Abstract
Diisocyanates (dNCOs) are low molecular weight chemical sensitizers that react with autologous proteins to produce neoantigens. dNCO-haptenated proteins have been used as immunogens for generation of dNCO-specific antibodies and as antigens to screen for dNCO-specific antibodies in exposed individuals. Detection of dNCO-specific antibodies in exposed individuals for diagnosis of dNCO asthma has been hampered by poor sensitivities of the assay methods in that specific IgE can only be detected in approximately 25 % of the dNCO asthmatics. Apart from characterization of the conjugates used for these immunoassays, the choice of the carrier protein and the dNCO used are important parameters that can influence the detection of dNCO-specific antibodies. Human serum albumin (HSA) is the most common carrier protein used for detection of dNCO specific-IgE and -IgG but the immunogenicity and/or antigenicity of other proteins that may be modified by dNCO in vivo is not well documented. In the current study, 2,4-toluene diisocyanate (TDI) and 1,6-hexamethylene diisocyanate (HDI) were reacted with HSA and human hemoglobin (Hb) and the resultant adducts were characterized by (i) HPLC quantification of the diamine produced from acid hydrolysis of the adducts, (ii) 2,4,6-trinitrobenzene sulfonic acid (TNBS) assay to assess extent of cross-linking, (iii) electrophoretic migration in polyacrylamide gels to analyze intra- and inter-molecular cross-linking, and (iv) evaluation of antigenicity using a monoclonal antibody developed previously to TDI conjugated to Keyhole limpet hemocyanin (KLH). Concentration-dependent increases in the amount of dNCO bound to HDI and TDI, cross-linking, migration in gels, and antibody-binding were observed. TDI reactivity with both HSA and Hb was significantly higher than HDI. Hb-TDI antigenicity was approximately 30 % that of HSA-TDI. In conclusion, this data suggests that both, the extent of haptenation as well as the degree of cross-linking differs between the two diisocyanate species studied, which may influence their relative immunogenicity and/or antigenicity.
Keywords: 2,4-toluene diisocyanate; 1,6-hexamethylene diisocyanate; occupational asthma; haptenation
Introduction
Diisocyanates (dNCOs) are highly reactive chemicals used as cross-linking agents in the manufacture of polyurethane products such as paints, elastomers, and adhesives1–3. They are potent sensitizers and are a commonly reported cause of occupational chemical hypersensitivity reactions including asthma4,5. 2, 4-Toluene diisocyanate (2, 4-TDI) and 1, 6-hexamethylene diisocyanate (HDI) are among the most widely used isocyanates. Both have high vapor pressures6 and exposure often occurs through inhalation of vapors and aerosols during spraying operations at workplaces.
Immune-mediated hypersensitivity reactions to dNCOs include allergic rhinitis7, asthma8, hypersensitivity pneumonitis9,10 and allergic contact dermatitis11. Although most reported cases of isocyanate sensitization occur at workplaces13,14, it has been suggested that non-occupational exposure to the general public may also occur through use of “do-it-yourself” free diisocyanate containing commercial products such as polyurethane foams and sprays15,16. Once allergic sensitization to isocyanates occurs, asthmatic reactions may be triggered by exceedingly minute concentrations of isocyanates5,17,18.
Diisocyanates are low-molecular-weight compounds that must first react with autologous proteins to produce a functional antigen19. The fate of the dNCO in the body and the protein adducts responsible for immunological sensitization remain unknown8. Apart from reacting with proteins at the site of exposure, protein conjugation by dNCOs may also occur via glutathione (GSH) thiocarbamate intermediates. GSH is abundant in the airways and Wisnewski and colleagues demonstrated that albumin can be conjugated to TDI and HDI by GSH-TDI and GSH-HDI, respectively20. HSA is the most common carrier protein used for dNCO antibody immunoassays21 due to its prevalence in plasma22 to form dNCO adducts. Other molecules, such as keratin 1822, tubulin23, and the peptide glutathione24, have been found to be modified by dNCO exposure. Sabbioni and coworkers reported MDI bound to the N-terminal valine of Hb in MDI exposed rats and proposed Hb–MDI as a biological marker of MDI exposure25.
There is currently no simple diagnosis for dNCO-induced occupational asthma (OA)26. One approach that can potentially be used is testing for dNCO-specific IgE from a worker’s serum. For confirmation of the diagnosis of dNCOas the etiological agent of the occupational asthma, these assays have been reported to be specific (96–98%), but not sensitive (18–27%)27. These low sensitivities have been attributed to both assay limitations and potential IgE-independent dNCO asthma mechanisms21. Immunoassay standardization is critical for improvement of immunoassay sensitivity and comparison of results across studies19. A number of factors that may confound results from these immunoassays include the choice of dNCO used, the carrier protein employed, dNCO–protein reaction conditions, and post-reaction processing and characterization of the haptenated protein.
Wisnewski et al, in separate studies, reported differences in reactivity between TDI and HDI toward glutathione (GSH). Albumin was conjugated to TDI and HDI by GSH-TDI28 and GSH-HDI20, respectively. From these two reports, the kinetics of GSH-HDI mediated albumin carbamoylation was substantially slower compared with those of GSH-TDI. The hydrolysis of aliphatic isocyanates is also much slower than aromatic isocyanates. However, the nature and extent of HDI and TDI conjugation, in vivo, to serum proteins has not yet been reported. Diisocyanate haptenated proteins have been used both to produce specific antibodies29,30 and to screen for dNCO specific antibodies in workers’ sera for diagnosis of OA31.However, these conjugates are often poorly characterized and non-standardized.
Our previous work focused on characterization of methylene diphenyl diisocyanate (MDI)-HSA and MDI-Hb conjugates32. Although HSA is the most common carrier protein used for dNCO antibody immunoassays21,22, other proteins, however, have also been found to be potentially modified by dNCO exposure22–25,33,34but the immunogenicity of adducted proteins other than albumins has not been reported.
One hypothesis is that the lack of a standard characterization protocol for conjugates used to screen for dNCO specific antibodies in workers' sera is contributing to the reported low sensitivities and variability of these assay methods. Our previous work on MDI shed light on the need to use multiple methods to characterize these conjugates. In the present study we extend the characterization of the dNCO-protein conjugates from MDI to understand reactivity differences among dNCOs such as TDI and HDI that can impact assay sensitivities and standardization protocols. Quantification of the amount of TDI and HDI bound per mole protein was conducted by analyses of the corresponding hydrolysis products following assay hydrolysis of the conjugate, derivitization of the diamines and HPLC florescence detection32. Cross linking was evaluated using the 2, 4, 6-trinitrobenzene sulphonic acid (TNBS) assay, which is a primary amine-specific spectrophotometric probe. TNBS reacts with primary amines in proteins to produce a complex that absorbs at 420 nm. Loss of TNBS reactivity in dNCO-conjugated proteins occurs only when the dNCO cross-links two amine sites. This method, though not very sensitive, is very specific because only primary amines, the predominant sites found to be conjugated and cross-linked by dNCOs, react with TNBS. Gel electrophoresis was also used to qualitatively evaluate the extent of conjugation and cross linking in dNCO conjugated proteins. Under denaturing conditions intermolecular cross-linked proteins and highly substituted proteins have a larger molecular size in comparison to unconjugated protein and these tend to migrate slower. On the other hand, intramolecular cross-linking may prevent complete protein denaturation resulting in the migration similar to that of a smaller molecule that migrates faster. Proteomic mass spectrometry was employed to delineate TDI binding sites on Hb. Acrylonitrile adducted Hb and trimellitic anhydride (TMA)-adducted Hb were demonstrated to be antigenic35,36, so it is crucial to understand the reactivity of Hb to different dNCOs as well as to dNCO specific monoclonal antibodies relative to a well-documented dNCO reactive protein HSA.
Materials and methods
Chemicals
Unless otherwise specified, all reagents were acquired from Sigma–Aldrich (St. Louis, MO, USA) and used without further purification. Dichloromethane (reagent grade) was purchased from J.T. Baker/Avantor Performance Materials (Center Valley, PA, USA). Sodium tetra borate, sodium hydroxide, hydrochloric acid, 98 % sulfuric acid, and N-acetyl glycine were purchased from Fisher Scientific (Fair Lawn, NJ, USA).
Preparation of TDI–HSA/Hb adducts
TDI–protein adducts were prepared as described previously for MDI-HSA/Hb conjugates32. Briefly, 0.5 mg/ml protein solutions were prepared in 0.01 M PBS (pH 7.4). TDI (42.3 µl) was dissolved in 1 ml dry acetone and diluted ten times to make stock solution for 40:1 TDI: protein. Serial dilutions of TDI in acetone were performed to make stock solutions for 10:1, 5:1 and 1:1 TDI:protein. Fifty µL TDI stock solution was added to 5 ml of 0.5 mg/ml protein with mixing, resulting in TDI:protein molar conjugation ratios of 1:1, 5:1, 10:1, and 40:1. Samples were then incubated at room temperature (RT) for 1 h with mixing. Following incubation, samples were dialyzed for 18 h at 4°C against 4 L of distilled deionized water using 12,000–14,000 MWCO dialysis tubing (Spectrum Laboratories, Inc., Rancho Dominguez, CA) and stored at 4°C until analysis.
Preparation of HDI–HSA/Hb adducts
For preparation of HDI–protein adducts, 0.5 mg/ml protein solutions were prepared in 0.01 M PBS (pH 7.4). HDI (47.3 µL) was dissolved in 1 ml dry acetone and diluted ten times to make stock solution for the 40:1 HDI:protein conjugation ratio. Serial dilutions of HDI in acetone were prepared to make solutions for 10:1, 5:1 and 1:1 HDI:protein conjugation ratios. Conjugations, dialysis and sample storage were performed as described for TDI samples.
Analysis of number of moles of dNCO bound per mole protein
TDI/HDI-conjugated proteins (2 ml aliquots) were hydrolyzed by incubating with 1 ml of 3 M H2SO4 at 100°C for 16 h. Toluene diamine (TDA) and hexamethylene diamine (HDA) standards (Sigma–Aldrich, St. Louis, MO, USA) were spiked into protein standards (1–16,000 ng/ml) and were run in parallel with conjugates. Following hydrolysis, samples and standards were cooled to RT and 5 ml of saturated sodium hydroxide was added. Samples were vortexed, and put in an ice bath to cool for 10 min. The resulting TDA and HDA from samples and standards were extracted into 6 ml of dichloromethane and the solvent was subsequently evaporated at 40°C under N2 to 1 ml. The dichloromethane extracts were then back-extracted into 500 µl of 0.5 % H2SO4. Saturated borate buffer (250 µl, pH 8.5) and 450 µl of acetonitrile were added to 250 µl of H2SO4 extract and vortexed for 1 min. Fluorescamine (50 µl of 14.4 mg/ml in acetonitrile) was added. This was vortexed for 1 min, and 100 µl was injected onto a Supelco LC-SI C18 column (25 cm 4.6 mm, 5 µm, Supelco, Bellefonte, PA, USA). Samples and standards were analyzed on a Shimadzu Prominence high-performance liquid chromatography system (HPLC) (Shimadzu, Columbia, MD, USA) consisting of an online vacuum degasser (model DGU-20A5), a quaternary pump (model LC-20AT), an auto sampler (model SIL-10AD-VP), and a fluorescence detector (model RF-10AXL). The HPLC system was controlled by EZ Start software version 7.3 (Lab Alliance, State College, PA, USA). Samples and standards were eluted from the column at 1 ml/min over 20 min using a linear gradient of 10% to 50% acetonitrile/water over 13 min and held at 50% for 5 min. The resulting TDA/HDA–fluorescamine complex was excited at 410 nm, and emission was measured at 510 nm.
Assessment of cross-linking: TNBS assay
The trinitrobenzene sulfonic acid (TNBS) assay was used to evaluate the extent of cross-linking in TDI–HSA and HDI-HSA conjugates37. TNBS (5%, w/v) was diluted 1:5.48 with 0.1 M borate buffer, pH 9.3. To 500 µl of sample, 12.5 µl of diluted TNBS was added, mixed and incubated for 30 min at RT. Absorbance at 420 nm was measured on a Beckman Coulter spectrophotometer (model DU 800, Beckman Coulter, Somerset, NJ, USA).
Assessment of cross-linking: Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
For denaturing gels, HSA, Hb, and TDI/HDI–HSA/Hb conjugates were mixed with Laemmli sample buffer containing 2-mercaptoethanol. Samples were run on 8% and 12% polyacrylamide gels. Following electrophoretic separation of proteins, the gels were stained with Imperial™ protein stain (Pierce, Rockford, IL, USA) and destained in water. Unmodified/unconjugated HSA, Hb and Bio-Rad pre-stained molecular weight markers (Life Science, Hercules, CA) were used for relative molecular weight determination.
Trypsin digestion of hemoglobin samples
For identification of TDI conjugation sites on Hb by ultra-performance liquid chromatography quadruple time-of-flight mass spectrometry (UPLC–qTOF MS), 200-µl aliquots of TDI–Hb samples were incubated with tributyl phosphine for 30 min at RT to reduce the disulfide bonds, followed by alkylation with iodoacetamide for 1 h at RT. Alkylation was quenched by further addition of tributyl phosphine for 15 min at RT. Porcine trypsin in 25 mM NH4HCO3 was then added at a 40:1 (protein/trypsin) ratio. Samples were incubated overnight at 37°C. The next day, 12 µL of 10 % triflouroacetic acid (TFA) was added to stop trypsin digestion.
Ultra-performance liquid chromatography (UPLC)
Tryptic peptides of Hb and TDI–Hb were separated on a Waters nanoACQUITY UPLC system (Waters, Milford, MA, USA). Aliquots (1 µl) of the digest mixture were injected and trapped/desalted on a 5-µm Symmetry C18 trapping column (180 µm × 20 mm) with 99.5/0.5 A/B (A: 0.1% formic acid; B: 0.1% formic acid in acetonitrile) at a flow rate of 15 µl/min for 1 min. Separation was performed on a 1.7-µm BEH130 C18 analytical column (100 µm × 100 mm) using gradient elution at a flow rate of 400 nl/min and a gradient of 99:1 to 60:40 A/B over 90 min.
Tandem Mass Spectrometry (MS/MS) analysis of Hb peptides
The eluent from the UPLC system was directed to the nano-electrospray source of a Waters SYNAPT MS qTOF mass spectrometer. Positive ion nano-electrospray was performed using 10-µm Pico-Tip (Waters) emitters held at a potential of +3.5 kV. The cone voltage was held constant at +40 V for all experiments. Dry nitrogen desolvation gas was supplied to the instrument via a nitrogen generator (NitroFlowLab, Parker Hannifin, Haverhill, MA, USA). [Glu] 1-Fibrinopeptide B (100 fmol/µl in 75:25 A/B) was supplied to an orthogonal reference probe, and the [M+2H]2+ ion (m/z 785.84265u) was measured as an external calibrant at 30-s intervals. Ultra-high purity (UHP) argon was used as collision gas. Spectra were acquired in an “MSe” fashion38,39. Alternating 1-s mass spectra were acquired. The collision energy was set to 6 eV (1-s low energy scan) and a 15- to 30-eV ramp (1-s high energy scan).
Data analysis for TDI binding sites on Hb
Data were analyzed with BioPharmaLynx version 1.2 (Waters), a software program for analysis of peptide mass maps and identification of sites of modification on known protein sequences. Identification of an isocyanate binding site involved observing a potential peptide–dNCO conjugation product with less than 30 ppm m/Dm mass error in the analyte peptide mass map, comparing analyte and control peptide mass map from unmodified Hb showing that observed m/z and chromatographic retention time are unique to analyte, and observing MS/MS data containing bn- and yn-type ions consistent with the assigned sequence and modifier.
Immunoassay for conjugates: ELISA for TDI-HSA/Hb
Binding of IgG1 monoclonal antibody (mAb) 60G2 raised against TDI-KLH30 to TDI-conjugated HSA and Hb was analyzed using an indirect enzyme-linked immunosorbent assay (ELISA). The development and characterization of the 60G2 mAb has been previously described by Ruwona et al30. Ninety-six-well plates (Corning, NY, USA) were coated with TDI-protein conjugates overnight at 4°C. After washing three times with PBST (PBS with 0.05% Tween 20), plates were blocked with 3% skim milk/PBST (SMPBST) for 1 h at 37°C. Plates were then incubated on a shaker for 1 h with 2 µg/ml 60G2 mAb at RT, washed three times with PBST and incubated for 1 h at 37°C with alkaline phosphatase conjugated AffiniPure goat, anti-mouse IgG (H+L) (Promega, Madison, WI) diluted 1:5000 (v/v) in SMPBST. Following incubation, plates were washed 3 times with PBST and binding of the 60G2 mAb to the conjugates was visualized using 0.5 mg/ml p-nitrophenyl phosphate (Sigma–Aldrich, CAS Number 4264-83-9) in alkaline phosphatase substrate. The optical density was measured at 405 nm after 30 min using a Molecular Devices SpectraMax M4 Multi-mode Micro plate Reader (Sunnyvale, CA, USA)
Statistical analysis
Data are presented as mean and standard deviation (SD). Analysis of variance (ANOVA) was employed for comparing the effect of dNCO and protein on the extent of conjugation and crosslinking on proteins. Differences were considered significant at a p < 0.05. N = 3/group, where N is number of replicates.
Results
Mapping TDI binding sites on Hb
TDI-Hb conjugates were digested with trypsin, and resultant peptides were analyzed by UPLC–MS/MS to determine TDI binding sites. Examination of the tandem mass spectra of the tryptic peptides allowed assignment of conjugation sites on Hb as previously described32. Hb has 2 alpha and 2 beta subunits and mass spectrometry allowed identification of the parent subunit from which each binding site originated. Table 1 shows the concentration-dependent specific binding sites identified for TDI on Hb.
Table 1.
Amino acid specific binding sites observed for TDI on hemoglobin
| 1:1 | 5:1 | 10:1 | 40:1 | |
|---|---|---|---|---|
| Alpha subunit | ||||
| Val 1 | x | x | x | x |
| Lys 11 | x | x | x | |
| Lys 16 | x | x | ||
| Lys 40 | x | |||
| Beta subunit | ||||
| Val 1 | x | x | x | x |
| Lys 17 | x | x | ||
| Lys 144 | x | x | ||
| Lys 61 | x |
A TDI concentration dependent increase in the number of binding sites was observed and a total of eight binding sites were identified at the highest concentration of TDI used, including the N-terminal valine on both the alpha and beta chains. TDI bound to three lysines on the alpha chain and three additional lysines on the beta chain. At the lowest TDI concentration used, only the two N-terminal valines of the alpha and beta chains were bound. Increasing TDI concentrations increased the number of sites bound to a maximum of eight at 40:1 TDI:Hb.
Quantification of TDI and HDI binding in Hb and HSA
TDI and HDI-conjugated HSA and Hb were hydrolyzed under strong acidic conditions and the resultant TDA and HDA were derivatized with fluorescamine and quantified using HPLC. Quantification of the number of moles of TDI and HDI bound to Hb and HSA is reported in Table 2. On a per mole basis, TDI was more reactive to both Hb and HSA than HDI over the entire concentration ranges used in this study. This agrees with findings from Wisnewski et al. who found that the rate of HSA carbamoylation from TDI-GSH derived TDI was higher than carbamoylation from HDI-GSH derived HDI20,28. Table 2 also demonstrates that HSA was more reactive to TDI than Hb. A similar trend was noted for HDI.
Table 2.
Moles of TDI and HDI bound per mole of Hb or HSA quantified using HPLC
| dNCO: protein | Average moles of dNCO bound | Average moles of dNCO bound | ||
|---|---|---|---|---|
| TDI: HSA | HDI: HSA | TDI: Hb | HDI: Hb | |
| 1:1 | 0.51 ± 0.56 | 0.04 ± 0.41** | 0.15 ± 0.15## | 0.02 ± 0.21** |
| 5:1 | 2.65 ± 0.23 | 0.44 ± 0.45** | 0.57 ± 0.02## | 0.13 ± 0.23** |
| 10:1 | 5.06 ± 0.43 | 0.82 ± 0.36* | 1.48 ± 0.09## | 0.32 ± 0.60** |
| 40:1 | 12.86 ± 0.56 | 2.79 ± 0.40* | 4.31 ± 0.06## | 1.03 ± 0.59** |
Extent of HDI binding to HSA is statistically different than TDI binding (*P<0.05; **P<0.01) Comparison of dNCO binding to HSA vs. to Hb.
(##P<0.01)
Cross-linking in TDI–and HDI-HSA: TNBS assay
Table 3 shows a concentration-dependent loss of available primary amines with increasing TDI and HDI concentrations and, thus, an increase in the amount of dNCO cross-linking of protein residues32. At TDI and HDI concentrations ranging from 1:1 to 10:1, the degree of cross linking is not statistically different between the 2 diisocyanates. However at 40:1 dNCO: HSA, TDI has a significantly higher degree of cross linking than HDI (P-value < 0.01). The TNBS assay could not be used to evaluate cross-linking in Hb conjugates because of spectral interference at 420 nm, the wavelength at which the absorbance of the complex between TNBS and primary amine is measured.
Table 3.
Cross-linking in TDI-HSA and HDI-HSA using loss of absorbance: TNBS assay.
| dNCO: protein molar ratio | Loss of TNBS Amine reactivity (%) | |
|---|---|---|
| HDI: HSA | 2,4-TDI: HSA | |
| Negative control | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 1:1 | 25.46 ± 2.34 | 18.04 ± 3.98 |
| 5:1 | 30.24 ± 2.89 | 31.47 ± 3.57 |
| 10:1 | 32.88 ± 3.14 | 39.92 ± 4.14 |
| 40:1 | 48.35 ± 3.76 | 62.42 ± 4. 18** |
Extent of TDI cross-linking statistically different than HDI (P<0.01)
Qualitative assessment of conjugation and crosslinking in TDI/HDI–HSA and TDI/HDI– Hb: Gel electrophoresis
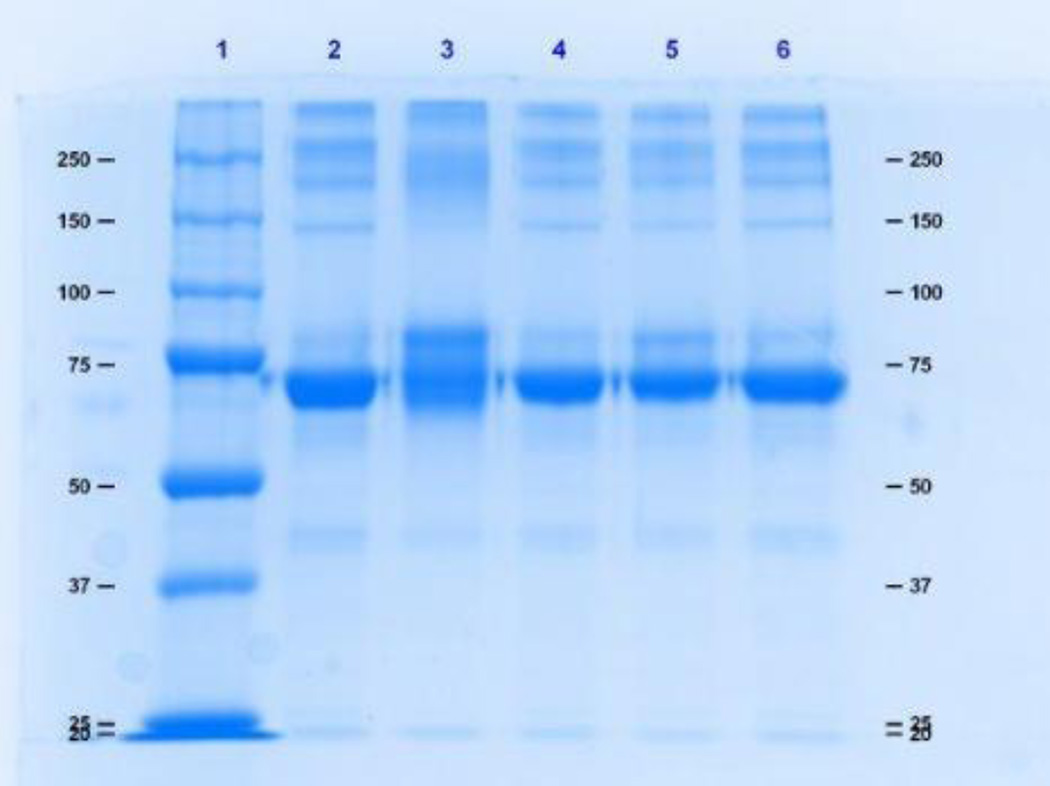
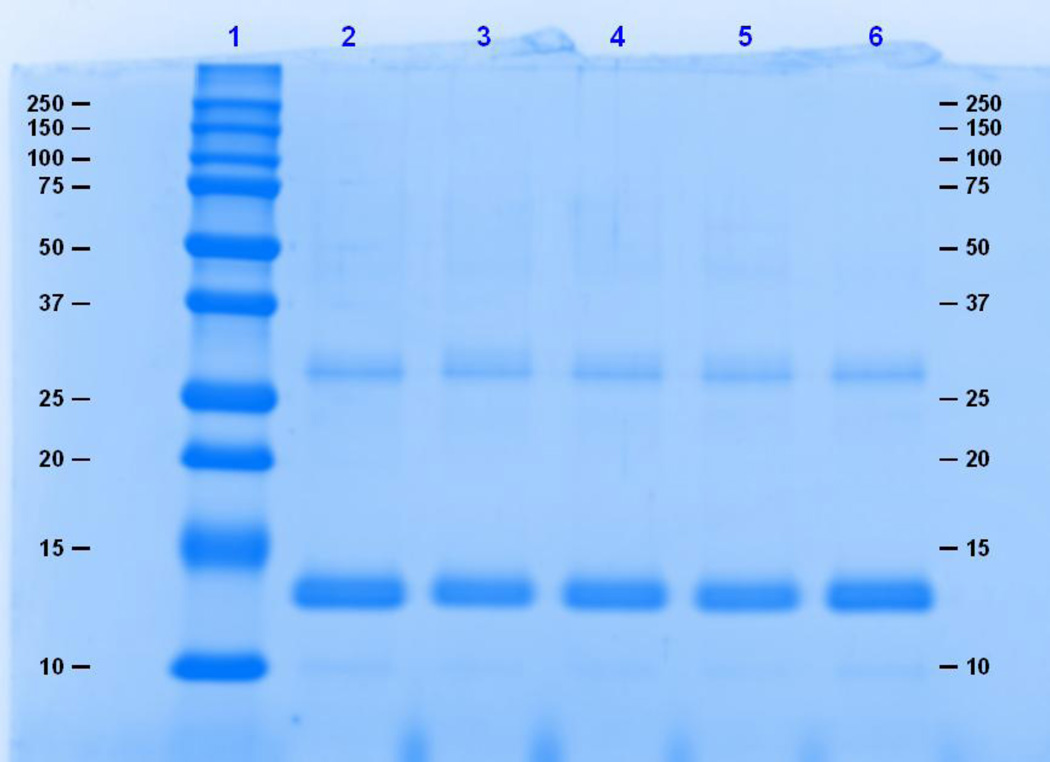
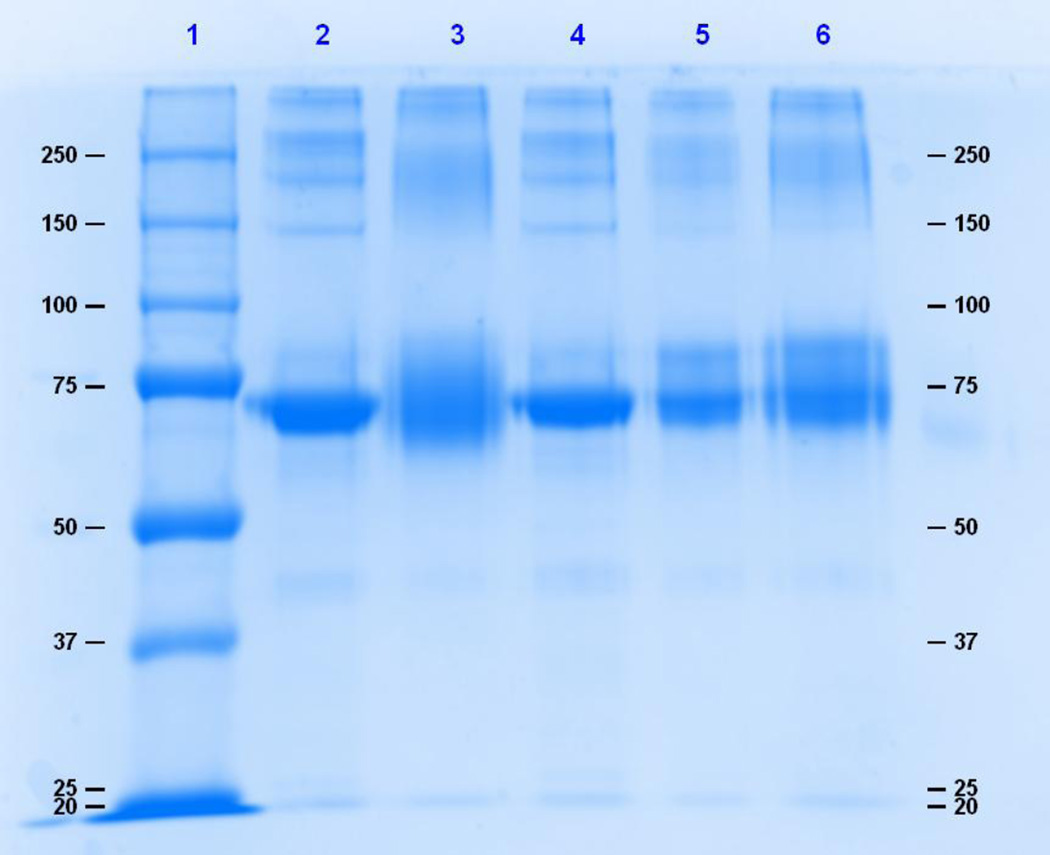
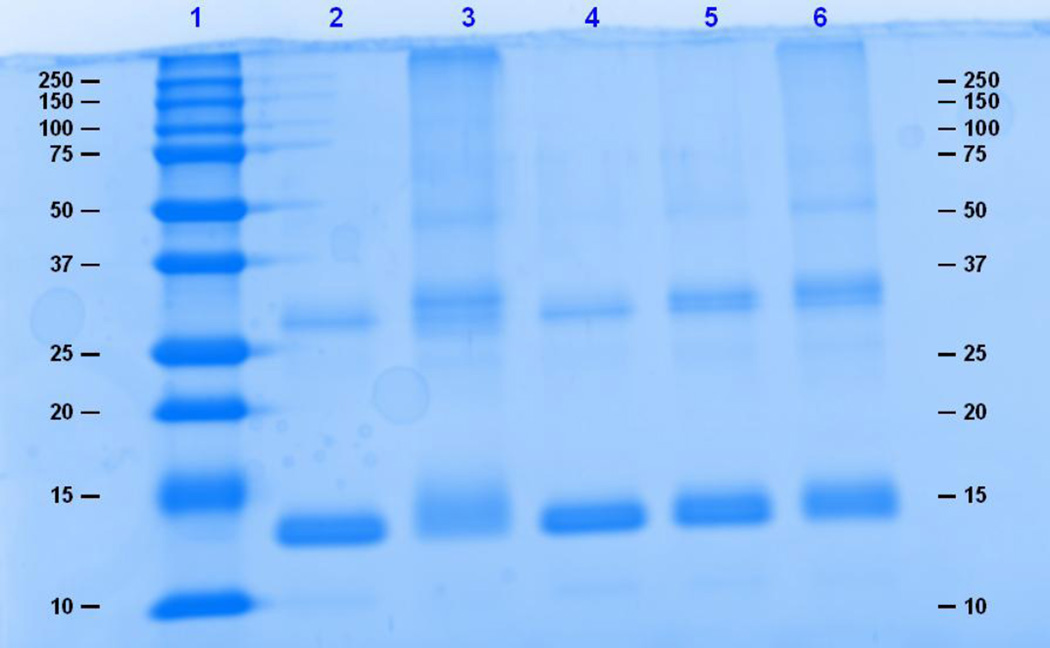
Polyacrylamide gel electrophoresis was used to evaluate the extent of binding and cross-linking. Intermolecular cross-linked and highly substituted proteins will migrate at a slower rate than the unconjugated protein, whereas extensive intramolecular cross-linking may prevent complete protein denaturation, causing an apparent migration of a molecule smaller that the unconjugated protein. Figure 1 shows an 8% SDS– PAGE gel of 0.5 mg/ml HSA reacted to TDI. Significant spreading of the HSA band was observed at the 40:1 TDI:HSA conjugation ratio. Figure 2 is a 12% denaturing gel of 0.5 mg/ml Hb reacted to TDI. Denaturation of Hb resulted in the incomplete dissociation of the alpha and beta subunits that migrated at molecular weights of approximately 14 kDa 28 kDa. Shift in migration due to conjugation to TDI was not observed. In contrast, HDI-HSA and HDI-Hb conjugates produced band spreading and shifts in migration/band spreading for both HSA and Hb conjugates. (Fig. 3 and 4).
Figure 1.
An 8% SDS– PAGE denaturing gel of 0.5 mg/ml HSA reacted to TDI. Lane 1 is the molecular weight marker, lane 2 is HSA, and lanes 3, 4, 5, and 6 are 40:1, 10:1, 5:1, and 1:1 TDI: HSA, respectively.
Figure 2.
A 12% denaturing gel of 0.5 mg/ml Hb reacted to TDI. Lane 1 is the molecular weight marker, lane 2 is Hb, and lanes 3, 4, 5, and 6 are 40:1, 10:1, 5:1, and 1:1 TDI: Hb, respectively.
Figure 3.
An 8% denaturing gel of 0.5 mg/ml HSA reacted to HDI. Lane 1 is the molecular weight marker, lane 2 is HSA, and lanes 3, 4, 5, and 6 are 40:1, 10:1, 5:1, and 1:1 HDI: HSA, respectively.
Figure 4.
A 12% denaturing gel of 0.5 mg/ml Hb reacted to HDI. Lane 1 is the molecular weight marker, lane 2 is Hb, and lanes 3, 4, 5, and 6 are 40:1, 10:1, 5:1, and 1:1 HDI: Hb, respectively.
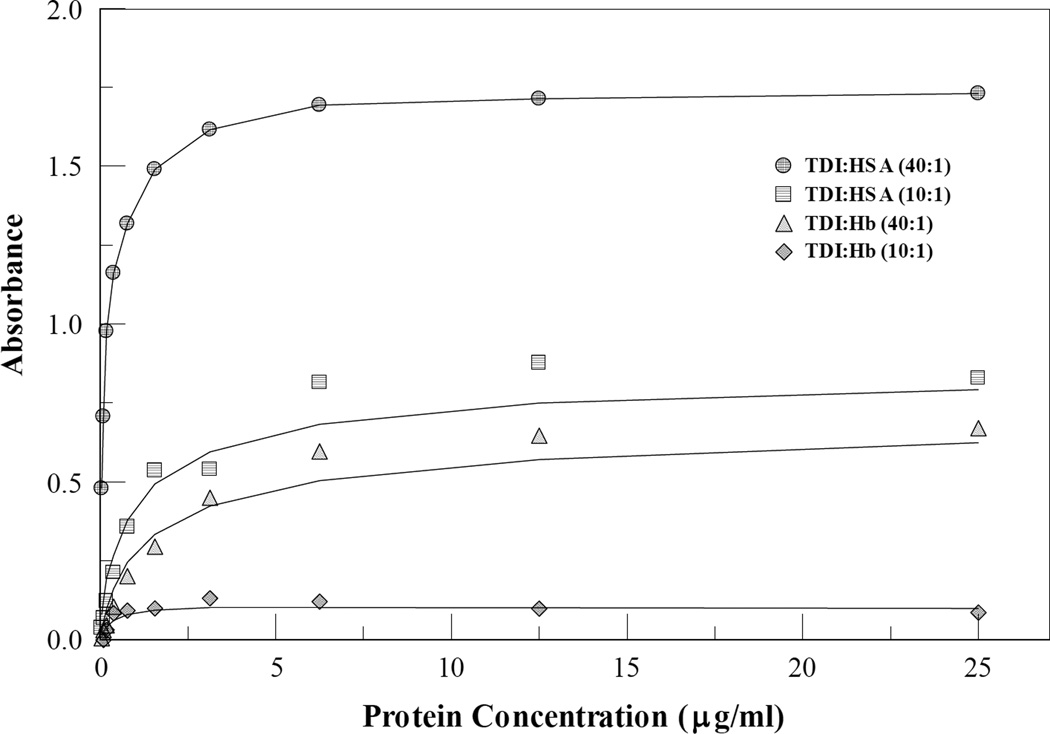
ELISA assessment of TDI-HSA and TDI-Hb
HDI-Hb conjugates reactivity to antibodies was not evaluated due to the lack of an HDI specific antibody. Although there is cross reactivity between HDI and monoclonal antibodies raised against TDI, the reactivity was too low and close to detection limit to make any quantitative analysis in agreement with HPLC results. Binding of IgG mAb 60G2 to TDI-conjugated HSA and Hb was analyzed using an indirect enzyme-linked immunosorbent assay (ELISA). Figure 5 shows the immunoassay results of conjugated proteins following titration into the ELISA plate at protein concentrations from 97.66 ng/ml to 25 µg/ml. Immuno reactivity of 60G2 to the conjugated Hb was higher at 40:1 TDI-protein than 10:1 in both HSA and Hb conjugates. 60G2 was more reactive to TDI-HSA than TDI-Hb at both 40:1 and 10:1 TDI-protein.
Figure 5.
Comparison of 60G2 mAb binding to TDI conjugated Hb and TDI conjugated HSA. ELISA absorbances curves for 40:1 and 10:1 TDI:protein conjugation ratios at multiple dilutions demonstrate superior binding of the mAb to TDI-HSA on a per mass basis..
Discussion
Our previous study employed several techniques to evaluate MDI-HSA and MDI-Hb conjugates32. In the current study, we extended use of this methodology to compare TDI–HSA, TDI–Hb, HDI-HSA, and HDI-Hb conjugates. The objective was to compare the extent of conjugation of TDI and HDI to proteins (HSA and Hb), evaluate differences in the extent of cross-linking using the TNBS assay between TDI and HDI on HSA, and assess reactivity of TDI conjugated HSA and Hb with a monoclonal antibody (IgG 60G2 mAb) that recognizes TDI conjugated proteins. The methodology used herein is relevant for the characterization and standardization of dNCO haptenated protein for specific antibody detection. Although knowledge of specific sights bound at lower conjugation ratios may have value in the development of biomonitoring of dNCO conjugates in biological fluids, the measurement and characterization of in vivo formed species is beyond the scope of the present work. Differences in reactivity between TDI and HDI conjugated to HSA and Hb were observed using the HPLC quantification of moles dNCO bound per mole protein. HSA was more reactive to both TDI and HDI than Hb. This may be indicative of the structural differences between the two proteins. Hb, with four polypeptide subunits (two alpha and two beta) and an iron-containing porphyrin ring, may mask potential binding sites, thus affecting its reactivity with dNCOs. This contrasts sharply with HSA, a single polypeptide with 17 pairs of disulfide bridges and 1 free cysteine. TDI was more reactive to both HSA and Hb than HDI at pH 7.4. These results agree with earlier findings where HSA was found to be the most modified protein in the blood of dNCO exposed subjects21. MS/MS was used to delineate specific TDI binding sites on Hb. A concentration-dependent increase in the number of binding sites was observed across the entire TDI concentration range employed. Only the N terminal valines on both the alpha and beta subunits were observed at 1:1 TDI: Hb and these were conserved at all concentrations studied, suggesting that these sites are the kinetically favored reactive sites. Non-terminal amino acids of the beta subunit were bound by TDI only from 10:1 TDI:Hb concentrations and higher, while non-terminal amino acid binding sites on the alpha subunit were observed at 5:1 TDI:Hb. The non-terminal TDI binding sites observed on Hb were all lysine residues, specifically lysines 11, 16, and 40 of the alpha subunit and lysines 17, 144, and 61 of the beta subunit. Some of the TDI binding sites observed in this study were comparable to the MDI binding sites reported in our previous study32. In addition to the N-terminal valines of the alpha and beta subunits, lysine 66 was also observed at 1:1 MDI:Hb. Only lysine 40 of the alpha subunit and lysine 61 of the beta subunit were bound by both MDI and TDI. Lysines 11 and 16 of the alpha subunit and lysines 17 and 144 of the beta subunit were only observed in TDI. In contrast, lysine 7 of the alpha subunit and lysines 8, 65 and 66 of the beta subunit were only observed with MDI. The differences in the binding sites between MDI and TDI can give an insight into the possibility for conformational and structural differences in the resultant conjugates, which may potentially affect their antigenicity and immunogenicity.
The TNBS assay, which has traditionally been used to assess chemical adduction with amino groups40, was employed in this study to evaluate cross-linking in TDI-HSA and HDI-HSA conjugates. A concentration dependent loss of available primary amines with increasing TDI and HDI concentrations was observed, indicating an increase in the amount of dNCO cross-linking of protein residues. At lower TDI and HDI concentrations (1:1–10:1 TDI/HDI: HSA), the degree of cross-linking was similar for both dNCOs. At 40:1 dNCO: HSA, TDI had a higher degree of cross-linking than HDI. A 62% loss of primary amine reactivity was observed at 40:1 TDI:HSA compared with a 48% loss of amine reactivity at 40:1 HDI:HSA (P < 0.01). A similar comparison could not be made for hemoglobin conjugates due to spectral interference at 420 nm, the wavelength at which the absorbance of the TNBS-amine complex was measured.
The ability of the TDI conjugates to be bound by TDI-specific antibody was evaluated using an indirect ELISA format. The ELISA format employed in the current study used an anti TDI–protein IgG that was produced in our lab against TDI-KLH17 as the primary antibody and an alkaline phosphatase-labeled anti-IgG as the detection antibody. Both TDI-HSA and TDI-Hb reacted to the anti-TDI-protein monoclonal antibody, indicating that TDI conjugated Hb can be antigenic. TDI-Hb antigenicity with the 60G2 mAb was however 30% lower than that observed for TDI-HSA. This is in agreement with HPLC results where TDI binding to HSA was significantly higher than to Hb suggesting that the absolute number of moles of dNCO bound rather than the specific protein bound is a greater determinant for recognition by the 60G2 mAb. The immunogenicity or antigenicity of in vivo dNCO-adducted hemoglobin has not yet been reported in the literature, although our data suggest that dNCO haptenated HSA is superior to conjugated Hb for the detection of dNCO specific antibody.
Cross-linking and extent of conjugation was visualized using denaturing gel electrophoresis. Alteration of migration and band spreading was evident for both HDI and TDI conjugated HSAs at the highest conjugation ratio, however, a clear migration shift/band spreading was only evident in HDI:HSA at the lower binding ratios (Figures 1 and 3). Hemoglobin subunits did not completely dissociate under denaturing conditions as evidenced by the protein band at approximately 28 kDa. Shift in migration of TDI bound Hb subunits was not observed, and only clearly observed at the highest HDI conjugation ratio of 40:1 (Figures 2 and 4). These finding are in contrast to what was previously observed for MDI, where clear conjugation-dependent shifts in migration were observed down to a 1:1 conjugation ratio.32 One possible explanation for the differences seen between SDS-Page between that observed for HDI, MDI and TDI bound proteins may be that location of the 2 TDI NCO groups located on the benzene ring are spatially closer to each other than in MDI or HDI which may produce differences in comparative migration of the conjugates in the SDS-Page gels.
Increasing molar ratio for conjugation increased extent of conjugation, degree of cross-linking, gel migration and reactivity with dNCO specific monoclonal antibody binding. It is therefore difficult to dissect the specific influences of intra- and intermolecular cross-linking from total dNCO bound on the overall antigenicity of the resultant conjugates.
The bifunctional electrophilic nature of the diisocyanates make it very difficult to dissect out the components critical to dNCO specific antibody recognition.
Increases in total amount of dNCO bound, intra- and intermolecular crosslinking, and dNCO self-polymerization on proteins, and as well as recognition by the 60G2 mAb all increase were demonstrated with increasing conjugation ratios of dNCO:protein. The mAb 60G2 is extremely well characterized with respect to binding specificity (30). It recognizes both 2,4-TDI and 2,6-TDI bound HSA, bound mouse serum albumin, and bound keratin. It has slight reactivity toward MDI-HSA, HDI-HSA and HSA conjugated to 2,5- and 3,4- dimethyl phenylisocyanate. It has no reactivity toward phenyl isocyanate, 2-toluene isocyanate, 4-toluene isocyanate or toluene diisothiocyanates. Although, dNCO specific IgE and IgG from dNCO exposed individuals was not tested against the various dNCO conjugated proteins, others have reported that recognition by patient sera antibodies is dependent on immunoassay procedure, conjugation method and predominant exposure dNCO form to that individual41,42. Comparison of specific antibody prevalence in dNCO workers is difficult in the absence of detailed dNCO-HSA characterization. Until the relative contribution of the multiple dNCO conjugation products to dNCO immunogenicity and antigenicity can be determined, we believe that dNCO antigen preparations used for standardized screening of workers’ sera or research applications should undergo as complete quantitative chemical characterization as possible similar to that outlined in the present manuscript.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institute for Occupational Safety and Health. This work was partially supported by an Interagency Agreement with the NIEHS (IAG#Y1-ES-0001-12) and by Grant Number CHE 1056311 from the National Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Vangronsveld E, Berckmans S, Spence M. Toluene diisocyanate emission to air and migration to a surface from a flexible polyurethane foam. Ann. Occup. Hyg. 2013;57(5):650–661. doi: 10.1093/annhyg/mes105. [DOI] [PubMed] [Google Scholar]
- 2.Petsonk EL, Wang ML, Lewis DM, Siegel PD, Husberg BJ. Asthma-like symptoms in wood product plant workers exposed to methylene diphenyl diisocyanate. Chest. 2000;118(4):1183–1193. doi: 10.1378/chest.118.4.1183. [DOI] [PubMed] [Google Scholar]
- 3.Arnold SM, Collins MA, Graham C, Jolly AT, Parod RJ, Poole A, Schupp T, Shiotsuka RN, Woolhiser MR. Risk assessment for consumer exposure to toluene diisocyanate (TDI) derived from polyurethane flexible foam. Regul. Toxicol. Pharmacol. 2012;64(3):504–515. doi: 10.1016/j.yrtph.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Buyantseva LV, Liss GM, Ribeiro M, Manno M, Luce CE, Tarlo SM. Reduction in diisocyanate and non-diisocyanate sensitizer-induced occupational asthma in Ontario. J. Occup. Environ. Med. 2011;53(4):420–426. doi: 10.1097/JOM.0b013e3182122376. [DOI] [PubMed] [Google Scholar]
- 5.Ribeiro M, Tarlo SM, Czyrka A, Vernich L, Luce CE, Liss GM. Diisocyanate and nondiisocyanate sensitizer-induced occupational asthma frequency during 2003 to 2007 in Ontario, Canada. J Occup Environ Med. 2014;56(9):1001–1007. doi: 10.1097/JOM.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan JB, Krieger GR. Clinical environmental health and toxic exposures. Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 7.Matheson JM, Johnson VJ, Vallyathan V, Luster MI. Exposure and immunological determinants in a murine model for toluene diisocyanate (TDI) asthma. Toxicol. Sci. 2005;84(1):88–98. doi: 10.1093/toxsci/kfi050. [DOI] [PubMed] [Google Scholar]
- 8.Mapp CE. Agents, old and new, causing occupational asthma. Occup. Environ. Med. 2001;58(5):354–360. 290. doi: 10.1136/oem.58.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baur X. Hypersensitivity pneumonitis (extrinsic allergic alveolitis) induced by isocyanates. J. Allergy Clin. Immunol. 1995;95(5 Pt 1):1004–1010. doi: 10.1016/s0091-6749(95)70101-x. [DOI] [PubMed] [Google Scholar]
- 10.Charles J, Bernstein A, Jones B, Jones DJ, Edwards JH, Seal RM, Seaton A. Hypersensitivity pneumonitis after exposure to isocyanates. Thorax. 1976;31(2):127–136. doi: 10.1136/thx.31.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aalto-Korte K, Suuronen K, Kuuliala O, Henriks-Eckerman ML, Jolanki R. Occupational contact allergy to monomeric isocyanates. Contact Dermatitis. 2012;67(2):78–88. doi: 10.1111/j.1600-0536.2011.02049.x. [DOI] [PubMed] [Google Scholar]
- 12.Hur GY, Koh DH, Choi GS, Park HJ, Choi SJ, Ye YM, Kim KS, Park HS. Clinical and immunologic findings of methylene diphenyl diisocyanate-induced occupational asthma in a car upholstery factory. Clin. Exp. Allergy. 2008;38(4):586–593. doi: 10.1111/j.1365-2222.2008.02935.x. [DOI] [PubMed] [Google Scholar]
- 13.Rudzinski WE, Yin J, Norman SH, Glaska DA. Determination of hexamethylene-based isocyanates in spray-painting operations. Part 1. Evaluation of a polyurethane foam sponge sampler. Analyst. 1998;123(10):2079–2083. doi: 10.1039/a803942i. [DOI] [PubMed] [Google Scholar]
- 14.Redlich CA, Karol MH. Diisocyanate asthma: clinical aspects and immunopathogenesis. Int. Immunopharmacol. 2002;2(2–3):213–224. doi: 10.1016/s1567-5769(01)00174-6. [DOI] [PubMed] [Google Scholar]
- 15.Krone CA. Diisocyanates and nonoccupational disease: a review. Arch. Environ. Health. 2004;59(6):306–316. [PubMed] [Google Scholar]
- 16.Wilder LC, Langley RL, Middleton DC, Ernst K, Lummus ZL, Streicher RP, Campbell DS, Wattigney WA, Bernstein JA, Bernstein DI, Dearwent SM. Communities near toluene diisocyanate sources: an investigation of exposure and health. J Expo Sci Environ Epidemiol. 2011;21(6):587–594. doi: 10.1038/jes.2011.5. [DOI] [PubMed] [Google Scholar]
- 17.Ruwona TB, Johnson VJ, Schmechel D, Simoyi RH, Beezhold D, Siegel PD. Monoclonal antibodies against toluene diisocyanate haptenated proteins from vapor-exposed mice. Hybridoma (Larchmt.) 2010;29(3):221–229. doi: 10.1089/hyb.2009.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wisnewski AV, Stowe MH, Nerlinger A, Opare-Addo P, Decamp D, Kleinsmith CR, Redlich CA. Biomonitoring Hexamethylene diisocyanate (HDI) exposure based on serum levels of HDI-specific IgG. Ann. Occup. Hyg. 2012;56(8):901–910. doi: 10.1093/annhyg/mes024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wisnewski AV, Stowe MH, Cartier A, Liu Q, Liu J, Chen L, Redlich CA. Isocyanate vapor-induced antigenicity of human albumin. J. Allergy Clin. Immunol. 2004;113(6):1178–1184. doi: 10.1016/j.jaci.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Wisnewski AV, Mhike M, Hettick JM, Liu J, Siegel PD. Hexamethylene diisocyanate (HDI) vapor reactivity with glutathione and subsequent transfer to human albumin. Toxicol. In Vitro. 2013;27(2):662–671. doi: 10.1016/j.tiv.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budnik LT, Preisser AM, Permentier H, Baur X. Is specific IgE antibody analysis feasible for the diagnosis of methylenediphenyl diisocyanate-induced occupational asthma? Int. Arch. Occup. Environ. Health. 2013;86(4):417–430. doi: 10.1007/s00420-012-0772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wisnewski AV, Srivastava R, Herick C, Xu L, Lemus R, Cain H, Magoski NM, Karol MH, Bottomly K, Redlich CA. Identification of human lung and skin proteins conjugated with hexamethylene diisocyanate in vitro and in vivo. Am. J. Respir. Crit Care Med. 2000;162(6):2330–2336. doi: 10.1164/ajrccm.162.6.2002086. [DOI] [PubMed] [Google Scholar]
- 23.Lange RW, Lantz RC, Stolz DB, Watkins SC, Sundareshan P, Lemus R, Karol MH. Toluene diisocyanate colocalizes with tubulin on cilia of differentiated human airway epithelial cells. Toxicol. Sci. 1999;50(1):64–71. doi: 10.1093/toxsci/50.1.64. [DOI] [PubMed] [Google Scholar]
- 24.Lantz RC, Lemus R, Lange RW, Karol MH. Rapid reduction of intracellular glutathione in human bronchial epithelial cells exposed to occupational levels of toluene diisocyanate. Toxicol. Sci. 2001;60(2):348–355. doi: 10.1093/toxsci/60.2.348. [DOI] [PubMed] [Google Scholar]
- 25.Sabbioni G, Hartley R, Henschler D, Hollrigl-Rosta A, Koeber R, Schneider S. Isocyanate-specific hemoglobin adduct in rats exposed to 4, 4'-methylenediphenyl diisocyanate. Chem. Res. Toxicol. 2000;13(2):82–89. doi: 10.1021/tx990096e. [DOI] [PubMed] [Google Scholar]
- 26.Wisnewski AV. Developments in laboratory diagnostics for isocyanate asthma. Curr. Opin. Allergy Clin. Immunol. 2007;7(2):138–145. doi: 10.1097/ACI.0b013e3280895d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ott MG, Jolly AT, Burkert AL, Brown WE. Issues in diisocyanate antibody testing. Crit Rev. Toxicol. 2007;37(7):567–585. doi: 10.1080/10408440701419553. [DOI] [PubMed] [Google Scholar]
- 28.Wisnewski AV, Hettick JM, Siegel PD. Toluene diisocyanate reactivity with glutathione across a vapor/liquid interface and subsequent transcarbamoylation of human albumin. Chem Res. Toxicol. 2011;24(10):1686–1693. doi: 10.1021/tx2002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemons AR, Siegel PD, Mhike M, Law BF, Hettick JM, Bledsoe TA, Nayak AP, Beezhold DH, Green BJ. A murine monoclonal antibody with broad specificity for occupationally relevant diisocyanates. J. Occup. Environ. Hyg. 2014;11(2):101–110. doi: 10.1080/15459624.2013.843783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruwona TB, Johnson VJ, Hettick JM, Schmechel D, Beezhold D, Wang W, Simoyi RH, Siegel PD. Production, characterization and utility of a panel of monoclonal antibodies for the detection of toluene diisocyanate haptenated proteins. J. Immunol. Methods. 2011;373(1–2):127–135. doi: 10.1016/j.jim.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Tee RD, Cullinan P, Welch J, Burge PS, Newman-Taylor AJ. Specific IgE to isocyanates: a useful diagnostic role in occupational asthma. J. Allergy Clin. Immunol. 1998;101(5):709–715. doi: 10.1016/S0091-6749(98)70181-2. [DOI] [PubMed] [Google Scholar]
- 32.Mhike M, Chipinda I, Hettick JM, Simoyi RH, Lemons A, Green BJ, Siegel PD. Characterization of methylene diphenyl diisocyanate-haptenated human serum albumin and hemoglobin. Anal. Biochem. 2013;440(2):197–204. doi: 10.1016/j.ab.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabbioni G, Beyerbach A. Haemoglobin adducts of aromatic amines: diamines and polyaromatic amines. J. Chromatogr. B Biomed. Sci. Appl. 2000;744(2):377–387. doi: 10.1016/s0378-4347(00)00265-6. [DOI] [PubMed] [Google Scholar]
- 34.Sabbioni G, Hartley R, Schneider S. Synthesis of adducts with amino acids as potential dosimeters for the biomonitoring of humans exposed to toluenediisocyanate. Chem. Res. Toxicol. 2001;14(12):1573–1583. doi: 10.1021/tx010053+. [DOI] [PubMed] [Google Scholar]
- 35.Wong JL, Liu DZ, Zheng YT. Lysine conjugate of acrylonitrile as antigenic sites in hemoglobin adducts. J. Pept. Res. 2004;63(2):171–174. doi: 10.1111/j.1399-3011.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 36.Pien LC, Zeiss CR, Leach CL, Hatoum NS, Levitz D, Garvin PJ, Patterson R. Antibody response to trimellityl hemoglobin in trimellitic anhydride-induced lung injury. J. Allergy Clin. Immunol. 1988;82(6):1098–1103. doi: 10.1016/0091-6749(88)90149-2. [DOI] [PubMed] [Google Scholar]
- 37.Snyder SL, Sobocinski PZ. An improved 2,4,6-trinitrobenzenesulfonic acid method for the determination of amines. Anal. Biochem. 1975;64(1):284–288. doi: 10.1016/0003-2697(75)90431-5. [DOI] [PubMed] [Google Scholar]
- 38.Hettick JM, Siegel PD, Green BJ, Liu J, Wisnewski AV. Vapor conjugation of toluene diisocyanate to specific lysines of human albumin. Anal. Biochem. 2012;421(2):706–711. doi: 10.1016/j.ab.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hettick JM, Siegel PD. Determination of the toluene diisocyanate binding sites on human serum albumin by tandem mass spectrometry. Anal. Biochem. 2011;414(2):232–238. doi: 10.1016/j.ab.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 40.Lemus R, Lukinskeine L, Bier ME, Wisnewski AV, Redlich CA, Karol MH. Development of immunoassays for biomonitoring of hexamethylene diisocyanate exposure. Environ. Health Perspect. 2001;109(11):1103–1108. doi: 10.1289/ehp.011091103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campo P, Wisnewski AV, Lummus Z, Cartier A, Malo J, Boulet LP, Bernstein DI. Diisocyanate conjugate and immunoassay characteristics influence detection of specific antibodies in HDIΓÇÉexposed workers. Clinical & Experimental Allergy. 2007;37(7):1095–1102. doi: 10.1111/j.1365-2222.2007.02745.x. [DOI] [PubMed] [Google Scholar]
- 42.Ye YM, Kim CW, Kim HR, Kim HM, Suh CH, Nahm DH, Park HS, Redlich CA, Wisnewski AV. Biophysical determinants of toluene diisocyanate antigenicity associated with exposure and asthma. J. Allergy Clin. Immunol. 2006;118(4):885–891. doi: 10.1016/j.jaci.2006.06.026. [DOI] [PubMed] [Google Scholar]