Abstract
Critical illness is a major cause of morbidity and mortality around the world. While obesity is often detrimental in the context of trauma, it is paradoxically associated with improved outcomes in some septic patients. The reasons for these disparate outcomes are not well understood. A number of animal models have been used to study the obese response to various forms of critical illness. Just as there have been many animal models that have attempted to mimic clinical conditions, there are many clinical scenarios that can occur in the highly heterogeneous critically ill patient population that occupies hospitals and intensive care units. This poses a formidable challenge for clinicians and researchers attempting to understand the mechanisms of disease and develop appropriate therapies and treatment algorithms for specific subsets of patients, including the obese. The development of new, and the modification of existing animal models is important in order to bring effective treatments to a wide range of patients. Not only do experimental variables need to be matched as closely as possible to clinical scenarios, but animal models with pre-existing comorbid conditions need to be studied. This review briefly summarizes animal models of hemorrhage, blunt trauma, traumatic brain injury, and sepsis. It also discusses what has been learned through the use of obese models to study the pathophysiology of critical illness in light of what has been demonstrated in the clinical literature.
Keywords: obese, trauma, hemorrhage, sepsis, critical illness, animal model
INTRODUCTION
Critical illness is a major cause of morbidity and mortality in the United States and around the world (86). It is associated with significant social, physical, and functional impairments that can lead to decreased quality of life, financial stress, and psychological struggles not only for patients but also for their families (22). An increasing amount of research has demonstrated that obesity, a growing problem in developed countries around the world (35) that is associated with the development of several chronic medical conditions (81), may affect outcomes in critically ill patients (89). While it is well known that obese critically ill patients present healthcare professionals with unique treatment challenges (88), it is also becoming increasingly realized that adipose tissue, which has many unique endocrine, immune, cardiovascular, and metabolic functions (81), may undergo distinct alterations during critical illness (51). While larger, well-designed clinical trials are needed, an expansion in the use of preclinical models is necessary to better understand the pathophysiological mechanisms and therapeutic options in the critical care of the obese patient.
This review will discuss the animal models of critical illness that have been used, with a focus on hemorrhage, trauma, and sepsis. Special attention will be given to the models that have been applied to the study of obesity during these critical illnesses. The findings of these animal studies will be highlighted in light of what the clinical literature has demonstrated.
OBESITY AND ANIMAL MODELS OF CRITICAL ILLNESS
Obesity
Biology of obesity
Both animal and clinical studies have helped shed light on physiological and pathological changes that often accompany obesity. The development of obesity is multifactorial, with lifestyle and environmental factors, genetics, and epigenetics all playing an important role. Obesity is associated with numerous pathophysiological changes and/or diseases, including type 2 diabetes, cardiovascular diseases such as stroke and coronary heart disease, various cancers, sleep apnea, hypertension, and others (81). While strong basic science and clinical evidence supports these associations, causality has not yet been firmly established. Adipose tissue is the source of numerous adipokines and inflammatory cytokines, and has been shown to induce a chronic, low-grade state of inflammation. This inflammatory state is thought to be integrally linked to many of the metabolic and vascular abnormalities that occur concomitantly with obesity (21). Some of the aberrations that frequently occur in the context of obesity include insulin resistance, atherogenic dyslipidemia, hypertriglyceridemia, hyperleptinemia, and endothelial dysfunction (81). Recent evidence has highlighted that adipose tissue may undergo significant alterations and interfere with immune responses during critical illness (51). For example, in the acute phase of critical illness, adipose tissue may undergo an increase in pro-inflammatory cytokine secretion and M1 macrophage accumulation, as well as a downregulation in the secretion of adiponectin. In the chronic, prolonged phase of critical illness, adiponectin secretion may be increased, and the insulin-sensitizing, anti-inflammatory M2 macrophage may predominate (81). In addition, accumulating studies indicate that the “metaflammation” (metabolic disorder-associated chronic inflammation) in obesity interacts and/or correlates with multiple physiological changes such as altered autonomic activity, mitochondrial dysfunction, and increased oxidative and endoplasmic reticulum (ER) stress. For example, high fat diet-treated obese mice exhibit increased mitochondrial DNA (mtDNA)-induced damage, which is correlated with elevated mitochondrial dysfunction, oxidative stress, ER stress, and apoptosis (99). These obesity-associated abnormalities are thought to affect cellular/organ function, immune responses, and outcomes in obese patients with critical illness, while the mechanisms have not been thoroughly investigated.
The obesity paradox and nutrition status
An interesting trend in the literature is that obesity has recently been shown to be associated with poor outcomes in certain subsets of critically ill patients, such as those with severe trauma (19), while also appearing to be protective in other patients such as those with sepsis (the “obesity paradox”) (27, 62). These disparate findings are most likely due to artifacts of data collection or differences in pre-existing conditions, medical treatments, and inconsistent classifications of obesity and critical illness. For example, the curve for the obesity-mortality relationship in critical illness is U-shaped, where class I and II obese patients exhibit lower mortality but patients with low BMI (<20) or class III obesity exhibit higher mortality (33). It has been recently suggested that, due to insulin resistance and decreased energy uptake in the liver and skeletal muscle, the immune and central nervous systems in obesity are able to use more energy during acute immune or anti-inflammatory responses (78). If this is the case, obese patients may be protected against adverse outcomes due to reserved energy supply for inflammatory responses in the setting of critical illness. Indeed, the contributions of nutritional status have been emphasized during the treatment of critical illness. It has been shown that obese patients with a good nutritional status exhibit lower mortality, while worse outcomes and higher mortality are found in the obese patients with malnutrition (67). However, several limitations have been noticed from nutritional studies, such as potential biases regarding sample collection, the definition of malnutrition, and the prevalence of malnutrition in obesity (41, 57). Regarding its interactions with inflammation, malnutrition is suggested to be separated into three categories: starvation-associated, chronic disease-associated, and acute disease or injury-associated (31). The malnutrition found in obese patients with critical illness could be both chronic and acute disease-associated, and thus might be both a cause and a consequence of the adverse outcomes. Therefore, a better understanding of the prevalence and impacts of malnutrition during critical illness is important to ensure the effectiveness of nutritional interventions and related medical therapies, especially in a setting of obesity.
Animal models of obesity
The two major categories of obese animal models include 1) monogenic and 2) polygenic. Monogenic models have frequently utilized a mutation in the leptin pathway, although it has been shown that the single manipulation of nearly 250 different genes in mice can lead to obesity (76). Depending on the genetic manipulation or environmental and dietary factors, the severity of the obesity and the comorbid metabolic alterations can vary greatly (60). While monogenic models have been extremely helpful in understanding obesity, the mechanisms underlying many associated pathologies, monogenic obesity in humans is rare (14). Thus, polygenic models more closely mimic human obesity and should be used for preclinical testing of therapeutic agents. In polygenic models, obesity is often induced by feeding animals a hypercaloric diet. Two common variations include the high-fat diet (24), which causes obesity due to its high energy density, and the cafeteria diet, which stimulates hyperphagia (and subsequent obesity) due to its palatability (76). As would be expected, different strains of animals have a variable response to these diets, with some being prone and others being more resistant to the development of obesity (48). Because of this and the logistical difficulty of long-term feeding with a special diet, morbid obesity is not easy to replicate in diet-induced obesity models. Other less common models of obesity include those induced by chemical or surgical methods. While a comprehensive review of these approaches is outside of the scope of this article, the end result in each model is phenotypic obesity. The most common species of animals used for obesity studies are mice and rats, but many others have been used, including other rodents, larger animals like pigs, sheep, and primates, and even more exotic species such as seals and bats (76). Table 1 summarizes commonly used obese animal models including inherent leptin dysfunction and diet-induced obesity. These models can be greatly different from each other due to the methods used to induce obesity. For example, the polygenic obesity models may exhibit less hyperphagia as compared to monogenic models, and many obese animal models do not have increased fasting glucose levels (Table 1, indicated as “+/−”) despite similar levels of insulin resistance. Thus, it is vital to be aware of the baseline characteristics in an obese animal when choosing a model for a critical illness study, and potential non-specific impacts such as hyperglycemia and hyperleptinemia on innate immune responses from these different baseline parameters, for example, the fasting hyperglycemia in Zucker diabetic fatty rats vs. normal fasting glucose in obese Zucker rats, and the lack of leptin in ob-/ob-mice vs. high leptin in db-/db-mice. Also, investigators should be aware of the impact of aging on cardiovascular and immune systems in the obese animals models (17). Regardless these discrepancies, metabolic disorders such as hyperlipidemia and postprandial hyperglycemia are commonly observed in obese models and appear to play a critical role in the development of chronic metaflammation and cardiovascular dysfunction. We have demonstrated that decreasing the insulin resistance and postprandial hyperglycemia in obese Zucker rats significantly improves vascular oxidative stress and function (71, 94). The obese Zucker rat is a model of severe obesity due to leptin receptor mutation (Table 1). Although these animal models may not perfectly mimic every facet of human obesity, they have been helpful in understanding why and how comorbid conditions develop along with obesity and contribute to obesity-associated morbidity and mortality.
Table 1.
Summary of commonly used animal models of obesity
| Model name | Mutation | Hyperphagia | Hyperglycemia | Insulin Resistance |
|---|---|---|---|---|
| MONOGENIC MUTATIONS IN THE LEPTIN PATHWAY | ||||
| ob/ob mouse | Lepob/Lepob(leptin deficiency) |
+ | +/− | + |
| db/db mouse | Lepdb/Lepdb(leptin receptor) |
+ | + | + |
| Zucker rat | mutated leptin receptor (fa/fa) |
+ | +/− | + |
| ZDF rat | mutated leptin receptor (fa/fa) |
+ | + | + |
| POMC knockout mouse |
POMC deficiency | + | +/− | + |
| POMC/AgRP double knockout mice |
POMC and AgRP deficiency |
+ | +/− | + |
| MC4R knockout mouse |
melanocortin 4 receptor |
+ | +/− | + |
| MC4R knockout rat | melanocortin 4 receptor |
+ | +/− | + |
| MC4/MC3 receptor double knockout mouse |
+ | +/− | + | |
| DIET-INDUCED MODELS; POLYGENIC MODELS | ||||
| Diet-induced obese (DIO) rat |
Polygenic | + | +/− | + |
| Cafeteria diet induced obesity |
Polygenic | + | +/− | + |
| High-fat diet-induced obesity |
Polygenic | + | +/− | + |
| New Zealand obese mouse |
Polygenic | + | + | + |
Hemorrhage
Animal models of hemorrhage
The two most commonly utilized methods for inducing hemorrhage in animals include 1) uncontrolled and 2) controlled, with the latter then subdivided into a fixed-volume or fixed-pressure hemorrhage. Excellent reviews of these methods have been published previously (15, 82), as has a description of the advantages and disadvantages of each method of hemorrhage (46). Uncontrolled hemorrhages are elicited by tearing organs with a major blood supply, or by disrupting a major vessel. While this method most closely mimics clinical hemorrhage, it is difficult to control the degree of blood loss. Fixed-volume hemorrhages typically entail the loss of 25–45% of blood volume via arterial catheter or cardiac puncture, and while this allows for the study of compensatory mechanisms, hemodynamic responses are highly variable. In fixed-pressure hemorrhage or shock models, blood pressure is controlled at 20–50 mmHg for anywhere from 30 minutes to three hours by continually withdrawing blood. The fixed-pressure method is advantageous in that experimental variables (e.g. length and severity of hypotension) can be tightly controlled, but it comes at the expense of not closely mirroring hemorrhages seen in the clinical setting. Both large and small animals have been used for hemorrhage research. The choice of animal type for experimentation depends on many factors (Table 2).
Table 2.
Factors to consider when choosing an animal model of critical illness
| Small animals (mice, rats, guinea pigs) vs. large animals (dogs, pigs, sheep, monkeys) |
| Costs, staffing, and facilities |
| Sample (e.g. blood, tissue) sizes |
| Ease of procedures/transport/care |
| Physiological/genetic similarities to humans |
| Availability of transgenic models |
| Ethical considerations |
| Availability of reagents |
| Interanimal variation |
| Need for functional/behavioral outcome measures |
| Healthy vs. comorbid conditions |
| Modified and updated from Tsukamoto and Pape (16) |
The type of resuscitation used to control hemorrhage is just as important as the way it is induced in animal models. The most commonly used resuscitation fluids have been blood, saline, or lactated Ringer’s, but colloids have also been used. There is active clinical discussion about the merits and detriments of each fluid type and also about when and how much resuscitation is ideal (55). Other important protocol issues include the presence or absence of anesthesia, which can affect hemodynamic, neurological, and other physiological responses, as well as the use of anticoagulants such as heparin (Table 3) (49). To mimic clinical traumatic injuries, experimental hemorrhage methods have often been combined with additional blunt traumatic insults, including those to the head, chest, abdomen, and/or extremities (82). These models will be discussed in the blunt trauma section below.
Table 3.
Overview of animal models of critical illness
| Type of critical illness | Experimental variables to consider |
|---|---|
| Hemorrhage | Controlled (fixed volume or fixed pressure) vs. uncontrolled Length of bleed, duration kept at low volume/pressure Type and extent of resuscitation +/− anticoagulant use |
|
Blunt trauma (with or without hemorrhage) |
Extent and location of soft tissue injury, fracture, or chest trauma +/− hemorrhage |
| Traumatic brain injury | Mechanism and severity of injury +/− hemorrhage |
| Sepsis | Method for sepsis induction Dose/route/virulence of bacteria/toxin used for induction Type and extent of resuscitation +/− antibiotic use |
| All types | Clinically relevant timeline of measurements Outcomes and variables measured Reversible vs. irreversible insult Reproducibility of insult Type of anesthesia/analgesia Single vs. multiple pharmacological agents Sex, age, strain, species of animals Sources of experimental bias Consistency in animal husbandry and care Appropriate statistical testing and power |
Obesity and hemorrhage
Because hemorrhage typically occurs in the context of other injuries, only a limited number of studies have investigated outcomes in obese patients after hemorrhage. This was first shown in a 2012 report, which demonstrated that mortality from traumatic hemorrhage was increased in a stepwise fashion with increasing body mass index (59), which may have been related to inadequate resuscitation in these obese patients. Similarly, a recently published study utilizing the Glue Grant database showed that the incidence of multiple organ failure increased following hemorrhage in those with higher body weight, and found an increased mortality in those with morbid obesity (29). These clinical data suggest obesity increases the incidence of multiple organ failure and the risk of mortality after blood loss.
A few hemorrhage studies using obese animal models have helped shed light on obesity-related pathophysiology following traumatic blood loss. Obesity may decrease the ability to maintain blood pressure and organ perfusion in response to a hemorrhage. Frisbee (16) subjected anesthetized lean and obese rats to repetitive 10% withdrawals of blood volume, and found that obese rats had an earlier decompensation of blood pressure. This may have been due to the increased sympathetic vascular tone at baseline, limiting vasoconstriction and the buffering capacity to tolerate hemorrhage (16). In anesthetized obese, type II diabetic mice (db/db) (13), hypotension maintained at 35 mmHg for 90 minutes caused greater renal dysfunction and exacerbated renal hypoxia more than in nondiabetic mice. Matheson et al. conducted studies using anesthetized lean and obese rats with a fixed-pressure hemorrhage at 40% of the baseline MAP and sustained this pressure for 60 min, and demonstrated that obesity was associated with impaired hepatic blood flow, increased systemic inflammation, as well as increased liver and kidney injury (52, 53).
While many hemorrhage studies were performed in anesthetized animals, it should be realized that anesthesia blunts sympathetic activity, and obese rats have been shown to have an altered hemodynamic and adrenergic response to anesthesia (5). Thus, it is encouraged to study the responses after hemorrhage under conscious conditions. We found that conscious obese Zucker rats with 35% blood volume loss had an impaired ability to maintain their blood pressure, associated with decreases in baroreflex regulation of heart rate, renin activity, and vasopressin release, which synergistically suppressed the increase in total peripheral resistance after hemorrhage (93). This animal evidence suggests that the increased morbidity and mortality in obese patients after a severe hemorrhage could be due to impaired cardiovascular and hemodynamic regulatory responses that may lead to underperfusion of critical organs (Figure 1).
Figure 1.
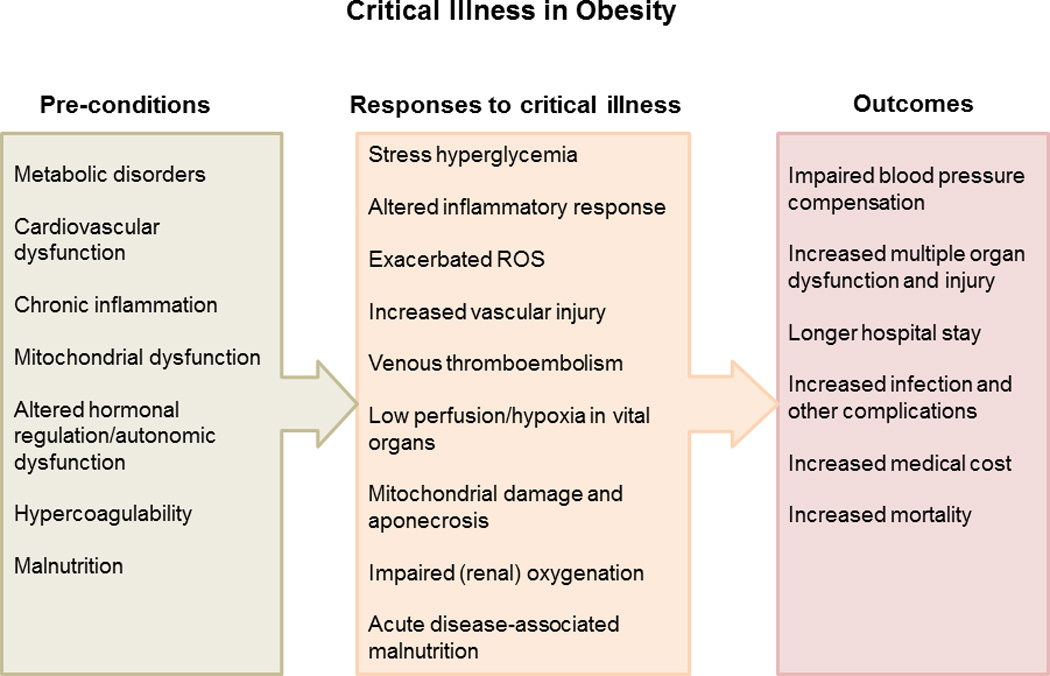
An overview of risk factors that may contribute to adverse outcome in obesity during critical illness. ROS – reactive oxygen species
Other co-morbidities seen with obesity that may play a role in the worsened outcomes after hemorrhage include hyperglycemia, insulin resistance, and coagulopathy. Post-traumatic stress hyperglycemia has been shown to be important not only in mediating patient outcomes but also because of its pro-inflammatory and pro-oxidant effects (2). We showed that obese Zucker rats have an exaggerated hyperglycemic response to hemorrhage, which was associated with larger suppression of insulin release and increased adrenergic-mediated hepatic glycogenolysis (7). Dysregulation of the autonomic nervous system was also shown in Wistar rats fed a high-calorie diet, who exhibited higher plasma glucose levels after hemorrhage as compared to their lean counterparts (9). Additionally, obesity creates a hypercoagulable, physiological milieu at baseline. Venous thromboembolism can be a major cause of organ failure after hemorrhage, and the risk of developing this complication is increased in obese trauma patients (68). For example, in Sprague-Dawley rats subjected to an uncontrolled hemorrhage (54), those fed a high-fat diet remained in a hypercoagulable state as compared to those fed a normal diet, which may play a role increasing the risk of thromboembolism. Characterized by insulin resistance and a hypercoagulable state, obese animals could be used to determine the impacts of stress hyperglycemia and thromboembolism on outcomes following hemorrhage. However, it should be noted that the hypercoagulable state is absent in db-/db- and ob-/ob- obese mice (25), and thus a validation of the coagulation state is recommended before using an obese animal model if thromboembolism is involved in the hypothesis.
Notably, adipose tissue requires relatively less blood supply as compared to lean mass. There is evidence that the total blood volume is not different between age-matched lean and obese rodents, despite the difference in body weight (16, 70). Thus, a volume- or pressure-controlled hemorrhage should be induced by removing the same (absolute) amount of blood volume from age-matched lean and obese animals (16, 93). It may be misleading to adjust the percent of blood loss or degree of shock by their body weights. For example, Matheson’s group revealed that in order to maintain the blood pressure at 40% of baseline, 3.0±0.1 mL blood per 100g body mass was removed in the lean animals in contrast to 2.5±0.2 mL/100g in obese animals (52, 53). In fact, the absolute amount of blood loss was larger in obese animals as compared to their age-matched lean animals, which may have skewed the results. Moreover, obese animals have altered blood pressure regulation systems in response to hemorrhage, as we have discussed previously (16, 93). Although the blood pressures in lean and obese animals were similar following a pressure-controlled hemorrhage, the obese animals may have undergone a different amount of blood loss and disparate neurohormonal regulation as compared to lean animals. Therefore, pressure-controlled hemorrhage is not likely to be an ideal model if comparison is made between lean and obese groups, unless the blood distribution and blood pressure regulation in obesity are more clearly elucidated in future studies.
In summary, in response to hemorrhage, obese animals may exhibit impaired organ perfusion and greater organ injury, increased inflammation, increased stress hyperglycemia, and altered autonomic, hormonal, and coagulation responses as compared to lean animals. These variables could certainly contribute to the worse outcomes that have been observed in hemorrhaged patients in the context of obesity (Figure 1), whereas more obese animal studies are still needed to verify these mechanisms and to probe optimal resuscitation strategies. In addition, the confounding effects of anesthesia and anticoagulants on hemodynamic factors, immune responses, metabolism, and nervous activity after hemorrhage warrant further consideration.
Blunt trauma
Animal models of blunt trauma
Various methods have attempted to mirror clinical blunt traumatic injuries. Excluding traumatic brain and spinal injury, which will be discussed later in this review, these blunt injuries include 1) bone fracture(s), 2) soft tissue injury, 3) chest trauma, and 4) polytrauma (82). Trauma in animals necessitates anesthesia during the insult and analgesic administration after the animals have awakened, both of which can affect physiological, metabolic, and immune variables. Fracture models have often involved the fracture of long bones like the femur and/or the tibia, and for ethical purposes, this requires fracture fixation using internal or external fixation methods (15). Much information has been gleaned from the use of animal models of fracture, and it is now well recognized that early operative fixation of fractures in the setting of polytrauma creates a “second hit” that exacerbates the inflammatory response following trauma (34, 43). To circumvent this issue, some investigators have attempted to mimic a severe long-bone fracture by injuring the soft tissue adjacent to the femur and infusing homogenized bone components into the region, and this has resulted in similar inflammatory responses and target organ failure as compared to a bilateral femur fracture in patients (38, 56). Other soft tissue injury models that have been used include abdominal laparotomy and crush injury to the rectus abdominus (82). Chest trauma models have involved lung contusion induced by clamping, bolt gun, or blast injury (82). Polytrauma models include a combination of a number of the above techniques with or without concomitant hemorrhage. While every attempt has been made to mimic injuries that occur commonly in the clinical setting, the variety and combination of clinical traumatic injuries is impossible to perfectly model. In theory, polytrauma models are likely the closest representation of severe clinical trauma, but for ethical reasons these animals often require early euthanasia, which may confound physiological responses and limit their utility. Despite the limitations, animal models have been extremely helpful in elucidating the pathophysiological response to trauma, particularly in relation to the inflammatory and compensatory anti-inflammatory responses (34, 47).
Many types of animals have been used in blunt trauma research studies. Pigs have been extensively studied in models of chest trauma combined with hemorrhage, whereas most of the fracture and soft tissue injury experiments have been conducted in rats, mice, and pigs (82). Because of their similar immune system to humans, the use of non-human primates for trauma studies has been encouraged, particularly for the preclinical study of therapeutic agents (66, 85). However, ethical considerations and costs have thus far limited their widespread use.
Obesity and blunt trauma
Since an initial report in 1991 by Choban and colleagues demonstrating increased mortality in obese patients with blunt trauma (6), many studies have compared responses and outcomes in lean and obese trauma patients, with varying results. Because obesity creates a chronic state of low-grade inflammation, it has been hypothesized that obese individuals may be more prone to an exaggerated acute-phase inflammatory response to trauma. However, the clinical evidence on this has been sparse and the results have been controversial. One study showed that two days after trauma, no differences were seen in the plasma levels of pro-inflammatory cytokines between patients with visceral versus subcutaneous obesity (8). Similarly, another study showed that the genomic blood leukocyte response to trauma was not different between lean and obese humans (91). Two other studies were in disagreement with one another, with one showing decreased cytokine levels in obese blunt trauma patients (90) and another showing increased levels (1). These inconsistencies may be due to variations in comorbid conditions of the patients, patterns of trauma, and differences in post-trauma treatments and in the time points at which plasma collections are made. In addition, as discussed previously, the outcomes may be also confounded by the presence of malnutrition. Unfortunately, to avoid these confounding variables is not possible in the clinical setting, and thus using animal models for this study area is becoming increasingly important. While there is still not a consensus on this issue, recent reviews and a meta-analysis concluded that obesity has a detrimental effect in the context of trauma (19, 45, 89). Similarly, a few recent large cohort studies have demonstrated that obese patients have prolonged stays in the intensive care unit as well as an increased risk of organ injury and failure, other in-hospital complications, and death (11, 20) (Table 4).
Table 4.
Outcomes following critical illness in obese as compared to lean patients and animals
| Hemorrhage | Blunt trauma | TBI | Sepsis | |||||
|---|---|---|---|---|---|---|---|---|
| Obese patients |
Obese animals |
Obese patients |
Obese animals |
Obese patients |
Obese animals |
Obese patients |
Obese animals |
|
| Inflammatory responses |
↑ | ↑ | ↑↓ | ↑ | ? | ? | ↓ | ↑↓ |
| Complications and organ injury |
↑ | ↑ | ↑ | ↑ | ↑ → | ↑ | ? | ↑ |
| Mortality | ↑ | ↑ | ↑ | ? | ↑ → | ? | ↓ | ↑ |
Despite the strong clinical interest in the differences in blunt trauma outcomes between lean and obese patients, there is a surprising dearth of animal studies on this topic. There have been a plethora of studies investigating patterns of inflammation and remote organ failure after blunt traumatic injury in lean animals, but little has been done outside of our laboratory with obese animals. Based on a mouse model of “pseudofracture” by Kobbe et al. (37), we developed a severe, non-hemorrhagic lower extremity trauma model in obese Zucker rats in order to study the disparate responses to trauma between lean and obese animals. Our trauma procedure involves a bilateral hind limb soft tissue injury, injection of homogenized bone components, and fibula fracture (95). The goal in developing this model was to mimic a bilateral femur fracture but to obviate the need for fixation surgery, which can be a second hit that can both exacerbate the inflammatory response and worsen outcomes. We have shown that the day after orthopedic trauma, obese rats exhibit increased incidences of acute lung and acute kidney injury, the two most-frequent organ injuries observed in trauma patients. This is evidenced by the presence of greater lung inflammation and pulmonary edema, decreased glomerular filtration rate and renal plasma flow, and increased urine excretion of renal damage markers in obese Zucker rats as compared to their lean counterparts (58, 96). This is in agreement with the clinical literature, which has consistently demonstrated an increased risk of multiple organ (especial lung and kidney) dysfunction, injury, and failure in obese blunt trauma patients (11, 20).
Additionally, we observed significant increases in systemic, lung, and renal inflammation and oxidative stress the day after trauma in obese rats, and the inhibition of NADPH oxidase (NOX), a major source of superoxide, prevented the development of both lung and kidney injury in the obese Zucker rats but had no effect in lean rats (58, 96). These results suggest that trauma increases the incidence of kidney and lung injury in obesity due, at least in part, to exacerbated oxidative stress. Reactive oxygen species play an important role in mediating innate immune responses, including activation of damage-associated molecular pattern molecules, neutrophil priming, stimulation of oxidant-related transcription factors and molecular events, and production of cytokines and chemokines. Interestingly, we found that antioxidant treatment decreases lung neutrophil numbers, myeloperoxidase activity, and plasma interleukin-6 levels in obese Zucker rats following trauma, suggesting that exacerbated oxidative stress in obesity facilitates or exaggerates immune responses (58, 96). In addition, we found that the pulmonary microvasculature in obese Zucker rats exhibit increased basal permeability and is more vulnerable to elevated oxidative stress as compared to lean Zucker rats (58, 96). These alterations in obese rats likely contribute to the increased incidence of acute lung injury. Together, these findings may provide insights into the area of redox-based therapeutics following trauma in obesity. However, it is unknown whether other obese animal models also have these pathophysiological responses after trauma.
We have also observed that the obese Zucker rats have an exaggerated hyperglycemic response after trauma as compared to lean rats (97), mainly due to pre-existing insulin resistance, which is consistent with the evidence that obese critically ill patients have an increased need for insulin (63). Inhibition of this stress hyperglycemic response attenuates the development of acute lung injury (97). Since acute hyperglycemia is known to have both pro-inflammatory and pro-oxidant effects (50), we postulate that the early stress hyperglycemia in obesity may modulate innate immune responses and increase the incidence of downstream remote organ injury. The mechanisms by which these pathological changes occur are an active area of investigation. Recent studies show that following trauma, increased circulating levels of mtDNA, a type of damage associated molecular pattern, can manipulate innate immune pathways identical to those activated in sepsis to create a sepsis-like state, and thus increase the incidence of organ injury (100). This might be an important mechanism for the adverse outcomes in obese subjects and animals with pre-existing mitochondria dysfunction and increased mtDNA damage (99). An expansion in the use of blunt trauma models in obese animals is needed to better understand these pathophysiological differences and mechanisms underlying the current disparate outcomes in the literature.
Traumatic brain injury (TBI)
Animal models of TBI
While many methods have been used to reproduce TBI in animals, the four most common ways to induce injury are the following: 1) fluid percussion; 2) controlled cortical impact; 3) weight-drop impact acceleration; and 4) blast injury (15, 82, 98). Animal models of TBI have been used not only for the study of structural damage but also functional deficits. The fluid percussion and cortical impact models are advantageous because they are easily reproducible, but the downside is that they have a high mortality rate and require a craniotomy. The weight-drop models mimic clinical TBI better than most other methods, but the injury is often not reproducible. Blast injuries are excellent for recapitulating what might be observed in a military combat setting, but the limitations in reproducibility and the difficulties in comparing blast injuries from different laboratories have been cited as a concern (98).
Mice and rats have been the species most commonly used for weight-drop models, while a combination of these and large animals like pigs have been used for the other methods of TBI induction (98). As with other types of traumatic injury, TBI in the clinical setting is extremely heterogeneous in regards to both patients and injury mechanisms. Thus, variables in animal studies should be matched as closely as possible to mimic clinical injuries while at the same time balancing the need for appropriate experimental controls. Because there have been many failed clinical trials using therapies shown to be effective in animals, it has been recognized that both short- and long-term animal studies of TBI should be conducted with increased rigor, and animals with co-morbid conditions should be studied.
Obesity and TBI
The clinical literature on obesity and TBI is sparse. In a study of patients who had undergone a frontal motor vehicle collision, obese individuals were two times as likely to sustain a severe head injury and more likely to die, as compared to those who were not obese (79). Another study showed that obese patients have increased complication and mortality rates as compared to lean patients after TBI, but once age, admission blood pressure, and chest injuries were controlled for, the differences in outcomes were no longer significant (3). Very little has been done to explore the causality of these findings. Notably, in one study, obese patients with TBI were found to have reduced brain oxygen tension as compared to lean patients, but the mechanisms have not been explored (39).
Only a few studies of TBI have been conducted in obese animals. Sprague-Dawley rats fed a high-fat sucrose diet and subjected to bilateral frontal cortical contusion had a greater loss of cortical tissue as well as impaired functional testing as compared to rats fed a standard diet (26). Other investigators also observed impaired cognitive function in Sprague-Dawley rats subjected to mild fluid percussion injury that had been fed a high-fat sucrose diet as compared to their lean counterparts fed a regular diet (92). The decrease in cognitive function in the rats fed a high-fat sucrose diet was attributed to decreases in brain-derived neurotrophic factor, which is thought to help the brain endure traumatic injury by modulating synaptic transmission. An expansion in the depth and breadth of animal studies will help uncover disparate pathophysiological responses to TBI that may occur in the context of obesity.
Sepsis
Animal models of sepsis
Animal models of sepsis have been used extensively for many years, with three main methods being used to induce the condition: 1) administration of a toxin such as lipopolysaccharide (LPS); 2) administration of a bacterium such as E. coli; and 3) puncture of the alimentary tract to allow for translocation of bacteria (10). As would be expected, each model has it merits and limitations. The LPS model replicates the shock-like state observed in septic patients and has the advantage of being easy to perform and reproduce. Most LPS models mimic the late, hypodynamic phase of sepsis well. However, because many animals are resistant to LPS, large doses are often required, and most animal studies fail to replicate the early hyperdynamic phase (4). The bacterial administration models have been helpful in studying the bodily response to bacteria, but they fail to recapitulate many of the clinical features of sepsis. Cecal ligation and puncture (CLP) and colon ascendens stent peritonitis are two methods whereby the alimentary tract has been disrupted. The CLP model is often thought to be an excellent clinically applicable sepsis model, but this model has drawn criticism for the highly variable responses observed between animal species and by different laboratories (4). Most sepsis research has been conducted in rodents because of the advantages that have been previously discussed, but larger animals such as sheep and pigs have also been used to study the effects of therapies and volume resuscitation (44). Because of the heterogeneity and complexity of sepsis (30), finding appropriate animal models has been challenging. Many clinical trials for sepsis therapies have failed, possibly due to faulty and overly optimistic conclusions drawn from poorly designed animal studies (10). Recommendations for the improvement of animal studies of sepsis have included the following: 1) both positive and negative effects of drug administration should be reported; 2) high-quality preclinical studies should be conducted prior to moving a therapy to a clinical trial; 3) experimental groups with antibiotic and other clinically used therapies should be included (4, 10); and 4) special attention should be paid to confounding variables, including sex, age, obesity, and differences in the animal and human immune systems.
Obesity and sepsis
As discussed before, although obesity is shown to be associated with poor outcomes in trauma patients (19), it may be protective in critically ill patients with sepsis (27, 62). However, more studies are needed to confirm these observations. Few studies have focused specifically on obese septic patients, with most evidence indicating that obesity is protective in the context of sepsis. Studies in hospitalized septic patients show that obese patients have lower in-hospital (40), 28-day (87), and 1-year (65) mortality rates, respectively, as compared to nonobese patients. One of these studies also examined plasma pro-inflammatory cytokine levels, with decreased interleukin-6 levels being found in obese patients (87). Conversely, one small study (149 patients) did identify obesity as a strong risk factor for 30-day mortality in patients with bacteremia (28).
Although the clinical literature suggests that obese patients, if subjected to isolated sepsis, may have decreased inflammatory responses and mortality, most sepsis animal studies have observed worse outcomes in obese as compared to lean animals. Studies have shown that obese animals have increased mortality as compared to lean animals in LPS-(69), S. aureus- (77), and CLP-induced (32) sepsis models, while one showed that survival was improved in the obese in a CLP model (73). In an LPS model, obese pigs had increased pulmonary hypertension and hypoxemia as well as an impaired cardiac index as compared to lean pigs (12). C57 mice fed a high-fat diet and subjected to CLP had worse lung injury than those consuming a regular diet (32). Increased liver injury was observed in Wistar rats fed a high-fat diet and given LPS as compared to rats fed a regular diet (69). Additionally, sepsis often causes weight loss, which was shown to be exacerbated in LPS models in ob/ob mice (42) and diet-induced obese mice (64) as compared to wild type controls. Behavioral deficits were also found to be greater in ob/ob mice with CLP-induced sepsis as compared to wild type mice with the condition (84).
Because of the massive systemic inflammatory response that often occurs in sepsis (30), the immune system and inflammation have been major areas of focus in nearly every sepsis study. The results from animal studies have been far from consistent with regard to these variables. Many have shown that obesity increases cytokine expression during sepsis (12, 32, 64, 69), while others have shown either no difference or decreased levels of cytokines in obese animals (36, 73). In CLP models of sepsis, obese mice were found to have increased leukocyte and platelet adhesion in the microcirculation of the brain (83, 84) and intestines (74), creating a more prothrombogenic environment. The precise mechanisms by which dysfunction of the inflammatory response can lead to death or other complications is a current area of investigation, but it is generally agreed that excesses in either the systemic inflammatory response syndrome (SIRS) or compensatory anti-inflammatory response syndrome (CARS) can be problematic (30, 61).
Acute lung and kidney injury are the most common and earliest fatal complication after sepsis. Sepsis-induced acute lung injury is characterized by exacerbated lung neutrophil retention and oxidative stress, which impair the regulation and integrity of endothelial cell junctions and thus increase capillary permeability and the resultant risk of non-cardiogenic pulmonary edema (23). The accumulation and activation of neutrophils in the lung facilitate production of reactive oxygen species due to increased NOX-dependent superoxide (respiratory burst) and myeloperoxidase. As mentioned previously, we found that the lung microvasculature in obese rats is more vulnerable to oxidative stress as compared to lean rats. If this is the case in patients, special attention may be needed during the treatment of sepsis in the obese population despite the controversial findings following antioxidant trials in lean subjects (18, 23). Sepsis-induced acute kidney injury is also widely studied in human and animal models, and one of the most important mechanisms appears to be blunted oxygen supply coupled with increased oxygen consumption, resulting in impaired renal oxygenation and outer medullary hypoxia (75). Renal microvascular damage and hypercoagulability could potentially decrease oxygen delivery. On the other hand, the increased renal oxygen consumption may be caused by mitochondrial dysfunction instead of increased sodium uptake (75). Obese subjects and animals are usually characterized by elevated basal levels of oxidative stress, a hypercoagulable state, and mitochondrial dysfunction. These pre-existing factors may increase the risk for renal injury following sepsis and other critical illnesses. However, there are currently no studies investigating renal hemodynamics and oxygenation after sepsis in obese animal models.
Contrary to what has been shown clinically, animal models of sepsis have demonstrated that obesity is often associated with increased mortality, a greater number of complications, and an altered systemic inflammatory response as compared to what is observed in lean animals. The fact that the clinical literature points towards obesity being protective in sepsis and the opposite is observed in animals highlights the need for an improvement in both clinical and animal experimental design (Table 4). As previously discussed, sepsis is a highly heterogeneous and complex condition, so it is not surprising that animal and clinical studies have not yielded clear and consistent findings. Future studies should be geared towards clarifying the discrepancies between animal and human studies, improving animal models, and standardizing experimental protocols (44).
CONCLUSIONS AND FUTURE DIRECTIONS
Based on observations from clinical and animal studies, obesity appears to have a disparate impact on outcomes following critical illness depending on the type of disease or insult, the experimental protocol, and the animal model used (Table 4). A large and diverse group of animal models have been used to study the responses to various forms of critical illness. Due to the inherent limitations of conducting research in humans, these models are necessary and have been helpful in understanding the impact of different factors in response to injury or illness. Because trauma and sepsis in humans are highly heterogeneous in nature, it should be realized that there is no animal model that can perfectly mimic what occurs in the clinical setting. Many clinical trials of therapies that were proven to be effective in animal models have failed in the clinical setting. Thus, testing should be conducted in a rigorous way in multiple animal models, with varying degrees of illness or injury severity (44, 98). There is ongoing debate regarding the advantages and disadvantages of using animal models in trauma research, and this is appropriate (72, 80). The development of new, and the modification of existing animal models is important in order to bring effective treatments to a wide range of patients.
More animal studies are needed to better understand the role of obesity-associated inflammation, mitochondrial dysfunction, malnutrition, and cardiovascular, metabolic, and autonomic disorders and how these factors may affect the pathophysiological responses to critical illness. Additionally, studies should seek to determine why obesity might be protective in certain critical illnesses while it appears to be detrimental following trauma. With the current focus on personalized medicine, it will be increasingly important to treat individual critically ill patients with special attention to their comorbid conditions. Because of the sustained increase in the prevalence of obesity and associated metabolic dysfunction, this will continue to be an important area of future research.
Acknowledgments
SOURCES OF SUPPORT—
Peter N. Mittwede: American Heart Association (14PRE17810005); Orthopaedic Trauma Association
John S. Clemmer: American Heart Association (14PRE20380069)
Patrick F. Bergin: Depuy-Synthes, Acumed, National Institutes of Health (P20GM104357)
Lusha Xiang: National Institutes of Health (P20GM104357); American Heart Association (12SDG12050525); Orthopaedic Trauma Association
REFERENCES
- 1.Andruszkow H, Veh J, Mommsen P, Zeckey C, Hildebrand F, Frink M. Impact of the body mass on complications and outcome in multiple trauma patients: what does the weight weigh? Mediators Inflamm. 2013;2013:345702. doi: 10.1155/2013/345702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosarge PL, Kerby JD. Stress-induced Hyperglycemia: Is It Harmful Following Trauma? Adv Surg. 2013;47:287–297. doi: 10.1016/j.yasu.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Brown CV, Rhee P, Neville AL, Sangthong B, Salim A, Demetriades D. Obesity and traumatic brain injury. J Trauma. 2006;61:572–576. doi: 10.1097/01.ta.0000200842.19740.38. [DOI] [PubMed] [Google Scholar]
- 4.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 5.Bussey CT, de Leeuw AE, Lamberts RR. Increased haemodynamic adrenergic load with isoflurane anaesthesia in type 2 diabetic and obese rats in vivo. Cardiovasc Diabetol. 2014;13:161. doi: 10.1186/s12933-014-0161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choban PS, Weireter LJ, Jr, Maynes C. Obesity and increased mortality in blunt trauma. J Trauma. 1991;31:1253–1257. doi: 10.1097/00005373-199109000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Clemmer J, Xiang L, Lu S, Mittwede P, Hester R. B2-adrenergic regulation of stress hyperglycemia following hemorrhage in the obese Zucker rat. Physiol Rep. 2014 doi: 10.14814/phy2.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collier B, Dossett L, Shipman J, Day M, Lawson G, Sawyer R, May A. Visceral adiposity is not associated with inflammatory markers in trauma patients. J Trauma. 2010;68:57–61. doi: 10.1097/TA.0b013e3181c40262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lima DC, Silveira SA, Haibara AS, Coimbra CC. The enhanced hyperglycemic response to hemorrhage hypotension in obese rats is related to an impaired baroreflex. Metab Brain Dis. 2008;23:361–373. doi: 10.1007/s11011-008-9101-x. [DOI] [PubMed] [Google Scholar]
- 10.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Ditillo M, Pandit V, Rhee P, Aziz H, Hadeed S, Bhattacharya B, Friese RS, Davis K, Joseph B. Morbid obesity predisposes trauma patients to worse outcomes: a National Trauma Data Bank analysis. J Trauma Acute Care Surg. 2014;76:176–179. doi: 10.1097/TA.0b013e3182ab0d7c. [DOI] [PubMed] [Google Scholar]
- 12.Duburcq T, Hubert T, Saint-Leger P, Mangalaboyi J, Favory R, Gmyr V, Quintane L, Tailleux A, Staels B, Tournoys A, Pattou F, Jourdain M. Impact of endotoxin challenge in obese pigs. Shock. 2014;41:546–553. doi: 10.1097/SHK.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 13.Dupuy V, Mayeur N, Buleon M, Jaafar A, Al Saati T, Schaak S, Praddaude F, Minville V, Tack I. Type 2 diabetes mellitus in mice aggravates the renal impact of hemorrhagic shock. Shock. 2012;38:351–355. doi: 10.1097/SHK.0b013e318268810f. [DOI] [PubMed] [Google Scholar]
- 14.Farooqi IS, O’Rahilly S. Monogenic obesity in humans. Annu Rev Med. 2005;56:443–458. doi: 10.1146/annurev.med.56.062904.144924. [DOI] [PubMed] [Google Scholar]
- 15.Frink M, Andruszkow H, Zeckey C, Krettek C, Hildebrand F. Experimental trauma models: an update. J Biomed Biotechnol. 2011;2011:797383. doi: 10.1155/2011/797383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frisbee JC. Impaired hemorrhage tolerance in the obese Zucker rat model of metabolic syndrome. J Appl Physiol. 2006;100:465–473. doi: 10.1152/japplphysiol.01062.2005. [DOI] [PubMed] [Google Scholar]
- 17.Frisbee JC, Goodwill AG, Frisbee SJ, Butcher JT, Brock RW, Olfert IM, DeVallance ER, Chantler PD. Distinct temporal phases of microvascular rarefaction in skeletal muscle of obese Zucker rats. Am J Physiol Heart Circ Physiol. 2014;307:H1714–H1728. doi: 10.1152/ajpheart.00605.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gadek JE, DeMichele SJ, Karlstad MD, Pacht ER, Donahoe M, Albertson TE, Van Hoozen C, Wennberg AK, Nelson JL, Noursalehi M. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid antioxidants in patients with acute respiratory distress syndrome Enteral Nutrition in ARDS Study Group. Crit Care Med. 1999;27:1409–1420. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Gharib M, Kaul S, LoCurto J, Perez M, Hajri T. The obesity factor in critical illness: Between consensus and controversy. J Trauma Acute Care Surg. 2015;78:866–873. doi: 10.1097/TA.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 20.Glance LG, Li Y, Osler TM, Mukamel DB, Dick AW. Impact of obesity on mortality and complications in trauma patients. Ann Surg. 2014;259:576–581. doi: 10.1097/SLA.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 21.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 22.Griffiths J, Hatch RA, Bishop J, Morgan K, Jenkinson C, Cuthbertson BH, Brett SJ. An exploration of social and economic outcome and associated health-related quality of life after critical illness in general intensive care unit survivors: a 12-month follow-up study. Crit Care. 2013;17:100. doi: 10.1186/cc12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo RF, Ward PA. Role of oxidants in lung injury during sepsis. Antioxid Redox Signal. 2007;9:1991–2002. doi: 10.1089/ars.2007.1785. [DOI] [PubMed] [Google Scholar]
- 24.Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23:270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 25.Henry ML, Davidson LB, Wilson JE, McKenna BK, Scott SA, McDonagh PF, Ritter LS. Whole blood aggregation and coagulation in db/db and ob/ob mouse models of type 2 diabetes. Blood Coagul Fibrinolysis. 2008;19:124–134. doi: 10.1097/MBC.0b013e3282f41e56. [DOI] [PubMed] [Google Scholar]
- 26.Hoane MR, Swan AA, Heck SE. The effects of a high-fat sucrose diet on functional outcome following cortical contusion injury in the rat. Behav Brain Res. 2011;223:119–124. doi: 10.1016/j.bbr.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogue CW, Jr, Stearns JD, Colantuoni E, Robinson KA, Stierer T, Mitter N, Pronovost PJ, Needham DM. The impact of obesity on outcomes after critical illness: a meta-analysis. Intensive Care Med. 2009;35:1152–1170. doi: 10.1007/s00134-009-1424-5. [DOI] [PubMed] [Google Scholar]
- 28.Huttunen R, Laine J, Lumio J, Vuento R, Syrjanen J. Obesity and smoking are factors associated with poor prognosis in patients with bacteraemia. BMC Infect Dis. 2007;7:13. doi: 10.1186/1471-2334-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwabejire JO, Nembhard CE, Obirieze AC, Oyetunji TA, Tran DD, Fullum TM, Siram SM, Cornwell EE, 3rd, Greene WR. Body mass index in blunt trauma patients with hemorrhagic shock: opposite ends of the body mass index spectrum portend poor outcome. Am J Surg. 2015;209:659–665. doi: 10.1016/j.amjsurg.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Iskander KN, Osuchowski MF, Stearns-Kurosawa DJ, Kurosawa S, Stepien D, Valentine C, Remick DG. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol Rev. 2013;93:1247–1288. doi: 10.1152/physrev.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen GL, Wheeler D. A new approach to defining and diagnosing malnutrition in adult critical illness. Curr Opin Crit Care. 2012;18:206–211. doi: 10.1097/MCC.0b013e328351683a. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan JM, Nowell M, Lahni P, O’Connor MP, Hake PW, Zingarelli B. Short-term high fat feeding increases organ injury and mortality after polymicrobial sepsis. Obesity (Silver Spring) 2012;20:1995–2002. doi: 10.1038/oby.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapoor JR, Heidenreich PA. Obesity and survival in patients with heart failure and preserved systolic function: a U-shaped relationship. Am Heart J. 2010;159:75–80. doi: 10.1016/j.ahj.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36:691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 35.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 36.Khan M, Patrick AL, Fox-Robichaud AE. Development of a murine model of early sepsis in diet-induced obesity. Biomed Res Int. 2014;2014:719853. doi: 10.1155/2014/719853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobbe P, Kaczorowski DJ, Vodovotz Y, Tzioupis CH, Mollen KP, Billiar TR, Pape HC. Local exposure of bone components to injured soft tissue induces Toll-like receptor 4-dependent systemic inflammation with acute lung injury. Shock. 2008;30:686–691. doi: 10.1097/SHK.0b013e31816f257e. [DOI] [PubMed] [Google Scholar]
- 38.Kobbe P, Vodovotz Y, Kaczorowski DJ, Mollen KP, Billiar TR, Pape HC. Patterns of cytokine release and evolution of remote organ dysfunction after bilateral femur fracture. Shock. 2008;30:43–47. doi: 10.1097/SHK.0b013e31815d190b. [DOI] [PubMed] [Google Scholar]
- 39.Kumar MA, Chanderraj R, Gant R, Butler C, Frangos S, Maloney-Wilensky E, Faerber J, Kofke WA, Levine JM, LeRoux P. Obesity is associated with reduced brain tissue oxygen tension after severe brain injury. Neurocrit Care. 2012;16:286–293. doi: 10.1007/s12028-011-9576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuperman EF, Showalter JW, Lehman EB, Leib AE, Kraschnewski JL. The impact of obesity on sepsis mortality: a retrospective review. BMC Infect Dis. 2013;13:377. doi: 10.1186/1471-2334-13-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lasocki S. The true obesity paradox: obese and malnourished? Crit Care Med. 2015;43:240–241. doi: 10.1097/CCM.0000000000000646. [DOI] [PubMed] [Google Scholar]
- 42.Lawrence CB, Brough D, Knight EM. Obese mice exhibit an altered behavioural and inflammatory response to lipopolysaccharide. Dis Model Mech. 2012;5:649–659. doi: 10.1242/dmm.009068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lichte P, Kobbe P, Dombroski D, Pape HC. Damage control orthopedics: current evidence. Curr Opin Crit Care. 2012;18:647–650. doi: 10.1097/MCC.0b013e328359fd57. [DOI] [PubMed] [Google Scholar]
- 44.Lilley E, Armstrong R, Clark N, Gray P, Hawkins P, Mason K, Lopez-Salesansky N, Stark AK, Jackson SK, Thiemermann C, Nandi M. Refinement of animal models of sepsis and septic shock. Shock. 2015;43:304–316. doi: 10.1097/SHK.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 45.Liu T, Chen JJ, Bai XJ, Zheng GS, Gao W. The effect of obesity on outcomes in trauma patients: a meta-analysis. Injury. 2013;44:1145–1152. doi: 10.1016/j.injury.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 46.Lomas-Niera JL, Perl M, Chung CS, Ayala A. Shock and hemorrhage: an overview of animal models. Shock 24 Suppl. 2005;1:33–39. doi: 10.1097/01.shk.0000191411.48719.ab. [DOI] [PubMed] [Google Scholar]
- 47.Lord JM, Midwinter MJ, Chen YF, Belli A, Brohi K, Kovacs EJ, Koenderman L, Kubes P, Lilford RJ. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. 2014;384:1455–1465. doi: 10.1016/S0140-6736(14)60687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lutz TA, Woods SC. Overview of animal models of obesity. Curr Protoc Pharmacol Chapter. 2012;5 doi: 10.1002/0471141755.ph0561s58. Unit5 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Majde JA. Animal models for hemorrhage and resuscitation research. J Trauma. 2003;54:S100–S105. doi: 10.1097/01.TA.0000064503.24416.F4. [DOI] [PubMed] [Google Scholar]
- 50.Mariappan MM, DeSilva K, Sorice GP, Muscogiuri G, Jimenez F, Ahuja S, Barnes JL, Choudhury GG, Musi N, DeFronzo R, Kasinath BS. Combined acute hyperglycemic and hyperinsulinemic clamp induced profibrotic and proinflammatory responses in the kidney. Am J Physiol Cell Physiol. 2014;306:C202–C211. doi: 10.1152/ajpcell.00144.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marques MB, Langouche L. Endocrine, metabolic, and morphologic alterations of adipose tissue during critical illness. Crit Care Med. 2013;41:317–325. doi: 10.1097/CCM.0b013e318265f21c. [DOI] [PubMed] [Google Scholar]
- 52.Matheson PJ, Franklin GA, Hurt RT, Downard CD, Smith JW, Garrison RN. Direct peritoneal resuscitation improves obesity-induced hepatic dysfunction after trauma. Journal of the American College of Surgeons. 2012;214:517–528. doi: 10.1016/j.jamcollsurg.2011.12.016. discussion 528–530. [DOI] [PubMed] [Google Scholar]
- 53.Matheson PJ, Hurt RT, Franklin GA, McClain CJ, Garrison RN. Obesity-induced hepatic hypoperfusion primes for hepatic dysfunction after resuscitated hemorrhagic shock. Surgery. 2009;146:739–747. doi: 10.1016/j.surg.2009.06.037. discussion 747–738. [DOI] [PubMed] [Google Scholar]
- 54.McCully BH, Dean RK, McCully SP, Schreiber MA. Diet-induced obesity prevents the development of acute traumatic coagulopathy. J Trauma Acute Care Surg. 2014;77:873–877. doi: 10.1097/TA.0000000000000461. discussion 878. [DOI] [PubMed] [Google Scholar]
- 55.Medby C. Is there a place for crystalloids and colloids in remote damage control resuscitation? Shock 41 Suppl. 2014;1:47–50. doi: 10.1097/SHK.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 56.Menzel CL, Pfeifer R, Darwiche SS, Kobbe P, Gill R, Shapiro RA, Loughran P, Vodovotz Y, Scott MJ, Zenati MS, Billiar TR, Pape HC. Models of lower extremity damage in mice: time course of organ damage and immune response. J Surg Res. 2011;166:e149–e156. doi: 10.1016/j.jss.2010.11.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mittwede PN, Bergin PF, Clemmer JS, Xiang L. Obesity, Malnutrition, and the Response to Critical Illness. Crit Care Med. 2015;43:321. doi: 10.1097/CCM.0000000000000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mittwede PN, Xiang L, Lu S, Clemmer JS, Hester RL. Oxidative stress contributes to orthopedic trauma-induced acute kidney injury in obese rats. Am J Physiol Renal Physiol. 2015;308:157–163. doi: 10.1152/ajprenal.00537.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nelson J, Billeter AT, Seifert B, Neuhaus V, Trentz O, Hofer CK, Turina M. Obese trauma patients are at increased risk of early hypovolemic shock: a retrospective cohort analysis of 1,084 severely injured patients. Crit Care. 2012;16:77. doi: 10.1186/cc11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nilsson C, Raun K, Yan FF, Larsen MO, Tang-Christensen M. Laboratory animals as surrogate models of human obesity. Acta Pharmacol Sin. 2012;33:173–181. doi: 10.1038/aps.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 62.Pickkers P, de Keizer N, Dusseljee J, Weerheijm D, van der Hoeven JG, Peek N. Body mass index is associated with hospital mortality in critically ill patients: an observational cohort study. Crit Care Med. 2013;41:1878–1883. doi: 10.1097/CCM.0b013e31828a2aa1. [DOI] [PubMed] [Google Scholar]
- 63.Pieracci F, Hydo L, Eachempati S, Pomp A, Shou J, Barie PS. Higher body mass index predicts need for insulin but not hyperglycemia, nosocomial infection, or death in critically ill surgical patients. Surg Infect (Larchmt) 2008;9:121–130. doi: 10.1089/sur.2007.039. [DOI] [PubMed] [Google Scholar]
- 64.Pini M, Castellanos KJ, Rhodes DH, Fantuzzi G. Obesity and IL-6 interact in modulating the response to endotoxemia in mice. Cytokine. 2013;61:71–77. doi: 10.1016/j.cyto.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prescott HC, Chang VW, O’Brien JM, Jr, Langa KM, Iwashyna TJ. Obesity and 1-year outcomes in older Americans with severe sepsis. Crit Care Med. 2014;42:1766–1774. doi: 10.1097/CCM.0000000000000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Redl H, Bahrami S. Large animal models: baboons for trauma, shock, and sepsis studies. Shock 24 Suppl. 2005;1:88–93. doi: 10.1097/01.shk.0000191339.46777.63. [DOI] [PubMed] [Google Scholar]
- 67.Robinson MK, Mogensen KM, Casey JD, McKane CK, Moromizato T, Rawn JD, Christopher KB. The relationship among obesity, nutritional status, and mortality in the critically ill. Crit Care Med. 2015;43:87–100. doi: 10.1097/CCM.0000000000000602. [DOI] [PubMed] [Google Scholar]
- 68.Rogers FB, Shackford SR, Horst MA, Miller JA, Wu D, Bradburn E, Rogers A, Krasne M. Determining venous thromboembolic risk assessment for patients with trauma: the Trauma Embolic Scoring System. J Trauma Acute Care Surg. 2012;73:511–515. doi: 10.1097/ta.0b013e3182588b54. [DOI] [PubMed] [Google Scholar]
- 69.Sakai S, Iizuka N, Fujiwara M, Miyoshi M, Aoyama M, Maeshige N, Hamada Y, Usami Y, Usami M. Mild obesity reduces survival and adiponectin sensitivity in endotoxemic rats. J Surg Res. 2013;185:353–363. doi: 10.1016/j.jss.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 70.Schreihofer AM, Hair CD, Stepp DW. Reduced plasma volume and mesenteric vascular reactivity in obese Zucker rats. American journal of physiology Regulatory, integrative and comparative physiology. 2005;288:R253–R261. doi: 10.1152/ajpregu.00498.2004. [DOI] [PubMed] [Google Scholar]
- 71.Sebai M, Lu S, Xiang L, Hester RL. Improved functional vasodilation in obese Zucker rats following exercise training. Am J Physiol Heart Circ Physiol. 2011;301:H1090–H1096. doi: 10.1152/ajpheart.00233.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siegl D, Annecke T, Johnson BL, 3rd, Schlag C, Martignoni A, Huber N, Conzen P, Caldwell CC, Tschop J. Obesity-induced hyperleptinemia improves survival and immune response in a murine model of sepsis. Anesthesiology. 2014;121:98–114. doi: 10.1097/ALN.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 74.Singer G, Stokes KY, Terao S, Granger DN. Sepsis-induced intestinal microvascular and inflammatory responses in obese mice. Shock. 2009;31:275–279. doi: 10.1097/SHK.0b013e3181834ab3. [DOI] [PubMed] [Google Scholar]
- 75.Singh P, Ricksten SE, Bragadottir G, Redfors B, Nordquist L. Renal oxygenation and haemodynamics in acute kidney injury and chronic kidney disease. Clin Exp Pharmacol Physiol. 2013;40:138–147. doi: 10.1111/1440-1681.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Speakman J, Hambly C, Mitchell S, Krol E. Animal models of obesity. Obes Rev 8 Suppl. 2007;1:55–61. doi: 10.1111/j.1467-789X.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 77.Strandberg L, Verdrengh M, Enge M, Andersson N, Amu S, Onnheim K, Benrick A, Brisslert M, Bylund J, Bokarewa M, Nilsson S, Jansson JO. Mice chronically fed high-fat diet have increased mortality and disturbed immune response in sepsis. PLoS One. 2009;4:7605. doi: 10.1371/journal.pone.0007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Straub RH. Insulin resistance, selfish brain, and selfish immune system: an evolutionarily positively selected program used in chronic inflammatory diseases. Arthritis Res Ther 16 Suppl. 2014;2:4. doi: 10.1186/ar4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tagliaferri F, Compagnone C, Yoganandan N, Gennarelli TA. Traumatic brain injury after frontal crashes: relationship with body mass index. J Trauma. 2009;66:727–729. doi: 10.1097/TA.0b013e31815edefd. [DOI] [PubMed] [Google Scholar]
- 80.Takao K, Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2015;112:1167–1172. doi: 10.1073/pnas.1401965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 82.Tsukamoto T, Pape HC. Animal models for trauma research: what are the options? Shock. 2009;31:3–10. doi: 10.1097/SHK.0b013e31817fdabf. [DOI] [PubMed] [Google Scholar]
- 83.Vachharajani V, Vital S, Russell J, Granger DN. Hypertonic saline and the cerebral microcirculation in obese septic mice. Microcirculation. 2007;14:223–231. doi: 10.1080/10739680601139153. [DOI] [PubMed] [Google Scholar]
- 84.Vachharajani V, Vital S, Russell J, Scott LK, Granger DN. Glucocorticoids inhibit the cerebral microvascular dysfunction associated with sepsis in obese mice. Microcirculation. 2006;13:477–487. doi: 10.1080/10739680600777599. [DOI] [PubMed] [Google Scholar]
- 85.Valparaiso AP, Vicente DA, Bograd BA, Elster EA, Davis TA. Modeling acute traumatic injury. J Surg Res. 2015;194:220–232. doi: 10.1016/j.jss.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 86.Vincent JL, Marshall JC, Namendys-Silva SA, Francois B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli M, Pickkers P, Njimi H, Jimenez E, Sakr Y. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2:380–386. doi: 10.1016/S2213-2600(14)70061-X. [DOI] [PubMed] [Google Scholar]
- 87.Wacharasint P, Boyd JH, Russell JA, Walley KR. One size does not fit all in severe infection: obesity alters outcome, susceptibility, treatment, and inflammatory response. Crit Care. 2013;17:122. doi: 10.1186/cc12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Winfield RD. Caring for the critically ill obese patient: challenges and opportunities. Nutr Clin Pract. 2014;29:747–750. doi: 10.1177/0884533614553234. [DOI] [PubMed] [Google Scholar]
- 89.Winfield RD, Bochicchio GV. The critically injured obese patient: a review and a look ahead. J Am Coll Surg. 2013;216:1193–1206. doi: 10.1016/j.jamcollsurg.2013.01.055. [DOI] [PubMed] [Google Scholar]
- 90.Winfield RD, Delano MJ, Cuenca AG, Cendan JC, Lottenberg L, Efron PA, Maier RV, Remick DG, Moldawer LL, Cuschieri J. Obese patients show a depressed cytokine profile following severe blunt injury. Shock. 2012;37:253–256. doi: 10.1097/SHK.0b013e3182449c0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Winfield RD, Delano MJ, Dixon DJ, Schierding WS, Cendan JC, Lottenberg L, Lopez MC, Baker HV, Cobb JP, Moldawer LL, Maier RV, Cuschieri J. Differences in outcome between obese and nonobese patients following severe blunt trauma are not consistent with an early inflammatory genomic response. Crit Care Med. 2010;38:51–58. doi: 10.1097/CCM.0b013e3181b08089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu A, Molteni R, Ying Z, Gomez-Pinilla F. A saturated-fat diet aggravates the outcome of traumatic brain injury on hippocampal plasticity and cognitive function by reducing brain-derived neurotrophic factor. Neuroscience. 2003;119:365–375. doi: 10.1016/s0306-4522(03)00154-4. [DOI] [PubMed] [Google Scholar]
- 93.Xiang L, Clemmer JS, Lu S, Mittwede PN. Impaired blood pressure compensation following hemorrhage in conscious obese Zucker rats. Life Sciences. 2013;93:214–219. doi: 10.1016/j.lfs.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xiang L, Dearman J, Abram SR, Carter C, Hester RL. Insulin resistance and impaired functional vasodilation in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2008;294:H1658–H1666. doi: 10.1152/ajpheart.01206.2007. [DOI] [PubMed] [Google Scholar]
- 95.Xiang L, Hester RL, Fuller WL, Sebai ME, Mittwede PN, Jones EK, Aneja A, Russell GV. Orthopedic trauma-induced pulmonary injury in the obese Zucker rat. Microcirculation. 2010;17:650–659. doi: 10.1111/j.1549-8719.2010.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xiang L, Lu S, Mittwede PN, Clemmer JS, Hester RL. Inhibition of NADPH oxidase prevents acute lung injury in obese rats following severe trauma. Am J Physiol Heart Circ Physiol. 2014;306:H684–H689. doi: 10.1152/ajpheart.00868.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiang L, Lu S, Mittwede PN, Clemmer JS, Husband GW, Hester RL. beta2-Adrenoreceptor blockade improves early posttrauma hyperglycemia and pulmonary injury in obese rats. Am J Physiol Heart Circ Physiol. 2014;307:H621–H627. doi: 10.1152/ajpheart.00208.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yuzefovych LV, Musiyenko SI, Wilson GL, Rachek LI. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One. 2013;8:54059. doi: 10.1371/journal.pone.0054059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]


