Abstract
Laboratory rodents can adopt the pain or fear of nearby conspecifics. This phenotype conceptually lies within the domain of empathy, a bio-psycho-social process through which individuals come to share each other’s emotion. Using a model of cue-conditioned fear, we show here that the expression of vicarious fear varies with respect to whether mice are raised socially or in solitude during adolescence. The impact of the adolescent housing environment was selective: i) vicarious fear was more influenced than directly acquired fear, ii) ‘Long-term’ (24-h post-conditioning) vicarious fear memories were stronger than ‘short-term’ (15-min post-conditioning) memories in socially reared mice whereas the opposite was true for isolate mice, and iii) Females were more fearful than males. Housing differences during adolescence did not alter the general mobility of mice or their vocal response to receiving the unconditioned stimulus. Previous work with this mouse model underscored a genetic influence on vicarious fear learning, and the present study complements these findings by elucidating an interaction between the adolescent social environment and vicarious experience. Collectively, these findings are relevant to developing models of empathy amenable to mechanistic exploitation in the laboratory.
Keywords: empathy, emotion, mouse, rodent, behavior, conditioning
Introduction
Survival and reproduction require that an animal engage in social interactions. Such actions always include mating-related behaviors, but can extend to nurturance of offspring, allo-parenting, cooperative defense and hunting, as well as formation and maintenance of hierarchies. Social participants can benefit in such contexts when aware of the affective state of others; for instance, if a calm infant becomes agitated, if companions increase vigilance to a threat or if a peer changes its receptivity toward play solicitations. Empathy, or expressing “an affective response more appropriate to another’s situation than to one’s own” (Hoffman, 2001), is a mechanism through which emotions can be shared (Preston & de Waal, 2002). Behavioral mimicry is another way by which individuals can learn from others, but does not require emotion, and may be less beneficial than empathy when the social environment is variable or difficult to predict (Nakahashi & Ohtsuki, 2015).
Comparative and evolutionary approaches to understanding empathy (Panksepp & Lahvis, 2011; de Waal, 2012; Mogil, 2012; Panksepp & Panksepp, 2013) indicate that it manifests as a compilation of sub-processes (Preston & de Waal, 2002). In this regard studies have demonstrated that laboratory mice express such ‘endophenotypes’ (Langford, Crager, Shehzad, Smith, Sotocinal, Levenstadt et al., 2006; Chen, Panksepp & Lahvis, 2009; Jeon, Kim, Chetana, Jo, Ruley, Lin et al., 2010; Sanders, Mayford, & Jeste, 2013; Gonzalez-Liencres, Juckel, Tas, Friebe, & Brüne, 2014). For example, observer C57BL/6J mice exhibit vicarious behavioral (Chen et al., 2009; Jeon et al., 2010), physiological (Chen et al., 2009) and neural responses (Jeon et al., 2010) to other’s expressions of fear. These studies and others using laboratory rats (Atsak, Orre, Bakker, Cerliani, Roozendaal, Gazzola et al., 2011; Jones, Riha, Gore, & Monfils, 2013; Ben-Ami Bartal, Rodgers, Bernardez Sarria, Decety, & Mason, 2014) additionally support the notion that social relationships, sexual characteristics, stress and prior experience are potent modulators of empathic responsiveness (Christov-Moore, Simpson, Coudé, Grigaityte, Iacoboni & Ferrari, 2014; Freidin, Carballo, & Bentosela, 2015; Martin, Hathaway, Isbester, Mirali, Acland, Niederstrasser et al., 2015).
Social exclusion can also influence empathy (Twenge, Baumeister, DeWall, Ciarocco & Bartels, 2007) particularly during adolescent development (Eisenberg, 1982). In rodents, manipulation of the social housing environment is used as an experimental procedure to restrict or augment social interaction during adolescence, and can profoundly affect maturation of the brain and behavior (Yang, Perry, Weber, Katz, & Crawley, 2010; Liu, Dietz, DeLoyht, Pedre, Kelkar, Kaur et al. 2012; Makinodan, Rosen, Ito, & Corfas, 2012; Gan, Bowline, Lourenco, & Pickel, 2014; Adams & Rosenkranz, 2015). In the present study, we hypothesized that social housing during mouse adolescence would have a supportive effect on vicarious fear relative to isolate housing. Females and males were also compared because sexual identity can influence empathic responding. Moreover, responses were assessed 15-min and 24-h post-conditioning to distinguish between ‘short-term and ‘long-term’ memories, respectively, the latter of which may be less sensitive to the acute experience associated with conditioning.
Methods
Mice from the BALB/cJ (‘BALB’) and C57BL/6J (‘B6’) mice were bred within our own colony. At weaning B6 mice were pooled from 2-4 litters (Figure 1A), and then randomly selected for housing in either a social group of 2 males and 2 females (see Panksepp, Jochman, Kim, Koy, Wilson, Chen et al., 2007 for rationale) or in complete social isolation (Figure 1B). These B6 mice then became observers of target mice undergoing fear conditioning (i.e., vicarious fear) or were directly conditioned (see Figure 1C and below). Mice remained in their respective housing conditions throughout conditioning and testing. To control for familiarity with strain-specific communication modalities, such as vocalizations or volatile odorants, target mice during vicarious fear conditioning were age matched male-female pairs of unfamiliar mice derived from reciprocal BALB × B6 (F1) crosses (see Chen et al., 2009 for rationale).
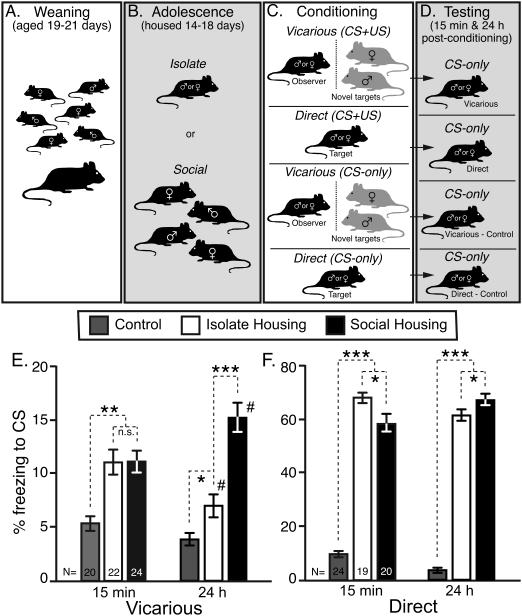
Figure 1. Animal husbandry timeline, behavioral procedure, and the effects of differential social housing on the expression of vicarious and directly acquired fear.
(A) Litters of B6 juveniles were raised in their natal litter without the sire. (B) Juveniles were then weaned into either mixed-sex groups or social isolation where they were housed throughout the experiment. (C) B6 observers experienced unfamiliar F1 targets undergo cued-fear conditioning whereas B6 targets were directly conditioned. These experimental groups were compared to control groups that did not directly experience the US. (D) B6 observers and targets were then tested for the expression of a fear response as a function of differential social housing at 15-min and 24-h post-conditioning. (E) Isolate and socially housed mice expressed equivalent vicarious fear responses at 15-min, but this phenotype decreased and increased, respectively, at 24-h (see Results). (F) Isolate mice exhibited longer freezing responses than socially housed mice at 15-min post-conditioning, but this difference was reversed at 24-h. N’s for (E) and (F) are provided within each respective bar. Control mice from the ISO and SOC groups were pooled in (E) and (F) as there were no differences between them (statistics not shown). Note that the ordinates for the vicarious and direct groups are on different scales. Data are presented as the mean ± std. error. Asterisks represent significant orthogonal contrasts. *P<0.05, **P<0.01, ***P<0.001. #P<0.05 for comparisons between the 15-min and 24-h time points.
On Day 1, B6 observers and targets were allowed 300-s to freely explore the fear-conditioning compartment of the experimental apparatus (www.cleversysinc.com/products/hardware). To familiarize observers with a distressful stimulus, they were exposed to a single, unconditioned stimulus (US; 3-s, 1mA scrambled shock) halfway through this habituation period (see Chen et al., 2009 for rationale; also see Sanders et al., 2013 for empirical support). Note that directly conditioned B6 targets did not receive US pre-exposure, but were habituated to the conditioning compartment. Exposure of observers to the US during the habituation period did not change subsequent baseline freezing when re-exposed to the conditioning chamber for testing (see Results), indicating that this experience did not engender contextual fear (for an example of vicarious fear using contextual conditioning see Jeon et al., 2010). The inefficacy of US pre-exposure to produce contextual freezing during testing is consistent with previous findings (Chen et al., 2009), and may be partially accounted for by the fact that the ‘observation’ chambers and conditioning compartment shared a common feature (i.e., stainless steel dowels; see below), which may lead to partial extinction of contextual fear that results from US pre-exposure. Additionally, observers received a single US during habituation and were handled by the experimenter during the intervening cued-conditioning protocols (see below), which are aspects of this procedure that deviate from more traditional contextual-conditioning paradigms and may explain why US pre-exposure in observers did not increase baseline freezing during testing.
Fifteen-min after the habituation period B6 observers and targets were subjected to vicarious and direct cued-fear conditioning, respectively (Figure 1C). During vicarious conditioning, 2 observers (a male and female) were isolated individually into observation chambers adjacent to the conditioning compartment and separated from each other by opaque Plexiglas. The floor and one wall of the observation chambers were composed of inactive stainless steel dowels, identical to the active dowels of the conditioning compartment on the floor and on the wall facing the observation chambers. Conditioning and testing was conducted under infrared LED arrays during the middle 6-h of the dark phase when laboratory mice are generally awake and active, so observers could hear and smell F1 targets, but could not touch or see them. Dim red lighting in the colony and testing room during experimental procedures was ≈15 lux.
After 120-s in the conditioning compartment pairs of F1 targets used for vicarious fear conditioning, or an individual B6 target for direct fear conditioning, were exposed to 10 presentations of a 30-s auditory stimulus (1kHz pure tone, 85dB) that co-terminated with the US (3-s, 1mA scrambled shock). US-CS pairings were separated by 90-s intervals. The conditioning protocol was repeated on Day 2. Although this conditioning protocol is somewhat extreme for the direct conditioning groups, it is used for vicarious conditioning to elicit robust activation of social communication modalities in observers (e.g., see Jeon et al., 2010). Mice in control groups were treated exactly as described above except that F1 targets and B6 targets were not exposed to the US (see Figure 1C). Vocalizations at frequencies below 150kHz were recorded during all conditioning sessions on Day 2 with the UltraSoundGate 416H recording system and CM16 condenser microphones (Avisoft Bioacoustics).
Testing entailed placing an individual B6 observer or target into the conditioning compartment 15-min (Day 2) and 24-h (Day 3) after the second conditioning session (Figure 1D). These testing time points were selected due to their standard use as respective short-term and long-term assays of classical conditioned memory. After 120-s in the conditioning compartment each mouse was exposed to 5 presentations of the CS (each spaced by a 90-s interval). All components of the experimental apparatus were thoroughly cleaned with 70% ethanol and water, and dried between each phase of the experiment. Administration of all conditioning stimuli was controlled automatically (FreezeScan, Cleversys Inc.). Infrared video cameras recorded mouse behaviors during the test sessions. Freezing was defined as the complete absence of movement other than respiratory movements and was assessed with computer-assisted software (ButtonBox v.5.0, Behavioral Research Solutions) for the duration of each CS presentation (i.e., cued fear) and for the 60-s leading up to the first CS (i.e., pre-cue, baseline fear). Each measurement was repeated by the first author—blinded to the experimental condition—and all data presentation and statistical outcomes were based on the average of these 2 measurements. ‘Intra-rater’ reliability was high for both cued (Pearson’s correlation, R=0.99, d.f.=1,289) and pre-CS measurements (R=0.99, d.f.=257). A subset of the cued trials (59% of the data) were also evaluated for ‘inter-rater’ reliability by comparing the measurements of the first author to those of a blinded laboratory technician (R=0.98, d.f.=765). Distress vocalizations (DVs) during US administration (see Chen et al., 2009) were tallied using the ‘interacting labeling’ function in the SASLab Pro software package (Avisoft Bioacoustics). DVs were identified by their characteristic resonant energies above the CS and above background noise. For each conditioning session on Day 2, DVs were quantified two times in a blinded fashion by the first author (R=0.91, d.f.=57) and averaged across the session. Data presentation and statistical outcomes were based on the average of these 2 measurements. The ‘automatic parameters’ function was also used to extract the fundamental frequency (pitch), amplitude and duration of each DV.
Results
Conditioned mice expressed higher levels of CS-induced freezing relative to control mice when they were tested 15-min after the last vicarious conditioning experience (Figure 1E; F[2,327]=10.5, P<0.0001), but there was no difference between isolate and socially housed mice (orthogonal contrast, F[1,324]<0.1, P=0.96). A main effect of conditioning was also found 24-h following the last vicarious conditioning trial (F[2,327]=30.2, P<0.0001). Compared to 15-min post-conditioning, CS-induced freezing was more sensitive to housing conditions of mice at this time point: Socially housed mice expressed higher levels of freezing than isolate mice (orthogonal contrast, F[1,324]=30.3, P<0.0001), a difference attributable to a decrease in isolated mice (two-tailed paired t-tests; t=|2.6|, P=0.01, d.f.=218) and an increase in socially housed mice (t=|2.8|, P=0.02, d.f.=238) relative to the 15-min time point. Isolated mice expressed marginally higher levels of CS-induced freezing relative to their controls at the 24-h time point (orthogonal contrast, F[1,324]=4.1, P=0.04).
For mice receiving vicarious conditioning CS-induced freezing was not sensitive to their sexual identity at either time point nor was there a housing-by-sex interaction at 15-min post-conditioning (statistics not shown). However, a housing-by-sex interaction was found at the 24-h time point (F[2,327]=5.4, P<0.01). Males (Table 1; orthogonal contrast, F[1,324]=3.1, P=0.08) were much less influenced by the housing conditions than females (F[1,324]=36.3, P<0.0001) when tested 24-h after the last vicarious conditioning experience.
Table 1.
Freezing responses of observers and target mice 24-h post-conditioning as a function of housing condition and sex.
| Housing-type | Conditioning-type | Sex | % freezing |
|---|---|---|---|
| isolate | direct | M | 67.9±2.23 |
| social | direct | M | 63.9±3.40 |
| isolate | direct | F | 53.0±2.88 |
| social | direct | F | 70.0±2.49*** |
| isolate | vicarious | M | 7.8±1.74 |
| social | vicarious | M | 11.5±1.33 |
| isolate | vicarious | F | 6.2±1.16 |
| social | vicarious | F | 18.8±2.26*** |
Freezing responses of directly and vicariously conditioned B6 mice are displayed relative to their housing context and sex. Data are presented as the mean ± std. error. Asterisks represent significant orthogonal contrasts comparing isolate versus social housing contexts-by-sex for each type of conditioning.
P<0.001.
Mice conditioned by direct exposure to the US-CS paring exhibited robust CS-induced freezing responses compared to control mice (F[2,297]=225.3, P<0.0001). Fifteen-min after conditioning isolate mice were more likely to freeze than socially housed mice (Figure 1F; orthogonal contrast, F[1,294]=9.1, P<0.01). Twenty-four hours after direct fear conditioning mice were also substantially more likely to freeze than their respective controls (F[2,307]=532.7, P<0.0001). However, while isolate mice were more likely to freeze than socially housed mice when tested 15-min after conditioning, they exhibited less CS-induced freezing than socially housed mice when tested 24-h post-conditioning (Figure 1F; orthogonal contrast, F[1,304]=7.0, P<0.01).
Similar to mice conditioned vicariously, the sexual identity of mice did not affect freezing 15-min or 24-h after direct conditioning nor was there a housing-by-sex interaction detected when mice were tested 15-min post-conditioning (statistics not shown). A housing-by-sex interaction was present 24-h after direct fear conditioning (F[2,307]=11.4, P<0.0001), with socially housed females expressing higher levels of freezing than their isolated counterparts (Table 1; F[1,304]=25.6, P<0.0001). By contrast, isolated males and socially housed males exhibited similar levels of CS-induced freezing (F[1,304]=1.6, P=0.21) following direct fear conditioning.
Because socially housed females from both the vicarious and direct groups expressed higher levels of freezing than isolated females 24-h post-conditioning we used the average freezing time of isolate females as a baseline to assess the magnitude to which social housing affected the vicarious and direct fear phenotypes. There was a larger difference between isolate and socially housed females that underwent vicarious conditioning (mean change from isolation ± std. error, +213.5 ± 37.65%) compared to females with directly acquired fear (+32.0 ± 4.69%; F[1,108]=19.1, P<0.0001).
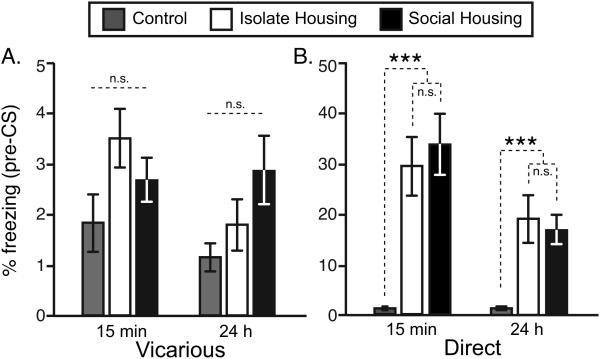
Social influences on CS-induced freezing were not attributable to factors unrelated to the US-CS pairing during vicarious or direct conditioning. For instance, differences in pre-CS freezing were not detected for vicariously conditioned mice relative to controls at the short-term or long-term testing time points (Figure 2A; both P’s>0.10). Although pre-CS freezing was higher at both time points for mice that were directly conditioned relative to controls (Figure 2B; both P’s<0.0001), there were no differences in pre-CS freezing between directly conditioned isolate and socially housed mice at either post-conditioning time point (both P’s>0.25).
Figure 2. Pre-cue freezing after placement into the conditioning chamber following vicarious or direct fear conditioning.
(A) Baseline freezing upon placement into the conditioning chamber was minimal following vicarious conditioning and did not differ between groups at 15-min or 24-h post-conditioning. (B) Increased baseline freezing responses following direct fear conditioning were detected, and were similar in isolate and socially housed mice at both time points. Note that the ordinates for the vicarious and direct groups are on different scales. Data are presented as the mean ± std. error. Asterisks represent significant orthogonal contrasts. ***P<0.001.
The number of DVs emitted during direct conditioning did not differ as a function of social housing (Table 2; F[1,33]=1.8, P=0.19) nor did DV pitch, amplitude or duration differ between isolate and socially housed mice (all P’s>0.55). Effects of sex or housing-by-sex interactions were not detected for any of the DV variables (statistics not shown). DV emission, pitch, amplitude and duration were also similar between target F1 mice used for vicarious conditioning of isolate and socially housed observer mice (all P’s>0.54).
Table 2.
Distress vocalizations of fear conditioned mice.
| Housing-type | Conditioning-type | DVs |
|---|---|---|
| isolate | direct | 9.5±0.65 |
| social | direct | 8.6±0.29 |
| isolate | vicarious | 12.0±0.91* |
| social | vicarious | 12.5±0.70* |
The number of DVs emitted by directly conditioned B6 mice and F1 target-pairs from vicarious conditioning are displayed. Note that DVs in the vicarious condition represent the acoustic emissions of 2 mice versus 1 during direct conditioning. Data are presented as the mean ± std. error. Asterisks represent significant Tukey-Kramer post-hoc tests comparing direct versus vicarious conditioning for each housing context.
P<0.05.
Discussion
Empathy is a complex social ability mediated by interactions between several sub-processes, but it is fundamentally governed by emotional substrates. Advances in understanding the biological underpinnings of empathy have come from careful laboratory studies of rodents (for a review see Panksepp & Lahvis, 2011; Panksepp & Panksepp, 2013) that complement those in other species (Decety, 2011). In this respect, we described the (short-term) vicarious fear phenotype in adolescent mice over six years ago at our former laboratory site, with a different batch of B6 mice, and using a slightly modified conditioning procedure (Chen et al., 2009), which suggests this behavioral response is relatively stable despite potential sources of uncontrolled variability.
Our current findings provide further evidence that this mouse vicarious fear phenotype models some basic features of empathy. For instance, in humans strong attachment to peers during adolescence can be influential for empathic responding (Laible, Carlo, & Raffaelli, 2000) and female empathy moderates social relationships with peers (Laible, Carlo, & Roesch, 2004). Consistent with these studies we found adolescent mice restricted from social interactions (and thus social relationships with cage mates) express blunted vicarious fear 24-h post-conditioning and females were more affected than male observers. The finding that vicarious fear in female mice was more sensitive to the adolescent housing environment than males is consistent with the idea that sex differences in empathy likely have deep phylogentic and ontogenetic underpinnings (Christov-Moore, Simpson, Coudé, Grigaityte, Iacoboni & Ferrari, 2014).
Had social isolation induced changes in stress reactivity to conspecific exposure, we expected to find group differences in freezing shortly after (i.e., 15-min) vicarious conditioning, but we did not. By contrast isolate mice expressed higher levels of freezing at the short-term testing time point after direct conditioning, indicating that social isolation increases fearfulness for oneself rather than for others at 15-min post-conditioning. Furthermore, isolation did not alter the general mobility of mice, as there were no housing-based differences in baseline freezing prior to CS administration during testing. The emission of DVs (i.e., ‘audible squeaks’) to the US during conditioning, which communicates fear to observers (Chen et al., 2009) and is an index of sensitivity to US administration in rodents (e.g., Ji, Fu, Adwanikar & Neugebauer, 2013)—was similarly unchanged by social versus isolate housing. Collectively these findings indicate that social contact can enhance the learning abilities of adolescent female mice particularly when the task is focused on the emotional state of others.
The vicarious fear phenotype was increased and decreased in the social versus isolate housing conditions, respectively, only 24-h after conditioning, which raises questions about the events that occur between conditioning and the long-term testing time point. After conditioning, socially reared mice may communicate with each other via ultrasonic vocalizations and/or their level of arousal in the home cage, and this could foster the expression of vicarious fear. Therefore lack of a supportive influence that is characteristic of social housing might also result in a reduction of vicarious fear when individuals are isolated after conditioning. By contrast, growing up in a social group versus solitude may influence processes associated with memory consolidation, an interpretation that we favor due to the selectivity of the adolescent housing environment on affecting vicarious fear 24-h (but not 15-min) post-conditioning. Such hypotheses regarding the time-sensitivity of social versus isolate housing on expression of the vicarious fear phenotype can be further explored experimentally. Nevertheless, adolescent mice developing in a group presumably have more social competence than those raised in isolation (e.g., Liu et al., 2012; Makinodan et al., 2012). If this is the case, isolated mice would likely be more influenced in the vicarious conditioning paradigm, which involves social communication, than by direct conditioning, which does not include a social component, as was found here.
It is especially noteworthy that ‘observational fear’ between adolescent rats, but not directly acquired fear, is similarly affected by social versus isolate housing during adolescence (Yusufishaq & Rosenkranz, 2013), which suggests that across species this period of development is important for the maturation of empathy. Whether adolescence constitutes a ‘critical period’ for emergence of the capacity for vicarious fear remains an open issue. Moreover, the distinct pattern of increased/decreased fear across housing contexts, conditioning procedures and testing times that we observed beckons an additional point to consider: These differential and interactive influences on CS-induced freezing suggest socially acquired fear may have biological mechanisms (genomic, synaptic or neuro-anatomical) different than those involved in directly acquired fear, which may have important implications for understanding the neurobiology of empathy in animals.
References
- Adams T, Rosenkranz JA. Social isolation during postweaning development causes hypoactivity of neurons in the medial nucleus of the male rat amygdala. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.364. Epub ahead of print. doi:10.1038/npp.2015.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsak P, Orre M, Bakker P, Cerliani L, Roozendaal B, Gazzola V, et al. Experience modulates vicarious freezing in rats: a model for empathy. Public Library of Science One. 2011;6:e21855. doi: 10.1371/journal.pone.0021855. doi:10.1371/journal.pone.0021855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami Bartal I, Rodgers DA, Bernardez Sarria MS, Decety J, Mason P. Pro-social behavior in rats is modulated by social experience. Elife. 2014;3 doi: 10.7554/eLife.01385. doi:10.7554/elife.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. Public Library of Science One. 2009;4:e4387. doi: 10.1371/journal.pone.0004387. doi:10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov-Moore L, Simpson EA, Coudé G, Grigaityte K, Iacoboni M, Ferrari PF. Empathy: Gender effects in brain and behavior. Neuroscience & Biobehavioral Reviews. 2014;46:604–627. doi: 10.1016/j.neubiorev.2014.09.001. doi.org/10.1016/j.neubiorev.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal FB. The antiquity of empathy. Science. 2012;336:874–876. doi: 10.1126/science.1220999. doi:10.1126/science.1220999. [DOI] [PubMed] [Google Scholar]
- Decety J. The neuroevolution of empathy. Annals of the New York Academy Science. 2011;1231:35–45. doi: 10.1111/j.1749-6632.2011.06027.x. doi:10.1111/j.1749-6632.2011.06027.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg N. The development of prosocial behavior. Academic Press; New York: 1982. [Google Scholar]
- Freidin E, Carballo F, Bentosela M. Direct reciprocity in animals: The roles of bonding and affective processes. International Journal of Psychology. 2015 doi: 10.1002/ijop.12215. Epub ahead of print. doi.org/10.1002/ijop.12215. [DOI] [PubMed] [Google Scholar]
- Gan J, Bowline E, Lourenco F, Pickel V. Adolescent social isolation enhances the plasmalemmal density of NMDA NR1 subunits in dendritic spines of principal neurons in the basolateral amygdala of adult mice. Neuroscience. 2014;258:174–183. doi: 10.1016/j.neuroscience.2013.11.003. doi:10.1016/j.neuroscience.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Liencres C, Juckel G, Tas C, Friebe A, Brüne M. Emotional contagion in mice: the role of familiarity. Behavioural Brain Research. 2014;263:16–21. doi: 10.1016/j.bbr.2014.01.020. doi:10.1016/j.bbr.2014.01.020. [DOI] [PubMed] [Google Scholar]
- Hoffman M. Empathy and moral development. Cambridge University Press; Cambridge, UK: 2001. [Google Scholar]
- Ji G, Fu Y, Adwanikar H, Neugebauer V. Non-pain-related CRF1 activation in the amygdala facilitates synaptic transmission and pain responses. Molecular Pain. 2013;9:2. doi: 10.1186/1744-8069-9-2. doi:10.1186/1744-8069-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nature Neuroscience. 2010;13:482–488. doi: 10.1038/nn.2504. doi:10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CE, Riha PD, Gore AC, Monfils M-H. Social transmission of Pavlovian fear: fear-conditioning by-proxy in related female rats. Animal Cognition. 2013;17:827–834. doi: 10.1007/s10071-013-0711-2. doi:10.1007/s10071-013-0711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laible DJ, Carlo G, Raffaelli M. The differential relations of parent and peer attachment to adolescent adjustment. Journal of Youth Adolescence. 2000;29:45–59. doi:10.1023/A:1005169004882. [Google Scholar]
- Laible DJ, Carlo G, Roesch SC. Pathways to self-esteem in late adolescence: the role of parent and peer attachment, empathy, and social behaviours. Journal of Adolescence. 2004;27:703–716. doi: 10.1016/j.adolescence.2004.05.005. doi:10.1016/j.adolescence.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, et al. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. doi:10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- Langford DJ, Tuttle AH, Brown K, Deschenes S, Fischer DB, Mutso A, et al. Social approach to pain in laboratory mice. Social Neuroscience. 2010;5:163–170. doi: 10.1080/17470910903216609. doi:10.1080/17470910903216609. [DOI] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht J, Pedre X, Kelkar D, Kaur J, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nature Neuroscience. 2012;15:1621–1623. doi: 10.1038/nn.3263. doi:10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. doi:10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Hathaway G, Isbester K, Mirali S, Acland EL, Niederstrasser N, et al. Reducing social stress elicits emotional contagion of pain in mouse and human strangers. Current Biology. 2015;25:326–332. doi: 10.1016/j.cub.2014.11.028. doi.org/10.1016/j.cub.2014.11.028. [DOI] [PubMed] [Google Scholar]
- Mogil J. The surprising empathic abilities of rodents. Trends in Cognitive Science. 2012;16:143–144. doi: 10.1016/j.tics.2011.12.012. doi:10.1016/j.tics.2011.12.01. [DOI] [PubMed] [Google Scholar]
- Nakahashi W, Ohtsuki H. When is emotional contagion adaptive? Journal of Theoretical Biology. 2015;380:480–488. doi: 10.1016/j.jtbi.2015.06.014. doi:10.1016/j.jtbi.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, et al. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. Public Library of Science One. 2007;2:e351. doi: 10.1371/journal.pone.0000351. doi:10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Rodent empathy and affective neuroscience. Neuroscience & Biobehavioral Reviews. 2011;35:1864–1875. doi: 10.1016/j.neubiorev.2011.05.013. doi:10.1016/j.neubiorev.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Panksepp JB. Toward a cross-species understanding of empathy. Trends in Neuroscience. 2013;36:489–496. doi: 10.1016/j.tins.2013.04.009. doi:10.1016/j.tins.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, de Waal FB. Empathy: Its ultimate and proximate bases. Behavioral Brain Science. 2002;25:1–20. doi: 10.1017/s0140525x02000018. doi:10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- Sanders J, Mayford M, Jeste D. Empathic fear responses in mice are triggered by recognition of a shared experience. Public Library of Science One. 2013;8:e74609. doi: 10.1371/journal.pone.0074609. doi:10.1371/journal.pone.0074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twenge JM, Baumeister RF, DeWall CN, Ciarocco NJ, Bartels JM. Social exclusion decreases prosocial behavior. Journal of Personality and Social Psychology. 2007;92:56–66. doi: 10.1037/0022-3514.92.1.56. doi.org/10.1037/0022-3514.92.1.56. [DOI] [PubMed] [Google Scholar]
- Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Research. 2010;4:17–27. doi: 10.1002/aur.163. doi.org/10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusufishaq S, Rosenkranz JA. Post-weaning social isolation impairs observational fear conditioning. Behavioural Brain Research. 2013;242:142–149. doi: 10.1016/j.bbr.2012.12.050. doi:10.1016/j.bbr.2012.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]