Abstract
Aims
To determine the role of sialylation on α5β1 and α2β1 integrins in the regulation of adhesion between breast cancer cells and extracellular matrix (ECM).
Main methods
Static cell adhesion assays were performed to quantify avidity of breast cancer cells to ECM. The effects of sialidases on α2,6 sialylation was assessed by flow cytometry using biotin conjugated sambucus nigra lectin. Lectin affinity assays were used to determine expression of α2,6 sialylated integrins. Cell migration and invasion were investigated by wound healing and transwell invasion assays.
Key findings
α2, α5 and β1 integrins had considerable α2,6 sialylation on MDA-MB-231 cells, whereas signals from MCF-7 cells were undetectable. Cleavage of α2,6 sialylation increased adhesion of MDA-MB-231 cells to ECM, while adhesion of MCF-7 cells was unaffected, consistent with the latter’s lack of endogenous α2,6 sialylated surface integrins. Neither surface expression of α2β1 and α5β1 integrins, nor activated β1 integrin, changed in MDA-MB-231 cells after sialidase treatment. However, sialidase treatment did not have significant impact on migration or invasion of MDA-MB-231 cells.
Significance
Cell adhesion is an important early step of cancer metastasis, yet the roles of sialylation in regulating integrin-mediated breast cancer cell adhesion in comparison to migration and invasion are not well-understood. Our data suggest desialylation of α2,6-sialylated integrins increases adhesion, but not migration or invasion, of MDA-MB-231 cells to ECM without altering integrin expression. It should be considered that α2,6 sialylation may play different roles in regulating cell adhesion of different cancer cells when developing potential therapeutics targeting α2,6 sialylation.
Keywords: cell adhesion, extracellular matrix, integrins, N-Acetylneuraminic acid, breast neoplasms
Introduction
The avidity of integrins to their ligands is not only affected by surface expression level, but also regulated by glycosylation. Integrins, glycoproteins composed of α and β subunits, are known receptors of extracellular matrix (ECM) (Hynes, 2002). The interactions between integrins and ECM have been shown to mediate diverse physiological functions such as cell adhesion, development and carcinogenesis (Bianchi-Smiraglia et al., 2012). As a basic function carried out by integrins, adhesion to ECM on endothelial and stromal cells is thought to participate in cancer cell metastasis by facilitating extravasation of circulating cancer cells (Lafrenie et al., 1992). In addition, integrin mediated adhesion also contributes to the resistance of cancer cells to chemotherapeutic drugs (Aoudjit and Vuori, 2012) and ionizing radiation (Eke et al., 2012). The function of integrins is not only regulated by surface protein expression, but is also controlled by signaling pathways, which confer conformation changes between low and high affinity states (Arnaout et al., 2007, Askari et al., 2009).
The alteration of integrin sialylation is also an important regulator of cell adhesion, especially sialylation of β1 integrin. Mitogen-activated protein kinase/ERK kinase (MEK) activation in U937 myeloid cells induced hyposialylation of β1 integrin and increased the avidity of U937 cell adhesion to fibronectin (Seales et al., 2005b). In contrast, upregulation of ST6Gal-I in metastatic colon cancer cells led to the elevation of α2,6 sialylated β1 integrin, yet also enhanced cell adhesion, migration and metastasis (Seales et al., 2005a, Shaikh et al., 2008). Ras activation increased α2,6 sialylation of β1 integrin of HD3 colonocytes, and desialylation by sialidase inhibited cell adhesion to collagen I (Seales et al., 2003). Application of a fluorinated sialic acid analogue has shown great potential in reducing B16F10 melanoma cell adhesion to ECM and tumor growth in mice, which is encouraging for developing drugs targeting hypersialylation in cancer (Bull et al., 2013). In light of the importance of α2,6 sialylation of integrins in the regulation of adhesion of these types of cells, we sought to determine the role of α2,6 sialylation of integrins in regulating adhesion and invasion of breast cancer cells to ECM. Our data indicate that integrins are highly α2,6 sialylated on MDA-MB-231 cells but not on MCF-7 cells. Desialylation of integrins increases adhesion of MDA-MB-231 cells to ECM without alteration of integrin expression. Moreover, the desialylation-increased adhesion does not correlate with the migration and invasiveness of the cells.
Materials and methods
Cell Culture
The breast cancer cell line MDA-MB-231 was kindly provided by Dr. Jianjian Li (University of California Davis Cancer Center, CA), and the breast cancer cell line MCF-7 was obtained from the American Type Culture Collection (Manassas, VA). MDA-MB-231 cells were cultured in minimal essential medium (MEM) (Corning, Manassas, VA), containing 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 1 mM sodium pyruvate, 1X non-essential amino acids (NEAA) and 1% penicillin and streptomycin. MCF-7 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin.
Antibodies and Reagents
Sialidase from Vibrio cholerae (Ada et al., 1961) (VC, broad substrate specificity) was purchased from Roche (Indianapolis, IN). Clostridium perfringens sialidase (Cassidy et al., 1965, St Geme, 1994) (CP, specificity of cleavage: α2,3 > α2,6 sialylation) and Arthrobacter ureafaciens sialidase (Corfield et al., 1983, Saito et al., 1979) (AU, specificity of cleavage: α2,6 > α2,3 sialylation) were purchased from Sigma-Aldrich (St. Louis, MO) and Prozyme (Hayward, CA), respectively. An ECM screening kit containing fibronectin and collagen IV pre-coated strips was obtained from EMD Millipore (Billerica, MA). The following anti-human primary antibodies were purchased from BD Biosciences (San Jose, CA): CD29 (Mab13) mAb, CD15 (HI98) mAb, CD15s (CSLEX1) mAb, and CD49b (12F1) mAb. The following anti-human primary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA): CD49e (JBS5) mAb, CD49e (H-104) polyclonal antibody, CD49b (H-293) polyclonal antibody and CD29 (N-20) polyclonal antibody. Anti-activated integrin β1 antibody (HUTS-4) was purchased from EMD Millipore (Billerica, MA). Biotin conjugated Sambucus nigra lectin (SNA) was obtained from EY labs (San Mateo, CA). Corresponding isotype control antibodies, fluorescein isothiocyanate (FITC) conjugated secondary antibodies, and FITC-conjugated streptavidin were purchased from BD Biosciences. Horse radish peroxidase (HRP) conjugated secondary antibodies were obtained from Santa Cruz Biotechnology. Streptavidin agarose resin was obtained from Thermo Scientific (Rockford, IL).
Western Blotting and Lectin Affinity Assay
For western blotting, MDA-MB-231 and MCF-7 cells were first lysed in 2% NP-40 buffer containing protease inhibitor on ice. Supernatant was collected by centrifugation. Proteins (100 μg) were separated in 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) resolving gel and transferred to a nitrocellulose membrane (Pall, Port Washington, NY). After blocking for 1 h with 5% non-fat milk in Tris-buffered saline with Tween (TBS-T) at room temperature, the membrane was incubated with anti-CD49b (H293), anti-CD49e (H-104), or anti-CD29 (N-20) antibodies at 4°C overnight. After washing with TBS-T three times, the membrane was incubated with corresponding HRP conjugated secondary antibodies for 30 min at room temperature. Blots were washed with TBS-T and Tris-buffered saline (TBS) twice respectively and visualized using West Pico Supersignal chemiluminescent substrate (Pierce, Rockford, IL). β-actin was used as the loading control.
For the lectin affinity assay, cell lysates (300 μg) of MDA-MB-231 and MCF-7 cells were incubated with 50 μg/ml biotinylated SNA, a specific lectin for α2,6 sialylation (Shibuya et al., 1987, Taatjes et al., 1988), at 4°C overnight. Streptavidin agarose resin (Thermo Scientific, Waltham, MA) was added to cell lysates for 4 h rotation at 4°C. The beads were then washed once with TBS/1% Triton X-100 and twice with TBS/0.5% Triton X-100. Bead-protein complexes were boiled in SDS-PAGE loading buffer, and released proteins were electrophoresed on an 8% SDS-PAGE as described above. Since β-actin cannot be precipitated by the resin, the loading control (β-actin) for lectin affinity assay was run in a separate gel using the cell lysates before adding biotinylated SNA.
Flow Cytometry
Surface sialylation and integrin expression were assessed by flow cytometry. MDA-MB-231 and MCF-7 cells were collected in phosphate buffered saline (PBS) and washed twice. Cells were then fixed in 4% formaldehyde for 10 min. After being chilled on ice for 60 s, cells were washed once in PBS, and twice in 0.5% bovine serum albumin (BSA) before resuspension in 0.5% BSA for blocking. 1×106 cells were incubated with primary mAb anti-CD15s (CSLEX1), anti-CD15 (HI98), anti-CD49b (12F1), anti-CD49e (JBS5), anti-CD29 (Mab13), anti-CD29 (HUTS-4), biotinylated SNA and corresponding isotype control antibodies for 1 h at room temperature. For biotinylated SNA, the FITC-conjugated streptavidin was used as background control. For CD15 and CD15s, the secondary antibody alone was used as background. After washing with 0.5% BSA twice, cells were incubated with corresponding FITC conjugated secondary antibodies for 30 min at room temperature. Cells were washed twice with 0.5% BSA, resuspended in 0.5 ml PBS and analyzed on a FACSAria Special Order Research Product flow cytometer (BD Biosciences).
Cell Adhesion Assay
Adhesion of MDA-MB-231, MCF-7 and HT-29 cells to fibronectin was measured using ECM coated strips (EMD Millipore) per the manufacturer’s instructions. Briefly, cells were collected, washed and resuspended in culture medium. 2×105 MDA-MB-231 cells, 3 × 105 MCF-7 cells and 1 × 105 HT-29 cells were seeded onto fibronectin coated strips. Cells were incubated for 30 min at 37°C. Strips were washed by DPBS to remove non-adherent cells. 0.2% Crystal violet in 10% ethanol was used to stain attached cells and the cells were finally dissolved in 2% SDS after washing. Absorbance at 540 nm was measured by a microplate reader (Molecular Devices, Sunnyvale, CA). Absorbance was normalized to the control cells incubated in DPBS without sialidase.
Cell Migration and Invasion Assay
For migration, a wound-healing assay was performed as previously described with modifications (Cheng et al., 2012). A 96-well plate for suspension culture (Greiner Bio-One, Monroe, NC) was coated with 2 μg/cm2 fibronectin overnight at 4°C. Then the cells were mock treated or treated with sialidases, and monolayer wounds were created using a 200 μL pipette tip. The cells were washed once with serum free medium and allowed to migrate for 7 h in FBS free DMEM. The wound healing was visualized using an Olympus IX70 inverted microscope (Olympus, Tokyo, Japan) at 4X magnification. Photographs were taken by a Retiga 1300 CCD Camera (Qimaging, Burnaby, B.C., Canada) using iVision-Mac™ software (BioVision Technologies, Exton, PA). The width of the wound (gap distance) was measured by ImageJ (National Institutes of Health, Bethesda, MD). Gap distances were shown as the ratio of 7 h to 0 h.
For the invasion assay, 8 μm pore Polycarbonate Membrane Transwell® Inserts in 24-well plates (Corning, Tewksbury, MA) were used per the manufacturer’s instructions. Inner chambers of transwell inserts were first coated with 20 μg/ml fibronectin overnight at 4°C. Cells were mock treated or treated with sialidases. After washing once with serum free medium, the cells (1×105) were suspended in 100 μL FBS free medium and seeded onto each inner chamber. 600 μL DMEM with 10% FBS was added to the outer chambers. Transwell inserts were incubated for 24 h at 37°C. Cells that migrated into the lower surface of the membrane were fixed by 4% formaldehyde and stained with crystal violet. In each experiment, cells were counted in 4 different fields. Cells were imaged by the same digital camera and software as described above.
Statistical Analysis
The data are presented as mean ± SD of at least 3 independent experiments. Student’s t-test was used in comparing control group and each treatment. Only p<0.05 was considered statistically significant.
Results
α5β1 and α2β1 integrins on MDA-MB-231 but not MCF-7 cells are α2,6 sialylated
To determine the role of sialylation of integrin in breast cancer cell adhesion, we first determined the expression and sialylation on α5β1 and α2β1 integrin, which play critical roles in regulation of adhesion of breast cancer MDA-MB-231 or MCF-7 cells to ECM proteins, specifically collagen IV (Col IV) and fibronectin (Lee et al., 2014). Our data indicate that α2, α5 and β1 integrins were expressed in both cell lines, and MDA-MB-231 expressed a higher level of each integrin compared to MCF-7 cells (Fig 1, right panel). In view of the importance of α2,6 sialylation in regulating cell adhesion, lectin SNA was used as an α2,6 sialylation marker to compare the sialylation level between the two breast cancer cell lines. However, only the integrins on MDA-MB-231 were heavily α2,6 sialylated; the α2,6 sialylation of integrins on MCF-7 cells was nearly undetectable by lectin affinity assay (Fig 1, left panel).
Fig. 1.
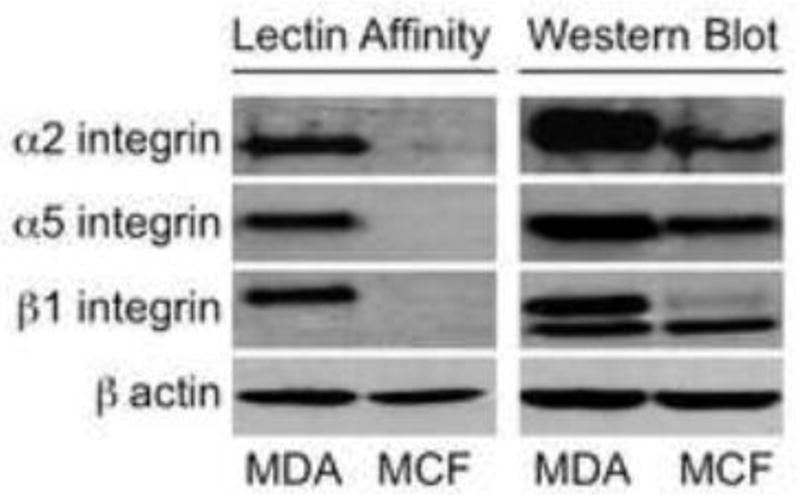
Sialylation of relevant integrins on MDA-MB-231 and MCF-7 cells. For lectin affinity assay, 300 μg cell lysate of MDA-MB-231 and MCF-7 cells were harvested and incubated with 50 μg/ml biotinylated SNA at 4°C overnight. Streptavidin agarose beads were then added for additional 4 hours incubation at 4°C. Beads were washed and boiled before loading to the gel. Loading control for lectin affinity assay was collected before adding biotinylated SNA. For western blotting, 100 μg cell lysate was loaded. This figure is representative of 3 independent experiments.
Desialylation of α2,6-sialylated integrins increases adhesive affinity between MDA-MB-231 cells and ECM proteins
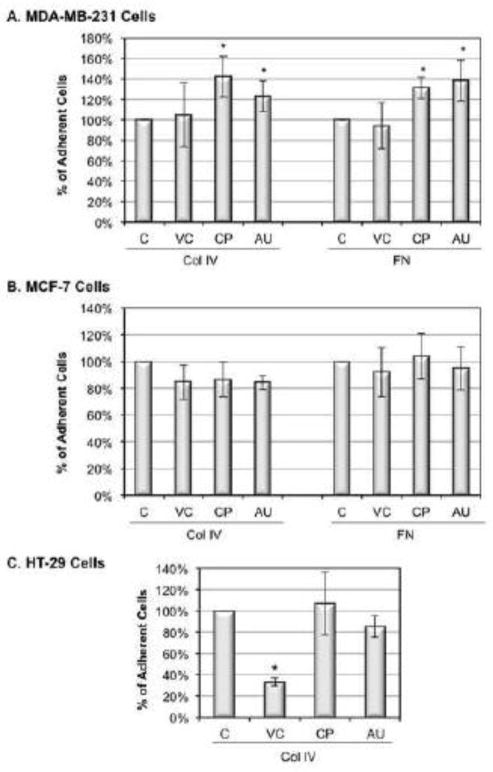
To determine the involvement of α2,6 sialylation in mediating breast cancer cell adhesion, we tested the effect of three sialidases with different specificities on adhesive affinities between α2β1 integrin and Col IV, and between α5β1 and fibronectin. V. cholera (VC) sialidase has broad cleavage activity (i.e., no specificity preference for sialic acid linkages) (Ada et al., 1961); and C. perfringens (CP) and A. ureafaciens (AU) sialidases have a preference towards α2,3 sialylation (Cassidy et al., 1965, St Geme, 1994) and α2,6 sialylation (Corfield et al., 1983, Saito et al., 1979), respectively. Our data showed that the attachment of MDA-MB-231 cells to collagen IV was increased to 142.2%±19.9% or 123.1%±15.4% after CP sialidase or AU sialidase treatment, respectively (Fig 2A). The attachment of MDA-MB-231 cells to fibronectin was increased to 131.3%±10.7% and 138.1%±19.7% after CP sialidase and AU sialidases treatment, respectively (Fig 2A). As expected, under the same conditions, the attachment of MCF-7 cells to collagen IV or fibronectin did not increase (Fig 2B). However, it was a surprise that VC sialidase had no statistically significant effect on breast cancer cell adhesion, given its ability to cleave sialic acids of multiple linkages (Fig 2A). Further analysis using a sialylated integrins-expressing colon cancer cell line HT-29 (Vercoutter-Edouart et al., 2008) demonstrated that the same VC sialidase treatment decreased adhesion to collagen IV by 70%±1.9% (Fig 2C), which is in agreement with a previous report (Kemmner et al., 1992). Interestingly CP and AU sialidases had no statistically significant effects on HT-29 cell adhesion (Fig 2C), suggesting the specificity of sialidases might be cell line dependent.
Fig. 2.
Effect of sialidase on adhesion of MDA-MB-231 cells, MCF-7 cells and HT-29 cells to ECM. (A) MDA-MB-231 cells, (B) MCF-7 cells and (C) HT-29 cells were harvested and treated with 0.1U/ml V. cholera (VC), C. perfringens (CP), and A. ureafaciens (AU) sialidase in DPBS for 30 min at 37°C. Cells were seeded into each well of fibronectin (FN) or collagen IV (Col IV) pre-coated strips. Cells were washed and stained after 30 min incubation at 37°C. Absorbance was normalized to the control cells incubated in DPBS without sialidase. Duplicate samples were prepared in each experiment. Data shown are the means ± SD, n=5. *: p<0.05 versus untreated control group.
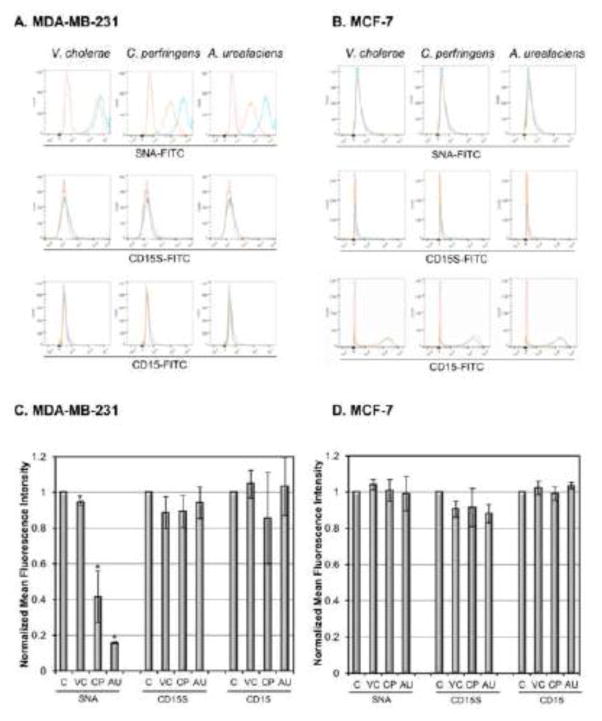
Since VC sialidase, which should cleave both α2,6 and α2,3 linked sialic acids, did not have a significant effect on the cell adhesion, we examined specificities of the sialidases using lectin SNA as α2,6 sialylation marker and CD15s/CD15 ratio as α2,3 sialylation markers, in removing sialic acids. Our data showed that lectin SNA-binding on MDA-MB-231 cells was reduced by 5.8%±1.3%, 58.5%±14.5% and 84.3%±0.5% after treating with VC, CP and AU sialidase respectively (Figs. 3A, 3C). This result confirmed that CP and AU sialidases are more efficient in cleaving α2,6 sialylation than VC sialidase. Our data also showed that endogenous CD15S expression on MDA-MB-231 cells was very weak and none of the sialidase treatments had any effect on CD15S/CD15 ratio on the cells (Figs. 3A, 3C). As for MCF-7 cells, the lectin SNA-binding and CD15S expression were hardly detected. Thus the sialidase treatment did not have any effect on these markers as expected (Figs. 3B, 3D).
Fig. 3.
Detection of sialic acid cleavage on MDA-MB-231 and MCF-7 cells by flow cytometry after sialidase treatment. (A) MDA-MB-231 and (B) MCF-7 cells were harvested and treated with 0.1U/ml V. cholera (VC), C. perfringens (CP), or A. ureafaciens (AU) sialidase in DPBS for 30 min at 37°C. SNA binding, CD15 expression and CD15s expression on MDA-MB-231 and MCF-7 cells were measured by flow cytometric analysis. Red dotted lines represent background (secondary antibody alone), blue lines represent control cells incubated in DPBS without sialidase and orange lines represent sialidase treated cells. Quantification of flow cytometry by normalizing mean fluorescence intensity relative to control cells is shown in C and D. These figures are representative of 3 independent experiments.
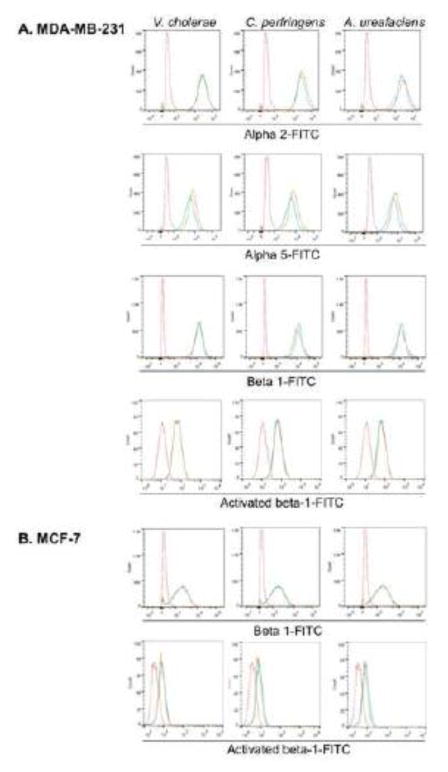
To eliminate the possibility that the changes in adhesion to Col IV and fibronectin were due to the surface integrin expression level changes after sialidase treatment, flow cytometry was used to analyze cells. In MDA-MB-231 cells, there was no significant change in any integrins (Fig 4A). In MCF-7 cells, β1 integrin level did not change after sialidase treatment (Fig 4B). In addition, activated β1 integrin was also tested under the same conditions, and no significant change was observed in either cell line (Fig 4). These data indicate that the increased adhesion resultant with sialidase treatments is not due to a change in surface expression or activation of β1 integrin.
Fig. 4.
Surface expression of relevant integrins on MDA-MB-231 and MCF-7 cells after sialidase treatment. (A) MDA-MB-231 cells and (B) MCF-7 cells were harvested and treated with 0.1U/ml V. cholerae, C. perfringens, and A. ureafaciens sialidase in DPBS for 30 min at 37°C. Expression of α5, β1 and activated β1 integrin on MDA-MB-231 cells, and β1 integrin and activated β1 integrin on MCF-7 cells were analyzed by flow cytometry. Red dotted lines represent isotype control, blue lines represent control cells incubated in DPBS without sialidase treatment and orange lines represent sialidase treated cells. These figures are representative of 3 independent experiments.
Sialidase-catalyzed integrin desialylation does not correlate with MDA-MB-231 cell migration and invasion
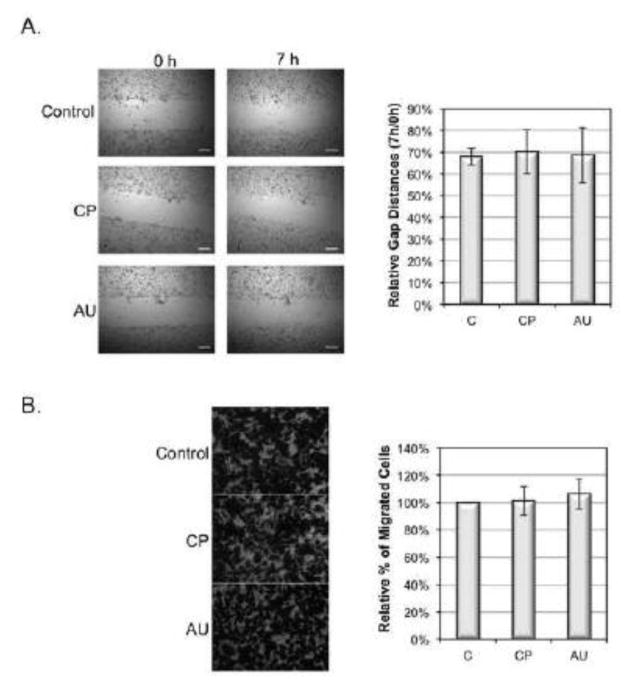
To determine whether the desialylation and increased adhesion of MDA-MB-231 cells are correlated to the later metastatic cascade steps of migration and invasion, we examined migration and invasion of desialylated cells on fibronectin-coated surface using wound healing assay and transwell invasion assay. Our data indicate that CP or AU sialidase treatments have no statistically significant effects on migration and invasiveness of the cells (Fig 5). As CP and AU were confirmed to be the most efficient sialidases cleaving α2,6 sialylation on MDA-MB-231 cells, these data together indicate that decreasing α2,6 sialylation by sialidase treatment does not affect cell migration and invasion, regardless of the influence on cell adhesion.
Fig. 5.
Effect of sialidase on migration and invasion of MDA-MB-231 cells. (A) MDA-MB-231 cells were grown on a fibronectin-coated plate to reach confluence and were treated with 0.1U/ml C. perfringens (CP), and A. ureafaciens (AU) sialidase in DPBS for 30 min at 37°C. The wound was then made using a 200 μl pipette tip. Cells were incubated in FBS free medium for 7 h at 37°C. Photographs were taken at 0 h and 7 h. Gap distances were shown as the ratio of 7 h to 0 h. Duplicate samples were prepared in each experiment. Data shown are the means ± SD, n=4. *: p<0.05 versus control group. Scale bar, 250 μm. (B) MDA-MB-231 cells were harvested and treated with sialidases as described in (A). 1×105 MDA-MB-231 cells were seeded into inner chamber of transwell inserts coated with fibronectin (FN). After 24 h incubation at 37°C, cells migrated to the lower surface were fixed, stained and then counted under a microscope from 4 different fields. Duplicate samples were prepared in each experiment. Data shown are the means ± SD, n=3. *: p<0.05 versus control group. Scale bar, 50 μm.
Discussion
Altered glycosylation of integrins plays roles in cancer cell adhesion, migration and invasion during metastasis (Almaraz et al., 2012, Christie et al., 2008, Lee et al., 2010, Reddy and Kalraiya, 2006, Uemura et al., 2009). As one of a diverse family of sialylated integrins, α2,6 sialylated β1 integrin has been identified in many types of cancer cells, such as ovarian cancer cells (SKOV3 and PA1) (Christie et al., 2008), colon cancer cells (SW480, Lovo) (Lee et al., 2010), and melanoma cells (WM9 and WM239) (Kremser et al., 2008). However, the role of sialylation on breast cancer cells in regulation of cell adhesion is not fully understood. It has been reported that antisense transfection of ST6 Gal-I decreased adhesion of MDA-MB-435 breast cancer cells to collagen IV (Lin et al., 2002). But MDA-MB-435 cells were later found to originate from M14 melanoma cells (Rae et al., 2007), leaving a need to investigate the differential sialylation of integrins on widely used breast cancer cells, for instance MDA-MB-231 and MCF-7 cells, and the role(s) in cell adhesion. In this study, we determined the regulatory roles of sialylation on α5β1 and α2β1 integrins, which mediate the adhesion of breast cancer cells to ECM proteins (Lee et al., 2014). We demonstrated that MDA-MB-231, but not MCF-7, cells are highly sialylated with α2,6 linked sialic acids on α2, α5 and β1 subunits of the integrins (Figs. 1 and 3); and that α2,6 sialylation reduces the affinity between MDA-MB-231 cells to collagen IV and fibronectin (Fig 2). These results agree with previous findings with leukemic cells (Pretzlaff et al., 2000, Seales et al., 2005b, Semel et al., 2002). Nevertheless, the results contrast with large amount of reports based on the studies of colon cancer cells indicating α2,6 sialylation on β1 integrin promotes the cell adhesion to ECM proteins laminin and collagens (Kemmner et al., 1992, Seales et al., 2005a, Shaikh et al., 2008). Thus, our results further confirm that the regulation of integrin function by sialylation is not uniform across different cancer cell types. Moreover, our results also demonstrate that the sialylation-increased adhesion of MDA-MB-231 cells is not correlated with migration and invasiveness of the cells (Fig 5). These results, together with previous reports (Gu and Taniguchi, 2008, Lawrence and Springer, 1991, Yamamoto et al., 1997, Yamamoto et al., 2001), strongly indicate that sialylated integrin-mediated cancer adhesion does not have a direct correlation with cancer cell migration and invasiveness.
Acknowledgments
This work was supported in part by NSF CBET-1039869 (to MMB and SW), NIH RO1CA086928 (to SW) and graduate assistantship (to Y. Yuan) from the Department of Chemistry and Biochemistry, Ohio University. The authors thank Dr. Shinhee Lee, Curran Rhodes, Tiantian Liu and Michelle Pate for their technical assistance, as well as Grady Carlson for technical assistance and critical review of the manuscript.
Footnotes
Conflict of Interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ada GL, French EL, Lind PE. Purification and properties of neuraminidase from Vibrio cholerae. J Gen Microbiol. 1961;24:409–25. doi: 10.1099/00221287-24-3-409. [DOI] [PubMed] [Google Scholar]
- Almaraz RT, Tian Y, Bhattarcharya R, Tan E, Chen SH, Dallas MR, et al. Metabolic flux increases glycoprotein sialylation: implications for cell adhesion and cancer metastasis. Mol Cell Proteomics. 2012;11:M112 017558. doi: 10.1074/mcp.M112.017558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoudjit F, Vuori K. Integrin signaling in cancer cell survival and chemoresistance. Chemother Res Pract. 2012;2012:283181. doi: 10.1155/2012/283181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout MA, Goodman SL, Xiong JP. Structure and mechanics of integrin-based cell adhesion. Curr Opin Cell Biol. 2007;19:495–507. doi: 10.1016/j.ceb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askari JA, Buckley PA, Mould AP, Humphries MJ. Linking integrin conformation to function. J Cell Sci. 2009;122:165–70. doi: 10.1242/jcs.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi-Smiraglia A, Paesante S, Bakin AV. Integrin beta5 contributes to the tumorigenic potential of breast cancer cells through the Src-FAK and MEK-ERK signaling pathways. Oncogene. 2012;32:3049–58. doi: 10.1038/onc.2012.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull C, Boltje TJ, Wassink M, de Graaf AM, van Delft FL, den Brok MH, et al. Targeting aberrant sialylation in cancer cells using a fluorinated sialic acid analog impairs adhesion, migration, and in vivo tumor growth. Molecular cancer therapeutics. 2013;12:1935–46. doi: 10.1158/1535-7163.MCT-13-0279. [DOI] [PubMed] [Google Scholar]
- Cassidy JT, Jourdian GW, Roseman S. The sialic acids. VI. Purification and properties of sialidase from Clostridium perfringens. J Biol Chem. 1965;240:3501–6. [PubMed] [Google Scholar]
- Cheng H, Mollica MY, Lee SH, Wang L, Velazquez-Martinez CA, Wu S. Effects of nitric oxide-releasing nonsteroidal anti-inflammatory drugs (NONO-NSAIDs) on melanoma cell adhesion. Toxicology and applied pharmacology. 2012;264:161–6. doi: 10.1016/j.taap.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie DR, Shaikh FM, Lucas JAt, Lucas JA, 3rd, Bellis SL. ST6Gal-I expression in ovarian cancer cells promotes an invasive phenotype by altering integrin glycosylation and function. J Ovarian Res. 2008;1:3. doi: 10.1186/1757-2215-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield AP, Higa H, Paulson JC, Schauer R. The specificity of viral and bacterial sialidases for alpha(2-3)- and alpha(2-6)-linked sialic acids in glycoproteins. Biochim Biophys Acta. 1983;744:121–6. doi: 10.1016/0167-4838(83)90080-8. [DOI] [PubMed] [Google Scholar]
- Eke I, Dickreuter E, Cordes N. Enhanced radiosensitivity of head and neck squamous cell carcinoma cells by beta1 integrin inhibition. Radiother Oncol. 2012;104:235–42. doi: 10.1016/j.radonc.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Gu J, Taniguchi N. Potential of N-glycan in cell adhesion and migration as either a positive or negative regulator. Cell Adh Migr. 2008;2:243–5. doi: 10.4161/cam.2.4.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Kemmner W, Morgenthaler J, Brossmer R. Alterations in cell surface carbohydrate composition of a human colon carcinoma cell line affect adhesion to extracellular matrix components. Biochimie. 1992;74:117–22. doi: 10.1016/0300-9084(92)90191-g. [DOI] [PubMed] [Google Scholar]
- Kremser ME, Przybylo M, Hoja-Lukowicz D, Pochec E, Amoresano A, Carpentieri A, et al. Characterisation of alpha3beta1 and alpha(v)beta3 integrin N-oligosaccharides in metastatic melanoma WM9 and WM239 cell lines. Biochim Biophys Acta. 2008;1780:1421–31. doi: 10.1016/j.bbagen.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Lafrenie RM, Podor TJ, Buchanan MR, Orr FW. Up-regulated biosynthesis and expression of endothelial cell vitronectin receptor enhances cancer cell adhesion. Cancer Res. 1992;52:2202–8. [PubMed] [Google Scholar]
- Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–73. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Lee M, Lee HJ, Seo WD, Park KH, Lee YS. Sialylation of integrin beta1 is involved in radiation-induced adhesion and migration in human colon cancer cells. Int J Radiat Oncol Biol Phys. 2010;76:1528–36. doi: 10.1016/j.ijrobp.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Lee SH, Cheng H, Yuan Y, Wu S. Regulation of ionizing radiation-induced adhesion of breast cancer cells to fibronectin by alpha5beta1 integrin. Radiat Res. 2014;181:650–8. doi: 10.1667/RR13543.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Kemmner W, Grigull S, Schlag PM. Cell surface alpha 2,6 sialylation affects adhesion of breast carcinoma cells. Exp Cell Res. 2002;276:101–10. doi: 10.1006/excr.2002.5521. [DOI] [PubMed] [Google Scholar]
- Pretzlaff RK, Xue VW, Rowin ME. Sialidase treatment exposes the beta1-integrin active ligand binding site on HL60 cells and increases binding to fibronectin. Cell Adhes Commun. 2000;7:491–500. doi: 10.3109/15419060009040306. [DOI] [PubMed] [Google Scholar]
- Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD. MDA-MB-435 cells are derived from M14 melanoma cells--a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res Treat. 2007;104:13–9. doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- Reddy BV, Kalraiya RD. Sialilated beta1,6 branched N-oligosaccharides modulate adhesion, chemotaxis and motility of melanoma cells: Effect on invasion and spontaneous metastasis properties. Biochim Biophys Acta. 2006;1760:1393–402. doi: 10.1016/j.bbagen.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Saito M, Sugano K, Nagai Y. Action of Arthrobacter ureafaciens sialidase on sialoglycolipid substrates. Mode of action and highly specific recognition of the oligosaccharide moiety of ganglioside GM1. J Biol Chem. 1979;254:7845–54. [PubMed] [Google Scholar]
- Seales EC, Jurado GA, Brunson BA, Wakefield JK, Frost AR, Bellis SL. Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 2005a;65:4645–52. doi: 10.1158/0008-5472.CAN-04-3117. [DOI] [PubMed] [Google Scholar]
- Seales EC, Jurado GA, Singhal A, Bellis SL. Ras oncogene directs expression of a differentially sialylated, functionally altered beta1 integrin. Oncogene. 2003;22:7137–45. doi: 10.1038/sj.onc.1206834. [DOI] [PubMed] [Google Scholar]
- Seales EC, Shaikh FM, Woodard-Grice AV, Aggarwal P, McBrayer AC, Hennessy KM, et al. A protein kinase C/Ras/ERK signaling pathway activates myeloid fibronectin receptors by altering beta1 integrin sialylation. J Biol Chem. 2005b;280:37610–5. doi: 10.1074/jbc.M508476200. [DOI] [PubMed] [Google Scholar]
- Semel AC, Seales EC, Singhal A, Eklund EA, Colley KJ, Bellis SL. Hyposialylation of integrins stimulates the activity of myeloid fibronectin receptors. J Biol Chem. 2002;277:32830–6. doi: 10.1074/jbc.M202493200. [DOI] [PubMed] [Google Scholar]
- Shaikh FM, Seales EC, Clem WC, Hennessy KM, Zhuo Y, Bellis SL. Tumor cell migration and invasion are regulated by expression of variant integrin glycoforms. Exp Cell Res. 2008;314:2941–50. doi: 10.1016/j.yexcr.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya N, Goldstein IJ, Broekaert WF, Nsimba-Lubaki M, Peeters B, Peumans WJ. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2-6)Gal/GalNAc sequence. J Biol Chem. 1987;262:1596–601. [PubMed] [Google Scholar]
- St Geme JW., 3rd The HMW1 adhesin of nontypeable Haemophilus influenzae recognizes sialylated glycoprotein receptors on cultured human epithelial cells. Infect Immun. 1994;62:3881–9. doi: 10.1128/iai.62.9.3881-3889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taatjes DJ, Roth J, Peumans W, Goldstein IJ. Elderberry bark lectin--gold techniques for the detection of Neu5Ac (alpha 2,6) Gal/GalNAc sequences: applications and limitations. Histochem J. 1988;20:478–90. doi: 10.1007/BF01002646. [DOI] [PubMed] [Google Scholar]
- Uemura T, Shiozaki K, Yamaguchi K, Miyazaki S, Satomi S, Kato K, et al. Contribution of sialidase NEU1 to suppression of metastasis of human colon cancer cells through desialylation of integrin beta4. Oncogene. 2009;28:1218–29. doi: 10.1038/onc.2008.471. [DOI] [PubMed] [Google Scholar]
- Vercoutter-Edouart AS, Slomianny MC, Dekeyzer-Beseme O, Haeuw JF, Michalski JC. Glycoproteomics and glycomics investigation of membrane N-glycosylproteins from human colon carcinoma cells. Proteomics. 2008;8:3236–56. doi: 10.1002/pmic.200800151. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kaneko Y, Rebbaa A, Bremer EG, Moskal JR. alpha2,6-Sialyltransferase gene transfection into a human glioma cell line (U373 MG) results in decreased invasivity. J Neurochem. 1997;68:2566–76. doi: 10.1046/j.1471-4159.1997.68062566.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Oviedo A, Sweeley C, Saito T, Moskal JR. Alpha2,6-sialylation of cell-surface N-glycans inhibits glioma formation in vivo. Cancer Res. 2001;61:6822–9. [PubMed] [Google Scholar]