Abstract
Background
The integrity of endothelial monolayer is a sine qua non for vascular homeostasis and maintenance of tissue fluid balance. However, little is known about the signaling pathways regulating regeneration of the endothelial barrier following inflammatory vascular injury.
Methods and Results
Employing genetic and pharmacological approaches, we demonstrated that endothelial regeneration selectively requires activation of p110γPI3K signaling, which thereby mediates the expression of the endothelial reparative transcription factor FoxM1. We observed that FoxM1 induction in the pulmonary vasculature was inhibited in mice treated with p110γ-selective inhibitor and in Pik3cg−/− mice following LPS challenge. Pik3cg−/− mice exhibited persistent lung inflammation induced by sepsis and sustained increase in vascular permeability. Restoration of expression of either p110γ or FoxM1 in pulmonary endothelial cells of Pik3cg−/− mice restored endothelial regeneration and normalized the defective vascular repair program. We also observed diminished expression of p110γ in pulmonary vascular endothelial cells of ARDS patients, suggesting that impaired p110γ-FoxM1 vascular repair signaling pathway is a critical factor in persistent leaky lung microvessels and edema formation in the disease.
Conclusions
We identify p110γ as the critical mediator of endothelial regeneration and vascular repair following sepsis-induced inflammatory injury. Thus, activation of p110γ-FoxM1 endothelial regeneration may represent a novel strategy for the treatment of inflammatory vascular diseases.
Keywords: Acute lung injury, endovascular repair, inflammation, sepsis, vascular disease, vascular endothelium
Journal Subject Terms: Vascular Disease, Cell Signalling/Signal Transduction, Endothelium/Vascular Type/Nitric Oxide, Inflammation, Pulmonary Biology
INTRODUCTION
The integrity of endothelial monolayer is a sine qua non for vascular homeostasis and maintenance of tissue fluid balance.1, 2 The key function of the endothelial barrier is to maintain fluid balance between the blood plasma and interstitium.3, 4 Endothelial injury results in most of the complications associated with inflammation: increased vascular permeability to protein, diapedesis of erythrocytes and transmigration of inflammatory cells, tissue edema and microthrombi formation.5–7 In acute lung injury (ALI) and its severe form, acute respiratory distress syndrome (ARDS), vascular endothelial injury leads to extravasation of neutrophils and monocytes and accumulation and intractability of protein-rich edema.8–10 After vascular injury, repair of the endothelial monolayer through activation of intrinsic endothelial repair programs is a prerequisite for restoring vascular homeostasis.11–14
Endothelial regeneration occurs due to endothelial proliferation and re-annealing of endothelial junctions to form the characteristic restrictive endothelial barrier.11, 15–17 We showed that Forkhead box M1 (FoxM1), belonging to fox family of transcriptional factors sharing homology in winged helix DNA-binding domains,18 is required for vascular endothelial regeneration.11 FoxM1 mediated G1/S and G2/M transition secondary to transcriptional control of cell cycle progression genes.19–22 FoxM1 expression was upregulated in endothelial cells (ECs) only during the repair phase following vascular injury induced by lipopolysaccharide (LPS).11 In mice with EC-restricted disruption of FoxM1, endothelial barrier recovery following LPS challenge was severely impaired due to defective EC proliferation11 and re-annealing of endothelial adherens junctions.15
Phosphoinositide 3-kinases (PI3Ks) are cellular lipid kinases that phosphorylate the 3 position hydroxyl group of the inositol ring of phosphatidylinositol to generate the lipid second messenger phosphatidylinositol 3,4,5-triphosphate.23–25 Studies have focused on class I PI3K comprising the class IA (p110α, β, and δ) and class IB (p110γ, encoded by Pik3cg) isoforms. Class IA kinases forming a complex with SH2-conatining regulatory p85-related subunits are in general activated through receptor tyrosine kinases whereas class IB, p110γ is activated by G protein-coupled receptors (GPCR) through its regulatory subunit p101 or p84 and G-protein subunits.24,25 p110β is also activated by GPCR signaling in p110γ-deficient cells.26 p110γ is expressed in leukocytes and at low levels in ECs and smooth muscle cells. In neutrophils, eosinophils and macrophages, p110γ mediates chemokine-induced migration of these cells.27, 28 It has been shown that p110γ plays an important role in the initial inflammatory responses (at 1 or 6h post-challenge) to sepsis challenge via regulating neutrophil recruitment and migration.29, 30 However, little is known about the role of p110γ in regulating endothelial regeneration and vascular repair during the repair phase. Here we focused on the fundamental role of endothelial p110γ based on our observation that p110γ expression was markedly reduced in pulmonary vascular ECs of ARDS patients (see Results). In the mouse model of endotoxemia, we demonstrated that p110γ deficiency severely impaired vascular repair following lipopolysaccharide (LPS) challenge and that restoration of endothelial expression of either p110γ or FoxM1 normalized defective endothelial regeneration in Pik3cg−/− mice. Thus, selectively targeting p110γ to promote FoxM1-mediated vascular repair represents a novel therapeutic strategy for the treatment of inflammatory vascular diseases such as acute lung injury.
METHODS
Please see the online-only Data Supplement for full details of Methods
Mice
Pik3cg−/− mice were obtained from Dr. Joseph Penninger (Amgen Institute, Canada).29,31 FoxM1 transgenic mice were obtained from Dr. Robert H. Costa at the University of Illinois College of Medicine.20 Littermate WT mice (C57BL/6 background) were used as controls. All mice were bred and maintained in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facilities at the University of Illinois at Chicago according to National Institutes of Health guidelines. All animal experiments were performed in accordance with protocols approved by the University of Illinois at Chicago Animal Care and Use Committee.
Human lung tissues
Human lung tissues were obtained from ARDS patients (n = 6) and unused donor lungs (n=8) under supervision of Kurt Albertine. Informed consents and approval from Ethics Committee of the University of Utah (IRB #5632) were obtained prior to tissue collection.
Statistical analysis
Data are expressed as mean ± SD. Statistical significance was determined by one-way ANOVA with a Games-Howell post hoc analysis for multiple group comparisons. Two-group comparisons were analyzed by the two-tailed unpaired Student’s t test or Mann-Whitney (nonparametric) test depending on the data distribution. Statistical analysis of the mortality study was performed with the Log–rank (Mantel-Cox) test. The P-values are two-sided. P < 0.05 denoted the presence of a statistically significant difference.
An expanded Materials and Methods section containing detailed description of induction of polymicrobial sepsis, lung transvascular albumin flux assessment, scanning electron microscopy, intravital microscopy, myeloperoxidase assay, histology and imaging, in situ cell proliferation and apoptosis assay, primary cultures of human lung microvascular ECs, molecular analysis, liposome-mediated transduction of cDNA into mouse lung vascular ECs is provided in the online-only Data Supplement.
RESULTS
p110γPI3K isoform mediates FoxM1 expression in the pulmonary vasculature
We first used the pan-PI3K inhibitor wortmannin to determine the role of PI3K signaling in regulating FoxM1 expression in the pulmonary vasculature following inflammatory injury. At 12h post-LPS challenge, WT mice were treated with wortmannin (i.p., 0.05mg/kg BW, every 12h) or the vehicle DMSO. There was little FoxM1 induction at 24h post-LPS challenge in lungs of either DMSO- or wortmannin-treated mice, whereas FoxM1 mRNA expression in DMSO-treated lungs was upregulated at 48 and 72h post-LPS (Figure 1A). This time course directly paralleled the time course of lung vascular repair seen in this model.11 Western blotting demonstrated decreased FoxM1 protein expression in the wortmannin-treated mice (Figure 1B).
Figure 1.
p110γ mediates FoxM1 expression during the repair phase following LPS challenge. (A) FoxM1 mRNA expression in lungs. At 12h post-LPS, WT mice were administered either DMSO (CTL) or wortmannin (Wor, 0.05mg/kg BW, i.p.) every 12 h. Lung tissue was collected for QRT-PCR analysis. n = 5 mice/group. *, P < 0.05; **, P < 0.01 (Student t test). (B) Representative Western blotting demonstrating decreased FoxM1 protein expression induced by wortmannin treatment. The experiment was repeated twice with similar results. (C) Inhibition of p110γ but not p110α decreased FoxM1 expression. At 12 h post-LPS, WT mice were administered either DMSO (CTL), the p110γ inhibitor AS-605240 (AS) (30 mg/kg, per os.), or the p110α inhibitor PI-103 (10 mg/kg, i.p.) at 12 h intervals. Lungs at 72h post-LPS were collected for QRT-PCR analysis. n = 5. *, P < 0.05 (ANOVA). (D) Inhibition of FoxM1 expression in Pik3cg−/− mouse lungs following LPS challenge. n = 5. *, P < 0.05; **, P < 0.01 (Student’s t test).
To identify the PI3K isoform mediating FoxM1 upregulation, mice were treated at 12h post-LPS challenge with either PI-103, a p110α inhibitor,32 or AS-605240, a p110γ inhibitor.33 Quantitative real-time RT-PCR (QRT-PCR) analysis showed that only p110γ inhibition decreased FoxM1 expression at 72h post-LPS (Figure 1C). Using lungs from Pik3cg−/− mice, we confirmed that FoxM1 expression was inhibited post-LPS in these lungs (Figure 1D). These data demonstrate that FoxM1 induction in the pulmonary vasculature following LPS challenge selectively requires the p110γPI3K isoform.
p110γ is required for vascular repair following endotoxin-induced lung inflammation
We next determined alterations in vascular permeability by assessing pulmonary transvascular flux of Evans blue-conjugated albumin (EBA).34 EBA flux increased 3–4 fold and peaked within 24h post-LPS challenge in Pik3cg−/− lungs similar to WT lungs (Figure 2A). EBA flux in WT lungs returned to basal levels at 60h post-LPS indicating full recovery whereas Pik3cg−/− lungs exhibited persistent vascular leakiness indicating defective vascular repair. Lung microvascular integrity was also examined by scanning electron microscopy after filling vessels with the tracer methyl methacrylate. At 60h post-LPS challenge, WT lungs had minimal tracer extravasation as seen in WT and Pik3cg−/− lungs basally whereas Pik3cg−/− lungs exhibited striking leakage (Figure 2B) consistent with the albumin permeability data above. TUNEL staining also revealed similar rate of apoptosis in Pik3cg−/− lungs compared to WT at 24h post-LPS challenge (Supplemental Figure 1), further demonstrating similar injury in Pik3cg−/− and WT lungs induced by LPS challenge. Thus, the critical difference is impaired vascular repair in Pik3cg−/− lungs.
Figure 2.
Impairment of vascular repair in Pik3cg−/− mice secondary to inhibition of FoxM1 expression. (A) Pulmonary transvascular EBA flux demonstrating defective vascular repair in Pik3cg−/− mouse lungs which was rescued by overexpression of FoxM1 in Pg−/−/Tg mice. n = 5. *, P < 0.05; *, P < 0.01 (ANOVA). (B) Scanning electron microscopy demonstrating p110γ-mediated FoxM1 expression is required for vascular repair. Representative micrographs of lung sections from mice challenged with LPS for 60h are shown. Pik3cg−/− mouse lungs had extensive extravasation (Ex) of methacrylate tracer on the cut surfaces and many alveoli were filled with the tracer. However, Pg−/−/Tg lungs displayed normal profile similar to WT lungs. A, airways. The experiment was performed 3 times with similar data. Scale bar, 20μm. (C) Representative Western blotting of p110γ and FoxM1 in lung lysates at basal. Experiment were performed three times with similar results. (D) Representative micrographs of cremaster muscle venule demonstrating marked leakage of FITC-conjugated dextran in Pik3cg−/− mice in contrast to WT and Pg−/−/Tg at 48h post-LPS. Thirty min post-administration of FITC-conjugated dextran (i.v.), vascular permeability in the cremaster muscle venule was monitored by the FITC signal in an area of 0.02 mm2. The vessel walls were indicated in white lines. Scale bar, 10μm. (E) Graphic presentation of prominent vascular leakiness in Pik3cg−/− mice at 48h post-LPS challenge, which was rescued by FoxM1 overexpression in Pg−/−/Tg mice. n = 6 venules in 3 mice/group. *, P < 0.0001 (ANOVA).
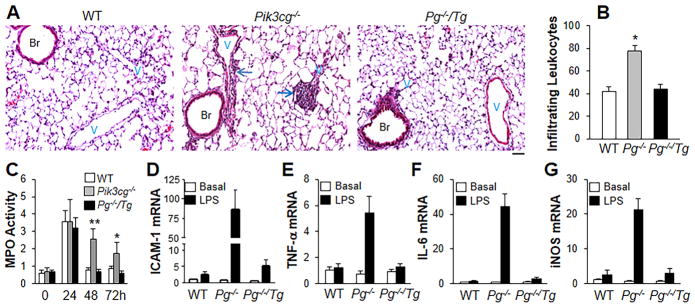
Lungs from Pik3cg−/− mice also showed greater perivascular infiltration of leukocytes at 48h post-LPS challenge compared to WT lungs (Figure 3A and 3B). Lung myeloperoxidase (MPO) activity11 showed similar neutrophil infiltration in WT and Pik3cg−/− lungs during the injury phase up to 24h post-LPS except p110γ deficiency induced an early decrease of neutrophil infiltration at 2h post-LPS (Figure 3C and Supplemental Figure 2). By 48h post-LPS challenge, MPO activity in WT lungs returned to basal level whereas MPO activity in Pik3cg−/− lungs remained elevated (Figure 3C). QRT-PCR analysis demonstrated increased expression of ICAM-1 and pro-inflammatory cytokines as well as iNOS at 48h post-LPS in Pik3cg−/− lungs (Figure 3, D–G) consistent with persistence of lung inflammation in Pik3cg−/− mice. Given that p110γ deficiency itself affects the initial neutrophil recruitment following LPS challenge (Supplemental Figure 2), we treated WT mice with the p110γ inhibitor at 12h post-LPS challenge. As shown in Supplemental Figure 3, p110γ inhibitor-treated mice exhibited similar lung vascular permeability and inflammation responses at 24h post-LPS challenge as the controls. However, the p110γ inhibitor-treated mice showed greater EBA extravasation and MPO activity at 48h post-LPS challenge, supporting the role of p110γ deficiency in impairing the lung vascular repair program.
Figure 3.
Restoration of FoxM1 expression in Pg−/−/Tg mice mitigates lung inflammation. (A) H & E staining of lung sections (of 3 independent experiments) showing perivascular leukocyte infiltration in Pik3cg−/− mouse lungs at 48h post-LPS challenge. Arrows indicate leukocyte infiltration. Scale bar, 50 μm. Br, bronchia; V, vessel. (B) Analysis of infiltrating leukocytes in lungs at 48h post-LPS challenge. Bar graphs show infiltrating leukocytes per vessels (> 30μm in diameter). n = 5. *, P < 0.05(ANOVA). P−/−, Pik3cg−/−. (C) Time course of MPO activity in mouse lungs following LPS challenge (7.5 mg/kg, i.p.). n =5. *, P < 0.05; **, P < 0.001 (ANOVA). (D–G) QRT-PCR analysis of expression of proinflammatory mediators in mouse lungs. AT 48h post-LPS challenge, mouse lungs were collected for QRT-PCR analysis. n = 5. Elevated expression of pro-inflammatory mediators seen in Pik3cg−/− mouse lungs was inhibited in Pg−/−/Tg mouse lungs.
Defective vascular repair in Pik3cg−/− mice results from impaired FoxM1 expression
To address at the genetic level the function of p110γ upstream of FoxM1, we generated a mouse model with genetic deletion of Pik3cg and transgenic expression of human FOXM1 (Pik3cg−/−/FOXM1Tg) (Pg−/−/Tg). FoxM1 transgenic mice in which FOXM1 expression was under control of the Rosa promoter20 were bred into the genetic background of Pik3cg−/−. Pg−/−/Tg lungs showed increased FoxM1 expression at basal (Figure 2C) and 72h post-LPS challenge (Supplemental Figure 4). Pg−/−/Tg mice exhibited the same maximal increases in vascular permeability as WT and Pik3cg−/− lungs at 18h post-LPS (Figure 2A). However, in contrast to Pik3cg−/− lungs, vascular permeability in Pg−/−/Tg mouse lungs during the repair phase was markedly reduced; that is, Pg−/−/Tg mouse lungs showed similar vascular leakage as WT mouse lungs at 48, 60, and 72h post-LPS challenge (Figure 2A). Scanning electron microscopy also demonstrated normal vascular barrier integrity in Pg−/−/Tg mouse lungs at 60h post-LPS as seen in WT lungs (Figure 2B).
We also performed real-time intravital microscopy to assess vascular permeability in live animals. FITC-conjugated dextran was injected through tail vein and cremaster muscle was then exteriorized onto an intravital microscopy tray to image the vascular permeability of cremaster muscle venules. As shown in Figure 2D and 2E, Pik3cg−/− mice exhibited similar basal permeability as WT and Pg−/−/Tg mice. However, at 48h post-LPS challenge, Pik3cg−/− mice exhibited marked increase of vascular permeability compared to WT mice, whereas Pg−/−/Tg mice exhibited similar vascular permeability as seen in WT mice. These data suggest p110γ-mediated vascular repair via FoxM1 is a generalized process following inflammatory vascular injury.
p110γ signaling of FoxM1 expression reduces lung inflammation
To determine whether persistent lung inflammation in Pik3cg−/− mice was also the result of decreased FoxM1 expression, we assessed leukocyte infiltration and MPO activity in Pg−/−/Tg mice. H & E staining revealed that perivascular infiltration of leukocytes in Pik3cg−/− mouse lungs during the repair phase (e.g., 48h post-LPS) was normal in Pg−/−/Tg mouse lungs, and similar to WT lungs (Figure 3A and 3B). During the lung vascular injury and inflammation phase (up to 24h) post-LPS, Pg−/−/Tg mouse lungs exhibited similar MPO activity as WT and Pik3cg−/− mouse lungs (Figure 3C and Supplemental Figure 2). In contrast to Pik3cg−/− mouse lungs, MPO activity during the repair phase in Pg−/−/Tg lungs returned to basal levels (Figure 3C). Elevated expression levels of ICAM-1 and various pro-inflammatory mediators seen in Pik3cg−/− mouse lungs at 48h post-LPS were also restored in Pg−/−/Tg mouse lungs (Figure 3, D–G).
p110γ is required for endothelial regeneration
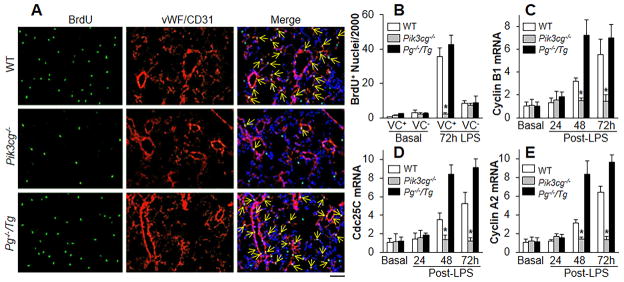
We next determined whether p110γ-induced FoxM1 expression was required for EC proliferation as measured by BrdU incorporation.11 As shown in Figure 4A and 4B, WT mouse lungs exhibited marked increase in cell proliferation at 72h post-LPS compared to WT lungs at basal as also previously shown.11 However, we observed decreased cell proliferation in Pik3cg−/− mouse lungs, which was the result of reduced EC proliferation as demonstrated by co-localization of EC markers vWF/CD31 and BrdU immunostaining. This defective response was restored in Pik3cg−/−/Tg mouse lungs.
Figure 4.
Impairment in endothelial cell proliferation in Pik3cg−/− lungs is rescued by expression of FoxM1. (A) Representative micrographs showing EC proliferation. Cryosections of lungs (3–5μm thick), collected at 72h following LPS challenge, were stained with FITC-conjugated anti-BrdU antibody to identify proliferating cells (green) and with anti-vWF and anti-CD31 antibodies to identify EC (red). Nuclei were counterstained with DAPI (blue). Arrows indicate proliferating lung ECs. Scale bar, 50 μm. (B) Graphic presentation of decreased proliferating ECs in Pik3cg−/− lungs. Three consecutive cryosections from each mouse lung were examined and average number of BrdU-positive nuclei was used. n = 5. *, P < 0.0001 (ANOVA). VC+, vWF+/CD31+ cells. (C–E) QRT-PCR analysis of expression of FoxM1 target genes essential for cell cycle progression. n = 5, *, P < 0.01 (ANOVA).
To address the basis of decreased endothelial proliferation in Pik3cg−/− lungs, we determined expression of FoxM1 target genes essential for cell cycle progression. QRT-PCR analysis showed that expression of Cdc25C, Cyclin B1 and Cyclin A2 was upregulated in WT lungs during the repair phase at 48 and 72h post-LPS challenge but not in Pik3cg−/− lungs (Figure 4, C–E). Overexpression of FoxM1 in Pg−/−/Tg lungs however significantly increased the expression of these genes indicating that FoxM1 is the effector of p110γ-mediated EC proliferation in vivo.
Restoration of endothelial cell expression of p110γ induces FoxM1 expression and endothelial regeneration in Pik3cg−/− lungs
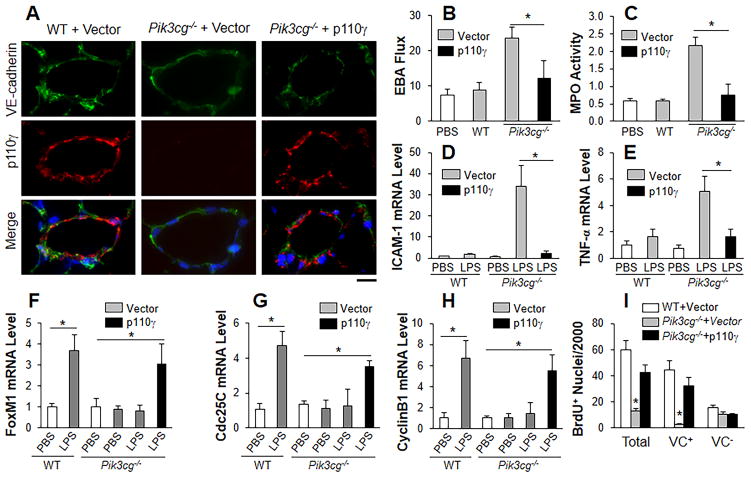
We next employed liposome-mediated gene transduction15 to address whether forced expression of p110γ in pulmonary vascular ECs of Pik3cg−/− mice will reverse the defective vascular repair. Transduction of plasmid DNA expressing human p110γ under the control of the 3.5kb CDH5 (encoding VE-cadherin) promoter35 restored p110γ expression in lung ECs of these mice (Figure 5A and Supplemental Figure 5). In Pik3cg−/− mouse lungs at 60h post-LPS challenge, restored endothelial expression of p110γ markedly reduced pulmonary transvascular EBA flux and MPO activity compared to control Pik3cg−/− mouse lungs (Figure 5B and 5C). These lungs also expressed low levels of pro-inflammatory molecules during vascular repair phase at 60h post-LPS challenge in contrast to control Pik3cg−/− lungs transduced with empty vector DNA (Figure 5, D–E). However, restored p110γ expression in ECs of Pik3cg−/− lungs didn’t normalize the initial defective recruitment and migration of neutrophils at 2h post-LPS whereas transplantation of WT bone marrow cells to Pik3cg−/− mice resulted in normal neutrophil infiltration (Supplemental Figure 6). Thus, defective vascular repair in Pik3cg−/− mice is ascribed to diminished expression of p110γ in ECs whereas impaired neutrophil recruitment and migration during the early response to LPS challenge is owing to p110γ deficiency in neutrophils.
Figure 5.
Restored expression of p110γ in lung endothelial cells of Pik3cg−/− mice induces FoxM1 expression and normalizes endothelial regeneration. (A) Representative micrographs showing endothelial expression of p110γ in pulmonary vascular ECs of Pik3cg−/− mice at 30h following liposome-mediated transduction of p110γ plasmid DNA (driven by human CDH5 promoter). Scale bar, 30μm. (B) Endothelial expression of p110γ normalized the defective vascular repair phenotype of Pik3cg−/− mouse lungs. At 30h post-plasmid DNA transduction, mice were challenged with LPS and lungs were collected for assessing pulmonary transvascular EBA flux at 60h post-LPS. n = 5. *, P < 0.01 (t test). (C) Lung tissue MPO activity indicating resolved lung inflammation in Pik3cg−/− mice transduced with p110γ plasmid DNA at 60h post-LPS. *, P < 0.01(t test). (D, E) QRT-PCR analysis of expression of pro-inflammatory molecules in mouse lungs. *, P < 0.01 (t test). (F–H) QRT-PCR analysis of FoxM1 expression and its downstream target genes in Pik3cg−/− lungs transduced with p110γ plasmid DNA. *, P < 0.01 (t test). (I) Endothelial expression of p110γ normalized lung EC proliferation in Pik3cg−/− mice at 60h post-LPS. *, P < 0.001 (ANOVA). VC, vWF/CD31.
We next addressed whether restoration of endothelial p110γ expression in Pik3cg−/− mouse lungs induced FoxM1 expression and thereby endothelial regeneration after injury. In the Pik3cg−/− lungs in which p110γ had been restored in ECs, FoxM1 mRNA expression was induced similarly in WT lungs (Figure 5F). The downstream targets of FoxM1 responsible for cell cycle progression Cdc25C and Cyclin B1 were also upregulated in these lungs in contrast to the control Pik3cg−/− mouse lungs (Figure 5G and 5H). BrdU labeling demonstrated normal proliferation rate in the mouse lung ECs in which p110γ was introduced (Figure 5I and Supplemental Figure 7). These data demonstrate that endothelial expression of p110γ is required for FoxM1 induction and resulting endothelial regeneration following inflammatory vascular injury.
Endothelial cell expression of FoxM1 in Pik3cg−/− lungs is sufficient to rescue defective vascular repair
Given that FoxM1 is ubiquitously expressed in all cell types in Pg−/−/Tg mice, it is unclear whether the normalized endothelial regeneration and vascular repair is ascribed to FoxM1 expression in ECs or other cells through a parallel pathway. To address this important question, we employed a similar liposome-mediated gene transduction approach to restore FoxM1 expression specifically in lung ECs of Pik3cg−/− mice. Plasmid DNA expressing human FOXM1 under the control of CDH5 promoter was transduced in Pik3cg−/− lungs at 12h post-LPS challenge. Liposome-mediated expression of FoxM1 in Pik3cg−/− lung ECs (Figure 6A) resulted in normalized vascular repair response (Figure 6B and 6C). Pulmonary transvascular EBA flux and MPO activity were markedly reduced in Pik3cg−/− mouse lungs transduced with the FOXM1 plasmid, in contrast to non-FOXM1-transduced Pik3cg−/− lungs (Figure 6B and 6C). Together, these data in Figures 5 and 6 demonstrate that p110γ and FoxM1 are acting specifically in ECs in vivo to mediate endothelial regeneration and vascular repair.
Figure 6.
Endothelial expression of FoxM1 rescued the defective vascular repair phenotype of Pik3cg−/− mouse lungs. (A) QRT-PCR analysis demonstrating restored expression of FoxM1 in Pik3cg−/− mouse lungs. At 12h post-LPS, plasmid DNA expressing human FOXM1 under control of the CDH5 promoter (FOXM1) or empty vector was transduced in Pik3cg−/− lungs. At 60h post-LPS, lung tissues were collected for QRT-PCR analysis. n = 4. *, P < 0.01. (B) Pulmonary transvascular EBA flux demonstrating decreased lung vascular permeability of Pik3cg−/− mice transduced with FOXM1 plasmid DNA. At 60h post-LPS challenge, lungs were collected for EBA assay. n = 4. *, P < 0.01. (C) Normalized resolution of lung inflammation in Pik3cg−/− mice transduced with FOXM1 plasmid DNA. n = 4. *, P < 0.001. All statistical analyses were performed with t test.
p110γ-mediated FoxM1 expression is required for lung vascular repair in septic mice
We next addressed the pathophysiological relevance of these findings using the mouse cecal ligation and puncture (CLP) model, which causes polymicrobial septicemia accompanied by ALI. CLP similarly increased pulmonary vascular EBA flux at 24h post-challenge in WT, Pik3cg−/−, and Pg−/−/Tg mice (Figure 7A). However during the recovery phase at 48 and 72h post-CLP, EBA flux was markedly decreased in both WT and Pg−/−/Tg mouse lungs whereas Pik3cg−/− mouse lungs showed persistent increase (Figure 7A). Pik3cg−/− mouse lungs also showed impaired resolution of neutrophilic inflammation (assessed by MPO activity) (Figure 7B). Strikingly, Pik3cg−/− mice exhibited a greater mortality rate (60%). Similar to WT mice, less than 20% of Pg−/−/Tg mice died at the same period (Figure 7C).
Figure 7.
Reduced expression of p110γ in pulmonary vascular endothelial cells of ARDS patients. (A–C) Normalization of vascular repair and improved survival of Pik3cg−/−/Tg mice following CLP sepsis. EBA extravasation demonstrating defective vascular repair in Pik3cg−/− lungs following CLP sepsis, which was rescued by overexpression of FoxM1 in Pg−/−/Tg mouse lungs (A). Time course of lung tissue MPO activity (B). *, P < 0.01 (ANOVA). Improved survival of Pg−/−/Tg mice (C). Mortality rate was monitored for 5 days following CLP. Approximately 60% of the Pik3cg−/− mice died within 72h post-CLP. *, P < 0.01 (Mantel-Cox). (D) Representative micrographs of immunostaining demonstrating diminished expression of p110γ in pulmonary vascular ECs of ARDS patients. Arrows, ECs expressing p110γ. Scale bar, 40 μm. V, vessel. (E) Quantification of p110γ expression in pulmonary vascular ECs of human lung samples. The fluorescence intensity of p110γ staining in pulmonary vascular ECs was scored from 1 to 5, with 5 as the highest. *, P = 0. 01 (Mann-Whitney). A.U, arbitrary units.
p110γ expression is diminished in pulmonary vascular endothelial cells of ARDS patients
To further address the clinical relevance of these observations in mice to the pathogenesis of ARDS in patients, we examined p110γ protein levels in lung sections from ARDS patients.36 As shown in Figure 7D and 7E, p110γ expression is markedly diminished in pulmonary vascular ECs from ARDS patients.
p110γ-mediated FoxM1 expression in endothelial cells is activated by SDF-1α signaling
Different from other class I PI3K isoforms, p110γ is GPCR-dependent23–25. To determine which GPCR signaling activates p110γ and thereby induces FoxM1 expression in ECs, human lung microvascular ECs were treated with two well-known GPCR agonists SDF-1α (also called CXCL12) and sphingosine-1-phosphate, respectively. As shown in Figure 8A, SDF-1α induced FoxM1 expression in ECs. However, sphingosine-1-phosphate had no effect on FoxM1 expression (data not shown). Inhibition of p110γ but not p110β blocked SDF-1α-induced FoxM1 expression (Figure 8B).
Figure 8.
p110γ mediates SDF-1α-induced FoxM1 expression in lung endothelial cells through inactivation of FoxO1. (A) SDF-1α-induced FoxM1 expression in human lung microvascular ECs. Lung ECs were treated with recombinant human SDF-1α (50 ng/ml) for various times and then collected for QRT-PCR analysis. n=3 experiments. *, P < 0.01 (ANOVA). (B) p110γ inhibition abrogated SDF-1α-induced FoxM1 expression in ECs. AS, AS-605240 (10 μM); TGX, TGX-221 (p110β inhibitor, 1 μM). n=3. *, P < 0.01 (t test). (C) Representative images of immunostaining demonstrating p110γ-mediated FoxO1 translocation out of nucleus induced by SDF-1α (SDF) treatment. (D) FoxO1 is a negative regulator of FoxM1 expression. Co-treatment of ECs with inhibitors for p110γ (AS) and FoxO1 (Oi) (5μM) reversed the inhibitory effect of p110γ inhibitor on SDF-1α-induced FoxM1 expression. Inhibition of nuclear export of FoxO1 by psammaphysene (SAM) (5μM) inhibited SDF-1α-induced FoxM1 expression. The inhibitor(s) was/were added to the cells 2h prior to SDF-1α addition and the cells were collected for QRT-PCR analysis of FoxM1 expression at 4h post-SDF-1α treatment. n=3. *, P < 0.01 (t test). (E) SDF-1α expression in mouse lungs following LPS challenge (7.5 mg/kg, i.p.). At times indicated, WT mouse lungs were collected for RNA isolation and QRT-PCR analysis. n=3–5 mice/time point. *, P < 0.01 (t test). (F) Our studies delineated an important role of the GPCR-activated p110γ expressed in ECs in mediating FoxM1-dependent endothelial regeneration and vascular repair and thereby promoting resolution of inflammatory injury.
Next we examined the signaling mediator downstream of p110γ. FoxO is inactivated by PI3K through phosphorylation-induced translocation out of nucleus36. As shown in Figure 8C, SDF-1α treatment induced FoxO1 translocation out of nucleus in a p110γ-dependent manner. Thus, we determined whether FoxO1 is a negative regulator of p110γ-dependent FoxM1 expression. Co-treatment with p110γ inhibitor and FoxO1-selective inhibitor AS184285637 reversed p110γ inhibition-mediated blockade of SDF-1α-induced FoxM1 expression whereas FoxO1 activator psammaphysene A which inhibits FoxO1 nuclear export38 inhibited SDF-1α-induced FoxM1 expression (Figure 8D).
To determine whether SDF-1α was induced in mouse lungs responsible for activation of p110γ in vivo, we examined SDF-1α mRNA expression in WT mouse lungs at different times following LPS challenge. SDF-1α was initially decreased and then returned to basal levels at 24h post-LPS challenge (Figure 8E). Importantly, SDF-1α expression was markedly induced at 48h post-LPS challenge during the recovery phase (Figure 8E), indicating the important role of SDF-1α signaling-activated p110γ in mediating FoxM1 expression and thereby endothelial regeneration following inflammatory vascular injury (Figure 8F).
DISCUSSION
We have identified a novel function of p110γ expressed in ECs in mediating endothelial regeneration and vascular repair through the activation of the endothelial reparative transcription factor FoxM1. We showed that endothelial p110γ activation following endotoxemia-induced vascular injury was required for FoxM1 activation and the resulting repair of the endothelial barrier. We also identified SDF-1α as a critical agonist to activate the GPCR-dependent p110γPI3K and thereby induces FoxM1 expression in ECs through inactivation of FoxO1. The finding that p110γ expression was reduced in pulmonary vascular ECs of ARDS patients, suggests that impaired p110γ function itself is a fundamental determinant of persistent lung vascular leakiness and the poorly resolving inflammation seen in these patients.
We have shown previously role of FoxM1 in ECs in mediating endothelial regeneration and vascular repair.11, 15 As little is known about the upstream signaling mechanisms mediating FoxM1 induction in ECs, here we focused on the PI3K isoform p110γ that was found to have reduced expression in pulmonary vascular ECs of ARDS patients. Several lines of evidence in the present study support the requirement of endothelial p110γ in mediating FoxM1 expression and resultant vascular repair. Treatment of mice with the p110γ-inhibitor AS-605240 at 12h post-LPS challenge (i.e., after the induction of lung inflammation and vascular injury) markedly decreased FoxM1 expression in mouse lungs and failed to restore vascular integrity. In addition, FoxM1 activation was blocked in Pik3cg−/− mouse lungs in response to LPS challenge and this was accompanied by persistent lung inflammation and vascular injury. Endothelial-specific expression of p110γ in lung vessels of Pik3cg−/− mouse was sufficient to induce FoxM1 expression and activate vascular repair. These data collectively suggest that activation of endothelial p110γ mediates FoxM1 expression in ECs and thereby activates the endothelial regeneration and vascular repair program following sepsis-induced inflammatory vascular injury. Consistent with our observation, previous study has shown that PI3K signaling is involved in regulation of FoxM1 expression in cancer cells.39 Overexpression of epidermal growth factor receptor pathway substrate 8 induces FoxM1 expression in cancer cells through PI3K activation. However, the specific PI3K isoform was never identified. The present study provide clear evidence that FoxM1 is induced in the pulmonary vasculature in a p110γ-dependent manner following inflammatory vascular injury. Our studies demonstrate the GPCR agonist SDF-1α is a critical upstream activator of the GPCR-dependent PI3K isoform p110γ in human lung ECs. Activation of p110γ results in FoxO1 nuclear export and inactivation and thereby induction of FoxM1 expression. We observed that inhibition of FoxO1 reversed p110γ-inhibition-mediated blockade of SDF-1α-induced FoxM1 expression whereas inhibition of FoxO1 nuclear export, i.e. activation of FoxO1 inhibited SDF-1α-induced FoxM1 expression in ECs. Importantly, SDF-1α is induced in mouse lungs only in the recovery phase following LPS challenge. A recent study shows that p110γ cooperating with the class II PI3K-C2β plays a role in sphingosine-1-phosphate-induced EC migration.40 However, we observed no induction of FoxM1 expression in ECs by sphingosine-1-phosphate. Thus, sphingosine-1-phosphate is not the upstream activator of p110γ to activate FoxM1-dependent endothelial regeneration. Taken together, our data suggest an important role of SDF-1α signaling in activating the GPCR-dependent p110γ PI3K and thereby promoting FoxM1-dependent endothelial regeneration and vascular repair.
p110γ, which is highly expressed in hematopoietic cells,41 regulates the early phase of transendothelial leukocyte migration occurring within minutes to hours.42,43 Consistently, we also observed an early (2h post-LPS) decrease of neutrophil infiltration in Pik3cg−/− mouse lungs. However, the subsequent neutrophil uptake in Pik3cg−/− mouse lungs (up to 24h post-LPS) was the same as in WT lungs. This increase paralleled the vascular permeability values at this time in both groups. At 48h and 72h post-LPS, the increases in neutrophil uptake persisted in Pik3cg−/− mouse lungs in which vascular permeability was also increased, whereas neutrophil uptake was restored to basal level in WT mouse lungs in which permeability had fully recovered. Restoring the expression of either p110γ or FoxM1 in ECs of Pik3cg−/− mouse lungs fully normalized vascular repair and resolved lung inflammation. Given that FoxM1 is essential for endothelial regeneration,11 the impaired resolution of lung inflammation in Pik3cg−/− mouse lungs is likely the result of failure of vascular integrity to recover in these lungs. In support of the concept that recovery of endothelial barrier function is required to resolve lung inflammation, studies have shown strong causal relationship between leakiness of the microvessel barrier and transendothelial migration of neutrophils.2,44,45 Additionally, we observed elevated mRNA levels of ICAM-1, iNOS and various pro-inflammatory mediators (TNFα and IL-6) in Pik3cg−/− mouse lungs at 48h post-LPS in contrast to Pg−/−/Tg mouse lungs. Since these pro-inflammatory pathways are not the direct transcriptional targets of FoxM1, it is unlikely that the persistent lung inflammation seen in Pik3cg−/− mouse lungs was the result of activation of these pathways. Our results support the essential role of impaired p110γ-FoxM1 signaling as being responsible for the defective vascular repair and thereby impaired resolution of inflammation.
Employing the mouse model of ALI induced by intratracheal instillation of LPS, Kim et al. observed decreased lung inflammation and vascular permeability in mice pretreated with p110γ inhibitor AS605240.46 The discrepancy of this observation with ours may ascribe to the different models of lung injury. In contrast to systemic administration of LPS which induces systemic inflammation and multiple organ injury, intratracheal instillation of LPS-induced injury is limited in the lung. As p110γ plays a critical role in neutrophil recruitment and migration, pretreatment with AS605240 to inhibit p110γ impairs neutrophil infiltration into the lung and thus may result in reduced lung inflammation and vascular injury in the lung injury model induced by intratracheal instillation of LPS. Consistent with the findings in LPS-induced endotoxemia, we also observed that p110γ deficiency impairs endothelial regeneration and induces persistent lung vascular injury and sustained lung inflammation as well as increased mortality following CLP-induced polymicrobial sepsis. In agreement with our observation, previous studies have shown that PI3K signaling plays an important protective role in the response to polymicrobial sepsis.47,48 Inhibition of PI3K with pan-PI3K inhibitors (wortmannin or LY294002) results in increased susceptibility to polymicrobial sepsis in CLP mice and induced a marked increase of mortality.47,48 In a separate report, Maus, et al. observed increased lung injury and mortality of Pik3cg−/− mice following pneumococcal infection.49 However, Martin, et al. demonstrates that p110γ deficiency or p110γ inhibition reduces multiorgan damage and improves survival.50 In the present study, we showed that p110γ was indispensible for endothelial regeneration and vascular repair following injury induced by endotoxemia and polymicrobial sepsis. Our studies for the first time have identified FoxM1 as the critical downstream effector of endothelial p110γ. We have shown that transgenic expression of FoxM1 normalizes vascular repair and reduces CLP-induced mortality. Importantly, we also observed decreased p110γ expression in the pulmonary vascular ECs of ARDS patients, supporting the concept that impaired p110γ-FoxM1 endothelial regeneration signaling pathway is a critical factor in persistent leaky lung microvessels and edema formation in the disease.
In summary, our studies provide unequivocal evidence that endothelial p110γ is required for FoxM1 expression in ECs in vivo and thereby mediates the endothelial regeneration and vascular repair program following sepsis-induced inflammatory vascular injury. Our data also showed decreased expression of p110γ in pulmonary vascular ECs of ARDS patients. Thus, activation of p110γ signaling may represent a novel therapeutic strategy for restoring vascular integrity and resolving lung inflammation associated with ALI/ARDS.
Supplementary Material
Clinical Perspectives.
The integrity of endothelial monolayer is essential for vascular homeostasis. However, little is known about the signaling pathways regulating regeneration of the endothelial barrier following inflammatory vascular injury. In this study, we have identified a novel function of p110γPI3K expressed in ECs in mediating endothelial regeneration and vascular repair. We showed that endothelial p110γ activation by GPCR signaling following sepsis-induced vascular injury was required for FoxM1 activation and the resulting repair of the endothelial barrier and resolution of inflammation. Our data also provide evidence of the clear association of ARDS with diminished expression of p110γPI3K in pulmonary vascular ECs of ARDS patients. Thus, development of means of activation of p110γPI3k-FoxM1 signaling may represent a novel strategy for treatment of inflammatory vascular diseases such as ALI/ARDS.
Acknowledgments
We thank Dr. Philippe Huber from the Department of Cellular Responses and Dynamics, CEA-INSERM-Joseph Fourier University, France for his generosity of providing the human CDH5 promoter and Dr. Robert G. Kalb from the Department of Neurology, University of Pennsylvania for providing FoxO1 nuclear export inhibitor psammaphysene A.
Funding Sources: This work was supported in part by NIH grants R01HL123957, R01HL125350, and P01HL077806 (Project 3) to Y.Y.Z.
Footnotes
Disclosures: None.
References
- 1.Aird WC. Endothelium in health and disease. Pharmacol Rep. 2008;60:139–143. [PubMed] [Google Scholar]
- 2.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 3.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 4.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 5.Austin GE, Ratliff NB, Hollman J, Tabei S, Phillips DF. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985;6:369–375. doi: 10.1016/s0735-1097(85)80174-1. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz RS, Holmes DR, Jr, Topol EJ. The restenosis paradigm revisited: an alternative proposal for cellular mechanisms. J Am Coll Cardiol. 1992;20:1284–1293. doi: 10.1016/0735-1097(92)90389-5. [DOI] [PubMed] [Google Scholar]
- 7.Mc Fadden EP, Bauters C, Lablanche JM, Quandalle P, Leroy F, Bertrand ME. Response of human coronary arteries to serotonin after injury by coronary angioplasty. Circulation. 1993;88(5 Pt 1):2076–2085. doi: 10.1161/01.cir.88.5.2076. [DOI] [PubMed] [Google Scholar]
- 8.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 10.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 11.Zhao YY, Gao XP, Zhao YD, Mirza MK, Frey RS, Kalinichenko VV, Wang IC, Costa RH, Malik AB. Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest. 2006;116:2333–2343. doi: 10.1172/JCI27154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minamino T, Komuro I. Regeneration of the endothelium as a novel therapeutic strategy for acute lung injury. J Clin Invest. 2006;116:2316–2319. doi: 10.1172/JCI29637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.London NR, Zhu W, Bozza FA, Smith MC, Greif DM, Sorensen LK, Chen L, Kaminoh Y, Chan AC, Passi SF, Day CW, Barnard DL, Zimmerman GA, Krasnow MA, Li DY. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med. 2010;2:23ra19. doi: 10.1126/scitranslmed.3000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363:689–691. doi: 10.1056/NEJMcibr1007320. [DOI] [PubMed] [Google Scholar]
- 15.Mirza MK, Sun Y, Zhao YD, Potula HH, Frey RS, Vogel SM, Malik AB, Zhao YY. FoxM1 regulates re-annealing of endothelial adherens junctions through transcriptional control of beta-catenin expression. J Exp Med. 2010;207:1675–1685. doi: 10.1084/jem.20091857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 17.Goldenberg NM, Steinberg BE, Slutsky AS, Lee WL. Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med. 2011;3:88ps25. doi: 10.1126/scitranslmed.3002011. [DOI] [PubMed] [Google Scholar]
- 18.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- 19.Costa RH. FoxM1 dances with mitosis. Nat Cell Biol. 2005;7:108–110. doi: 10.1038/ncb0205-108. [DOI] [PubMed] [Google Scholar]
- 20.Kalinichenko VV, Gusarova GA, Tan Y, Wang IC, Major ML, Wang X, Yoder HM, Costa RH. Ubiquitous expression of the forkhead box M1B transgene accelerates proliferation of distinct pulmonary cell types following lung injury. J Biol Chem. 2003;278:37888–37894. doi: 10.1074/jbc.M305555200. [DOI] [PubMed] [Google Scholar]
- 21.Laoukili J, Kooistra MR, Bras A, Kauw J, Kerkhoven RM, Morrison A, Clevers H, Medema RH. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7:126–136. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- 22.Ye H, Kelly TF, Samadani U, Lim L, Rubio S, Overdier DG, Roebuck KA, Costa RH. Hepatocyte nuclear factor 3/fork head homolog 11 is expressed in proliferating epithelial and mesenchymal cells of embryonic and adult tissues. Mol Cell Biol. 1997;17:1626–1641. doi: 10.1128/mcb.17.3.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitman M, Kaplan DR, Schaffhausen B, Cantley L, Roberts TM. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985;315:239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- 24.Leevers SJ, Vanhaesebroeck B, Waterfield MD. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr Opin Cell Biol. 1999;11:219–225. doi: 10.1016/s0955-0674(99)80029-5. [DOI] [PubMed] [Google Scholar]
- 25.Stephens LR, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, Smrcka AS, Thelen M, Cadwallader K, Tempst P, Hawkins PT. The G beta gamma sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 1997;89:105–114. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 26.Guillermet-Guibert J, Bjorklof K, Salpekar A, Gonella C, Ramadani F, Bilancio A, Meek S, Smith AJ, Okkenhaug K, Vanhaesebroeck B. The p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proc Natl Acad Sci U S A. 2008;105:8292–8297. doi: 10.1073/pnas.0707761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson GJ, Milne L, Kulkarni S, Sasaki T, Walker S, Andrews S, Crabbe T, Finan P, Jones G, Jackson S, Camps M, Rommel C, Wymann M, Hirsch E, Hawkins P, Stephens L. PI(3)Kgamma has an important context-dependent role in neutrophil chemokinesis. Nat Cell Biol. 2007;9:86–91. doi: 10.1038/ncb1517. [DOI] [PubMed] [Google Scholar]
- 29.Ong E, Gao XP, Predescu D, Broman M, Malik AB. Role of phosphatidylinositol 3-kinase-gamma in mediating lung neutrophil sequestration and vascular injury induced by E. coli sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1094–1103. doi: 10.1152/ajplung.00179.2005. [DOI] [PubMed] [Google Scholar]
- 30.Yum HK, Arcaroli J, Kupfner J, Shenkar R, Penninger JM, Sasaki T, Yang KY, Park JS, Abraham E. Involvement of phosphoinositide 3-kinases in neutrophil activation and the development of acute lung injury. J Immunol. 2001;167:6601–6608. doi: 10.4049/jimmunol.167.11.6601. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak TW, Ohashi PS, Suzuki A, Penninger JM. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 32.Hers I. Insulin-like growth factor-1 potentiates platelet activation via the IRS/PI3Kalpha pathway. Blood. 2007;110:4243–4252. doi: 10.1182/blood-2006-10-050633. [DOI] [PubMed] [Google Scholar]
- 33.Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Francon B, Martin T, Gretener D, Perrin D, Leroy D, Vitte PA, Hirsch E, Wymann MP, Cirillo R, Schwarz MK, Rommel C. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 34.Huang X, Zhao YY. Transgenic expression of FoxM1 promotes endothelial repair following polymicrobial sepsis in mice. PLoS ONE. 2012;7:e50094. doi: 10.1371/journal.pone.0050094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prandini MH, Dreher I, Bouillot S, Benkerri S, Moll T, Huber P. The human VE-cadherin promoter is subjected to organ-specific regulation and is activated in tumour angiogenesis. Oncogene. 2005;24:2992–3001. doi: 10.1038/sj.onc.1208483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albertine KH, Soulier MF, Wang Z, Ishizaka A, Hashimoto S, Zimmerman GA, Matthay MA, Ware LB. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol. 2002;161:1783–1796. doi: 10.1016/S0002-9440(10)64455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagashima T, Shigematsu N, Maruki R, Urano Y, Tanaka H, Shimaya A, Shimokawa T, Shibasaki M. Discovery of novel forkhead box O1 inhibitors for treating type 2 diabetes: improvement of fasting glycemia in diabetic db/db mice. Mol Pharmacolo. 2010;78:961–970. doi: 10.1124/mol.110.065714. [DOI] [PubMed] [Google Scholar]
- 38.Schroeder FC, Kau TR, Silver PA, Clardy J. The pasammaplysenes, specific inhibitors of FOXO1a nuclear export. J Nat Prod. 2005;68:574–576. doi: 10.1021/np049624z. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Teh MT, Ji Y, Patel V, Firouzabadian S, Patel AA, Gutkind JS, Yeudall WA. EPS8 upregulates FoxM1 expression, enhancing cell growth and motility. Carcinogenesis. 2010;31:1132–1141. doi: 10.1093/carcin/bgq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tibolla G, Piñeiro PR, Chiozzotto D, Mavrommati I, Wheeler AP, Norata GD, Catapano AL, Maffucci T, Falasca M. Class II phosphoinositide 3-kinases contribute to endothelial cells morphogenesis. PLoS ONE. 2013;8:e53808. doi: 10.1371/journal.pone.0053808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kok K, Geering B, Vanhaesebroeck B. Regulation of phosphoinositide 3-kinase expression in health and disease. Trends Biochem Sci. 2009;34:115–127. doi: 10.1016/j.tibs.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Ruckle T, Schwarz MK, Rommel C. PI3Kgamma inhibition: towards an ‘aspirin of the 21st century’? Nat Rev Drug Discov. 2006;5:903–918. doi: 10.1038/nrd2145. [DOI] [PubMed] [Google Scholar]
- 43.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 44.Cepinskas G, Noseworthy R, Kvietys PR. Transendothelial neutrophil migration. Role of neutrophil-derived proteases and relationship to transendothelial protein movement. Circ Res. 1997;81:618–626. doi: 10.1161/01.res.81.4.618. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez-Borja M, van Buul JD, Hordijk PL. The regulation of leucocyte transendothelial migration by endothelial signalling events. Cardiovasc Res. 2010;86:202–210. doi: 10.1093/cvr/cvq003. [DOI] [PubMed] [Google Scholar]
- 46.Kim DI, Kim SR, Kim HJ, Lee SJ, Lee HB, Park SJ, Im MJ, Lee YC. PI3K-gamma inhibition ameliorates acute lung injury through regulation of IkappaBalpha/NF-kappaB pathway and innate immune responses. J Clin Immunol. 2012;32:340–351. doi: 10.1007/s10875-011-9628-1. [DOI] [PubMed] [Google Scholar]
- 47.Williams DL, Li C, Ha T, Ozment-Skelton T, Kalbfleisch JH, Preiszner J, Brooks L, Breuel K, Schweitzer JB. Modulation of the phosphoinositide 3-kinase pathway alters innate resistance to polymicrobial sepsis. J Immunol. 2004;172:449–456. doi: 10.4049/jimmunol.172.1.449. [DOI] [PubMed] [Google Scholar]
- 48.Wrann CD, Tabriz NA, Barkhausen T, Klos A, van Griensven M, Pape HC, Kendoff DO, Guo R, Ward PA, Krettek C, Riedemann NC. The phosphatidylinositol 3-kinase signaling pathway exerts protective effects during sepsis by controlling C5a-mediated activation of innate immune functions. J Immunol. 2007;178:5940–5948. doi: 10.4049/jimmunol.178.9.5940. [DOI] [PubMed] [Google Scholar]
- 49.Maus UA, Backi M, Winter C, Srivastava M, Schwarz MK, Ruckle T, Paton JC, Briles D, Mack M, Welte T, Maus R, Bohle RM, Seeger W, Rommel C, Hirsch E, Lohmeyer J, Preissner KT. Importance of phosphoinositide 3-kinase gamma in the host defense against pneumococcal infection. Am J Respir Crit Care Med. 2007;175:958–966. doi: 10.1164/rccm.200610-1533OC. [DOI] [PubMed] [Google Scholar]
- 50.Martin EL, Souza DG, Fagundes CT, Amaral FA, Assenzio B, Puntorieri V, Del Sorbo L, Fanelli V, Bosco M, Delsedime L, Pinho JF, Lemos VS, Souto FO, Alves-Filho JC, Cunha FQ, Slutsky AS, Ruckle T, Hirsch E, Teixeira MM, Ranieri VM. Phosphoinositide-3 kinase gamma activity contributes to sepsis and organ damage by altering neutrophil recruitment. Am J Respir Crit Care Med. 2010;182:762–773. doi: 10.1164/rccm.201001-0088OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.