Abstract
Introduction
Brain injury is the leading cause of morbidity and death following pediatric cardiac arrest. Serum biomarkers of brain injury may assist in outcome prognostication. The objectives of this study were to evaluate the properties of serum ubiquitin carboxyl-terminal esterase-L1 (UCH-L1) and glial fibrillary acidic protein (GFAP) to classify outcome in pediatric cardiac arrest.
Methods
Single center prospective study. Serum biomarkers were measured at 2 time points during the initial 72 h in children after cardiac arrest (n=19) and once in healthy children (controls, n=43). We recorded demographics and details of the cardiac arrest and resuscitation. We determined the associations between serum biomarker concentrations and Pediatric Cerebral Performance Category (PCPC) at 6 months (favorable (PCPC 1–3) or unfavorable (PCPC 4–6)).
Results
The initial assessment (time point 1) occurred at a median (IQR) of 10.5 (5.5–17.0) h and the second assessment (time point 2) at 59.0 (54.5–65.0) h post-cardiac arrest. Serum UCH-L1 was higher among children following cardiac arrest than among controls at both time points (p<0.05). Serum GFAP in subjects with unfavorable outcome was higher at time point 2 than in controls (p<0.05). Serum UCH-L1 at time point 1 (AUC 0.782) and both UCH-L1 and GFAP at time point 2 had good classification accuracy for outcome (AUC 0.822 and 0.796), p<0.05 for all.
Conclusion
Preliminary data suggest that serum UCH-L1 and GFAP may be of use to prognosticate outcome after pediatric cardiac arrest at clinically-relevant time points and should be validated prospectively.
Keywords: Biomarker, pediatric, brain injury, outcomes, cardiac arrest
Introduction
Neurologic injury is the leading cause of death in children with cardiac arrest1,2. Children surviving cardiac arrest are at increased risk of neurologic morbidity, leading to emotional, cognitive, and functional disabilities3. A reliable test that could inform medical decision-making and/or provide families with meaningful information regarding prognosis would be extremely valuable. Challenges to early prognostication include the ambiguity of the early neurologic examination due to developmental stage, provision of analgesics, sedatives, and neuromuscular blocking agents, safety concerns using advanced technology to assess physiologically unstable patients (i.e., magnetic resonance imaging (MRI)), and that clinically, final outcome may not be conclusive for months or years4–6. Although clinical variables have been associated with outcome after pediatric cardiac arrest, an individual child’s risk of neurologic disability is not yet accurately ascertained early after resuscitation.
Serum biomarkers of brain injury can objectively estimate severity of brain injury. Data can inform specific disease pathophysiology and potentially identify new therapeutic targets, examine response to therapy, and assist in outcome prognostication7–9. There are accumulating reports that concentrations of brain-specific biomarkers ubiquitin carboxyl-terminal esterase L1 (UCH-L1), a protein and component of the ubiquitin–proteasome system in neurons and glial fibrillary acidic protein (GFAP), a type III neurofilament protein present in astrocytes can be used to accurately classify outcome following an acute brain insult, but there are no data in pediatric cardiac arrest10–17.
In this single center exploratory study, we examined serum concentrations of UCH-L1 and GFAP in children with cardiac arrest at two time points after return of spontaneous circulation (ROSC) and in a pediatric control group without cardiac arrest and tested their ability to classify favorable vs. unfavorable outcome at 6 months. We hypothesized that children with unfavorable outcomes would have increased serum biomarker concentrations versus children with favorable outcome.
Methods
Design and Setting
The study was approved by the University of Pittsburgh Institutional Review Board and informed consent was obtained from the subject’s parent or guardian. Between November 2009 and September 2011, 19 subjects with cardiac arrest were prospectively enrolled in an RCT (NCT00797680) at the Children’s Hospital of Pittsburgh of UPMC for which results have not yet become unblinded (therefore data from both groups were pooled). Standard post-resuscitation care was provided to all children at the discretion of the treating clinicians. Banked serum samples from 43 healthy children without brain insults or other acute illness who received outpatient phlebotomy for routine laboratory testing were used as control group.
Inclusion and Exclusion Criteria
We studied children between the ages 1 week and 17 years who were admitted to the ICU with ROSC after in- or out-of-hospital cardiac arrest. Cardiac arrest was defined as receipt of chest compressions for pulselessness by a healthcare worker. Subjects were included if they had an indwelling arterial or venous catheter for phlebotomy. Subjects were excluded if they had a do not resuscitate status, were pregnant, any contraindication for MRI, had other simultaneous acute brain disease (i.e., trauma), or were undergoing brain death evaluation. Subjects were included if Glasgow coma scale score ≤ 8 after ROSC and had therapeutic hypothermia initiated by their ICU attending. Subjects were also excluded from the RCT if they had active hemorrhage or a pre-existing anti-coagulation defect. Post-resuscitation care guidelines used at our institution are published elsewhere18.
Serum Biomarkers
Three milliliters of blood were collected twice daily (days 1–4) and once on day 7 after ROSC for the parent study. Samples were centrifuged, aliquoted, frozen at −70°C, and batched for analysis. Serum samples for this analysis were taken closest to but not after 24 and 72 hours post-ROSC. Serum UCH-L1 and GFAP were measured in the banked serum samples in duplicate using proprietary ELISAs as previously described (Banyan Biomarkers, Florida, USA)19. An experienced technician blinded to subject treatment and outcome performed all biomarker measurements. Clinical team members were unaware of the biomarker results. The limits of detection were 0.05 ng/ml for UCH-L1 and 0.1 ng/ml for GFAP.
Data Collection
Data were collected from medical charts using the Utstein template for cardiac arrest, including subject demographics, details about the cardiac arrest and resuscitation, and post-resuscitation care20. We documented the subject’s temperature at the time of the blood draw.
Outcome Measures
Subjects were followed until 6 months post-cardiac arrest. The primary objective of this study was to determine the accuracy of serum brain biomarker concentrations to predict favorable (Pediatric Cerebral Performance Category (PCPC) score 1–3) or unfavorable (PCPC 4–6) outcome (including death [PCPC=6]) 21. Pre-arrest PCPC was assigned based on medical records, and 6 month outcomes were obtained either the phone or in person.
Data analysis
Data are presented as median (interquartile range [IQR]) or mean + standard deviation (SD), as appropriate. The data were analyzed for outcome group differences with Fisher’s exact tests for categorical variables. Median serum biomarkers were represented graphically by outcome group. Serum biomarker levels were correlated with each other and with subject age and temperature using the Spearman rho test. The Wilcoxon rank sum was used to compare serum biomarker concentration and outcome. Receiver operating characteristic (ROC) analyses were used to evaluate the sensitivity and specificity of the biomarkers. There were no missing biomarker data points. All tests were two-sided and a p-value of < 0.05 was considered to be significant. Data analysis was performed using SPSS version 18.
Results
Study subject and biomarker characteristics
We studied 19 subjects with cardiac arrest and 43 control subjects. Cardiac arrest subjects were older than control subjects (mean ± SD 6.1 ± 6.7 vs. 3.7 ± 3.6 years) (Table 1). Most cardiac arrest events were due to asphyxia and most subjects presented with pulseless electrical activity or asystole as the initial cardiac rhythm. Ten (53%) of the cardiac arrest subjects had unfavorable outcome, including 5 who died. Subjects with unfavorable outcome were more likely to have had unwitnessed events than subjects with favorable outcome (p=0.001).
Table 1.
Subject demographics, cardiac arrest details, and outcomes at 6 months.
| Mean±SD or n (%) |
Controls (n=43) |
Cardiac Arrest Overall (n=19) |
Cardiac Arrest Favorable (n=9) |
Cardiac Arrest Unfavorable (n=10) |
p-value Favorable vs. Unfavorable |
|---|---|---|---|---|---|
| Age, y | 3.7 ± 3.6 | 6.1 ± 6.7 | 7.0 ±6.8 | 5.4 ± 6.8 | 0.905 |
|
Sex Female Male |
18 (41.9) 25 (58.1) |
6 (31.6) 13 (68.4) |
3 (33.3) 6 (66.7) |
3 (30.0) 7 (70.0) |
0.630 |
|
Race White Nonwhite |
25 (58.1) 18 (41.9) |
12 (63.2) 7 (36.8) |
5 (55.6) 4 (44.4) |
7 (70.0) 3 (30.0) |
0.430 |
|
Duration of CPR to ROSC (min) |
n/a | 16.5 ± 12.4 | 12.0 ± 6.4 | 20.0 ± 16.1 | 0.489 |
|
Doses of Epinephrine |
n/a | 3.0 ± 1.9 | 3.0 ± 2.4 | 2.0 ± 1.3 | 0.497 |
| Hospital LOS, d | n/a | 23.0 ± 15.7 | 16.0 ± 8.6 | 28.0 ± 20.0 | 0.549 |
| ICU LOS, d | n/a | 11.0 ± 13.9 | 11.0 ± 8.2 | 24.0 ± 16.2 | 0.243 |
|
First Rhythm PEA Asystole Sinus VT/VF Other |
n/a |
9 (47.4) 8 (41.8) 0 (0.0) 1 (5.3) 1 (5.3) |
5 (55.6) 2 (22.2) 0 (0.0) 1 (11.1) 1 (11.1) |
4 (40.0) 6 (60.0) 0 (0.0) 0 (0.0) 0 (0.0) |
0.057 |
|
Etiology Asphyxia Cardiac |
n/a |
16 (84.2) 3 (15.8) |
6 (66.7) 3 (33.3) |
10 (100.0) 0 (0.0) |
0.087 |
| Defibrillated | n/a | 3 (15.8) | 6 (66.7) | 0 (0.0) | 0.087 |
|
Witnessed event |
n/a | 9 (47.4) | 8 (88.9) | 1 (10.0) | 0.001 |
| Bystander CPR | n/a | 14 (73.7) | 6 (66.7) | 8 (80.0) | 0.444 |
| Died | n/a | 5 (26.3) | 0 (0.0) | 5 (50.0) | n/a |
SD, standard deviation; ROSC, return of spontaneous circulation; PEA, pulseless electrical activity; VT/VF, ventricular tachycardia or fibrillation; CPR, cardiopulmonary resuscitation; LOS, length of stay; ICU, intensive care unit; n/a, not applicable.
Serum was taken at median (IQR) 10.5 (5.5–17.0) hours post-ROSC for time point 1 and at 59.0 (54.5–65.0) hours post-ROSC for time point 2. Serum UCH-L1 and GFAP concentrations were not correlated with each other (ρ=0.004, p=0.980). Neither biomarker was associated with subject age (p>0.05). Neither biomarker was associated with age (UCH-L1: ρ = −0.23, p = 0.22; GFAP: ρ = −0.30, p = 0.06) or temperature (UCH-L1: ρ = 0.25, p = 0.30; GFAP: ρ = −0.04, p = 0.86) at the time of biomarker assessment.
Serum UCH-L1 performance
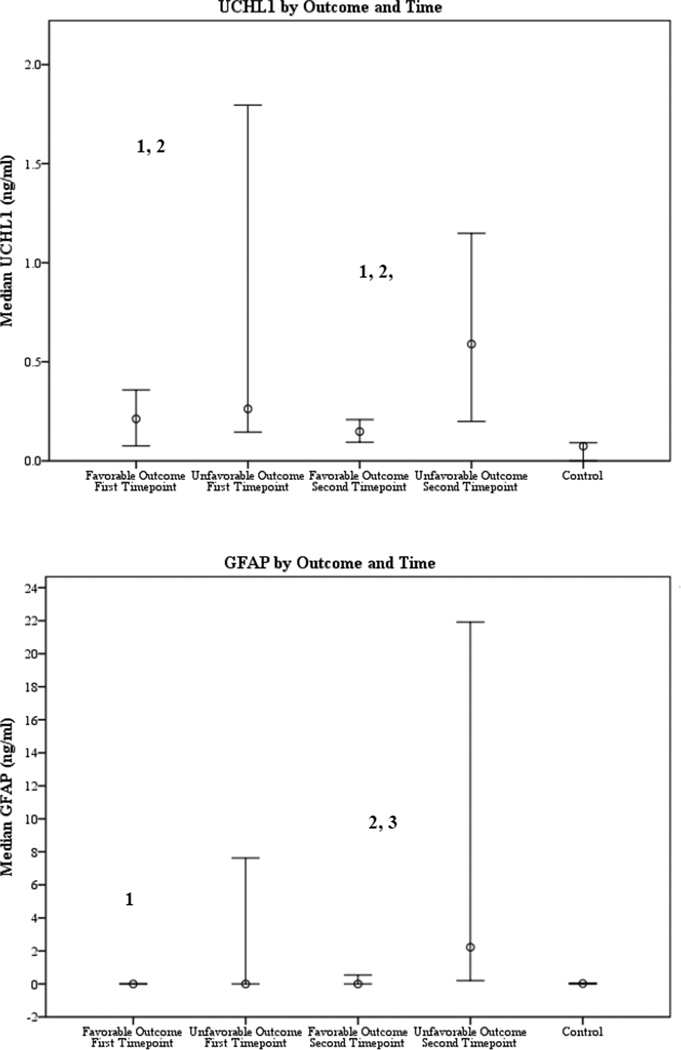
Serum UCH-L1 concentration was increased in subjects with cardiac arrest with favorable and unfavorable outcome versus controls at both time points (both p < 0.05) (Table 2 and Figure 1a).
Table 2.
Serum biomarker concentrations by outcome group and time point.
| Median [IQR], ng/ml |
Controls (n=43) |
Cardiac arrest Overall (n=19) |
Cardiac arrest Favorable (n=9) |
Cardiac arrest Unfavorable (n=10) |
p-values: Control vs. Fav Control vs. Unfav Fav vs. Unfav |
|---|---|---|---|---|---|
|
Time point 1 UCH-L1 |
0.07 [0.00,0.10] | 0.21 [0.14,0.38] | 0.21 [0.11,0.23] | 0.26 [0.15,0.85] |
0.002 <0.001 0.315 |
|
Time point 2 UCH-L1 |
0.21[0.13,0.94] | 0.15 [0.12,0.18] | 0.59 [0.27,1.12] |
0.001 <0.001 0.006 |
|
|
Time point 1 GFAP |
0.02 [0.00,0.05] | 0.00 [0.00,0.00] | 0.00 [0.00,0.00] | 0.00 [0.00,2.13] |
0.0161 0.632 0.156 |
|
Time point 2 GFAP |
0.54 [0.00,3.11] | 0.00 [0.00,0.49] | 2.23 [0.73,16.52] | 0.515 <0.001 0.001 |
UCH-L1, ubiquitin carboxyl-terminal esterase L1; GFAP, glial fibrillary acidic protein; IQR, interquartile range; Fav, favorable outcome 6 months after cardiac arrest; Unfav, unfavorable outcome 6 months after cardiac arrest
Serum GFAP concentrations for subjects with cardiac arrest and favorable outcome and controls had levels below detection for the assay
Figure 1.
1a and 1b. Median serum biomarker concentrations (ng/ml) by outcome and time point. UCH-L1, ubiquitin carboxyl-terminal esterase L1; GFAP, glial fibrillary acidic protein
1 Control vs. cardiac arrest with favorable outcome, p<0.05
2 Control vs. cardiac arrest with unfavorable outcome, p<0.05
3 Cardiac arrest with favorable vs. unfavorable outcome, p<0.05
Among subjects with cardiac arrest, serum UCH-L1 was not different among outcome groups at time point 1 (0.26 [0.15, 0.85] vs. 0.21 [0.11, 0.23] ng/ml, p=0.315). Serum UCH-L1 was increased in subjects with unfavorable versus favorable outcome at time point 2 (0.59 [0.27, 1.12] vs. 0.15 [0.12, 0.18] ng/ml, p=0.006).
Serum GFAP performance
Levels of serum GFAP at time point 1 were increased in controls vs. cardiac arrest subjects with favorable outcome (0.02 [0.00, 0.05] vs. 0.00 [0.00, 0.00] ng/ml, p=0.016) but concentrations were below the limits of detection for both groups (Table 2, Figure 1b). Serum GFAP in subjects with unfavorable outcome did not differ from controls at time point 1 (0.00 [0.00, 2.13] ng/ml, p=0.632) but was increased vs. controls at time point 2 (2.23 [0.73, 16.52] ng/ml, p < 0.001).
Serum GFAP was not different between outcome groups at time point 1, but subjects with unfavorable outcome had increased serum GFAP versus subjects with favorable outcome at time point 2 (2.23 [0.73, 16.52] vs. 0.00 [0.00, 0.49] ng/ml, p = 0.001).
Receiver operating characteristics of serum UCH-L1 and GFAP to prognosticate outcome
The area under the curve (AUC) [95% CI] for serum UCH-L1 to classify 6 month outcome at time points 1 and 2 were 0.782 [0.638–0.925] and 0.822 [0.702–0.942], respectively (p<0.05 for both) (Table 3). The AUC for serum GFAP was 0.408 [0.238–0.578] (p>0.05) and 0.796 [0.642–0.950] at time points 1 and 2 (p<0.05).
Table 3.
Receiver operator characteristic data for serum UCH-L1 and GFAP by time point.
| Area | p- value |
Asymptotic 95% Confidence Interval |
||
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
|
Time point 1 UCH-L1 |
0.782 | 0.001 | .638 | .925 |
|
Time point 1 GFAP |
0.408 | 0.256 | .238 | .578 |
|
Time point 2 UCH-L1 |
0.822 | <0.001 | .702 | .942 |
|
Time point 2 GFAP |
0.796 | <0.001 | .642 | .950 |
UCH-L1, ubiquitin carboxyl-terminal esterase L1; GFAP, glial fibrillary acidic protein
Discussion
This is the first study assessing the use of the neuron biomarker UCH-L1 and the astrocyte biomarker GFAP in children after cardiac arrest. We found that serum UCH-L1 in the first 3 days post-cardiac arrest distinguished children having cardiac arrest from healthy children while serum GFAP discerned cases versus controls only in children with unfavorable outcomes at the second time point. Similar to serum neuron specific enolase (NSE)’s time trajectory after pediatric cardiac arrest, both serum UCH-L1 and GFAP significantly discerned favorable versus unfavorable outcome groups at the later sampling time point.
Prior small clinical studies show that UCH-L1 and GFAP have promise in assessing the severity of illness and classifying outcome across various age groups and types of brain insults. In one of the earliest clinical studies to assess GFAP concentrations after birth asphyxia, CSF concentrations of GFAP as well as astrocyte marker S100b and neuronal marker NSE distinguished infants with hypoxic ischemic encephalopathy (HIE) (n=22) from healthy infants (n=8) and levels correlated significantly with outcome at one year22. In a study matching neonates with and without birth asphyxia, serum GFAP levels in the first four days were increased in neonates with HIE versus those without HIE and levels also correlated with brain MRI lesions consistent with HIE13. Similarly, in another pilot study of infants with HIE, umbilical cord GFAP and UCH-L1 concentrations discerned between babies with mild, moderate, and severe HIE23. In this study, serum GFAP levels peaked between 78–96 hours while UCH-L1 was highest at birth in cord blood. Only serum GFAP significantly predicted neurological outcome in this study. Next, Douglas-Escobar, et al found that early (first 24 h) serum UCH-L1 levels were increased in infants with moderate-severe HIE compared to healthy infants and that levels correlated with the 10 minute Apgar score, a component of the Sarnat score that categorizes severity of encephalopathy, lending support to its use as stratification tool for prospective research studies16. There are no early correlates to the Sarnat score that predict severity of encephalopathy for cardiac arrest outside of the newborn period, but a panel of serum biomarkers and clinical variables known to be associated with outcome would be useful to clinicians and researchers5,9.
Serum GFAP has been investigated for prognostication qualities in adults with cardiac arrest. Serum GFAP distinguished subjects with favorable vs. unfavorable outcome in 44 adults with cardiac arrest, with best prediction accuracy occurring at 24 hours in adult kept normothermic and at 48 hours in adults who received hypothermia post-cardiac arrest14. In another pilot study (n=31) of adults with cardiac arrest, serum S100b had superior prognostication accuracy versus NSE and GFAP24. Serum biomarker trajectories in this study were similar to our findings in pediatric cardiac arrest in that serum S100b peaked early (24 h) and NSE and GFAP performed better at later time points (48–72 h)18. Of note, Mortberg et al did not report the number of patient samples available at later time points, in which a smaller sample size due to drop out from early mortality may have contributed to its weaker performance since there was a trend of higher GFAP levels in subjects with worse outcomes. Similarly, in Hayashida et al, serum GFAP within first 24 hours of cardiac arrest was not predictive of outcome, but the sample size was small in addition to the relatively early time sampling time25. A larger, more recent study of 125 adults with cardiac arrest showed that while serum GFAP predicted outcomes, it was not superior to NSE or S100b, and when placed in an inclusive model, addition of GFAP did not improve predictive accuracy26. It should be noted that although neurologic injury is the leading cause of death after cardiac arrest, subjects also succumb from cardiovascular failure or multiorgan dysfunction, so a prognostication panel may benefit from inclusion of other organ biomarkers. In summary, serum GFAP on days 2–3 shows promise across various age groups with hypoxic-ischemic insult to prognosticate outcome. To our knowledge, there are no studies of serum UCH-L1 in adults with cardiac arrest.
For comparison, serum and cerebrospinal fluid (CSF) GFAP and UCH-L1 prognostication accuracy has been examined subjects with traumatic brain injury (TBI). In general, peak biomarker concentrations occur earlier after TBI versus hypoxia-ischemia. Serum and CSF UCH-L1 levels were predictive of outcome and concentrations highest at 6 hours post-TBI in adults27,28. Serum GFAP levels in adults with severe TBI were associated with severity of illness as measured by Glasgow Coma Scale score, and increased levels were associated with the presence of brain CT lesions, mortality, and the need for neurosurgical intervention15,19. Further illustrating differences between brain pathophysiology and biomarker trajectory, serum UCH-L1 and GFAP peaked earlier after pediatric TBI patients versus cardiac arrest29. This suggests that the timing, ROC cutoffs, and/or specific biomarkers used may differ between hypoxic-ischemic and traumatic brain insults.
Serum UCH-L1 and GFAP performance characteristics at the two time points assessed in this study were similar to those seen for serum NSE in children with cardiac arrest in prior reports by our group and others, with best performance occurring between days 2 and 3 post-ROSC8,18,30, paralleling the time period of early and delayed neuronal death and influx of and activation of microglia following hypoxic-ischemic insult31–34. Interestingly, serum GFAP performed best on day 2–3 as compared to another astroglial protein S100b serum level, which peaks on day 1–2. These differences could relate to a number of factors such as 1) the initial concentration of each marker present in the tissue, 2) differences in biomarker location in the astroglia, 3) differences in biomarker half-life, 4) differential contributions of astrocyte death vs biomarker release, 4) a possible contribution of GFAP induction in injured glia to the ultimate levels observed, and 5) differential effects of tissue perfusion and/or glymphatic clearance on biomarker levels35–37.
Serum GFAP was undetectable in control subjects and cardiac arrest subjects with favorable outcome while increased in subjects with unfavorable outcome, demonstrating potential utility as an early biomarker for prognostication or for severity of illness stratification; its use as a theragnostic tool is unknown. A clinical research study is underway to validate a point of care test for serum UCH-L1 and GFAP to predict brain CT lesions in adult TBI (NCT01426919). Relating to our current report, positive findings would strongly support evaluation in a panel with clinical variables and tests for use in prognostication in children or adults after cardiac arrest.
Study limitations
This preliminary study used banked serum from a parent study. We did not assess the performance of the all time points collected, which could better inform on potential utility for either theragnostic use or to stratify subjects for clinical trials, but instead chose clinically relevant time points for practical and financial constraints. PCPC was assigned in a non-blinded fashion by the study PI and is a gross measure of function. Due to the limited sample size, were unable to compare the ability of serum biomarkers to classify outcome compared with clinical variables such as blood lactate or duration of resuscitation. Our study does not include data on long-term outcomes or detailed cognitive function. This study combined treatment groups that were not yet unblinded; therefore any treatment effect on biomarker levels cannot be excluded.
Conclusion
The data in this exploratory study suggest that serum UCH-L1 and GFAP measured at a clinically-relevant time point show promise in prognosticating outcome after pediatric cardiac arrest. Validation in a large, prospective trial and correlation with other clinical variables associated with outcome after pediatric cardiac arrest is needed.
Acknowledgments
Special thanks to Michelle Dragotta, Christine Kyper, and Alan Abraham for assistance in data acquisition. We are grateful to the staff, nurses, and physicians of the ICU for their efforts in subject recruitment and provision of excellent clinical care. Thank you to the staff and research scientists at Banyan Biomarkers, Inc. for providing excellent technical and financial assistance to this study.
Dr. Fink reports grants from National Institutes of Health, Laerdal Foundation, and biomarker assay measurement Banyan Biomarkers, Inc., during the conduct of the study. Dr. Mondello reports personal fees from Banyan Biomarkers, Inc., during the conduct of the study. Dr. Hayes reports grants from U.S. Department of Defense during the conduct of the study, grants from W81XWH-06-1-0517 outside the submitted work, and a patent issued.
Funding Sources
NICHD K12 HD047349 (E.L.F.), NINDS K23 NS065132 (E.L.F.), the Laerdal Foundation (E.L.F.), and Banyan Biomarkers, Inc. (E.L.F.). The project described was supported by the National Institutes of Health through Grant Numbers UL1 RR024153 and UL1TR000005. Banyan Biomarkers, Inc. performed all biomarker measurements without charge.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The other co-authors have nothing to disclose.
References
- 1.Moler FW, Meert K, Donaldson AE, et al. In-hospital versus out-of-hospital pediatric cardiac arrest: a multicenter cohort study. Crit Care Med. 2009;37:2259–2267. doi: 10.1097/CCM.0b013e3181a00a6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young KD, Gausche-Hill M, McClung CD, Lewis RJ. A prospective, population-based study of the epidemiology and outcome of out-of-hospital pediatric cardiopulmonary arrest. Pediatrics. 2004;114:157–164. doi: 10.1542/peds.114.1.157. [DOI] [PubMed] [Google Scholar]
- 3.Bedell GM. Functional outcomes of school-age children with acquired brain injuries at discharge from inpatient rehabilitation. Brain Inj. 2008;22:313–324. doi: 10.1080/02699050801978948. [DOI] [PubMed] [Google Scholar]
- 4.Lieh-Lai MW, Theodorou AA, Sarnaik AP, Meert KL, Moylan PM, Canady AI. Limitations of the Glasgow Coma Scale in predicting outcome in children with traumatic brain injury. J Pediatr. 1992;120:195–199. doi: 10.1016/s0022-3476(05)80426-3. [DOI] [PubMed] [Google Scholar]
- 5.Abend NS, Licht DJ. Predicting outcome in children with hypoxic ischemic encephalopathy. Pediatr Crit Care Med. 2008;9:32–39. doi: 10.1097/01.PCC.0000288714.61037.56. [DOI] [PubMed] [Google Scholar]
- 6.Fugate JE, Wijdicks EF, Mandrekar J, et al. Predictors of neurologic outcome in hypothermia after cardiac arrest. Ann Neurol. 2010;68:907–914. doi: 10.1002/ana.22133. [DOI] [PubMed] [Google Scholar]
- 7.Tiainen M, Roine RO, Pettila V, Takkunen O. Serum neuron-specific enolase and S-100B protein in cardiac arrest patients treated with hypothermia. Stroke. 2003;34:2881–2286. doi: 10.1161/01.STR.0000103320.90706.35. [DOI] [PubMed] [Google Scholar]
- 8.Berger RP, Adelson PD, Richichi R, Kochanek PM. Serum biomarkers after traumatic and hypoxemic brain injuries: insight into the biochemical response of the pediatric brain to inflicted brain injury. Dev Neurosci. 2006;28:327–335. doi: 10.1159/000094158. [DOI] [PubMed] [Google Scholar]
- 9.Czeiter E, Mondello S, Kovacs N, et al. Brain injury biomarkers may improve the predictive power of the IMPACT outcome calculator. J Neurotrauma. 2012;29:1770–1778. doi: 10.1089/neu.2011.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eng LF. Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J Neuroimmunol. 1985;8:203–214. doi: 10.1016/s0165-5728(85)80063-1. [DOI] [PubMed] [Google Scholar]
- 11.Day IN, Thompson RJ. UCHL1 (PGP 9.5): neuronal biomarker and ubiquitin system protein. Prog Neurobiol. 2010;90:327–362. doi: 10.1016/j.pneurobio.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Berger RP, Hayes RL, Richichi R, Beers SR, Wang KK. Serum concentrations of ubiquitin C-terminal hydrolase-L1 and alphaII-spectrin breakdown product 145 kDa correlate with outcome after pediatric TBI. J Neurotrauma. 2012;29:162–167. doi: 10.1089/neu.2011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ennen CS, Huisman TA, Savage WJ, et al. Glial fibrillary acidic protein as a biomarker for neonatal hypoxic-ischemic encephalopathy treated with whole-body cooling. Am J Obstet Gynecol. 2011;205:e1–e7. doi: 10.1016/j.ajog.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaneko T, Kasaoka S, Miyauchi T, et al. Serum glial fibrillary acidic protein as a predictive biomarker of neurological outcome after cardiac arrest. Resuscitation. 2009;80:790–794. doi: 10.1016/j.resuscitation.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Papa L, Lewis LM, Falk JL, et al. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann Emerg Med. 2012;59:471–483. doi: 10.1016/j.annemergmed.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douglas-Escobar M, Yang C, Bennett J, et al. A pilot study of novel biomarkers in neonates with hypoxic-ischemic encephalopathy. Pediatr Res. 2010;68:531–536. doi: 10.1203/PDR.0b013e3181f85a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bembea MM, Savage W, Strouse JJ, et al. Glial fibrillary acidic protein as a brain injury biomarker in children undergoing extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12:572–579. doi: 10.1097/PCC.0b013e3181fe3ec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fink EL, Berger RP, Clark RSB, et al. Serum Biomarkers of Brain Injury to Classify Outcome after Pediatric Cardiac Arrest. Critical Care Medicine. 2014;42:664–674. doi: 10.1097/01.ccm.0000435668.53188.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mondello S, Papa L, Buki A, et al. Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit Care. 2011;15:R156. doi: 10.1186/cc10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langhelle A, Nolan J, Herlitz J, et al. Recommended guidelines for reviewing, reporting, and conducting research on post-resuscitation care: the Utstein style. Resuscitation. 2005;66:271–283. doi: 10.1016/j.resuscitation.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 22.Blennow M, Savman K, Ilves P, Thoresen M, Rosengren L. Brain-specific proteins in the cerebrospinal fluid of severely asphyxiated newborn infants. Acta Paediatr. 2001;90:1171–1175. doi: 10.1080/080352501317061594. [DOI] [PubMed] [Google Scholar]
- 23.Chalak LF, Sanchez PJ, Adams-Huet B, Laptook AR, Heyne RJ, Rosenfeld CR. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J Pediatr. 2014;164:468–474 e1. doi: 10.1016/j.jpeds.2013.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mortberg E, Zetterberg H, Nordmark J, Blennow K, Rosengren L, Rubertsson S. S-100B is superior to NSE, BDNF and GFAP in predicting outcome of resuscitation from cardiac arrest with hypothermia treatment. Resuscitation. 2011;82:26–31. doi: 10.1016/j.resuscitation.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Hayashida H, Kaneko T, Kasaoka S, et al. Comparison of the predictability of neurological outcome by serum procalcitonin and glial fibrillary acidic protein in postcardiac-arrest patients. Neurocrit Care. 2010;12:252–257. doi: 10.1007/s12028-009-9318-5. [DOI] [PubMed] [Google Scholar]
- 26.Larsson IM, Wallin E, Kristofferzon ML, Niessner M, Zetterberg H, Rubertsson S. Post-cardiac arrest serum levels of glial fibrillary acidic protein for predicting neurological outcome. Resuscitation. 2014;85:1654–1661. doi: 10.1016/j.resuscitation.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Mondello S, Linnet A, Buki A, et al. Clinical utility of serum levels of ubiquitin C-terminal hydrolase as a biomarker for severe traumatic brain injury. Neurosurgery. 2012;70:666–675. doi: 10.1227/NEU.0b013e318236a809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papa L, Akinyi L, Liu MC, et al. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit Care Med. 2010;38:138–144. doi: 10.1097/CCM.0b013e3181b788ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fraser DD, Close TE, Rose KL, et al. Severe traumatic brain injury in children elevates glial fibrillary acidic protein in cerebrospinal fluid and serum. Pediatr Crit Care Med. 2011;12:319–324. doi: 10.1097/PCC.0b013e3181e8b32d. [DOI] [PubMed] [Google Scholar]
- 30.Topjian AA, Lin R, Morris MC, et al. Neuron-specific enolase and S-100B are associated with neurologic outcome after pediatric cardiac arrest. Pediatr Crit Care Med. 2009;10:479–490. doi: 10.1097/PCC.0b013e318198bdb5. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Nagayama T, Jin K, et al. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci. 1998;18:4914–4928. doi: 10.1523/JNEUROSCI.18-13-04914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horn M, Schlote W. Delayed neuronal death and delayed neuronal recovery in the human brain following global ischemia. Acta Neuropathol. 1992;85:79–87. doi: 10.1007/BF00304636. [DOI] [PubMed] [Google Scholar]
- 33.Jin K, Chen J, Nagayama T, et al. In situ detection of neuronal DNA strand breaks using the Klenow fragment of DNA polymerase I reveals different mechanisms of neuron death after global cerebral ischemia. J Neurochem. 1999;72:1204–1214. doi: 10.1046/j.1471-4159.1999.0721204.x. [DOI] [PubMed] [Google Scholar]
- 34.Northington FJ, Ferriero DM, Graham EM, Traystman RJ, Martin LJ. Early Neurodegeneration after Hypoxia-Ischemia in Neonatal Rat Is Necrosis while Delayed Neuronal Death Is Apoptosis. Neurobiol Dis. 2001;8:207–219. doi: 10.1006/nbdi.2000.0371. [DOI] [PubMed] [Google Scholar]
- 35.Kochanek PM, Berger RP, Fink EL, et al. The potential for bio-mediators and biomarkers in pediatric traumatic brain injury and neurocritical care. Frontiers in neurology. 2013;4:40. doi: 10.3389/fneur.2013.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iliff JJ, Lee H, Yu M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plog BA, Dashnaw ML, Hitomi E, et al. Biomarkers of Traumatic Injury Are Transported from Brain to Blood via the Glymphatic System. J Neurosci. 2015;35:518–526. doi: 10.1523/JNEUROSCI.3742-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]