Abstract
Accumulating evidence indicates that inflammatory signals required for maximizing effector T cell generation have opposing effects on the development of memory T cell precursors. Toll-Like receptor (TLR)2, and TLR9 significantly contribute to the inflammatory milieu and therefore in this study we examined whether the absence of TLR9 alone or the combined absence of TLR2 and TLR9 would affect vaccine-mediated immunity to Mtb. We found that TLR9KO and TLR2/9DKO mice vaccinated with a live Mtb auxotroph, akin to vaccinated WT mice, exhibited early control of Mtb growth in the lungs compared to their naïve counterparts. The granulomatous response, IFNγ production and cellular recruitment to the lungs were also similar in all the vaccinated groups of mice. These findings indicate that there is minimal contribution from TLR2 and TLR9 in generating memory immunity to Mtb with live vaccines. Defining the innate milieu that can drive maximal memory T cell generation with a tuberculosis vaccine needs further inquiry.
Keywords: Mycobacterium tuberculosis, vaccination, Toll-like Receptor 9, IL-12, memory immunity1
1. INTRODUCTION
Mycobacterium bovis, Bacillus Calmette-Guérin (BCG) is the only vaccine currently used against tuberculosis (TB). Whereas, BCG is clearly effective in preventing disseminated TB in children, the protection conferred in adults has been variable, ranging from 0 to 80% in different studies [1]. In the last decade, therefore, a great deal of research effort in TB was invested in generating new TB vaccines [2]. This concerted effort from many TB investigators has produced several new vaccine candidates including live recombinant BCG, viral vector-based and subunit vaccines [3]. In the pipe line are several live vaccine candidates in clinical trials [4, 5]. The recent failure of MVA85A booster immunization to provide better protection than BCG vaccination alone [6, 7], strongly indicates the need to continue efforts to understand the complexity of Mtb-specific memory T cell development, especially at the molecular level.
There is now accumulating evidence that inflammatory signals from the environment regulate the magnitude of effector and memory T cell generation. For example, high levels of Interleukin (IL)-12 leads to high T box transcription factor (T-bet) expression and induction of short-lived effector T cells while low expression favors the generation of memory precursor effector T cells [8]. Transcription factors such as Eomesodermin (Eomes) and B cell lymphoma 6 favor the development of memory T cells [9, 10]. Cytokine levels dictate the ratio between T-bet and Eomes and this maintains the balance between effector and memory T cells [11]. Consistent with the notion that curbing inflammatory response is conducive to memory T cell development, it was found that IL-21/IL-10/Signal transducer and activator of transcription 3 signaling axis is critical for the maturation of memory CD8+ T cells [12].
Mycobacterium tuberculosis (Mtb) expresses several ligands that stimulate TLRs on macrophages and dendritic cells (DCs) [13]. In particular, Mtb has been shown to stimulate signaling through TLR2 and TLR9 [14–16]. We and others have previously shown that although TLR2 is not essential for protection against acute infection [17], it is required in maintaining the granulomatous response and in controlling inflammation during chronic Mtb infection [18, 19]. We have also reported the redundant role of TLR2 in mediating protective immunity to secondary Mtb infection [17]. However, the requirement of TLR9 in the generation of protective immune response to Mtb has not been addressed. Based on the growing evidence that decreased IL-12 levels favor central memory T cell (TCM) generation [8, 11] and our finding that DCs lacking TLR9 have diminished IL-12 production [20], we posited that memory immune response against Mtb would be enhanced in TLR9KO mice. The main approach to improve BCG vaccine efficacy has been to modify the vaccine by inclusion of different Mtb antigens [21–23]. However, in this study, the approach was to modify the host innate environment and determine whether vaccine efficacy would improve. We therefore examined memory immunity against Mtb in the presence and absence of TLR9 signaling in a live auxotroph vaccination-challenge model.
2. MATERIALS AND METHODS
2.1. Mice and Mtb
Breeding pairs of TLR9KO and TLR2/9DKO were obtained from Alan Sher’s laboratory, NIH, Bethesda, MD [14, 24]. These mice were bred under pathogen free conditions at the animal facility at Rutgers-New Jersey Medical School, Newark, NJ. C57Bl/6 WT mice were purchased from National Cancer Institute. Mtb infected mice were housed in the BSL3 facility at Rutgers, New Jersey Medical School. Mice protocols used in this study have been approved by the Rutgers-Institutional Animal Care and Use Committee. ΔleuCΔpanCD double auxotroph mutant of H37Rv, mc26206 was obtained from the laboratory of William Jacobs at Albert Einstein College of Medicine, NY. The virulent strain of Mtb Erdman (Trudeau Institute, Saranac Lake, NY) was prepared as previously described [17].
2.2. Establishment of auxotroph induced memory immunity
Age matched 6–8 week old WT, TLR9KO and TLR2/9DKO mice were vaccinated subcutaneously with 4×106 colony forming units (CFU) of mc26206. After 9 weeks [25, 26], vaccinated mice of all genotypes and an equal number of genotype-matched unvaccinated (naïve) mice were challenged with a low dose of Mtb Erdman (50–100 CFU) by aerosol infection (Glas-Col) as previously described [19].
2.3. Bacterial burden
Superior lobe of lung or spleen tissue was homogenized in 1ml of PBS with 0.05% Tween-80. Serial dilutions of the tissue lysates were plated on 7H11 plates and CFU was enumerated after incubation at 37°C for 14–21 days.
2.4. Lung cell preparation and flow cytometry
The middle and left lobes of the lungs were perfused with 10ml sterile PBS, and digested with 2mg/ml collagenase D at 37°C for 30minutes. Reaction was stopped with the addition of 20µl of 5mM EDTA. Digested tissue was mashed through a 40µm nylon membrane filter to obtain single cell suspensions. Red blood cells were lysed using ACK lysis buffer. Single cell suspensions were surface stained with directly conjugated fluorochrome labeled anti-mouse CD4-V450 (clone RM4-5; BD Horizon), anti-mouse CD8-AF488 (clone 53-6.7; BD Pharmingen), anti-mouse CD44-AF700 (clone IM7; BD Pharmingen) and anti-mouse CD62L-PerCPCy5.5 (clone MEL-14; BD Pharmingen) antibodies. For tetramer staining, lung cells were first surface stained with ESAT6(1–20)-PE tetramer (NIH Tetramer Facility) for 1hr at 37°C, followed by surface staining for T cell markers. Following staining, cells were washed with FACS buffer, fixed in 4% paraformaldehyde and acquired on LSR-II (BD Biosciences). Frequency of specific cell types was calculated using Flow Jo software.
2.5. Detection of IFNγ by ELISPOT
Mtb specific IFNγ producing cells in the lungs were determined by the enzyme linked immunospot assay (ELISPOT). 96 well MultiScreen HTS filter plates (Millipore) were coated overnight with 8µg/ml anti-IFNγ antibody (clone R4-6A2; BD Biosciences). Lung cells were seeded at a concentration of 0.025–0.1×106 per well. The cells were re-stimulated ex vivo with Mtb infected bone marrow derived dendritic cells (BMDC) overnight at a ratio of 1:2. Cultures were supplemented with 20U/ml IL-2 and incubated at 37°C for 2 days. Post incubation period, the plates were washed, treated sequentially with biotinylated secondary antibody (clone XMG1.2; BD Biosciences), streptavidin HRP and the substrate: 3-amino-9-ethyl-carbzole. Spot forming units were determined using an ELISPOT plate reader (Cellular Technology).
2.6. Gene expression by real time PCR
Post caval lobe of the lung from infected mice was homogenized in 1ml of TRIzol reagent (Invitrogen) and total RNA was extracted using RNeasy column (Qiagen). cDNA was prepared from the total RNA by reverse transcription using Superscript II (Invitrogen). Real time PCR was performed using TaqMan probes. Beta-actin was used as the endogenous control. (Anti-mouse IL-12p40 accession number: Mm00434174_m1; anti-mouse beta actin accession number: Mm00607939_s1; anti-mouse IL-12p35 accession number: Mm00434169_m1; anti-mouse IL-23p19 accession number: Mm01160011_g1; Life Technologies). Total RNA from uninfected naïve lung was used as calibrators. Relative gene expression was expressed as 2−ΔΔCt.
2.7. Histological examination of lung tissue
Inferior lobe of the lung was perfused with 10ml of PBS and fixed in 4% paraformaldehyde for 4 days. Paraffin embedded-tissue sections were cut and stained with haematoxylin (H) and eosin (E). Stereoscopic images of H&E stained sections of whole lungs were obtained using Act-1 software from Nikon.
2.8. Statistical analysis
Graph pad Prism software was used. One or Two way ANOVA with Bonferroni’s correction was used depending on the number of groups compared.
3. RESULTS
3.1. Increased IFNγ secreting T cells in the lungs of auxotroph-immunized TLR2/9DKO mice
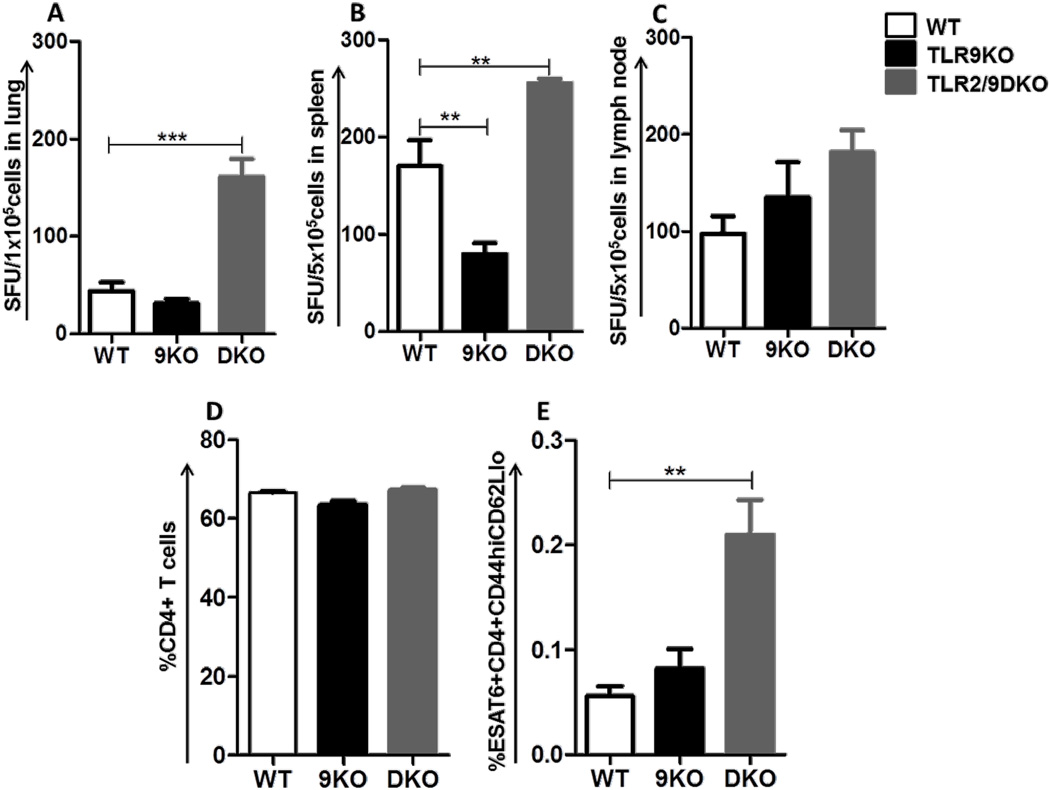
9 weeks following auxotroph immunization, the percentage of IFNγ producing cells in the lungs, spleen and draining lymph nodes from WT, TLR9KO and TLR2/9DKO mice were analyzed by ELISPOT. Interestingly, we observed an increased frequency of IFNγ producing cells in the lungs and spleen of TLR2/9DKO mice compared to WT mice (Fig 1A,B). Consistent with the increased IFNγ producing cells, flow cytometric analysis showed a higher percentage of ESAT6-tetramer+ CD4+ T effector memory cells (TEM: CD44hi CD62Llo) in the lungs of TLR2/9DKO mice when compared to WT mice (Fig 1E). A similar increase was not observed in the lungs of TLR9KO mice (Fig 1A,B,E). The total percentage of CD4+ T cells in the lungs was similar in the three groups of vaccinated mice (Fig 1D). Increased IFNγ producing cells were also present in the lymph nodes of the TLR2/9DKO mice (Fig 1C), although not statistically significant. These data indicate that auxotroph immunization induces a higher frequency of IFNγ producing cells when both TLR2 and TLR9 are absent.
Fig. 1. Increase frequency of IFNγ producing T cells in the lungs of auxotroph immunized TLR2/9DKO mice.
WT, TLR9KO and TLR2/9DKO mice were vaccinated subcutaneously with Mtb-auxotroph. 9 weeks post-vaccination, single cell suspension was prepared from lungs, spleen and draining lymph nodes. Frequency of IFNγ+ cells was measured as number of spots upon ex vivo stimulation with Mtb-infected dendritic cells from lungs (a), spleen (b) and lymph nodes (c). Percent ESAT6+CD4+CD44hiCD62Llo T cells from the lungs were quantitated (d, e). Each group includes 4–5 mice per time point. (Data represented as mean+/− SEM, *** p<0.001 and ** p<0.01)
3.2. Similar induction of memory immunity in TLR9KO and TLR2/9DKO mice compared to WT mice
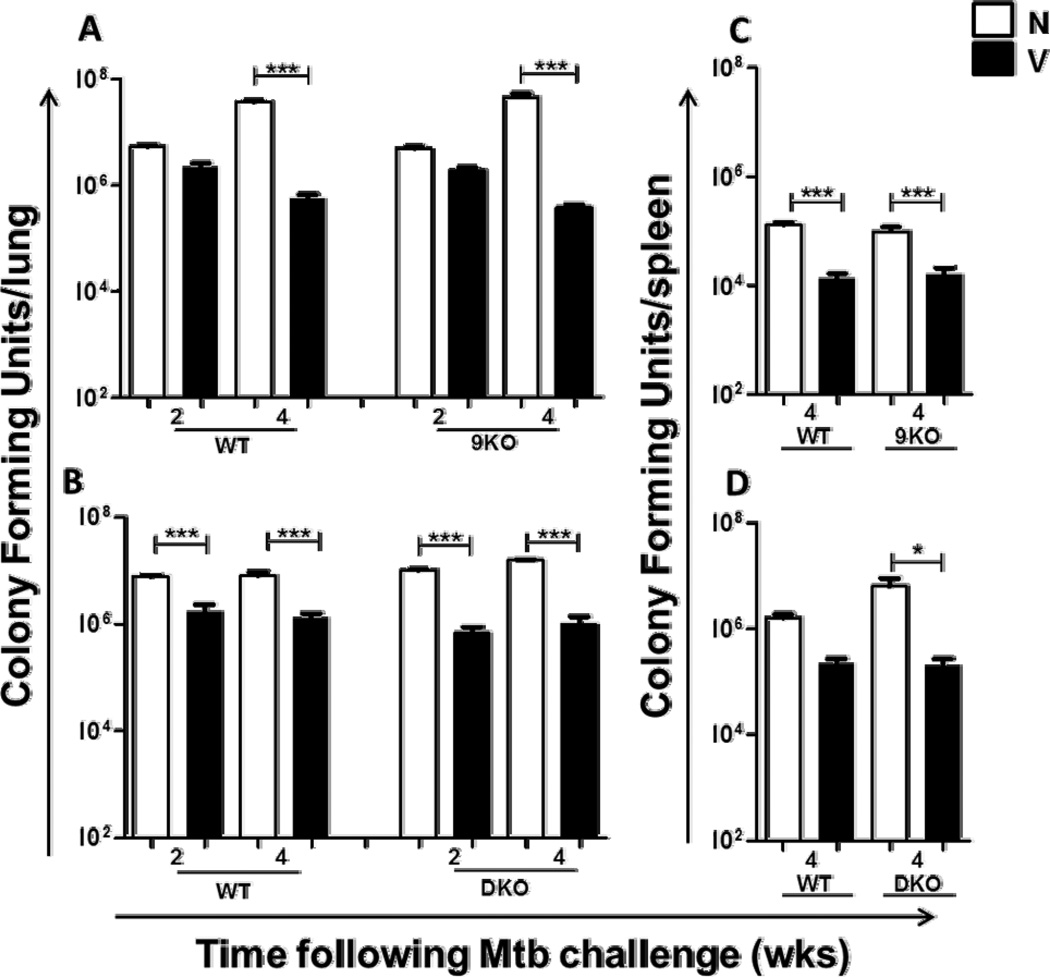
Since there was a higher frequency of IFNγ producers and TEM in the lungs of TLR2/9DKO mice, we hypothesized that this would lead to enhanced protection in these mice upon challenge with Mtb. Consistent with an earlier study [27], WT mice vaccinated with mc26206 were able to control bacterial burden in the lungs at a significantly lower number than their naïve counterparts at 2 and 4 weeks following Mtb challenge (Fig. 2A,B). Similarly, significant reduction in Mtb load in the lungs was also observed in the vaccinated TLR9KO (Fig. 2A) and TLR2/9DKO mice (Fig. 2B) at both 2 and 4 weeks, in comparison to their naive counterparts. It is worthy of note that the presence of increased IFNγ producers in the lungs of TLR2/9DKO mice did not improve their ability to control Mtb infection. Bacterial burden in the spleens of all groups of vaccinated mice was also significantly lower compared to corresponding naïve mice (Fig. 2C,D). These data indicate that signaling through TLR2 and TLR9 is not required to generate memory immunity and concomitant enhanced restriction of Mtb growth in the lungs and spleens of vaccinated mice.
Fig. 2. Similar induction of memory immunity in TLR9KO and TLR2/9DKO mice compared to WT mice.
WT, TLR9KO and TLR2/9DKO mice were vaccinated subcutaneously with Mtb-auxotroph. 9 weeks post-vaccination, naïve and vaccinated mice of all three genotypes were challenged with a low dose of Mtb-Erdman via aerosol infection. Serial dilutions of lung and spleen homogenates were plated on 7H11 agar plates and the bacterial load was determined after 14–21 days of incubation at 37°C. Bacterial burdens in the lungs at 2 and 4 weeks (a and b) and spleens at 4 weeks (c and d) are compared. Each group includes 4–5 mice per time point. (Naïve: N, Vaccinated: V, Data represented as mean +/− SEM, *** p<0.001 and * p<0.05)
3.3. Granulomatous inflammation is comparable between vaccinated WT, TLR9KO and TLR2/9DKO mice
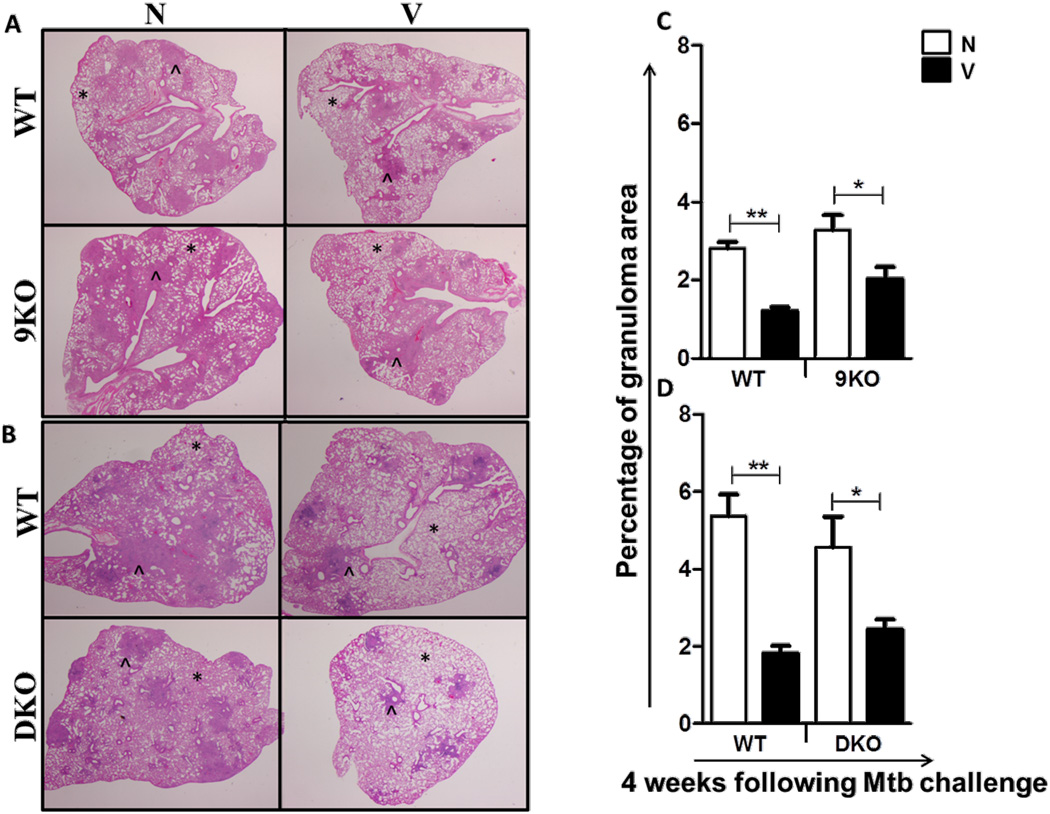
The enhanced control of bacterial burden in the lungs of vaccinated mice is typically associated with smaller granulomatous lesions [28]. As expected, WT naïve mice exhibited extensive granulomatous inflammation in the lungs at 4 weeks post challenge. In contrast, the vaccinated WT mice displayed compact and localized granuloma with more clear alveolar spaces (Fig 3A,B). Vaccinated TLR9KO (Fig 3A) and TLR2/9DKO (Fig 3B) mice also presented with smaller granulomatous inflammation than their naïve counterparts. Also, the total granulomatous area in the lungs of vaccinated WT, TLR9KO and TLR2/9DKO mice was significantly lower in comparison to their naïve counterparts (Fig 3C,D).
Fig. 3. Granulomatous inflammation in the lungs is comparable between the vaccinated WT, TLR9KO and TLR2/9DKO mice.
Formalin-fixed, paraffin-embedded lung tissue was obtained at 4 weeks following infection, and sections were stained using a standard H&E protocol. WT and 9KO (a) and WT and DKO (b). (*: clear alveolar spaces, ^: tubercle granuloma). Each group includes 4–5 mice per time point and the stereoscopic images are representative of a section per group. The granulomatous area was compared between the naïve and vaccinated groups of mice at 4 weeks post challenge (c and d). (5–10 granulomas per section, Naïve: N, Vaccinated: V, data represented as mean +/− SEM, ** p<0.01 and * p<0.05)
3.4. Vaccinated TLR9KO and TLR2/9DKO mice exhibit early recruitment of IFNγ secreting T cells and have equivalent IL-12 expression
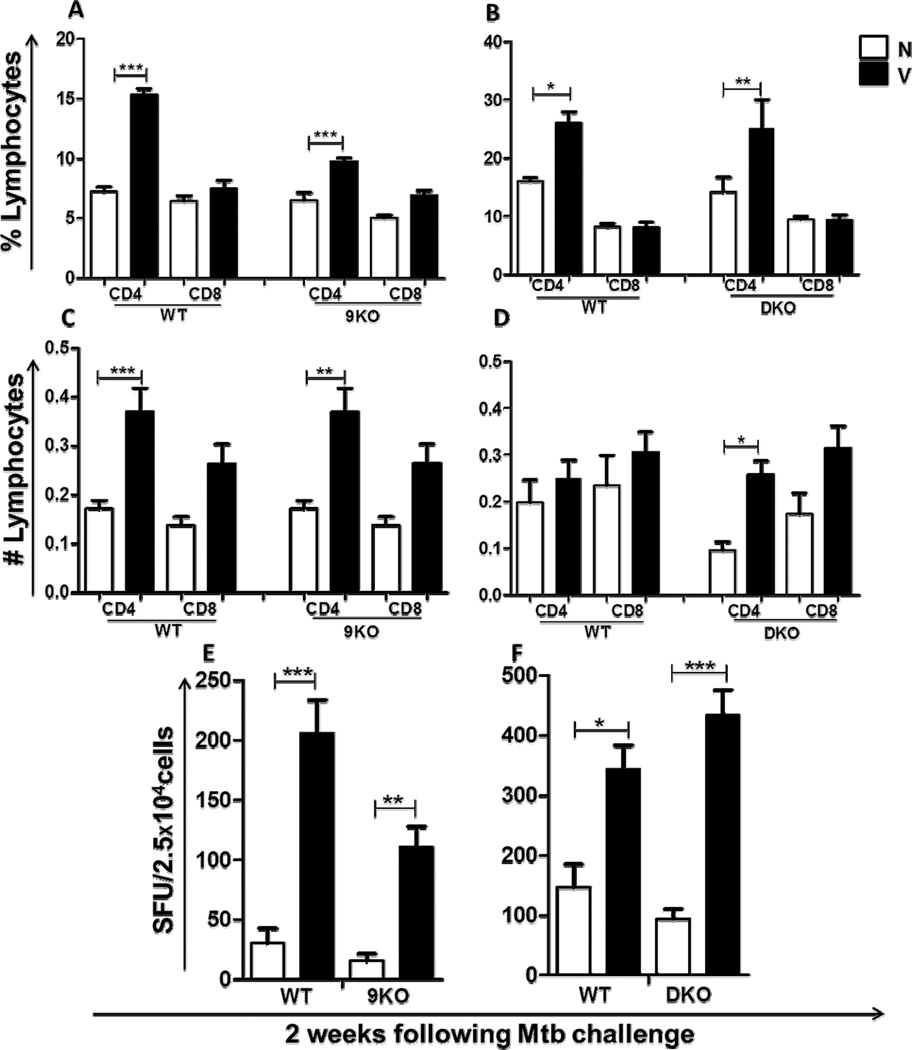
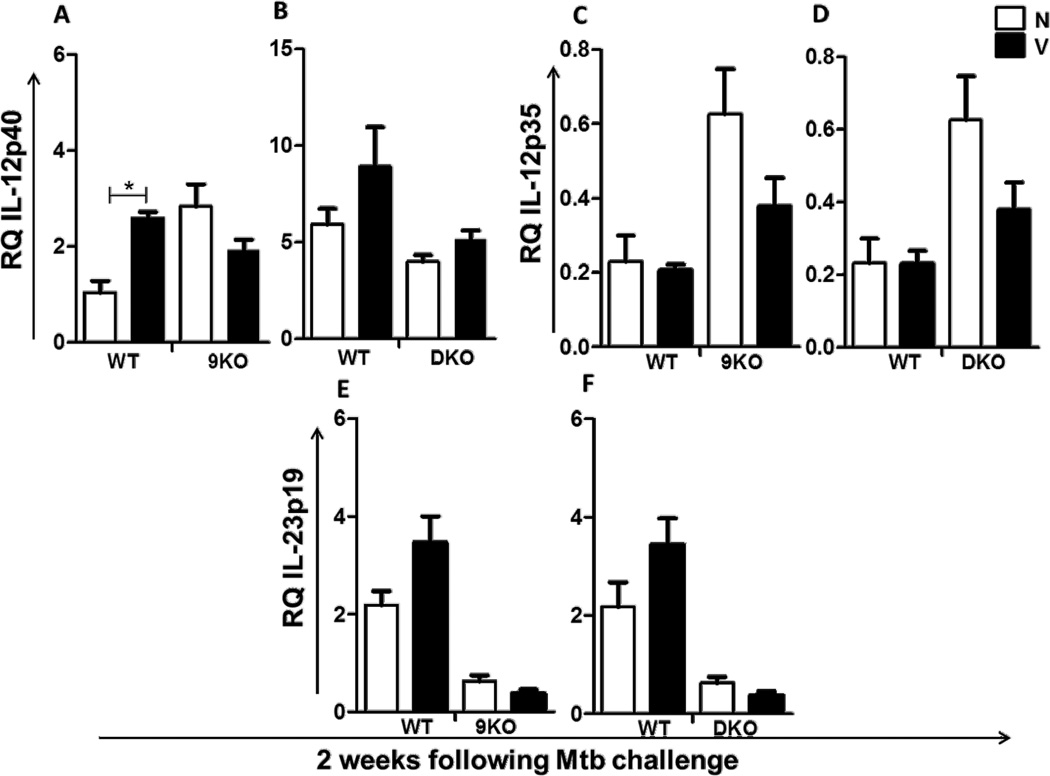
As expected, in comparison to their naïve counterparts, vaccinated WT mice had a significantly higher recruitment of CD4+ T cells to the lungs 2 weeks following Mtb challenge (Fig. 4A–D). A similar increased recruitment was also observed in vaccinated TLR9KO (Fig. 4A,C) and TLR2/9DKO (Fig. 4B,D) mice when compared with the naïve controls. The recruitment of CD8+ T cells however did not significantly differ in the three groups of vaccinated mice (Fig. 4A–D). Consistent with the earlier recruitment of CD4+ T lymphocytes to the lungs, we observed a higher frequency of IFNγ producing cells in the lungs of vaccinated WT, TLR9KO and TLR2/9DKO mice when compared to their naïve counterparts (Fig. 4E,F). Interestingly, we found a lower frequency of IFNγ producers in the lungs of vaccinated TLR9KO mice when compared to vaccinated WT mice (Fig 4E), although there was no difference in bacterial burden between the two vaccinated groups of mice. We also found that the expression of IL-12p40 (Fig. 5A,B), IL-12p35 (Fig. 5C,D) and IL-23p19 (Fig. 5E,F) in the lungs following Mtb challenge was not affected either in TLR9KO or TLR2/9DKO mice. Taken together, these findings suggest that the early recruitment of IFNγ secreting CD4+ T cells to the lungs and in vivo expression of IL-12 upon Mtb challenge are not affected by the loss of TLR2 and TLR9 signaling.
Fig. 4. Early lymphocytic infiltration and IFNγ recall response in the lungs of vaccinated WT, TLR9KO and TLR2/9DKO mice.
CD4+ T cells and CD8+ T cells in the lungs of WT and 9KO mice (a and c) and WT and DKO mice (b and d) at 2 weeks post-Mtb challenge were determined. Frequency of IFNγ positive lung cells from WT and 9KO mice (e) and WT and DKO mice (f) was measured as number of spots upon ex vivo stimulation with Mtb-infected dendritic cells. Each group includes 4–5 mice per time point. (Naïve: N, Vaccinated: V, Data represented as mean +/− SEM,*** p<0.001 and * p<0.05)
Fig. 5. In vivo expression of IL-12 in the lungs is not compromised in TLR9KO and TLR2/9DKO mice.
RNA was extracted from the lungs of naïve and vaccinated WT, 9KO and DKO mice at 2 weeks post-Mtb challenge and gene expression of IL-12p40 (a and b), IL-12p35 (c and d) and IL-23p19 (e and f) was determined by real time RT-PCR using TaqMan probes. Each group includes 4–5 mice per time point. (Naïve: N, Vaccinated: V, Data represented as mean +/− SEM,* p<0.05)
4. DISCUSSION
In an earlier in vitro study we had found that IL-12 production from macrophages and DCs is reliant on the innate receptors, TLR2 and TLR9 [20]. Based on that finding, we argued that the production of IL-12 in vivo would be significantly reduced in the absence of TLR2 and TLR9 and this would in turn favor an enhanced memory response, analogous to what has been reported with other pathogens [29, 30]. However, findings from the current study indicate that TLR2 and TLR9, are dispensable for a live vaccineinduced memory immune response against Mtb. Furthermore, examination of lung gene expression revealed that TLR9KO and TLR2/9DKO naïve and vaccinated mice were fully capable of expressing IL-12 in the lungs. This suggests that other innate receptors such as the C-type lectin receptors (CLRs) and Nucleotide-binding Oligomerization domain (NOD) like receptors (NLRs) could compensate for the lack of TLR2 and TLR9 in IL-12 induction in vivo during Mtb infection. Indeed, Mtb induced signaling through Dectin-1 (type II CLR) on splenic DCs has been shown to enhance IL-12 production in vitro [31]. Despite the requirement for NLRs on macrophages for IL-12 production in vitro, NOD-2 deficient mice can control Mtb infection similar to WT mice. [32], suggesting that during Mtb infection in vivo, multiple signaling pathways induce IL-12.
Importance of IFNγ in protection against Mtb infection is clear since mice lacking the cytokine are highly susceptible to Mtb infection [33, 34]. However, recent studies indicate that CD4+ T effector cells can mediate protection against Mtb in an IFNγ independent manner [35, 36]. It is possible that in the absence of IFNγ, Th17 cells may confer protection early on during Mtb infection [37]. In this study we found that either increase or decrease in IFNγ producing cells in the TLR-deficient mice, in comparison to WT mice, did not influence the disease outcome. These data support the growing consensus that IFNγ is not the critical effector cytokine mediating the control of Mtb infection.
TLR ligands have been widely used in various subunit and protein based vaccines. Using adjuvants such as Lipokel, a TLR2 ligand in a subunit vaccine preparation [38] and CpG-complexed liposomes with ESAT6 protein [39] have been demonstrated to mediate protection to Mtb challenge. These studies suggest a critical role for TLR stimulation at the time of vaccination. In our study, mice vaccinated with mc26206 were able to protect against Mtb infection even in the absence of TLR2 and TLR9 signaling. This indicates that live vaccines, unlike subunit or peptide/protein vaccines, can bypass the requirement for TLR in generating memory immunity to Mtb.
Based on the differential expression of a number of cell surface markers, antigen-experienced CD4+ and CD8+ T cells can be separated into TCM cells that chiefly migrate to lymphoid organs and TEM cells that largely migrate to extra-lymphoid organs [40, 41]. While both TCM and TEM provide protection, only TCMs are long-lived [42] and also provide better and long-term protection [43–45]. BCG vaccination induces predominantly TEMs, [46–48], which could partly explain its variable efficacy in protection. Consistent with this theory, BCG ΔureC::hly vaccine, a recombinant variant of BCG, induces an increased TCM response in the vaccinated mice and confers better protection against challenge Mtb infection in comparison to BCG which induces predominantly a TEM response [49]. Mechanistically, the enhanced protection afforded by BCG ΔureC::hly vaccine was shown to be at the level of AIM2 inflammasome activation with a concomitant increase in production of IL-1β and IL-18 [50]. These findings suggest that perhaps skewing the innate response towards enhanced IL-1β and IL-18 may promote TCM development and enhance vaccine efficacy. Clearly, future studies aimed at defining the innate milieu that can drive maximal memory T cell generation with a TB vaccine candidate are needed.
5. CONCLUSION
We conclude that vaccine mediated immunity to Mtb is not dependent on TLR2 and TLR9 signaling.
HIGHLIGHTS.
Vaccination with Mtb auxotroph protects mice from virulent Mtb infection.
Vaccine mediated immunity does not depend on TLR2 and TLR9 signaling.
IL-12 expression is not impaired in the lungs of TLR9KO mice.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grant AI084822. We thank Dr. Sher for TLR9KO and TLR2/9DKO mice, Dr. Stockinger for the Granulocyte colony stimulating factor-secreting cell line (X63) Dr. Jacobs for the Mtb auxotroph, mc26206 and John Altman, NIH Tetramer Facility for the ESAT6 tetramer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: TLR, Toll-like receptor; TB, tuberculosis; BCG, Bacillus Calmette-Guérin; IL, Interleukin; T-bet, T box transcription factor; Eomes, Eomesodermin; Mtb, Mycobacterium tuberculosis; DC, dendritic cells; T central memory, TCM; CFU, colony forming units; ELISPOT, enzyme linked immunospot assay; BMDC; bone marrow derived dendritic cell; H, hematoxylin; E, eosin; T effector memory, TEM; CLR, Ctype lectin recpetors; NOD, Nucleotide-binding Oligomerization domain; NLR, Nod-like receptors.
REFERENCES
- 1.Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, Rodrigues LC, Smith PG, Lipman M, Whiting PF, Sterne JA. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58:470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 2.Ottenhoff TH, Kaufmann SH. Vaccines against Tuberculosis: Where Are We and Where Do We Need to Go? PLoS pathogens. 2012;8:e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufmann SH. Tuberculosis vaccine development: strength lies in tenacity. Trends in immunology. 2012 doi: 10.1016/j.it.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann SH, Gengenbacher M. Recombinant live vaccine candidates against tuberculosis. Current opinion in biotechnology. 2012;23:900–907. doi: 10.1016/j.copbio.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Andersen P, Kaufmann SH. Novel vaccination strategies against tuberculosis. Cold Spring Harbor perspectives in medicine. 2014;4 doi: 10.1101/cshperspect.a018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, Mahomed H, McShane H, Team MATS. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381:1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, Fletcher HA, Hill AVS. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nature medicine. 2004;10:1240–1244. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 8.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nature immunology. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 10.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. Journal of immunology. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 12.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2011;35:792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryffel B, Fremond C, Jacobs M, Parida S, Botha T, Schnyder B, Quesniaux V. Innate immunity to mycobacterial infection in mice: critical role for toll-like receptors. Tuberculosis. 2005;85:395–405. doi: 10.1016/j.tube.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. The Journal of experimental medicine. 2005;202:1715–1724. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 16.Harding CV, Boom WH. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nature reviews. Microbiology. 2010;8:296–307. doi: 10.1038/nrmicro2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McBride A, Bhatt K, Salgame P. Development of a secondary immune response to Mycobacterium tuberculosis is independent of Toll-like receptor 2. Infection and immunity. 2011;79:1118–1123. doi: 10.1128/IAI.01076-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drennan MB, Nicolle D, Quesniaux VJ, Jacobs M, Allie N, Mpagi J, Fremond C, Wagner H, Kirschning C, Ryffel B. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. The American journal of pathology. 2004;164:49–57. doi: 10.1016/S0002-9440(10)63095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McBride A, Konowich J, Salgame P. Host defense and recruitment of Foxp3(+) T regulatory cells to the lungs in chronic Mycobacterium tuberculosis infection requires toll-like receptor 2. PLoS pathogens. 2013;9:e1003397. doi: 10.1371/journal.ppat.1003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pompei L, Jang S, Zamlynny B, Ravikumar S, McBride A, Hickman SP, Salgame P. Disparity in IL-12 release in dendritic cells and macrophages in response to Mycobacterium tuberculosis is due to use of distinct TLRs. Journal of immunology. 2007;178:5192–5199. doi: 10.4049/jimmunol.178.8.5192. [DOI] [PubMed] [Google Scholar]
- 21.Orme IM. Tuberculosis vaccine types and timings. Clinical and vaccine immunology : CVI. 2015;22:249–257. doi: 10.1128/CVI.00718-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Junqueira-Kipnis AP, Marques Neto LM, Kipnis A. Role of Fused Mycobacterium tuberculosis Immunogens and Adjuvants in Modern Tuberculosis Vaccines. Frontiers in immunology. 2014;5:188. doi: 10.3389/fimmu.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Costa AC, Nogueira SV, Kipnis A, Junqueira-Kipnis AP. Recombinant BCG: Innovations on an Old Vaccine. Scope of BCG Strains and Strategies to Improve Long-Lasting Memory. Frontiers in immunology. 2014;5:152. doi: 10.3389/fimmu.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 25.Sambandamurthy VK, Wang X, Chen B, Russell RG, Derrick S, Collins FM, Morris SL, Jacobs WR., Jr A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis. Nature medicine. 2002;8:1171–1174. doi: 10.1038/nm765. [DOI] [PubMed] [Google Scholar]
- 26.Hondalus MK, Bardarov S, Russell R, Chan J, Jacobs WR, Jr, Bloom BR. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infection and immunity. 2000;68:2888–2898. doi: 10.1128/iai.68.5.2888-2898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampson SL, Dascher CC, Sambandamurthy VK, Russell RG, Jacobs WR, Jr, Bloom BR, Hondalus MK. Protection elicited by a double leucine and pantothenate auxotroph of Mycobacterium tuberculosis in guinea pigs. Infection and immunity. 2004;72:3031–3037. doi: 10.1128/IAI.72.5.3031-3037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruppo V, Turner OC, Orme IM, Turner J. Reduced up-regulation of memory and adhesion/integrin molecules in susceptible mice and poor expression of immunity to pulmonary tuberculosis. Microbiology. 2002;148:2959–2966. doi: 10.1099/00221287-148-10-2959. [DOI] [PubMed] [Google Scholar]
- 29.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. Journal of immunology. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 30.Wilson DC, Grotenbreg GM, Liu K, Zhao Y, Frickel EM, Gubbels MJ, Ploegh HL, Yap GS. Differential regulation of effector- and central-memory responses to Toxoplasma gondii Infection by IL-12 revealed by tracking of Tgd057-specific CD8+ T cells. PLoS pathogens. 2010;6:e1000815. doi: 10.1371/journal.ppat.1000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothfuchs AG, Bafica A, Feng CG, Egen JG, Williams DL, Brown GD, Sher A. Dectin-1 interaction with Mycobacterium tuberculosis leads to enhanced IL-12p40 production by splenic dendritic cells. Journal of immunology. 2007;179:3463–3471. doi: 10.4049/jimmunol.179.6.3463. [DOI] [PubMed] [Google Scholar]
- 32.Gandotra S, Jang S, Murray PJ, Salgame P, Ehrt S. Nucleotide-binding oligomerization domain protein 2-deficient mice control infection with Mycobacterium tuberculosis. Infect Immun. 2007;75:5127–5134. doi: 10.1128/IAI.00458-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. The Journal of experimental medicine. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. The Journal of experimental medicine. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallegos AM, van Heijst JW, Samstein M, Su X, Pamer EG, Glickman MS. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS pathogens. 2011;7:e1002052. doi: 10.1371/journal.ppat.1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cowley SC, Elkins KL. CD4+ T cells mediate IFN-gamma-independent control of Mycobacterium tuberculosis infection both in vitro and in vivo. Journal of immunology. 2003;171:4689–4699. doi: 10.4049/jimmunol.171.9.4689. [DOI] [PubMed] [Google Scholar]
- 37.Wozniak TM, Saunders BM, Ryan AA, Britton WJ. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infection and immunity. 2010;78:4187–4194. doi: 10.1128/IAI.01392-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyne AS, Chan JG, Shanahan ER, Atmosukarto I, Chan HK, Britton WJ, West NP. TLR2-targeted secreted proteins from Mycobacterium tuberculosis are protective as powdered pulmonary vaccines. Vaccine. 2013;31:4322–4329. doi: 10.1016/j.vaccine.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 39.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, Bosio C, Dow S. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176:7335–7345. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 40.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 41.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 43.Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nature medicine. 2004;10:1104–1110. doi: 10.1038/nm1108. [DOI] [PubMed] [Google Scholar]
- 44.Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L, Yang ZY, Chakrabarti B, Rao SS, Schmitz JE, Montefiori DC, Barker BR, Bookstein FL, Nabel GJ. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312:1530–1533. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaveh DA, Carmen Garcia-Pelayo M, Hogarth PJ. Persistent BCG bacilli perpetuate CD4 T effector memory and optimal protection against tuberculosis. Vaccine. 2014;32:6911–6918. doi: 10.1016/j.vaccine.2014.10.041. [DOI] [PubMed] [Google Scholar]
- 47.Henao-Tamayo MI, Ordway DJ, Irwin SM, Shang S, Shanley C, Orme IM. Phenotypic definition of effector and memory T-lymphocyte subsets in mice chronically infected with Mycobacterium tuberculosis. Clinical and vaccine immunology : CVI. 2010;17:618–625. doi: 10.1128/CVI.00368-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaveh DA, Bachy VS, Hewinson RG, Hogarth PJ. Systemic BCG immunization induces persistent lung mucosal multifunctional CD4 T(EM) cells which expand following virulent mycobacterial challenge. PloS one. 2011;6:e21566. doi: 10.1371/journal.pone.0021566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogelzang A, Perdomo C, Zedler U, Kuhlmann S, Hurwitz R, Gengenbacher M, Kaufmann SH. Central memory CD4+ T cells are responsible for the recombinant Bacillus Calmette-Guerin DeltaureC::hly vaccine's superior protection against tuberculosis. The Journal of infectious diseases. 2014;210:1928–1937. doi: 10.1093/infdis/jiu347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saiga H, Nieuwenhuizen N, Gengenbacher M, Koehler AB, Schuerer S, Moura-Alves P, Wagner I, Mollenkopf HJ, Dorhoi A, Kaufmann SH. The Recombinant BCG DeltaureC::hly Vaccine Targets the AIM2 Inflammasome to Induce Autophagy and Inflammation. The Journal of infectious diseases. 2015;211:1831–1841. doi: 10.1093/infdis/jiu675. [DOI] [PubMed] [Google Scholar]