Abstract
BACKGROUND
Artemisinins, commonly used to treat malaria, have shown activity against cytomegalovirus (CMV) in vitro, in an animal model, and in case reports; however, the in vivo anti-CMV activity has not been well investigated.
OBJECTIVES
To evaluate whether artemisinins affect CMV shedding among subjects co-infected with CMV and malaria.
STUDY DESIGN
A prospective observational study of children in Mali (6 mo-10 yr) presenting with fever. Urine samples were collected at day 0, 3, and 14 from children treated with artemether-lumefantrine (Coartem®) for malaria and those who had other illnesses not treated with Coartem. CMV DNA was quantified using a real-time PCR. Resulting urine viral loads were compared between the groups at three time points.
RESULTS
164 malaria cases and 143 non-malaria comparisons were enrolled. Eighty-one (49%) cases and 88 (62%) comparisons shed CMV at day 0. Day 0 and day 3 viral loads were similar, but at day 14 the median viral load of cases was lower than that of comparisons (360 versus 720 copies/mL or 2.56 versus 2.86 log10), p=0.059. A stratified analysis of day 0 high viral shedders (defined as >1000 copies/mL) showed significantly lower median viral load at day 14 among cases (490 copies/mL, 2.69 log10) versus comparisons (1200 copies/mL, 3.08 log10), p=0.045.
CONCLUSION
A high rate of urinary CMV shedding was found in a malaria-endemic area. Among high virus shedders artemether-lumefantrine decreased urine viral load, but the effect was not observed when analysis of both high and low shedders was performed. These results support additional studies of artemisinin dosing and duration in CMV infection.
Keywords: cytomegalovirus, malaria, artemether-lumefantrine, urine viral load
BACKGROUND
Human cytomegalovirus is a common and important pathogen capable of establishing lifelong persistent infection, which usually remains asymptomatic (1). However, CMV can cause severe disease in immunocompromised individuals and when transmitted during pregnancy to the fetus (1–3). Most available anti-CMV drugs target the viral DNA polymerase and their use is associated with considerable side effects such as bone marrow toxicity (ganciclovir) and nephrotoxicity (foscarnet and cidofovir) (4, 5). Drug resistance also develops when prolonged or repeated ganciclovir treatment is used in the transplant population (5, 6). New compounds are needed to treat CMV infection, especially non-toxic, low-cost anti-CMV drugs with high oral bioavailability.
Artemisinins are well established for the treatment of malaria (7). Artemisinin-combination therapies (for example, artemether-lumefantrine, known by the trade name Coartem) are the World Health Organization’s recommended treatment for uncomplicated malaria. More recently, artemisinin and its derivatives have shown anti-viral activities (8–13). Artesunate inhibited CMV replication in vitro with similar efficacy to ganciclovir, without cytotoxicity (8). Several artemisinin derivatives including artemether and novel artemisinin-derived dimers were shown to inhibit CMV (9, 10). Research on the use of artemisinins for CMV infection is of major importance because these compounds are known to be well-tolerated, safe, and orally available (14). While there is compelling in vitro data that these compounds are highly effective against CMV, in vivo data supporting the use of artemisinin derivatives is scattered and consists largely of case reports and one observational clinical study (15, 16). A trial of Ugandan children receiving either artesunate plus amodiaquine or sulfadoxine-pyrimethamine plus amodiaquine showed no difference in CMV viral loads measured from dried blood spots at day one or day three following therapy (17).
OBJECTIVES
To determine the effect of artemether-lumenfantrine on urine CMV shedding in children treated with the drug for malaria, as compared to those without malaria and not treated with the drug.
STUDY DESIGN
Subjects and recruitment
This was a prospective collaborative study with the Malaria Research and Training Center (MRTC) in Bamako, Mali. Children (6 months - 10 years) were recruited from November 2013 to January 2015 in Faladje, Mali, a rural village located 80 km northwest of the capital Bamako. Urine samples were collected during periods of peak malaria transmission. Parents of children who sought medical care at the local clinic were consented to participate in the study. Exclusion criteria were: recent treatment of malaria (<30 days), allergy to artemisinin-based therapies, inability to attend follow up screening, and inability to identify medical treatments a child had received in the past 30 days. If children were eligible, parents were presented written informed consent translated into the local languages and read aloud for those parents who were not literate. The Institutional Review Board and Ethic Committees at the University of Science, Techniques, and Technologies in Bamako, the MRTC, and the Johns Hopkins Medical Institutions approved the consent procedures and the research protocol.
Data Collection
Upon enrollment, screening procedures included review of medical history, obtaining vital signs, and measuring growth parameters. The following data were collected from each patient: age, sex, reason for visit, duration of fever, degree of parasitemia and hemoglobin (if malaria positive), and treatments received for any medical indication in the last 30 days. Urine samples were collected at day 0 prior to treatment, day 3, and day 14, a timeline that corresponds to anti-malaria drug monitoring protocols. Children diagnosed with malaria received directly observed therapy of artemether-lumefantrine and blood smears were obtained at day 0, 3, and 14 to document parasite clearance.
Children not diagnosed with malaria were followed with clinical data and urine sampling only at the same time points. If during follow up children in the comparison group became positive for malaria, they were dropped from further follow up and appropriately treated.
Urine samples were collected into a sterile cup or adherent infant bag and transferred to two separate 2 mL tubes labeled with the unique study number and collection date. Samples were transported by courier on ice to the main laboratory in Bamako where they were stored at −80ºC. Samples were de-identified, batched, and shipped on liquid nitrogen to Johns Hopkins Hospital. They were stored at −80ºC prior to undergoing CMV detection by real-time PCR and subsequent data analysis.
Sample Size
In a previous CMV study in Mali (unpublished data), we found that 30% of the pediatric population shed CMV in their urine. Sample size was based on the following conservative assumptions that if 30% of screened children would shed CMV in their urine, for 90% power to detect at least a 20% difference between groups, and a two-sided test with alpha error of 0.05, 62 CMV positive children would be required (31 case, 31 comparison). The comparison group was drawn from children who were evaluated for other illnesses or complaints, did not have malaria, and met the remaining inclusion criteria Therefore, to enroll 62 CMV-infected children, the total number of children screened and tested was estimated at 206. Our achieved sample size of 81 malaria cases and 88 non-malaria cases exceeds this minimum number.
Laboratory methods
DNA was isolated from urine using automated DNA extraction on the BioRobot M48 instrument (Qiagen, Valencia, CA). Urine input and elution volumes were 400 and 125 μL, respectively. The real-time PCR assay targets 151-bp from the highly conserved US17 region of the CMV genome (18). The primers and probe for US17 are: forward- 5’ GCGTGCTTTTTAGCCTCTGCA-3’, reverse 5’-AAAAGTTTGTGCCCCAACGGTA-3’ and US17 probe FAM- 5’ TGATCGGGCGTTATCGCGTTCT-3’. The limit of detection of the assay is 100 copies/mL, (2.0 log10) and the measurable range of the assay is 2.0–8.0 log10 copies/mL. Intra- and inter-run precision at 2.0 log10 copies/mL was identical (standard deviations = 0.09 and percent coefficient of variation = 4.2). This assay has been used extensively in our laboratory to measure viral loads in different body fluids (12, 19).
Statistical Analysis
Continuous clinical and demographic variables were reported as mean and standard error, viral loads were reported as medians and interquartile range (IQR) and categorical variables as frequency counts and percentages. Normality of variables was assessed and tested using the Shapiro Wilk test, and proper transformation was performed if necessary. CMV copies per mL were right-skewed and some measurements were below the level of detection of the assay. We added a value of √2*(minimum detectable level of 100 copies/mL) before logarithmic transformation of this variable for adjusted regression analysis. For normally distributed continuous variables, mean difference between groups was performed using two-sample t-tests (all two-sided tests). Wilcoxon rank-sum tests were used for non-normally distributed variables. Comparison of proportions between groups was performed using chi-squared test, or Fischer’s exact test as appropriate. Bivariate, cross sectional associations of viral load with clinical and demographic variables were explored using scatter plots, Pearson correlations, linear regression or analysis of variance (ANOVA) as appropriate. All statistical tests with 2-sided p-value of <0.05 were considered significant. Regression analysis included controlling for age, sex, and baseline CMV copies/mL shedding. Groups were further stratified into high and low shedders based on a cut off of 1000 CMV copies/mL (3.0 log10). Changes in viral shedding over time were measured by taking the ratio from two different time points (ie copies/mL from Day 3/Day 0, Day 14/ Day 0, and Day 3/ Day 14.
RESULTS
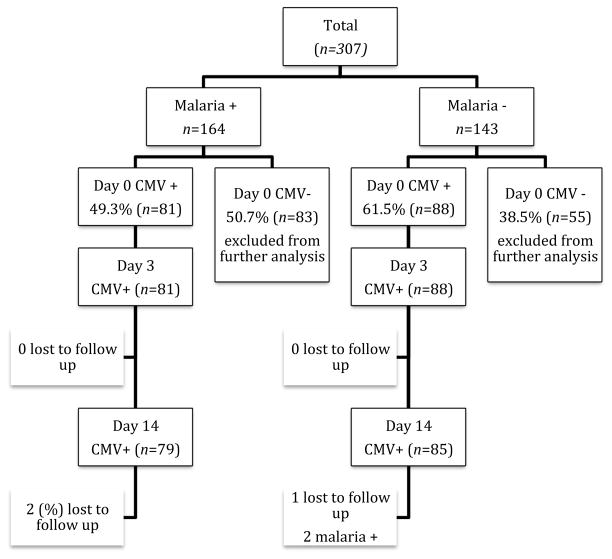
A total of 307 subjects (164 malaria positive and 143 malaria negative) were screened and enrolled in the study from November 2013 to January 2015. Study retention was 98% among cases (two children were lost to follow up) and 97% in the comparison group (one child was lost to follow up and two others became positive for malaria during the 14 day follow up period) (Figure 1). The demographic characteristics of the initial enrollees are shown in Table 1. Of the total 307 subjects screened for CMV infection, 81 (49%) malaria positive subjects (referred to as cases) and 88 (62%) of malaria negative subjects (referred to as comparisons) had evidence of CMV shedding in their urine. All children that were treated with artemether-lumefantrine had clearance of malaria parasites by study day 3.
Figure 1.
Recruitment and Follow up of Study Subjects
Table 1.
Baseline Characteristics of cases and comparisons
| Malaria Positive (n=164) |
p CMV+ vs CMV- among Malaria Positives |
Malaria Negative (n=143) |
p CMV+ vs CMV- among Malaria Negatives |
p malaria positives vs. malaria negatives among CMV Positives |
|||
|---|---|---|---|---|---|---|---|
| CMV (−) | CMV + | CMV (−) | CMV + | ||||
| N | 83 (51%) | 81 (49%) | 55 (38%) | 88 (62%) | 0.033 | ||
| % female | 46 (55%) | 39 (48%) | 0.35 | 37 (67%) | 44 (50%) | 0.043 | 0.13 |
| Years of age (SE) | 7.1 (2.3) | 5.7 (2.1) | <0.001 | 5.9 (2.8) | 4.0 (2.1) | <0.001 | <0.001 |
| Weight in kg (SE) | 21.7 (6.1) | 18.5 (5.0) | <0.001 | 19.3 (6.8) | 14.8 (4.6) | <0.001 | <0.001 |
| Height in cm (SE) | 121.1 (14.0) | 111.4 (14.3) | <0.001 | 111.9 (18.8) | 99.2 (14.9) | <0.001 | <0.001 |
| Malaria Parasites/μL median [IQR] | 23550 [6950 to 43500] | 30350 [1720 to 57300] | 0.059 | - | - | - | - |
| Hemoglobin mg/dL day 0 (SE) | 10.3 (1.4) | 10.5 (1.5) | 0.39 | - | - | - | - |
| Day 0 median viral load copies/mL [IQR] | - | 920 [320 to 3800] | - | - | 945 [490 to 3350] | - | 0.97 |
| N (%) High viral shedders (>1000 copies/mL) | - | 37 (46%) | - | - | 42 (48%) | - | 0.79 |
IQR: Interquartile Range, SE standard error
- Data not collected on this group
CMV shedding was less frequent at day 0 in cases than in comparisons in this cohort (p=0.033). In both groups, day 0 CMV positive children were younger than children who did not shed CMV. Cases were older on average than the comparison group (5.7 years v. 4.0 years p<0.001). Among those who screened positive for CMV at day 0, the median viral loads and the percentage of high shedders were similar (Table 1).
Table 2 shows the median viral loads, interquartile range (IQR), and ratios measuring viral load change for day 0, 3, and 14. There was a general trend of lower viral loads among the cases after therapy but these trends did not meet statistical significance among all shedders (p=0.059). However, a subgroup analysis of children with > 1,000 copies/mL or > 3.0 log10 copies/mL (high shedders) revealed a significant drop in viral loads from day 0 to day 14 (comparison 0.355 versus case 0.100, p = 0.045). After adjusting for age and sex a trend was observed, but it did not reach statistical significance (p=0.088). Although a 0.3 log10 difference may be within the error of the assay for an individual, our statistical tests estimate the probability that the observed differences between groups (rather than differences between individuals) have occurred by chance. The assay performed on a large number of patients would need to exhibit a systematic error to have the medians of the groups separated by 0.3 log10 (malaria treated vs comparison) or 0.39 log10 copies/mL (in the high shedders subgroup). This statistical calculation incorporates all measures of random error, including the within-person error of the assay and between-person error into a single p-value. The median value of a group has much lower error rate than individual measurements.
Table 2.
Quantitative real-time PCR results comparing cases and comparisons at day 3 and day 14
| Cases median [IQR] | Comparison median [IQR] | p (unadjusted using rank sum test) | Adjusted Linear regression Exp (beta) and 95% CI | p age, sex, day 0 viral load adjusted | |
|---|---|---|---|---|---|
| Day 3 | n=81 | n=88 | |||
|
| |||||
| Median Copies/mL | 390 [0 – 1400] | 425 [0 – 1650] | 0.733 | 1.565 [0.835 – 2.933] | 0.161 |
| *Day 0 High Shedders | 590 [150 – 1800] | 625 [280 – 2800] | 0.379 | 1.108 [0.432 – 2.842] | 0.829 |
| Ratio of Day 3 to Day 0 Copies/mL | 0.235 [0.055 – 1.585] | 0.231 [0.075 – 1.335] | 0.536 | 0.949 [0.478 – 1.884] | 0.88 |
| *Day 0 High Shedders | 0.074 [0.011 – 0.421] | 0.217 [0.071 – 0.667] | 0.058 | 0.473 [0.173 – 1.296] | 0.143 |
|
| |||||
| Day 14 | n=79 | n=85 | |||
|
| |||||
| Median Copies/mL | 360 [0 –1400] | 720 [200 –3200] | 0.059 | 0.983 [0.506 – 1.908] | 0.959 |
| *Day 0 High Shedders | 490 [160 –4600] | 1200 [310 –3900] | 0.243 | 0.899 [0.298 – 2.711] | 0.848 |
| Ratio of Day 14 to Day 0 Copies/mL | 0.264 [0.068 – 1.118] | 0.684 [0.115 – 3.260] | 0.058 | 0.615 [0.290 – 1.303] | 0.203 |
| *Day 0 High Shedders | 0.100 [0.019 – 0.490] | 0.355 [0.064 – 1.714] | 0.045 | 0.353 [0.107 to 1.173] | 0.088 |
| Ratio of Day 14 to Day 3 Copies/mL | 1.000 [0.244 to 4.848] | 1.000 [0.512 to 6.471] | 0.255 | 0.661 [0.331 to 1.323] | 0.241 |
| *Day 0 High Shedders | 1.000 [0.306 to 4.333] | 1.000 [0.447 to 4.792] | 0.709 | 0.833 [0.308 to 2.251] | 0.715 |
ANOVA and Linear Regression analysis adjusted for age, sex, and day 0 viral loads.
Subanalysis restricted to only those that were shedding at least 1000 copies/mL on day 0. High shedder cases N at day 3 = 37 and day 14 = 35. High shedder comparison N day 3 = 42 and N day 14 = 42.
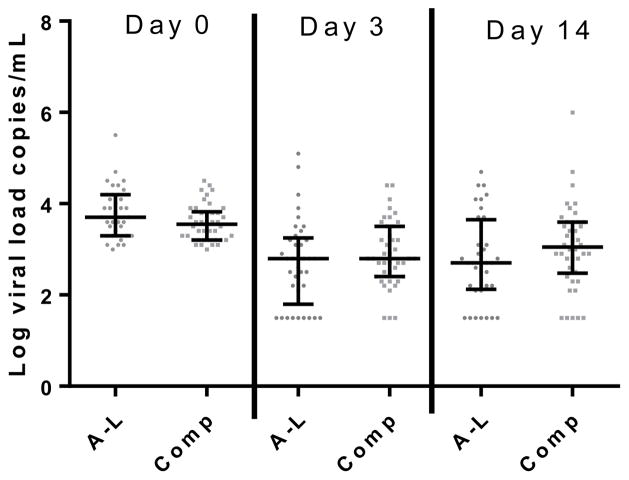
Viral loads varied widely during the study in both groups (Figure 2). The percentage of subjects with viral loads falling below the limit of detection during follow up were similar between the two groups, but a trend towards a greater percentage of cases dropping below the limit of detection at day 3 was found among the high shedders (p=0.06, Table 3).
Figure 2.
Changes in Viral Load Among Children Shedding >1000 copies/mL at Presentation
A-L: Artemether-Lumefantrine exposed group
Comp: Comparison Group, (not A-L exposed)
Error Bars represent Median and Interquartile Range
A-L N = 37, Comp N = 42
Table 3.
Percent of subjects converting to undetectable CMV levels during follow up among all subjects and among subgroup shedding >1000 or 3.0 log10 copies/mL at day 0
| All subjects CMV positive at Day 0 | High viral shedders at Day 0 | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Day of Follow and Group | Percent undetectable | SE | p (χ2 test) | Percent undetectable | SE | p (Fisher exact) | |
| Day 3 | Cases
|
31/81 (36%) | 5.3% | 0.39 | 9/38 (22%) | 6.8% | 0.06 |
| Comparisons | 26/88 (29%) | 4.9% | 3/42 (7%) | 4.0% | |||
|
| |||||||
| Day 14 | Cases
|
22/79 (28%) | 5.0% | 0.17 | 6/35 (17%) | 6.4% | 0.53 |
| Comparisons | 16/85 (19%) | 4.2% | 5/42 (12%) | 5.0% | |||
Left- results drawn from the total cohort. Right – children with viral shedding greater than 1000 copies/mL at day 0.
SE=Standard Error
DISCUSSION
The use of artemisinins for CMV therapy is attractive, since these agents are orally available, well-tolerated and safe (7). Thus, one might expect similar safety in treatment of CMV disease. These properties are important because prolonged therapy is required for CMV disease. A multi-center phase III trial comparing six weeks to six months of oral val-ganciclovir in newborns suggests that 6 months of therapy may be preferred to 6 weeks based on improved neurodevelopmental outcome (20, 21). Prolonged therapy carries the risk of adverse effects and may lead to emergence of drug resistant strains (6). Thus, additional drugs and consideration for combination therapy are both needed.
Our study reports two key findings. First, the short course of artemether-lumefantrine was associated with lower CMV shedding in the urine among children with high day 0 viral loads (greater than 1000 copies/mL or 3.0 log10) at follow up day 14. This is illustrated by a greater drop in median viral loads among cases from day 0 to day 14 and a trend towards lower DNA copy numbers at day 14 among cases. However, after adjustment for age and sex these observations did not meet our predetermined threshold of a statistical significance (p=0.088). These trends may suggest initial virus suppression by artemether-lumefantrine followed by sustained virus suppression by host immune responses. Notably, artemether-lumefantrine has a half-life of at most several hours with low levels of urinary excretion (22). Because of this, it is possible that the dosing and duration of therapy were inadequate for CMV therapy. Moreover, anti-CMV drugs generally require prolonged courses of therapy and it is not uncommon to see initial viral load spikes during therapy prior to observing viral suppression (23, 24). Finally, as with any observational trial, these findings need experimental studies to corroborate these data.
Gantt et. al did not observe a difference in their 3-day follow up study in which dried blood spots were used to evaluate viremia in patients receiving artemisinin combination therapy (ACTs) for malaria (17). Since viremia is likely uncommon in the normal host and typically at low levels, (only 11% of participants in that study were found to have low level viremia) changes in viral loads during artemisinin therapy may have been difficult to detect (25). Our study used urine specimens as opposed to dried blood spots, which may have improved the sensitivity for CMV detection and evaluation of trends in viral loads over time (26). The half-life of CMV in plasma is estimated to be 1.5 to 2 days (27), thus, we would not be able to observe the full effect of therapy by day 3 of follow up. The additional time point of day 14 allowed us to observe changes in viral loads after completion of therapy.
A limitation of our study is that that we were unable to have a control group of children with malaria receiving non-artemisinin derived therapy (such as sulfadoxine/pyrimethamine); or alternatively a comparable treatment group, i.e., CMV-shedding children without malaria who were treated with artemisinin. The standard of care for the treatment of malaria in Mali is ACT and alternative therapies are only used in cases of allergy or intolerance. Therefore, use of an alternative regimen would not have been appropriate in an observation trial. Furthermore, artemether-lumefantrine is not labeled for use in non-malaria patients. Thus, we are unable to definitively ascertain whether the trend towards lower CMV loads was a result of malaria parasite clearance or the drug treatment. While a higher percentage of children without malaria were shedding CMV than those with the disease (62% versus 49%); both groups of CMV positive children had no difference in the median viral load at the time of presentation. In addition, the number of high CMV shedders was similar between the groups. Thus, malaria on its own may not affect CMV shedding, and the effect observed at day 14 could be attributed to artemether-lumefantrine therapy.
An important secondary finding of our work was the unexpectedly high rate of CMV shedding in this population. Our unpublished prior work in Mali showed rates of shedding of approximately 30% in a different village with a similar aged cohort (6 months to 10 years). The Faladje cohort presented here had greater than 50% of subjects shedding CMV. Further investigations may help determine the role of CMV in this population.
In summary, children treated with artemether-lumefantrine had lower median viral loads at the 14-day follow up. We propose that further studies investigating longer courses and higher doses of artemisinins may help determine its role in CMV therapy.
Highlights.
CMV shedding is common in Mali, a malaria-endemic area
Most children have low level of CMV shedding in the urine
Artemisinin-based therapy for malaria may decrease CMV shedding in urine
Acknowledgments
We thank the people of the village of Faladje, Mali for their participation in this study and the physicians, nurses, and laboratory personnel who provided invaluable assistance with the study. Funding information: This study was funded by the Thrasher Foundation Career Development Grant to Breanna Barger-Kamate, the Johns Hopkins Center for Child and Community Health Research – BEAD Core, the National Institutes of Health training grant (T32 AI052071), and the Fogarty International Clinical Research Scholars and Fellows Program at Vanderbilt University (R24 TW007988) and the American Recovery and Reinvestment Act. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Conflict of Interest Statement: The authors in this paper have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lumbreras C, Manual O, Len O, ten Berge IJ, Sgarabotto D, Hirsch HH. Cytomegalovirus infection in solid organ transplant recipients. Clin Microbiol Infect. 2014;20(Suppl 7):19–26. doi: 10.1111/1469-0691.12594. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton ST, van Zuylen W, Shand A, Scott GM, Naing Z, Hall B, Craig ME, Rawlinson WD. Prevention of congenital cytomegalovirus complications by maternal and neonatal treatments: a systematic review. Rev Med Virol. 2014;24:420–433. doi: 10.1002/rmv.1814. [DOI] [PubMed] [Google Scholar]
- 3.Naing ZW, Scott GM, Shand A, Hamilton ST, van Zuylen WJ, Basha J, Hall B, Craig ME, Rawlinson WD. Congenital cytomegalovirus infection in pregnancy: a review of prevalence, clinical features, diagnosis and prevention. Aust N Z J Obstet Gynaecol. 2015 doi: 10.1111/ajo.12408. [DOI] [PubMed] [Google Scholar]
- 4.Mareri A, Lasorella S, Iapadre G, Maresca M, Tambucci R, Nigro G. Antiviral therapy for congenital cytomegalovirus infection: pharmacokinetics, efficacy and side effects. J Matern Fetal Neonatal Med. 2015 doi: 10.3109/14767058.2015.1058774:1-8. [DOI] [PubMed] [Google Scholar]
- 5.Tan BH. Cytomegalovirus Treatment. Curr Treat Options Infect Dis. 2014;6:256–270. doi: 10.1007/s40506-014-0021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakki M, Chou S. The biology of cytomegalovirus drug resistance. Curr Opin Infect Dis. 2011;24:605–611. doi: 10.1097/QCO.0b013e32834cfb58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, White N International Artemisinin Study G. Artesunate combinations for treatment of malaria: meta-analysis. Lancet. 2004;363:9–17. doi: 10.1016/s0140-6736(03)15162-8. [DOI] [PubMed] [Google Scholar]
- 8.Efferth T, Marschall M, Wang X, Huong SM, Hauber I, Olbrich A, Kronschnabl M, Stamminger T, Huang ES. Antiviral activity of artesunate towards wild-type, recombinant, and ganciclovir-resistant human cytomegaloviruses. J Mol Med (Berl) 2002;80:233–242. doi: 10.1007/s00109-001-0300-8. [DOI] [PubMed] [Google Scholar]
- 9.Efferth T, Romero MR, Wolf DG, Stamminger T, Marin JJ, Marschall M. The antiviral activities of artemisinin and artesunate. Clin Infect Dis. 2008;47:804–811. doi: 10.1086/591195. [DOI] [PubMed] [Google Scholar]
- 10.Arav-Boger R, He R, Chiou CJ, Liu J, Woodard L, Rosenthal A, Jones-Brando L, Forman M, Posner G. Artemisinin-derived dimers have greatly improved anti-cytomegalovirus activity compared to artemisinin monomers. PLoS One. 2010;5:e10370. doi: 10.1371/journal.pone.0010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He R, Sandford G, Hayward GS, Burns WH, Posner GH, Forman M, Arav-Boger R. Recombinant luciferase-expressing human cytomegalovirus (CMV) for evaluation of CMV inhibitors. Virol J. 2011;8:40. doi: 10.1186/1743-422X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He R, Park K, Cai H, Kapoor A, Forman M, Mott B, Posner GH, Arav-Boger R. Artemisinin-derived dimer diphenyl phosphate is an irreversible inhibitor of human cytomegalovirus replication. Antimicrob Agents Chemother. 2012;56:3508–3515. doi: 10.1128/AAC.00519-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mott BT, He R, Chen X, Fox JM, Civin CI, Arav-Boger R, Posner GH. Artemisinin-derived dimer phosphate esters as potent anti-cytomegalovirus (anti-CMV) and anti-cancer agents: a structure-activity study. Bioorg Med Chem. 2013;21:3702–3707. doi: 10.1016/j.bmc.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribeiro IR, Olliaro P. Safety of artemisinin and its derivatives. A review of published and unpublished clinical trials. Med Trop (Mars) 1998;58:50–53. [PubMed] [Google Scholar]
- 15.Shapira MY, Resnick IB, Chou S, Neumann AU, Lurain NS, Stamminger T, Caplan O, Saleh N, Efferth T, Marschall M, Wolf DG. Artesunate as a potent antiviral agent in a patient with late drug-resistant cytomegalovirus infection after hematopoietic stem cell transplantation. Clin Infect Dis. 2008;46:1455–1457. doi: 10.1086/587106. [DOI] [PubMed] [Google Scholar]
- 16.Germi R, Mariette C, Alain S, Lupo J, Thiebaut A, Brion JP, Epaulard O, Saint Raymond C, Malvezzi P, Morand P. Success and failure of artesunate treatment in five transplant recipients with disease caused by drug-resistant cytomegalovirus. Antiviral Res. 2014;101:57–61. doi: 10.1016/j.antiviral.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Gantt S, Huang ML, Magaret A, Bunts L, Selke S, Wald A, Rosenthal PJ, Dorsey G, Casper C. An artesunate-containing antimalarial treatment regimen did not suppress cytomegalovirus viremia. J Clin Virol. 2013;58:276–278. doi: 10.1016/j.jcv.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka N, Kimura H, Iida K, Saito Y, Tsuge I, Yoshimi A, Matsuyama T, Morishima T. Quantitative analysis of cytomegalovirus load using a real-time PCR assay. J Med Virol. 2000;60:455–462. doi: 10.1002/(sici)1096-9071(200004)60:4<455::aid-jmv14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 19.He R, Forman M, Mott BT, Venkatadri R, Posner GH, Arav-Boger R. Unique and highly selective anticytomegalovirus activities of artemisinin-derived dimer diphenyl phosphate stem from combination of dimer unit and a diphenyl phosphate moiety. Antimicrob Agents Chemother. 2013;57:4208–4214. doi: 10.1128/AAC.00893-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimberlin DW, Aban I, Acosta EP. Valganciclovir for Congenital Cytomegalovirus. N Engl J Med. 2015;372:2463. doi: 10.1056/NEJMc1504937. [DOI] [PubMed] [Google Scholar]
- 21.Kimberlin DW, Jester PM, Sanchez PJ, Ahmed A, Arav-Boger R, Michaels MG, Ashouri N, Englund JA, Estrada B, Jacobs RF, Romero JR, Sood SK, Whitworth MS, Abzug MJ, Caserta MT, Fowler S, Lujan-Zilbermann J, Storch GA, DeBiasi RL, Han JY, Palmer A, Weiner LB, Bocchini JA, Dennehy PH, Finn A, Griffiths PD, Luck S, Gutierrez K, Halasa N, Homans J, Shane AL, Sharland M, Simonsen K, Vanchiere JA, Woods CR, Sabo DL, Aban I, Kuo H, James SH, Prichard MN, Griffin J, Giles D, Acosta EP, Whitley RJ National Institute of A, Infectious Diseases Collaborative Antiviral Study G. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med. 2015;372:933–943. doi: 10.1056/NEJMoa1404599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anonymous. Coartem [drug insert] Novartis Pharmaceuticals Corporation; East Hanover, New Jersey 07936: 2015. [Google Scholar]
- 23.Buyck HC, Griffiths PD, Emery VC. Human cytomegalovirus (HCMV) replication kinetics in stem cell transplant recipients following anti-HCMV therapy. J Clin Virol. 2010;49:32–36. doi: 10.1016/j.jcv.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Nichols WG, Corey L, Gooley T, Drew WL, Miner R, Huang M, Davis C, Boeckh M. Rising pp65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood. 2001;97:867–874. doi: 10.1182/blood.v97.4.867. [DOI] [PubMed] [Google Scholar]
- 25.La Rosa C, Diamond DJ. The immune response to human CMV. Future Virol. 2012;7:279–293. doi: 10.2217/fvl.12.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boppana SB, Ross SA, Novak Z, Shimamura M, Tolan RW, Jr, Palmer AL, Ahmed A, Michaels MG, Sanchez PJ, Bernstein DI, Britt WJ, Fowler KB National Institute on D, Other Communication Disorders CMV, Hearing Multicenter Screening S. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA. 2010;303:1375–1382. doi: 10.1001/jama.2010.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emery VC, Cope AV, Bowen EF, Gor D, Griffiths PD. The dynamics of human cytomegalovirus replication in vivo. J Exp Med. 1999;190:177–182. doi: 10.1084/jem.190.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]