Abstract
Background
NSABP B-35 is a phase III trial comparing anastrozole (A) to tamoxifen (T) for breast cancer-free interval (BCFI), defined as time from randomization to any breast cancer event: local, regional, distant, or contralateral, invasive or DCIS.
Methods
Postmenopausal women with hormone-positive DCIS treated by lumpectomy with clear resection margins and whole breast irradiation were randomized to receive either 20mg/day T or 1mg/day A (blinded) for 5 years. Stratification was by <60 versus ≥60 years.
Findings
From 1/6/2003–6/15/2006, 3,104 patients were entered and randomized. As of 2/28/15, follow-up information was available on 3,083 patients for OS and 3,077 for all other disease-free endpoints, with median follow-up of 9 years. There were 212 BCFI events, 122 in the T group and 90 in the A group (HR, 0.73; 95% CI, 0.56–0.96; p=0.0234). 10-year estimates for BCFI were 89.1% (95% CI, 86.8–91.0) for T and 93.1% (95% CI, 91.5–94.5) for A. A significant time-by-treatment interaction (p=0.0410) became evident later in the study. There was a significant interaction between treatment and age group (p=0.0379); A is superior only in women <60 years. There were 495 DFS events: 260 in the T group, 235 in the A group (HR, 0.89; 95% CI, 0.75–1.07; p=0.21). 10-year DFS estimates were 77.9% (95% CI, 75.0–80.6) for T and 82.7% (95% CI, 80.4–84.7) for A. There were 186 deaths: 88 in the T group, 98 in the A group (HR, 1.11; 95% CI, 0.83–1.48; p=0.48). 10-year OS estimates were 92.1% (95% CI, 90.1–93.7) for T, 92.5% (95% CI, 90.8–93.9) for A. Eight deaths were due to breast cancer in the T group and five in the A. There were 69 cases of invasive breast cancer in the T group and 43 in the A group (HR, 0.62; 95% CI, 0.42–0.90; p=0.0123). A significant reduction in contralateral breast cancers with A (HR, 0.64; 95% CI, 0.43–0.96; p=0.0322) was identified.
Interpretation
Anastrozole provided a significant improvement compared to tamoxifen for BCFI, primarily in women <60 years.
Funding
US NCI; AstraZeneca Pharmaceuticals LP.
Keywords: DCIS, Aromatase inhibitors, Tamoxifen, Breast cancer prevention
Introduction
With continuous improvements in screening mammography and diagnostic breast imaging, DCIS has been identified more frequently, and its management has become an increasingly challenging problem. Originally called early or minimal breast cancer, DCIS is now classified as stage 0 breast cancer and considered by some to be a precancerous entity. As a result, there is ongoing debate as to whether DCIS should be treated as malignancy or as a precursor of cancer.
Randomized clinical trials of IBC established that breast-conserving surgery (BCT) and whole breast irradiation (WBI) provided the same long-term survival rates as total mastectomy.1,2 This shift in surgical management was soon adopted also for DCIS. However, BCT left open the chance for a local recurrence, which can manifest itself as another DCIS or as invasive recurrence.
Seeking to minimize these events, adjuvant therapies were then tested. NSABP B-243 was based on previous reports that adjuvant T decreased the incidence of tumor recurrence in the affected breast of patients with invasive breast cancer (IBC) and reduced the rate of new primary tumors in the contralateral breast (CB). This suggested that T can interfere with the development of primary IBC or with the progression from DCIS to IBC.
In B-24, women with DCIS were randomly assigned to receive 5 years of adjuvant T or placebo following BCT+WBI. A total of 1804 women were randomized. At 83 months follow-up, women in the T group had fewer breast cancer events (10.3% versus 16.9%, P=0.0003). The cumulative incidence of all breast cancers in the T group was 4.8% at seven years: 2.6% in the ipsilateral breast, 1.8% in the CB, and 0.4% at regional and distant sites.3
Wapnir4 evaluated long-term outcomes for ipsilateral breast tumor recurrences (IBTR) in the NSABP B-17 and B-24 studies. Of 490 events, 263 (53.7%) were invasive (I-IBTR). Addition of WBI reduced the risk compared to patients receiving lumpectomy alone. (HR=0.48). I-IBTR was associated with increased mortality risk (HR=1.75), but recurrence of DCIS was not. In B-24, L-RT+T reduced I-IBTR by 32% compared to L-RT+placebo.
Despite these benefits, some women still relapsed or suffered serious side effects such as endometrial cancer, vascular complications, and bothersome menopausal symptoms, which affect compliance. We hypothesized that the partial agonist properties of T and the lack of complete suppression of estrogen receptor signaling may limit the benefits of such treatment.
The advent of third-generation aromatase inhibitors provided the possibility to reduce or eliminate signaling through the estrogen receptor pathway with treatments that do not have estrogen agonist effects.
In the treatment of ER-positive postmenopausal patients with metastatic disease, the aromatase inhibitor A was superior to T in overall response rates and time to progression.5 Side effects and toxicities were similar, but A was associated with fewer thromboembolic events.
Results of the ATAC6 trial for women with early-stage, IBC demonstrated superiority for A over T. This multicenter randomized, double-blind study involved 9,366 postmenopausal women randomly assigned to take A or T, or a combination of both. There was a 17% reduction in relative risk of disease recurrence with A (P=.0129), and an absolute risk reduction of 2%. Among women with confirmed ER-positive tumors, the reduction in risk of recurrence was 22%. There was no additional benefit seen in the combination group. There were significantly fewer reports of endometrial cancer, deep vein thrombosis, stroke, and hot flashes, with A, but the A group had more fractures, predominantly wrist, when compared to T. Additionally, A significantly reduced the risk of developing CBC (odds ratio 0·42 [0·22–0·79], p=0·007).
Based on this rationale, we undertook a prospective, double-blind randomized trial in postmenopausal ER-positive DCIS patients. The primary aim of B-35 was to compare the value of A vs. T, given for 5 years in preventing subsequent occurrence of breast cancer (local, regional, and distant recurrences, and CBC) following L-RT.
Materials and Methods
Study Population
Postmenopausal women with DCIS or mixed DCIS and lobular carcinoma in situ (LCIS) who were ER or PgR+, with no invasive component, were eligible for B-35. Participants had to have undergone a lumpectomy with clear margins and negative nodes (if biopsied), followed by WBI and no systemic therapy for the current DCIS. Patients requiring a mastectomy or those who had a history of IBC, DCIS, or a cancer not of the breast within 5 years prior to randomization were ineligible except for carcinoma in situ of the cervix, carcinoma in situ of the colon, melanoma in situ, and basal cell and squamous cell carcinoma of the skin. Patients receiving raloxifene, other SERMs, or any sex hormonal therapy were ineligible. Patients with a history of thromboembolic disease, cerebral vascular accident or transient ischemia attack, uncontrolled hypertension, uncontrolled diabetes, or uncontrolled atrial fibrillation were ineligible.
Methods
All participating clinical centers obtained approval from institutional review boards, and all participants provided written informed consent. Treatment assignment was double-blinded and eligible patients were randomly assigned on a 1:1 basis to receive either 1 mg per day of A plus a placebo for T or 20 mg per day of T plus a placebo for A. Because age is a prognostic factor for both breast cancer and other concomitant diseases that affect survival, participants were stratified (<60 versus ≥60) to assure balance between treatment groups. Randomization was performed centrally by the statistical center using minimization.
A/T therapy was to begin within 30 days following random assignment and end 5 years from the date of first dose, regardless of any missed doses. All patients received WBI. Patients were followed via physical examinations every 6 months for the first 5 years and every 12 months thereafter. Patients also received annual bilateral mammograms.
The primary endpoint was breast cancer-free interval (BCFI), the time from randomization to any breast cancer event including local, regional, or distant recurrence or contralateral disease, invasive or DCIS. It was censored for deaths. Disease-free survival (DFS) was a secondary endpoint, defined as time to any recurrence (excluding LCIS), second primary cancer (excluding basal cell or squamous cell carcinoma of the skin, or various carcinomas in situ) and death from any cause. Other secondary endpoints included ipsilateral breast cancer, CBC, non-breast second primary cancers osteoporotic fractures, and overall survival (OS). B-35 also included quality of life components (results reported separately).*
Statistical Analysis
The expected rate of breast cancer events was 0.015 per person year of follow-up. The study was designed to a sample size of at least 3,000 patients to provide at least 80% power to detect a 33% reduction in breast cancer event rates. This required 199 BCFI events to be observed before final analysis. Three pre-planned interim analyses were conducted. To account for alpha spending and preserve the overall type I error at 0.05, the adjusted significance criterion for the final analysis was 0.04838.
All analyses followed an intention-to-treat principle and included all at-risk women with available follow-up information. Distributions of time to any breast cancer as well as DFS and OS for each treatment group were estimated by the Kaplan-Meier method and compared between treatments by log-rank tests stratified by age. Hazard ratios (HRs) and 95% confidence intervals (CIs) for any breast cancer, DFS, and OS were calculated from Cox models stratified by age. As pre-specified in the protocol, tests for interactions between treatment and specific covariates including age group, comedo necrosis, and palpable mass at presentation were conducted. Significant interactions prompted separate tests within each covariate group. Invasive and non-invasive breast cancers, ipsilateral recurrence, and CBC were compared using Cox models controlling for age to obtain HRs, 95% CIs, and p-values. Non-breast second primary cancers and osteoporotic fractures were compared by calculating average annual rates and risk ratios (RRs). CIs for RRs were computed assuming a Poisson distribution and conditioning on the total number of events and person-years at risk. Analyses were performed using SAS version 9.4 (SAS Institute, Inc.).
Results
Between January 6, 2003, and June 15, 2006, 3,104 patients were entered into B-35 and randomly assigned to T (n=1,552) or A (n=1,552) (Figure 1; See also Appendix Suppl ST1, Reasons for Stopping Therapy). The current analysis used data collected through February 28, 2015. A total of 3,083 patients (99.3%) had follow-up information available for survival, with 3,077 (99.1%) also having follow-up information for all other disease-free endpoints. Median time of follow-up was 9 years. Distribution of all patient and tumor characteristics is presented in Table 1. All characteristics were well balanced by treatment group. There was no difference in treatment compliance between the T and A groups, with about 70% of participants in each group completing 5 years of therapy. The mean duration of treatment was 46.8 months in the T group and 47.1 months in the A group.
Figure 1.

CONSORT diagram: NSABP B-35
Table 1.
Patient and tumor characteristics for all randomly assigned patients NSABP protocol B-35
| Patient or Tumor Characteristic | Tamoxifen | Anastrozole | Total | |||
|---|---|---|---|---|---|---|
| n | % | n | % | N | % | |
| Age (Years) | ||||||
| < 60 | 730 | 47.0 | 731 | 47.1 | 1,461 | 47.1 |
| ≥ 60 | 822 | 53.0 | 821 | 52.9 | 1,643 | 52.9 |
| Race | ||||||
| White | 1,352 | 87.1 | 1,361 | 87.7 | 2,713 | 87.4 |
| Black | 135 | 8.7 | 124 | 8.0 | 259 | 8.3 |
| Pacific Islander | 6 | 0.4 | 6 | 0.4 | 12 | 0.4 |
| Asian | 39 | 2.5 | 34 | 2.2 | 73 | 2.4 |
| Native American/Alaskan | 3 | 0.2 | 5 | 0.3 | 8 | 0.3 |
| Multi-racial | 4 | 0.3 | 5 | 0.3 | 9 | 0.3 |
| Unknown | 13 | 0.8 | 17 | 1.1 | 30 | 1.0 |
| Ethnicity | ||||||
| Non-Hispanic | 1,407 | 90.7 | 1,421 | 91.6 | 2,828 | 91.1 |
| Hispanic or Latino | 51 | 3.3 | 45 | 2.9 | 96 | 3.1 |
| Unknown | 94 | 6.1 | 86 | 5.5 | 180 | 5.8 |
| Tumor evident on mammogram | ||||||
| Yes | 1,488 | 95.9 | 1,513 | 97.5 | 3,001 | 96.7 |
| No | 59 | 3.8 | 33 | 2.1 | 92 | 3.0 |
| Unknown | 5 | 0.3 | 6 | 0.4 | 11 | 0.4 |
| Comedo necrosis | ||||||
| Absent | 728 | 46.9 | 669 | 43.1 | 1,397 | 45.0 |
| Present | 592 | 38.1 | 669 | 43.1 | 1,261 | 40.6 |
| Unknown | 232 | 14.9 | 214 | 13.8 | 446 | 14.4 |
| Tumor palpable | ||||||
| Yes | 135 | 8.7 | 121 | 7.8 | 256 | 8.2 |
| No | 1,412 | 91.0 | 1,425 | 91.8 | 2,837 | 91.4 |
| Unknown | 5 | 0.3 | 6 | 0.4 | 11 | 0.4 |
| Pathological tumor size | ||||||
| < 1.0 | 556 | 35.8 | 528 | 34.0 | 1,084 | 34.9 |
| 1.0+ | 370 | 23.8 | 389 | 25.1 | 759 | 24.5 |
| Unknown | 626 | 40.3 | 635 | 40.9 | 1,261 | 40.6 |
| Body mass index | ||||||
| <25.0 | 405 | 26.1 | 376 | 24.2 | 781 | 25.2 |
| 25–29.9 | 518 | 33.4 | 503 | 32.4 | 1021 | 32.9 |
| ≥30.0 | 629 | 40.5 | 673 | 43.4 | 1302 | 41.9 |
|
| ||||||
| Total | 1,552 | 100.0 | 1,552 | 100.0 | 3,104 | 100.0 |
As of February 28, 2015, we observed a total of 212 BCFI events, with 122 in the T group and 90 in the A group. As shown in Figure 2 and Table 2, A resulted in an overall statistically significant decrease in BCFI compared to T (HR, 0.73; 95% CI, 0.56 to 0.96; p=0.0234). The 5-year BCFI estimates were 96.3% (95% CI, 95.2 to 97.2) and 96.3% (95% CI, 95.3 to 97.2) for the T and A arms, respectively. The 10-year BCFI estimates were 89.1% (95% CI, 86.8 to 91.0) and 93.1% (95% CI, 91.5 to 94.5) for the T and A arms, respectively. As illustrated in the Kaplan-Meier curves, there is a divergence after the first 5 years. This divergence was a statistically significant time-by-treatment interaction (p=0.0410). There was also a statistically significant interaction between treatment and age group categorized as <60 vs. ≥60 years (p=0.0379). The number of breast cancer events, HRs, CIs, and p-values by age group are presented in the top of Table 3. The beneficial effect of A is significant only among women <60 years.
Figure 2.

Breast cancer-free interval: NSABP B-35
Table 2.
Breast cancer first events NSABP Protocol B-35
| Number of Events
|
Hazard Ratio (HR) | 95% Confidence Interval | P-value | ||
|---|---|---|---|---|---|
| Tamoxifen (n=1,538) |
Anastrozole (n=1,539) |
||||
| All breast cancers | |||||
| Total | 122 | 90 | 0.73 | (0.56 – 0.96) | 0.0234 |
| Invasive | 69 | 43 | 0.62 | (0.42 – 0.90) | 0.0123 |
| Ductal carcinoma in situ | 53 | 47 | 0.88 | (0.59 – 1.30) | 0.52 |
|
| |||||
| Ipsilateral recurrence | |||||
| Total | 55 | 46 | 0.83 | (0.56 – 1.22) | 0.34 |
| Invasive | 22 | 17 | 0.76 | (0.40 – 1.43) | 0.39 |
| Ductal carcinoma in situ | 33 | 29 | 0.87 | (0.53 – 1.43) | 0.59 |
|
| |||||
| Contralateral breast cancer | |||||
| Total | 60 | 39 | 0.64 | (0.43 – 0.96) | 0.0322 |
| Invasive | 40 | 21 | 0.52 | (0.31 – 0.88) | 0.0148 |
| Ductal carcinoma in situ | 20 | 18 | 0.90 | (0.47 – 1.69) | 0.73 |
|
| |||||
| Breast cancer at distant sites | 7 | 4 | 0.57 | (0.17 – 1.95) | 0.37 |
|
| |||||
| Breast second primary cancer* | 0 | 1 | – | – | – |
Angiosarcoma in the ipsilateral breast
Table 3.
Breast cancer-free interval and disease-free survival by age group: NSABP Protocol B-35
| N | Number of Events
|
Hazard Ratio (HR) | HR 95% Confidence Interval | P-value | ||
|---|---|---|---|---|---|---|
| Tamoxifen | Anastrozole | |||||
| Breast Cancer-free interval | ||||||
| <60 years | 1,447 | 63 | 34 | 0.53 | 0.35 – 0.80 | 0.0026 |
| ≥60 years | 1,630 | 59 | 56 | 0.95 | 0.66 – 1.37 | 0.78 |
|
| ||||||
| Disease-free survival | ||||||
| <60 years | 1,447 | 104 | 74 | 0.69 | 0.51 – 0.93 | 0.0151 |
| ≥60 years | 1,630 | 156 | 161 | 1.03 | 0.83 – 1.28 | 0.79 |
The rates for the individual events contributing to BCFI are presented in Table 2. There was a statistically significant difference between treatment groups for all IBC, with 69 occurring in the T group and 43 in the A group (HR, 0.62; 95% CI, 0.42 to 0.90; p=0.0123). There was also a statistically significant reduction in CBC with A (HR, 0.64; 95% CI, 0.43 to 0.96; p=0.0322), and a statistically significant reduction in invasive CBC with A (HR, 0.52; 95% CI, 0.31 to 0.88; p=0.0148). The other endpoints did not reach statistical significance.
There have been 495 DFS events observed, with 260 in the T group and 235 in the A group (Figure 3, HR, 0.89; 95% CI, 0.75–1.07; p=0.21). The 5-year estimates for DFS were 91.6% (95% CI, 90.0 to 92.9) and 91.5% (95% CI, 89.9 to 92.8) for the T and A arms, respectively. The 10-year estimates for DFS were 77.9% (95% CI, 75.0 to 80.6) and 82.7% (95% CI, 80.4 to 84.7) for the T and A arms, respectively. Although this difference is not statistically significant, there does appear to be a trend similar to that seen for BCFI in which the Kaplan-Meier curves separate in the second half of the study but at a later point in time. Again, there was a statistically significant interaction between treatment group and age (p=0.0331), with a significant effect found only among those < 60 years (Table 3).
Figure 3.
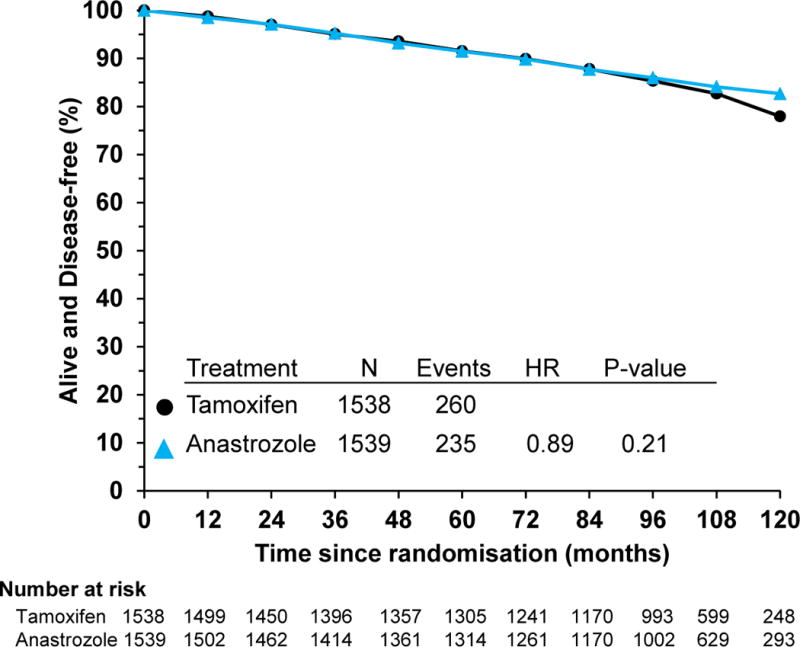
Disease-free survival: NSABP B-35
There were 209 patients (102 in the T group and 107 in the A group) who experienced a non-breast second primary cancer as a first event (Table 4). Inspection of the individual sites of second primary cancers revealed no notable differences by treatment arm, except for uterine cancer, a known side effect of T; there was a nonsignificant difference (RR, 0.47; 95% CI, 0.18–1.15) with 17 cases of uterine cancer reported in the T group and eight in the A group.
Table 4.
Non-breast cancer events: NSABP Protocol B-35
| Number of Events
|
Risk Ratio (RR)* | RR 95% Confidence Interval | ||
|---|---|---|---|---|
| Tamoxifen | Anastrozole | |||
| Non-breast second primary cancer | 102 | 107 | 1.04 | 0.78 – 1.37 |
| Uterine cancer | 17 | 8 | 0.47 | 0.18 – 1.15 |
| Osteoporotic fractures | 50 | 69 | 1.38 | 0.95 – 2.03 |
Risk ratio for women in the anastrozole group compared to those in the tamoxifen group
There were more osteoporotic fractures, defined as fractures of the hip, spine, and wrist reported in the A group than the T group (69 versus 50, respectively), but this difference was not statistically significant (Table 4).
A total of 186 deaths were reported, with 88 in the T group and 98 in the A group (data not shown). This difference was not statistically significant (HR, 1.11; 95% CI, 0.83–1.48; p=0.48). The 5-year estimates for OS were 98.0% (95% CI, 97.2 to 98.6) and 97.9% (95% CI, 97.0–98.5) for the T and A arms, respectively. The 10-year estimates for OS were 92.1% (95% CI, 90.1 to 93.7) and 92.5% (95% CI, 90.8–93.9) for the T and A arms, respectively. There was no statistically significant interaction between treatment and age group for OS (p=0.38). There were 8 breast cancer deaths in the T group and 5 in the A group.
Information regarding adverse events (AE) was available for 3,070 (98.9%) randomized patients (Table 5). Except for thrombosis/embolism, which is a known side effect of T (T group toxicities=17; A group=four (three Grade 4, one Grade 5), there were no striking differences by treatment group overall or for any specific type of AE reported. Women in the A group experienced slightly more instances of arthralgia and myalgia, but the percentages were comparable between treatment groups. However, these AEs underestimate the difference in severity of patient-reported symptoms for both treatments, as is described in more detail in our report of the quality of life outcomes.
Table 5.
Adverse events by treatment group: NSABP B-35
| Characteristic | Tamoxifen (n=1,535) |
Anastrozole (n=1,535) |
|---|---|---|
| Overall toxicity | ||
| Grade 0/1 | 312 (20.3%) | 318 (20.7%) |
| Grade 2 | 771 (50.2%) | 771 (50.2%) |
| Grade 3 | 380 (24.8%) | 384 (25.0%) |
| Grade 4 | 59 (3.8%) | 50 (3.3%) |
| Grade 5 | 13 (0.8%) | 12 (0.8%) |
|
| ||
| Thromboembolic event | ||
| Grade 0/1 (none/superficial thrombosis) | 1,494 (97.3%) | 1,522 (99.2%) |
| Grade 2 (deep vein thrombosis) | 4 (0.3%) | 1 (0.1%) |
| Grade 3 (uncomplicated pulmonary embolism) | 20 (1.3%) | 8 (0.5%) |
| Grade 4 (life-threatening pulmonary embolism) | 17 (1.1%) | 3 (0.2%) |
| Grade 5 (death) | 0 | 1 (0.1%) |
|
| ||
| Arthralgia | ||
| Grade 0/1 (none/mild pain) | 1,177 (76.7%) | 1,031 (67.2%) |
| Grade 2 (moderate pain) | 302 (19.7%) | 427 (27.8%) |
| Grade 3 (severe pain) | 55 (3.6%) | 77 (5.0%) |
| Grade 4 (disabling) | 1 (0.1%) | 0 |
|
| ||
| Myalgia | ||
| Grade 0/1 (none/mild pain) | 1,367 (89.1%) | 1,317 (85.8%) |
| Grade 2 (moderate pain) | 150 (9.8%) | 187 (12.2%) |
| Grade 3 (severe pain) | 18 (1.2%) | 30 (2.0%) |
| Grade 4 (disabling) | 0 | 1 (0.1%) |
Discussion
This trial can be viewed as part of a step-wise evaluation by the NSABP of new treatments for patients with DCIS. The NSABP B-177,8 trial established lumpectomy and WBI as an appropriate, optional treatment for DCIS. The B-24 trial3 showed that the addition of adjuvant T following L+WBI resulted in fewer recurrences (invasive or non-invasive), in either breast, compared to placebo. Results from B-35 now show that A compared to T resulted in further improvement in BCFI, especially in younger postmenopausal patients.
Limitations of study: our rate of fractures is less than other reports. All AE were reported by participating centers.
The observed difference in BCFI in B-35 was almost entirely attributable to the younger postmenopausal patients. There is no evident biologic explanation for this difference. Study drug compliance, body mass index, tumor characteristics, and deaths from other causes were reviewed and not found to contribute to this finding.
There was no significant decrease in ipsilateral cancer, either invasive or noninvasive. This might be a result of radiotherapy to the ipsilateral breast, but overall recurrence rates for both were low.
The difference in the treatments did not become apparent until after 5 years of followup, which may be explained by the low rate of breast cancer events in both groups.
The AEs common to these drugs were similar to previous experiences. The incidence of thromboembolic events with T was 2.7% vs 0.8% for A.
There were 17 cases of uterine cancer reported in the T group and eight in the A group, (RR, 0.47; 95% CI, 0.18–1.15) similar to B-24 results, although B-24 also included premenopausal women. Arthralgias grade 2 or higher were reported in 23–32% of patients, with higher severity among the A treatment group. Myalgias were less frequently reported but were more severe among the A treatment group. These findings are comparable to AEs for these symptoms reported in previous studies.6 However, observer rating of these symptoms and others are known to underestimate the severity experienced by patients. One of the strengths of our study is the systematic patient self assessment with standardized questionnaires, as is reported in our companion manuscript. (SABC abstract no.S6-04; Lancet, submitted) Specifically, musculoskeletal pain severity was significantly greater among the A-treated patients (p=.001)
We found a non significant excess of fractures in the anastrazole group. Osteoporosis was not an eligibility issue. One possible limitation of study is our unexplained report of fewer fractures than in other reports. All fractures were reported by participating institutions and were defined as fractures of hip, spine, and wrist, which may cause under-reporting. However, considering the overall pattern of serious AEs and uterine cancer, Anastrazole appears to have a preferable safety profile for serious events.
A was more effective than T in postmenopausal women <60 years and would be the preferred treatment for those patients. For those ≥60 years, there was no evidence of a significant difference in outcomes. Thus, decisions for adjuvant therapy in this age group should be based on the safety profile of each drug. T should be avoided in women with a history of DVT or uterine problems. Women with osteoporosis would probably be more safely treated with T. Based on our findings, women who develop AEs or uncomfortable symptoms on one drug would have the option of treatment with the other agent without compromising efficacy.
One of the most notable findings in our trial was the reduction of contralateral IBC with Anastrazole. Previous studies showed that women with a history of DCIS were at significant risk for future IBC; DCIS has long been recognized as a precursor of invasive cancer.9,10 suggesting a role for anastrazole in breast cancer prevention. Examining these findings against the background of the Breast Cancer Prevention Trial (P-1)11 supports this view. If DCIS is a risk factor for future breast cancer, then intervention for the prevention of future IBC may be the most useful aspect of treatment with Anastrazole.
In the P-1 study T, compared to placebo, reduced the incidence of breast cancer by 48%. In B-35, Anastraszole further reduced the rate of contralateral invasive cancer significantly compared to Tamoxifen. However, B-35 was not a trial of cancer prevention in high-risk women. Although DCIS is one risk factor for the future development of breast cancer, a group of women with high risk would also include those with strong family history or those with previous biopsies showing atypical hyperplasia. These findings cannot be extrapolated to all high-risk patients based on cross-protocol comparisons, but in the absence of a direct trial, the choice could reasonably be made on an individualized basis. If DCIS is a risk factor for future breast cancer, then intervention for the prevention of future IBC may be the most useful aspect of treatment of DCIS with Anastrazole.
Further support comes from IBIS-II12, a trial comparing A to placebo for cancer prevention in high-risk women. Participants treated with A were 53% less likely to develop breast cancer than those receiving placebo (40 versus 85 cases). IBIS-II-DCIS, comparing A to T in women with DCIS, is expected to be reported shortly.
The B-35 results confirm the excellent overall prognosis for women treated for DCIS. There were 8 breast cancer deaths in the T group and 5 in the A group. A recent SEER data analysis13 of patients with DCIS concluded that radiotherapy did not improve OS. However, a local recurrence of invasive cancer in either breast carries an increased risk of death3 and is, at the very least, a serious event, usually leading to further breast and possibly axillary surgery and additional adjuvant systemic therapies.
The SEER data analysis did not include a comparison of patients who received hormone therapy, which was shown in Protocol B-24 to provide a significant benefit. With modern therapies, the outcomes for patients with DCIS have improved. In protocol B-17 there was a 25% incidence of any breast cancer event in the lumpectomy-alone arm.7 For patients treated with WBI, this fell to 13%, and for those receiving T in B-24 it was 8%.3,7 This is a 77% reduction in all events with the combined treatment. With B-35 we report a further decrease in events, particularly contralateral IBC, in postmenopausal women <60 years. With the main concern in treating DCIS being the prevention of invasive cancer with the possibility of metastases and death, adjuvant hormone treatment following DCIS can be seen as an important prevention issue. The recent SEER data analysis indicates that younger women and African American women with DCIS are at higher risk for invasive cancer. Future research should focus on identifying molecular or genetic subgroups that would or would not benefit from adjuvant treatments.
Treatment of DCIS patients with A provides a significant decrease in breast cancer events and the unwanted treatments that would follow. B-35 documents the potential AEs and toxicities so that women and their doctors can make appropriate choices for treatment of DCIS. Our companion report (SABC abstract No. S6-04; Lancet, submitted) on patient-reported quality of life and symptoms provides a more detailed evaluation of the comparative symptom profiles of these two therapies, and highlights differences in symptom patterns by age group and treatment, allowing integration of outcome results and adverse effects analysis to help decision making. Currently, the use of anastrozole provides a distinct benefit for the treatment for DCIS and should be offered as an option when appropriate.
Supplementary Material
Research in context.
In addition to the review of published clinical trials evaluating tamoxifen in breast cancer and DCIS, we conducted ongoing literature surveys annually throughout the term of this study. These results were interpreted and evaluated by the senior authors and incorporated in our annual reports
Systematic review
A thorough review of relevant studies of adjuvant endocrine therapy for breast cancer and DCIS was conducted. B-35 was based on previous NSABP studies B-06, B-17, and B-24. This sequence of studies showed that lumpectomy and radiotherapy was appropriate for the management of DCIS, and that adjuvant tamoxifen improved outcomes.
Added value
This is the first prospective randomized trial demonstrating additional efficacy for treating DCIS with anastrozole compared to tamoxifen. Comprehensive quality of life analyses were also performed because of the high likelihood that participants in both arms of the study would do well and that evaluating adverse effects would be important.
Interpretation of totality of evidence
Anastrozole is more effective than tamoxifen in reducing the incidence of invasive cancer. Severe adverse reactions were less frequent with anastrozole. Both drugs have now been shown to be effective and women with DCIS who desire adjuvant therapy now have the choice of medication. This decision can be aided by the integration of the efficacy and adverse effect information.
Acknowledgments
This clinical trial was conducted through the support of AstraZeneca Pharmaceuticals LP. The study sponsor had no role in neither the design nor conduct of the study; collection, management, analysis, nor interpretation of the data; preparation, review, nor approval of the manuscript; nor decision to submit the manuscript for publication. Drs. Margolese and Cecchini had full access to all of the data in the study and take responsibility for the integrity of the data, the accuracy of the data analysis, and take responsibility for the work as a whole, from inception to published article.
Support: NCI U10CA-180868, 180822, 189867, 196067, 114732; AstraZeneca Pharmaceuticals LP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov: NCT00053898
Declarations of Potential Conflicts of Interest
The following authors declare the following potential conflict(s) of interest:
MEC declares research support from Novartis, fees Biotheranostics’ Advisory Board, and fees from Roche and Abbvie outside of submitted work.
All other authors declare no conflicts of interest.
Author contributions
RM: Contributed to literature search; study design; data interpretation; writing; Final approval.
RSC: Contributed to the conception/design of the work; acquisition, analysis, interpretation of data; drafting and revising it critically for important intellectual content; final approval of the version to be published; agree to be accountable for all aspects of the work.
TBJ: Contributed to conception/design; or acquisition, analysis, interpretation of data; revision of manuscript; final approval of version to be published; agree to be accountable for all aspects of the work.
PAG: Contributed to study design, data collection, data interpretation, writing.
JPC: Contributed to study design, data collection, data interpretation, writing of manuscript.
LAV: Contributed to acquisition of data; drafting/revising work; final approval; agree to be accountable for aspects of work.
KSA: Contributed to study design on behalf of SWOG Breast Committee; patient accrual; review and approval of manuscript.
PWW: Contributed to
MEC: Substantial contributions to the acquisition of data; reviewed draft of manuscript; approved version to be published; agree to be accountable for all aspects of the work.
AMB: Contributed to acquisition and analysis of data and patients; drafting of work; final approval of manuscript.
HMG: Contributed to study design, data analysis and interpretation, patient accrual, and editing of manuscript.
GSS: Contributed to acquisition and analysis of data and patients, drafting of work, and final approval of manuscript.
JOH: Contributed to design; reviewed intellectual content; final approval of version to be published; agree to be accountable for all aspects of work.
LF: Contributed to acquisition, analysis, and interpretation of work; critical revision of draft of work including intellectual; approval of the final version; agree to be accountable for all aspects of the work.
KS: Contributed to patient accrual; data collection; local study research oversight.
TFW: Participated in the Breast Cancer Committee and Prevention Trial implementation; promoted patient enrollment at the Christiana Care CCOP as local Principal Investigator; contributed the majority of patients from the CC CCOP for this trial and the preceding trial that was the basis for the control arm in the study; reviewed and approved the final manuscript for publication; accepts accountability for accuracy and integrity of the work.
TES: Helped with acquisition of data with patient enrollment; contributed to review of work; final approval of publishable version; agree with data interpretation, conclusions, and recommendations.
EPM: Contributed to study design, data collection, data interpretation, writing final manuscript, approval.
NW: Contributed to the conception/design of the work; revision; final approval of the version to be published; Agree to be accountable for all aspects of the work.
References
- 1.Fisher B, Costantino J, Redmond C, Fisher E, Margolese R, Dimitrov N, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328(22):1581–1586. doi: 10.1056/NEJM199306033282201. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Dignam J, Wolmark N, Mamounas E, Costantino J, Poller W, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16(2):441–452. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Dignam J, Wolmark N, Wickerham DL, Fisher ER, Mamounas E, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 4.Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103(6):478–488. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nabholtz JM, Buzdar A, Pollak M, Harwin W, Burton G, Mangalik A, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol. 2000;18(22):3758–3767. doi: 10.1200/JCO.2000.18.22.3758. [DOI] [PubMed] [Google Scholar]
- 6.Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, et al. ATAC Trialists’ Group Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: First results of the ATAC randomised trial. Lancet. 2002;359(9324):2131–2139. doi: 10.1016/s0140-6736(02)09088-8. Erratum in: Lancet 2002 Nov 9;360(9344):1520. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Costantino J, Redmond C, Fisher E, Margolese R, Dimitrov N, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328(22):1581–1586. doi: 10.1056/NEJM199306033282201. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Dignam J, Wolmark N, Mamounas E, Costantino J, Poller W, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16(2):441–452. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 9.Page DL, Dupont WD, Rogers LW, Jensen RA, Schuyler PA. Continued local recurrence of carcinoma 15–25 years after a diagnosis of low grade ductal carcinoma in situ of the breast treated only by biopsy. Cancer. 1995;76(7):1197–200. doi: 10.1002/1097-0142(19951001)76:7<1197::aid-cncr2820760715>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Eusebi V, Feudale E, Foschini MP, Micheli A, Conti A, Riva C, et al. Long-term follow-up of in situ carcinoma of the breast. Semin Diagn Pathol. 1994;11(3):223–235. [PubMed] [Google Scholar]
- 11.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 12.Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al. IBIS-II investigators Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383(9922):1041–8. doi: 10.1016/S0140-6736(13)62292-8. [DOI] [PubMed] [Google Scholar]
- 13.Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1(7):888–896. doi: 10.1001/jamaoncol.2015.2510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


