Abstract
Technological advances over the last decade are changing the face of behavioral neuroscience research. Here we review recent work on the use of one such transformative tool, chemogenetics (or Designer Receptors Exclusively Activated by Designer Drugs, DREADDS), in behavioral neuroscience research. As transformative technologies such as DREADDs are introduced, applied, and refined, their utility in addressing complex questions on behavior and cognition become clear and exciting. In the behavioral neuroscience field, remarkable new findings now regularly appear as a result of the ability to monitor and intervene in neural processes with high anatomical precision as animals behave in complex task environments. As these new tools are applied to behavioral questions, individualized procedures for their use find their way into diverse labs. Thus, “tips of the trade” become important for wide dissemination not only for laboratories that are using the tools but also those that are interested in incorporating them into their own work. Our aim is to provide an up-to-date perspective on how the DREADD technique is being used for research on learning and memory, decision making, and goal-directed behavior, as well as to provide suggestions and considerations for current and future users based on our collective experience.
Keywords: chemogenetics, reward, motivation, learning and memory, addiction
This review focuses on the use and application of a powerful new technology, chemogenetics, to behavioral studies. The approach uses synthetically derived receptors and selective ligands for transient activation or inactivation of targeted brain areas, often abbreviated as DREADDs (Designer Receptors Exclusively Activated by Designer Drugs). DREADD receptors can be introduced into neural tissue through a range of gene transfer strategies, allowing for transient and repeatable interventions in brain dynamics upon application of otherwise inert exogenous ligands, for example clozapine-n-oxide (CNO). The rapid expansion of studies using DREADDs has accompanied the development of diverse procedural strategies, both published and transmitted through word-of-mouth. Here we seek to highlight the tremendous potential of the DREADD approach in many behavioral neuroscience research areas – potential that is already clear in the published behavioral literature – and to provide an overview of considerations in using DREADDs in behavioral studies. We have gathered this material from our experiences; while there remain uncertainties and questions surrounding any new application of DREADD technology, the goal here is to share our own assessment of how the technology can be used effectively for behavioral research.
We begin in Section 1 by outlining key advantages of a DREADD-based approach to behavior research compared to traditional or alternative technologies for neural manipulations. In Section 2 we highlight advances in a number of behavioral neuroscience research areas that have emerged by leveraging these advantages, and then discuss in Section 3 various considerations and tips for using and applying DREADD technologies to behavioral neuroscience questions. We conclude in Section 4 with comments on pressing questions for continued DREADD-based research.
Section 1 – Advantages for Behavioral Neuroscience
DREADDs offer distinct advantages for gain-of-function and loss-of-function studies over conventional methodologies. We focus here on how these advantages can be leveraged specifically in the context of behavioral neuroscience research, and thus will only briefly review the cellular and molecular mechanisms underlying the use of DREADDs. We refer readers seeking more detailed information on the development and details of DREADD molecules for perturbing neural activity to several excellent reviews (Armbruster, Li, Pausch, Herlitze, & Roth, 2007; Ferguson & Neumaier, 2012; Rogan & Roth, 2011; Sternson & Roth, 2014).
In brief, DREADDs involve the use of receptor proteins derived from targeted mutagenesis of endogenous G-protein coupled receptor DNA to yield synthetic receptors. These receptors are readily expressed in neuronal membranes, but lack an endogenous ligand to activate them. However, they are sensitive to the otherwise inert drug CNO, which can be delivered systemically and binds to DREADD receptors. One popular variant is hM4Di, which is an engineered version of the M4 muscarinic acetylcholine receptor. When bound by CNO, membrane hyperpolarization results through a decrease in cAMP signaling and increased activation of inward rectifying potassium channels (Armbruster et al., 2007; Rogan & Roth, 2011). This yields a temporary suppression of neuronal activity similar to that seen after endogenous activation of the M4 receptor. A commonly-used variant for neuronal excitation is hM3Dq, an engineered version of the M3 muscarinic receptor. When activated by CNO, this receptor leads to activation of the phospholipase C cascade altering intracellular calcium and leading to burst-like firing of neurons (Armbruster et al., 2007; Rogan & Roth, 2011). Other options for neuronal excitation are rM3Ds, which similarly result in neuronal depolarization based on G-protein signaling (e.g., cAMP increases) (Dong, Allen, Farrell, & Roth, 2010; Ferguson, Phillips, Roth, Wess, & Neumaier, 2013), and Rq(R165L), which can modulate neuronal activity through arrestin-based signaling processes instead of G-protein signaling (Nakajima & Wess, 2012).
A new variant of note is a mutated form of the Gi-coupled kappa opioid receptor (KORD), which is activated by the otherwise inert ligand salvinorin B (SalB), but not the endogenous kappa ligand dynorphin, and leads to a decrease in neuronal activity (Vardy et al., 2015). Time from KORD vector injection to expression is comparable to other DREADDs (ca. 2-3 weeks) (Vardy et al., 2015). In addition to utility in its own right, the distinct ligand for KORD (SalB, notably soluble only in 100% DMSO) makes it usable in conjunction with other DREADDs that require CNO. For example KORD could be used together with hM3Dq for bidirectional modulation of activity in brain areas (i.e., KORD-salvinorin-mediated inhibition and hM3Dq-CNO-mediated inhibition), or used with hM4Di for targeting inhibitions to different brain areas at different times in the same animal (e.g., KORD in area X, hM4Di in area Y) (Marchant et al., 2015; Vardy et al., 2015). Such work is rapidly moving the field toward a truly rich set of DREADD tools. As demonstrated by the success of related research technologies such as optogenetics, having a diverse set of methods will facilitate the application of DREADDs to a wide range of behavioral neuroscience questions (Fenno, Yizhar, & Deisseroth, 2011; Sternson & Roth, 2014). As illustrated below, DREADDs have many of the same advantages as optogenetics for probing neural circuit function in behavior. While they lack the temporal precision of optogenetics, they offer advantages in other domains including their relative non-invasiveness and plausibly more naturalistic effects on brain activity. We and others regard these technologies to be companion tools alongside traditional methods (e.g., lesions or drug microinjections) or newer-generation methods (e.g. optogenetics) for studying behaviorally relevant research questions. Here, we compare and contrast common techniques for manipulating the brain in behavioral neuroscience experiments (see also link for optogenetics/chemogenetics online comparison in Resource List).
Minimal Invasiveness and Maximal Control of Anatomical Coverage
Arguably the greatest advantage of DREADDs for behavioral neuroscience is the ability to manipulate brain systems through systemic injection of the activating ligand, constituting a “remote control” of neural activity (Rogan & Roth, 2011). For most applications, an initial surgery is required for intracranial delivery of viral constructs carrying the DREADD transgene, a promoter, and a fluorescent reporter (see Fig. 1 for example DREADD expression). Using transgenic mice that express DREADD receptors in subclasses of neurons circumvents even that requirement (e.g., (Farrell et al., 2013)). Once DREADD expression is achieved in the brain, reversible inhibition or activation of the transfected neurons occurs shortly after intraperitoneal (i.p.) injection of the designer ligand. In this way, the ongoing dynamics of targeted neural systems can be modulated as the experimental conditions require with only a minor injection-time delay. Moreover, this procedure does not necessarily require any tethering or cranial implants. Thus the animal can navigate essentially any task environment, including those involving ceilings or guillotine doors, without resorting to modifying the task apparatus to accommodate cranial hardware and connector elements. A related benefit is that the number of subjects that can be tested simultaneously is limited only by the time needed for injections and the number of testing chambers available. In experiments using operant behavioral procedures, for example, it is common to have a dozen or more conditioning chambers that can be programmed simultaneously and thus used to evaluate the effects of brain manipulations in an entire cohort of animals in parallel. The CNO injection procedure is faster than intracranial microinjections (e.g., of the GABAA agonist muscimol for neural inactivation), providing an additional advantage over those approaches in terms of personnel time. An additional benefit in this context is the relative ease with which DREADD-based manipulations can be combined with other neuroscience approaches, for instance, intracranial cannulation or electrophysiological recordings (below).
Figure 1. Examples of hM4Di DREADD expression in different brain areas.
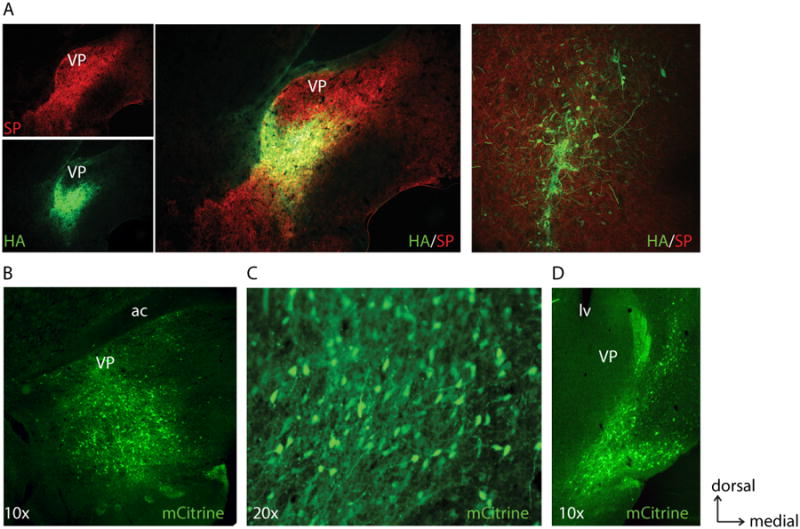
A, Lentiviral expression of hM4Di under a synapsin (Syn) promoter (HA-tag immunostain) in a 5× coronal view of rostral ventral pallidum (VP), Substance P (SP) counter-immunostain to define ventral pallidum borders. Top left, SP only. Bottom left, HA only. Middle, merge. Right, 20× view of hM4Di expression within VP in a different animal injected with the same vector and stains. B-D, Expression of hM4Di-mCitrine natural fluorescence using AAV8-synapsin also in the ventral pallidum (B), in the orbitofrontal cortex (C), and in the nucleus accumbens shell (D). ac, anterior commissure. lv, lateral ventricle.
Further, as a result of the use of a systemically deliverable ligand, DREADDs can be used to transiently perturb neural activity in large or elongated brain structures. Such regions are impossible to effectively target with arrays of fiberoptic or cannulae implants without causing excessive damage, but can be readily targeted using multiple injections of DREADD vectors along the region's axes. For example, recent work by DJB and colleagues used this to perturb activity in the retrosplenial cortex, which is ca. 8 mm along the rostro-caudal axis of the rat brain, during behavior (below) (S. Robinson et al., 2014). We note that new tools are available in the optogenetics domain to modulate larger brain areas, including the use of red-shifted opsins as red light can penetrate tissue due to its low scattering (e.g., (Chuong et al., 2014; Klapoetke et al., 2014; Lin, Knutsen, Muller, Kleinfeld, & Tsien, 2013; Zhang et al., 2008)), which carry great promise.
Repeatability
While DREADDs can be used to modulate neural activity transiently in single test sessions, they are particularly well-suited for studies in which a brain area must be manipulated repeatedly and transiently over days. In many regards, this has been impossible before the last decade or so. Chemical or electrical lesions are valuable for loss-of-function studies, but they are permanent. Thus, it is difficult to parse out lesion effects from compensatory changes that may have occurred in other brain areas. In addition, it is often difficult to distinguish between lesion effects on planning, performance, and post-performance (consolidation) aspects of a behavior, or to study acquisition effects without impacting performance too. Although traditional intracranial microinjections (e.g., muscimol) circumvent several of these problems, it is difficult to determine the precise time-course and neural spread of their effects, though substantial efforts have been made (e.g., (Edeline, Hars, Hennevin, & Cotillon, 2002; Peciña & Berridge, 2000)). More significantly, a major drawback of microinjection methods is that the repeated infusion of the drug accrues damage at the target site and the cannulae can often become unusable over time due to clogging. Indeed, most studies using this approach are restricted to only about five microinjections per animal, which produces a significant barrier to studying the role of a brain circuit in behavior that occurs over longer periods of time. For instance, a current question in the field of reward learning is how complex Pavlovian motivational behaviors (e.g., sign-tracking) are acquired over weeks. To address that, KSS has used DREADDs to transiently inactivate target brain areas each day during a two-week acquisition period (Chang, Todd, Bucci, & Smith, 2015). In our and others' experiences, CNO has been repeatedly used in the same animals to acutely activate DREADDs (with CNO doses up to 20 mg/kg by SVM) repeatedly, without evidence of decaying effects, hangover-like effects, or lingering behavioral issues that may confound subsequent behavior(Mahler, Vazey, & Aston-Jones, 2013; Mahler et al., 2014; S. Robinson et al., 2014). This repeatability feature of the DREADD approach allows researchers to also test for acquisition versus expression effects, for example, by training under DREADD activation and testing without further activation (or vice versa). Still, caution is warranted (particularly when CNO is administered chronically, e.g., in animals' drinking water), since systematic characterizations of behavioral and neural responses to repeated or chronic CNO deliveries, across doses, are lacking in the literature.
Ability to Precisely Measure Changes in Neural Activity
A great advantage of DREADDs, as noted, is the ability to readily characterize their physiological effects (see Fig. 2 for examples). For example, in in vitro recording preparations using direct application of CNO onto cells, the effect of CNO on hM4Di receptors has been shown to occur within milliseconds. hM3Dq exhibits similar temporal dynamics, including millisecond latencies and effects that last as long as cells are maintained in the whole cell configuration. One disadvantage of direct CNO application, as is often the case for at least slightly hydrophobic drugs, is the very slow rate of wash out. We (BWL) typically observe a very slow reversal of the CNO response over tens of minutes with responses never returning baseline levels after 1 hour of washout. Thus for slice-physiology and bath-application reversibility studies are impractical. Assessments of the KORD DREADD have been made as well (Vardy et al., 2015). Physiological effects in slice recordings are evident within minutes of salvinorin-B application. Behavioral effects exhibit a 10-15 min latency (Vardy et al., 2015) and roughly 60 min duration after systemic salvinorin-B injection, with some variation across different behaviors. This time-course is relatively shorter lasting than hM4Di, which may be due to the distinct kinetics of the salvinorin-B ligand compared to CNO (Vardy et al., 2015).
Figure 2. Physiological assays of DREADD effects.
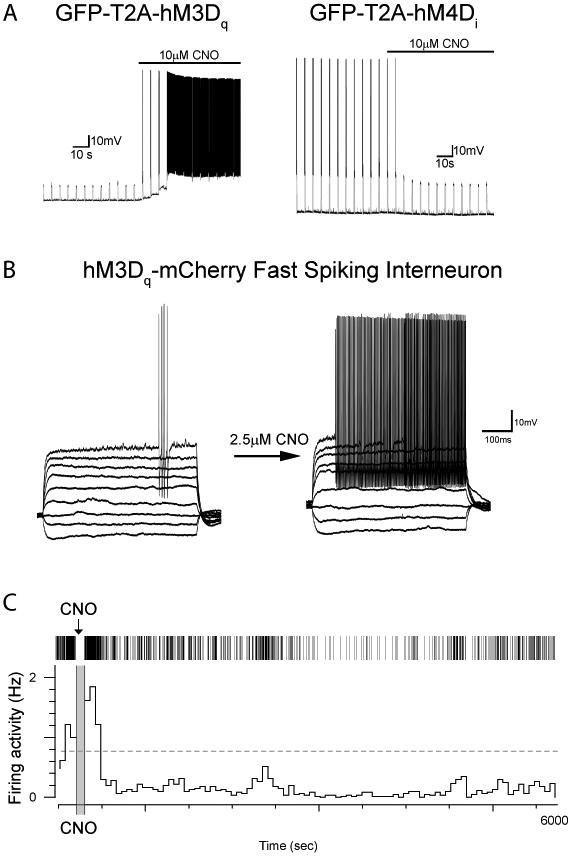
A, Dentate gyrus granule neurons were infected with lentivirus expressing DREADD receptors. Left, GFP-T2A-HM3Dq expressing neuron recorded in slice given subthreshold current pulses every 5 seconds exhibiting robust bursting response to bath CNO application. Right, HM4Di expressing neuron receiving suprathreshold current injections every 5 seconds stops firing in response to bath application of CNO. B, Entorhinal cortical fast spiking interneuron infected with AAV HM3Dq-mCherry under current clamp showing minimal spiking in response to current steps (left) then a robust excitatory response to CNO application (right). In this case, the CNO was produced internally rather than acquired from an external vendor. C, Example of ventral pallidum neuron recorded in a behaving animal using tetrodes following earlier injection of AAV8-hM4Di; this neuron exhibited a short-latency, long-duration suppression of spontaneous firing activity following i.p. CNO application (short pause in recording for injection). Top, raster of spiking activity. Bottom, histogram in 5-min time windows.
Efforts to study neural response dynamics in vivo have begun to make strides in characterizing the temporal properties and efficacy of DREADD procedures. For example, Vazey and Aston-Jones (Vazey & Aston-Jones, 2014) have characterized responses of hM3Dq-expressing norepinephrine cells in the locus coeruleus. They found that 63% of recorded cells showed excitation following locally applied CNO, with an average of 150% excitation above baseline firing rates. When delivering CNO systemically, they found nearly all recorded cells exhibited a firing increase, with an average excitation of 225% over baseline. Interestingly, the effect on firing rate was no different at doses of 0.1 mg/kg or 10 mg/kg of CNO. In work from the Gary Aston-Jones laboratory, recordings from hM4Di-expressing neurons (in the ventral pallidum) in anesthetized animals following locally applied CNO revealed that firing rates were suppressed to around 60% of pre-CNO rates, with 79% of cells affected, suggesting a robust but not complete suppression of firing in the intact brain after systemic CNO delivery (see below discussion). KSS and DJB have found a similar circa 60% suppression of activity in awake-behaving animals expressing hM4Di in the same pallidal structure upon i.p. CNO administration (Chang, Todd, Bucci, et al., 2015). In that work, an average onset latency of the change in firing was 12 minutes after CNO delivery and response offset (i.e., return to baseline) occurred at ca. 70 min, though some neurons exhibited longer-duration responses (e.g., Fig. 2C). This response onset latency is similar to the latency of firing suppression of hM4Di-expressing dopaminergic neurons that the Aston-Jones group has observed (Mahler et al., 2013). In another study, two-photon imaging of calcium in somatostatin-expressing motor cortex interneurons of mice expressing hM4Di, revealed a similar significant but incomplete suppression of calcium transients following i.p. injection of CNO (Cichon & Gan, 2015). However, one effect of inhibiting these interneurons was to increase the amount of overlap that occurred between calcium responses on different dendritic elements of pyramidal neurons as rats ran forwards versus backwards on a treadmill; the increase in overlap was comparable to that observed after genetic deletion of the interneurons, suggesting robust circuit-level effects of the DREADD-mediated suppression of interneuron activity despite it being non-complete. Finally, in an interesting test of neural responsivity, Katzel et al. (Katzel, Nicholson, Schorge, Walker, & Kullmann, 2014) used hM4Di to disrupt cortical seizure activity in rat motor cortex evoked by pilocarpine or picrotoxin microinjection. They found that cortical EEG responses were attenuated within 10 min of i.p. CNO delivery, which lasted for the ca. 40-70 min duration of the seizure response.
An important consideration in deciding whether or not to use DREADDs is that DREADD-mediated suppression of neural activity is unlikely to be as robust or complete as suppression resulting from traditional intracranial microinfusion procedures (i.e., muscimol or lidocaine administration). Thus, inhibitory DREADDs may dampen ongoing neural activity rather than eliminating it. Similarly, Gq DREADDs are thought to facilitate endogenous input control over cell firing rather than simply causing action potentials, and may not lead to as robust of a response as other stimulation methods (e.g., electrical stimulation). These features can be regarded as a disadvantage if total elimination or vigorous stimulation of activity is desired. However, generally it is regarded as an advantage as this likely leads to a more naturalistic up-weighting or down-weighting of neural activity, which is an attractive goal for many behavioral applications. Indeed, the attractiveness of DREADDs rests partly on the way in which they work through endogenous intracellular signaling mechanisms (i.e., similar to endogenous GPCR activations by neurotransmitters), leading to more physiologically meaningful changes in the patterns of activity, activity timing, synaptic input modulation, etc., in a manner likely more complex than we are currently aware. We add as well that it is possible, even likely, that axon terminals of DREADD-expressing neurons will be affected when the ligand is administered systemically. This is because of the axonal trafficking of DREADD receptors that is known to occur through many virus constructs (Mahler et al., 2014; Stachniak, Ghosh, & Sternson, 2014), leading to potential changes in synaptic transmission beyond changes in cell body signaling.
In addition, DREADDS may not affect as many of the neurons in a target region as traditional inactivation procedures. It is important to note that not all cells will express the DREADD receptor in most applications; the common approach of using viruses to introduce DREADD receptors into cells does not lead to 100% penetrance even with very effective vectors. We have estimated that ca. 2/3 of neurons express the DREADD receptor using common AAV8-synapsin vectors in several structures. This is consistent with a study demonstrating a reported 60% of recorded orbitofrontal cortex cells being inhibited by CNO delivery in an in vivo preparation (Gremel & Costa, 2013).
One aspect of the less-than-total penetrance is that the non-DREADD-expressing cells may still show changes in activity despite not being directly affected by the ligand. For example, in the behaving animal in which recordings were made from pallidal neurons after viral delivery of the hM4Di receptor, we have observed that a proportion of pallidal neurons were excited by CNO application (Chang, Todd, Bucci, et al., 2015), which we hypothesize are cells that do not express the DREADD receptor but that are modulated through circuit changes or through DREADD inhibition of inhibitory interneurons. Similarly, in the Vazey and Aston-Jones (2014) study cited above using hM3Dq to excite neurons, a subset of neurons were found to instead exhibit a decrease in firing, which were hypothesized to be neurons weakly expressing the hM3Dq receptor and being modulated by other cells more robustly expressing the receptor. This seemingly opposite effect has been observed in optogenetic experiments as well (Anikeeva et al., 2011; Smith, Virkud, Deisseroth, & Graybiel, 2012). As with any perturbation method, including cell-type specific methods, it can be unclear to what extent behavioral effects arise from manipulations of the targeted cells versus other cells that are impacted as a result of the perturbation. Examining these effects may lead to insights on local circuit activity related to behavioral function.
An overall message is that rather than assuming that a behavioral result is due to neural activation or silencing, it is highly valuable to include a measurement of how DREADDs impact the brain in a given experiment, in order to accurately interpret a behavioral effect. Examples would include recordings or imaging of neural activity, or non-invasive immunohistochemical staining of activity markers (e.g., Fos, phosphorylated ribosomal protein S6). This offers researchers a means to understand behavioral consequences in relation to brain function in a manner that generally surpasses traditional transient manipulation approaches, where effects on brain activity are often presumed. Other temporary inactivation tools, such as muscimol injection, can be assessed for physiological consequences in vivo as well, but it is a far greater technical challenge to do so (e.g., combining microinjections with recording wires, dealing with fluid-related pressure changes at recording sites, etc.). Substantial strides have been made in integrating recording devices (including tetrodes, silicon probes and single wires) with light delivery devices to evaluate physiological activity in behaving animals during optogenetic manipulations (e.g., (Anikeeva et al., 2011; Buzsaki et al., 2015; Cohen, Haesler, Vong, Lowell, & Uchida, 2012; Smith et al., 2012)), but the technical challenges to acquire them are comparatively high. Similar applications are possible in combining DREADD activation/inactivation with microinjections to enhance or suppress particular transmitter receptors, or with optogenetics to transiently perturb those or other cell types.
Anatomical Specificity
DREADDs can be inserted into particular subclasses of cells and pathways, providing tremendous spatial and phenotypic selectivity (Ferguson & Neumaier, 2012; Sternson & Roth, 2014). Virus promoter elements help dictate the type of cells that express DREADD and tag proteins, and they have included those that are non-specific (e.g. CAG), neuronal-specific (e.g., human synapsin; hSyn) or preferential to cell types (e.g., dynorphin or enkephalin; CaMKII, which is preferential cortical glutamatergic cells but can also target subcortical GABAergic cells). Often, however, greater selectivity is desired than what minimal promoters currently allow, because promoter regions of genes that define cell types are often too large to be effectively packaged into viral particles. Transgenic animals have provided a route for achieving this selectivity. For example, a double-floxed inverted open reading frame (DIO) system incorporates the DREADD gene in an antisense configuration, which can be flipped for sense translation in neurons expressing the enzyme Cre recombinase (Atasoy, Aponte, Su, & Sternson, 2008). Therefore, in neurons expressing Cre linked to a gene of choice, LoxP sites are cleaved and the inverted gene is flipped into its functional orientation, allowing the DREADD gene to be transcribed with little to no leakage when Cre is not present (Atasoy et al., 2008). This tool provides a means for inactivating or activating particular cell types of interest. A wide range of transgenic mice, and some rats, exist in which Cre expression occurs in particular subsets of cells. Some examples that have been commonly used in behavioral neuroscience experiments include dopamine cells (tyrosine hydroxylase (TH) promoter), GABAergic cells (parvalbumin promoter), cholinergic cells (ChAT promoter), and striatum medium spiny neurons in direct or indirect basal ganglia pathways (D1/2 dopamine receptor promoters). Figure 3 shows an example expression profile of DREADDs using TH-Cre rats.
Figure 3. Example of cell type-specific expression of DREADDs.
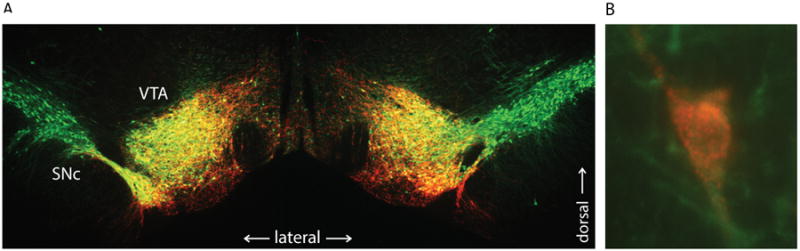
A, Tiled 5× coronal images showing bilateral expression of hM3Dq in the midbrain (AAV2-DIO vector in TH-Cre rat). mCherry (red; DS Red immunostain) shows DREADD expression superimposed on tyrosine hydroxylase-positive (TH+) neurons (green; TH immunostain) in the ventral midbrain. Overlap indicated in yellow, showing restricted DREADD expression in ventral tegmental area (VTA) and not in lateral substantia nigra pars compacta (SNc). B, 60× image of neuron co-expressing hM3Dq (red) and TH (green). Note membrane bound, punctate staining of mCherry-fused hM3Dq receptors.
Viral delivery procedures also provide the means to use DREADDs to manipulate particular brain pathway. Different strategies exist for this: One involves using DREADD-containing retrograde viruses (e.g., rabies), which will lead to expression of DREADDs in cells projecting to an area of interest. Delivery of CNO will then, in theory, modulate these cells only. A major drawback of this system is toxicity of the rabies virus; thus there is a very short time-window after infection when the experiments must be performed. A related promising strategy is to use a Canine Adenovirus (CAV)-Cre retrograde virus (Bru, Salinas, & Kremer, 2011) to cause Cre expression in cells in a pathway-defined manner, and then introduce the Cre-dependent DREADD construct in the cell body area (Boender et al., 2014; Carter, Soden, Zweifel, & Palmiter, 2013; Nair, Strand, & Neumaier, 2012). We (KSS) have had early success with this approach (Figure 4A). Although in its current form this approach requires multiple injections, it may be advantageous over the more cytotoxic rabies and pseudorabies, and HSVs, which can take prolonged time periods for retrograde expression (Nieh et al., 2015). Additionally, some AAV vectors, such as AAV2/5 and AAV9, can also lead to retrograde transport of genes (Christoffel et al., 2015; Walsh et al., 2014). For DREADD applications, a recent proof-of-principle study (Oguchi et al., 2015) used a combination of a Cre-dependent DREADD construct (AAV5-hSyn-DIO-hM4Di-mCherry) delivered to lateral prefrontal cortex and a retrogradely transported AAV9 construct carrying Cre (AAV9-hSyn-GFP-Cre) delivered to the frontal eye fields, which led to hM4Di expression in the frontal eye field-projecting cortical cells. This study also achieved putative hM4Di expression in cortical cells projecting to the caudate nucleus using a different retrograde virus, HiRet, which is an HIV-1 based lentiviral vector pseudotyped with the rabies virus envelope.
Figure 4. Example of axonal expression of DREADDs.
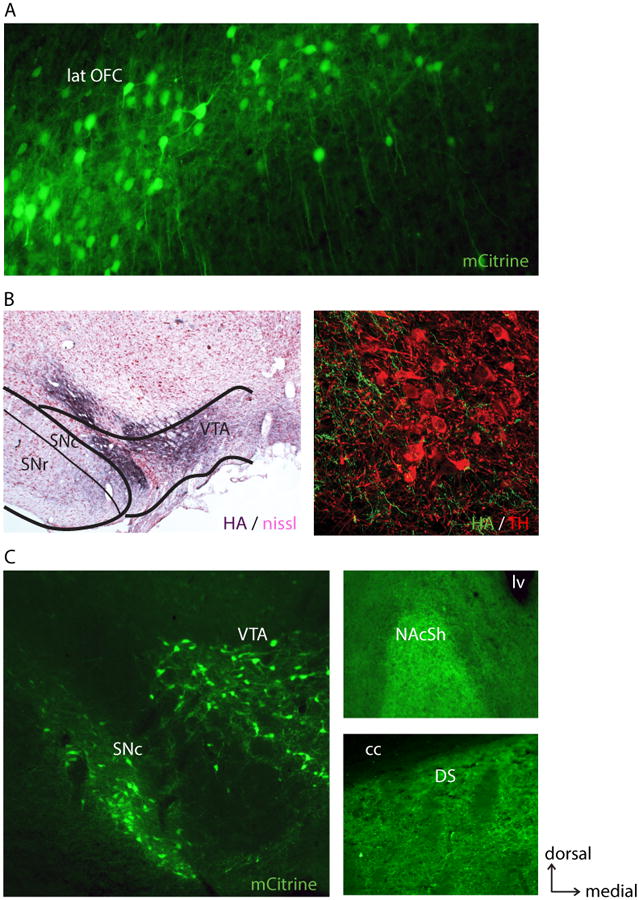
A, 10× coronal image of lateral orbitofrontal cortex (lat OFC) neurons that project to the striatum expressing the Gi DREADD (natural mCitrine fluorescence). CAV-Cre injections were made in a focal point of the striatum and Cre-dependent hM4Di delivered to the lat OFC. B, Left, 5× coronal image example of axonal expression (HA-tag immunostain, dark purple) of hM4Di in Nissl-counterstained ventral tegmental area (VTA) and substantia nigra pars compacta (SNc), following lentiviral syn-hM4Di injection in the rostral ventral pallidum (example pallidum injection in Fig. 1). Right, 60× confocal image showing comingling of hM4Di-expressing fibers (green; HA-tag immunostain) and tyrosine hydroxylase-positive (TH+) neurons (red; TH immunostain). C, Left, coronal image of midbrain VTA and SNc expressing hM4Di in TH+ neurons in the TH-Cre rat (natural mCitrine fluorescence). Right, labeled fibers in the nucleus accumbens (NAc; predominantly shell region; top) and fibers in the dorsal striatum (DS) arising from dopaminergic neurons (bottom) in the same brain. cc, corpus callosum. lv, lateral ventricle.
A different way of targeting pathways takes advantage of the fact that DREADDs are expressed not only in the infected cell bodies but are also trafficked down the axon and into axon terminals using common viral vectors (e.g., AAV, lentivirus). Figure 4B-C shows examples of axonal trafficking of DREADD molecules in wild type and TH-Cre rats. The same axonal transport is true of optogenetic constructs, and researchers have leveraged this to shine light onto the axon terminals to modulate their activity, thus modulating only the pathway connecting one area to another area, leaving activity from the first area to a distinct third area undisturbed (however, note that antidromic activation of collaterals from targeted axons may occur) (Stuber, Britt, & Bonci, 2012; Tye & Deisseroth, 2012). DREADDs require a ligand (e.g., CNO), and so the analogous experiment is to implant microinjection cannulae and inject the CNO directly on the axon terminal area, which has been done with success in several studies (Mahler et al., 2014; Stachniak et al., 2014). A simpler but less specific method to dissect pathway mechanisms that support a particular behavior is to use DREADDs following traditional contralateral disconnection procedures, in which, for example, hM4Di is expressed contralaterally in two connected areas and CNO delivered systemically to disconnect their influence on one another (Chang, Todd, & Smith, 2015; Mahler et al., 2014).
The promise for DREADD applications concerning spatial specificity is perhaps best exemplified by the recent surge in feeding research centered on hypothalamic systems. Small hypothalamic nuclei, cell types, and circuit connections are being manipulated with DREADDs and transgenic targeting systems to powerfully change feeding behaviors and in so doing are uncovering highly complex hypothalamic feeding mechanisms (Jennings et al., 2015; Stachniak et al., 2014). For further details see: (Krashes & Kravitz, 2014). These efforts complement a larger body of work on tractable homeostatic processes like hunger/satiety and can serve as useful reference points for designing similar mechanistic investigations into complex behaviors.
Histological Assessment
The use of reporter molecules allows for accurate histological localization of exogenous proteins like DREADDs in individual neurons and in circuits (Figs. 1, 3, 4). Presently, University vector cores (Resource List) offer several vectors expressing DREADDs receptors fused with fluorophores, or expressing DREADD receptors as well as internal ribosomal entry sequences (IRES) enhanced fluorophores. Fluorophores come in a wide variety of emission spectra that can be detected under conventional or confocal fluorescent microscopes. In the case of DREADDs, this gives researchers several valuable insights. Under high-magnification, the membrane expression of fluorophore-DREADD fusion proteins can be used to characterize the subcellular localization of the DREADD (e.g., (Vazey & Aston-Jones, 2014)). Under lower magnification, the expression pattern reveals the general topography of DREADD expression. This mapping procedure is much like procedures used for lesion reconstruction, and is immensely useful in interpreting the effect of DREADDs on behavior in relation to the brain areas manipulated in individual animals. Fluorescently tagged antibodies can be used to amplify the endogenous fluorescent signal (e.g., GFP antibodies; see Resources List), while simultaneously assessing other proteins present in infected cells with additional antibodies/fluorophores. This can be useful for determining the proportion of neurons expressing the DREADD (e.g., with co-staining for NeuN) or confirming that DREADDs are expressed in the class of neurons that was targeted (e.g., dopaminergic). Combining such histological maps with assays of the DREADD effect on neural activity can provide a compelling picture of where the DREADDs were expressed to influence behavior of animals.
Although fused DREADD/fluorophore constructs can be used to precisely localize DREADDs subcellularly, one disadvantage of this system is that fluorophore expression is regulated as the cell regulates the DREADD receptor. Thus the expression levels of the fluorophore tend to be low, making the cells somewhat difficult to visualize. Inefficiency of IRES sequences can also sometimes result in modest fluorophore expression. Even with immunohistochemical amplification of the signal, the present systems are not adequate for fluorescence intensity-dependent morphological evaluations such as whole-cell reconstructions and dendritic spine imaging. It is possible that higher efficiency systems (i.e., T2A/P2A) for the expression of fluorophore independent of the DREADD receptors will overcome this limitation. However, we note that cellular function can be compromised if proteins, including fluorophores or DREADDs, are over-expressed.
As an added benefit, the axonal trafficking of reporter molecules can also provide anatomical information on where a targeted population of neurons projects in the brain (Fig. 4). If sufficient time is allowed for the reporter to be transported axonally (often >4 weeks), one can observe fluorescent axons and putative terminals downstream from the injection site. For example, SVM used this to assess the projection pattern of different ventral pallidal subregions to the ventral tegmental area, which was then used to guide pathway-defined DREADD manipulations (below; Fig. 4A) (Mahler et al., 2014). Parnaudeau and colleagues (Parnaudeau et al., 2013) were similarly able to interpret neural recordings of prefrontal cortex in relation to projection patterns arising from mediodorsal thalamus using this approach. The incubation time required for axon tracing varies depending on the virus and distance between structures of interest, but generally ca. 4-9 weeks is sufficient for common AAV vectors in the pathways we have examined. This “free” anterograde tracing information is valuable not just for mapping connection patterns, but also for informing the stereotaxic coordinates used to target circuit perturbations using DREADDs or similar approaches in behavioral work. The power of this approach is shown, for example, in recent anatomical studies of the basal ganglia, in which viral mediated gene transfer of reporter molecules in specific cell types have provided new views on the connectivity and molecular makeup of behavior-related circuits (e.g., (Friedman et al., 2015; Wall, De La Parra, Callaway, & Kreitzer, 2013; Watabe-Uchida, Zhu, Ogawa, Vamanrao, & Uchida, 2012)).
Feasibility
The cost of setting up and maintaining a new research methodology is not lost on researchers making decisions on what tools to employ in their labs. With DREADDs, the cost in supplies and experimenter time is surprisingly minimal. Beyond what already exists in a typical behavioral neuroscience lab and imaging facility, the required supplies are: viral vectors purchased from a vector core, a stereotactic injection apparatus, and CNO (Resources List). A transgenic animal colony in which DREADDs are endogenously expressed also circumvents the virus need. In short, DREADDs are a cost-effective and efficient method for many behavioral neuroscience applications. Their use involves procedures that are familiar to most researchers, but which garner unprecedented advantages in selectivity and precision over conventional methodologies.
Translational Potential
A final point to highlight is the potential advantages of DREADDs as a potential neuropsychiatric treatment of tomorrow. Chemogenetic approaches have been used in preclinical models of widely varied psychiatric disorders (e.g., (Dell'Anno et al., 2014; Fortress et al., 2015; Penagarikano et al., 2015; Perova, Delevich, & Li, 2015; Sachs, Ni, & Caron, 2015; Soumier & Sibille, 2014; Vazey & Aston-Jones, 2014)), and in theory, DREADDs could offer relatively non-invasive, selective, reversible, and titratable (via dosage of the orally available agonist) inhibition or excitation of neuronal populations and pathways in psychiatric patients. Compared to routinely employed interventions for severe psychiatric disorders like surgical transection, deep brain stimulation, and psychopharmaceuticals, a chemogenetic approach targeting only the disordered neuronal population might offer increased efficacy and decreased side effects. Of course, it is far too early to know whether DREADDs or related technologies will translate well to the human brain, but initial experiments in non-human primates show similar patterns of DREADD expression, modulation of neuronal activity, and behavioral effects as seen in rodents (M.A. Eldridge, Lerchner, Minamimoto, Saunders, & Richmond, 2014; M. A. Eldridge et al., 2016; Lerchner et al., 2014; Oguchi et al., 2015; Schnebelen et al., 2015). Notably, AAV vectors appear to be well tolerated in nearly 20 years of human gene therapy clinical trials (clinicaltrials.gov; “AAV”), including in neural tissue in Alzheimer's and Parkinson's Diseases, and these same vectors are routinely used to express DREADDs in preclinical experiments.
Section 2 – Highlights of DREADD Applications in Behavioral Neuroscience
Learning, Memory and Decision Making Applications
The advent of chemogenetic methods has made it possible to address new and previously intractable issues questions in the domain of learning and memory research. For example, several reports have capitalized on the unique properties of DREADDs to ask new questions about memory circuits. Garner et al (Garner et al., 2012) recently combined chemogenetic techniques and transgenic techniques (using a Fos promoter strategy) to introduce an excitatory hM3Dq DREADD receptor into the specific population of neurons that were active as mice underwent fear conditioning. This network of neurons could be subsequently activated by injecting the mice with CNO. By gaining experimental control of this set of ‘encoding’ neurons, the representation of the fear memory could be artificially activated a under a variety of circumstances. For example, reactivating the encoding network was necessary for memory retrieval, and reactivation of the original memory during the formation of a new memory produced a mixed-memory that contained elements of both conditioning experiences. In addition, reactivation also produced deficits in retrieval when the original population of neurons was not involved in encoding the new learning experience. These observations provide valuable new insights into the nature of memory representations in distributed networks of neurons and would not have been possible without chemogenetics.
Yau and McNally (Yau & McNally, 2015) recently demonstrated that chemogenetic excitation of neurons in the medial prefrontal cortex (mPFC) can renew prediction error and promote new learning. In that study, a blocking procedure was used to retard learning to a stimulus that provided no new information (i.e., another cue already successfully predicted an outcome). Although blocking occurred as expected in the control groups, the authors demonstrated that exciting neurons in mPFC by injecting CNO during the conditioning phase permitted conditioning to the otherwise redundant cue. Importantly, the chemogenetic approach facilitated the activation of a large population of neurons in mPFC, which is not possible with traditional electrical stimulation techniques that affect only a circumscribed area of neurons. Similarly, in a recent study by Koike et al. (Koike et al., 2015), chemogenetic tools enabled the first investigation of cell-specific inactivation of anterior cingulate neurons on attentional function.
Another growing area of memory research concerns the mechanisms that underlie the allocation of specific neurons to memory formation. In other words, it has remained unclear how and why certain neurons are recruited when new memories are formed, while other neurons remain uninvolved. Chemogenetic approaches have been applied to this to test the notion that activity-dependent expression of particular molecules, such as CREB, are responsible for neuronal allocation to memory formation. Using a conditioned taste aversion procedure, Sano and colleagues (Sano et al., 2014) trained mice that were infected with a lentivirus vector to overexpress CREB and also express the hM4Di DREADD receptor. Activation of the inhibitory DREADD receptor with CNO silenced the CREB-overexpressing neurons that were recruited during memory formation and blocked memory retrieval, supporting the notion that CREB-expressing neurons are preferentially recruited during memory formation.
Chemogenetic approaches are also being used to probe the contribution of glial cells to information processing and plasticity. A recent study by Orr and colleagues (Orr et al., 2015) used a DREADD receptor that can be specifically targeted to astrocytes by using a GFAP promotor. In that study, an excitatory DREADD receptor was preferentially expressed in astrocytes and was activated during different phases of a spatial memory task. Interestingly, activation of the DREADD receptor during acquisition of the task was without effect, but activation during a memory probe trial impaired spatial memory in that rats failed to spend more time in the area that previously contained the escape platform compared to other parts of the pool. Given that persons with Alzheimer's disease exhibit changes in the expression of astrocytic Gs-coupled adenosine receptors, (Albasanz, Perez, Barrachina, Ferrer, & Martin, 2008; Kalaria, Sromek, Wilcox, & Unnerstall, 1990), this is an important demonstration that alterations in G-protein signaling in astrocytes can compromise learning.
Other studies have demonstrated the utility of chemogenetics to reversibly manipulate neurons in brain regions that have proven to be inaccessible using traditional inactivation approaches, such as cannulation. For example, the retrosplenial cortex is among the largest cortical regions in the rat, extending ∼8mm along the rostro-caudal axis of the brain. Using cannula to infuse pharmacological agents along the entire length of retrosplenial cortex is not feasible as it would require the implantation of several cannula on each side of the brain in order to infuse an agent throughout the region while sparing adjacent cortical regions. The resulting permanent mechanical damage is substantial and precludes ascribing any behavioral effects to the temporary drug effects. This is surmountable using chemogenetics as shown in a recent study by Robinson, DJB, and colleagues (S. Robinson et al., 2014). In that study, a vector containing the gene for the hM4Di receptor was infused at various sites in retrosplenial cortex. Compared to vehicle-infused rats, those expressing hM4Di exhibited a deficit in sensory preconditioning when retrosplenial cortex neurons were silenced following CNO administration. The resulting data demonstrated that unlike hippocampus (Iordanova, Good, & Honey, 2011; Wimmer & Shohamy, 2012), retrosplenial cortex in necessary for forming associations between neutral sensory stimuli prior to the introduction of any reinforcement.
Similar advancements on long-standing issues have been made using DREADDs in the domain of reinforcement learning and reward seeking behavior. For example, the striatum is regarded as a major hub for reinforcement learning in the brain, with the dorsomedial aspects being implicated in processing action-outcome learning and behavioral flexibility (Balleine & O'Doherty, 2010; Bradfield, Bertran-Gonzalez, Chieng, & Balleine, 2013; Graybiel, 2008; Packard, 2009; Ragozzino, 2007). However, the medium spiny neurons (MSNs) that comprise the output cells of the dorsal striatum can have distinct projections to either the internal globus pallidus/substantia nigra (direct basal ganglia pathway) or to the external globus pallidus (indirect pathway), and these cell populations are intermingled and difficult to target in behavioral studies without new molecular-genetic strategies (Cui et al., 2013; Ferguson & Neumaier, 2012; Kravitz, Owen, & Kreitzer, 2013). Prior studies on appetitive decision making have capitalized on the distinct molecular composition of these cell types, as direct pathway cells express D1 receptors and dynorphin while indirect pathway cells express D2 receptors and enkephalin, but it has remained difficult to gain causal control over these striatal pathways themselves to study their necessary and sufficient roles in reward-guided behaviors. In one study, to selectively and bidirectionally control the direct pathway cells only, Ferguson et al. (Ferguson et al., 2013) engineered an HSV vector containing either hM4Di or hM3Dq DREADDs and a dynorphin promoter, which led to DREADD receptor expression in the dynorphin-rich direct-pathway neurons. Rats were trained on forced and free choice trials in which they were required to perform an effortful lever-press task to receive, depending on lever, high or low reward amounts. Levers were reversed across sessions. DREADD-mediated suppression (or activation) of direct-pathway striatal cells did not affect task performance, but, interestingly, decreased (or increased) rats' ability to use their learning to inform choice for the high-reward lever when tested a week later. Effects were specific to manipulations during, but not after, daily training. These findings use the cell-type targeting and acquisition/expression test advantages of chemogenetics to demonstrate a surprising role for medial-striatonigral projections in forming stable representations of reinforced action strategies.
As another example, a set of recent studies have used DREADDs to investigate behavioral flexibility based on reinforcement contingencies. Parnaudeah et al. (Parnaudeau et al., 2015) expressed hM4Di in mouse mediodorsal thalamus to study its potential roles in processing the reinforcement outcomes of a learned behavior across different task conditions in single animals. The authors found that disrupting the thalamic nucleus with DREADDs did not disrupt extinction of a discriminative operant behavior but did impair post-extinction reversal learning. Also disrupted were animals' normal reduction in performance when reward is delivered less reliably upon behavior and the acquisition of outcome-specific Pavlovian-instrumental transfer. Such results offer an interesting dissociation between tasks involving behavioral adjustment to outcome changes, in this case, supporting a conclusion that mediodorsal thalamus may play a preferential role in behavior flexibility based on changes in outcome contingency, but less role in conditions of outcome loss. This conclusion finds support in a notable prior study demonstrating a role for thalamic projections to striatal cholinergic cells in outcome contingency processing (Bradfield et al., 2013), though it will be interesting in future work to parse out the contributions of thalamic projections to striatum and prefrontal cortex in this kind of behavioral flexibility. On this point, prior work by Parnaudeau et al. (Parnaudeau et al., 2015) used DREADDs in combination with recordings to demonstrate a role for mediodorsal thalamus and thalamic-prefrontal beta oscillation synchrony in working memory tasks for reward. Also, related work used DREADDs to demonstrate that orbitofrontal cortex is necessary for the use of reward probability information when performing on a cued response task (Ward, Winiger, Kandel, Balsam, & Simpson, 2015), in line with suggestions that this cortical region processes information about specific reward outcomes for decision making (McDannald et al., 2012).
As a final example, we (KSS, DJB) have used the hM4Di DREADD to examine the involvement of the ventral pallidum in motivational attraction to reward cues (Chang, Todd, Bucci, et al., 2015). Animals often approach and attempt to consume discrete cues paired with rewards, such as insertion of a lever, in a behavior called sign-tracking. This is thought to reflect the attribution of incentive value to the reward cues (Berridge, 2004; Robinson, Yager, Cogan, & Saunders, 2014). While several brain sites have been demonstrated to be necessary for the expression of sign-tracking, it has difficult to causally manipulate sign-tracking acquisition due to the length of time (ca. 1 week) over which the behavior is acquired. DREADDs circumvented this problem by providing a way to transiently inactivate the ventral pallidum each day during acquisition. This allowed us to assess not only sign-tracking acquisition curves but also the extent to which sign-tracking had been acquired but not expressed, which we did by conducting an expression test without DREADD-inactivation in the same animals at the end of the study. We found that inhibiting the ventral pallidum stunted sign-tracking acquisition, which was not due to a deficit in performance or expression. This finding shows a necessary role for the ventral pallidum in the attribution of incentive value to reward cues. It also provides a compelling twist on the traditional notion that the ventral pallidum functions primarily as an expression area for motivated behaviors (Mogenson, Jones, & Yim, 1980), suggesting instead it is critical acquiring them in the first place (Smith, Tindell, Aldridge, & Berridge, 2009).
Applications to Models of Addiction and other Disorders
DREADDs have proved useful in an increasing number of investigations into addiction-related brain structures and circuits. Initial experiments showed roles for D1 and D2-expressing striatal neurons in sensitization to the locomotor activation effects of amphetamine, but not acute locomotor activation caused by amphetamine. In a pioneering study, Ferguson et al. (Ferguson et al., 2011) found that hM4Di DREADD inhibition of D2/enkephalin MSNs increased, while D1/dynorphin MSN inhibition slightly decreased amphetamine sensitization. These effects persisted following CNO washout, showing that D2 inhibition increases, and D1 inhibition decreases plasticity related to repeated amphetamine exposure and locomotor sensitization. Complementing this, Farrell et al. (Farrell et al., 2013) showed that hM3Dq-mediated excitation of all D2-expressing expressing striatal MSNs in a transgenic D2-hM3Dq mouse model reduced locomotor exploration of a novel environment, and prevented locomotor sensitization to repeated amphetamine injections. Both of these studies employed DREADDs to support the notion that MSNs in thee D1 direct-pathway, and D2 indirect-pathway, play a crucial role in activating, and inhibiting, reward-related locomotor activation. Also related to the influence of psychostimulants on neuronal function, Mizoguchi et al (Mizoguchi et al., 2015) found using hM4Di and hM3Dq DREADDs that risky behavior in a rat gambling task involved insular cortex, as does methamphetamine-induced increases in risky behavior.
Two studies have also used DREADDs to explore the functions of little-studied astrocyte signaling in nucleus accumbens core regulation of drug seeking. Bull et al (Bull et al., 2014) expressed Gq DREADDs in nucleus accumbens core astrocytes, which increased astrocytic expression of cytosolic calcium (confirming DREADD activation of Gq signaling), decreased intracranial self-stimulation thresholds (indicating facilitation of reward), and reduced motivation for ethanol in a progressive ratio test conducted following ethanol withdrawal. Along the same lines, Scofield et al. (Scofield et al., 2015) found that accumbens astrocyte stimulation with hM3Dq DREADDs increased extrasynaptic glutamate, reversing an accumbens hypoglutamatergic state associated with cocaine exposure. Nucleus accumbens astrocyte DREADD stimulation also reduced cue-induced reinstatement of cocaine seeking via activation of presynaptic group 2 metabotropic glutamate receptors thought to inhibit reinstatement-related synaptic glutamate inputs from medial PFC (Scofield & Kalivas, 2014).
DREADDs have also been used to examine alcohol seeking behavior. Cassatero et al. (Cassataro et al., 2014) examined effects of co-administration of two AAV viruses into the nucleus accumbens core expressing, 1) Cre recombinase, and 2) a floxed cre-dependent hM4Di or hM3Dq gene, which causes expression of inhibitory or excitatory DREADDs in all accumbens neurons. They found that while Gq-mediated stimulation of nucleus accumbens core neurons with CNO did not affect alcohol consumption, Gi-mediated inhibition of these same neurons reduced binge drinking in a limited access “drinking in the dark” paradigm, but did not similarly reduce sucrose or water consumption. Pleil et al. (Pleil et al., 2015) also examined the roles of bed nucleus of the stria terminalis (BNST) corticotropin releasing factor (CRF)+ neurons in binge alcohol drinking. They found that while neuropeptide Y (NPY) signaling at the Y1 receptor in BNST reduces ethanol binge drinking via Gi inhibition of protein kinase A (PKA), concurrent Gs DREADD-mediated stimulation of the same intracellular signaling pathway in these neurons reverses NPY inhibition of both drinking and PKA signaling. DREADD-mediated inhibition of BNST CRF neurons, like endogenous Y1 Gi activation, also decreases alcohol drinking, and slightly reduces anxiety as measured by increased time spent in the center of an open field. This finding highlights the usefulness of DREADDs to interrogate endogenous signaling at GPCRs with DREADDs, here by replicating and reversing intracellular and behavioral effects of endogenous Gi signaling at the Y1 receptor in BNST CRF neurons.
Anderson et al (Anderson et al., 2013) employed DREADDs in a novel way, which they termed DREADD-assisted metabolic mapping (DREAMM; see also (Michaelides et al., 2013)), to interrogate wider circuit activity after hM4Di DREADD inhibition of dynorphin-expressing neurons in rat periamygdaloid cortex (PAC). They found that dynorphin mRNA in PAC is higher in humans and rats with heroin self-administration history, so they measured effects of DREADD-mediated inhibition of PAC dynorphin neurons on behavior, and on wider brain activity as measured by radiolabeled glucose utilization with PET imaging. PAC dynorphin neuron inhibition increased blood corticosterone levels, decreased voluntary sucrose intake, increased freezing behavior, and increased metabolic activity in extended amygdala structures including medial and central amygdala, accumbens shell, and BNST. This group has since used the same DREAMM technique to examine circuit effects of manipulating D1/D2 nucleus accumbens neurons, and dorsal raphe serotonin neurons (Michaelides et al., 2013; Urban et al., 2015). These findings highlight the usefulness of DREADDs to non-invasively perturb nodes in behavior-related brain circuits while simultaneously examining wider neuronal circuit activity in vivo.
We (SVM) have also employed DREADDs to explore the roles of ventral pallidum subregions and their projections to midbrain dopamine (DA) populations in cocaine seeking (Mahler et al., 2014). We employed a self-administration/reinstatement paradigm where rats are trained to self-administer i.v. cocaine and a discrete tone/light cue, extinguished of this behavior, then are re-exposed to cocaine cues or a priming injection of cocaine itself to reinstate seeking. This models conditioned and primed drug seeking in humans, both of which are major risk factors for relapse to drug use in addiction. Based on previous reports from KSS and others that rostral (RVP) and caudal (CVP) regions of ventral pallidum play different roles in sucrose hedonic, motivation, and conditioned responses (Johnson, Stellar, & Paul, 1993; Smith & Berridge, 2005), we wondered whether VP subregions differentially participate in reinstatement of cocaine seeking when elicited by either cocaine cues or a cocaine prime. When we re-examined cue reinstatement-related Fos expression in RVP and CVP neurons that project to VTA (Mahler & Aston-Jones, 2012), we found that Fos in RVP, but not CVP, efferents to VTA was elevated in proportion to cocaine seeking, relative to several behavioral control conditions.
Therefore, we next asked whether RVP and/or CVP, or their projections to VTA, are necessary for cocaine reinstatement. Using a lentivirus causing expression of an hM4Di DREADD under a hSyn promoter, we were able to precisely target inhibitory DREADDs selectively to RVP or CVP, confirmed by mapping expression of immunohistochemically labeled HA-tagged DREADDs compared to VP subregion borders. We found that inhibiting RVP, but not CVP, via i.p. injections of CNO (1-20mg/kg) suppressed cue-induced, but not cocaine primed reinstatement. However, similar CVP DREADD inhibition instead blocked primed, but not cue-induced reinstatement. This novel double-dissociation in RVP and CVP roles in cued and primed reinstatement was facilitated by the precise localization of manipulation sites, and the repeatability of neuronal inhibition allowed by DREADD technology.
Next, we sought to determine the necessity of RVP and CVP projections to VTA in cued and primed cocaine reinstatement. Again targeting hM4Di DREADDs to bilateral RVP or CVP neurons, we implanted cannulae into VTA (or nearby substantia nigra as a control), allowing specific inactivation of RVP and CVP projections to midbrain DA-containing nuclei. We found that DREADD inhibition of RVP, but not CVP, efferents to VTA blocked cue-induced cocaine seeking without affecting primed reinstatement. Inactivation of CVP-VTA projections had no effect on cue- or primed reinstatement, suggesting that CVP's role in primed reinstatement does not require VTA projections. Similarly, inactivation of RVP projections to subsantia nigra did not affect reinstatement of either type.
Having determined the necessity of RVP efferents to VTA, we sought to identify the circuit connectivity of VP-VTA projections. First, we confirmed that RVP inputs to VTA are predominantly GABAergic, as CNO application to VTA slices expressing VP axonal DREADDs inhibited iPSC frequency in VTA DA neurons. In anesthetized animals, we also found that intra-VTA CNO application onto VP DREADD expressing axons disinhibited DA-like neuron firing. It is not likely that this disinhibition of DA neurons mediates reinstatement deficits seen after RVP-VTA DREADD inactivation, however. When VTA DA neurons were disinhibited with microinjections of the GABA-A receptor antagonist gabazine, cue-induced reinstatement was instead increased.
We also found that intra-VTA CNO inhibited the firing of another population of fast-spiking, short waveform VTA neurons. This would be consistent with DREADD inhibition of VP glutamatergic projections to VTA (35% of VP efferents to VTA are vGlut2+ (Geisler, Derst, Veh, & Zahm, 2007)), but we did not see reduced cued reinstatement after unilateral RVP DREADD inhibition, coupled with contralateral VTA glutamate antagonist microinjections. This modified asymmetric inhibition technique blocks VP glutamatergic projections to VTA bilaterally, arguing against a reinstatement-related role for RVP glutamate projections to VTA. Clearly, VP inputs regulate VTA neuronal activity in a complex manner, depending upon still-unknown interactions between heterogeneous VP and VTA populations.
Although the complex circuitry by which RVP inputs modulate VTA during reinstatement remains unclear, we did discover that both RVP and VTA DA neuron activity is simultaneously required for cues to elicit reinstatement. We again employed a modified asymmetric inhibition technique, in which Gi DREADDs were expressed in unilateral VP (hSyn-hM4Di lentivirus), and also in VTA dopamine neurons of the contralateral hemisphere (DIO-hM4Di AAV2 in TH:Cre rats). Disrupting connectivity between VP and VTA DA neurons bilaterally in this manner reduced cued reinstatement. Unilateral inhibition of VP or VTA DA neurons alone did not similarly reduce reinstatement, meaning that coordinated activity between dopamine neurons and their VP afferents are required for cues to elicit reinstatement of cocaine seeking.
These experiments employed DREADD technology to discover a crucial pathway of cue-triggered cocaine seeking involving projections from RVP to VTA that modulate multiple types of VTA cells, and requiring both VP and DA neuron activity. We also dissociated roles of rostral and caudal VP in cue- vs. prime-elicited reinstatement, facilitated by our ability to map the precise localization of inhibited VP neurons.
Section 3 – Technical Considerations for Using Dreadds in Behavioral Neuroscience
Such work offers examples of knowledge gained by using DREADD methods to address difficult behavioral questions. However, for current and future users, the diversity of viral vectors, specific procedures being used, and terminologies in the field can lead to some uncertainty. Below we offer a set of considerations and recommendations in this regard.
Virus and serotype choice
AAVs, probably the most common vectors in behavioral research, are used for long-lasting expression in a relatively wide area. This makes them attractive for research involving chemogenetic manipulations of most brain areas. The common incubation time that researchers use is 2-3 weeks following virus injection, at which point robust DREADD expression exists in most brain areas we and others have studied. The DREADD constructs for AAVs are available in a variety of serotypes, or variations of the virus species. In our experience and that of others, the specific serotype can have several influences on using the DREADD approach. In our own work, for example, one serotype-promoter combination (e.g., AAV serotype 8 of hM4Di-hSyn-mCitrene) may be effective for infection of cortical neurons or ventral striatum neurons, but result in little or no expression of the reporter in dorsal striatum neurons (Fig. 1). Common AAV serotypes used for central nervous system cells include AAV1, 2, 5, 8, 9, and the serotype chimera “DJ”, each of which can lead to different anatomical spread of DREADD expression, and/or expression levels, in infected neurons. The extent to which each serotype is most effective in a brain area of interest is an ongoing question (Aschauer, Kreuz, & Rumpel, 2013). Serotypes 1-4 tend to be larger viral particles with decreased anatomical spread while 5-9 tend to be compact particles that spread long ranges through brain tissue. The newer DJ serotype appears to have the greatest anatomical spread of all serotypes ((Grimm et al., 2008); unpublished observation BWL). Some AAVs can lead to retrograde expression as well (e.g., 9), which could carry experimental consequences, wanted or unwanted. In all, pilot studies are highly advisable to test expression at key time points after viral delivery for any new virus and brain area under investigation, as it may be difficult to predict the consequences based on prior studies.
Concerning recommended AAV injection volume, of course the size of the target area will influence how much virus to deliver. Virus family and serotype, and titer (viral particle concentration), will dictate the extent to which a given injection volume will transduce neurons and spread through tissue. KSS and DJB have had success in cortical, striatal and pallidal regions with volumes of ca. 0.8 μl per hemisphere using non-specific promoters and AAV 8 (titer ca. 1×1012), and waiting ≥ 3 weeks after virus injection prior to CNO tests. The spread of expression is robust, and roughly 1 mm3 from a 0.8 μl injection at a 0.15 μl/min rate (Chang, Todd, Bucci, et al., 2015), though expression varies across brain areas in our experience even when using similar injection volumes. Note also that it may be advisable in transgenic Cre lines to use larger volumes as spread is often ineffective if it extends beyond a localized region of Cre-expressing cells (e.g. TH+ cells in the ventral midbrain).
HSVs can be used for faster, but shorter duration, transgene expression (Neve & Carlezon Jr, 2002). These may be desirable in applications where rapid DREADD expression is useful based on experimental time-courses that are short or otherwise time-sensitive. Using ubiquitous promoters, the area of expression using HSVs tends to be sizable but typically less than AAVs. For applications in which small expression areas are desired (e.g., for small nuclei or subregions of areas), AAV/HSV volume or titer may be decreased, or, alternatively, lentivirus vectors used. Lentivirus vectors have proven useful across a range of behavioral neuroscience applications. Lentivirus tends to not spread much beyond the injection site. For example, SVM has used 1 microliter (synapsin promoter) to achieve a small expression area in ventral pallidal subregions (Mahler et al., 2014) (Fig. 1A). This can be immensely useful, considering that restricted expression of DREADD transgenes is critical for targeting manipulations to brain areas of interest, and may be uniquely suited to small brain areas.
As noted above, pathway-defined manipulations can be achieved using methods for retrograde transport of genes from axonal terminal areas to cell bodies. Rabies and pseudorabies viruses are useful as they can carry DREADD or Cre molecules to sites projecting to cells of interest (e.g., (Lammel et al., 2012; Wall et al., 2013; Watabe-Uchida et al., 2012; Wickersham et al., 2015)). As noted, one caveat to this approach is that cell health deteriorates leading to toxicity after a short period of time, which limits their use in longer-duration behavioral experiments. An attractive alternative is the CAV2-Cre method for retrograde DREADD delivery which leads to stable expression over long periods of time (Boender et al., 2014; Gore, Soden, & Zweifel, 2013; Nair et al., 2012), and which we and others find can be used for DREADD expression (Nair et al., 2012) (Figure 4A). Additional diverse vector options exist for retrograde delivery of genes, and carry promise for DREADD research. These include HSVs (e.g., (Barot, Ferguson, & Neumaier, 2007; Ferguson et al., 2011; Nieh et al., 2015; Znamenskiy & Zador, 2013)), AAVs (e.g., (Christoffel et al., 2015; Oguchi et al., 2015; Walsh et al., 2014)), high-titer lentivirus (Kinoshita et al., 2012; Wickersham et al., 2015), and pseudotyped HIV vectors (S. Kato et al., 2011; Oguchi et al., 2015).
Reporter Sensitivity
A variety of fluorescent reporter molecules can be used to aid visualization of DREADD expression in the brain. Some are more easily visible than others. For example, reporters such as EGFP, EYFP, and mCherry are readily visible with commonly used fluorescent microscope filter cubes (e.g., GFP or YFP, Texas Red cubes), while other reporters, such as mCitrine tend to produce a weaker signal. We note that the visibility of the reporter is also affected by post-fixation processes. Indeed, in our hands, more than 2 hours of post-perfusion fixation in paraformaldehyde greatly reduces the visibility of mCitrine as well as GFP. Antibodies can be used to enhance the reporter signal through immunostaining, which can increase the visibility of weaker reporter molecules and (using a DAB reaction) more permanently label the reporters as fluorescent signals can wash out over time (see Resources List). For example, antibodies to mCherry exist for enhancing its signal (e.g., for the structurally similar DS Red protein) and antibodies for GFP work well for enhancing EGFP, EYFP and mCitrine signals. The hemagglutanin tag for the hM4Di and hM3Dq proteins can also be stained to aid visualization (e.g., (Ferguson et al., 2011; Mahler et al., 2014)).
The reporters we have used exhibit a remarkable stability over time, such that microscope visualizations can be made months after tissue sectioning and mounting. Storage in a refrigerator and protection from light (during both storage and microscope work) is essential for extending the lifetime of the fluorescent signal, should that be desired. However, unless immunostaining procedures are used, it is generally advisable to make histological assessments soon after tissue mounting.
CNO Care, Use, and Sources
We continue to wrestle with determining the most ideal and appropriate conditions for storing and using the designer drug, CNO, which is used to activate most DREADD receptors. In our experience, CNO becomes harder to dissolve in aqueous solution as the solid ages. Indeed, freshly obtained CNO goes into solution in water or 0.9% saline with the help of sonication up to a concentration of 2mg/ml. However, if stored at room temperature for more than 1 week, it is increasingly difficult to put into solution and DMSO must be used to initially dissolve the solid. Efforts to restrict CNO exposure to air and light can help, but do not circumvent the aging issue in our experience. Thus, it seems best to store the solid below 0°C until it is needed for use. It can also be effective to dissolve the solid into a mixture of DMSO in 0.9% saline, store it in subzero conditions, and dilute the stock solution to the target dosage on experiment days (see Resources list). Concerning specifics, each of us has a subtly distinct approach, which all appear to work well. KSS dissolves CNO (source: NIMH) in water using sonication at each use (1 mg/ml; milky product). SVM creates a final CNO (source: NIMH, NIDA) concentration of 5% DMSO/saline (generally 5-10mg/ml/kg; cloudy yellow product) and stores it in room temperature outside of light, shaking it for before each use for higher concentrations. DJB freezes the solid CNO (source: NIMH, Sigma) at -20°C and then dissolves 5mg in 100ul DMSO and then sterile water to achieve a concentration of 1mg/ml. BWL dissolves 5mg CNO (source: NIMH) in 200μl DMSO then adds 1250μl H2O to generate at 10mM CNO in 13.8% DMSO stock solution which is stored as aliquots at -20°C. This is then diluted in ACSF for final concentrations of 1-10μM for slice electrophysiology or diluted in injection saline (5.78μL 10mM stock to 192.4μL saline) for IP injection of 10μL per gram of animal weight in vivo (1mg/Kg).
Suitable CNO dosages for affecting behaviors continue to be investigated, and may vary based on DREADD receptor expression levels, cell types infected, duration of DREADD activation desired, and species examined. KSS and DJB have found robust results with 1 mg/kg in several task conditions (Chang, Todd, Bucci, et al., 2015; S. Robinson et al., 2014), and SVM finds that up to 20 mg/kg can be used effectively and without apparent non-specific effects (Mahler et al., 2014). His experience is that 5-10 mg/kg produces maximal behavioral effects through a number of assays, viruses, and neuronal pathways. As with most brain manipulation strategies, it is generally advisable to work with maximal effective (and specific) doses, which may need to be resolved through dose-response pilot studies. Concerning CNO injection time related to task time, most of the literature ranges from delivering CNO ca. 20-60 min prior to the onset of a behavioral task. The most common is 30 min prior to task, with which we have all had success.
Concerning CNO sources, NIMH and NIDA operate dispensary programs that produce CNO for investigators conducting work relevant to the institutes' missions. Commercial vendors such as Sigma also produce CNO, but it can be prohibitively expensive for experiments requiring a large CNO quantity (e.g. systemic CNO administration in adult rats). Several groups are taking to manufacturing their own CNO, which may be an advisable approach – if feasible – to minimize cost and avoid any future issues that may arise from increased CNO demand. DJB, KSS and BWL have collaborated to do this in-house with chemists, with early success (Fig. 2B). See Resources List for additional CNO source information.
Appropriate Control Conditions
Proper controls are essential for conducting interpretable behavioral studies using DREADDs, and this can lead to a larger cohort of animals compared to traditional approaches to neural manipulation. Ideal conditions are a 2-×-2 design covering the combinations of experimental and control viruses with CNO and vehicle delivery. In this design, conditions might be (1) hM4Di+CNO, (2) hM4Di+vehicle, (3) GFP+CNO, (4) GFP+vehicle. However, an appropriate alternative in our view can be to conduct two experiments, such as comparing hM4Di+CNO to hM4Di+vehicle, and the other comparing hM4Di+CNO to GFP+CNO. This alternative has the advantage of replicating main experimental results and can still be used for within-subject designs using both CNO and vehicle.
One consideration for the control viruses is that they may not lead to identical changes in the protein expression structure of neuronal membranes. For example, the control virus AAV-hSyn-mCherry will lead to mCherry expression, while the DREADD virus AAV-hSyn-hM4Di-mCherry will lead to expression of the larger DREADD-reporter fusion protein. Following the logic of similar efforts on the optogenetics field incorporating crystal structure characterization of opsins (H. E. Kato et al., 2012), one potential future direction to building ideal controls would be to incorporate a non-functional DREADD receptor based on structural mutations into the control vector.
We note that CNO by itself from NIMH, NIDA or Sigma (Resource List) has not been reported to produce overt effects on behavior during its delivery or on subsequent days. For example, SVM has extensively checked for CNO effects in behavior and neuronal activity at 10 and 20 mg/kg without any signs (Mahler et al., 2013; Mahler et al., 2014). Nevertheless, control conditions as above where nonspecific CNO effects can be pulled out, such as by comparing GFP-CNO and hM4Di-vehicle, make checking this quite feasible and advisable. As CNO characterizations evolve, in the future we foresee the most prudent use of subjects being to have two conditions, DREADDs+CNO and Control+CNO. This type of comparison is common with other tools, such as optogenetics (e.g., lacking an opsin without light delivery control). For Cre driver system experiments, active DIO virus in Cre-negative animals, yielding viral infection but no functional DREADD protein expression, is another type of control that is common and suggested.
To the extent that spread of DREADD expression is noted beyond the targeted area, off-site controls may be warranted. Intracranially injected compounds like muscimol can spread effectively at considerable distances from the injection site (Edeline et al., 2002), including up the cannulae track, and thus it is common to include off-site injections to rule out non-targeted areas as contributing to behavioral effects. Through fluorescent reporter expression mapping, the area of DREADD expression can be well characterized. Thus, off-site targets can be clearly defined based on where the DREADDs may have happened to consistently spread in a group of subjects. However, DJB and KSS have noted limited spread (and, in most cases, nearly zero dorsal spread) of DREADDs (AAV8) by using long injection durations (e.g., 10-20 min) and small-gauge (e.g., 33 ga) injector needles, which may be an advantage over intracranial injections in behaving animals that require shorter infusion durations and larger cannulae. SVM has similarly used picospritzer/glass pipette injections for deep structures, again with negligible dorsal spread of DREADDs (lentivirus, AAV2).
Section 4: Forecast
The stage has been set for multiplexed modulation of brain circuits using combinations of DREADDs (e.g., Gq and KORD), or DREADDs in combination with other tools including optogenetics. For example, the effort to develop KORD underscores an important next step for chemogenetics, which is to engineer hM3Dq and hM4Di DREADD receptors that can be bound by ligands other than CNO, as CNO carries the disadvantage of at least minimal amounts being metabolized into the psychoactive molecule clozapine in primates, including in humans (however, note that new ligands being developed may circumvent this issue (Chen et al., 2015)). Likewise, synapse-specific expression of DREADDs could carry research into compelling new directions regarding functionality of pathways (e.g., genetic Cre driver+floxed rabies+glycoprotein strategy to target inputs to a neuronal population (e.g., (Lammel et al., 2012)). Exciting progress has been made in this realm chiefly in the domain of unconditioned behaviors such as feeding, and so it will be an important step to determine the utility of such approaches for applications involving complex behavior – but the potential is high.
Similarly, as behavioral neuroscience rests heavily on a history of research with rats, the expansion of transgenic rat lines will be essential to capitalize on the cell-type-specificity that can be obtained in order to gain an ever-more mechanistic understanding of complex behaviors. Several Cre transgenic lines exist for using DREADDs in behavioral neuroscience, including TH-Cre and ChAT-Cre (Witten et al., 2011), and NIDA is in the process of developing more through their “Transgenic Rat Project” (http://irp.drugabuse.gov/OTTC/rats.php). However, at the time of writing, there is a substantial gap in what can be accomplished between mouse and rat species for genetically-defined DREADD manipulations.
We are still at the stage of needing to pilot DREADD expression for most studies and brain areas, due in part to a lack of a good understanding of the heterogeneity of DREADD expression in different brain areas and cell types (e.g., why a vector would be effective in one area but not in another, or even in a different subregion). This is likely a feature dependent on the internal promoters of a particular viral vector and differences in tropism of different serotype AAV particles and pseudotyped lentiviral particles. In addition, the ability of DREADD receptors to modulate activity in a particular brain region or cell-type must be evaluated empirically. DREADD receptors work by “tuning” intracellular signaling pathways; thus the endogenous intracellular signaling status of the cell and differences in molecular signaling components will affect how a cell responds when a particular signaling pathway is activated. The response to increasing levels of IP3, via hM3Dq activation for example, is dependent on the relative contribution and compliment of all receptors impinging on IP3 production. Further, molecular components of signaling pathways present can differ among cell types resulting in varied downstream effects in response to the activation of hM3Dq. On this point, DREADDs might potentially interfere in unknown ways with endogenous GPCR signaling, an issue that deserves experimental attention. We do not as yet have a good sense of whether endogenous signaling processes are normal or abnormal, which is a major question and relevant to long term DREADD use in experiments or in human applications. Finally, it is unclear in the intact brain how much the extent of receptor expression on a given neuron affects its response to CNO. Would a neuron that expresses just a few receptors exhibit the same response to CNO as would one that expresses dozens or hundreds of the receptors? These are questions that may be difficult to resolve in behavioral preparations. However, there is some evidence that lower DREADD expression levels require larger CNO doses for neuronal modulation (Farrell & Roth, 2013). These issues are not the least bit unique to DREADD research, but touch on important methodological questions that researchers foraying into chemogenetics are likely to have.
To end, it is important to place chemogenetics back in context of the technical renaissance that grows to define modern behavioral neuroscience. Ongoing research into brain systems for complex behavior will benefit not just from chemogenetics for the advantages highlighted here, but also from tools for monitoring and characterizing neuronal responses, for intervening in them at physiological realistic timescales, and for monitoring and perturbing particular transmitter systems or molecular signaling processes. In this context, the use of DREADDs allows us to address longstanding questions about brain function related to behavior that have not been possible before, and we anticipate a great deal of continued progress in the future as they are more widely integrated into behavioral studies.
Acknowledgments
Research leading to this work was funded by the Whitehall Foundation (KSS), National Science Foundation (IOS1353137 and IOS0922075, DJB), and National Institutes of Health (R01DA027688, DJB; R01MH097949, BWL; R00DA035251, SVM). We thank Jeffrey Stott, Stephen Chang, and Jennifer Kaufling for research leading to the figures.
Resource List
Dreadd Information and Protocols
DREADD Wiki (operated by B. Roth)
DREADD Blog (operated by B. Roth)
http://chemogenetic.blogspot.com/ (comparison of optogenetics and chemogenetics: http://chemogenetic.blogspot.com/2015/03/dreadds-vs-opto-does-it-matter-to-neuron.html)
Instructional video
Robinson SA, Adelman JS (2015). A method for remotely silencing neural activity in rodents during discrete phases of learning. J. Vis. Exp. (100), e52859, doi:10.3791/52859.
CNO Resources
Dispensary operated by the National Institute of Mental Health
Dispensary operated by the National Institute of Drug Abuse
http://www.drugabuse.gov/ordering-guidelines-research-chemicals-controlled-substances
CNO from Sigma Aldrich
Product number: C0832
CAS number: 34233-69-7
MDL number: MFCD00210190
CNO (and other agonists) from Tocris
CNO product number: 4936
Salvinorin B product number: 5611
hM3Dq DREADD Agonist 21 product number: 5548
hM3Dq DREADD agonist Perlapine: 5549
CNO from Cayman Chemical
Product number: 12059
Resources for Viral Delivery and Histological Assessment of DREADD Vectors
Virus Injection Supplies
Stereotactic Pressure Injections (used by KSS and DJB)
Stereotaxic motorized syringe pump: Quintessential Stereotactic Injector (Stoelting, item 53311)
Syringe: NanoFil 10 μl syringe (World Precision Instruments, item: NANOFIL)
Needle: 36 gauge beveled removable needle (World Precision Instruments, item: NF36BV-2)
Viral Vector Supplies
Sample of Virus Cores
University of North Carolina Vector Core: http://www.med.unc.edu/genetherapy/vectorcore
University of Pennsylvania Vector Core: http://www.med.upenn.edu/gtp/vectorcore/
MIT Viral Core: https://mcgovern.mit.edu/technology/viral-core-facility
Antibodies
Commonly Used Antibodies for Enhancing Signals
Anti-hemagglutinin (e.g., Covance MMS-101P). Labels the HA-tag.
Anti-GFP (e.g., Abcam ab13970). Labels mCitrine, YFP and GFP.
Anti-Ds-Red (e.g., Clontech 632496); Anti-mCherry (Abcam ab167453). Labels mCherry.
References
- Albasanz JL, Perez S, Barrachina M, Ferrer I, Martin M. Up-regulation of adenosine receptors in the frontal cortex in Alzheimer's disease. Brain Pathol. 2008;18(2):211–219. doi: 10.1111/j.1750-3639.2007.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Michaelides M, Zarnegar P, Ren Y, Fagergren P, Thanos PK, et al. Hurd YL. Impaired periamygdaloid-cortex prodynorphin is characteristic of opiate addiction and depression. J Clin Invest. 2013;123(12):5334–5341. doi: 10.1172/JCI70395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anikeeva P, Andalman AS, Witten I, Warden M, Goshen I, Grosenick L, et al. Deisseroth K. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat Neurosci. 2011;15(1):163–170. doi: 10.1038/nn.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104(12):5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschauer DF, Kreuz S, Rumpel S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One. 2013;8(9):e76310. doi: 10.1371/journal.pone.0076310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28(28):7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35(1):48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barot SK, Ferguson SM, Neumaier JF. 5-HT(1B) receptors in nucleus accumbens efferents enhance both rewarding and aversive effects of cocaine. Eur J Neurosci. 2007;25(10):3125–3131. doi: 10.1111/j.1460-9568.2007.05568.x. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81(2):179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Boender AJ, de Jong JW, Boekhoudt L, Luijendijk MC, van der Plasse G, Adan RA. Combined use of the canine adenovirus-2 and DREADD-technology to activate specific neural pathways in vivo. PLoS One. 2014;9(4):e95392. doi: 10.1371/journal.pone.0095392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield LA, Bertran-Gonzalez J, Chieng B, Balleine BW. The thalamostriatal pathway and cholinergic control of goal-directed action: interlacing new with existing learning in the striatum. Neuron. 2013;79(1):153–166. doi: 10.1016/j.neuron.2013.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bru T, Salinas S, Kremer EJ. An update on canine adenovirus type 2 and its vectors. Viruses. 2011;2(9):2134–2153. doi: 10.3390/v2092134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull C, Freitas KC, Zou S, Poland RS, Syed WA, Urban DJ, et al. Bowers MS. Rat nucleus accumbens core astrocytes modulate reward and the motivation to self-administer ethanol after abstinence. Neuropsychopharmacology. 2014;39(12):2835–2845. doi: 10.1038/npp.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Stark E, Berenyi A, Khodagholy D, Kipke DR, Yoon E, Wise KD. Tools for probing local circuits: high-density silicon probes combined with optogenetics. Neuron. 2015;86(1):92–105. doi: 10.1016/j.neuron.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503(7474):111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassataro D, Bergfeldt D, Malekian C, Van Snellenberg JX, Thanos PK, Fishell G, Sjulson L. Reverse pharmacogenetic modulation of the nucleus accumbens reduces ethanol consumption in a limited access paradigm. Neuropsychopharmacology. 2014;39(2):283–290. doi: 10.1038/npp.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Todd TP, Bucci DJ, Smith KS. Inhibiting ventral pallidum with DREADDs impairs sign-tracking in rats European Journal of Neuroscience. 2015;42(12):3105–3116. doi: 10.1111/ejn.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Todd TP, Smith KS. Disconnection of ventral pallidum and nucleus accumbens shell with DREADDs enhances sign-tracking in rats. Society for Neuroscience Abstracts. 2015;255.08 [Google Scholar]
- Chen X, Choo H, Huang XP, Yang X, Stone O, Roth BL, Jin J. The first structure-activity relationship studies for designer receptors exclusively activated by designer drugs. ACS Chem Neurosci. 2015;6(3):476–484. doi: 10.1021/cn500325v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Walsh JJ, Guise KG, Heshmati M, Friedman AK, et al. Russo SJ. Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nat Neurosci. 2015;18(7):962–964. doi: 10.1038/nn.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong AS, Miri ML, Busskamp V, Matthews GA, Acker LC, Sorensen AT, et al. Boyden ES. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat Neurosci. 2014;17(8):1123–1129. doi: 10.1038/nn.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichon J, Gan WB. Branch-specific dendritic Ca(2+) spikes cause persistent synaptic plasticity. Nature. 2015;520(7546):180–185. doi: 10.1038/nature14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482(7383):85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494(7436):238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Anno MT, Caiazzo M, Leo D, Dvoretskova E, Medrihan L, Colasante G, et al. Broccoli V. Remote control of induced dopaminergic neurons in parkinsonian rats. J Clin Invest. 2014;124(7):3215–3229. doi: 10.1172/JCI74664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Allen JA, Farrell M, Roth BL. A chemical-genetic approach for precise spatio-temporal control of cellular signaling. Mol Biosyst. 2010;6(8):1376–1380. doi: 10.1039/c002568m. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Hars B, Hennevin E, Cotillon N. Muscimol diffusion after intracerebral microinjections: a reevaluation based on electrophysiological and autoradiographic quantifications. Neurobiol Learn Mem. 2002;78(1):100–124. doi: 10.1006/nlme.2001.4035. [DOI] [PubMed] [Google Scholar]
- Eldridge MA, Lerchner W, Minamimoto T, Saunders RC, Richmond BJ. Reversible DREADD inactivation of orbitofrontal cortex neurons in rhesus monkeys with contralateral rhinal cortex removal disrupts cued reward discrimination. I. Behavioral analysis. Society for Neuroscience Abstracts. 2014;462.23 [Google Scholar]
- Eldridge MA, Lerchner W, Saunders RC, Kaneko H, Krausz KW, Gonzalez FJ, et al. Richmond BJ. Chemogenetic disconnection of monkey orbitofrontal and rhinal cortex reversibly disrupts reward value. Nat Neurosci. 2016;19(1):37–39. doi: 10.1038/nn.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MS, Pei Y, Wan Y, Yadav PN, Daigle TL, Urban DJ, et al. Roth BL. A Galphas DREADD mouse for selective modulation of cAMP production in striatopallidal neurons. Neuropsychopharmacology. 2013;38(5):854–862. doi: 10.1038/npp.2012.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MS, Roth BL. Pharmacosynthetics: Reimagining the pharmacogenetic approach. Brain Res. 2013;1511:6–20. doi: 10.1016/j.brainres.2012.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, et al. Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14(1):22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Neumaier JF. Grateful DREADDs: engineered receptors reveal how neural circuits regulate behavior. Neuropsychopharmacology. 2012;37(1):296–297. doi: 10.1038/npp.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Phillips PE, Roth BL, Wess J, Neumaier JF. Direct-pathway striatal neurons regulate the retention of decision-making strategies. J Neurosci. 2013;33(28):11668–11676. doi: 10.1523/JNEUROSCI.4783-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Hamlett ED, Vazey EM, Aston-Jones G, Cass WA, Boger HA, Granholm AC. Designer receptors enhance memory in a mouse model of Down syndrome. J Neurosci. 2015;35(4):1343–1353. doi: 10.1523/JNEUROSCI.2658-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A, Homma D, Gibb LG, Amemori K, Rubin SJ, Hood AS, et al. Graybiel AM. A Corticostriatal Path Targeting Striosomes Controls Decision-Making under Conflict. Cell. 2015;161(6):1320–1333. doi: 10.1016/j.cell.2015.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner AR, Rowland DC, Hwang SY, Baumgaertel K, Roth BL, Kentros C, Mayford M. Generation of a synthetic memory trace. Science. 2012;335(6075):1513–1516. doi: 10.1126/science.1214985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27(21):5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore BB, Soden ME, Zweifel LS. Manipulating gene expression in projection-specific neuronal populations using combinatorial viral approaches. Curr Protoc Neurosci. 2013;4(435):4 35 31–34 35 20. doi: 10.1002/0471142301.ns0435s65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Costa RM. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat Commun. 2013;4:2264. doi: 10.1038/ncomms3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA, Kay MA. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol. 2008;82(12):5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanova MD, Good M, Honey RC. Retrieval-mediated learning involving episodes requires synaptic plasticity in the hippocampus. J Neurosci. 2011;31(19):7156–7162. doi: 10.1523/JNEUROSCI.0295-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Ung RL, Resendez SL, Stamatakis AM, Taylor JG, Huang J, et al. Stuber GD. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell. 2015;160(3):516–527. doi: 10.1016/j.cell.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PI, Stellar JR, Paul AD. Regional reward differences within the ventral pallidum are revealed by microinjections of a mu opiate receptor agonist. Neuropharmacology. 1993;32(12):1305–1314. doi: 10.1016/0028-3908(93)90025-x. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Sromek S, Wilcox BJ, Unnerstall JR. Hippocampal adenosine A1 receptors are decreased in Alzheimer's disease. Neurosci Lett. 1990;118(2):257–260. doi: 10.1016/0304-3940(90)90641-l. [DOI] [PubMed] [Google Scholar]
- Kato HE, Zhang F, Yizhar O, Ramakrishnan C, Nishizawa T, Hirata K, et al. Nureki O. Crystal structure of the channelrhodopsin light-gated cation channel. Nature. 2012;482(7385):369–374. doi: 10.1038/nature10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Kuramochi M, Takasumi K, Kobayashi K, Inoue K, Takahara D, et al. Kobayashi K. Neuron-specific gene transfer through retrograde transport of lentiviral vector pseudotyped with a novel type of fusion envelope glycoprotein. Hum Gene Ther. 2011;22(12):1511–1523. doi: 10.1089/hum.2011.111. [DOI] [PubMed] [Google Scholar]
- Katzel D, Nicholson E, Schorge S, Walker MC, Kullmann DM. Chemical-genetic attenuation of focal neocortical seizures. Nat Commun. 2014;5:3847. doi: 10.1038/ncomms4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Matsui R, Kato S, Hasegawa T, Kasahara H, Isa K, et al. Isa T. Genetic dissection of the circuit for hand dexterity in primates. Nature. 2012;487(7406):235–238. doi: 10.1038/nature11206. [DOI] [PubMed] [Google Scholar]
- Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, et al. Boyden ES. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11(3):338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Demars MP, Short JA, Nabel EM, Akbarian S, Baxter MG, Morishita H. Chemogenetic Inactivation of Dorsal Anterior Cingulate Cortex Neurons Disrupts Attentional Behavior in Mouse. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Kravitz AV. Optogenetic and chemogenetic insights into the food addiction hypothesis. Front Behav Neurosci. 2014;8:57. doi: 10.3389/fnbeh.2014.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Owen SF, Kreitzer AC. Optogenetic identification of striatal projection neuron subtypes during in vivo recordings. Brain Res. 2013;1511:21–32. doi: 10.1016/j.brainres.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, et al. Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491(7423):212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerchner W, Eldridge MAG, Saunders RC, Kaneko H, Higuchi M, Minamimoto T, Richmond BJ. Reversible DREADD inactivation of less than 10% of Orbitofrontal cortex neurons in interconnection with rhinal cortex is sufficient to disrupt cue discrimination in monkeys. Society for Neuroscience Abstracts 2014 [Google Scholar]
- Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci. 2013;16(10):1499–1508. doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones GS. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2012;32(38):13309–13326. doi: 10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Aston-Jones G. Dopamine DREADDs: Chemicogenetic control of midbrain dopamine activity during reinstatement of cocaine seeking. Society for Neuroscience Abstracts. 2013;256.03 [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, et al. Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci. 2014 doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Whitaker LR, Bossert JM, Harvey BK, Hope BT, Kaganovsky K, et al. Shaham Y. Behavioral and Physiological Effects of a Novel Kappa-Opioid Receptor-Based DREADD in Rats. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannald MA, Takahashi YK, Lopatina N, Pietras BW, Jones JL, Schoenbaum G. Model-based learning and the contribution of the orbitofrontal cortex to the model-free world. Eur J Neurosci. 2012;35(7):991–996. doi: 10.1111/j.1460-9568.2011.07982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelides M, Anderson SA, Ananth M, Smirnov D, Thanos PK, Neumaier JF, et al. Hurd YL. Whole-brain circuit dissection in free-moving animals reveals cell-specific mesocorticolimbic networks. J Clin Invest. 2013;123(12):5342–5350. doi: 10.1172/JCI72117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi H, Katahira K, Inutsuka A, Fukumoto K, Nakamura A, Wang T, et al. Yamada K. Insular neural system controls decision-making in healthy and methamphetamine-treated rats. Proc Natl Acad Sci U S A. 2015;112(29):E3930–3939. doi: 10.1073/pnas.1418014112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14(2-3):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Nair SG, Strand NS, Neumaier JF. DREADDing the lateral habenula: a review of methodological approaches for studying lateral habenula function. Brain Res. 2012;1511:93–101. doi: 10.1016/j.brainres.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Wess J. Design and functional characterization of a novel, arrestin-biased designer G protein-coupled receptor. Mol Pharmacol. 2012;82(4):575–582. doi: 10.1124/mol.112.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve RL, Carlezon WA., Jr . Gene Delivery into the Brain Using Viral Vectors. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia, Pennsylvania: Lippincott, Williams, & Wilkins; 2002. [Google Scholar]
- Nieh EH, Matthews GA, Allsop SA, Presbrey KN, Leppla CA, Wichmann R, et al. Tye KM. Decoding neural circuits that control compulsive sucrose seeking. Cell. 2015;160(3):528–541. doi: 10.1016/j.cell.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguchi M, Okajima M, Tanaka S, Koizumi M, Kikusui T, Ichihara N, et al. Sakagami M. Double Virus Vector Infection to the Prefrontal Network of the Macaque Brain. PLoS One. 2015;10(7):e0132825. doi: 10.1371/journal.pone.0132825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AG, Hsiao EC, Wang MM, Ho K, Kim DH, Wang X, et al. Mucke L. Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat Neurosci. 2015;18(3):423–434. doi: 10.1038/nn.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG. Exhumed from thought: basal ganglia and response learning in the plus-maze. Behav Brain Res. 2009;199(1):24–31. doi: 10.1016/j.bbr.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Parnaudeau S, O'Neill PK, Bolkan SS, Ward RD, Abbas AI, Roth BL, et al. Kellendonk C. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron. 2013;77(6):1151–1162. doi: 10.1016/j.neuron.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnaudeau S, Taylor K, Bolkan SS, Ward RD, Balsam PD, Kellendonk C. Mediodorsal thalamus hypofunction impairs flexible goal-directed behavior. Biol Psychiatry. 2015;77(5):445–453. doi: 10.1016/j.biopsych.2014.03.020. d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res. 2000;863(1-2):71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Penagarikano O, Lazaro MT, Lu XH, Gordon A, Dong H, Lam HA, et al. Geschwind DH. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7(271):271ra278. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perova Z, Delevich K, Li B. Depression of excitatory synapses onto parvalbumin interneurons in the medial prefrontal cortex in susceptibility to stress. J Neurosci. 2015;35(7):3201–3206. doi: 10.1523/JNEUROSCI.2670-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil KE, Rinker JA, Lowery-Gionta EG, Mazzone CM, McCall NM, Kendra AM, et al. Kash TL. NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat Neurosci. 2015;18(4):545–552. doi: 10.1038/nn.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Robinson S, Todd TP, Pasternak AR, Luikart BW, Skelton PD, Urban DJ, Bucci DJ. Chemogenetic silencing of neurons in retrosplenial cortex disrupts sensory preconditioning. J Neurosci. 2014;34(33):10982–10988. doi: 10.1523/JNEUROSCI.1349-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology. 2014;76 Pt B:450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63(2):291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD, Ni JR, Caron MG. Brain 5-HT deficiency increases stress vulnerability and impairs antidepressant responses following psychosocial stress. Proc Natl Acad Sci U S A. 2015;112(8):2557–2562. doi: 10.1073/pnas.1416866112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y, Shobe JL, Zhou M, Huang S, Shuman T, Cai DJ, et al. Silva AJ. CREB regulates memory allocation in the insular cortex. Curr Biol. 2014;24(23):2833–2837. doi: 10.1016/j.cub.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnebelen W, Browning PGF, Croxson PL, Rudebeck PH, Brookshire SW, Baxter MG. Chemogenetic suppression of neural activity in dorsolateral prefrontal cortex impairs spatial working memory in rhesus monkeys. Society for Neuroscience Abstracts. 2015;356.11 [Google Scholar]
- Scofield MD, Boger HA, Smith RJ, Li H, Haydon PG, Kalivas PW. Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking. Biol Psychiatry. 2015;78(7):441–451. doi: 10.1016/j.biopsych.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Kalivas PW. Astrocytic dysfunction and addiction: consequences of impaired glutamate homeostasis. Neuroscientist. 2014;20(6):610–622. doi: 10.1177/1073858413520347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci. 2005;25(38):8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res. 2009;196(2):155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Virkud A, Deisseroth K, Graybiel AM. Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proc Natl Acad Sci U S A. 2012;109(46):18932–18937. doi: 10.1073/pnas.1216264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumier A, Sibille E. Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacology. 2014;39(9):2252–2262. doi: 10.1038/npp.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachniak TJ, Ghosh A, Sternson SM. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→midbrain pathway for feeding behavior. Neuron. 2014;82(4):797–808. doi: 10.1016/j.neuron.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci. 2014;37:387–407. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Britt JP, Bonci A. Optogenetic modulation of neural circuits that underlie reward seeking. Biol Psychiatry. 2012;71(12):1061–1067. doi: 10.1016/j.biopsych.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13(4):251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban DJ, Zhu H, Marcinkiewcz CA, Michaelides M, Oshibuchi H, Rhea D, et al. Roth BL. Elucidation of The Behavioral Program and Neuronal Network Encoded by Dorsal Raphe Serotonergic Neurons. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy E, Robinson JE, Li C, Olsen RH, DiBerto JF, Giguere PM, et al. Roth BL. A New DREADD Facilitates the Multiplexed Chemogenetic Interrogation of Behavior. Neuron. 2015;86(4):936–946. doi: 10.1016/j.neuron.2015.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazey EM, Aston-Jones G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc Natl Acad Sci U S A. 2014;111(10):3859–3864. doi: 10.1073/pnas.1310025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall NR, De La Parra M, Callaway EM, Kreitzer AC. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron. 2013;79(2):347–360. doi: 10.1016/j.neuron.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, et al. Han MH. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci. 2014;17(1):27–29. doi: 10.1038/nn.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Winiger V, Kandel ER, Balsam PD, Simpson EH. Orbitofrontal cortex mediates the differential impact of signaled-reward probability on discrimination accuracy. Front Neurosci. 2015;9:230. doi: 10.3389/fnins.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74(5):858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Sullivan HA, Pao GM, Hamanaka H, Goosens KA, Verma IM, Seung HS. Lentiviral vectors for retrograde delivery of recombinases and transactivators. Cold Spring Harb Protoc. 2015;2015(4):368–374. doi: 10.1101/pdb.prot075879. [DOI] [PubMed] [Google Scholar]
- Wimmer GE, Shohamy D. Preference by association: how memory mechanisms in the hippocampus bias decisions. Science. 2012;338(6104):270–273. doi: 10.1126/science.1223252. [DOI] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, et al. Deisseroth K. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72(5):721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JO, McNally GP. Pharmacogenetic excitation of dorsomedial prefrontal cortex restores fear prediction error. J Neurosci. 2015;35(1):74–83. doi: 10.1523/JNEUROSCI.3777-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Prigge M, Beyriere F, Tsunoda SP, Mattis J, Yizhar O, et al. Deisseroth K. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat Neurosci. 2008;11(6):631–633. doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znamenskiy P, Zador AM. Corticostriatal neurons in auditory cortex drive decisions during auditory discrimination. Nature. 2013;497(7450):482–485. doi: 10.1038/nature12077. [DOI] [PMC free article] [PubMed] [Google Scholar]


