Abstract
Background
HIV infected (HIV+) individuals may be more susceptible to alcohol-related harm than uninfected individuals.
Methods
We analyzed data on HIV+ and uninfected individuals in the Veterans Aging Cohort Study (VACS) with an Alcohol Use Disorders Identification Test-Consumption AUDIT-C score from 2008–2012. We used Cox proportional hazards models to examine the association between alcohol exposure and mortality through July, 2014; and linear regression models to assess the association between alcohol exposure and physiologic injury based on VACS Index Scores. Models were adjusted for age, race/ethnicity, smoking, and Hepatitis C infection.
Results
The sample included 18,145 HIV+ and 42,228 uninfected individuals. Among HIV+ individuals, 76% had undetectable HIV-1 RNA (<500 copies/ml). The threshold for an association of alcohol use with mortality and physiologic injury differed by HIV status. Among HIV+ individuals, AUDIT-C score ≥4 (hazard ratio [HR] 1.25, 95% CI 1.09–1.44) and ≥30 drinks per month (HR, 1.30, 95% CI 1.14–1.50) were associated with increased risk of mortality. Among uninfected individuals, AUDIT-C score ≥5 (HR, 1.19, 95% CI 1.07–1.32) and ≥70 drinks per month (HR 1.13, 95% CI 1.00–1.28) were associated with increased risk. Similarly, AUDIT-C threshold scores of 5–7 were associated with physiologic injury among HIV+ individuals (beta 0.47, 95% CI 0.22, 0.73) and a score of 8 or more was associated with injury in uninfected (beta 0.29, 95% CI 0.16, 0.42) individuals.
Conclusions
Despite antiretroviral therapy, HIV+ individuals experienced increased mortality and physiologic injury at lower levels of alcohol use compared with uninfected individuals. Alcohol consumption limits should be lower among HIV+ individuals.
Keywords: alcohol, mortality, morbidity, VACS Index, AUDIT-C, veteran
1. INTRODUCTION
The health impact of alcohol use is well described (Dawson et al., 2008; Rehm et al., 2003; Saitz, 2005). While the impact of alcohol on cause-specific mortality can vary by condition, most large observational studies demonstrate a dose-dependent association between higher levels of alcohol consumption and all-cause mortality (Di Castelnuovo et al., 2006; Harris et al., 2010; Knott et al., 2015; Plunk et al., 2014; Rogers et al., 2015). However, alcohol use is common among those with HIV infection (HIV+ individuals) and may be uniquely harmful in this population (Braithwaite and Bryant, 2010; Braithwaite et al., 2008, 2005; Cook et al., 2001). Among other effects, alcohol use is associated with poor adherence to life preserving combination antiretroviral treatment (ART; Braithwaite and Bryant, 2010; Braithwaite et al., 2008, 2005; Cook et al., 2001; Samet et al., 2004) and increases risk for liver fibrosis (Lim et al., 2014), a leading cause of death. Further, compared with uninfected individuals, HIV+ individuals report fewer drinks required to experience intoxication (McGinnis et al., 2015). As a result, some have suggested that “safe limits” for alcohol use among those with HIV infection should be lower than among uninfected individuals (Samet et al., 2007, 2004).
Further, health effects of alcohol among HIV+ individuals appear to vary depending on whether viral suppression is attained. Given a unit dose of alcohol, HIV+ individuals experience higher blood alcohol concentrations before, compared to after, initiating ART (McCance-Katz et al., 2012). In addition, having a detectable HIV viral load is associated with a lower number of drinks to feel intoxicated among HIV+ individuals (McGinnis et al., 2015). Since many the prior studies were dominated by HIV+ individuals who had not achieved viral suppression, it is important to determine whether differential harms from alcohol use persist among a more current sample of HIV+ individuals, most of whom have achieved HIV-1 RNA suppression on ART, compared with uninfected individuals.
Finally, all-cause mortality is an important health outcome, but it is not the only one of interest (Kinder et al., 2009). HIV+ individuals in care are aging (Justice, 2010) and experiencing increasing physiologic injury resulting in cumulative frailty or a greater vulnerability to stressors (Clegg et al., 2013). The Veterans Aging Cohort Study (VACS) Index incorporates weighted values of routinely available clinical biomarkers including age, CD4 count, HIV-1 RNA, hemoglobin, platelets, aspartate transaminase, alanine transaminase, creatinine, and Hepatitis C sero-status (Justice et al., 2013; Tate et al., 2013). The Index provides reliable estimates of five-year mortality in HIV + individuals (Tate et al., 2013) and has been shown to predict hospitalization (Akgun et al., 2013a), physical and cognitive performance (Marquine et al., 2014; Oursler et al., 2013), and fragility fractures (Womack et al., 2013). The Index also predicts hospitalization and mortality among uninfected individuals (Akgun et al., 2013a). As a result, the VACS Index is a clinically applicable measure of overall physiologic injury and may be a meaningful indicator of frailty (Akgun et al., 2013a; Escota et al., 2014; Shaper, 1990; Shaper and Wannamethee, 1998; Womack et al., 2013). The Alcohol Use Disorders Identification Test - Consumption (AUDIT-C) is a commonly used tool to screen for alcohol use in large clinical populations (Bradley et al., 2007). We use mortality, the VACS Index, and self-reported alcohol data, to compare health outcomes associated with patterns of alcohol use among HIV+ and uninfected individuals. Our first aim is to determine whether level of alcohol use is associated with all-cause mortality and physiologic injury as measured by the VACS Index. Our second aim is to determine whether AUDIT-C thresholds for physiologic injury differ by HIV status.
2. METHODS
2.1 Data
We used data from the Veterans Aging Cohort Study (VACS), a large electronic medical record (EMR) based cohort study of 47,805 HIV + and 99,061 uninfected patients receiving care in the Veterans Health Administration (VHA) from fiscal years 1997 to 2014 (Conigliaro et al., 2004; Justice et al., 2006a, 2006b). We identified those who reported alcohol use based on having a non-zero AUDIT-C score after enrollment into the cohort and from October, 2008 to March, 2012. Observations with an AUDIT-C score of zero were excluded since those who abstain from alcohol may be an inappropriate referent group (Knott et al., 2015) as they often include individuals who became abstinent due to comorbidities and other health issues (Fillmore et al., 2007; Shaper, 1990; Shaper and Wannamethee, 1998). We elected not to use alcohol-related diagnoses from the EMR for the current analysis due to concerns over low sensitivity (Kim et al., 2012; Quan et al., 2008). Women were excluded because they represent less than 3% of VACS subjects and sensitivity to the effects of alcohol varies by gender (Midanik, 1999).
2.2 Main Outcomes
Outcomes included: 1) all-cause mortality and 2) physiologic injury as measured by the VACS Index. Mortality rates were calculated using follow-up time starting with date of first AUDIT-C and ending with date of death or July 31, 2014, whichever came first. Deaths were identified from four sources: 1) the Patient Treatment File, which records hospital deaths in the VHA healthcare system, 2) the Beneficiary Identification Records Locating System, which tracks VHA death benefits, 3) the Medicare Vital Status File, and 4) the Social Security National Death Index. The VACS Index was calculated as previously reported using laboratory values from closest to (±90 days) date of AUDIT-C measurement. Of note, VACS Index is calculated for uninfected individuals by assuming a normal CD4 count (i.e., greater than 500 cells/mm3) and an undetectable HIV-1 RNA VL (Akgun et al., 2013a; Akgun et al., 2013b).
2.3 Main Predictor
Alcohol exposure was assessed based on self-reported responses to the AUDIT-C. AUDIT-C data are collected nationally in the VHA using automated clinical reminders in the EMR that providers must complete on their patients on a regular basis (Bradley et al., 2006). For each of the 3 questions in the AUDIT-C, 0–4 points are assigned based on the response and summed for a total score ranging from 0–12. Item 1 is: “How often do you have a drink containing alcohol?” Choices (points) include: never (0), monthly or less (1), 2–4 times a month (2), 2–3 times a week (3); or 4 or more times a week (4). Item 2 is: “How many standard drinks containing alcohol do you have on a typical day?” Choices (points) include: 0 (0), 1 or 2 (0); 3 or 4 (1); 5 or 6 (2); 7 to 9 (3); 10 or more (4). The original AUDIT-C Item 2 had no option for 0 number of drinks; therefore, we combined the responses of 0 and 1 or 2. Item 3 is: “How often do you have six or more drinks on one occasion?” Choices (points) include: never (0); less than monthly (1); monthly (2); weekly (3); daily or almost daily (4).
AUDIT-C has been evaluated using multiple cutoffs including 3+, 4+, and 5+ (Bradley et al., 2007; Bush et al., 1998; Gordon et al., 2001; Gual et al., 2002). Heavy episodic drinking (HED) has been assessed using a cutoff based on any positive response to AUDIT-C item 3 (McGinnis et al., 2013). Further, recommended cutoffs for AUDIT-C vary based on the population (male vs. female, 65 years), the different modes in which the AUDIT-C is asked (face to face vs. confidential survey), and the purpose for using the AUDIT-C (initial screening prior to further assessment vs. the only assessment). Because of the diversity of cutoffs used in prior literature, we initially employed integer categories of AUDIT-C to evaluate multiple candidate cutoff(s) (1, 2, 3, 4, 5–7, and 8+) for predicting mortality and physiologic frailty.
Alcohol use is often evaluated using quantity-frequency measures (i.e., total number of drinks over a certain timeframe) and an average of more than 2 drinks per day is considered potentially unhealthy alcohol use for men (Saitz, 2005; NIAAA, 2005). Therefore, we also created a variable for estimated total number of drinks per month using the first two items in the AUDIT-C. Categories included 1–2, 3–7, 8–29, 30–69, and 70+ drinks per month. We also considered HED (Smith et al., 2009) frequency separately.
2.4 Covariates
Covariates assessed include HIV status, age, race/ethnicity, smoking status, drug dependence-related diagnosis, and Hepatitis C. HIV status is based on a previously validated group of International Classification of Diseases, Ninth Revision (ICD-9) codes. Like AUDIT-C for alcohol, smoking status (current/past/never) is an automated EMR-based clinical reminder; drug dependence-related diagnoses are based on ICD-9 codes, and Hepatitis C is based on a combination of ICD-9 codes and laboratory data. Age was measured at the time of AUDIT-C and used as a continuous variable for descriptive statistics and categorized as <50, 50–64 and 65 years and over for regression models.
2.5 Analysis
We compared characteristics of participants by HIV status using chi-square tests, t-tests and Wilcoxon rank-sum tests. We compared number of participants identified with unhealthy alcohol use based on AUDIT-C 4+ vs. a combination of HED and 30+ drinks per month. Mortality rates were summarized and compared graphically by HIV status and alcohol use levels (AUDIT-C scores, estimated quantity-frequency, and HED) and using Cox proportional hazard (PH) models. Multivariate Cox PH models for time to death were adjusted for age, race/ethnicity, smoking status, and Hepatitis C infection. Interactions between HIV and alcohol use levels were tested. Drug dependence-related diagnoses were not associated with the outcomes in the multivariate models, nor did their exclusion change the association of interest, so they were not included in the final models. VACS Index Scores were summarized graphically by HIV status and alcohol exposure (AUDIT-C scores, estimated quantity-frequency, and HED). Ordinary least squares linear regression models were used to quantify the association between alcohol exposure and VACS Index Scores. Multivariate models were adjusted for age, race/ethnicity, smoking status, and Hepatitis C. Since sensitivity to alcohol varies with age, and recommended drinking limits are lower for those older than 65 years of age, we repeated all analyses excluding those over age 65.
3. RESULTS
3.1 Demographics and Characteristics
There were 18,145 HIV+ and 42,228 uninfected individuals with an AUDIT-C score greater than zero. Mean age was 52.5 years for HIV+ and 54.0 years for uninfected individuals. The distribution of race/ethnicity and AUDIT-C scores was generally similar by HIV status. Hepatitis C was more prevalent among HIV+ (31%) than uninfected individuals (16%, p<.001). Among HIV+ and uninfected individuals, 16% and 13% had a drug dependence-related diagnosis code (p<.001), respectively. Most HIV+ individuals (76%) had achieved HIV-1 RNA suppression demonstrating effective ART. Mean VACS index was 30 for HIV+ and 21 for uninfected individuals (p<.001) (Table 1). Among HIV+ individuals, considering HED and drinks per month as separate criteria results in identifying 30% with unhealthy alcohol use compared to 24% using the overall AUDIT-C threshold of 4 or higher.
Table 1.
Demographic Characteristics of HIV Infected and Uninfected Men in VACS with an AUDIT-C score of at least 1
| Characteristic | HIV Infected | Uninfected | P-Value |
|---|---|---|---|
|
| |||
| N | 18,145 | 42,228 | |
|
| |||
| Mean Age (SD) | 52.5 (10.5) | 54.0 (10.0) | <.001 |
|
| |||
| Race/Ethnicity (%) | <.001 | ||
| White | 40.7 | 38.6 | |
| African-American | 47.1 | 48.5 | |
| Hispanic | 7.5 | 8.2 | |
| Other/Unknown | 4.8 | 4.6 | |
|
| |||
| AUDIT-C Scores (%) | <.001 | ||
| 1 | 42.2 | 37.8 | |
| 2 | 20.9 | 19.7 | |
| 3 | 13.1 | 13.7 | |
| 4 | 9.2 | 10.0 | |
| 5–7 | 8.6 | 10.1 | |
| 8–12 | 6.2 | 8.8 | |
| Median (IQR) | 2 (1,3) | 2 (1,4) | |
|
| |||
| Number of drinks containing alcohol consumed per month (%) | <.001 | ||
| 1–2 | 45.7 | 41.5 | |
| 3–7 | 21.1 | 19.6 | |
| 8–29 | 15.5 | 16.7 | |
| 30–69 | 10.1 | 12.1 | |
| 70+ | 7.5 | 10.1 | |
|
| |||
| Heavy Episodic Drinking* (define below) HED | <.001 | ||
| Never | 77.5 | 74.4 | |
| <Monthly | 11.2 | 10.8 | |
| Monthly | 3.8 | 4.6 | |
| Weekly | 3.9 | 5.4 | |
| Daily | 3.6 | 4.9 | |
|
| |||
| Drug Related Diagnosis Code (%) | 16.3 | 13.3 | <.001 |
|
| |||
| Hepatitis C infection (%) | 31.2 | 16.1 | <.001 |
|
| |||
| Smoking (%) | <.001 | ||
| Never | 27.5 | 26.6 | |
| Past | 13.8 | 16.9 | |
| Current | 58.7 | 56.5 | |
|
| |||
| Deaths (%) | 7.2 | 4.7 | |
|
| |||
| Deaths/100 Person | 2.7 | 1.8 | <.001 |
|
| |||
| Year | |||
|
| |||
| Median Follow-Up Time (IQR) | 4.7 (3.5–5.3) | 4.8 (3.7–5.3) | <.001 |
|
| |||
| *N | 9,216 | 12,841 | |
|
| |||
| Mean VACS Index Score (SD) | 29.8 (21.4) | 20.7 (16.2) | <.001 |
|
| |||
| Mean Estimated 5 Year Mortality Based on VACS Index Score (SD) | 15.0 (14.6) | 9.4 (9.6) | <.001 |
|
| |||
| Hemoglobin | <.001 | ||
| <10 | 2.0 | 1.7 | |
| 10–11.9 | 8.8 | 6.8 | |
| 12–13.9 | 32.2 | 29.9 | |
| 14+ | 57.0 | 61.7 | |
|
| |||
| Estimated glomerular filtration rate | .03 | ||
| <30 | 1.7 | 1.8 | |
| 30–44.9 | 1.8 | 1.9 | |
| 45–59.9 | 6.5 | 5.5 | |
| 60+ | 90.1 | 90.8 | |
|
| |||
| FIB4 | <.001 | ||
| <1.45 | 52.1 | 65.0 | |
| 1.45–3.249 | 38.1 | 29.5 | |
| 3.25+ | 9.8 | 5.5 | |
|
| |||
| CD4 | - | ||
| <50 | 2.9 | ||
| 50–99 | 3.3 | ||
| 100–199 | 9.6 | ||
| 200–349 | 20.5 | ||
| 350–499 | 21.8 | ||
| 500+ | 41.8 | ||
|
| |||
| HIV viral load | - | ||
| <500 | 75.7 | ||
| 500–99,999 | 20.0 | ||
| 100,000+ | 4.3 | ||
Includes those who had VACS Index Score w/in 90 days after their AUDIT-C
3.2 Mortality
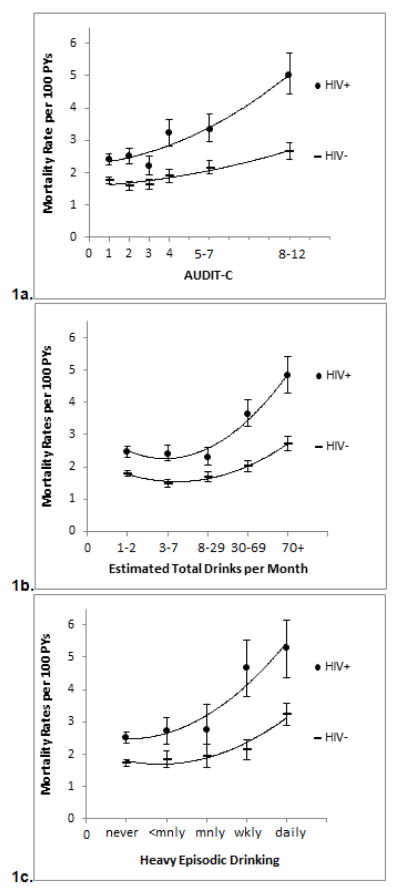
Median follow-up was 4.8 years (interquartile range (IQR) 3.6 to 5.3). Mortality per 100 person-years was 2.7 among HIV+ and 1.8 among uninfected individuals (p<.001, Table 1). Mortality rates increased with increasing AUDIT-C scores for both HIV+ and uninfected individuals (Figure 1a). However, the difference in mortality rates between HIV+ and uninfected individuals widened with higher AUDIT-C scores (interaction term from Cox PH model: p=.02). Similar patterns and significant interactions were found using number of drinks per month (Figure 1b) or HED (Figure 1c) as the measure of alcohol exposure.
Figure 1.
Figure 1a–c. Mortality per 100 Person Years (PYs) by AUDIT-C Level and HIV Status
In multivariable models, based on hazard ratios (HRs), the association between AUDIT-C scores of one, two, and three with the outcomes were similar, so the categories were combined so that the AUDIT-C referent group contains individuals with an AUDIT-C of 1–3. Among HIV+ individuals, adjusted HRs for mortality were significantly higher for those with AUDIT-C of 4, 5–7, and 8–12 compared to those with AUDIT-C of 1–3. Among uninfected individuals, HRs were significantly higher for those with AUDIT-C scores of 5–12 (Table 2). In a combined model HIV infection was associated with mortality (HR 1.35: 95% CI 1.26–1.45) and the interaction term for HIV and AUDIT-C was significant (p=.02). Age, smoking, and hepatitis C infection were also significant predictors of mortality in all three models.
Table 2.
Adjusted Mortality by AUDIT-C Level Among HIV Infected and Uninfected Men
| HIV Infected (n=18,145) | Uninfected (n=42,228) | |||
|---|---|---|---|---|
| Variable | HR | 95% CI | HR | 95% CI |
|
| ||||
| AUDIT-C score (reference is 1–3) | ||||
| 4 | 1.25 | 1.09–1.44 | 1.01 | 0.90–1.13 |
| 5–7 | 1.29 | 1.12–1.48 | 1.19 | 1.07–1.32 |
| 8–12 | 1.71 | 1.49–1.98 | 1.35 | 1.22–1.50 |
|
| ||||
| Age (reference is <50 years) | ||||
| 50–64 years | 2.40 | 2.13–2.71 | 2.94 | 2.63–3.28 |
| 65+ years | 5.92 | 5.11–6.85 | 7.29 | 6.39–8.26 |
|
| ||||
| Race/Ethnicity (reference is white) | ||||
| African-American | 0.94 | 0.85–1.03 | 0.83 | 0.77–0.89 |
| Hispanic | 0.78 | 0.64–0.93 | 0.73 | 0.64–0.85 |
| Other | 0.88 | 0.69–1.12 | 0.71 | 0.58–0.87 |
|
| ||||
| Smoking (ref is never smoked) | ||||
| Current | 1.85 | 1.64–2.10 | 2.21 | 1.99–2.45 |
| Past | 1.22 | 1.04–1.44 | 1.39 | 1.23–1.58 |
| Unknown/Missing | 1.81 | 1.31–2.50 | 1.54 | 1.17–2.03 |
|
| ||||
| Hepatitis C infection | 1.90 | 1.74–2.09 | 1.89 | 1.75–2.05 |
Number of drinks per month and HED in the past 12 months were also important predictors of mortality. Among HIV+ individuals, HRs for mortality were significantly higher for those drinking an estimated 30 or more drinks per month compared to those drinking 1–2 drinks per month (Table 3). Among uninfected individuals, HRs were significantly higher for those drinking an estimated 70 or more drinks per month compared to those drinking 1–2 drinks per month. Notably, a “J-shaped” relationship between drinks per month and risk of mortality was only observed for the uninfected, whereby 3–7 drinks per month (compared to 1–2 drinks per month) was found to be protective (HR 0.86: 95% CI 0.78–0.95). In combined models, HIV infection was associated with higher risk of mortality (HR 1.28: 95% CI 1.18, 1.40). Both drinks per month and HED in the past 12 months were independently associated with mortality and the interaction term for HIV and drinks per month was significant (p<.001). Age, smoking, and Hepatitis C infection were also significant predictors of mortality in all three models.
Table 3.
Adjusted Mortality by Level of Alcohol Exposure Among HIV Infected and Uninfected Men
| HIV Infected (n=18,145) | Uninfected (n=42,228) | |||
|---|---|---|---|---|
|
| ||||
| Variable | HR | 95% CI | HR | 95% CI |
|
| ||||
| Number of alcoholic drinks per month (reference is 1–2) | ||||
| 3–7 | 1.03 | 0.91–1.16 | 0.86 | 0.78–0.95 |
| 8–29 | 0.96 | 0.83–1.10 | 0.92 | 0.83–1.02 |
| 30–69 | 1.30 | 1.14–1.50 | 0.96 | 0.86–1.07 |
| 70+ | 1.50 | 1.28–1.76 | 1.13 | 1.00–1.28 |
|
| ||||
| HED Weekly or More | 1.12 | 1.00–1.25 | 1.17 | 1.07–1.27 |
|
| ||||
| Age (reference is <50 years) | ||||
| 50–64 years | 2.39 | 2.12–2.69 | 2.93 | 2.62–3.28 |
| 65+ years | 5.86 | 5.06–6.79 | 7.27 | 6.39–8.26 |
|
| ||||
| Race/Ethnicity (reference is white) | ||||
| African-American | 0.94 | 0.85–1.03 | 0.83 | 0.77–0.89 |
| Hispanic | 0.78 | 0.65–0.94 | 0.73 | 0.63–0.84 |
| Other | 0.88 | 0.70–1.12 | 0.71 | 0.58–0.87 |
|
| ||||
| Smoking (ref is never smoked) | ||||
| Current | 1.86 | 1.64–2.10 | 2.21 | 1.99–2.45 |
| Past | 1.22 | 1.04–1.44 | 1.39 | 1.23–1.58 |
| Unknown/Missing | 1.82 | 1.32–2.51 | 1.54 | 1.17–2.04 |
|
| ||||
| Hepatitis C infection | 1.91 | 1.74–2.09 | 1.89 | 1.75–2.05 |
3.3 Physiologic Injury
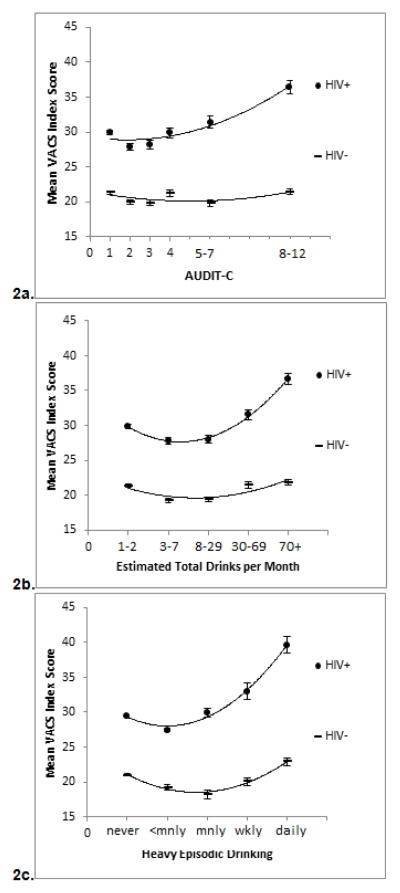
Among HIV+ individuals, physiologic injury, as measured by VACS Index, increased with increasing AUDIT-C scores (Figure 2a). Similarly, injury increased with increasing number of drinks per month and increasing frequency of HED (Figures 2b–c). Additionally, the difference in physiologic injury between HIV+ and uninfected individuals was greater as all three measures of alcohol use increased (Figures 2a–c). In linear regression models adjusting for the same factors as models predicting all-cause mortality, the levels of alcohol use associated with injury were lower among HIV+ than among uninfected individuals (Tables 4 and 5). AUDIT-C scores of 5–7 and 8–12 were significantly associated with physiologic injury among HIV+ individuals compared to those with AUDIT-C 1–3 (beta 0.47: 95% CI 0.22, 0.73; and beta 1.05: 95% CI 0.77, 1.34, respectively). In contrast, only a score of 8–12 was associated with injury among uninfected individuals (beta 0.29: 95% CI 0.16, 0.42) (Table 4). Interestingly, after adjusting for HED (which was not independently associated with VACS Index), drinking between 3 and 29 drinks a month was protective against injury among uninfected individuals (beta −0.23: 95% CI −0.33- (−0.12) and beta −0.16: 95% CI −0.27-(−0.04), respectively) whereas no level of use was protective among HIV+ individuals. Drinking 70 or more drinks per month was associated with injury among HIV+ individuals (beta 0.99: 95% CI 0.68–1.29) and HIV uninfected individuals (beta 0.19: 95% CI 0.04–0.34). The J-shaped associated between alcohol and physiologic injury was only observed for the uninfected group.
Figure 2.
Figure 2a–c. Mean VACS Index by AUDIT-C Level and HIV Status
Table 4.
Adjusted VACS Index, by AUDIT-C Level Among HIV Infected and Uninfected Men
| HIV Infected (n=9,216) | Uninfected (n=12,833) | |||
|---|---|---|---|---|
|
| ||||
| Variable | Beta Coefficient | 95% CI | Beta Coefficient | 95% CI |
|
| ||||
| AUDIT-C (ref 1) | ||||
| 4 | 0.10 | −0.14–0.35 | 0.08 | −0.06–0.22 |
| 5–7 | 0.47 | 0.22–0.73 | 0.04 | −0.09–0.17 |
| 8–12 | 1.05 | 0.77–1.34 | 0.29 | 0.16–0.42 |
|
| ||||
| Age (ref <50 yrs) | ||||
| 50–64 years | 3.29 | 3.14–3.44 | 3.26 | 3.17–3.36 |
| 65+ years | 7.26 | 7.00–7.52 | 7.63 | 7.48–7.78 |
|
| ||||
| Race/Eth (ref white) | ||||
| Af-Am | 1.28 | 1.13–1.43 | 0.67 | 0.58–0.75 |
| Hispanic | 0.18 | −0.09–0.45 | −0.03 | −0.17–0.11 |
| Other | 0.40 | 0.03–0.76 | 0.01 | −0.23–0.24 |
|
| ||||
| Smoking (ref never) | ||||
| Current | 0.14 | −0.03–0.30 | −0.16 | −0.25–(−0.06) |
| Past | −0.04 | −0.28–0.19 | 0.16 | 0.03–0.29 |
| Unknown/Missing | −0.40 | −0.99–0.20 | −0.31 | −0.66–0.03 |
|
| ||||
| Hepatitis C | 2.20 | 2.05–2.36 | 1.98 | 1.88–2.08 |
|
| ||||
| Constant | 1.92 | 1.75–2.10 | 0.58 | 0.47–0.70 |
Table 5.
Adjusted VACS Index, by Level of Alcohol Exposure Among HIV Infected and Uninfected Men
| HIV Infected (n=9,216) | Uninfected (n=12,833) | |||
|---|---|---|---|---|
|
| ||||
| Variable | Beta Coefficient | 95% CI | Beta Coefficient | 95% CI |
|
| ||||
| # Drinks/Mn (ref 1–2) | ||||
| 3–7 | −0.14 | −0.32–0.05 | −0.23 | −0.33–(−0.12) |
| 8–29 | −0.03 | −0.24–0.17 | −0.16 | −0.27–(−0.04) |
| 30–69 | 0.22 | −0.03–0.47 | −0.05 | −0.19–0.08 |
| 70+ | 0.99 | 0.68–1.29 | 0.19 | 0.04–0.34 |
|
| ||||
| HED Weekly or More | 0.05 | −0.14–0.24 | 0.08 | −0.02–0.18 |
|
| ||||
| Age (ref <50 yrs) | ||||
| 50–64 years | 3.27 | 3.12–3.43 | 3.26 | 3.17–3.35 |
| 65+ years | 7.23 | 6.97–7.49 | 7.62 | 7.47–7.77 |
|
| ||||
| Race/Ethnicity (ref white) | ||||
| Af-Am | 1.28 | 1.13–1.43 | 0.68 | 0.59–0.76 |
| Hispanic | 0.20 | −0.07–0.47 | −.03 | −0.17–0.12 |
| Other | 0.40 | 0.03–0.77 | 0.02 | −0.22–0.26 |
|
| ||||
| Smoking (ref never) | ||||
| Current | 0.15 | −0.02–0.31 | −0.15 | −0.25–(−0.05) |
| Past | −0.04 | −0.28–0.19 | 0.16 | 0.03–0.29 |
| Unknown/Missing | −0.38 | −0.98–0.21 | −0.30 | −0.65–0.04 |
|
| ||||
| Hepatitis C | 2.21 | 2.06–2.36 | 1.98 | 1.88–2.08 |
|
| ||||
| Constant | 1.97 | 1.78–2.15 | 0.65 | 0.53–0.77 |
4. DISCUSSION
Compared with uninfected individuals, mortality and physiologic injury associated with discrete levels of alcohol exposure were higher among HIV+ individuals. While we found evidence of a J-shaped association between alcohol use, mortality, and physiologic injury among uninfected subjects, there was little evidence suggesting a protective effect of alcohol at any level of use among HIV+ individuals. Further, lower levels of alcohol exposure were associated with more harm among HIV+ individuals. Thresholds for risk were also lower among HIV+ individuals when physiologic injury was estimated using the VACS Index. Based on our findings, HIV+ individuals consuming more than 30 drinks per month are at increased risk of all-cause mortality and physiologic frailty. This would translate to a recommended drinking limit for HIV+ individuals of no more than 1 drink containing alcohol per day. This is lower than the current limits by the National Institute on Alcohol Abuse and Alcoholism recommended for men, which is no more than 14 drinks per week (equivalent to 2 drinks per day), and similar to the recommendations for women and those over 65 years pf age (NIAAA, 2005). This is the first study to demonstrate an association between low levels of alcohol use and mortality or physiologic injury among HIV+ individuals in the modern antiretroviral treatment era.
This is the most comprehensive study of the question of unhealthy alcohol consumption among HIV+ individuals to date. The VHA benefits from one of the largest and most comprehensive electronic health information systems in the world with some of the longest follow up (Affairs, 2006; Corrigan et al., 2003; McQueen et al., 2004). Our group has developed and validated a national multisite cohort of HIV+ individuals engaged in HIV treatment with demographically matched uninfected comparators. Drawing from the VHA EMR, we have clinical data supporting careful adjustment for important confounders including tobacco exposure and Hepatitis C infection. We were able to use national AUDIT-C data collected in the course of routine clinical care to assess varying levels and patterns of alcohol exposure.
There are several, likely overlapping, reasons why HIV+ individuals might be more susceptible to physiologic harm from alcohol. First, they may experience higher blood alcohol levels given a unit exposure. This has been demonstrated among those with untreated HIV infection (McCance-Katz et al., 2013, 2012). Recent analyses in VACS have demonstrated that HIV+ individuals with unsuppressed HIV-1 RNA report the lowest number of drinks required to feel intoxicated, HIV+ individuals with suppressed HIV-1 RNA report a higher number to experience intoxication and uninfected individuals report an even higher number of drinks to experience intoxication (McGinnis et al., 2015). Further, even modest alcohol use is associated with poorer adherence to antiretroviral therapy (Braithwaite and Bryant, 2010; Braithwaite et al., 2008, 2010) which can in turn increase susceptibility to harm. Collectively, these data suggest that HIV+ individuals are more susceptible to physiologic harm from alcohol. Our results support this hypothesis.
The VACS Index, an integrated measure of physiologic injury, has been widely recognized as a means of estimating physiologic injury and risk of frailty related outcomes among HIV+ individuals in care (Akgun et al., 2014; Escota et al., 2014; Womack et al., 2013). The VACS Index includes many biomarkers known to be directly or indirectly influenced by alcohol exposure. CD4 count and HIV VL are altered by non-adherence to antiretroviral therapy which is strongly associated with alcohol use (Braithwaite and Bryant, 2010; Braithwaite et al., 2008). Hemoglobin, platelets, aspartate transaminase and alanine transaminase are altered by direct toxicity of alcohol and by non-adherence to antiretroviral treatment (Anderson et al., 2014; Conigliaro et al., 2003; Lo et al., 2014; Schmitt et al., 1999; Sullivan et al., 2008). Given this dual effect of alcohol among HIV+ individuals, we might expect the VACS Index to be sensitive to adverse health effects from increasing alcohol exposure in this population and that is what we observed. Importantly, 76% of HIV+ individuals had achieved HIV virus suppression, suggesting that this susceptibility to alcohol does not disappear once suppressed. HIV+ individuals, even those on ART, are more susceptible to physiologic harm from alcohol.
An AUDIT-C score of 4 or more is often considered consistent with unhealthy alcohol use (Gordon et al., 2001) and 24% of HIV+ individuals in our study had scores at or above this threshold. However we found that HED and drinks per month were also independently associated with mortality suggesting that these criteria should be considered individually. When these were considered as separate criteria, 30%, or an additional 6%, of HIV+ individuals in VACS were identified with unhealthy alcohol use.
4.1 Limitations
Accurate measurement of exposure to alcohol is challenging. While self-report has limitations, it remains the standard in clinical settings. We used the AUDIT-C from the VHA EMR. At most sites AUDIT-C is asked face to face by a healthcare provider or health technician who records the patient’s response, an approach that may be subject to social desirability bias. There may also be quality issues related to how screening is conducted in routine clinical settings (non-verbatim screening, assumptions/inferences being made about patient responses, and inputting responses not reported; Williams et al., 2015). When compared with responses on a confidential survey, clinical AUDIT-C data tends to under report alcohol exposure equally by HIV status (Bradley et al., 2011). In addition, we did not separate out patients with alcohol use disorder in the current analysis. Because detection of alcohol use disorder in routine clinical settings is poor we were not confident that separating out those assigned a diagnosis of alcohol use disorder based on ICD-9 codes would help (Kim et al., 2012; Quan et al., 2008). While higher AUDIT-C scores have been proposed for this purpose, the AUDIT-C is not intended as a diagnostic tool for alcohol use disorder (Johnson et al., 2013; McGinnis et al., 2013; Rubinsky et al., 2010). These limitations would introduce misclassification which would dampen observed associations suggesting that the differences we were able to observe by HIV status may be under estimated. Finally, these data reflect information available to the clinician in the course of care.
Another important limitation was the need to exclude those who reported no exposure to alcohol in the past 12 months. By excluding non-drinkers, we are not able to determine whether there is a level of alcohol exposure measured by AUDIT-C that is “safe”. All our analyses were compared with the lowest score on the AUDIT-C among those reporting current alcohol use (a score of 1). Non-drinkers were excluded because non-drinkers are often people who quit drinking because they experienced adverse outcomes related to alcohol (so called, “sick quitters”; Fillmore et al., 2007; Shaper, 1990; Shaper and Wannamethee, 1998). For instance, in a recent VACS survey, 29% of subjects reported that they stopped drinking because they had problems due to alcohol (data not otherwise shown). Our results may only apply to men and those receiving care in the Veterans Healthcare System.
4.2 Conclusion
In conclusion, among HIV+ individuals on ART, lower thresholds of alcohol use are associated with mortality and physiologic injury than among uninfected individuals. In both groups HED and drinks per month are independently associated with mortality and considering each as separate criteria identifies more individuals with unhealthy alcohol use.
Supplementary Material
Highlights.
Individuals with HIV on antiretroviral treatment (ART) experience mortality at lower levels of alcohol use.
Individuals with HIV experience physiologic frailty at lower levels of alcohol use.
Alcohol consumption limits should be lower among HIV+ individuals.
Acknowledgments
Role of funding source
The funders of this study had no further role in its design, collection, analysis and interpretation of data, writing of the report, or in the decision to submit the paper for publication.
National Institutes of Health: NIAAA: (U10-AA13566), (U24 AA022000) (U24AA02002). NIA (R01-AG029154), NHLBI (R01-HL095136; R01-HL090342; RCI-HL100347), NIAID (U01-A1069918), and the Veterans Health Administration Office of Research and Development (VA REA 08-266) (CDA 12-276) and Office of Academic Affiliations (Medical Informatics Fellowship)
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:
Conflicts of interest
The manuscript has not been previously published and is not being considered for publication elsewhere. The authors have all approved the manuscript and have no conflicts of interest to declare.
- Amy C. Justice-Dr. Justice’s roles for this submission were conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, obtaining funding, administrative, technical or material support and supervision.
- Kathleen A. McGinnis-Dr. McGinnis’ contributions to this submission were conception and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content and statistical analysis.
- Janet P. Tate-Dr. Tate’s contributions to this submission were conception and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and supervision.
- R. Scott Braithwaite-Dr. Braithwaite’s contributions to this submission were analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
- Kendall J. Bryant-Dr. Bryant’s contributions to this submission were conception and design, analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
- Robert L. Cook-Dr. Cook’s contributions to this submission were analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
- E. Jennifer Edelman-Dr. Edelman’s contributions to this submission were analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
- Lynn E. Fiellin-Dr. Lynn Fiellin’s contribution to this submission was critical revision of the manuscript for important intellectual content.
- Matthew S. Freiberg-Dr. Freiberg’s contributions to this submission were analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
- Adam J. Gordon-Dr. Gordon’s contribution to this submission was critical revision of the manuscript for important intellectual content.
- Kevin L. Kraemer-Dr. Kraemer’s contributions to this submission were analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
- Brandon D.L. Marshall-Dr. Marshall’s contributions to this submission were analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
- Emily C. Williams-Dr. Williams contributions to this submission were analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
- David A. Fiellin-Dr. David Fiellin’s contributions to this submission were conception and design, analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Veterans Health Administration; Department of Veterans Affairs, editor. VistA. 2006. Innovations in American Government Award Fact Sheet; pp. 1–3. [Google Scholar]
- Akgun KM, Gordon K, Pisani M, Fried T, McGinnis KA, Tate JP, Butt AA, Gibert CL, Huang L, Rodriguez-Barradas MC, Rimland D, Justice AC, Crothers K. Risk factors for hospitalization and medical intensive care unit (MICU) admission among HIV infected Veterans. J Acquir Immune Defic Syndr. 2013a;62:52–59. doi: 10.1097/QAI.0b013e318278f3fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgun KM, Tate JP, Crothers K, Crystal S, Leaf DA, Womack JA, Brown TT, Justice AC, Oursler KK. An adapted frailty-related phenotype and the VACS index as predictors of hospitalization and mortality in HIV-infected and uninfected individuals. J Acquir Immune Defic Syndr. 2014;67:397–404. doi: 10.1097/QAI.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgun KM, Tate JP, Pisani M, Fried T, Butt AA, Gibert CL, Huang L, Rodriguez-Barradas MC, Rimland D, Justice AC, Crothers K. Medical ICU admission diagnoses and outcomes in human immunodeficiency virus-infected and virus-uninfected veterans in the combination antiretroviral era. Crit Care Med. 2013b;41:1458–1467. doi: 10.1097/CCM.0b013e31827caa46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JP, Tchetgen Tchetgen EJ, Lo RV, III, Tate JP, Williams PL, Seage GR, III, Horsburgh CR, Lim JK, Goetz MB, Rimland D, Rodriguez-Barradas MC, Butt AA, Klein MB, Justice AC. Antiretroviral therapy reduces the rate of hepatic decompensation among HIV- and hepatitis C virus-coinfected veterans. Clin Infect Dis. 2014;58:719–727. doi: 10.1093/cid/cit779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31:1208–1217. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Lapham GT, Hawkins EJ, Achtmeyer CE, Williams EC, Thomas RM, Kivlahan DR. Quality concerns with routine alcohol screening in VA clinical settings. J Gen Intern Med. 2011;26:299–306. doi: 10.1007/s11606-010-1509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, Williams EC, Achtmeyer CE, Volpp B, Collins BJ, Kivlahan DR. Implementation of evidence-based alcohol screening in the Veterans Health Administration. Am J Manag Care. 2006;12:597–606. [PubMed] [Google Scholar]
- Braithwaite RS, Bryant KJ. Influence of alcohol consumption on adherence to and toxicity of antiretroviral therapy and survival. Alcohol Res Health. 2010;33:280–287. [PMC free article] [PubMed] [Google Scholar]
- Braithwaite RS, Conigliaro J, McGinnis KA, Maisto SA, Bryant K, Justice AC. Adjusting alcohol quantity for mean consumption and intoxication threshold improves prediction of nonadherence in HIV patients and HIV-negative controls. Alcohol Clin Exp Res. 2008;32:1645–1651. doi: 10.1111/j.1530-0277.2008.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite RS, Fiellin DA, Nucifora K, Bryant K, Roberts M, Kim N, Justice AC. Evaluating interventions to improve antiretroviral adherence: how much of an effect is required for favorable value? Value Health. 2010;13:535–542. doi: 10.1111/j.1524-4733.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, Cook RL, Gordon A, Bridges MW, Seiler JF, Justice AC. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29:1190–1197. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conigliaro J, Gordon AJ, McGinnis KA, Rabeneck L, Justice AC. How harmful is hazardous alcohol use and abuse in HIV infection: do health care providers know who is at risk? J Acquir Immune Defic Syndr. 2003;33:521–525. doi: 10.1097/00126334-200308010-00014. [DOI] [PubMed] [Google Scholar]
- Conigliaro J, Madenwald T, Bryant K, Braithwaite S, Gordon A, Fultz SL, Maisto S, Samet J, Kraemer K, Cook R, Day N, Roach D, Richey S, Justice A. The Veterans Aging Cohort Study: observational studies of alcohol use, abuse, and outcomes among human immunodeficiency virus-infected veterans. Alcohol Clin Exp Res. 2004;28:313–321. doi: 10.1097/01.alc.0000113414.73220.21. [DOI] [PubMed] [Google Scholar]
- Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. J Gen Intern Med. 2001;16:83–88. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan JM, Eden J, Smith BM. Leadership by Example: Coordinating Government Roles in Improving Health Care Quality (Quality Chasm) National Academies Press; Washington, DC: 2003. [Google Scholar]
- Dawson DA, Li TK, Grant BF. A prospective study of risk drinking: at risk for what? Drug Alcohol Depend. 2008;95:62–72. doi: 10.1016/j.drugalcdep.2007.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- Escota G, Patel P, Brooks JT, Bush T, Conley L, Baker J, Kojic EM, Hammer J, Onen N, Investigators SS. The VACS Index is an effective tool to assess baseline frailty status in a contemporary cohort of HIV-infected persons. AIDS Res Hum Retroviruses. 2014;31:313–317. doi: 10.1089/AID.2014.0225. [DOI] [PubMed] [Google Scholar]
- Fillmore KM, Stockwell T, Chikritzhs T, Bostrom A, Kerr W. Moderate alcohol use and reduced mortality risk: systematic error in prospective studies and new hypotheses. Ann Epidemiol. 2007;17:S16–23. doi: 10.1016/j.annepidem.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Gordon AJ, Maisto SA, McNeil M, Kraemer KL, Conigliaro RL, Kelley ME, Conigliaro J. Three questions can detect hazardous drinkers. J Fam Pract. 2001;50:313–320. [PubMed] [Google Scholar]
- Gual A, Segura L, Contel M, Heather N, Colom J. Audit-3 and audit-4: effectiveness of two short forms of the alcohol use disorders identification test. Alcohol Alcohol. 2002;37:591–596. doi: 10.1093/alcalc/37.6.591. [DOI] [PubMed] [Google Scholar]
- Harris AH, Bradley KA, Bowe T, Henderson P, Moos R. Associations between AUDIT-C and mortality vary by age and sex. Popul Health Manag. 2010;13:263–268. doi: 10.1089/pop.2009.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, Lee A, Vinson D, Seale JP. Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: a validation study. Alcohol Clin Exp Res. 2013;37(Suppl 1):E253–259. doi: 10.1111/j.1530-0277.2012.01898.x. [DOI] [PubMed] [Google Scholar]
- Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep. 2010;7:69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, Goulet J, Simberkoff M, Butt AA, Rimland D, Rodriguez-Barradas MC, Gibert CL, Oursler KA, Brown S, Leaf DA, Goetz MB, Bryant K. Veterans Aging Cohort Study (VACS): overview and description. Med Care. 2006a;44:S13–S24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, Lasky E, McGinnis KA, Skanderson M, Conigliaro J, Fultz SL, Crothers K, Rabeneck L, Rodriguez-Barradas M, Weissman SB, Bryant K. Medical disease and alcohol use among veterans with human immunodeficiency infection: a comparison of disease measurement strategies. Med Care. 2006b;44:S52–S60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- Justice AC, Modur SP, Tate JP, Althoff KN, Jacobson LP, Gebo KA, Kitahata MM, Horberg MA, Brooks JT, Buchacz K, Rourke SB, Rachlis A, Napravnik S, Eron J, Willig JH, Moore R, Kirk GD, Bosch R, Rodriguez B, Hogg RS, Thorne J, Goedert JJ, Klein M, Gill J, Deeks S, Sterling TR, Anastos K, Gange SJ. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62:149–163. doi: 10.1097/QAI.0b013e31827df36c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Smith EG, Stano CM, Ganoczy D, Zivin K, Walters H, Valenstein M. Validation of key behaviourally based mental health diagnoses in administrative data: suicide attempt, alcohol abuse, illicit drug abuse and tobacco use. BMC Health Serv Res. 2012;12:18. doi: 10.1186/1472-6963-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinder LS, Bryson CL, Sun H, Williams EC, Bradley KA. Alcohol screening scores and all-cause mortality in male Veterans Affairs patients. J Stud Alcohol Drugs. 2009;70:253–260. doi: 10.15288/jsad.2009.70.253. [DOI] [PubMed] [Google Scholar]
- Knott CS, Coombs N, Stamatakis E, Biddulph JP. All cause mortality and the case for age specific alcohol consumption guidelines: pooled analyses of up to 10 population based cohorts. BMJ. 2015;350:h384. doi: 10.1136/bmj.h384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JK, Tate JP, Fultz SL, Goulet JL, Conigliaro J, Bryant KJ, Gordon AJ, Gibert C, Rimland D, Goetz MB, Klein MB, Fiellin DA, Justice AC, Lo RV., III Relationship between alcohol use categories and noninvasive markers of advanced hepatic fibrosis in HIV-infected, chronic hepatitis C virus-infected, and uninfected patients. Clin Infect Dis. 2014;58:1449–1458. doi: 10.1093/cid/ciu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo RV, III, Kallan MJ, Tate JP, Localio AR, Lim JK, Goetz MB, Klein MB, Rimland D, Rodriguez-Barradas MC, Butt AA, Gibert CL, Brown ST, Park L, Dubrow R, Reddy KR, Kostman JR, Strom BL, Justice AC. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: a cohort study. Ann Intern Med. 2014;160:369–379. doi: 10.7326/M13-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquine MJ, Umlauf A, Rooney AS, Fazeli PL, Gouaux BD, Paul WS, Letendre SL, Ellis RJ, Grant I, Moore DJ. The Veterans Aging Cohort Study Index is associated with concurrent risk for neurocognitive impairment. J Acquir Immune Defic Syndr. 2014;65:190–197. doi: 10.1097/QAI.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Gruber VA, Beatty G, Lum PJ, Rainey PM. Interactions between alcohol and the antiretroviral medications ritonavir or efavirenz. J Addict Med. 2013;7:264–270. doi: 10.1097/ADM.0b013e318293655a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Lum PJ, Beatty G, Gruber VA, Peters M, Rainey PM. Untreated HIV infection is associated with higher blood alcohol levels. J Acquir Immune Defic Syndr. 2012;60:282–288. doi: 10.1097/QAI.0b013e318256625f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis KA, Fiellin DA, Tate JP, Cook RL, Braithwaite RS, Bryant KJ, Edelman EJ, Gordon AJ, Kraemer KL, Maisto S, Justice AC. Number of drinks to “feel a buzz” varies by HIV Status and viral load in men. AIDS Behav. 2015 doi: 10.1007/s10461-015-1053-7. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis KA, Justice AC, Kraemer KL, Saitz R, Bryant KJ, Fiellin DA. Comparing alcohol screening measures among HIV-infected and -uninfected men. Alcohol Clin Exp Res. 2013;37:435–442. doi: 10.1111/j.1530-0277.2012.01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen L, Mittman BS, Demakis JG. Overview of the Veterans Health Administration (VHA) Quality Enhancement Research Initiative (QUERI) J Am Med Inform Assoc. 2004;11:339–343. doi: 10.1197/jamia.M1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midanik LT. Drunkenness, feeling the effects and 5+ measures. Addiction. 1999;94:887–897. doi: 10.1046/j.1360-0443.1999.94688711.x. [DOI] [PubMed] [Google Scholar]
- NIAAA. Helping Patients Who Drink Too Much. A Clinician’s Guide. 2005 http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf. accessed on.
- Oursler KK, Tate JP, Gill TM, Crothers K, Brown TT, Crystal S, Womack J, Leaf DA, Sorkin JD, Justice AC. Association of the veterans aging cohort study index with exercise capacity in HIV-infected adults. AIDS Res Hum Retroviruses. 2013;29:1218–1223. doi: 10.1089/aid.2012.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plunk AD, Syed-Mohammed H, Cavazos-Rehg P, Bierut LJ, Grucza RA. Alcohol consumption, heavy drinking, and mortality: rethinking the j-shaped curve. Alcohol Clin Exp Res. 2014;38:471–478. doi: 10.1111/acer.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan H, Li B, Saunders LD, Parsons GA, Nilsson CI, Alibhai A, Ghali WA. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43:1424–1441. doi: 10.1111/j.1475-6773.2007.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Room R, Graham K, Monteiro M, Gmel G, Sempos CT. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: an overview. Addiction. 2003;98:1209–1228. doi: 10.1046/j.1360-0443.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- Rogers RG, Boardman JD, Pendergast PM, Lawrence EM. Drinking problems and mortality risk in the United States. Drug Alcohol Depend. 2015;151:38–46. doi: 10.1016/j.drugalcdep.2015.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsky AD, Kivlahan DR, Volk RJ, Maynard C, Bradley KA. Estimating risk of alcohol dependence using alcohol screening scores. Drug Alcohol Depend. 2010;108:29–36. doi: 10.1016/j.drugalcdep.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitz R. Clinical practice. Unhealthy alcohol use N Engl J Med. 2005;352:596–607. doi: 10.1056/NEJMcp042262. [DOI] [PubMed] [Google Scholar]
- Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr. 2007;46:194–199. doi: 10.1097/QAI.0b013e318142aabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- Schmitt M, Gleiter CH, Nichol JL, Pralle L, Hasselblatt M, Poser W, Ehrenreich H. Haematological abnormalities in early abstinent alcoholics are closely associated with alterations in thrombopoietin and erythropoietin serum profiles. Thromb Haemost. 1999;82:1422–1427. [PubMed] [Google Scholar]
- Shaper AG. Alcohol and mortality: a review of prospective studies. Br J Addict. 1990;85:837–847. doi: 10.1111/j.1360-0443.1990.tb03710.x. discussion 849–861. [DOI] [PubMed] [Google Scholar]
- Shaper AG, Wannamethee SG. The J-shaped curve and changes in drinking habit. Novartis Found Symp. 1998;216:173–188. doi: 10.1002/9780470515549.ch11. discussion 188–192. [DOI] [PubMed] [Google Scholar]
- Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24:783–788. doi: 10.1007/s11606-009-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PS, Hanson DL, Brooks JT. Impact on hemoglobin of starting combination antiretroviral therapy with or without zidovudine in anemic HIV-infected patients. J Acquir Immune Defic Syndr. 2008;48:163–168. doi: 10.1097/QAI.0b013e3181685714. [DOI] [PubMed] [Google Scholar]
- Tate JP, Justice AC, Hughes MD, Bonnet F, Reiss P, Mocroft A, Nattermann J, Lampe FC, Bucher HC, Sterling TR, Crane HM, Kitahata MM, May M, Sterne JA. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS. 2013;27:563–572. doi: 10.1097/QAD.0b013e32835b8c7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Achtmeyer CE, Thomas RM, Grossbard JR, Lapham GT, Chavez LJ, Ludman EJ, Berger D, Bradley KA. Factors underlying quality problems with alcohol screening prompted by a clinical reminder in primary care: a multi-site qualitative study. J Gen Intern Med. 2015;30:1125–1132. doi: 10.1007/s11606-015-3248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack JA, Goulet JL, Gibert C, Brandt CA, Skanderson M, Gulanski B, Rimland D, Rodriguez-Barradas MC, Tate J, Yin MT, Justice AC. Physiologic frailty and fragility fracture in HIV-infected male veterans. Clin Infect Dis. 2013;56:1498–1504. doi: 10.1093/cid/cit056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.