Abstract
Background
Individuals who use methamphetamine chronically exhibit emotional and dopaminergic neurochemical deficits. Although the amygdala has an important role in emotion processing and receives dopaminergic innervation, little is known about how dopamine transmission in this region contributes to emotion regulation. This investigation aimed to evaluate emotion regulation in subjects who met DSM-IV criteria for methamphetamine dependence, and to test for a relationship between self-reports of difficulty in emotion regulation and D2-type dopamine receptor availability in the amygdala.
Method
Ninety-four methamphetamine-using and 102 healthy-control subjects completed the Difficulties in Emotion Regulation Scale (DERS); 33 of those who used methamphetamine completed the Addiction Severity Index (ASI). A subset of 27 methamphetamine-group and 20 control-group subjects completed positron emission tomography with [18F]fallypride to assay amygdala D2-type dopamine receptor availability, measured as binding potential (BPND).
Results
The methamphetamine group scored higher than the control group on the DERS total score (p < 0.001), with DERS total score positively correlated with the Drug Composite Score on the ASI (p = 0.02) in the methamphetamine group. The DERS total score was positively correlated with amygdala BPND in both groups and the combined group of participants (combined: r = 0.331, p = 0.02), and the groups did not differ in this relationship.
Conclusion
These findings highlight problems with emotion regulation linked to methamphetamine use, possibly contributing to personal and interpersonal behavioral problems. They also suggest that D2-type dopamine receptors in the amygdala contribute to emotion regulation in both healthy and methamphetamine-using subjects.
Keywords: methamphetamine, amygdala, emotion dysregulation, dopamine, [18F]fallypride, PET
1. INTRODUCTION
Individuals who use methamphetamine chronically exhibit anxiety, depression, aggression, hostility, and irritability during early abstinence from the drug whether or not they are seeking treatment for their addiction (London et al., 2004; Newton et al., 2004; Payer et al., 2011; Zweben et al., 2004). Such disturbances may reflect deficits in emotion regulation associated with substance abuse (Hodgins et al., 1995). In previous studies, which used the Difficulties in Emotion Regulation Scale (DERS), deficits in emotion regulation were shown in abstinent alcohol-dependent and cocaine-dependent (Fox et al., 2007, 2008) participants compared with healthy controls. Nonetheless, the relationships between deficits in emotion regulation and addiction have been understudied, with few studies of emotion regulation in methamphetamine users. One study indicated an inverse relationship between reduction of amygdala activation during emotion regulation and perpetrated aggression in methamphetamine users, suggesting that those with reduced amygdala activation during regulation show less aggressive behavior (Payer et al., 2011). Another study indicated that resting state functional connectivity of the amygdala with hippocampus in methamphetamine users is positively related to emotion dysregulation and trait anxiety, and negatively related to self-compassion and dispositional mindfulness (Dean et al., 2014), suggesting the importance of the amygdala for several behavioral constructs related to the regulation of emotions in this population.
Emotion regulation generally refers to any operation that influences responses generated during emotion processing (Gross, 2015), and it can take several forms, which may be explicit (e.g., cognitive reappraisal strategies in which emotional stimuli or experience are reinterpreted) or implicit (e.g., verbal labeling of emotions; see Gyurak et al., 2011 for review). The amygdala has an important role in emotion processing (Phelps and Anderson, 1997; Vuilleumier, 2005), including the coding of affective relevance (Murray et al., 2014), both explicit and implicit forms of emotion regulation result in reduction of amygdala responses (Kanske et al., 2011; McRae et al., 2010). In turn, emotion dysregulation represents poor modulation of an emotional response to provocative stimuli despite regulation attempts (Hilt et al., 2011). Emotion dysregulation is associated with higher propensity for aggression and violence (Scott et al., 2014), and contributes to greater emotional intensity and increased risk of substance abuse (Weiss et al., 2015). Amygdala hyper-reactivity has been associated with emotion dysregulation in various patient populations, including borderline personality disorder (Donegan et al., 2003), and it is plausible that abnormal functioning of the amygdala may contribute to emotion dysregulation in methamphetamine users. However, no studies have investigated the relationship between neurochemical changes in the amygdala associated with chronic methamphetamine use and emotion dysregulation.
Several lines of evidence have suggested that dopaminergic neurotransmission in the amygdala influences emotion processing. A dynamic positron emission tomographic (PET) study in humans showed reduction of radioligand binding to dopamine D2-type receptors in several brain regions, presumably due to competition with dopamine, during presentation of stimuli with emotional content (Badgaiyan et al., 2009). The effect, which is consistent with dopamine release during processing of the stimuli, was observed in the amygdala, medial temporal lobe and inferior frontal gyrus of the left hemisphere. When measured in the resting state, however, dopamine D1-type receptor availability in the amygdala, which like D2-type receptor availability, is an index of capacity for dopaminergic signaling, was correlated with change in amygdala blood oxygenation level-dependent signal, assessed using functional magnetic resonance imaging (fMRI) and induced by presentation of novel facial stimuli with either neutral or fearful expression (Takahashi et al., 2010). Moreover, administration of d-amphetamine-enhanced task-related activity in the right amygdala during a facial affect-matching task (Hariri et al., 2002), suggesting that enhanced dopaminergic signaling can increase the response of the amygdala to affective stimulation. In animals, the amygdala has been strongly linked to anxiety/fear specifically via D2-type receptors. D2 antagonists infused directly into the amygdala attenuate fear-potentiated startle (Greba et al., 2001) and conditioned freezing (Guarraci et al., 2000). In sum, human and animal studies indicate a positive link between dopaminergic neurotransmission in the amygdala and emotional response, in healthy humans and animals, with indices of greater dopaminergic neurotransmission linked to enhanced emotional response.
Studies of dopaminergic neurochemical markers as related to addiction have focused on the striatum, mainly due to its rich dopaminergic innervation, and have revealed deficits in dopamine D2-type receptor availability, measured as nondisplaceable binding potential (BPND) in methamphetamine users (Lee et al., 2009; London et al., 2014; Volkow et al., 2001), as well as in individuals with other addictions (Volkow et al., 2009). Inasmuch as BPND represents the receptor pool accessible to radiotracer, it can be influenced by both receptor density and the concentration of intrasynaptic dopamine (Ito et al., 2011). Nonetheless, deficit in striatal D2-type BPND, measured at rest in stimulant users, has been considered to reflect below-control concentrations of D2-type receptors and reduced capacity for signaling through D2-type dopamine receptors rather than enhanced resting intrasynaptic dopamine concentration (Volkow et al., 2009). Supporting this view was the demonstration that acute reduction in endogenous dopamine with α-methyl-para-tyrosine produced a substantially smaller increase in BPND measured with [11C]raclopride in cocaine-dependent subjects than in controls, indicating that cocaine-dependent subjects had lower levels of endogenous dopamine and that the group difference in BPND was not due to higher intrasynaptic dopamine in the cocaine-dependent subjects (Martinez et al., 2009).
The amygdala receives a substantial dopaminergic innervation from the ventral tegmental area of the midbrain (Ciliax et al., 1999; Fallon and Moore, 1978), and is considered part of the mesolimbic dopamine pathway heavily implicated in addictive behavior. As indicated above, the amygdala also shows evidence of dopaminergic involvement in emotion processing. Despite evidence of dopaminergic dysfunction as well as emotional problems in methamphetamine users, however, the potential link between amygdala D2-type receptor availability and emotion dysregulation in methamphetamine users has not been examined.
The goals of this study, therefore, were to evaluate emotion dysregulation in methamphetamine users and its association with addiction severity, and to assess the potential association of D2-type dopamine receptor BPND with emotion dysregulation. BPND measured in the resting state was taken as an index of capacity for signaling through dopamine D2-type receptors. Although it is possible to infer changes in dopamine release from changes in BPND induced by a cognitive or emotional challenge (Badgaiyan, 2014), even those changes are small but would be expected to exceed individual differences in intrasynaptic dopamine concentrations in the resting state. BPND in the amygdala was assessed in recently abstinent methamphetamine users and control subjects, and was tested for association with emotion dysregulation, measured using the DERS, which provides a comprehensive assessment of emotion dysregulation (Gratz and Roemer, 2004). Given prior evidence for positive association of dopaminergic transmission in the amygdala and emotional response (see above), it was hypothesized that D2-type BPND in the amygdala would be positively associated with the DERS in healthy control subjects, but given evidence for dopaminergic deficits, at least in the striatum of methamphetamine users, amygdala BPND would be lower in the methamphetamine users than in controls and an association involving a dopaminergic marker would not be found in the methamphetamine users.
2. METHOD
2.1. Participants
All procedures were approved by the University of California Los Angeles Office for the Protection of Research Subjects. Participants were recruited using Internet and local newspaper advertisements and were paid. They were either healthy volunteers (N = 102; 48 men, 54 women) or active methamphetamine users who were not seeking treatment (N = 94; 56 men, 38 women), 18 – 55 years of age. Of these, 20 control subjects (10 men, 10 women) and 27 methamphetamine users (14 men, 13 women) completed PET scanning. Some of the subjects receiving PET scans here (7 of 20 controls and 13 of 27 methamphetamine users) participated in a study of dopamine D2-type receptors and impulsiveness (Lee et al., 2009), and smaller groups participated in other studies (Brown et al., 2012; Ghahremani et al., 2012; Kohno et al., 2015; Zorick et al., 2012).
After receiving a complete explanation of the study, each participant provided written informed consent. Participants were screened for eligibility using questionnaires and interviews, including the Structured Clinical Interview for DSM-IV Axis-I Disorders, and accordingly were included in a methamphetamine or control group. Methamphetamine-group participants were required to meet DSM-IV criteria for current methamphetamine dependence and each provided a urine sample that was positive for methamphetamine at the initial screening. Any current Axis-I diagnosis other than methamphetamine dependence, substance-induced mood/anxiety disorder, marijuana abuse/dependence, or nicotine abuse/dependence was exclusionary for the methamphetamine group. Eight of the methamphetamine-group participants, none of the control-group participants, and none of the subjects who participated in PET scans met criteria for marijuana dependence. For the control group, any current Axis-I diagnosis other than nicotine abuse or dependence was exclusionary. For both groups, the following conditions were exclusionary: use of any psychotropic medications or substances, presence of central nervous system, cardiovascular, pulmonary or systemic disease, human immunodeficiency virus seropositive status, hepatic disease; pregnancy, lack of English fluency and MRI contraindications. All the PET-scan subjects were right-handed, as indicated by the Edinburgh Handedness Questionnaire (Oldfield, 1971). Methamphetamine-group participants maintained abstinence from all illicit drugs of abuse, verified by urine testing, during their participation in the study.
2.2. Self-report Assessments
All participants completed the DERS, which is divided into six-distinct subscales as follows: Nonacceptance of Emotional Responses (NONACCEPT); Difficulties Engaging in Goal-Directed Behavior (GOALS); Impulse Control Difficulties (IMPULSE); Lack of Emotional Awareness (AWARE); Limited Access to Emotion Regulation Strategies (STRATEGIES); and Lack of Emotional Clarity (CLARITY; Gratz and Roemer, 2004). On the DERS, participants rate statements, such as “When I’m upset, I take time to figure out what I’m really feeling”, with scores ranging from one, “almost never (0 – 10 %)”; two, “sometimes (11 – 35%)”; three, “about half the time (36 – 65%)”; four, “most of the time (66 – 90%)”; and five, “almost always (91 – 100%).” The range of possible total scores on the DERS is 30 to 180, with higher scores representing greater difficulties in emotion regulation (i.e., emotion dysregulation). Methamphetamine users were abstinent from illicit drugs of abuse for 4.5 ± 3.37 days (mean ± SD; range: 1 – 15 days) at the time of testing.
In order to evaluate the relationship of DERS total score with drug use, the Addiction Severity Index (ASI) (McLellan et al., 1992) was administered to a subsample of the methamphetamine group (n = 33: 20 men and 13 women). One of the ASI subscales, the Drug Composite Score, which assesses the severity of drug use, was used in statistical analysis.
2.3. Positron emission tomography (PET) Imaging
PET scans were conducted using [18F]fallypride, a radiotracer with sufficient affinity for dopamine D2-type receptors to allow measurement in extrastriatal regions as well as the striatum (Mukherjee et al., 2002, 1995). Although [11C]FLB 457 provides greater sensitivity to subtle changes in receptor availability in low-receptor-density extrastriatal regions (Narendran et al., 2009; Vandehey et al., 2010), [18F]fallypride was chosen for this study because the use of [11C]FLB457 leads to underestimation of BPND in high-receptor-density regions such as the striatum, because the half-life of 11C is too short for [11C]FLB457 to reach the required transient-equilibrium condition for calculating BPND (Olsson et al., 1999). PET data were acquired using a Siemens ECAT EXACT HR+ scanner, which has an in-plane resolution full-width at half-maximum (FWHM) of 4.6 mm, axial resolution FWHM of 3.5 mm, and an axial field of view of 15.52 cm in the three-dimensional scanning mode. Each participant was placed on the scanning bed in the supine position, with his or her head immobilized. A transmission scan was conducted using a rotating 68Ga 68Ge rod source for attenuation correction. Emission data were collected for 80 min after the radiotracer injection. Participants were then removed from the scanner for a 20-min break. They then returned to the scanner and were repositioned. After another transmission scan, emission data were collected for 80 min. Cigarette smoking was not allowed for 2 h before PET scanning.
To accommodate scheduling requirements, the PET scans and DERS assessments were performed on different days; with a mean intervening interval of 19.0 ± 11.15 days for the control group (range: 6 – 44 days) and 5.7 ± 3.14 days for the methamphetamine group (range: 1 – 14 days).
2.4. Magnetic resonance imaging (MRI)
Structural MRI scans of the brain were acquired on a 1.5-Tesla Siemens Sonata tomograph, for co-registration with PET images and definition of volumes of interest (VOIs) (see below for details). A high-resolution sagittal T1-weighted 3D volumetric scan was acquired using a whole-brain magnetization-prepared rapid acquisition with gradient echo (MPRAGE) sequence.
2.5. PET data processing
Reconstructed PET data were combined into 16 images, each reflecting data averaged over 10 minutes. FSL MCFLIRT (FMRIB Centre, Department of Clinical Neurology, University of Oxford, Oxford, UK) was used to correct for head motion. The images were then co-registered to the MPRAGE image using a 6-parameter, rigid-body spatial transformation (FSL FLIRT; Jenkinson et al., 2002).
A volume of interest (VOI) in the amygdala, including left and right sides, was used to test the primary question of the study; left and right amygdala VOIs were tested separately in post-hoc analyses. VOIs of the striatum (including the caudate nuclei and putamina of both hemispheres), as well as the cerebellar hemispheres, were used for modeling of the PET data. Other subcortical (hippocampus, globus pallidus, thalamus) and cortical regions (anterior cingulate cortex, insula and orbital frontal cortex) were also used as control regions in post-hoc tests. All of the subcortical VOIs were derived from individual MPRAGE images using auto-segmentation procedures in FSL FIRST software (Patenaude et al., 2011). Cortical VOIs as well as cerebellar hemispheres were created in MNI space, using the Harvard-Oxford Atlas distributed with the FSL software (Desikan et al., 2006), and transformed to each subject’s native space using FSL FNIRT.
Time-activity data within anatomically defined VOIs were extracted from motion-corrected, co-registered PET images and imported into PMOD Kinetic Modeling (PKIN; PMOD Technologies Ltd., Zurich, Switzerland). The simplified reference tissue model (SRTM; Lammertsma and Hume, 1996) was used to estimate k2′, the rate constant for the transfer of the radiotracer from the reference-region tissue compartment to the plasma. The cerebellum was used as the reference region because of its very low level of specific binding for [18F]fallypride (Mukherjee et al., 2002). The VOI time-activity curves were refit using the SRTM2 model (Wu and Carson, 2002) using PKIN with the k2′ value representing clearance rate constant from the reference region derived from striatum using SRTM applied to all VOIs. [18F]Fallypride BPND, which is an index of receptor availability and used as an index of D2-type receptor density, was then calculated as BPND = R1*k2′/k2a – 1, where R1 = K1/K1′ is the ratio of tracer-delivery parameters from plasma to tissues in the target region (K1) and reference region (K1′), and k2a is the single-compartment rate constant for transfer from the target-region tissue compartment to plasma.
2.6. Statistical analyses
Group differences in demographic characteristics were evaluated using Pearson’s Chi-square Test or independent t-tests, as appropriate. Relationships between demographic characteristics and the DERS and D2-type amygdala BPND were tested using Pearson’s correlation for continuous variables and ANOVA for categorical variables. ANCOVA was used to assess the relationship between DERS and group, amygdala BPND, the interaction of group with amygdala BPND, and the following covariates: striatal BPND, participant’s age, smoking status, recent marijuana use (yes/no), and the time between the PET scan and DERS assessment. The DERS subscales were subsequently tested with the same model. To evaluate the possibility of laterality in amygdala findings, correlations between DERS total score and BPND in both left and right amygdala were tested. Mediation analysis (Preacher and Hayes, 2008), based on 1000 bootstrapped samples using bias-corrected 95% confidence intervals, was performed to see if there was a mediation effect of DERS between amygdala BPND (independent variable) and ASI (dependent variable).
3. RESULTS
3.1. Demographic analyses (Table 1)
Table 1.
Demographic Characteristics of Research Participants.
| All participants (n = 196) | Participants in ASI (n =33) | Participants in PET scan and ASI (n = 20) | ||||||
|---|---|---|---|---|---|---|---|---|
| Control group (n = 102) | MA group (n = 94) | Group difference: p-value | Control group (n = 20) | MA group (n = 27) | Group difference: p-value | MA group | MA group | |
| Age (years) | 33.7 ± 8.94 | 34.2 ± 9.70 | 0.751 a | 36.3 ± 7.09 | 31.5 ± 8.65 | 0.048 a | 33.2 ± 9.62 | 30.0 ± 8.10 |
| Sex (Male/ Female) | 48 / 54 | 56 / 38 | 0.079 b | 10 / 10 | 14 / 13 | 0.900 b | 20 / 13 | 11 / 9 |
| Ethnicity | 45 Caucasian, 31 Hispanic, 13 African American, 5 Asian, 8 other | 45 Caucasian, 28 Hispanic, 7 African American, 3 Asian, 11 other | 0.264 b | 12 Caucasian, 3 Hispanic, 1 African American, 2 Asian, 2 other | 16 Caucasian, 7 Hispanic, 2 Asian, 2 other | 0.604 b | 15 Caucasian, 11 Hispanic, 2 Asian, 5 other | 9 Caucasian, 7 Hispanic, 2 Asian, 2 other |
| Education (years) | 12.9 ± 1.91 | 12.1 ± 1.67 | 0.001 a | 13.9 ± 2.03 | 12.3 ± 1.69 | 0.005 a | 12.1 ± 2.12 | 11.7 ± 2.17 |
| Tobacco Smokers | 32 | 76 | < 0.001 b | 11 | 23 | 0.045 b | 29 | 17 |
| Regular marijuana userc | 12 | 41 | < 0.001 b | 0 | 12 | < 0.001 b | 15 | 10 |
| Self-report of methamphetamine use | ||||||||
| Days abstinent before administration of DERS | - | 4.5 ± 3.37 | - | 4.0 ± 2.59 | 4.6 ± 3.62 | 4.1 ± 2.69 | ||
| Days abstinent before administration of PET scan | - | - | 6.9 ± 2.24 | 7.0 ± 2.45 | ||||
| Days of use in month before testing | - | 21.2 ± 8.46 | - | 21.3 ± 8.06 | 21.9 ± 7.73 | 22.7 ± 7.39 | ||
| Age of first use (years) | - | 21.0 ± 6.77 | - | 21.4 ± 6.97 | 19.0 ± 5.50 | 19.5 ± 5.00 | ||
Group difference evaluated by Student’s t-test.
Group difference in likelihood evaluated by Pearson’s Chi-square test.
Used marijuana within 1 month of enrolling in the study.
Data is shown as mean ± SD.
MA: methamphetamine.
The groups did not differ in age or ethnicity distribution, but showed a trend for a difference in sex distribution when considering the larger group that was tested with the DERS (p = 0.079), but not the subset that took part in PET (p = 0.90). Control participants, on average, had significantly more years of education, a larger time interval between the PET scan and DERS assessment (p < 0.001), a smaller proportion of smokers (p = 0.045 in the PET scan subset), and more participants who recently had used marijuana (p’s < 0.05).
DERS total score was negatively correlated with age across all participants (r = −0.187, p = 0.01; r = −0.328, p = 0.03 in the PET-scan subset), whereas other demographic characteristics were not significantly related to DERS (p’s > 0.05). Methamphetamine users exhibited significantly higher DERS total scores (76.2 ± 20.10, mean ± SD) than control participants (55.2 ± 12.53): t(153.44) = 8.678; p < 0.001 (equal variances not assumed), and this difference retained significance when covarying for age, sex, smoking status, recent marijuana use (yes/no), and years of education (p < 0.001). Age was negatively correlated with amygdala BPND (r = −0.509, p < 0.001), whereas other demographic characteristics including smoking status were not significantly associated with amygdala BPND (p’s > 0.05). Amygdala BPND did not differ significantly between groups (Control: 2.22 ± 0.37 versus Methamphetamine: 2.24 ± 0.34; t(45) = 0.22, p = 0.83 (equal variances assumed)).
3.2. Emotion Regulation and Addiction Severity
Among methamphetamine-group participants who completed the ASI, the ASI-Drug composite score was significantly correlated with DERS (r = 0.398, p = 0.02).
Subscale analysis
The ASI-Drug composite score was correlated with IMPULSE (r = 0.548, p < 0.001) and STRATEGY (r = 0.427, p = 0.01) respectively.
3.3. Relationship between DERS and amygdala BPND
Based on the results presented above, ANCOVA was performed with the following independent variables: subject group, amygdala BPND, striatal BPND, the interaction between subject group with amygdala BPND, age, smoking status, recent marijuana use (yes/no), and the time between the PET scan and DERS assessment. The ANCOVA model yielded p values of <0.01 and 0.451 respectively for amygdala BPND and the interaction, and showing other independent variables were non-significant suggesting all the covariates were not significant modulator. Thus we see a significant positive linear dependence of DERS on amygdala BPND but no group dependence of the slope.
Subscale analysis
Significant independent variables were not found in subscale analysis except for GOALS and IMPULSE. The same ANCOVA analysis for GOALS yielded p values of 0.02, 0.02 and 0.08 respectively for group, amygdala BPND and the interaction. For IMPULSE, the analysis yielded p values of 0.049 for amygdala BPND. Therefore, we see a positive linear dependence of DERS GOALS and IMPULSE on amygdala BPND. The different association of amygdala BPND with DERS GOALS between the subject groups was suggested. BPNDs in other controls regions were not found related with any DERS subscales.
3.4. Post-hoc analyses
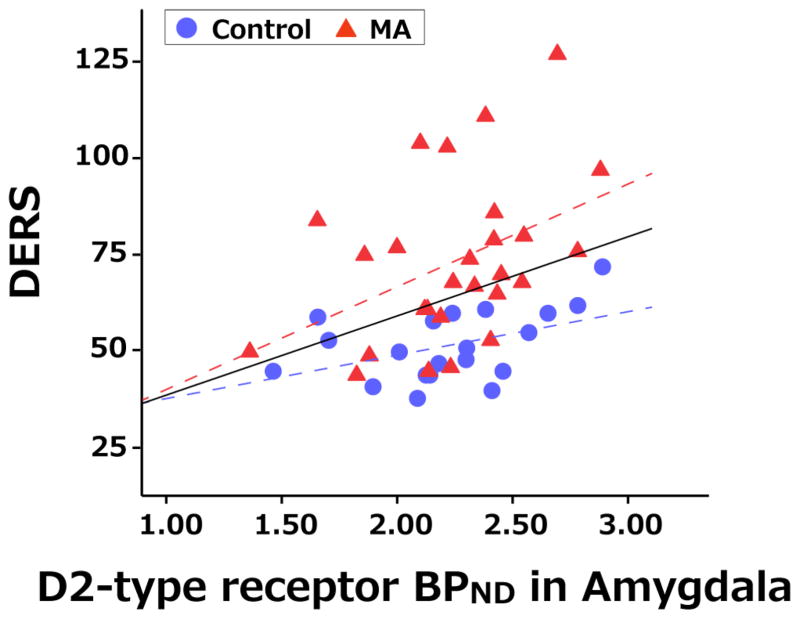
Separate tests of the relationship between DERS total score and right and left amygdala BPND revealed similar trends on both sides (left: r = 0.275, p = 0.078; right: r = 0.308, p = 0.047). Tests of the relationships between amygdala BPND and DERS total score in the combined and separate groups indicated significant correlations in the data of both groups; combined group (r = 0.331, p = 0.02), control group (r = 0.458, p = 0.04), and methamphetamine group (r = 0.424, p = 0.03). In regard to subscales, amygdala BPND was correlated with IMPULSE in combined group (r = 0.326, p = 0.03), control group (r = 0.465, p = 0.04), and methamphetamine group (r = 0.386, p = 0.047) and with GOALS in combined group (r = 0.307, p = 0.04) and control group (r = 0.763, p < 0.001) but not methamphetamine group (r = 0.06, p = 0.77). Figure 1 shows a plot of DERS total score against amygdala BPND with the overall best-fit regression line (solid) and dashed lines corresponding to the separate fits to the two groups. Amygdala BPND was not correlated with the ASI-Drug composite score.
Figure 1. Association of Amygdala Dopamine D2-type receptor BPND with DERS total score.
Scatter plots show individual data for the DERS scores (y-axis) and D2-type receptor binding potential (BPND) (x-axis) in amygdala for the Control group (blue circles) and MA group (red triangles). Black solid-line shows slope of relationship of all participants, while blue dashed- and red dashed- lines are for control group and MA group, respectively. DERS was correlated with amygdala BPND (control: r = 0.458, p = 0.042; MA: r = 0.424, p = 0.027).
A post-hoc mediation analysis was performed on data from the 20 methamphetamine-group subjects who had PET scans and ASI testing. In this limited sample, trends for an association of amygdala BPND to DERS and of DERS to ASI did not reach statistical significance (A = 33.17, SE = 15.89, p = 0.051; B = 0.0014, SE = 0.0008, p = 0.11 respectively).
BPND in the striatum was significantly lower in the methamphetamine group, as previously reported (Lee et al., 2009; Volkow et al., 2001). Other brain regions, tested as control regions, showed no significant group differences. BPND values in all the regions except for the amygdala were not associated with DERS (Table 2).
Table 2.
PET measures in various brain regions for group comparison and association with DERS
| Dopamine D2-type BPND | Correlation coefficients with DERS b | ||||
|---|---|---|---|---|---|
| Control group (n = 20) | MA group (n = 27) | Group difference a: p-value | Control group (n = 20) | MA group (n = 27) | |
| Amygdala | 2.22 ± 0.37 | 2.24 ± 0.34 | 0.83 | 0.458* | 0.424* |
| Striatum | 20.43 ± 3.11 | 18.03 ± 2.46 | 0.005 | 0.159 | 0.198 |
| Hippocampus | 0.93 ± 0.22 | 0.93 ± 0.17 | 0.89 | 0.304 | 0.301 |
| Globus pallidus | 10.76 ± 1.83 | 10.64 ± 1.43 | 0.80 | 0.209 | 0.265 |
| Thalamus | 2.06 ± 0.41 | 2.06 ± 0.34 | 0.99 | 0.310 | 0.145 |
| ACC | 0.55 ± 0.15 | 0.59 ± 0.15 | 0.40 | 0.212 | 0.237 |
| Insula | 1.23 ± 0.29 | 1.30 ± 0.30 | 0.44 | 0.180 | 0.327 |
| OFC | 0.38 ± 0.15 | 0.45 ± 0.48 | 0.15 | 0.071 | 0.260 |
Group difference evaluated by Student’s t-test.
Pearson’s correlation analysis was used to yield correlation coefficients.
p < 0.05
ACC: anterior cingulate cortex; OFC: orbitofrontal cortex; MA: methamphetamine.
4. DISCUSSION
Self-reported difficulty with emotion regulation was greater in participants who met DSM-IV criteria for methamphetamine dependence than in healthy controls; and it was positively associated with the severity of addiction in the methamphetamine-user group and with D2-type receptor availability in the amygdala across both methamphetamine and control groups. Although measures of D2-type receptor availability in the striatum and amygdala were correlated (r = 0.671, p < 0.001), striatal receptor availability was not significantly related to emotion dysregulation, and controlling for striatal receptor availability did not eliminate the association between amygdala BPND and difficulty in regulating emotion. BPND in other regions, tested in post-hoc analysis, did not show a relationship with DERS. These results support the view that dopamine D2-type receptor signaling in amygdala in contributes to emotional dysregulation.
Problems with emotion regulation can reflect deficits in coping with stress. In turn, stress-induced negative mood is strongly related to drug craving in individuals who use cocaine and heroin (Preston and Epstein, 2011), tobacco (al’Absi et al., 2007) and methamphetamine (Shen et al., 2012); and the involvement of several neurotransmitter systems in that relationship is suggested (Mantsch et al., 2015). In alcohol-dependent individuals, emotion regulation skills, evaluated using the Emotion-Regulation Skills Questionnaire, predicts dysfunctional alcohol use during the follow-up period after treatment (Berking et al., 2011). These findings are consistent with the correlation of the ASI-Drug score with the DERS total score in this study, suggesting that impaired emotion regulation may exacerbate addiction in those who use methamphetamine chronically, or alternatively, that drug use may lead to emotional difficulties.
The positive relationship between BPND and DERS total score, irrespective of subject group, suggests that greater dopaminergic transmission in the amygdala affects the ability to regulate emotion. DERS subscale analyses suggested the contribution of D2-type receptors in the amygdala to difficulties in motivated cognitive control over negative emotions, as the GOALS and IMPULSE subscales reflect the ‘ability to engage in goal-directed behavior and refrain from impulsive behavior when experiencing negative emotions’ (Gratz and Roemer, 2004). D2-type receptors in the amygdala are thought to contribute to enhanced neural activity associated with a negative emotional state (Schaefer et al., 2002). Blocking D2 receptors in the amygdala attenuates fear-potentiated startle (Greba et al., 2001) and conditioned freezing (Guarraci et al., 2000) in rats, supporting the view that signaling through D2-type receptors promotes negative emotion. Also, blocking the D3 receptors in the basolateral amygdala reduces anxiety-like behavior, and D3-receptor stimulation inhibits synaptic transmisison, both in basolateral amygdala feedback and feedforward GABAergic neuron populations (Bissiere et al., 2003; Diaz et al., 2011). Thus, signaling through dopamine D2-type receptors may attenuate the inhibitory function of GABA in the amygdala, and may contribute to increased neural activity in the amygdala in response to emotionally evocative stimuli. Considering the positive relationships observed here between amygdala D2-type BPND and the subscale scores in GOALS and IMPULSE, the present results suggest that dopaminergic transmission through D2-type receptors in amygdala contribute to bottom-up feedforward processing in response to emotional perceptual properties of the stimuli. Given the positive relationship of increase in amygdala activity with negative affect in response to bottom-up perception (Ochsner et al., 2009; Phelps, 2006) and that successful emotion regulation is accompanied by decreased neural activity in amygdala (see review (Frank et al., 2014)); these findings overall are consistent with the positive relationship between dopamine D2-type receptor availability and difficulty with emotion-regulation, observed here using self-report.
Although methamphetamine users reported higher DERS total scores than control-group participants, they did not differ from controls in suppression of amygdala activity during an emotion-regulation task paired with fMRI (Payer et al., 2011). The lack of physiological evidence for impaired amygdala downregulation during explicit emotion regulation, despite higher than control self-reports on the DERS, may reflect contributions of regions outside the amygdala, both to the process of emotion regulation, and to the participant’s subjective impression of success in this function.
Given the substantial evidence showing deficits in dopamine D2-type receptors in substance-use disorders (see review (Volkow et al., 2011)) we would expect lower receptor availability in the methamphetamine group. In our sample, this expectation was previously confirmed in the striatum (e.g., Lee et al., 2009) but not in other regions, including amygdala. For the same reason, we would expect no association of amygdala D2-type BPND and emotion dysregulation, although no difference in the association was found. The lack of a group difference in amygdala BPND and the absence of an interaction between group and amygdala BPND on DERS total suggest that the role of amygdala D2-type dopamine-receptor signaling in emotion dysregulation is not unique to methamphetamine users.
There are some limitations in this study. First of all, the methamphetamine and control groups were not well matched for education, tobacco smoking status and marijuana use. In addition, there was a relatively long interval, especially in the control group (range: 6 – 44 days), between the DERS assessment and PET scan. However, given the absence of an effect of this variable in the statistical analyses and the strong test-retest reliability of DERS with a 4 – 8 week interval (Gratz and Roemer, 2004), this should not affect the results. Another limitation relates to the low spatial resolution of PET (Prieto et al., 2010), which generally cannot distinguish between subnuclei of the amygdala that serve different functions and vary in the relative densities of D2 and D3 receptors (Alleweireldt et al., 2006; Grace and Rosenkranz, 2002; Rosenkranz and Grace, 1999). In addition, [18F]fallypride has almost equal affinity for D2 and D3 receptors (Jerjian et al., 2010), preventing a separate assessment of either receptor subtype. Finally, in a cross-sectional study, it is not possible to determine whether the association of DERS total score with the ASI-Drug Composite Score subscale reflects a causal effect of addiction on emotion regulation or the effect of emotion dysregulation to promote addiction. Also, the ASI interview was performed on only on a limited number of methamphetamine-group participants.
Despite these limitations, this study demonstrates that methamphetamine users suffer from heightened emotion dysregulation, and suggests that signaling through D2-type dopamine receptors in amygdala contributes to emotion dysregulation in healthy controls as well as methamphetamine users. These findings demonstrate the importance of amygdala D2-type receptors in emotion dysregulation and may potentially guide treatment strategies in clinical populations with substance-use or other psychiatric disorders.
Highlights.
Methamphetamine users versus healthy controls show impaired emotion regulation.
Emotion-dysregulation is related to dopamine receptor availability in amygdala.
That relationship is found in both of methamphetamine users and healthy controls.
Acknowledgments
The authors thank Kimberly Lin for recruiting the participants and scheduling the study procedures, and Hannah Jones and Brittany Reid for collecting psychological data.
Footnotes
Conflict of Interest
None
Contributors
KO and EL designed the study, and EL supervised all data collection. DG, DP and AD contributed to conceptual content. KO and CR performed PET data analyses. KO, AD and MM did the statistical analysis, and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Author Disclosures
This research was supported, in part, by grants from the National Institute on Drug Abuse (R01 DA015179, R01 DA020726, P20 DA022539, T32 DA024635, EDL; K23 DA027734, R21 DA034928, ACD) and the National Center for Research Resources (M01 RR00865), and endowments from the Thomas P and Katherine K Pike Chair in Addiction Studies and the Marjorie M Greene Trust. KO was partly supported by Department of Psychiatry, Chiba University, DOMONKAI fund.
Role of Funding Source
Nothing declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al’Absi M, Carr SB, Bongard S. Anger and psychobiological changes during smoking abstinence and in response to acute stress: prediction of smoking relapse. J Psychophysiol. 2007;66:109–115. doi: 10.1016/j.ijpsycho.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL. Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharmacology. 2006;31:363–374. doi: 10.1038/sj.npp.1300794. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD. Imaging dopamine neurotransmission in live human brain. Prog Brain Res. 2014;211:165–182. doi: 10.1016/B978-0-444-63425-2.00007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgaiyan RD, Fischman AJ, Alpert NM. Dopamine release during human emotional processing. Neuroimage. 2009;47:2041–2045. doi: 10.1016/j.neuroimage.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berking M, Margraf M, Ebert D, Wupperman P, Hofmann SG, Junghanns K. Deficits in emotion-regulation skills predict alcohol use during and after cognitive-behavioral therapy for alcohol dependence. J Consult Clin Psychol. 2011;79:307–318. doi: 10.1037/a0023421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissiere S, Humeau Y, Luthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- Brown AK, Mandelkern MA, Farahi J, Robertson C, Ghahremani DG, Sumerel B, Moallem N, London ED. Sex differences in striatal dopamine D2/D3 receptor availability in smokers and non-smokers. Int J Neuropsychopharmacol. 2012;15:989–994. doi: 10.1017/S1461145711001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, Mufson EJ, Mash DC, Levey AI. Immunocytochemical localization of the dopamine transporter in human brain. J Comp Neurol. 1999;409:38–56. doi: 10.1002/(sici)1096-9861(19990621)409:1<38::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Dean AC, Kohno M, Hellemann G, London ED. Childhood maltreatment and amygdala connectivity in methamphetamine dependence: a pilot study. Brain Behav. 2014;4:867–876. doi: 10.1002/brb3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Chappell AM, Christian DT, Anderson NJ, McCool BA. Dopamine D3-like receptors modulate anxiety-like behavior and regulate GABAergic transmission in the rat lateral/basolateral amygdala. Neuropsychopharmacology. 2011;36:1090–1103. doi: 10.1038/npp.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, Gore JC, Olson IR, McGlashan TH, Wexler BE. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Fox HC, Axelrod SR, Paliwal P, Sleeper J, Sinha R. Difficulties in emotion regulation and impulse control during cocaine abstinence. Drug Alcohol Depend. 2007;89:298–301. doi: 10.1016/j.drugalcdep.2006.12.026. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KA, Sinha R. Difficulties in emotion regulation and impulse control in recently abstinent alcoholics compared with social drinkers. Addict Behav. 2008;33:388–394. doi: 10.1016/j.addbeh.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, Hussein AA, Smart LM, Sabatinelli D. Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci Biobehav Rev. 2014;45:202–211. doi: 10.1016/j.neubiorev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, Brown AK, Monterosso JR, Aron AR, Mandelkern MA, Poldrack RA, London ED. Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J Neurosci. 2012;32:7316–7324. doi: 10.1523/JNEUROSCI.4284-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Rosenkranz JA. Regulation of conditioned responses of basolateral amygdala neurons. Physiol Behav. 2002;77:489–493. doi: 10.1016/s0031-9384(02)00909-5. [DOI] [PubMed] [Google Scholar]
- Gratz K, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: development, factor structure, and initial validation of the Difficulties in Emotion Regulation Scale. J Psychopathol Behav Assess. 2004;26:41–54. [Google Scholar]
- Greba Q, Gifkins A, Kokkinidis L. Inhibition of amygdaloid dopamine D2 receptors impairs emotional learning measured with fear-potentiated startle. Brain Res. 2001;899:218–226. doi: 10.1016/s0006-8993(01)02243-0. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Handbook of Emotion Regulation. Guilford Publications; 2015. [Google Scholar]
- Guarraci FA, Frohardt RJ, Falls WA, Kapp BS. The effects of intra-amygdaloid infusions of a D2 dopamine receptor antagonist on Pavlovian fear conditioning. Behav Neurosci. 2000;114:647–651. doi: 10.1037//0735-7044.114.3.647. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Gross JJ, Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cogn Emot. 2011;25:400–412. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Smith WG, Weinberger DR. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27:1036–1040. doi: 10.1016/S0893-133X(02)00373-1. [DOI] [PubMed] [Google Scholar]
- Hilt LM, Hanson JL, Pollak SD. Emotion Dysregulation. In: Prinstein BBBJ, editor. Encyclopedia of Adolescence. Academic Press; San Diego: 2011. pp. 160–169. [Google Scholar]
- Hodgins DC, el-Guebaly N, Armstrong S. Prospective and retrospective reports of mood states before relapse to substance use. J Consult Clin Psychol. 1995;63:400–407. doi: 10.1037//0022-006x.63.3.400. [DOI] [PubMed] [Google Scholar]
- Ito H, Kodaka F, Takahashi H, Takano H, Arakawa R, Shimada H, Suhara T. Relation between presynaptic and postsynaptic dopaminergic functions measured by positron emission tomography: implication of dopaminergic tone. J Neurosci. 2011;31:7886–7890. doi: 10.1523/JNEUROSCI.6024-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jerjian T, Constantinescu C, Sevrioukov E, Mukherjee J. Evaluation of dopamine D3 receptor binding of 18F-fallypride. J Nucl Med Meeting Abstracts. 2010;51:1762. [Google Scholar]
- Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex. 2011;21:1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kohno M, Ghahremani DG, Morales AM, Robertson CL, Ishibashi K, Morgan AT, Mandelkern MA, London ED. Risk-taking behavior: dopamine D2/D3 receptors, feedback, and frontolimbic activity. Cereb Cortex. 2015;25:236–245. doi: 10.1093/cercor/bht218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Kohno M, Morales AM, Ballard ME. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Res. 2014;1628:174–185. doi: 10.1016/j.brainres.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y. Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. 2015;41:335–356. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R, Slifstein M, Van Heertum R, Kleber HD. Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am J Psychiatry. 2009;166:1170–1177. doi: 10.1176/appi.ajp.2009.08121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. J Cogn Neurosci. 2010;22:248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, Mantil J. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002;46:170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Yang ZY, Das MK, Brown T. Fluorinated benzamide neuroleptics--III. Development of (S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3-[18F]fluoropropyl)-2, 3-dimethoxybenzamide as an improved dopamine D-2 receptor tracer. Nucl Med Biol. 1995;22:283–296. doi: 10.1016/0969-8051(94)00117-3. [DOI] [PubMed] [Google Scholar]
- Murray RJ, Brosch T, Sander D. The functional profile of the human amygdala in affective processing: insights from intracranial recordings. Cortex. 2014;60:10–33. doi: 10.1016/j.cortex.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Narendran R, Frankle WG, Mason NS, Rabiner EA, Gunn RN, Searle GE, Vora S, Litschge M, Kendro S, Cooper TB, Mathis CA, Laruelle M. Positron emission tomography imaging of amphetamine-induced dopamine release in the human cortex: a comparative evaluation of the high affinity dopamine D2/3 radiotracers [11C]FLB 457 and [11C]fallypride. Synapse. 2009;63:447–461. doi: 10.1002/syn.20628. [DOI] [PubMed] [Google Scholar]
- Newton TF, Kalechstein AD, Duran S, Vansluis N, Ling W. Methamphetamine abstinence syndrome: preliminary findings. Am J Addict. 2004;13:248–255. doi: 10.1080/10550490490459915. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, McRae K, Cooper JC, Weber J, Gabrieli JD, Gross JJ. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychol Sci. 2009;20:1322–1331. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olsson H, Halldin C, Swahn CG, Farde L. Quantification of [11C]FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab. 1999;19:1164–1173. doi: 10.1097/00004647-199910000-00013. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer DE, Lieberman MD, London ED. Neural correlates of affect processing and aggression in methamphetamine dependence. Arch Gen Psychiatry. 2011;68:271–282. doi: 10.1001/archgenpsychiatry.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Anderson AK. Emotional memory: what does the amygdala do? Curr Biol. 1997;7:R311–R314. doi: 10.1016/s0960-9822(06)00146-1. [DOI] [PubMed] [Google Scholar]
- Preacher K, Hayes A. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Preston KL, Epstein DH. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology. 2011;218:29–37. doi: 10.1007/s00213-011-2183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto E, Marti-Climent JM, Arbizu J, Garrastachu P, Dominguez I, Quincoces G, Garcia-Velloso MJ, Lecumberri P, Gomez-Fernandez M, Richter JA. Evaluation of spatial resolution of a PET scanner through the simulation and experimental measurement of the recovery coefficient. Comput Biol Med. 2010;40:75–80. doi: 10.1016/j.compbiomed.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. J Neurosci. 1999;19:11027–11039. doi: 10.1523/JNEUROSCI.19-24-11027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SM, Jackson DC, Davidson RJ, Aguirre GK, Kimberg DY, Thompson-Schill SL. Modulation of amygdalar activity by the conscious regulation of negative emotion. J Cogn Neurosci. 2002;14:913–921. doi: 10.1162/089892902760191135. [DOI] [PubMed] [Google Scholar]
- Scott LN, Stepp SD, Pilkonis PA. Prospective associations between features of borderline personality disorder, emotion dysregulation, and aggression. Personal Disord. 2014;5:278–288. doi: 10.1037/per0000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Liu Y, Li L, Zhang Y, Zhou W. Negative moods correlate with craving in female methamphetamine users enrolled in compulsory detoxification. Subst Abuse Treat Prev Policy. 2012;7:44–44. doi: 10.1186/1747-597X-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Takano H, Kodaka F, Arakawa R, Yamada M, Otsuka T, Hirano Y, Kikyo H, Okubo Y, Kato M, Obata T, Ito H, Suhara T. Contribution of dopamine D1 and D2 receptors to amygdala activity in human. J Neurosci. 2010;30:3043–3047. doi: 10.1523/JNEUROSCI.5689-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandehey NT, Moirano JM, Converse AK, Holden JE, Mukherjee J, Murali D, Nickles RJ, Davidson RJ, Schneider ML, Christian BT. High-affinity dopamine D2/D3 PET radioligands 18F-fallypride and 11C-FLB457: a comparison of kinetics in extrastriatal regions using a multiple-injection protocol. J Cereb Blood Flow Metab. 2010;30:994–1007. doi: 10.1038/jcbfm.2009.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci USA. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Weiss NH, Sullivan TP, Tull MT. Explicating the role of emotion dysregulation in risky behaviors: A review and synthesis of the literature with directions for future research and clinical practice. Curr Opin Psychol. 2015;3:22–29. doi: 10.1016/j.copsyc.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Carson RE. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cere Blood Flow Metab. 2002;22:1440–1452. doi: 10.1097/01.WCB.0000033967.83623.34. [DOI] [PubMed] [Google Scholar]
- Zorick T, Lee B, Mandelkern MA, Fong T, Robertson C, Ghahremani DG, Brown AK, Sumerel B, London ED. Low striatal dopamine receptor availability linked to caloric intake during abstinence from chronic methamphetamine abuse. Mol Psychiatry. 2012;17:569–571. doi: 10.1038/mp.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweben JE, Cohen JB, Christian D, Galloway GP, Salinardi M, Parent D, Iguchi M Methamphetamine Treatment P. Psychiatric symptoms in methamphetamine users. Am J Addict. 2004;13:181–190. doi: 10.1080/10550490490436055. [DOI] [PubMed] [Google Scholar]