Abstract
Background
Obesity is a public health problem that carries global and substantial social and economic burden. Relative to non-surgical interventions, bariatric surgery has the most substantial and lasting impact on weight loss. However, it leads to a number of nutritional deficiencies requiring long term supplementation.
Objectives
The aims of this paper are to review 25-hydroxyvitamin D [25(OH)D] status pre and post - bariatric surgery, describe the dose response of vitamin D supplementation, and assess the effect of the surgical procedure on 25(OH)D level following supplementation.
Methods
We searched Medline, PubMed, the Cochrane Library and EMBASE, for relevant observational studies published in English, from 2000–April 2015. The identified references were reviewed, in duplicate and independently, by two reviewers.
Results
We identified 51 eligible observational studies assessing 25(OH)D status pre and/or post bariatric surgery. Mean pre-surgery 25(OH)D level was below 30 ng/ml in 29 studies and 17 of these studies showed mean 25(OH)D levels ≤ 20 ng/ml. Mean 25(OH)D levels remained below 30 ng/ml following bariatric surgery despite various vitamin D replacement regimens, with only few exceptions. The increase in postoperative 25(OH)D levels tended to parallel increments in vitamin D supplementation dose but varied widely across studies. An increase in 25(OH)D level by 9–13 ng/ml was achieved when vitamin D deficiency was corrected using vitamin D replacement doses of 1,100–7,100 IU/day, in addition to the usual maintenance equivalent daily dose of 400 – 2,000 IU (total equivalent daily dose 1,500–9,150 IU). There was no difference in mean 25(OH)D level following supplementation between malabsorptive/ combination procedures and restrictive procedures.
Conclusion
Hypovitaminosisis D persists in obese patients undergoing bariatric surgery, despite various vitamin D supplementation regimens. Further research is needed to determine the optimal vitamin D dose to reach desirable 25(OH)D levels in this population, and to demonstrate whether this dose varies according to the surgical procedure.
Keywords: obesity, bariatric surgery, vitamin D deficiency, vitamin D dose, predictor
1. Introduction
The obesity epidemic is a worldwide public health problem [1–3]. Its overall prevalence reached about 35% in 2009 – 2010 among US adults, with a mean body mass index (BMI)1 of 28.7 kg/m2 (95% CI, 28.4 – 29.0) [4]. The prevalence of grades 2 and 3 obesity (BMI≥35 and 40≥kg/m2, respectively) reached 15.4% during the same time period, according to NHANES data [5,6]. While in the US the percent change was greater in the earlier years, before 2000, compared to the most recent years [5,6], the prevalence of obesity is still increasing in developing countries [1]. Obesity is a risk factor for several non-communicable diseases, including cardiovascular, metabolic, pulmonary, gastrointestinal, orthopedic, neurologic and psychological complications [7]. Therefore, obesity carries a significant socio-economic burden [1, 8], accounting for 0.7 – 2.8 % of healthcare expenditure of various countries [9]. Obesity is one the major global health targets set by the WHO in its 2013 World Health Assembly [8].
While medical therapy has limited effectiveness [10], ample evidence supports the efficacy of surgical intervention to treat this morbid condition [11,12]. Indeed, bariatric surgery results in a significant and sustained weight loss [13], a substantial reduction in cardiovascular risk factors [14], an improvement in diabetes control [15–17] and a decrease in mortality [18,19].
However, bariatric surgery leads to various nutritional and vitamin deficiencies, including vitamin D and others, requiring adequate follow up postoperatively [20,21]. Vitamin D deficiency in the bariatric surgery population is multifactorial, some factors being related to obesity, and might not resolve completely after surgery, and others may be related to the type of the surgical procedure and/or its consequences. The inverse relation between BMI and serum 25-hydroxyvitamin D [25(OH)D] level has been demonstrated in the large Framingham [22] and NHANES III [23] studies and confirmed in a meta-analysis of multiple cohorts, using Mendelian randomization [24]. In the latter analysis, an increasing BMI allele score (combining a battery of 12 BMI SNPs) was significantly associated with lower 25(OH)D level [24]. Decreased sun exposure, altered dietary habits, and lack of intake of adequate amounts of various minerals and vitamins in obese individuals are all contributing factors [25,26]. Furthermore, obese individuals have decreased bioavailability of vitamin D, secondary to its sequestration in subcutaneous and visceral fat, despite normal vitamin D cutaneous synthesis and gastrointestinal absorption, compared to lean control subjects [27]. Compared to lean subjects, obese individuals have decreased expression of the vitamin D metabolizing enzymes, 25-hydroxylase and 1α-hydroxylase in cutaneous and visceral adipose tissues [28]. A decrease in the activity of the hepatic 25-hydroxylase activity has been previously described, and may be secondary to non-alcoholic fatty liver disease [29]. Volumetric dilution, rather than sequestration, has been suggested to explain the low 25(OH)D level in obese. Therefore, it was proposed to adjust daily vitamin D supplementation according to body weight, in order to be able to reach desirable levels [30]. This would require supplementation of 70–80 IU/kg body weight to reach 30 ng/ml, or 30–40 IU/kg body weight to reach 20 ng/ml [30]. The role of obesity - associated inflammation on vitamin D level has been also suggested and needs to be further elucidated [31]. The surgical procedure per se affects 25(OH)D status. In malabsorptive procedures, fat malabsorption is a major contributor to deficiencies in liposoluble vitamins, secondary to bypass of primary absorption sites in the small intestine (duodenum, jejunum and ileum) and impaired digestion [32,33]. In fact, duodenal surgical bypass decreases cholecystokinin secretion which results in a reduction of the pancreatic lipolytic enzymes secretion and alteration in biliary salts, leading to alteration in fat digestion and steatorrhea [34]. Following both, malabsorptive and restrictive procedures, dietary intolerance with reduced intake of dairy products, vomiting, and non-adherence to supplement recommendations worsen 25(OH)D status further [32,33]. Finally, secondary hyperparathyroidism may be a contributory factor resulting in increased 25(OH)D hydroxylation, therefore decreasing 25(OH)D level [35].
The recommended 25(OH)D desirable level in the general population has been a matter of intense debate [36]. While the Institute Of Medicine (IOM) recommends a target of 20 ng/ml [37], the Endocrine Society aims at a higher level of 30 ng/ml [38]. Both societies based their recommendations on evidence related to musculo-skeletal outcomes. Despite the controversy around the desirable 25(OH)D cutoffs, it has been demonstrated that low 25(OH)D levels in bariatric surgery patients result in skeletal complications [39,40], including bone loss; the latter has been recently confirmed using quantitative computed tomography (QCT), that assesses volumetric bone mineral density (vBMD) [41,42]. Furthermore, in addition to the classically described secondary hyperparathyroidism [43,44], there have been reports of osteomalacia, following malabsorptive weight loss surgeries [45–47].
The aims of this paper are to review 25(OH)D status pre and post - bariatric surgery, describe the dose response of vitamin D supplementation in this specific population, and assess the effect of the surgical procedure on 25(OH)D level following supplementation.
2. Materials and methods
2.1. Literature search
We searched the following databases: Medline, PubMed, the Cochrane Library, and EMBASE. The search timeframe was 2000–April 2015, since the accuracy of vitamin D assays has improved in the last decade, following the introduction of the International Quality Assessment Scheme for Vitamin D metabolites (DEQAS), in 1997 [48]. We used the following MeSH terms: vitamin D, vitamin D deficiency, bariatrics, bariatric surgery, gastric bypass, gastroplasty, biliopancreatic diversion, anastomosis, Roux-en-Y, gastroenterostomy, pancreaticojejunostomy, gastrectomy, jejunoileal bypass, obesity, overweight. Recently retrieved review articles on this topic and others available in authors’ libraries were also considered.
2.2. Study selection
This review focuses on observational studies (prospective, retrospective and cross-sectional). We included studies conducted on obese adult patients undergoing bariatric surgery and assessing 25(OH)D status before and/or after any type of bariatric surgery, with or without vitamin D supplementation. We excluded studies that:
Were published as abstract only.
Had a number of participants undergoing bariatric surgery of < 50.
Did not specify the bariatric surgery type.
Did not report results for each bariatric surgery type (malabsorptive versus restrictive) separately.
Did not provide 25(OH)D levels, or the proportion of participants reaching a certain 25(OH)D level, or 25(OH)D level unit.
Did not define the cutoff for vitamin D deficiency, when the proportions of individuals with vitamin D deficiency were provided.
Did not define the dose of vitamin D administered post operatively, whether maintenance or treatment dose, while presenting results on 25(OH)D status post operatively.
2.3. Data abstraction
All retrieved references were screened by the first author (MC) and independently by another reviewer (KS or NN). Citations judged as potentially eligible by at least one of the reviewers were assessed in duplicate and independently, for inclusion in the systematic review. We compared results and resolved disagreements by discussion or with the help of a third reviewer (GEHF).
We also performed data abstraction in duplicate and independently. Data collected include: author’s name, year of publication, country, number of participants, type of surgical procedure, vitamin D supplementation dose, 25(OH)D levels, vitamin D assay (Appendix).
To assess vitamin D status before bariatric surgery, we compared 25(OH)D levels in different BMI categories: 40–45; 45–50 and >50 kg/m2. In each category, we calculated the weighted mean and pooled standard deviation of 25(OH)D level (ng/ml). Weights were based on sample size. Calculations were done using the following formulas:
| [49] |
(assuming equal variances, and by extrapolation from the weighted pooled standard deviation calculation of 2 independent samples) [50]. In the aforementioned formulas, “n” is the number of participants in each arm, “m” is the mean 25(OH)D level, “Sd” is the standard deviation of the level in each arm.
We assumed normality of distribution of 25(OH)D levels and using the mean 25(OH)D levels of included studies, and estimated the proportion of individuals reaching the target level of 20 ng/ml, as recommended by the IOM [37], in each dose category.
25(OH)D levels were reported in ng/ml; for conversion into nmol/l multiply by 2.496.
3. Results
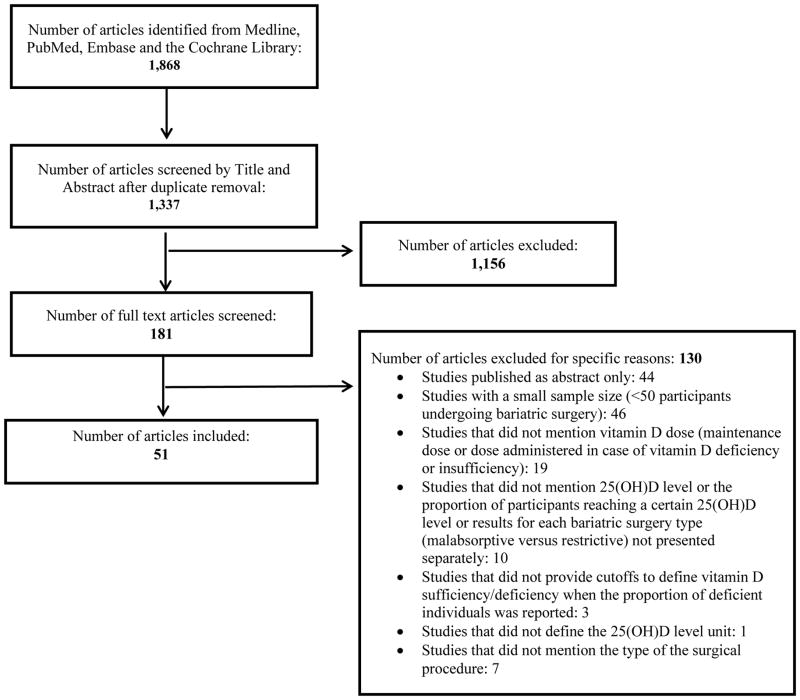
Figure 1 represents the study selection process. The search strategy identified 1,868 citations. Following duplicate removal, we were left with 1,337 citations. After title and abstract screening, we judged 181 articles as potentially eligible. We excluded 130 articles for the presence of one or more exclusion criteria. Therefore, we used data from 51 observational studies, illustrating 25(OH)D status before and/or after various types of bariatric surgery (Appendix). All studies were conducted in Europe and the United States, except one study conducted in Israel (Appendix).
Figure 1. Flow diagram for study selection for the period 2000–2015.
Following title and abstract and full text screening, 51 articles were included in the systematic review.
3.1. 25(OH)D status before bariatric surgery
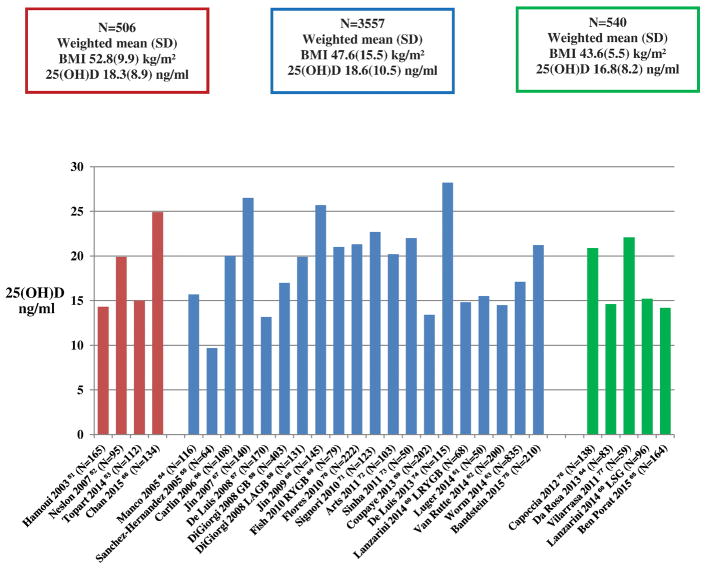
Thirty six studies assessed 25(OH)D status before bariatric surgery (Appendix). Figure 2 represents studies that have a number of participants of at least 50 per study group or subgroup, based on bariatric surgery type. It shows that mean 25(OH)D levels were ≤ 20 ng/ml in fifteen studies [51–65], and between 20 and 30 ng/ml in the others [66–77]. The weighted mean 25(OH)D levels (ng/ml) did not differ between studies, classified according to the baseline participants’ mean BMI (mean BMI ≥ 50 kg/m2, mean BMI between 45 and 50 kg/m2 and mean BMI ≤ 45 kg/m2) (Figure 2). Similarly, the weighted mean 25(OH)D levels of studies divided into 2 categories (mean BMI ≥ 50 kg/m2 versus mean BMI <50 kg/m2) also did not differ significantly (data not shown). Two other studies [78,79], in addition to the restrictive procedure arm of a third one [69] were not represented on Figure 2, as the number of participants per surgical procedure group was < 50. These studies also showed a mean 25(OH)D level of less than 20 ng/ml before bariatric surgery (Appendix). Mean 25(OH)D level was surprisingly high, reaching 39.4 ng/ml, in a group of obese individuals undergoing Roux-en-Y Gastric Bypass (RYGB) [80]; However, this study did not provide the mean BMI of participants, and therefore, it was not represented in Figure 2. Finally, four studies did not report 25(OH)D levels, but they provided the pre-operative proportion of individuals with 25(OH)D level < 20 ng/ml, of 66% [81], or < 30–32 ng/ml, varying from 23 to 98 % [82–84].
Figure 2. Mean 25(OH)D level (ng/ml), by BMI categories, before bariatric surgery in observational studies.
Studies represented are those that have a number of participants of at least 50 per study sub-group. Surgical procedures include malabsorptive and combination procedures, except DiGiorgi 2008, Laparoscopic Adjustable Gastric Banding arm [58], Lanzarini 2014, Laparoscopic Sleeve Gastrectomy (LSG) arm [60], Capoccia 2012, LSG [76], Van Rutte 2014, LSG [62], Ben Porat 2015, LSG [65]. Red bars represent studies with mean BMI ≥ 50 kg/m2; Blue bars represent studies with mean BMI between 45 and 50 kg/m2; Green bars represent studies with mean BMI ≤ 45 kg/m2. The difference between the weighted mean 25(OH)D levels in BMI categories was not clinically significant and did not show a decrease in 25(OH)D level with increasing BMI.
3.2. Vitamin D levels following bariatric surgery with and without vitamin D supplementation
Forty six studies assessed 25(OH)D status within 1 to 11 years following bariatric surgery (Appendix). These were divided into categories according to the type of the surgical procedure and the dose range of vitamin D supplementation. Only eight studies showed a mean 25(OH)D level > 30 ng/ml at 6 to 24 months post operatively [51,52,60,66,69,73,75,80]. Thirteen studies showed a mean 25(OH)D level < 20 ng/ml at 6 months to 11 years post operatively [51,54,55,64,78,85–92]. The remaining studies, almost half, reported a 25(OH)D level between 20 and 30 ng/ml [53,56–59,62,67–71,76,77,79,93–100].
3.3. Vitamin D supplementation dose and response in bariatric surgery
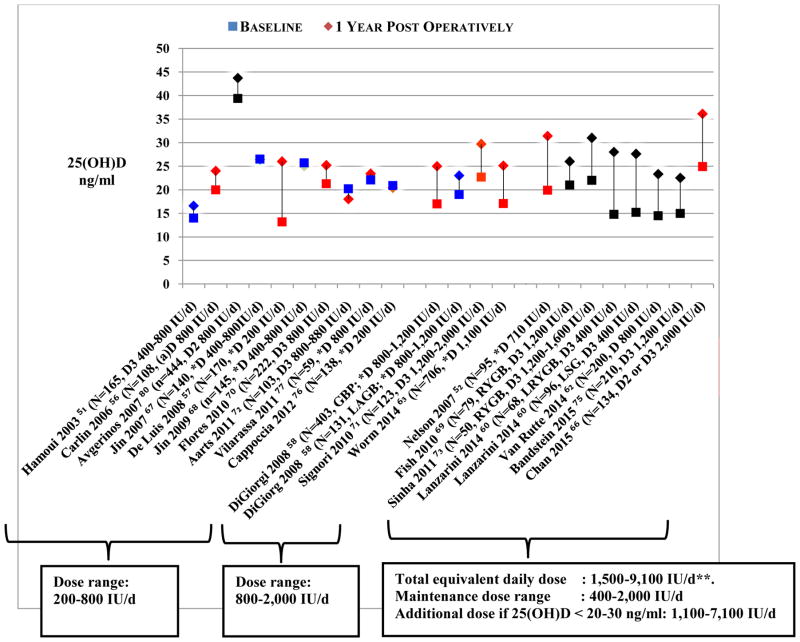
There was a high variability in vitamin D supplementation regimens administered post operatively, including enteral and parenteral preparations, daily and intermittent schedules (i.e. weekly, biweekly, monthly or every 3 months) and a wide range of equivalent daily doses, from 200 IU [57] to 28,500 IU [54]. Several studies used additional supplemental regimens in subjects who were deficient, as shown in Figure 3 and detailed in the Appendix. The timing of 25(OH)D status assessment also varied, from as early as 3 months [58–68], to as late as 11 years post operatively [87].
Figure 3. Mean 25(OH)D level before and 1 year after bariatric surgery in observational studies.
Studies represented are those that have a number of participants of at least 50 per study subgroup. Surgical procedures include malabsorptive and combination procedures, except DiGiorgi 2008, Laparoscopic Adjustable Gastric Banding arm [58], and Capoccia 2012, Laparoscopic Sleeve Gastrectomy [76]. Studies were divided according to vitamin D supplementation dose range. All subjects were allocated vitamin D supplementation post operatively at various doses, as mentioned in the figure; for full details see Appendix. 25(OH)D status following supplementation showed a trend for a larger increase in 25 (OH)D level with higher vitamin D supplementation doses.
The red color indicates a significant change in 25(OH)D level. The blue color indicates a non-significant change in 25(OH)D level. The black color indicates that statistical significance of the change in 25(OH)D status was not reported in the individual study.
* Type of vitamin D not provided.
**Excluding Fish et al
3.3.1. Dose response between the administered vitamin D dose and the increments in serum 25(OH)D levels
Thirty studies evaluated 25(OH)D level before and after bariatric surgery, at 3 months to 10 years postoperatively. Figure 3 represents mean 25(OH)D level in studies that included at least 50 participants in each surgical procedure group, and that assessed 25(OH)D status pre and 1 year post operatively. There was a trend for larger increments in mean 25(OH)D levels with higher doses. However, these increments were not consistently in concordance with the dose administered, and the statistical significance of the change in 25(OH)D level was not assessed in all the studies (Figure 3, see Appendix for studies not represented on Figure 3).
Starting at a mean baseline 25(OH)D level of 13–25 ng/ml, the change in 25(OH)D level did not exceed 8 ng/ml at 6 to 12 months post operatively, even with daily dosing up to 2,000 IU daily [51,56,58,63,70,71,79,94] (Figure 3, Appendix), with only one exception [57] (Appendix). Furthermore, 25(OH)D level did not change [67,68,76] or even decreased [64,72,78] in several studies at one year follow up, despite supplementation (equivalent daily dose range 200–800 IU) (Figure 3, Appendix). Avgerinos et al. showed an initial increase followed by a drastic decrease in 25(OH)D level at 1 year following RYGB, despite sustained vitamin D supplementation of 800 IU daily; findings that remain unexplained [80]. De Luis et al. showed an increase in mean 25(OH)D level of 15 ng/ml at 2 years after BPD, with administration of a small dose of vitamin D of 200 IU daily, followed by a decline and return to baseline level at 3 years [57]. Hamoui et al. assessed the effect of limb length in duodenal switch (DS) on 25(OH)D status, and demonstrated that a long common channel of 100 cm allowed a better improvement in 25(OH)D level at 18 months, compared to a short common channel of 75 cm (a difference in 25(OH)D level of 14.7 ng/ml between the 2 sub-groups at 18 months) [101].
Conversely, studies administering a maintenance vitamin D supplementation of 400–2,000 IU daily, and additional doses to deficient and/or insufficient individuals (additional equivalent dose range 1,100 IU–7,100 IU, total daily dose received 1,500–9,100 IU), with the exception of Fish et al. [69], consistently demonstrated an increase in mean 25(OH)D level of 9–13 ng/ml at six months [59] (Appendix), and at one year post operatively (Figure 3) [52,60,62,66,73,75]. The mean baseline 25(OH)D level in this group of studies was 13–25 ng/ml.
3.3.2. Relationship of vitamin D dose, type of bariatric surgery, and achievement of a desirable 25(OH)D level
In studies where no vitamin D supplement was administered, mostly following RYGB procedures, the mean 25(OH)D level achieved post-operatively was in low to mid-teens, in ng/ml, across the board [55,86,87,102,103].
Three studies conducted on patients undergoing laparoscopic sleeve gastrectomy (LSG) surgery suggest that a vitamin D dose less than 1,000 IU/d may not be sufficient to raise mean levels to above the desirable value of 20 ng/ml, even in restrictive procedures [76,81,90].
Studies of malabsorptive or combination procedures, with supplementation up to 2,000 IU of vitamin D daily, achieved mean 25(OH)D levels at or above 20 ng/ml, provided mean levels did not start below such cutoff pre-operatively [67,68,70,71,76,79,80,90,94,104]. When baseline 25(OH)D level was < 20 ng/ml, three studies showed an increase in mean 25(OH)D level to above the target [57,58,63], while three others did not [51,64,78].
Conversely, two studies that involved biliopancreatic diversion (BPD) and that administered very high doses of vitamin D (6,472 IU/d in one and the equivalent of 28,571 IU/d in another) showed mean 25(OH)D to remain in the low to mid-teens 5 years post-operatively [54,92].
Finally, studies that administered vitamin D, as a maintenance dose to all participants (≤2,000 IU daily), and additional doses (range of equivalent daily doses: 1,100–7,100 IU, total daily dose received 1,500–9,100 IU) to vitamin D deficient/insufficient individuals, following malabsorptive and combination procedures, achieved a mean 25(OH)D level above 20 ng/ml in five studies, with mean baseline 25(OH)D level 13–21 ng/ml [53,59,62,69,100], and above 30 ng/ml in six studies, with mean baseline 25(OH)D level 15–25 ng/ml [52,60,66,69,73,75] (Figure 3 and Appendix).
Assuming normality of the distribution of 25(OH)D levels, and considering studies of malabsorptive and combination procedures, we estimate that the proportion of participants achieving the target 25(OH)D level of 20 ng/ml, increased from 14–67% to 63–76%, on vitamin D doses of up to 2,000 IU daily [57,58,63,67,70,71,77,93,104]. Considering studies of vitamin D deficient individuals, these proportions increased from 25–55% at baseline, to 70–93% of at study completion, on replacement doses and add-on to the vitamin D maintenance regimen [52,53,59,60,62,69,73,75].
3.4. Comparative effect of restrictive versus malabsorptive and combination procedures on 25(OH)D status in individuals undergoing bariatric surgery
We identified only five studies comparing 25(OH)D status before and after vitamin D supplementation, in restrictive versus malabsorptive or combination procedures, within the same study, and we could not identify the emergence of a consistent trend in results (Table). DiGiorgi et al compared 25(OH)D levels following RYGB versus Laparoscopic Adjustable Gastric Banding (LAGB) in subjects who received vitamin D 800 – 1,200 IU daily and found no significant difference in mean levels both at 1 and 2 years post operatively, between the two groups [58]. Similarly, Lanzarini et al. compared prospectively LSG versus Laparoscopic Roux-en-Y gastric bypass (LRYGB) patients, when all participants received the same dose of vitamin D supplementation for a 6-month duration. A significantly higher 25(OH)D level in LSG compared to LRYGB was reported only at 2 years follow up [60]. A retrospective study showed lower 25(OH)D levels in RYGB compared to LAGB, when all participants received the same dose of vitamin D supplementation [69]. Coupaye et al. compared Adjustable Gastric Banding (AGB) to Gastric Bypass (GBP) procedures, and showed higher 25(OH)D levels in GBP patients [19.9(9.9) ng/ml], compared to AGB patients [11.9(4.2) ng/ml]; the GBP subgroup only received 500 IU of vitamin D3 daily [78]. Vilarrasa et al. showed no significant difference in 25(OH)D level between RYGB and Sleeve Gastrectomy (SG) groups when vitamin D supplementation doses were 800 – 1,200 IU daily in the former group and 400 IU in the latter group [79]. Three other studies were not included in our discussion; the first one did not provide 25(OH)D levels [82], the second did not report the standard deviation of 25(OH)D level following intervention [73] and the third one did not administer vitamin D supplementation [86].
4. Discussion
This systematic review underscores that all the literature describing 25(OH)D status pre and post-bariatric surgery comes from western populations, the United States and Europe. Data from non-western countries, which account for the fastest growing population worldwide, and for some of the fastest growing rates of obesity, are lacking.
Mean 25(OH)D levels in obese individuals before bariatric surgery were consistently below 30 ng/ml, and for almost half the studies at or below 20 ng/ml. Following bariatric surgery, mean 25(OH)D levels remained < 30 ng/ml, despite various vitamin D supplementation regimens, with the exception of few studies where vitamin D deficiency was corrected using vitamin D replacement equivalent daily doses of 1,100–7,100 IU, as add-on to the maintenance dose of 400 – 2,000 IU daily usually administered to all the study participants (total equivalent daily dose 1,500–9,150 IU). Studies using such regimens showed a substantial increase in mean 25(OH)D level, of 9–13 ng/ml and allowed to the majority of the study population to reach the desirable 25(OH)D level of 20 ng/ml [52,60,62,66,73,75], as recommended by the Institute Of Medicine (IOM) [37].
Although the increments in mean 25(OH)D levels following supplementation tended to parallel the increase in the vitamin D dose (Figure 3), they still remained below the expected range of increments, of 0.7–1 ng/ml for each 100 IU of vitamin D, depending on the baseline 25(OH)D level [105].
It is speculated that patients undergoing restrictive procedures would require less vitamin D supplementation, compared to malabsorptive and combination procedures, since the intestinal area responsible for vitamin D absorption is preserved in the former procedures [21]. However, the limited and inconsistent data available herein do not validate such expectations (Table). Two small randomized controlled trials administered the same vitamin D supplementation dose to SG and RYGB patients. They showed a significantly higher 25(OH)D level in SG groups, compared to RYGB groups, following 1 year of vitamin D supplementation [106,107]. Indeed, starting with a baseline 25(OH)D level of 20.1–24.3 ng/ml and with a supplementation dose of 600 IU daily, the difference in 25(OH)D levels achieved was 13 ng/ml in one study [106]. Conversely, with a similar baseline 25(OH)D level of 21.1–21.7 ng/ml, and a larger supplementation of 3,333 IU (daily equivalent), the difference was only of 1.5 ng/ml [107].
Our findings show that there is a high variability in the response to vitamin D supplementation in individuals undergoing bariatric surgery. In fact, in addition to the known predictors affecting the 25(OH)D level achieved in the general population, including baseline 25(OH)D levels and vitamin D dose, the effect of other predictors, specific to the bariatric surgery population, remain unknown. These predictors include: the amount of body fat in each patient, the type of surgery and the degree of malabsorption following surgery. Therefore, it may be difficult to recommend one vitamin D dose that would be optimal to all, and an individualized approach seem reasonable. Indeed, following malabsorptive and combination procedures, monitoring of 25(OH)D level to assess the response to therapy has been recommended by the Endocrine Society and the American Association of Clinical Endocrinologists (AACE)/The Obesity Society (TOS)/American Society for Metabolic and Bariatric Surgery (ASBMS) guidelines on the perioperative care of patients undergoing bariatric surgery [108,109]. While bi-annual monitoring was recommended by the ES guidelines [108], monitoring at 1, 3, 6 and 12 months was recommended by the AACE/TOS/ASBMS guidelines [109]. Noteworthy, vitamin D doses as high as 9,000 IU daily have been used following bariatric surgery (Appendix), and similar to doses up to 10,000 IU daily used in the general population, such doses have been shown to be safe [110,111]. Indeed, vitamin D toxicity did not occur until 25(OH)D level exceeded 100 ng/ml [110,111]. Active vitamin D supplementation is not recommended to patients undergoing bariatric surgery, but it has been suggested, exceptionally, in refractory cases with symptomatic hypocalcemia [108,109].
Strength and limitations
This is the first extensive systematic review of four major medical literature databases, addressing 25(OH)D status in patients undergoing bariatric surgery. One previous systematic review by Compher et al, published in 2008, was limited to only one database, PubMed. It showed a high prevalence of hypovitaminosis D pre and postoperatively, with no specific information on vitamin D supplementation dose, response to therapy, or analysis by type of surgery [112]. Several other reviews summarize the available evidence on various nutrient deficiencies following bariatric surgery, but none of them focused specifically on vitamin D nutritional status [20,21,34,113].
Although randomized controlled trials (RCTs) represent the highest quality of evidence, these are difficult to implement and take a long time to complete. Furthermore, they are quite expensive and in this specific instance, considering that the drug to be used is regular vitamin D, they also do not provide interest for pharma sponsored trials. Thus observational studies provide initial basis and the best initial readily available evidence to assess and to establish correlations between predictors and surrogate outcomes, as well as other important major outcomes [114]. Therefore, while awaiting the results of a systematic review of RCTs, our paper presents a thorough and updated overview of the available literature relevant to this topic to-date.
Our review has several limitations. We did not systematically assess the quality of the the included studies, but as we have described, in detail, the current available evidence. Indeed, the results are derived from observational studies, with the known drawbacks of difficulty in sorting out confounding factors, and most noteworthy, assessing compliance issues. In fact, only 6 studies presented details on participants’ compliance to vitamin D supplementation (Appendix). Furthermore, information related to vitamin D supplementation dose, type (D2 vs D3) and the exact duration of supplementation were lacking in many reports. Details on 25(OH)D assay were missing in some studies, and even when mentioned, the information obtained was limited by the wide variations in levels obtained with the various assays [115,116]. Seven studies used the more reliable high pressure liquid chromatography (HPLC) assay to measure 25(OH)D level (Appendix) [57,59,64,66,75,87,88] and only one study used the gold standard Liquid Chromatography Mass Spectrometry (LCMS) [52]. Finally, the timing of 25(OH)D level measurement differed between studies, which may affect the 25(OH)D level reached. In fact, a transient increase in 25(OH)D level by 2–4 ng/ml in the early post-operative phase (at 1 to 6 months) has been demonstrated, without vitamin D supplementation, an increase that was explained to occur due to the mobilization of 25(OH)D from adipose tissue [117]. However, others concluded that the contribution of fat mass to serum 25(OH)D level may not be significant, as the increase in 25(OH)D level falls within the coefficient of variation of the 25(OH)D assay [118]. Indeed, the higher compliance and the strict follow up in the immediate post-operative phase could explain, at least in part, such early transient improvement in vitamin D status.
Our findings shed light on the lack of high quality evidence, needed to define the optimal vitamin D dose needed in the bariatric surgery population. Furthermore, the role of vitamin D supplementation in improving patient important outcomes such as BMD, fracture prevention, cardiovascular benefits or others, in this population remains unknown.
5. Conclusion
Based on evidence derived from observational data, mean 25(OH)D level remains less than 30 ng/ml, before and after all types of bariatric surgery, and for several studies it is below 20 ng/ml, despite various vitamin D supplementation regimens. In addition to the maintenance dose, vitamin D replacement in insufficient and deficient individuals, using equivalent daily doses of 1,100 – 7,100 IU, is needed in order to allow for the majority of the population to reach a desirable 25(OH)D level of 20 ng/ml. The effect of the surgical procedure, restrictive versus malabsorptive, on the vitamin D dose response is inconsistent. Indeed, these findings have several limitations, related to the inherent drawbacks of observational studies, confounding factors and compliance problems.
Further research is needed to determine the optimal dose of vitamin D supplementation and replacement in individuals undergoing bariatric surgery, and whether this dose varies according to the surgical procedure.
Supplementary Material
Table.
Mean 25(OH)D level following restrictive procedures compared to malabsorptive and combination procedures within the same study, with or without vitamin D supplementation, in observational studies (prospective, retrospective and cross sectional).
| Author Year | Study design N*/surgery type | Vitamin D assay | Vitamin D Dose | 25(OH)D at baseline (ng/ml) | 25(OH)D following bariatric surgery (ng/ml) | Authors conclusion | ||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) or median (range) | Below cutoff % | Mean (SD) or median (range) | Below cutoff % | |||||
| DiGiorgi 2008 [58] | Prospective 403 GBP 131 LAGB |
NA | All D 800 – 1,200 IU/d (a) | GBP : 17(8) LAGB: 19(9) (b) |
< 20 GBP: 67% LAGB: 58% |
At 12 mo: GBP: 25(12); LAGB: 23(9) At 24 mo: GBP: 24(12); LAGB: 25(10)  No difference between groups(c). No difference between groups(c). |
< 20 At 12 mo: GBP: 37%; LAGB: 37% At 24 mo: GBP: 40%; LAGB: 33% |
Same vitamin D requirements may be needed in both groups. |
| Coupaye 2009 [78] | Prospective 21 AGB 49 GBP |
RIA | D3 500 IU/d in GBP only. | AGB: 13.4(8.6) GBP: 12.7(10.8)(c) |
- | At 12 mo AGB: 11.9(4.2); GBP: 19.9(9.9) 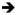 Significantly higher mean 25(OH)D level in GBP compared to AGB(c). Significantly higher mean 25(OH)D level in GBP compared to AGB(c). |
- | Results inconclusive. |
| Fish 2010 [69] | Retrospective 79 RYGB 48 LAGB |
NA | All: Pre op: D3 50,000 IU 3 times/week for 1 month for deficient. Post op: D3 1,200 IU/d (a,d) |
LAGB: 25(7–112) RYGB: 21(6–45) (b) |
< 30 LABG: 75% RYGB: 88% |
At 12 mo LAGB: 32(12–53); RYGB: 26(5–49) 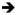 Significantly lower mean 25(OH)D level in RYGB compared to LAGB (b). Significantly lower mean 25(OH)D level in RYGB compared to LAGB (b). |
< 30 At 12 mo: LAGB: 41%; RYGB: 63% |
RYGB may require higher vitamin D supplementation dose. |
| Vilarrasa 2013 [79] | Prospective 33 RYGB 33 SG |
Electro-Chemiluminescence based immune analysis | D3 400 IU/d in SG and 400+800 IU/d in RYGB | RYGB: 20.1(8) SG: 17.6(8) (b) |
- | At 12 mo RYGB 21.6(8.4); SG 20.1(7.2) 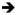 No significant difference between the 2 groups (b) No significant difference between the 2 groups (b)
|
- | RYGB may require higher vitamin D supplementation dose. |
| Lanzarini 2015 [60] | Prospective 96 LSG 68 LRYGB |
ECLIA | D3 400 IU/d Intervention group if 25(OH)D < 30 ng/ml at 3 or 6 mo follow up: D2 16,000 IU every 2 weeks; for a maximum of 6 mo |
LSG: 15.2 (7.0) LRYGB: 14.8 (2.8) (c) Intervention group: LSG: 15.0(7.0) LRYGB: 12.8(6.7) (c) Non-intervention group: LSG: 15.7 (7.1) LRYGB: 18.4(9.9) (c) |
- | At 12 mo LSG : 27.6 (16.6) LRYGB: 28.0 (14.2) Intervention group LSG : 28.0 (18.8) LRYGB: 28.8 (15.8) Non-intervention LSG : 26.8(11.4) LRYGB: 26.3 (10.6) At 24 mo LSG : 37.9 (18.4) LRYGB : 34.3 (19.0) Intervention group LSG : 41.0 (20.7) LRYGB: 39.0 (18.9) Non-intervention group LSG : 31.1 (9.1) LRYGB: 20.2 (11.0) 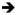 Significantly lower mean 25(OH)D in LRYGB only at 24 mo(c) Significantly lower mean 25(OH)D in LRYGB only at 24 mo(c)
|
< 30 mg/ml At 12 mo Intervention group LSG : 67 LRYGB: 66 Non-intervention group LSG : 63 LRYGB: 62 At 24 mo Intervention group LSG : 27 LRYGB: 37 Non-intervention group LSG : 40 LRYGB: 78 |
RYGB may require vitamin D supplements for a longer duration. |
Vitamin D type not specified;
No significant difference in 25(OH)D level between the 2 groups, based on our own calculations using mean and standard deviation data provided in studies;
No significant difference as provided in studies;
Previously deficient individuals received additional vitamin D3 800–1,600 IU daily.
Abbreviations: AGB: Adjustable Gastric Banding; ECLIA: competitive electrochemiluminescence protein binding assay; GBP: Gastric Bypass; LAGB: Laparoscopic Adjustable Gastric Banding; IU/d: International Unit per day; mo: months; NA: not available; RIA: Radioimmunoassay; RYGB: Roux–en-Y Gastric Bypass; SG: Sleeve Gastrectomy.
Acknowledgments
Funding:
This work was supported by a grant from the Medical Resource Plan at the American University of Beirut - Lebanon and made possible thanks to the Scholars in HeAlth Reseach Program (SHARP).
The authors would like to thank Miss Aida Farha, Medical Information Specialist, Saab Medical Library, for her advice and assistance in designing comprehensive and complex searches of the various medical literature resources and for the provision of select articles. The authors would like also to thank Dr Elie A Akl, MD, PhD, for his input regarding the methodology of this systematic review.
Footnotes
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; AACE, American Association of Clinical Endocrinologists; AGB, Adjustable gastric banding; BMI, body mass index; BPD, biliopancreatic diversion; GBP, Gastric Bypass; LAGB, laparoscopic adjustable gastric banding; LSG, laparoscopic sleeve gastrectomy; QCT, quantitative computed tomography; RYGB, Roux-en-Y gastric bypass; SG, Sleeve Gastrectomy; vBMD, volumetric bone mineral density.
Authors’ roles:
Study conception and design: Dr Marlene Chakhtoura, Dr Ghada El Hajj Fuleihan and Dr Christos Mantzoros. Title and abstract screening: Dr Marlene Chakhtoura, Dr Nancy Nakhoul and Dr Khaled Shawwa. Full text screening: Dr Marlene Chakhtoura, Dr Nancy Nakhoul and Dr Khaled Shawwa. Data abstraction: Dr Marlene Chakhtoura and Dr Nancy Nakhoul. Drafting the manuscript: Dr Marlene Chakhtoura, Dr Nancy Nakhoul, Dr Ghada El Hajj Fuleihan and Dr Christos Mantzoros. Revising the manuscript content and approving the final version of the manuscript: all authors.
Declaration of interest:
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Imes CC, Burke LE. The Obesity Epidemic: The United States as a Cautionary Tale for the Rest of the World. Curr Epidemiol Rep. 2014;1(2):82–8. doi: 10.1007/s40471-014-0012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10(1):22. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. [Accessed in October 2015];WHO website. Available from: http://www.who.int/gho/ncd/risk_factors/obesity_text/en/
- 4.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 7.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Resolutions and decisions, sixty-sixth World Health Assembly, 20–27 May 2013. Geneva: World Health Organization; 2013. WHA66/2013/REC/1. [Google Scholar]
- 9.Withrow D, Alter D. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev. 2011;12(2):131–41. doi: 10.1111/j.1467-789X.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- 10.McTigue KM, Harris R, Hemphill B, Lux L, Sutton S, Bunton AJ, et al. Screening and interventions for obesity in adults: summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2003;139(11):933–49. doi: 10.7326/0003-4819-139-11-200312020-00013. [DOI] [PubMed] [Google Scholar]
- 11.Padwal R, Klarenbach S, Wiebe N, Birch D, Karmali S, Manns B, et al. Bariatric surgery: a systematic review and network meta-analysis of randomized trials. Obes Rev. 2011;12(8):602–21. doi: 10.1111/j.1467-789X.2011.00866.x. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe BM, Belle SH. Long-term Risks and Benefits of Bariatric Surgery: A Research Challenge. JAMA. 2014;312(17):1792–3. doi: 10.1001/jama.2014.12966. [DOI] [PubMed] [Google Scholar]
- 13.Chang S-H, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surgery. 2014;149(3):275–87. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart. 2012;98(24):1763–77. doi: 10.1136/heartjnl-2012-301778. [DOI] [PubMed] [Google Scholar]
- 15.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–85. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 17.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370(21):2002–13. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pontiroli AE, Morabito A. Long-term prevention of mortality in morbid obesity through bariatric surgery: a systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann Surg. 2011;253(3):484–7. doi: 10.1097/SLA.0b013e31820d98cb. [DOI] [PubMed] [Google Scholar]
- 19.Kwok CS, Pradhan A, Khan MA, Anderson SG, Keavney BD, Myint PK, et al. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol. 2014;173(1):20–8. doi: 10.1016/j.ijcard.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 20.Levinson R, Silverman JB, Catella JG, Rybak I, Jolin H, Isom K. Pharmacotherapy prevention and management of nutritional deficiencies post Roux-en-Y gastric bypass. Obes Surg. 2013;23(7):992–1000. doi: 10.1007/s11695-013-0922-2. [DOI] [PubMed] [Google Scholar]
- 21.Sawaya RA, Jaffe J, Friedenberg L, Friedenberg FK. Vitamin, mineral, and drug absorption following bariatric surgery. Curr Drug Metab. 2012;13(9):1345. doi: 10.2174/138920012803341339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 2010;59(1):242–8. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88(2):558S–64S. doi: 10.1093/ajcn/88.2.558S. [DOI] [PubMed] [Google Scholar]
- 24.Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Medicine. 2013;10(2):e1001383. doi: 10.1371/journal.pmed.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ammor N, Berthoud L, Gerber A, Giusti V. Nutritional deficiencies in candidates for bariatric surgery. Revue Medicale Suisse. 2009;5(196):676–9. [PubMed] [Google Scholar]
- 26.Vanlint S. Vitamin D and obesity. Nutrients. 2013;5(3):949–56. doi: 10.3390/nu5030949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 28.Wamberg L, Christiansen T, Paulsen S, Fisker S, Rask P, Rejnmark L, et al. Expression of vitamin D-metabolizing enzymes in human adipose tissue—the effect of obesity and diet-induced weight loss. Int J Obes. 2012;37(5):651–7. doi: 10.1038/ijo.2012.112. [DOI] [PubMed] [Google Scholar]
- 29.Barchetta I, Angelico F, Ben MD, Baroni MG, Pozzilli P, Morini S, et al. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25 (OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Medicine. 2011;9(1):85. doi: 10.1186/1741-7015-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drincic AT, Armas LA, Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity. 2012;20(7):1444–8. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- 31.Earthman C, Beckman L, Masodkar K, Sibley S. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes. 2011;36(3):387–96. doi: 10.1038/ijo.2011.119. [DOI] [PubMed] [Google Scholar]
- 32.Saltzman E, Philip Karl J. Nutrient deficiencies after gastric bypass surgery. Ann Rev Nutr. 2013;33:183–203. doi: 10.1146/annurev-nutr-071812-161225. [DOI] [PubMed] [Google Scholar]
- 33.Shikora SA, Kim JJ, Tarnoff ME. Nutrition and gastrointestinal complications of bariatric surgery. Nutr Clin Pract. 2007;22(1):29–40. doi: 10.1177/011542650702200129. [DOI] [PubMed] [Google Scholar]
- 34.Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26(11):1031–7. doi: 10.1016/j.nut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Clements M, Davies M, Fraser D, Lumb G, Mawer EB, Adams P. Metabolic inactivation of vitamin D is enhanced in primary hyperparathyroidism. Clin Sci. 1987;73(6):659–64. doi: 10.1042/cs0730659. [DOI] [PubMed] [Google Scholar]
- 36.Fuleihan El Hajj, Bouillon R, Clarke B, Chakhtoura M, Cooper C, McClung M, et al. Serum 25-Hydroxyvitamin D Levels: Variability, Knowledge Gaps, and the Concept of a Desirable Range. J Bone Miner Res. 2015;30(7):1119–33. doi: 10.1002/jbmr.2536. [DOI] [PubMed] [Google Scholar]
- 37.IOM (Institute of Medicine) Dietary Reference Intakes for Calcium and Vitamin D. Vol. 2011. Washington, DC: The National Academies Press; 2011. [Accessed in October 2015]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK56070/pdf/Bookshelf_NBK56070.pdf. [Google Scholar]
- 38.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 39.Hage M, El Hajj Fuleihan G. Bone and mineral metabolism in patients undergoing Roux-en-Y gastric bypass. Osteoporos Int. 2014;25(2):423–39. doi: 10.1007/s00198-013-2480-9. [DOI] [PubMed] [Google Scholar]
- 40.Scibora LM, Ikramuddin S, Buchwald H, Petit MA. Examining the link between bariatric surgery, bone loss, and osteoporosis: a review of bone density studies. Obes Surg. 2012;22(4):654–67. doi: 10.1007/s11695-012-0596-1. [DOI] [PubMed] [Google Scholar]
- 41.Yu EW, Bouxsein ML, Putman MS, Monis EL, Roy AE, Pratt JS, et al. Two-year changes in bone density after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2015;100(4):1452–9. doi: 10.1210/jc.2014-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu EW, Bouxsein ML, Roy AE, Baldwin C, Cange A, Neer RM, et al. Bone loss after bariatric surgery: Discordant results between DXA and QCT bone density. J Bone Miner Res. 2014;29(3):542–50. doi: 10.1002/jbmr.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pugnale N, Giusti V, Suter M, Zysset E, Heraief E, Gaillard R, et al. Bone metabolism and risk of secondary hyperparathyroidism 12 months after gastric banding in obese pre-menopausal women. Int J Obes. 2003;27(1):110–6. doi: 10.1038/sj.ijo.0802177. [DOI] [PubMed] [Google Scholar]
- 44.Youssef Y, Richards W, Sekhar N, Kaiser J, Spagnoli A, Abumrad N, et al. Risk of secondary hyperparathyroidism after laparoscopic gastric bypass surgery in obese women. Surg Endosc. 2007;21(8):1393–6. doi: 10.1007/s00464-007-9228-6. [DOI] [PubMed] [Google Scholar]
- 45.Bhan A, Rao AD, Rao DS. Osteomalacia as a result of vitamin D deficiency. Endocrinol Metab Clin North Am. 2010;39(2):321–31. doi: 10.1016/j.ecl.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Al-Shoha A, Qiu S, Palnitkar S, Rao DS. Osteomalacia with bone marrow fibrosis due to severe vitamin D deficiency after a gastrointestinal bypass operation for severe obesity. Endocr Pract. 2009;15(6):528–33. doi: 10.4158/EP09050.ORR. [DOI] [PubMed] [Google Scholar]
- 47.Collazo-Clavell ML, Jimenez A, Hodgson SF, Sarr MG. Osteomalacia after Roux-en-Y gastric bypass. Endocr Pract. 2004;10(3):195–8. doi: 10.4158/EP.10.3.195. [DOI] [PubMed] [Google Scholar]
- 48.Carter G, Carter C, Gunter E, Jones J, Jones G, Makin H, et al. Measurement of vitamin D metabolites: an international perspective on methodology and clinical interpretation. J Steroid Biochem Mol Biol. 2004;89:467–71. doi: 10.1016/j.jsbmb.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 49. [accessed in October 2015];Weighted mean calculation. Available from: http://www.statisticshowto.com/weighted-mean/
- 50. [accessed in October 2015];Pooled Standard deviation calculation. Available from: http://wps.aw.com/wps/media/objects/15/15512/formulas.pdf.
- 51.Hamoui N, Kim K, Anthone G. The significance of elevated levels of parathyroid hormone in patients with morbid obesity before and after bariatric surgery. Arch Surg. 2003;138(8):891–7. doi: 10.1001/archsurg.138.8.891. [DOI] [PubMed] [Google Scholar]
- 52.Nelson ML, Bolduc LM, Toder ME, Clough DM, Sullivan SS. Correction of preoperative vitamin D deficiency after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2007;3(4):434–7. doi: 10.1016/j.soard.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Topart P, Becouarn G, Sallé A, Ritz P. Biliopancreatic diversion requires multiple vitamin and micronutrient adjustments within 2 years of surgery. Surg Obes Relat Dis. 2014;10(5):936–41. doi: 10.1016/j.soard.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 54.Manco M, Calvani M, Nanni G, Greco AV, Iaconelli A, Gasbarrini G, et al. Low 25-Hydroxyvitamin D Does Not Affect Insulin Sensitivity in Obesity after Bariatric Surgery. Obes Res. 2005;13(10):1692–700. doi: 10.1038/oby.2005.207. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez-Hernandez J, Ybarra J, Gich I, De Leiva A, Rius X, Rodriguez-Espinosa J, et al. Effects of bariatric surgery on vitamin D status and secondary hyperparathyroidism: A prospective study. Obes Surg. 2005;15(10):1389–95. doi: 10.1381/096089205774859182. [DOI] [PubMed] [Google Scholar]
- 56.Carlin AM, Rao DS, Meslemani AM, Genaw JA, Parikh NJ, Levy S, et al. Prevalence of vitamin D depletion among morbidly obese patients seeking gastric bypass surgery. Surg Obes Relat Dis. 2006;2(2):98–103. doi: 10.1016/j.soard.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 57.De Luis DA, Pacheco D, Izaola O, Terroba MC, Cuellar L, Martin T. Clinical results and nutritional consequences of biliopancreatic diversion: Three years of follow-up. Ann Nutr Metab. 2008;53(3–4):234–9. doi: 10.1159/000185641. [DOI] [PubMed] [Google Scholar]
- 58.DiGiorgi M, Daud A, Inabnet WB, Schrope B, Urban-Skuro M, Restuccia N, et al. Markers of bone and calcium metabolism following gastric bypass and laparoscopic adjustable gastric banding. Obes Surg. 2008;18(9):1144–8. doi: 10.1007/s11695-007-9408-4. [DOI] [PubMed] [Google Scholar]
- 59.Coupaye M, Breuil MC, Riviere P, Castel B, Bogard C, Dupre T, et al. Serum vitamin D increases with weight loss in obese subjects 6 months after roux-en-y gastric bypass. Obes Surg. 2013;23(4):486–93. doi: 10.1007/s11695-012-0813-y. [DOI] [PubMed] [Google Scholar]
- 60.Lanzarini E, Nogues X, Goday A, Benaiges D, de Ramon M, Villatoro M, et al. High-Dose Vitamin D Supplementation is Necessary After Bariatric Surgery: A Prospective 2-Year Follow-up Study. Obes Surg. 2015 doi: 10.1007/s11695-015-1572-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 61.Luger M, Kruschitz R, Langer F, Prager G, Walker M, Marculescu R, et al. Effects of Omega-Loop Gastric Bypass on Vitamin D and Bone Metabolism in Morbidly Obese Bariatric Patients. Obes Surg. 2014;25(6):1056–62. doi: 10.1007/s11695-014-1492-7. [DOI] [PubMed] [Google Scholar]
- 62.Van Rutte PWJ, Aarts EO, Smulders JF, Nienhuijs SW. Nutrient deficiencies before and after sleeve gastrectomy. Obes Surg. 2014;24(10):1639–46. doi: 10.1007/s11695-014-1225-y. [DOI] [PubMed] [Google Scholar]
- 63.Worm D, Madsbad S, Kristiansen VB, Naver L, Hansen DL. Changes in Hematology and Calcium Metabolism After Gastric Bypass Surgery-a 2-Year Follow-Up Study. Obes Surg. 2015;25(9):1647–52. doi: 10.1007/s11695-014-1568-4. [DOI] [PubMed] [Google Scholar]
- 64.Da Rosa CL, Dames Olivieri Saubermann AP, Jacqueline J, Pereira SE, Saboya C, Ramalho A. Routine supplementation does not warrant the nutritional status of vitamin d adequate after gastric bypass Roux-en-Y. Nutr Hosp. 2013;28(1):169–72. doi: 10.3305/nh.2013.28.1.6166. [DOI] [PubMed] [Google Scholar]
- 65.Ben-Porat T, Elazary R, Yuval JB, Wieder A, Khalaileh A, Weiss R. Nutritional deficiencies after sleeve gastrectomy: can they be predicted preoperatively? Surg Obes Relat Dis. 2015 doi: 10.1016/j.soard.2015.02.018. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 66.Chan LN, Neilson CH, Kirk EA, Colovos TF, Javelli DR, Khandelwal S. Optimization of Vitamin D Status After Roux-en-Y Gastric Bypass Surgery in Obese Patients Living in Northern Climate. Obes Surg. 2015 doi: 10.1007/s11695-015-1685-8. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 67.Jin J, Robinson AV, Hallowell PT, Jasper JJ, Stellato TA, Wilhem SM. Increases in parathyroid hormone (PTH) after gastric bypass surgery appear to be of a secondary nature. Surgery. 2007;142(6):914–20. doi: 10.1016/j.surg.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 68.Jin J, Stellato TA, Hallowell PT, Schuster M, Graf K, Wilhelm S. Utilization of preoperative patient factors to predict postoperative vitamin D deficiency for patients undergoing gastric bypass. J Gastrointest Surg. 2009;13(6):1052–7. doi: 10.1007/s11605-009-0847-1. [DOI] [PubMed] [Google Scholar]
- 69.Fish E, Beverstein G, Olson D, Reinhardt S, Garren M, Gould J. Vitamin D status of morbidly obese bariatric surgery patients. J Surg Res. 2010;164(2):198–202. doi: 10.1016/j.jss.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 70.Flores L, Osaba MJM, Andreu A, Moizé V, Rodríguez L, Vidal J. Calcium and vitamin D supplementation after gastric bypass should be individualized to improve or avoid hyperparathyroidism. Obes Surg. 2010;20(6):738–43. doi: 10.1007/s11695-010-0138-7. [DOI] [PubMed] [Google Scholar]
- 71.Signori C, Zalesin KC, Franklin B, Miller WL, McCullough PA. Effect of gastric bypass on vitamin D and secondary hyperparathyroidism. Obes Surg. 2010;20(7):949–52. doi: 10.1007/s11695-010-0178-z. [DOI] [PubMed] [Google Scholar]
- 72.Aarts EO, Berends FJ, Janssen IMC, Schweitzer DH. Semiquantitative assessment of bowel habits and its relation with calcium metabolism after gastric bypass surgery: a retrospective study. J Obes. 2011;2011:156164. doi: 10.1155/2011/156164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sinha N, Shieh A, Stein EM, Strain G, Schulman A, Pomp A, et al. Increased PTH and 1. 25 (OH) 2D levels associated with increased markers of bone turnover following bariatric surgery. Obesity. 2011;19(12):2388–93. doi: 10.1038/oby.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Luis DA, Pacheco D, Izaola O, Terroba MC, Cuellar L, Cabezas G. Micronutrient status in morbidly obese women before bariatric surgery. Surg Obes Relat Dis. 2013;9(2):323–7. doi: 10.1016/j.soard.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 75.Bandstein M, Schultes B, Ernst B, Thurnheer M, Schioth HB, Benedict C. The Role of FTO and Vitamin D for the Weight Loss Effect of Roux-en-Y Gastric Bypass Surgery in Obese Patients. Obes Surg. 2015;25(11):2071–7. doi: 10.1007/s11695-015-1644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Capoccia D, Coccia F, Paradiso F, Abbatini F, Casella G, Basso N, et al. Laparoscopic gastric sleeve and micronutrients supplementation: Our experience. J Obes. 2012;2012:672162. doi: 10.1155/2012/672162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vilarrasa N, San Jose P, Garcia I, Gomez-Vaquero C, Miras PM, De Gordejuela AGR, et al. Evaluation of bone mineral density loss in morbidly obese women after gastric bypass: 3-year follow-up. Obes Surg. 2011;21(4):465–72. doi: 10.1007/s11695-010-0338-1. [DOI] [PubMed] [Google Scholar]
- 78.Coupaye M, Puchaux K, Bogard C, Msika S, Jouet P, Clerici C, et al. Nutritional consequences of adjustable gastric banding and gastric bypass: A 1-year prospective study. Obes Surg. 2009;19(1):56–65. doi: 10.1007/s11695-008-9571-2. [DOI] [PubMed] [Google Scholar]
- 79.Vilarrasa N, de Gordejuela AGR, Gómez-Vaquero C, Pujol J, Elio I, San José P, et al. Effect of bariatric surgery on bone mineral density: comparison of gastric bypass and sleeve gastrectomy. Obes Surg. 2013;23(12):2086–91. doi: 10.1007/s11695-013-1016-x. [DOI] [PubMed] [Google Scholar]
- 80.Avgerinos DV, Leitman IM, Martinez RE, Liao EP. Evaluation of Markers for Calcium Homeostasis in a Population of Obese Adults Undergoing Gastric Bypass Operations. J Am Coll Surg. 2007;205(2):294–7. doi: 10.1016/j.jamcollsurg.2007.02.078. [DOI] [PubMed] [Google Scholar]
- 81.Våge V, Sande VA, Mellgren G, Laukeland C, Behme J, Andersen JR. Changes in obesity-related diseases and biochemical variables after laparoscopic sleeve gastrectomy: a two-year follow-up study. BMC Surgery. 2014;14(1):8. doi: 10.1186/1471-2482-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gehrer S, Kern B, Peters T, Christoffel-Courtin C, Peterli R. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB)-a prospective study. Obes Surg. 2010;20(4):447–53. doi: 10.1007/s11695-009-0068-4. [DOI] [PubMed] [Google Scholar]
- 83.Mahlay NF, Verka LG, Thomsen K, Merugu S, Salomone M. Vitamin D status before Roux-en-Y and efficacy of prophylactic and therapeutic doses of vitamin D in patients after Roux-en-Y gastric bypass surgery. Obes Surg. 2009;19(5):590–4. doi: 10.1007/s11695-008-9698-1. [DOI] [PubMed] [Google Scholar]
- 84.Cuesta M, Pelaz L, Perez C, Torrejon MJ, Cabrerizo L, Matia P, et al. Fat-soluble vitamin deficiencies after bariatric surgery could be misleading if they are not appropriately adjusted. Nutr Hosp. 2014;30(1):118–23. doi: 10.3305/nh.2014.30.1.7471. [DOI] [PubMed] [Google Scholar]
- 85.Ybarra J, Sánchez-Hernández J, Gich I, De Leiva A, Rius X, Rodríguez-Espinosa J, et al. Unchanged hypovitaminosis D and secondary hyperparathyroidism in morbid obesity after bariatric surgery. Obes Surg. 2005;15(3):330–5. doi: 10.1381/0960892053576758. [DOI] [PubMed] [Google Scholar]
- 86.Alexandrou A, Armeni E, Kouskouni E, Tsoka E, Diamantis T, Lambrinoudaki I. Cross-sectional long-term micronutrient deficiencies after sleeve gastrectomy versus Roux-en-Y gastric bypass: a pilot study. Surg Obes Relat Dis. 2014;10(2):262–8. doi: 10.1016/j.soard.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 87.Karefylakis C, Naslund I, Edholm D, Sundbom M, Karlsson FA, Rask E. Vitamin D Status 10 Years After Primary Gastric Bypass: Gravely High Prevalence of Hypovitaminosis D and Raised PTH Levels. Obes Surg. 2014;24(3):343–8. doi: 10.1007/s11695-013-1104-y. [DOI] [PubMed] [Google Scholar]
- 88.Granado-Lorencio F, Simal-Anton A, Blanco-Navarro I. Seasonal changes in serum 25-OH-vitamin D3 after bariatric surgery. e-SPEN Eur e-J Clin Nutr Metab. 2008;3(5):e208–e10. [Google Scholar]
- 89.Aarts E, Berends F, Janssen I, Schweitzer D. Semiquantitative assessment of bowel habits and its relation with calcium metabolism after gastric bypass surgery: a retrospective study. J Obes. 2010;2011 doi: 10.1155/2011/156164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aarts EO, Janssen IMC, Berends FJ. The gastric sleeve: losing weight as fast as micronutrients? Obes Surg. 2011;21(2):207–11. doi: 10.1007/s11695-010-0316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Slater GH, Ren CJ, Siegel N, Williams T, Barr D, Wolfe B, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8(1):48–55. doi: 10.1016/j.gassur.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 92.Abbasi AA, Amin M, Smiertka JK, Grunberger G, MacPherson B, Hares M, et al. Abnormalities of vitamin D and calcium metabolism after surgical treatment of morbid obesity: a study of 136 patients. Endocr Pract : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2007;13(2):131–6. doi: 10.4158/EP.13.2.131. [DOI] [PubMed] [Google Scholar]
- 93.Carlin AM, Yager KM, Rao DS. Vitamin D depletion impairs hypertension resolution after Roux-en-Y gastric bypass. Am J Surg. 2008;195(3):349–52. doi: 10.1016/j.amjsurg.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 94.Vilarrasa N, Gomez JM, Elio I, Gomez-Vaquero C, Masdevall C, Pujol J, et al. Evaluation of bone disease in morbidly obese women after gastric bypass and risk factors implicated in bone loss. Obes Surg. 2009;19(7):860–6. doi: 10.1007/s11695-009-9843-5. [DOI] [PubMed] [Google Scholar]
- 95.Dalcanale L, Oliveira CPMS, Faintuch J, Nogueira MA, Rondo P, Lima VMR, et al. Long-term nutritional outcome after gastric bypass. Obes Surg. 2010;20(2):181–7. doi: 10.1007/s11695-009-9916-5. [DOI] [PubMed] [Google Scholar]
- 96.Hewitt S, Søvik TT, Aasheim ET, Kristinsson J, Jahnsen J, Birketvedt GS, et al. Secondary hyperparathyroidism, vitamin D sufficiency, and serum calcium 5 years after gastric bypass and duodenal switch. Obes Surg. 2013;23(3):384–90. doi: 10.1007/s11695-012-0772-3. [DOI] [PubMed] [Google Scholar]
- 97.Costa TL, Paganotto M, Radominski RB, Kulak CM, Borba VC. Calcium metabolism, vitamin D and bone mineral density after bariatric surgery. Osteoporos Int. 2015;26(2):757–64. doi: 10.1007/s00198-014-2962-4. [DOI] [PubMed] [Google Scholar]
- 98.Balsa JA, Botella-Carretero JI, Peromingo R, Caballero C, Munoz-Malo T, Villafruela JJ, et al. Chronic increase of bone turnover markers after biliopancreatic diversion is related to secondary hyperparathyroidism and weight loss. Relation with bone mineral density. Obes Surg. 2010;20(4):468–73. doi: 10.1007/s11695-009-0028-z. [DOI] [PubMed] [Google Scholar]
- 99.Khandalavala BN, Hibma PP, Fang X. Prevalence and persistence of vitamin D deficiency in biliopancreatic diversion patients: a retrospective study. Obes Surg. 2010;20(7):881–4. doi: 10.1007/s11695-010-0185-0. [DOI] [PubMed] [Google Scholar]
- 100.Clements RH, Yellumahanthi K, Wesley M, Ballem N, Bland KI. Hyperparathyroidism and vitamin D deficiency after laparoscopic gastric bypass. Am Surg. 2008;74(6):469–74. discussion 74–5. [PubMed] [Google Scholar]
- 101.Hamoui N, Kim K, Anthone G, Crookes PF. The significance of elevated levels of parathyroid hormone in patients with morbid obesity before and after bariatric surgery. Arch Surg. 2003;138(8):891–7. doi: 10.1001/archsurg.138.8.891. [DOI] [PubMed] [Google Scholar]
- 102.Ybarra J, Sanchez-Hernandez J, Gich I, De Leiva A, Rius X, Rodriguez-Espinosa J, et al. Unchanged hypovitaminosis D and secondary hyperparathyroidism in morbid obesity after bariatric surgery. Obes Surg. 2005;15(3):330–5. doi: 10.1381/0960892053576758. [DOI] [PubMed] [Google Scholar]
- 103.Clements RH, Katasani VG, Palepu R, Leeth RR, Leath TD, Roy BP, et al. Incidence of vitamin deficiency after laparoscopic Roux-en-Y gastric bypass in a university hospital setting. Am Surg. 2006;72(12):1196–202. doi: 10.1177/000313480607201209. [DOI] [PubMed] [Google Scholar]
- 104.Carlin AM, Rao DS, Yager KM, Genaw JA, Parikh NJ, Szymanski W. Effect of gastric bypass surgery on vitamin D nutritional status. Surg Obes Relat Dis. 2006;2(6):638–42. doi: 10.1016/j.soard.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 105.Vieth R. Critique of the considerations for establishing the tolerable upper intake level for vitamin D: critical need for revision upwards. J Nutr. 2006;136(4):1117–22. doi: 10.1093/jn/136.4.1117. [DOI] [PubMed] [Google Scholar]
- 106.Nogués X, Goday A, Jesus Pena M, Benaiges D, de Ramón M, Crous X, et al. Bone mass loss after sleeve gastrectomy: a prospective comparative study with gastric bypass. Cir Esp (English Edition) 2010;88(2):103–9. doi: 10.1016/j.ciresp.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 107.Vix M, Liu K-H, Diana M, D’Urso A, Mutter D, Marescaux J. Impact of Roux-en-Y gastric bypass versus sleeve gastrectomy on vitamin D metabolism: short-term results from a prospective randomized clinical trial. Surg Endosc. 2014;28(3):821–6. doi: 10.1007/s00464-013-3276-x. [DOI] [PubMed] [Google Scholar]
- 108.Heber D, Greenway FL, Kaplan LM, Livingston E, Salvador J, Still C. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2010;95(11):4823–43. doi: 10.1210/jc.2009-2128. [DOI] [PubMed] [Google Scholar]
- 109.Mechanick JI, Youdim A, Jones DB, Timothy Garvey W, Hurley DL, Molly McMahon M, Heinberg LJ, Kushner R, Adams TD, Shikora S, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient-2013 update: Cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Endocr Pract. 2013;19(2):337–372. doi: 10.4158/EP12437.GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008 Aug;88(2):582S-586S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 111.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999 May;69(5):842–56. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 112.Compher CW, Badellino KO, Boullata JI. Vitamin D and the bariatric surgical patient: a review. Obes Surg. 2008;18(2):220–4. doi: 10.1007/s11695-007-9289-6. [DOI] [PubMed] [Google Scholar]
- 113.Stein J, Stier C, Raab H, Weiner R. Review article: the nutritional and pharmacological consequences of obesity surgery. Aliment Pharmacol Ther. 2014;40(6):582–609. doi: 10.1111/apt.12872. [DOI] [PubMed] [Google Scholar]
- 114.Schünemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, et al. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Res Synth Methods. 2013;4(1):49–62. doi: 10.1002/jrsm.1078. [DOI] [PubMed] [Google Scholar]
- 115.Barake M, Daher RT, Salti I, Cortas NK, Al-Shaar L, Habib RH, et al. 25-hydroxyvitamin D assay variations and impact on clinical decision making. J Clin Endocrinol Metab. 2012;97(3):835–43. doi: 10.1210/jc.2011-2584. [DOI] [PubMed] [Google Scholar]
- 116.Binkley N, Sempos CT. Standardizing Vitamin D assays: the way forward. J Bone Miner Res. 2014;29(8):1709–14. doi: 10.1002/jbmr.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin E, Armstrong-Moore D, Liang Z, Sweeney JF, Torres WE, Ziegler TR, et al. Contribution of adipose tissue to plasma 25-hydroxyvitamin D concentrations during weight loss following gastric bypass surgery. Obesity. 2011;19(3):588–94. doi: 10.1038/oby.2010.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pramyothin P, Biancuzzo RM, Lu Z, Hess DT, Apovian CM, Holick MF. Vitamin D in Adipose Tissue and Serum 25-Hydroxyvitamin D After Roux-en-Y Gastric Bypass. Obesity. 2011;19(11):2228–34. doi: 10.1038/oby.2011.170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.