8 days. Survival curves represent data compiled from 4 independent experiments. The survival curves were compared by log-rank test. *, p<0.05.
General discussion
In this study, we assessed the contributions of different catalases to H2O2 resistance in two Acinetobacter species. We found that KatG plays a predominant role in the resistance of Acinetobacter to H2O2 (Figs. 2 and 4), although KatE is the primary catalase responsible for H2O2 degradation in stationary phase bacteria (Fig. 1). Our studies also indicate that katA and katX do not play a detectable role in H2O2 resistance. The lack of a role for katA and katX may be explained by their expression pattern or protein structure. Although katA encodes a small-subunit monofunctional catalase, we were unable to detect a catalase activity corresponding to KatA protein (data not shown), suggesting that either katA is not expressed in normal culture conditions or its activity is very low, below the limit of detection. KatX is categorized as a “catalase domain-containing” protein, but does not have all the structural characteristics of a functional catalase, thus it is unlikely that KatX is an active catalase.
Acinetobacter species are major bacterial pathogens involved in nosocomial infections [42]. Due to their inherent resistance to multiple antibiotics, Acinetobacter infections are very difficult to prevent and treat. During the wars in Iraq and Afghanistan, large numbers of A. baumannii infection cases were observed in United States military medical facilities treating soldiers wounded in the Middle East [6, 36, 37], highlighting the challenge of this pathogen to the United States military medical service facilities. Acinetobacter is also a serious concern in civilian hospitals. Hospitalized patients, especially very ill patients on a ventilator and those with prolonged hospital stays, are particularly susceptible to Acinetobacter infections [2, 19, 26, 34]. Patients with open wounds or with invasive devices like urinary catheters are also at greater risk for Acinetobacter infection. Acinetobacter can be spread to susceptible patients by person-to-person contact or through contact with contaminated surfaces of medical devices or contaminated environments such as bedding and furniture. As Acinetobacter can survive on surfaces for a prolonged time, great emphasis has been placed on disinfection through aggressive and monitored cleaning of environmental reservoirs [1, 34]. H2O2 vapor has been found to be an effective decontamination agent [7, 33]. In this study, we found that KatG, and to a lesser extent KatE, confers Acinetobacter resistance to H2O2. Our studies suggest that compounds with catalase-inhibitory properties, if used in combination with H2O2, could enhance bacterial killing, and so increase the efficiency of Acinetobacter disinfection. While a variety of chemicals have been shown to inhibit catalase activity, including cyanide, azide, hydroxylamine, aminotriazole, and mercaptoethanol [40], most of the inhibitory compounds are either highly toxic or unpleasant, and so not suitable as disinfectants. However, non-toxic broad-specificity catalase inhibitors, if developed, would be very helpful when used in combination with H2O2 as disinfectants for the prevention or elimination of Acinetobacter pathogens in hospitals.
One of the unexpected findings is that Acinetobacter resistance to H2O2 is predominantly determined by KatG (Figs. 2 and 3), rather than KatE, the enzyme primarily responsible for the catalase activity during exponential growth and in stationary phase (Figs. 1 & 5C). One plausible explanation for the significantly increased H2O2 sensitivity of the katG mutant strains, but not the katE mutant strains, of Acinetobacter is that KatG could constitute a greater portion of the catalase activity during rapid exponential growth, particularly in the presence of H2O2. While we cannot completely rule out this possibility, we think that this is unlikely. Although katE expression levels in the exponential growth phase were substantially smaller than in the stationary phase in both A. baumanni and A. nosocomialis (Figs. 5A & B), at least in A. baumannii, KatE still constituted the vast majority of the catalase activity in the exponential growth phases (Fig. 5C). The almost complete absence of catalase activity in the A. baumannii katE mutant during exponential growth clearly indicates that even in this growth phase KatG does not constitute a significant portion of the catalase activity (Figs. 1 and 5C). The fact that the katG mutant of A. baumannii had catalase activity comparable to that of the WT parental strain, and yet exhibited elevated H2O2 sensitivity, strongly suggests that KatG confers H2O2 resistance through a mechanism independent of its catalase activity. We favor the idea that the KatG determines the H2O2 resistance due to its other enzymatic activity.
KatG is a bifunctional hydroperoxidase I enzyme, with both catalase and peroxidase activity, while KatE is a monofunctional catalase, ie. hydroperoxidase II [21]. The catalase activity uses one molecule of H2O2 as the electron donor and a second molecule of H2O2 as the electron acceptor, producing oxygen and water. At lower concentrations of H2O2, the peroxidase activity is able to utilize a suitable electron donor other than H2O2 [15]. For this reason, KatG can, at least in theory, detoxify other peroxide compounds, for example, peroxy acids and peroxy lipids quickly produced as the result of H2O2 exposure. It has been reported that H2O2 can directly oxidize a variety of cellular components, including DNA, protein, lipid and various cellular organelles [20, 41] as well as common metabolites such as carboxylic acids [18]. It is plausible that through such a mechanism KatG, but not KatE, is able to detoxify the peroxy compounds generated by H2O2 and confer cells resistance to H2O2. As KatE is capable of degrading H2O2, thus eliminating the root source of oxidative damage, it is not surprising that KatE also contributes to the H2O2 resistance of the bacteria. This idea is supported by the increased sensitivity in the halo assays demonstrated by the order of the diameters of the halos ΔkatGΔkatE mutant strain>Δ katG mutant strain>WT strain (Fig 4). Thus, we propose that both katE and katG are required for optimal H2O2 resistance. KatG is responsible for elimination of peroxy damage caused by H2O2, while KatE is primarily responsible for the degradation of H2O2.
A novel finding of this study is that KatE and KatG do not contribute to the virulence of A. nosocomialis (Fig. 7). This is surprising, given that catalase has been shown to contribute to the virulence of Staphylococcus aureus toward mice [25]. Mandell showed that a S. aureus strain with greater catalase activity is more resistant to neutrophil-mediated killing than S. aureus strain with less catalase activity. While the reason underlying such contradiction in these two bacterial species is unclear, we suggest that differential effects of catalase on phagocytic respiratory burst in the two different bacterial pathogens likely play an important role for the observed differences. Increased S. aureus virulence in the strain with greater catalase activity is correlated with decreased iodination of bacterial protein [25], presumably due to degradation of neutrophil-produced H2O2 by bacterial catalase. Unlike S. aureus, the ΔkatEΔkatG A. nosocomialis strain induced a far less robust production of ROS than did the parental strain (Fig. 6). Unlike the parent, which at an MOI less than 300, triggered a robust ROS production which should be able to overwhelm the bacteria’s catalase capacity, the ΔkatEΔkatG A. nosocomialis appeared to be able to thwart the potent respiratory burst of the neutrophils (Fig. 6C). In this sense, while KatE and KatG offer the bacteria protection against H2O2, they also make them more prone to killing by the neutrophils. On the other hand, the deletion of katE and katG makes the neutrophils less active against these bacteria, explaining why the A. nosocomialis ΔkatEΔkatG strain was more virulent in G. mellonella larvae (Fig. 7). While the mechanism underlying the attenuated ability of the ΔkatEΔkatG cells to induce respiratory burst remains unclear, we speculate that lack of the two catalases may have forced the bacteria to adapt compensatory changes, which may have helped the bacteria to stymie the killing by the respiratory burst of the neutrophils.
CONCLUSIONS
KatG is a more important determinant of resistance of Acinetobacter to H2O2 than KatE, despite KatE being the predominant catalase during exponential growth and in stationary phase.
KatA and KatX have no observable effect on peroxide resistance. katX encodes only a portion of the catalase protein which very likely lacks activity, while we found no evidence for katA expression.
Neither KatE nor KatG contribute to virulence in Acinetobacter, unlike in Staphylococcus aureus toward mice [25]. The latter situation was attributed to faster H2O2 degradation by catalase providing protection and survival advantage. The absence of such protection in Acinetobacter can be partially explained by the greater burst of H2O2 by neutrophils induced by catalase-containing cells compared to catalase-deficient cells. The mechanisms involved remains to be identified.
The combination of a KatG inhibitor with H2O2 might be developed as an effective disinfectant for the prevention or elimination of Acinetobacter pathogens in hospitals
Acknowledgments
We would like to thank Dr. David Denlinger and Mr. George Keeney for advices on the use and care of a G. mellonella system. We are also grateful to Drs. Brook Arivett and Luis Actis for teaching us the G. mellonella larvae injection techniques. This study was supported by a grant from NIAID (R21AI113930 to YL), the 2015 Qingdao Huimin Project of Science and Technology (Grant 15-9-2-82-NSH to DS), by a Discovery Grants 9600 and 2015–05550 from the Natural Sciences and Engineering Research Council (NSERC) of Canada (to PCL and AK respectively), the Canada Research Chair Program (to PCL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations used are: ACB, Acinetobacter calcocaceticus-baumannii; ICU, intensive care unit; kat, catalase; MOI, multiplicity of infection; OD, optical density; ROS, reactive oxygen species; WT, wildtype.
REFERENCES
- 1.Association for Professionals in Infection Control and Epidemiology. Guide to the Elimination of Multidrug-resistant Acinetobacter baumannii Transmission in Healthcare Settings. Washington, DC: APIC; 2010. [Google Scholar]
- 2.Babcock HM, Zack JE, Garrison T, Trovillion E, Kollef MH, Fraser VJ. Ventilator-associated pneumonia in a multi-hospital system: differences in microbiology by location. Infect. Control Hosp. Epidemiol. 2003;24(11):853–858. doi: 10.1086/502149. [DOI] [PubMed] [Google Scholar]
- 3.Bergogne-Berezin E, Towner KJ. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 1996;9(2):148–165. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calhoun JH, Murray CK, Manring MM. Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin. Orthop. Relat Res. 2008;466(6):1356–1362. doi: 10.1007/s11999-008-0212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carruthers MD, Harding CM, Baker BD, Bonomo RA, Hujer KM, Rather PN, Munson RS., Jr Draft Genome Sequence of the Clinical Isolate Acinetobacter nosocomialis Strain M2. Genome Announc. 2013;1(6) doi: 10.1128/genomeA.00906-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002–2004. MMWR Morb. Mortal. Wkly. Rep. 2004;53(45):1063–1066. [PubMed] [Google Scholar]
- 7.Chmielarczyk A, Higgins PG, Wojkowska-Mach J, Synowiec E, Zander E, Romaniszyn D, Gosiewski T, Seifert H, Heczko P, Bulanda M. Control of an outbreak of Acinetobacter baumannii infections using vaporized hydrogen peroxide. J. Hosp. Infect. 2012;81(4):239–245. doi: 10.1016/j.jhin.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Davis KA, Moran KA, McAllister CK, Gray PJ. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg. Infect. Dis. 2005;11(8):1218–1224. doi: 10.3201/1108.050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinauer MC. Chronic granulomatous disease and other disorders of phagocyte function. Hematology. Am. Soc. Hematol. Educ. Program. 2005:89–95. doi: 10.1182/asheducation-2005.1.89. [DOI] [PubMed] [Google Scholar]
- 10.Frazier WJ, Wang X, Wancket LM, Li XA, Meng X, Nelin LD, Cato AC, Liu Y. Increased inflammation, impaired bacterial clearance, and metabolic disruption after gram-negative sepsis in Mkp-1-deficient mice. J. Immunol. 2009;183(11):7411–7419. doi: 10.4049/jimmunol.0804343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallagher LA, Ramage E, Weiss EJ, Radey M, Hayden HS, Held KG, Huse HK, Zurawski DV, Brittnacher MJ, Manoil C. Resources for Genetic and Genomic Analysis of Emerging Pathogen Acinetobacter baumannii. J. Bacteriol. 2015;197(12):2027–2035. doi: 10.1128/JB.00131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glew RH, Moellering RC, Jr, Kunz LJ. Infections with Acinetobacter calcoaceticus (Herellea vaginicola): clinical and laboratory studies. Medicine (Baltimore) 1977;56(2):79–97. doi: 10.1097/00005792-197703000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Harding CM, Tracy EN, Carruthers MD, Rather PN, Actis LA, Munson RS., Jr Acinetobacter baumannii strain M2 produces type IV pili which play a role in natural transformation and twitching motility but not surface-associated motility. MBio. 2013;4(4) doi: 10.1128/mBio.00360-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison A, Santana EA, Szelestey BR, Newsom DE, White P, Mason KM. Ferric uptake regulator and its role in the pathogenesis of nontypeable Haemophilus influenzae. Infect. Immun. 2013;81(4):1221–1233. doi: 10.1128/IAI.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillar A, Peters B, Pauls R, Loboda A, Zhang H, Mauk AG, Loewen PC. Modulation of the activities of catalase-peroxidase HPI of Escherichia coli by site-directed mutagenesis. Biochemistry (Mosc) 2000;39(19):5868–5875. doi: 10.1021/bi0000059. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs AC, Thompson MG, Black CC, Kessler JL, Clark LP, McQueary CN, Gancz HY, Corey BW, Moon JK, Si Y, Owen MT, Hallock JD, Kwak YI, Summers A, Li CZ, Rasko DA, Penwell WF, Honnold CL, Wise MC, Waterman PE, Lesho EP, Stewart RL, Actis LA, Palys TJ, Craft DW, Zurawski DV. AB5075, a Highly Virulent Isolate of Acinetobacter baumannii, as a Model Strain for the Evaluation of Pathogenesis and Antimicrobial Treatments. MBio. 2014;5(3):e01076–e01014. doi: 10.1128/mBio.01076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klebanoff SJ, Clark RA. The neutrophil: Function and clinical disorders. Amsterdam: North-Holland Publishing Company; 1978. [Google Scholar]
- 18.Klenk H, Gotz PH, Siegmeier R, Mayr W. “Peroxy Compounds, Organic”, Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH; 2005. [Google Scholar]
- 19.Koh TH, Tan TT, Khoo CT, Ng SY, Tan TY, Hsu LY, Ooi EE, Van Der Reijden TJ, Dijkshoorn L. Acinetobacter calcoaceticus-Acinetobacter baumannii complex species in clinical specimens in Singapore. Epidemiol. Infect. 2012;140(3):535–538. doi: 10.1017/S0950268811001129. [DOI] [PubMed] [Google Scholar]
- 20.Linley E, Denyer SP, McDonnell G, Simons C, Maillard JY. Use of hydrogen peroxide as a biocide: new consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012;67(7):1589–1596. doi: 10.1093/jac/dks129. [DOI] [PubMed] [Google Scholar]
- 21.Loewen P. Probing the structure of catalase HPII of Escherichia coli--a review. Gene. 1996;179(1):39–44. doi: 10.1016/s0378-1119(96)00321-6. [DOI] [PubMed] [Google Scholar]
- 22.Loewen PC, Switala J, Triggs-Raine BL. Catalases HPI and HPII in Escherichia coli are induced independently. Arch. Biochem. Biophys. 1985;243(1):144–149. doi: 10.1016/0003-9861(85)90782-9. [DOI] [PubMed] [Google Scholar]
- 23.Loewen PC, Triggs BL. Genetic mapping of katF, a locus that with katE affects the synthesis of a second catalase species in Escherichia coli. J. Bacteriol. 1984;160(2):668–675. doi: 10.1128/jb.160.2.668-675.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manchanda V, Sanchaita S, Singh N. Multidrug resistant acinetobacter. J. Glob. Infect. Dis. 2010;2(3):291–304. doi: 10.4103/0974-777X.68538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandell GL. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J. Clin. Invest. 1975;55(3):561–566. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin. Infect. Dis. 2008;46(8):1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 27.Michan C, Manchado M, Dorado G, Pueyo C. In vivo transcription of the Escherichia coli oxyR regulon as a function of growth phase and in response to oxidative stress. J. Bacteriol. 1999;181(9):2759–2764. doi: 10.1128/jb.181.9.2759-2764.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulvey MR, Switala J, Borys A, Loewen PC. Regulation of transcription of katE and katF in Escherichia coli. J. Bacteriol. 1990;172(12):6713–6720. doi: 10.1128/jb.172.12.6713-6720.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013;13(5):349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemec A, Krizova L, Maixnerova M, Van Der Reijden TJ, Deschaght P, Passet V, Vaneechoutte M, Brisse S, Dijkshoorn L. Genotypic and phenotypic characterization of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex with the proposal of Acinetobacter pittii sp. nov. (formerly Acinetobacter genomic species 3) and Acinetobacter nosocomialis sp. nov. (formerly Acinetobacter genomic species 13TU) Res. Microbiol. 2011;162(4):393–404. doi: 10.1016/j.resmic.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 2008;21(3):538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2007;51(10):3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ray A, Perez F, Beltramini AM, Jakubowycz M, Dimick P, Jacobs MR, Roman K, Bonomo RA, Salata RA. Use of vaporized hydrogen peroxide decontamination during an outbreak of multidrug-resistant Acinetobacter baumannii infection at a long-term acute care hospital. Infect. Control Hosp. Epidemiol. 2010;31(12):1236–1241. doi: 10.1086/657139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rebmann T, Rosenbaum PA. Preventing the transmission of multidrug-resistant Acinetobacter baumannii: an executive summary of the Association for Professionals in infection control and epidemiology’s elimination guide. Am. J. Infect. Control. 2011;39(5):439–441. doi: 10.1016/j.ajic.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Rorth M, Jensen PK. Determination of catalase activity by means of the Clark oxygen electrode. Biochim. Biophys. Acta. 1967;139(1):171–173. doi: 10.1016/0005-2744(67)90124-6. [DOI] [PubMed] [Google Scholar]
- 36.Scott P, Deye G, Srinivasan A, Murray C, Moran K, Hulten E, Fishbain J, Craft D, Riddell S, Lindler L, Mancuso J, Milstrey E, Bautista CT, Patel J, Ewell A, Hamilton T, Gaddy C, Tenney M, Christopher G, Petersen K, Endy T, Petruccelli B. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin. Infect. Dis. 2007;44(12):1577–1584. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 37.Sebeny PJ, Riddle MS, Petersen K. Acinetobacter baumannii skin and soft-tissue infection associated with war trauma. Clin. Infect. Dis. 2008;47(4):444–449. doi: 10.1086/590568. [DOI] [PubMed] [Google Scholar]
- 38.Soares NC, Cabral MP, Gayoso C, Mallo S, Rodriguez-Velo P, Fernandez-Moreira E, Bou G. Associating growth-phase-related changes in the proteome of Acinetobacter baumannii with increased resistance to oxidative stress. J. Proteome. Res. 2010;9(4):1951–1964. doi: 10.1021/pr901116r. [DOI] [PubMed] [Google Scholar]
- 39.Storz G, Imlay JA. Oxidative stress. Curr. Opin. Microbiol. 1999;2(2):188–194. doi: 10.1016/s1369-5274(99)80033-2. [DOI] [PubMed] [Google Scholar]
- 40.Switala J, Loewen PC. Diversity of properties among catalases. Arch. Biochem. Biophys. 2002;401(2):145–154. doi: 10.1016/S0003-9861(02)00049-8. [DOI] [PubMed] [Google Scholar]
- 41.Thomas EL, Milligan TW, Joyner RE, Jefferson MM. Antibacterial activity of hydrogen peroxide and the lactoperoxidase-hydrogen peroxide-thiocyanate system against oral streptococci. Infect. Immun. 1994;62(2):529–535. doi: 10.1128/iai.62.2.529-535.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wisplinghoff H, Paulus T, Lugenheim M, Stefanik D, Higgins PG, Edmond MB, Wenzel RP, Seifert H. Nosocomial bloodstream infections due to Acinetobacter baumannii, Acinetobacter pittii and Acinetobacter nosocomialis in the United States. J. Infect. 2012;64(3):282–290. doi: 10.1016/j.jinf.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Yan J, Meng X, Wancket LM, Lintner K, Nelin LD, Chen B, Francis KP, Smith CV, Rogers LK, Liu Y. Glutathione reductase facilitates host defense by sustaining phagocytic oxidative burst and promoting the development of neutrophil extracellular traps. J. Immunol. 2012;188(5):2316–2327. doi: 10.4049/jimmunol.1102683. [DOI] [PMC free article] [PubMed] [Google Scholar]