Abstract
Chronic inflammation is known to play a critical role in the development of cancer. Recent evidence suggests that high salt in the tissue microenvironment induces chronic inflammatory milieu. In this report, using three breast cancer-related cell lines, we determined the molecular basis of the potential synergistic inflammatory effect of sodium chloride (NaCl) with interleukin-17 (IL-17). Combined treatment of high NaCl (0.15 M) with sub-effective IL-17 (0.1 nM) induced enhanced growth in breast cancer cells along with activation of reactive nitrogen and oxygen (RNS/ROS) species known to promote cancer. Similar effect was not observed with equi-molar mannitol. This enhanced of ROS/RNS activity correlates with upregulation of γENaC an inflammatiory sodium channel. The similar culture conditions have also induced expression of pro-inflammatory cytokines such as IL-6, TNFα etc. Taken together, these data suggest that high NaCl in the cellular microenvironment induces a γENaC mediated chronic inflammatory response with a potential pro-carcinogenic effect.
Keywords: Cytokine, Interleukin-17, Inflammation, Cancer, Epithelial Sodium Channel (ENaC)
Introduction
A large body of evidence suggests that chronic inflammation plays a critical role in the development of cancer [1]. Various inflammatory mediators, including cytokines, chemokines, and growth factors, establish an inflammatory milieu conducive for cancer growth [2]. Following tissue injury, resolution of inflammation is required for stable tissue functioning [3]. However, if inflammation resolution is dysregulated, cellular response changes to the pattern of chronic inflammation. In chronic inflammation, the recruited inflammatory cells generate a great amount of growth factors, cytokines, and reactive oxygen and nitrogen species that may cause DNA damage [4]. Microenvironments constituted by all the above elements induce a sustained cell proliferation with continued tissue damage, thus predisposing from chronic inflammation to neoplasia [5]. Several inflammatory cytokines have been correlated with the development of cancer [6]. Particularly, pro-inflammatory cytokine, interleukin (IL)-17, has gained recent prominence for its pro-tumor role.
The CD4+T lymphocytes are known to exert tumor immune-surveillance, a phenomenon wherein, they can act as tumor immune-suppressors throughTh1 response characterized by type 1 IFN-related cytokine secretion in inhibiting tumor development and growth [7]. Contrary to this traditional thinking, recent studies have demonstrated a critical role of certain phenotypes of immune cells towards promoting pro- tumor growth through production of specific cytokines and growth factors [8]. A pro-inflammatory cytokine of particular interest is IL-17, produced a subset of CD4+T cells called Th17 [9]. Although it is thought IL-17 promotes inflammation and therefore potentially tumor growth or tumor regression [8]. This dual antagonistic effect of IL-17 in the context of cancer has raised research in this direction. Studies by Cochaud et al [10], in human breast cancer issues demonstrated tumor infiltrating lymphocytes from triple negative breast cancers (ER-/PR-/Her-) have enhanced IL-17 expression which induces tumor progression through ERK-1/2 protein kinase. Further, IL-17-overexpression in cervical [11] and lung [12] cancers showed greater tumor formation ability in animal cancer models. Importantly, the underlying mechanisms of IL-17 in modulating tumor growth are still poorly understood.
Dietary high salt intake has been correlated with increase the incidence of cardiovascular disease and inflammatory injury in arteries [13]. In the animal models, high sodium chloride has been demonstrated to cause excessive inflammatory activation triggering ischemia injury and, end-organ stress mediated by reactive oxygen species and pro-inflammatory cytokine secretion leading irreversible cardiac cell damage [14, 15]. Similar effects were also reported from human studies on gastric cancer wherein excess sodium can cause inflammation and stomach ulcers, which can lead to gastric cancer [16]. In the context of breast cancer, the sodium content of mammary adenocarcinomas has been shown to be significantly higher than the normal lactating mammary epithelium [17]. However, in these studies it is unclear if the tumor activity is correlated with the extra-cellular or intracellular sodium concentration. Interestingly, studies by Wu et al, demonstrated that high salt-diet induced IL-17 secreting CD4+Th17 phenotype activation a murine autoimmune disease model through upregulation of a salt-sensing transcription factor serum-glucocorticoid kinase, SGK1 [18, 19]. Importantly, as IL-17 is shown to have pro-tumor effect and IL-17 secretion is induced in high salt micro-environment, in our current study, we examine the cellular response following synergistic pro-inflammatory effect of IL-17 with high concentration sodium chloride (NaCl) towards induction breast cancer cell proliferation potentially through epithelial sodium channel (ENaC), a known upstream target of transcription factor SGK-1.
Materials and Methods
Breast cancer cell lines
The breast cancer cell lines used are MDA-MB-231 (highly invasive) and MCF-7 (poorly invasive) and MCF10A (normalized) were obtained from the American Type Culture Collection (Manassas, VA). Breast cancer cell lines were cultured in RPMI 1640 supplemented with 10% FBS, 100 μg/mL streptomycin and 100 units/mL penicillin at 37 °C in 5% CO2 incubator until they reached an optimal 80% confluency. All chemicals unless mentioned were procured from Sigma-Aldrich (St Louis, MO). The cells were treated with varying concentrations of NaCl (0.1-0.3 M) and/or IL-17 (0.1 to 500 nM) for 12 hours in our molecular studies. It is important to note that RPMI media contains sodium chloride at 6 g/L (Sigma Aldrich, #R0883, St Louis, MO) which is equivalent of 0.1 M NaCl. This basal NaCl concentration of 0.1 M is essential for cell survival. In our current study, the final NaCl (0.1 to 0.3 M) concentrations is brought about by addition of NaCl (0-0.2 M) from a 5M stock to basal RPMI to make a final concentration of 0.1 to 0.3 M NaCl in our culture conditions. The negative control to rule-out of possibility if the observed effects were due to osmotic and/or intracellular cell volume/solute effect, we performed by using equi-molar mannitol (0 to 0.2 M mannitol in 0.1 M NaCl culture media).
MTT assay
Cell viability was measured by trypan blue dye exclusion (Sigma Aldrich, MO) and MTT assay (Life technologies, Grand Island, NY) as previously described [20]. The assay was performed as per manufacturer provided instructions and plates were read at 562 nm by the plate reader (EMax Plus spectrophotometer, Molecular Devices, Sunnyvale, CA). Viability was calculated as percentage compared to untreated cells in basal conditions.
NO/ROS /RNS Analysis
The analysis of the nitric oxide (NO), reactive oxygen and reactive nitrogen species (AbCam, Cambridge, MA) was performed in the cell lysate under various assay conditions as per manufacturer's instructions. The data analysis was performed based on a standard curve obtained using the positive controls provided by the manufacturer.
Protein extraction and Western blot analysis
Specific protein determination studies were performed by Western-blot on the total proteins were extracted from cells as described earlier [21]. Protein concentration was determined with a Bradford's assay kit from Bio-Rad (Hercules, CA). All primary and secondary Abs were obtained from Santa Cruz Biotech (Dallas, TX). The membranes were developed using the chemiluminescence kit (Fischer Sci, Pittsburgh, PA) and analyzed on using Bio-Rad Universal Hood II (Hercules, CA). Morphometric analysis was done using the software provided by the company.
Quantitative transcript expression
Expression profiles of intracellular signaling genes in the isolated from breast cancer cell lines were analyzed using the FAM-labeled RT-PCR primers for ENaC, VEGF, (Applied Biosystems, Foster City, CA) as per the manufacturer's recommendation and described earlier [22]. Briefly, total RNA was extracted from 106 cells using EZ-RNA extraction kit (Qiagen, Valencia, CA). RNA samples were quantified by absorbance at 260nm. The RNA was reverse-transcribed and RT-PCR was performed in a final reaction volume of 20μL using iCycler 480 Probes Master (Life Technologies, Grand Island, NY). Each sample was analyzed in triplicate. Cycling conditions consisted of an initial denaturation of 95°C for 15min, followed by 40 cycles of 95°C for 30s, followed by 61°C for 1min.
Cytokine/Chemokine ELISA
The secretory extracellular chemokines, cytokines, and growth factors in the supernatant from cells treated under various assay conditions was quantitated by ELISA as per the manufacturer's protocol (Life Technologies, Grand Island, NY). The protein supernatant was diluted 1:1000 and quantified with a standard curve using the manufacturer provided standards. Detection at 450 nm was performed using EMax Plus spectrophotometer and data analysis was carried out using software provided by the manufacturer (Molecular Devices, Sunnyvale, CA).
Statistical analysis
Data are expressed as mean ± SEM from five independent studies. Statistical differences between means were analyzed using a paired or unpaired Student's t test. A value of P less than 0.05 was considered significant. All data analysis was obtained using Origin 6 software (Origin Labs, Northampton, MA).
Results
IL-17 synergizes with high sodium chloride to induce reactive nitric oxide species in breast cancer cells
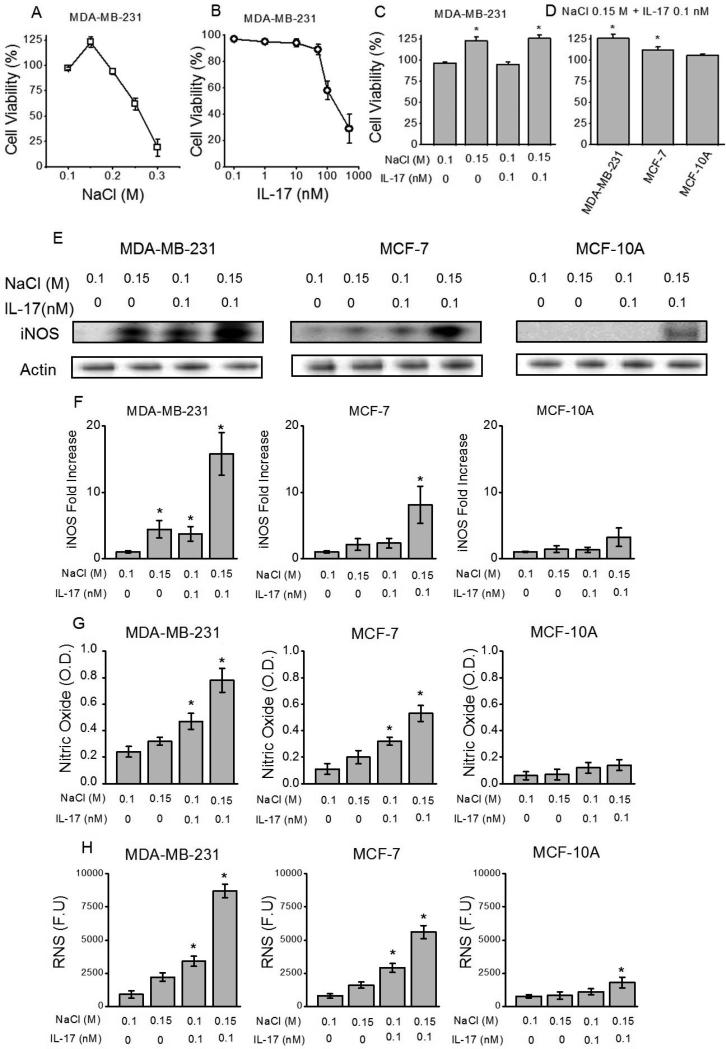
Chronic inflammation induced nitric oxide pathway upregulation has been shown to play a critical role in cancer development and progression [23]. To determine the inflammatory role of high concentration sodium chloride (NaCl) under sub-effective pro-inflammatory cytokine influence we analyzed for the induction of reactive nitric oxide species on breast cancer cell lines. We have utilized highly metastatic (MDA-MB-231), non-metastatic (MCF-7) and normal breast/ breast cancer cell lines. To ascertain the cytotoxicity, initially, we have evaluated the cell viability by dose-dependent studies at various concentrations of NaCl (0.1 M – 0.3 M) and interleukin-17 (IL-17, 0.1-500 nM) following culture for 72 hrs. It is important to note that 0.1 M NaCl refers to the sodium chloride concentration in the basal media and correspondingly culture conditions with 0.1 M NaCl represent basal media conditions. As shown in the figure 1A, NaCl concentrations at or above 0.25 M NaCl in our experiments were significantly cytotoxic. Also, for IL-17, concentrations at or above 350 nM were significantly cytotoxic (greater that 65% cytotoxicity). Further importantly, NaCl concentration at 0.15 M demonstrated a significant 23% (p<0.05) increase in proliferation. Therefore, to determine the role of high salt (0.15 M NaCl, i.e. addition of 0.05 M NaCl equivalent from 5 M stock to 0.1 M in basal media) under sub-effective IL-17 (0.1 nM) potentially mimicking tumor micro-environment, we have used these concentrations for further studies in this report. This combination of 0.15 M NaCl with sub-effective 0.1 nM IL-17 has not shown to be cytotoxic (Fig 1C,D) in the three cell lines used in our current studies. Nitric oxide (NO) pathway and reactive nitrogenous species have been shown to be upregulated during inflammatory injury. Western blot and qRT-PCR analysis demonstrated significantly increased expression of inducible nitric oxide synthetase (iNOS) (Fig 1E,F) following co-treatment with 0.15 M M NaCl+0.1 nM IL-17 in MDA-MB-231 (p<0.05)and MCF-7 (p<0.05) cell lines. However, there was no statistically significant expression of iNOS in normal breast cell line (MCF-10A). Further, ELISA based analysis of the NO content (Fig 1G) in the cell supernatant following co-treatment with 0.15 M M NaCl+0.1 nM IL-17 in MDA-MB-231 was determined to be 0.78 ± 0.09 OD (optical density units), which was higher than the treatment with 0.15 M M NaCl (0.32 ± 0.03 OD, p<0.05) or 0.1 nM IL-17 in basal 0.1 M NaCl media (0.47 ± 0.06 OD, p<0.05) individually. Similarly, enhanced cellular reactive nitrogen species (Fig 1H) were detected following co-treatment with 0.15 M M NaCl+0.1 nM IL-17 in MDA-MB-231 was determined to be 8700 ± 520 RFU (relative fluorescence units), which was higher than the treatment with 0.15 M M NaCl (2200 ± 310 RFU, p<0.05) or 0.1 nM IL-17 in basal 0.1 M NaCl media (3400 ± 370 RFU, p<0.05) individually. Equi-molar mannitol (0.1 M mannitol in 0.1 M NaCl) did not induce RNS response (data not shown). Interestingly, it is also important to note that this addition 0.05 M NaCl to induce ROS/RNS response in our breast cancer cell studies match well with previous salt studies performed by Wu et al [18] to stimulate T-lymphocytes, wherein the authors of used additional 0.04 M NaCl to induce Th17 phenotype switch in CD4+T cells. Taken together, these data demonstrate that high-dose NaCl induces inflammatory stress through enhanced nitric oxide pathway stress in established breast cancer cells. Further inflammatory milieu created in the context of co-treatment with pro-inflammatory cytokine, mimicking the in vivo tumor conditions, synergistically enhances inflammatory stress induced by high dose NaCl potentially playing a role in cancer progression under in vivo conditions.
Figure 1.
Induction of nitric oxide pathway by high sodium chloride in breast cancer cells. (A) Cell viability analysis of MDA-MB-231 in the presence of varying NaCl concentration (0.1 -0.3 mM). It is important to note that 0.1 M NaCl is the basal sodium chloride concentration in the RPMI1640 culture media and therefore 0.1 M NaCl refers to basal media treatment control group. (B) Cell viability analysis of MDA-MB-231 in the presence of varying interleukin (IL-)17 concentration (0.1 - 500 nM) in basal (0.1 M NaCl) media. (C) Lack of cell death with NaCl (0.15 M) and IL-17 (0.1 nM) combination. (D) Cell viability was similar among various breast cancer (MDA-MB-231, MCF-7) and normal breast (MCF10A) cell lines with NaCl (0.3 mM) and IL-17 (0.1 nM) combination; (E) iNOS, inducible nitric oxide synethsase (E), nitric oxide (F) and RNS, reactive nitrogen species (G) expression in all three cell lines mentioned above following treatment with NaCl (0.15 M) and/or IL-17 (0.1 nM in basal 0.1 M NaCl media). Data represented mean values ± SEM from five independent experiments. Student-t-test performed for statistical analysis (significance p<0.05).
IL-17 synergizes with high sodium chloride to induce reactive oxygen species in breast cancer cells
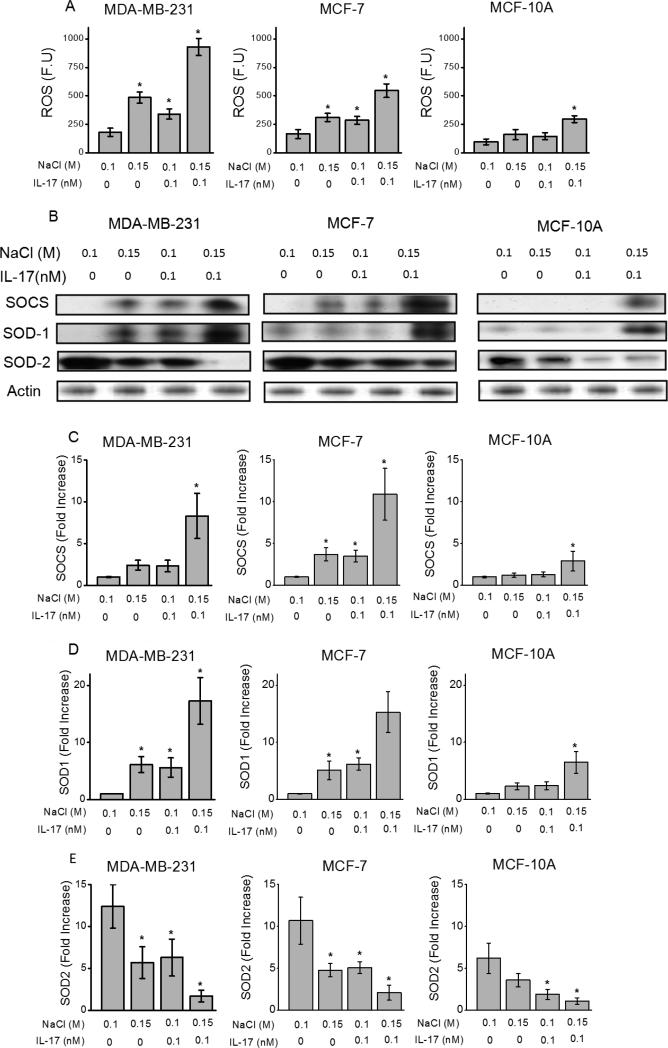
Along with nitric oxide pathway, chronic inflammatory stress is also known to induce reactive oxygen species/ free radical pathway which too plays an important role in cancer development and progression [24]. To determine the inflammatory role of high concentration sodium chloride (NaCl) under sub-effective pro-inflammatory cytokine influence we analyzed for the induction of reactive oxygen species (ROS) in breast cancer cell lines described above. As shown in the figure 2A, fluorimetric analysis demonstrated significantly increased expression of ROS following co-treatment with 0.15 M M NaCl+0.1 nM IL-17 in invasive cell line, MDA-MB-231 (930±75 FU vs 180 ±35 FU p<0.05); non-invasive, MCF-7 (545±60 FU vs 165 ±40 FU p<0.05) and normal MCF10A (295±30 FU vs 95 ±25 FU p<0.05) cell lines over control media treated cells, and also individually high salt (0.15 M NaCl) or sub-effective IL-17 in basal 0.1 M NaCl media cytokine treatment. Further, Western blot and qRT-PCR based analysis of ROS associated enzymes, namely, suppressors of cytokine signaling (SOCS) was also elevated (figure 2 C-D) following co-treatment with high salt and sub-effective IL-17 cytokine over control media treated cells, and also individually high salt or sub-effective IL-17 cytokine treatment. Interesting while protein levels of copper/zinc super oxide dismutase (SOD-1) associated with chronic inflammation were elevated, the protein levels of manganese superoxide dismutase (SOD-2), associated with acute inflammation were inhibited. This data is in line with the SOD2 to SOD1 switch observed in cancer [25]. Equi-molar mannitol (0.1 M mannitol in 0.1 M NaCl) did not induce ROS response (data not shown). Taken together, these data demonstrate that high-NaCl specifically induces a chronic inflammatory stress response through enhanced ROS pathway in established breast cancer cells and normal breast derived cells.
Figure 2.
High sodium chloride induced reactive oxygen species in breast cancer cells. (A) Western blot analysis of anti-oxidant enzyme (SOCS, SOD1, SOD2) expression following treatment with NaCl (0.15 M) and/or IL-17 (0.1 nM in basal 0.1 M NaCl media) in three cell lines; (B) ROS, reactive oxygen species expression in all three cell lines mentioned above following treatment with NaCl (0.15 M) and/or IL-17 (0.1 nM). (C-E) mRNA analysis for SOCS, SOD1, SOD2 expression in various breast cancer cell lines (MDA-MB-231, MCF-7 and MCF-10a) following co-treatment with high salt (0.15 M NaCl) and 0.1 nM IL-17. Data represented mean values ± SEM from five independent experiments. Student-t-test performed for statistical analysis (significance p<0.05).
Synergistic inflammatory effect of IL-17 and high sodium chloride is mediated by γENaC
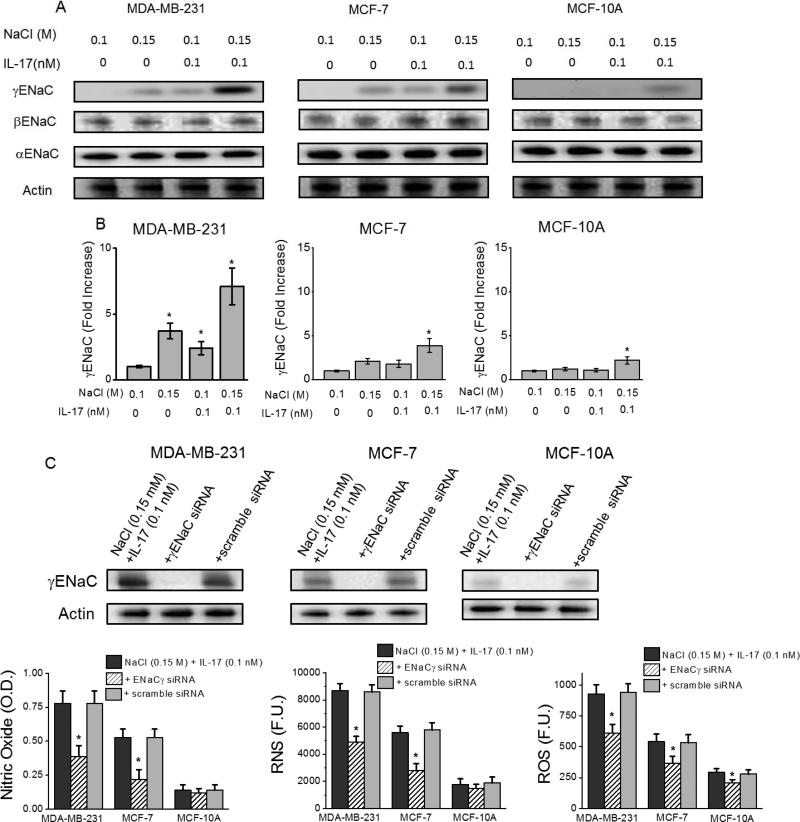
To determine the role of membrane channel proteins in the high sodium chloride mediated inflammatory response, we have studied the role of inflammatory endothelial associated sodium channel proteins (ENaCs) which were previously shown to be expressed in various breast cancers [26, 27]. As shown in figure 3A-B, qRT-PCR and Western-blot analysis demonstrated significantly increased expression of γENaC protein following co-treatment with 0.15 M M NaCl+0.1 nM IL-17 in invasive cell line, MDA-MB-231 (7.1±1.4 fold, p<0.05); non-invasive, MCF-7 (3.9±0.8 fold, p<0.05) and normal MCF10A (2.2±0.4 fold, p<0.05) cell lines over control media treated cells. Other ENaC group proteins, αENaC and βENaC, have not shown any changes in their expression pattern. To directly determine the role of γENaC in the inflammatory response, we have performed siRNA mediated knock-down experiments. As shown in figure 3C-E, stimulation of the three cell lines following co-treatment with 0.15 M NaCl+0.1 nM IL-17 under siRNA mediated knock-down of γENaC suppressed the reactive nitric oxide and reactive oxygen species synthesis. These data suggest that γENaC plays a direct role in high salt mediated inflammatory injury in breast cancer cell lines.
Figure 3.
γENaC mediates high sodium chloride induced inflammatory stress. (A) Western blot analysis of epithelial sodium channel (ENaC) isoforms (-α, -β, -γ) following treatment with NaCl (0.15 M) and/or IL-17 (0.1 nM in basal 0.1 M NaCl media) in three cell lines; (B) mRNA analysis for γENaC expression in various breast cancer cell lines (MDA-MB-231, MCF-7 and MCF-10a) following co-treatment with high salt (0.15 M M NaCl) and 0.1 nM IL-17. (C) Efficient knock-down of γENaC by specific siRNA, while scramble siRNA does not decrease expression of γENaC. (D-F) Inhibition of nitric oxide (C), RNS (D) and ROS (E) release following siRNA knock-down of γENaC. Data represented mean values ± SEM from five independent experiments. Student-t-test performed for statistical analysis (significance p<0.05).
Membrane lipid rafts play a critical role in γENaC mediated inflammatory stress
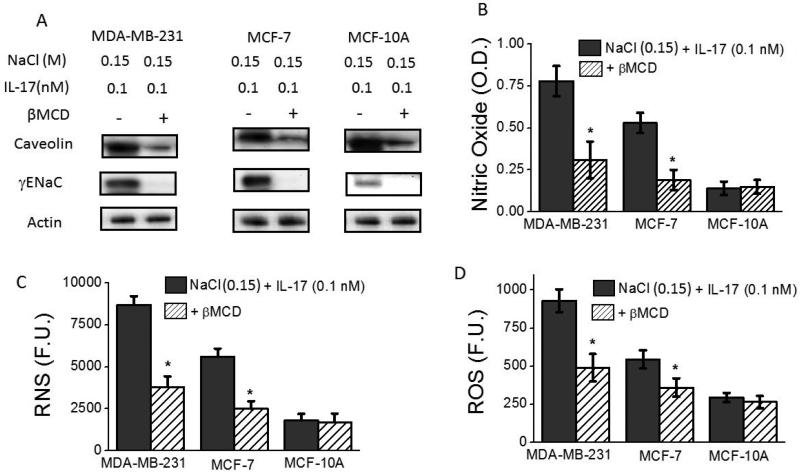
To determine the role of membrane characteristics in the inflammatory γENaC membrane sodium channel function, we have studied the effect of lipid rafts in our studies. Lipid rafts have been shown to play a critical in the functioning of several membrane proteins through modulation of membrane fluidity and liquid-phase of the cell membrane [28]. Modulation of lipid rafts in the cell membrane was achieved through treatment of cells with β-methyl cyclodextrin (βMCD) as determined by the quantitative membrane localization of caveolin, a lipid raft associated protein [29]. As shown in figure 4A-B, Westernblot analysis demonstrated significantly decreased membrane localization of γENaC protein following co-treatment with 0.15 M M NaCl+0.1 nM IL-17 in βMCD pre-treated cell lines, over control media (with basal 0.1 M NaCl) treated cells. Furthermore, specific inhibition of membrane localization of γENaC suppressed the reactive nitric oxide and reactive oxygen species synthesis (fugue 4 C-E). This data suggest that lipid raft play a crutial role in the membrane localization and inflammatory response mediated by γENaC in high salt mediated inflammatory injury of breast cancer cell lines.
Figure 4.
Lipid rafts play critical role in γENaC mediated inflammatory stress. (A) Western blot analysis of γENaC following depletion of lipid rafts in three cell lines; (B) (B-D) Inhibition of nitric oxide (B), RNS (C) and ROS (D) release following βMCD mediated removal of lipid rafts. Data represented mean values ± SEM from five independent experiments. Student-t-test performed for statistical analysis (significance p<0.05).
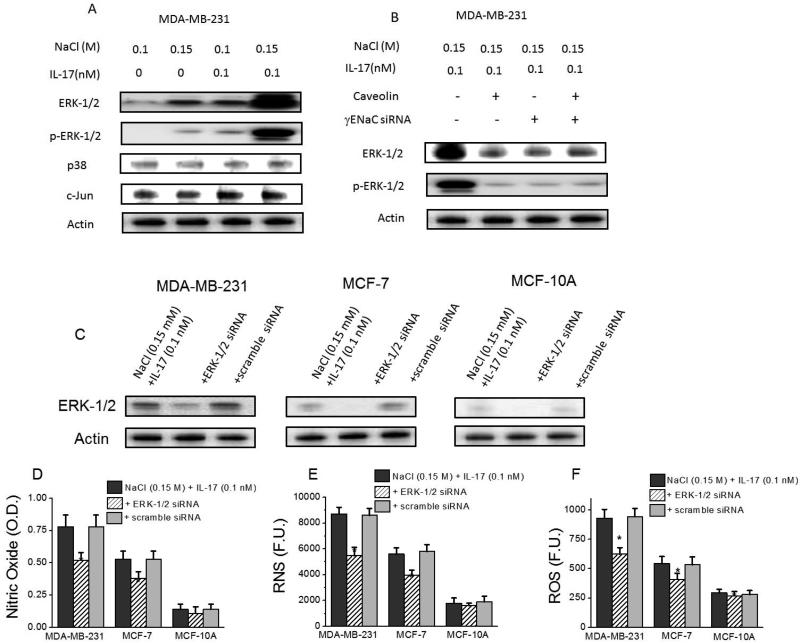
γENaC signals through ERK-1/2 activation in salt induced inflammatory stress
It has been shown in literature that γENaC induces MAP Kinase activation pathway [30]. We analyzed the possible role of MAPKinases in the high salt mediated γENaC induced inflammatory response. Towards this we tested for the protein expression changes of MAPkinase components namely, ERK-1/2, p38 and c-Jun. As shown in figure 5, following co-treatment with 0.15 M NaCl+0.1 nM IL-17 there is enhanced expression (p<0.05) and phosphorylation (p<0.05) of ERK-1/2 in MDA-MB-231 (invasive cell line) over control media (with basal 0.1 M NaCl) treated cell cultures. Similar effect was also seen in non-invasive MCF-7 cell line too (data not shown). Furthermore, siRNA knock-down of γENaC demonstrated inhibition of ERK-1/2 expression and also its phosphorylated form (figure 5B). A downstream role of ERK-1/2 protein kinase in the γENaC mediated inflammatory response, by reduced release of reactive nitric oxide and reactive oxygen species following siRNA ERK-1/2 knockdown (figure 5D-F). Control studies with scramble siRNA (figure 5C) demonstrated specificity of our ERK1/2 siRNA to significantly knockdown ERK1/2 expression. It is important to note that the cell proliferation affecting ERK1/2 [31, 32] has a varying inducible expression pattern among the three cell lines with highest expression in MDA-MB-231. These data demonstrate that ERK-1/2 protein kinase is potentially the downstream mediator of γENaC induced inflammatory response in high salt mediated injury of breast cancer cell lines. Furthermore, knockdown of caveolin by specific siRNA induced downregulation of ERK1/2, which strongly suggests that membrane fluidity affecting caveolin is an upstream factor to ERK1/2 in IL-17 synergized high salt mediated RNS/ROS response.
Figure 5.
ERK-1/2 protein kinase upregulation in γENaC induced inflammatory stress. (A) Western blot (phospho-blot) analysis of signaling factors (ERK-1/2, p-ERK-1/2, p38, c-Jun) following treatment with NaCl (0.15 M M) and/or IL-17 (0.1 nM in basal 0.1 M NaCl media) in three cell lines; (B) Western blot (phospho-blot) analysis of protein kinase, (p-)ERK-1/2, following siRNA knock-down of γENaC and/or lipid rafts in three cell lines; (C) Efficient knock-down of ERK-1/2 by specific siRNA, while scramble siRNA does not decrease expression of ERK-1/2. (D-F) Inhibition of nitric oxide (C), RNS (D) and ROS (E) release following siRNA knock-down of ERK-1/2. Data represented mean values ± SEM from five independent experiments. Student-t-test performed for statistical analysis (significance p<0.05).
Upregulation of inflammatory cytokines and chemokine following sodium chloride induced cancer cell stress
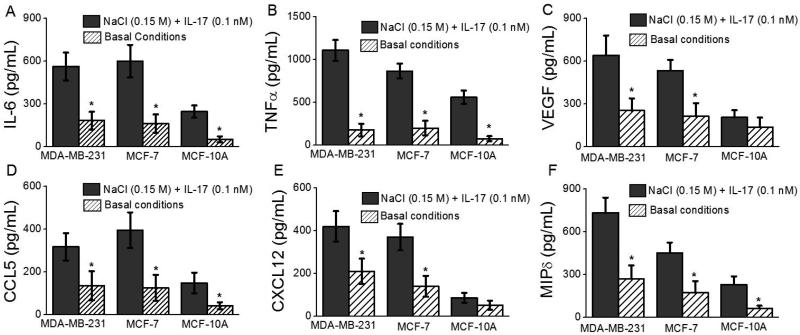
As cancer progression and metastasis is mediated by proinflammatory cytokines and chemokines [2] we performed gene expression analysis to quantitatively determine their expression pattern in our study conditions. As shown in figure 6, co-treatment with 0.15 M NaCl+0.1 nM IL-17 demonstrated significant (p<0.05) increase in the mRNA expression of cytokines, IL-6 (3 fold), TNFα (6.2 fold); chemokines, CCL5 (2.3 fold), CXCL-12 (2 fold), MIP-1δ (2.7 fold); and angiogenic growth factor VEGF (2.5 fold) over control media treated MDA-MD-231 cells. Other CXC and CC chemokines such as CXCL13, CCL 2,7,8, MIP-3 etc showed no statistically significant increase (data not shown). These data clearly demonstrate that high sodium chloride under sub-effective IL-17 stimulation induces expression of cytokines and chemokines which potentially favor cancer proliferation and metastasis.
Figure 6.
Upregulation of inflammatory cytokines and chemokine following sodium chloride induced cancer cell stress. ELISA based analysis of cytokines, IL-6 (A), TNFα (B); chemokines, CCL5 (C), CXCL-12 (D), MIP-1δ (E); and angiogenic growth factor VEGF (F) following treatment with NaCl (0.15 M mM) and IL-17 (0.1 nM) in three cell lines. Basal condition refers to 0.1 M NaCl which is the basal concentration of NaCl in regular RPMI1640 media. Data represented mean values ± SEM from five independent experiments. Student-t-test performed for statistical analysis (significance p<0.05).
Discussion
There is compelling evidence pointing out molecular pathways linking chronic inflammation and cancer. In the tumor microenvironment, smoldering inflammation contributes to proliferation and survival of malignant cells, along with evasion of adaptive immunity. Initial studies aiming at studying the relationship between inflammation and cancers led to the determination of reactive oxygen and nitrogen species generated within and from inflammatory cells recruited as the prime inducers of the assault resulting in tumor initiation and proliferation [2]. Among the family of nitric oxide synthases (NOS) specifically inducible NOS (iNOS) has shown to be involved in promoting the etiology of cancer [33]. Studies on iNOS expression in human breast cancer suggested that iNOS activity was higher in less differentiated tumors in a panel of 15 invasive breast carcinomas [34]. In line with this body of evidence, we demonstrate that high sodium chloride concentration under sub-effective cytokine stimulation in the cancer cellular environment was able to induce hazardous reactive nitrogen species (figure 1), thus clearly suggesting that high sodium chloride in the tumor microenvironment induces chronic inflammatory milieu leading to release of RNS species.
Similar to RNS, the role of reactive oxygen species (ROS) and its potential implications in cancer have been investigated for several decades [24]. Cancer cells are known to be metabolically active and under increased oxidative stress due to uncontrolled cell proliferation and dysfunction of metabolic regulation mainly mediated by increased generation of reactive oxygen species (ROS) [35]. ROS-mediated DNA lesions and mutations are likely to provide a mechanism through which drug-resistant variant cancers constantly evolve [36]. Several agents have been shown to induce this ROS stress in cancer cells. In our current report we demonstrate that high sodium chloride concentration the breast cancer cellular environment is potent inducer of ROS stress (figure 2). However, the relationship between intracellular sodium concentration and cancer remains elusive, although it may involve changes in cell volume and/or membrane potential. However, a potential explanation of high salt induced cell volume changes as the cause of inflammatory ROS and RNS response, does not seem possible as equi-molar mannitol (0.1 - 0.2 M mannitol + 0.1 M naCl) in our culture media did not elicit any inflammatory RNS and ROS response (data not shown). Thus we our data supports the conclusion that the inflammatory ROS and RNS responses were possibly a direct effect of high sodium chloride concentration. Furthermore in our current study, high salt in the culture media has demonstrated significant upregulation of pro-inflammatory chemokine, cytokine and angiogenic growth factors (figure 6).
The expression of antioxidant enzymes, such as superoxide dismutases (SOD), is regulated by complex mechanisms, oxidative stress being a major factor that induces the adaptive expression of these enzymes [37]. Increased SOD levels were observed in breast cancer tissue from 23 patients [38]. SOD has two isoforms – SOD1 (Cu/Zn SOD) and SOD2 (MnSOD) [37]. Emerging evidences from several groups indicate that SOD1 is over-expressed in cancers [39]. Further, it was also reported that SOD2 (MnSOD) is down-regulated in breast cancer cell lines [40]. Since in absence of SOD2, unrestricted SOD1 activity causes superoxide levels to elevate potentially leading to cancerous changes. Pathological studies have shown 87% reduction in the activity SOD2 breast cancers [40]. In line with this evidence our data (figure 2) shows that high salt conditions induce ROS enzymes SOCS and SOD1 while decreasing the activity of SOD2 thus potentially enhancing the inflammatory response in breast cancer cell lines.
In our current study as external sodium ions in the media require specialized membranous channels for their passage in the cells we checked for the known sodium channels with inflammatory downstream effector role. The epithelial sodium channel (ENaC) consisting of α-, β-, and γ-subunits modulates the rate-limiting step in sodium reabsorption [41]. These ENaCs are considered to exert an important regulatory effect on sodium transport of several epithelial tissues, including the colon, kidney, and lung [42]. Furthermore, genetic variants of ENaC were found to be associated with patients suffering with essential hypertension [43, 44]. Several studies have shown a positive correlation between expression of ENaC, increased sodium current, and increased metastatic invasiveness of breast cancer [45]. In line with this evidence, our current data (figure 3) demonstrates that following increased sodium concentration in the external environment there is specific increased expression of γENaC in the two breast cancer cell lines of our current study. Further, siRNA knock-down of γENaC expression significantly decreased the high sodium chloride induced RNS and ROS release, thus strongly suggesting that γENaC expression is directly correlated with the high salt induced inflammatory injury.
It is of interest to note that, the ENaC expression levels in breast cancer cell lines were upregulated by the mineralo-corticoid hormone, aldosterone, but not by other steroid hormones, such as glucocorticoids or progesterone [46]. This suggests a direct functional role of ENaC in high-salt mediated oncogenesis. Currently, there is not much published literature showing the factors which modulate ENaC expression in breast cancer. As ENaC is a membrane channel protein, we studied if lipid rafts play a role in ENaC membrane localization. As shown in figure 4, depletion of lipid rafts has inhibited stable ENaC membrane localization. Further, the enhanced membrane localization of ENaC has induced ERK-1/2 protein kinase signaling (figure 5). Of note, this is important as several human and animal studies have conclusively demonstrated an important role of ERK-1/2 in breast cancer [47]. Further, ERK-1/2 is a known protein kinase mediating cell growth [48].
Assessment of salt intake in human studies is difficult to study and is obviously prone to significant subjective biases. Many commonly used dietary assessment methods, such as food frequency questionnaires and diet records, have only limited reproducibility in epidemiological studies [49]. Although 24 hours urine collection method may be an optimal method in estimating routine salt intake [50], it is highly challenging to adopt this method for a large-scale population study. Therefore, a better molecular target which is can be used as a direct surrogate target for high salt tissue micro-environment will be more useful tool. In conclusion, in our current study we demonstrate an important role of epithelial sodium channel, γENaC, in high salt mediated inflammatory injury. We believe our current study supports the use of γENaC molecular target for possible future pre-clinical and clinical studies to determine the effect of salt on breast cancer staging and progression.
Highlights.
Inflammatory stress induces cancer proliferation
IL-17 synergizes with high salt to induce ROS/RNS activity
This synergistic inflammatory effect is mediated by γENaC
Lipid rafts and ERK-1/2 play a critical role in this inflammatory stress
Acknowledgement
Financial Support: This work was supported by NIH 5U54CA163066-04 Cancer partnership (Vanderbilt University and Tennessee State University. The authors thank Department of Biological Sciences, Tennessee State University for the kind support on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflict of interest.
References
- 1.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–6. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 4.Okada F. Inflammation and free radicals in tumor development and progression. Redox Rep. 2002;7:357–68. doi: 10.1179/135100002125001135. [DOI] [PubMed] [Google Scholar]
- 5.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 6.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braumuller H, Wieder T, Brenner E, Assmann S, Hahn M, Alkhaled M, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–5. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 8.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183:4169–75. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 9.Ernst M, Putoczki T. IL-17 cuts to the chase in colon cancer. Immunity. 2014;41:880–2. doi: 10.1016/j.immuni.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Cochaud S, Giustiniani J, Thomas C, Laprevotte E, Garbar C, Savoye AM, et al. IL-17A is produced by breast cancer TILs and promotes chemoresistance and proliferation through ERK1/2. Sci Rep. 2013;3:3456. doi: 10.1038/srep03456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tartour E, Fossiez F, Joyeux I, Galinha A, Gey A, Claret E, et al. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59:3698–704. [PubMed] [Google Scholar]
- 12.Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–89. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 13.Zhang F, Tang Z, Hou X, Lennartsson J, Li Y, Koch AW, et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci U S A. 2009;106:6152–7. doi: 10.1073/pnas.0813061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mozaffari MS, Patel C, Ballas C, Schaffer SW. Effects of excess salt and fat intake on myocardial function and infarct size in rat. Life Sci. 2006;78:1808–13. doi: 10.1016/j.lfs.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Ketonen J, Mervaala E. Effects of dietary sodium on reactive oxygen species formation and endothelial dysfunction in low-density lipoprotein receptor-deficient mice on high-fat diet. Heart Vessels. 2008;23:420–9. doi: 10.1007/s00380-008-1066-5. [DOI] [PubMed] [Google Scholar]
- 16.Tsugane S, Sasazuki S, Kobayashi M, Sasaki S. Salt and salted food intake and subsequent risk of gastric cancer among middle-aged Japanese men and women. Br J Cancer. 2004;90:128–34. doi: 10.1038/sj.bjc.6601511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sparks RL, Pool TB, Smith NK, Cameron IL. Effects of amiloride on tumor growth and intracellular element content of tumor cells in vivo. Cancer Res. 1983;43:73–7. [PubMed] [Google Scholar]
- 18.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–7. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leavy O. T cells: Salt promotes pathogenic TH17 cells. Nat Rev Immunol. 2013;13:225. doi: 10.1038/nri3432. [DOI] [PubMed] [Google Scholar]
- 20.Platt D, Amara S, Mehta T, Vercuyssee K, Myles EL, Johnson T, et al. Violacein inhibits matrix metalloproteinase mediated CXCR4 expression: potential anti-tumor effect in cancer invasion and metastasis. Biochem Biophys Res Commun. 2014;455:107–12. doi: 10.1016/j.bbrc.2014.10.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amara S, Lopez K, Banan B, Brown SK, Whalen M, Myles E, et al. Synergistic effect of pro-inflammatory TNFalpha and IL-17 in periostin mediated collagen deposition: potential role in liver fibrosis. Mol Immunol. 2015;64:26–35. doi: 10.1016/j.molimm.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiriveedhi V, Angaswamy N, Brand D, Weber J, Gelman AG, Hachem R, et al. A shift in the collagen V antigenic epitope leads to T helper phenotype switch and immune response to self-antigen leading to chronic lung allograft rejection. Clin Exp Immunol. 2012;167:158–68. doi: 10.1111/j.1365-2249.2011.04486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He D, Li H, Yusuf N, Elmets CA, Athar M, Katiyar SK, et al. IL-17 mediated inflammation promotes tumor growth and progression in the skin. PLoS One. 2012;7:e32126. doi: 10.1371/journal.pone.0032126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–96. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papa L, Hahn M, Marsh EL, Evans BS, Germain D. SOD2 to SOD1 switch in breast cancer. J Biol Chem. 2014;289:5412–6. doi: 10.1074/jbc.C113.526475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaug S, Hybiske K, Cohn J, Firestone GL, Machen TE, Miller SS. ENaC- and CFTR-dependent ion and fluid transport in mammary epithelia. Am J Physiol Cell Physiol. 2001;281:C633–48. doi: 10.1152/ajpcell.2001.281.2.C633. [DOI] [PubMed] [Google Scholar]
- 27.Boyd C, Naray-Fejes-Toth A. Steroid-mediated regulation of the epithelial sodium channel subunits in mammary epithelial cells. Endocrinology. 2007;148:3958–67. doi: 10.1210/en.2006-1741. [DOI] [PubMed] [Google Scholar]
- 28.Schilli R, Breuer RI, Klein F, Dunn K, Gnaedinger A, Bernstein J, et al. Comparison of the composition of faecal fluid in Crohn's disease and ulcerative colitis. Gut. 1982;23:326–32. doi: 10.1136/gut.23.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiriveedhi V, Angaswamy N, Weber J, Mohanakumar T. Lipid raft facilitated ligation of K-alpha1-tubulin by specific antibodies on epithelial cells: Role in pathogenesis of chronic rejection following human lung transplantation. Biochem Biophys Res Commun. 2010;399:251–5. doi: 10.1016/j.bbrc.2010.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicod M, Michlig S, Flahaut M, Salinas M, Fowler Jaeger N, Horisberger JD, et al. A novel vasopressin-induced transcript promotes MAP kinase activation and ENaC downregulation. Embo J. 2002;21:5109–17. doi: 10.1093/emboj/cdf509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–6. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 32.Ma Z, Webb DJ, Jo M, Gonias SL. Endogenously produced urokinase-type plasminogen activator is a major determinant of the basal level of activated ERK/MAP kinase and prevents apoptosis in MDA-MB-231 breast cancer cells. J Cell Sci. 2001;114:3387–96. doi: 10.1242/jcs.114.18.3387. [DOI] [PubMed] [Google Scholar]
- 33.Kostourou V, Cartwright JE, Johnstone AP, Boult JK, Cullis ER, Whitley G, et al. The role of tumour-derived iNOS in tumour progression and angiogenesis. Br J Cancer. 2011;104:83–90. doi: 10.1038/sj.bjc.6606034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomsen LL, Miles DW, Happerfield L, Bobrow LG, Knowles RG, Moncada S. Nitric oxide synthase activity in human breast cancer. Br J Cancer. 1995;72:41–4. doi: 10.1038/bjc.1995.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rios-Arrabal S, Artacho-Cordon F, Leon J, Roman-Marinetto E, Del Mar Salinas-Asensio M, Calvente I, et al. Involvement of free radicals in breast cancer. Springerplus. 2013;2:404. doi: 10.1186/2193-1801-2-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–5. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- 38.Punnonen K, Ahotupa M, Asaishi K, Hyoty M, Kudo R, Punnonen R. Antioxidant enzyme activities and oxidative stress in human breast cancer. J Cancer Res Clin Oncol. 1994;120:374–7. doi: 10.1007/BF01247464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papa L, Manfredi G, Germain D. SOD1, an unexpected novel target for cancer therapy. Genes Cancer. 2014;5:15–21. doi: 10.18632/genesandcancer.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell. 2011;19:416–28. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossier BC, Canessa CM, Schild L, Horisberger JD. Epithelial sodium channels. Curr Opin Nephrol Hypertens. 1994;3:487–96. doi: 10.1097/00041552-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Soundararajan R, Pearce D, Hughey RP, Kleyman TR. Role of epithelial sodium channels and their regulators in hypertension. J Biol Chem. 2010;285:30363–9. doi: 10.1074/jbc.R110.155341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang XF, Lu XM, Lin RY, Wang SZ, Zhang LP, Qian J, et al. Lack of association of functional variants in alpha-ENaC gene and essential hypertension in two ethnic groups in China. Kidney Blood Press Res. 2008;31:268–73. doi: 10.1159/000151286. [DOI] [PubMed] [Google Scholar]
- 44.Persu A, Barbry P, Bassilana F, Houot AM, Mengual R, Lazdunski M, et al. Genetic analysis of the beta subunit of the epithelial Na+ channel in essential hypertension. Hypertension. 1998;32:129–37. doi: 10.1161/01.hyp.32.1.129. [DOI] [PubMed] [Google Scholar]
- 45.Roger S, Besson P, Le Guennec JY. Involvement of a novel fast inward sodium current in the invasion capacity of a breast cancer cell line. Biochim Biophys Acta. 2003;1616:107–11. doi: 10.1016/j.bbamem.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest. 1999;104:R19–23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Svensson S, Jirstrom K, Ryden L, Roos G, Emdin S, Ostrowski MC, et al. ERK phosphorylation is linked to VEGFR2 expression and Ets-2 phosphorylation in breast cancer and is associated with tamoxifen treatment resistance and small tumours with good prognosis. Oncogene. 2005;24:4370–9. doi: 10.1038/sj.onc.1208626. [DOI] [PubMed] [Google Scholar]
- 48.Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–39. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 49.Kroke A, Klipstein-Grobusch K, Voss S, Moseneder J, Thielecke F, Noack R, et al. Validation of a self-administered food-frequency questionnaire administered in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: comparison of energy, protein, and macronutrient intakes estimated with the doubly labeled water, urinary nitrogen, and repeated 24-h dietary recall methods. Am J Clin Nutr. 1999;70:439–47. doi: 10.1093/ajcn/70.4.439. [DOI] [PubMed] [Google Scholar]
- 50.Tsubono Y, Takahashi T, Iwase Y, Iitoi Y, Akabane M, Tsugane S. Nutrient consumption and gastric cancer mortality in five regions of Japan. Nutr Cancer. 1997;27:310–5. doi: 10.1080/01635589709514542. [DOI] [PubMed] [Google Scholar]