Abstract
Background
Apolipoprotein E (ApoE) mediates potent anti-inflammatory and immunomodulatory properties in addition to its roles in regulating cholesterol transport and metabolism. However, its role in the intestine, specifically during inflammation is largely unknown.
Methods
Mice [C57BL/6 or ApoE deficient (ApoE-KO) mice] were administered either single or four injections (weekly) of anti-interleukin (IL)-10 receptor monoclonal antibody (1.0 mg/mouse; intraperitoneally) and euthanized one week after the last injection. 16S rRNA sequencing was performed in fecal samples to analyze the gut bacterial load and its composition. Microbiota was ablated by administration of broad-spectrum antibiotics in drinking water. IL-10KO mice were cohoused with ApoE-KO mice or their WT littermates to monitor the colitogenic potential of gut microbiota harbored in ApoE-KO mice.
Results
ApoE-KO mice developed severe colitis upon neutralization of IL-10 signaling as assessed by every parameter analyzed. 16S rRNA sequencing revealed that the ApoE-KO mice display elevated and altered gut microbiota that were accompanied with impaired production of intestinal antimicrobial peptides. Interestingly, microbiota ablation ameliorates the colitis development in ApoE-KO mice. Exacerbated and accelerated colitis was observed in IL-10KO mice when cohoused with ApoE-KO mice.
Conclusions
Our study highlights a novel interplay between ApoE and IL-10 in maintaining gut homeostasis and that such cross-talk may play a critical role in inflammatory bowel disease (IBD) pathogenesis. Gut sterilization and cohousing experiment suggests that microbiota play pivotal role in the development of IBD in mice lacking ApoE.
Keywords: ApoE, IL-10 receptor, intestinal inflammation, pro-inflammatory cytokines, gut epithelial permeability
Introduction
The host-gut microbiota metabolism and inflammatory pathways are highly inter-regulated whereas, the loss of this balance leads to the development of chronic inflammatory disorders or tissue pathology (1-3). Among such diseases, the inflammatory bowel diseases (IBD), encompassing Crohn’s disease (CD) (4) and ulcerative colitis (UC) (5), are currently estimated to affect more than 1-1.3 million people in the United States (6, 7). IBD are widely believed to be due to the breakdown of tolerance to commensal microbiota, resulting in dysregulated immune cell infiltration into the mucosa that drives intestinal pathology. The persistent secretion of pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, IL-8 and tumor necrosis alpha (TNFα) into the inflamed gut further exacerbate the disease (8, 9). Current strategies to treat IBD that involve the use of TNFα inhibitor (infliximab) (10) have been met with limited efficacy which are further complicated with undesired side effects (11, 12). Hence, there is a need for alternative therapeutic targets or strategies in treating IBD.
Apolipoprotein E (ApoE) is a 34-kDa secreted protein that belonged to the apolipoprotein superfamily whose members are involved in regulating cholesterol homeostasis and lipid metabolism (13). ApoE is primarily synthesized in the liver and macrophages, but is also produced in the brain, spleen, kidney, lung and muscle tissues as well (14, 15). It participates in the transport of plasma lipids by binding to very low-density lipoprotein (VLDL) and chylomicrons (13). As ApoE is also the ligand for the low-density lipoprotein (LDL) receptor, it plays an important role in lowering circulating cholesterol by facilitating its uptake by the liver (13). Due to its anti-atherogenic properties, ApoE deficient (ApoE-KO) mice develop spontaneous atherosclerosis and had become one of the widely utilized mouse model to study atherosclerosis.
In recent years, studies have begun to unravel a perhaps under-appreciated anti-inflammatory role of ApoE in the regulation of immune cells and their responses. In addition to its role in lipid metabolism, ApoE was shown to mediate the suppression of T cell proliferation (16) and maintain Th1/Th2 balance (17). Furthermore, ApoE modulates macrophage polarization, by favoring the establishment of alternatively activated anti-inflammatory M2 macrophages over the pro-inflammatory M1 macrophages (18). ApoE also counter-regulates the systemic upregulation of pro-inflammatory cytokines IL-6, IL-12, TNFα and interferon-gamma (IFNγ) in response to lipopolysaccharide (LPS) and polyinosine-polycytidylic acid challenge, which respectively activates the toll-like receptor (TLR)-4 and TLR3 responses in mice (19). These studies suggest that ApoE can be regarded as part of the innate immunity and may play important role in regulating inflammatory responses. Indeed, the loss of ApoE in mice increases their susceptibility to develop TLR4- and MyD88-dependent lung lipidosis and inflammation, suggesting the extrahepatic role of ApoE in tissue homeostasis (20). However, the roles of ApoE in intestinal homeostasis and inflammation are largely unknown and thus warrant further study.
Interleukin 10 (IL-10) is a pleiotropic anti-inflammatory cytokine that display inhibitory effects on the functions of TH1 cells, NK cells and macrophages (21). In the gut, IL-10 is produced by various immune cell types including T cells, B cells, macrophages, mast cells, NK cells, eosinophils and neutrophils (22). IL-10 is required to maintain immune tolerance in the gut and prevent unregulated/aberrant inflammatory responses and their consequent tissue damage (21, 23). This cytokine mediates its effect through binding to the IL-10 receptor whose downstream signaling inhibits NF-κB-dependent pathways and pro-inflammatory gene expression (24). The deficiency in intestinal IL-10 production, however, is known to disrupt gut homeostasis and potentiates the development of intestinal inflammation in a MyD88-dependent fashion (25, 26). Deletion of IL-10 in ApoE-KO mice exacerbate atherosclerosis (17), but it is not known whether IL-10/ApoE double knockout mice also succumb to IBD.
Herein, we investigated the interplay between intestinal ApoE and IL-10 and the extent to which the systemic subclinical inflammation due to dyslipidemia in ApoE-KO mice culminates into a robust chronic colitis upon breakdown of intestinal homeostasis. In the current study, we report that ApoE-KO mice are highly susceptible to the development of colitis upon neutralization of IL-10 signaling via the administration of anti-IL-10 receptor monoclonal antibody (αIL-10R). We further demonstrate that the colitis in αIL-10R-treated ApoE-KO mice are associated to a microbiotal dysbiosis that could have resulted from the loss of colonic ApoE. Collectively, our study highlights a novel role of ApoE in maintaining gut-microbiota homeostasis and the prevention of gut inflammation in an aberrant immune activation-induced colitis model.
Materials and Methods
Reagents
Rat anti-mouse IL-10 receptor (IgG1) monoclonal antibody (αIL-10R) and rat anti-mouse PD-1 monoclonal antibody were purchased from BioXcell (West Lebanon, NH). Hexadecyltrimethylammonium bromide, hydrogen peroxide, human neutrophil myeloperoxidase (MPO), lipopolysaccharide (LPS), RNAlater®, TRI Reagent® were purchased from Sigma (St. Louis, MO). Guaiacol (2-methoxyphenol) was obtained from Alfa Aesar (Ward Hill, MA). Mouse lipocalin 2 (Lcn2) and keratinocyte-derived chemokine CXCL1 (KC) duoset ELISA kits were procured from R&D Systems (Minneapolis, MN). SYBR® Green mix was procured from Quanta Biosciences (Gaithersburg, MD).
Mice
ApoE-KO, IL-10 deficient (IL-10KO) and stearoyl CoA desaturase-1 deficient (SCD1-KO) mice on C57BL/6 background were purchased from Jackson Laboratories. Toll-like receptor 5 deficient (TLR5-KO) mice were originally generated by Dr. Shizuo Akira, Japan. These mice were bred with wildtype (WT) C57BL/6 mice and the resulting offsprings were crossed to generate homozygous ApoE-KO, IL-10KO, SCD1-KO, TLR5-KO mice and their WT littermates. Mice were maintained in specific-pathogen free conditions in the animal house facility at Pennsylvania State University, PA. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Pennsylvania State University.
Dextran sodium sulfate (DSS)- and 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis
DSS- and TNBS-induced colitis were induced in eight-week-old male C57BL6 mice as described in Chassaing et al (27), and Scheiffele and Fuss (28), respectively. For DSS-induced colitis, mice were treated with 1.8% of DSS (MP Biomedical) in drinking water and euthanized at day 6 of treatment. For TNBS-induced colitis mice were administered TNBS (4mg/mouse prepared in 50% ethanol) intra-rectally using 3.5-French 38-cm, polyurethane catheter. Control mice received vehicle (50% ethanol) alone. After 3 days mice were euthanized to monitor colitis development.
IL-10R neutralization-induced colitis in mice
IL-10R neutralization-induced colitis was induced in ApoE-KO mice and their WT littermates as previously described (29). For the induction of acute colitis, eight-week-old male ApoE-KO mice and WT littermates (n=4) were administered single injection of αIL-10R (1.0 mg/mouse, intraperitoneally) and euthanized one week later. For the induction of chronic colitis, four-week-old ApoE-KO mice and WT littermates (n=4) were given four weekly intraperitoneal injections of αIL-10R and euthanized one week after the 4th injection. Control mice were given isotype control (anti-PD-1) antibody. Mice were monitored for body weights every two days. In some experiment, mice were maintained on a broad spectrum antibiotic cocktail (1.0 g/L ampicillin and 0.5 g/L neomycin) in drinking water two weeks before the first αIL-10R injection and maintained throughout duration of the study.
Cohousing
Four-week-old female IL-10KO mice (n=4) were cohoused with eight-week-old female ApoE-KO mice (n=4) or their WT littermates up to 6 weeks. Mice were constantly monitored for the onset of colitis by monitoring body weight, fecal occult blood, diarrhea and rectal prolapse.
Euthanasia and blood collection
At termination of experiment, mice were euthanized via CO2 asphyxiation and analyzed for standard colitic parameters as described previously (30). Blood was collected at the time of mice euthanasia in BD microtainer® (Becton, Dickinson, Franklin Lakes, NJ), via retro-orbital plexus. Hemolysis-free sera was obtained after centrifugation and stored at −80°C until further used.
Colonic myeloperoxidase (MPO) assay
MPO assay was performed according to the previously described method (31). Briefly, frozen or fresh colon tissue (50 mg) was homogenized in 1 mL of 50 mM potassium phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide, freeze-thawed 3x, sonicated, centrifuged (10000g, 4°C) and the clear supernatants were collected. The reaction was initiated by adding final concentrations of 50 mM guaiacol and 0.002% H2O2 to the clear supernatant prepared in 96-well plate (Corning). The change in absorbance at 470 nm was measured over a period of 10 minutes at 1 minute intervals. One unit of MPO activity is defined as the amount that increases absorbance at 470 nm by OD of 1.0 per minute at 25°C, calculated from the initial rate of reaction using guaiacol as the substrate.
Enzyme-linked immunosorbent assay (ELISA)
Serum Lcn2 and KC were measured by ELISA kits according to the manufacturer’s protocol. Frozen or freshly collected feces were reconstituted (100 mg/mL) in PBS containing 0.1% Tween 20, vortexed (30 min, room temperature) and centrifuged (10,000g, 4°C) to collect clear supernatants for fecal Lcn2 quantification as described previously (32). To monitor the systemic immunoreactivity to flagellin and LPS, serum samples were diluted (1:200) and analyzed by ELISA as described by Ziegler et al (33).
Quantitative RT-PCR
Mouse distal colons were collected in RNAlater® and stored in −80°C. Total colonic RNA was extracted using TRI Reagent® as per manufacturer's protocol. Purified RNA was used to synthesize cDNA for qRT-PCR using SYBR® Green mix according to the manufacturer's protocol. qRT-PCR was performed in StepOnePlusTM real time PCR instrument (Life technologies, Grand Island, NY). Sequence of primers used for qRT-PCR were (sense and antisense respectively): ApoE 5'-ACAGATCAGCTCGAGTGGCAAA-3’ and 5’-ATCTTGCGCAGGTGTGTGGAGA-3’; Lcn2 5’-AAGGCAGCTTTACGATGTACAGC-3’ and 5’-CTTGCACATTGTAGCTGTGTACC-3’; IFNγ 5’- TCAAGTGGCATAGATGTGGAAGAA-3’ and 5’-TGGCTCTGCAGGATTTTCATG-3’; TNFα 5’- ACTCCAGGCGGTGCCTATGT-3’ and 5’-AGTGTGAGGGTCTGGGCCAT-3’; IL-4 5’-TCGGCATTTTGAACGAGGTC-3’ and 5’-GAAAAGCCCGAAAGAGTCTC-3’; IL-10 5’ATTTGAATTCCCTGGGTGAGAAG-3’ and 5’-CACAGGGGAGAAATCGATGACA-3’; 36B4 5’- TCCAGGCTTTGGGCATCA and 5’-CTTTATTCAGCTGCACATCACTCAGA-3’. Thermal profile for the reaction was: initial denaturation at 95°C for 10 min, and 40 cycles of denaturation (95°C for 15 s) and annealing and extension (60°C for 1 min). Relative fold difference between groups was calculated using comparative Ct (2−ΔΔCt) method. Results obtained were normalized with the housekeeping 36B4 gene.
Histology
Colons were washed with cold PBS and opened longitudinally to make Swiss rolls. Subsequently Swiss rolls were transferred into 10% buffered formalin (Fisher Scientific) for 24 hours at room temperature. Paraffin embedding, slides preparation and hematoxylin & eosin (H&E) staining were performed at Animal Diagnostics Laboratories at the Pennsylvania State University using standard protocols. Histologic scoring was performed as described previously (34).
Total fecal microbiotal load
Total bacterial DNA was isolated from weighted feces using QIAamp DNA Stool Mini Kit (Qiagen). After 1/10 dilution, DNA was subjected to quantitative PCR using Quanti Fast SYBR Green PCR kit (Biorad) with universal 16S rRNA primers 8F: 5'-AGAGTTTGATCCTG GCTCAG-3' and 338R: 5'-CTGCTGCCTCCCGTAGGAGT-3' to measure total bacteria. Results were expressed as bacteria number per mg of stool, using a standard curve.
Sample collection and DNA isolation for 16S rRNA gene pyrosequencing
Fecal pellets from age and sex-matched ApoE-KO and WT littermates were collected under hygienic conditions and stored in sterile vials at −20°C for processing. DNA was extracted from 0.50g fecal material using the MO-BIO PowerSoil® DNA isolation kit (MO-BIO Laboratories, Carlsbad, California) according to the manufacturer’s instructions. DNA concentration was analyzed using the Qubit® 2.0 Fluorometer and related high-sensitivity double-stranded DNA kit, according to the manufacturer’s instructions (Life Technologies, New York, United States).
Illumina Tag PCR
DNA isolates were subject to duplicate 25 uL Illumina tag Polymerase Chain Reactions (PCR) to amplify the V4 region of the 16S rRNA gene. Each reaction contained final concentrations of 1X PCR buffer, 0.8uM dnTPs, 4uM 515F Illumina barcoded forward primers, 4 uM 806R reverse primers, 0.25 U Taq Polymerase, and 10 ng of template DNA. PCR was performed using the PTC-200 Thermocycler (MJ Research Incorporation, Massachusetts, United States). Reactions were held at 94°C for 3 minutes to allow for the DNA to denature, followed by 35 cycles at 94°C for 45s, 50°C for 60s, and 72°C for 90s, with a final extension time of 10 min at 72°C followed by holding at 4°C. PCR products were visualized on a 2% agarose E-gel (Life Technologies, Carlsbad, CA).
Library Preparation and Sequencing
The DNA concentration of successful PCR reaction products was analyzed using the Qubit® 2.0 Fluorometer, and equal-molar amounts of each PCR product were pooled and SPRI-bead purified using the Agencourt AMPure XP-PCR Purification Kit according to the manufacturer’s instructions (Beckman Coulter, Indiana, United States). Cleaned, pooled libraries were quality checked using the Agilent 2100 Bioanalyzer and the related Agilent High Sensitivity DNA Chip, according to the manufacturer’s instructions (Agilent Technologies, California, United States).
Pooled libraries were stored at −20°C until they were shipped on dry ice to the University of Tennessee-Knoxville (Knoxville, TN) for sequencing. Library pools were size verified using the Fragment Analyzer CE (Advanced Analytical Technologies Inc., Ames IA) and quantified using the Qubit high sensitivity dsDNA kit (Life Technologies, Carlsbad, CA). After dilution to a final concentration of 1nM containing 10% PhiX V3 library control (Illumina, San Diego CA), the library pools were denatured for 5 min in an equal volume of 0.1N NaOH, further diluted to 12 pM in HT1 buffer (Illumina) and sequenced using Illumina MiSeq V2 300 cycle kit cassette with 16S rRNA library sequencing primers and set for 150 base, paired-end reads.
Organ ex vivo culture
Two cm sections of mice ileum (10 cm above the cecum) were collected and cultured in serum-free DMEM media (Sigma) supplemented with 1% penicillin-streptomycin (Sigma). After two washes with sterile PBS, ileum was transferred to 12-well culture plate (Corning) containing 1 mL of serum-free DMEM media with 1% penicillin-ptreptomycin and incubated for 24 hours at 37°C in CO2 incubator. The cultures were then centrifuged (10000g, 4°C) to collect clear supernatant for ELISA and immunoblotting.
Immunoblotting
Ileal culture supernatants prepared in loading buffer were fractionated by 4-20% SDS-PAGE (Bio-Rad) and transferred to PVDF membrane (Bio-Rad). Next, PVDF membrane was stained with Ponceau S solution (Sigma) to confirm equal loading of samples and then washed with H2O. The blots were blocked for 1 hour in 5% non-fat milk (Bio-Rad) at room temperature and then incubated overnight at 4°C with rabbit anti-mouse Ang4 or RegIIIγ (antibodies are kind gift from Dr. Lora Hooper, UT Southwestern Medical Center). After 3 washes, blots were incubated with anti-rabbit HRP (Cell Signaling Technology) and developed by chemiluminiscent reagent.
Statistical Analysis
All values in the results are expressed as mean ± SEM. Statistical analysis for significance between two groups was determined using student’s t-test (unpaired, two-tailed) with p<0.05 (*) considered as significant. GraphPad Prism 6.0 software was used to calculate statistical significance.
Results
Colonic ApoE is elevated in various murine model of colitis
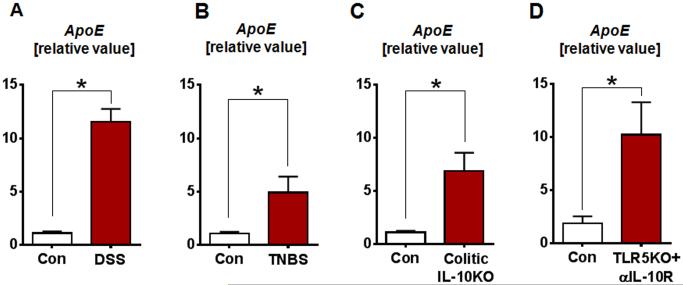
In recent years, studies have begun to elucidate a previously under-appreciated anti-inflammatory role of ApoE, highlighting the interrelation between lipid metabolism and inflammation (16-20). However, the role of ApoE in the regulation of inflammation, especially in the gut, has yet to be demonstrated. In this study, we first evaluated the expression of colonic ApoE in different murine models of colitis. ApoE expression was found to be significantly upregulated in the colon of DSS-induced and TNBS-induced colitic WT mice (Fig. 1A-B). Similarly, colonic ApoE expression was also elevated in spontaneously colitic IL-10KO mice with rectal prolapse (Fig. 1C). Besides that, colonic ApoE expression was also found to be considerably upregulated in IL-10R neutralization-induced colitic TLR5-KO mice (Fig. 1D). The upregulation of ApoE expression in the colon was consistently observed across multiple models colitis, suggesting that perhaps ApoE could play a yet unknown role in maintaining gut homeostasis.
FIGURE 1. Colonic ApoE transcripts are upregulated in various murine model of colitis.
qRT-PCR analysis was used to quantify mRNA levels of ApoE in the colon of (A) DSS-induced colitic WT mice, (B) TNBS-induced colitic WT mice, (C) spontaneously colitic IL-10KO mice and (D) αIL-10R-induced colitic TLR5-KO mice. mRNA values are represented as fold change normalized to 36B4 housekeeping gene and compared to the control group. Results presented as mean ± SEM. *p< 0.05.
Single dose of αIL-10R administration induces acute colitis in ApoE-KO mice
To further evaluate the role of ApoE in gut homeostasis, we employed the ApoE-KO mice in our study. Previously, we have described the feasibility of using IL-10R neutralization to induce colitis in genetically-susceptible mice (29). Hence, we asked whether ApoE-KO mice are susceptible to IL-10R neutralization-induced colitis by administering a single injection of αIL-10R followed by euthanasia one week later. Remarkably, αIL-10R-treated ApoE-KO mice developed classical symptoms of acute colitis as characterized by splenomegaly, colomegaly and increased neutrophil infiltration in the mucosa as measured by the colonic MPO activity (Fig. 2A-C). The severity of colitis in ApoE-KO mice was reflected in the increased levels of serum KC and Lcn2 (Fig. 2D-E). Histological analysis confirms that ApoE-KO mice developed colitis with increased immune cell infiltration in the colon (Fig. 2F). In contrast, WT mice displayed modest to no elevation in the above colitic parameters.
FIGURE 2. Single αIL-10R injection induces acute colitis in ApoE-KO mice.
ApoE-KO mice and WT littermates (n=4) were treated with a single injection of αIL-10R (1 mg/mouse, intraperitoneally) and euthanized one week later. The following colitis parameters were analyzed: (A) spleen weight, (B) colon weight, (C) colonic MPO activity, (D) serum KC, (E) serum Lcn2 and (F) histology of H&E-stained colon. Results presented as mean ± SEM. *p< 0.05.
Although αIL-10R treatment induced colitis development, the specificity of such phenotype may not be limited to only ApoE-KO mice. To rule out the possible non-specificity of our study approach, we tested αIL-10R on stearoyl-CoA desaturase deficient (SCD1-KO) mice. SCD-1 is known to be an obesogenic enzyme that synthesizes monounsaturated fatty acids palmitoleate and oleate (35). However, IL-10 neutralization did not induce any overt signs of colitis in SCD1-KO mice (Supplementary Fig. 1), which confirms that the αIL-10R-induced colitic phenotype is specific to ApoE-KO mice.
Multiple doses of αIL-10R administration induces chronic colitis in ApoE-KO mice
Considering that human IBD are mostly characterized as chronic disease of the intestines, we therefore proceed to evaluate the susceptibility of ApoE-KO mice in a chronic colitis model. Four weekly injections of αIL-10R induced robust chronic colitis in ApoE-KO mice, characterized by the gross phenotype, lack of body weight gain, splenomegaly and colomegaly (Fig. 3A-D). The indicators of acute colitis such as the colonic MPO activity and serum KC of αIL-10R-treated ApoE-KO mice are low and comparable to the control mice (Fig. 3E-F), indicating that the gut inflammation had established a chronic state known to be characterized by MPO-negative immune cells (i.e. T cells and macrophages). Nonetheless, αIL-10R-treated ApoE-KO mice exhibit highly elevated serum, fecal and colonic Lcn2 (Fig. 3G-I), a sensitive biomarker of gut inflammation (32). Additionally, the expression of pro-inflammatory genes IFNγ and TNFα were also significantly elevated in colitic ApoE-KO mice (Fig. 3J-K). The expression of the anti-inflammatory IL-4 and IL-10 mRNA were also elevated in αIL-10R-treated ApoE-KO mice (Fig. 3L-M), probably as a protective response against the loss of functional IL-10R. Histological analysis of mice colon confirmed the increased susceptibility of ApoE-KO mice to αIL-10R-induced chronic colitis as characterized by enhanced thickening of the mucosa (Fig. 3N-O). Consistent with our earlier observations, WT mice are resistant to IL-10R neutralization-induced chronic colitis; WT mice did not display significant changes in the analyzed colitic parameters, except in fecal Lcn2 in response to αIL-10R treatment.
FIGURE 3. Multiple injections of αIL-10R induce chronic colitis in ApoE-KO mice.
ApoE-KO mice and WT littermates (n=4) were treated with four weekly injections of αIL-10R (1 mg/mouse, intraperitoneally) and euthanized one week later. The following colitis parameters were analyzed: (A) gross colon, (B) body weight changes, (C) spleen weight, (D) colon weight, (E) colonic MPO activity, (F) serum KC, (G) serum Lcn2 and (H) fecal Lcn2. qRT-PCR analysis was used to quantify mRNA expression of colonic (I) Lcn2, (J) IFNγ, (K) TNFα, (L) IL-4 and (M) IL-10. mRNA values are represented as fold change normalized to 36B4 housekeeping gene and compared to the control group. (N) Histology images of H&E-stained colons and the corresponding (O) histological scores. Arrows indicate thickened mucosa. Results presented as mean ± SEM. *p< 0.05.
ApoE-KO mice harbor elevated microbiota load and altered composition
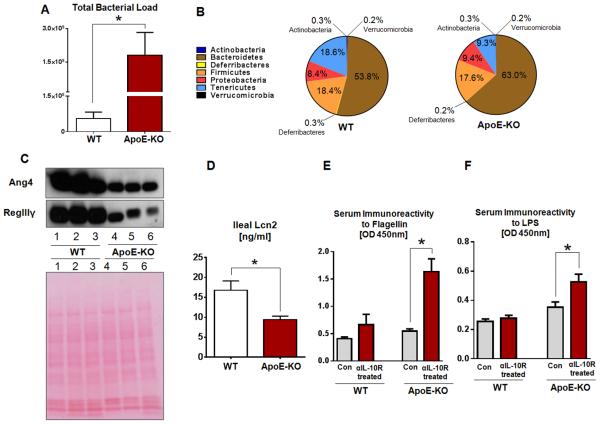
IL-10 is known to play central role in the maintenance of gut homeostasis whereby its deficiency predisposes the development of microbiota-dependent spontaneous colitis in IL-10KO mice (25, 36). As ApoE-KO mice were highly susceptible to IL-10R neutralization-induced colitis, we hypothesized that ApoE may play an equally important role in modulating the gut-microbiota homeostasis. To confirm this hypothesis, we performed fecal bacteria 16S rRNA sequencing to evaluate the gut microbiota in ApoE-KO mice. Interestingly, ApoE-KO mice were found to harbor an elevated gut bacterial load that is approximately 20-fold more compared to their WT littermates (Fig. 4A). Furthermore, ApoE-KO mice exhibit an altered microbiotal composition at the phyla level with a notable increase in the relative abundance of Bacteroidetes (~9.2% more) and a concomitant decrease in Tenericutes (~9.3% less) (Fig. 4B).
FIGURE 4. ApoE-KO mice exhibit gut microbiotal dysbiosis.
Mice fecal samples were collected from healthy ApoE-KO mice and WT littermates (n=4) for microbiota analysis by qRT-PCR and 16S rRNA sequencing. (A) Total fecal microbiota load. (B) Fecal bacteria composition. Sections of mice ileum were collected and cultured in serum-free DMEM media ex vivo. (C) Immunoblot of Ang4 and RegIIIγ in the supernatant of ileal cultures (upper panel) and Ponceau S staining as loading control (lower panel). (D) Ileal secretion of Lcn2 quantified by ELISA. Serum immunoreactivity to (E) flagellin and (F) LPS were quantified by ELISA. Results presented as mean ± SEM. *p< 0.05.
To evaluate the host response to intestinal microbiotal dysbiosis in ApoE-KO mice, we collected ileal sections from healthy mice, cultured them in serum-free DMEM media for 24 hours and measured the secretion of two potent antimicrobial peptides secreted by intestinal paneth cells: angiogenin 4 (Ang4; a ribonuclease) (37) and regenerating islet-derived 3 gamma (RegIIIγ; a C-type lectin) (38). We found ileal secretion of both Ang4 and RegIIIγ were substantially reduced in ApoE-KO mice compared to WT littermates (Fig. 4C). As shown in Figure 4D, the ileal sections from ApoE-KO mice also secreted a much reduced levels of Lcn2 that is also known for its antimicrobial properties in the gut (39). To assess the epithelial permeability or the extent to which gut luminal contents/microbial products disseminated to systemic circulation, we monitored the mice serum immunoreactivity to flagellin and LPS by ELISA. ApoE-KO mice displayed increased serum immunoreactivity to bacterial product flagellin and lipopolysaccharide (LPS) compared to WT mice, indicating increased dissemination of bacterial products across the gut mucosa which become more pronounced when mice were treated with αIL-10R (Fig. 4E-F).
Microbiota ablation protects ApoE-KO mice from IL-10R neutralization-induced chronic colitis
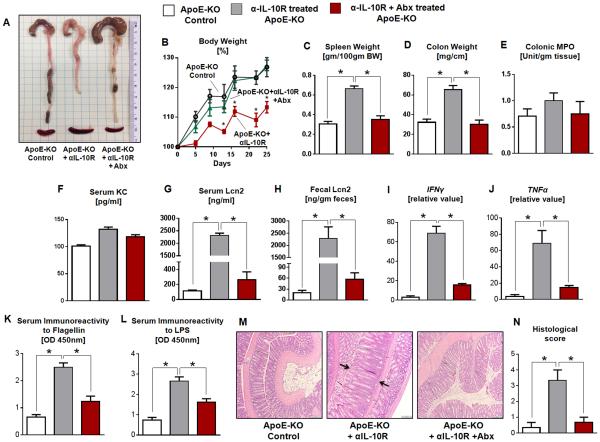
Having established that ApoE-KO mice are susceptible to gut microbiotal dysbiosis, we next asked whether microbiota ablation could prevent the development of αIL-10R-induced chronic colitis in these mice. ApoE-KO mice and their littermates receiving four weekly injections αIL-10R were administered a broad spectrum antibiotic cocktail (1.0 g/L ampicillin; 0.5 g/L neomycin) in the drinking water two weeks before the first injection and maintained throughout the duration of the study. This broad-spectrum antibiotics regimen (Abx) ablated 90% of the intestinal bacteria as measured by fecal 16S rRNA via qRT-PCR (data not shown). Interestingly, the Abx+αIL-10R-treated ApoE-KO mice were able to gain body weight normally over time and were significantly protected from developing colitis as evident by the lack of spleen enlargement and colon thickening (Fig. 5A-D). Other standard colitis parameters including colonic MPO activity, serum KC, serum Lcn2 and fecal Lcn2 of Abx+αIL-10R-treated ApoE-KO mice were comparable to the control group (Fig. 5E-H). The levels of colonic IFNγ and TNFα mRNA in Abx+αIL-10R-treated ApoE-KO mice were significantly mitigated compared to their counterparts without antibiotics treatment (Fig. 5I-J). Further, serum immunoreactivity to flagellin and LPS in Abx+αIL-10R-treated were substantially reduced (Fig. 5K-L). Histological analysis further confirmed that the colons from microbiota-ablated ApoE-KO mice were significantly protected from αIL-10R-induced thickening of the mucosa (Fig. 5M-N).
FIGURE 5. Antibiotics treatment ameliorates αIL-10R-induced chronic colitis in ApoE-KO mice.
ApoE-KO mice and WT littermates (n=4) were treated with or without antibiotics (1.0 g/L ampicillin and 0.5 g/L neomycin) in drinking water starting at two weeks before the first of the four weekly injections of αIL-10R and maintained throughout the duration of the study. The following colitis parameters were analyzed: (A) gross colon, (B) body weight changes, (C) spleen weight, (D) colon weight, (E) colonic MPO activity, (F) serum KC, (G) serum Lcn2 and (H) fecal Lcn2. qRT-PCR analysis was used to quantify mRNA expression of colonic (I) IFNγ, (J) TNFα. mRNA values are represented as fold change normalized to 36B4 housekeeping gene and compared to the control group. Serum immunoreactivity to (K) flagellin and (L) LPS were quantified by ELISA. (M) Histology images of H&E-stained colons and the corresponding (N) histological scores. Arrows indicate thickened mucosa. Results presented as mean ± SEM. *p< 0.05.
Cohousing with ApoE-KO mice exacerbated spontaneous colitis in IL-10KO mice
Our results suggest that ApoE-KO mice display gut microbiotal dysbiosis and harbor potentially colitogenic microbiota. To confirm that the gut microbiota in ApoE-KO mice was responsible for their higher susceptibility to colitis, we cohoused IL-10KO mice with either WT or ApoE-KO mice (cohoused IL-10KO mice are hereafter named as KO/CoH-WT and KO/CoH-ApoE-KO). Since mice are coprophagic in nature, the sharing of cages would allow the transmission of the colitogenic microbiota between cohoused mice. After four weeks of cohousing, 50% of the KO/CoH-ApoE-KO mice developed rectal prolapse, a severe irreversible form of colitis. KO/CoH-ApoE-KO mice also developed classical symptoms of colitis including lack of body weight gain, splenomegaly and colomegaly (Fig. 6A-D). Serum and fecal Lcn2 was significantly elevated in KO/CoH-ApoE-KO mice, but no detectable difference was observed in the levels of serum KC and colonic MPO activity, indicating the chronic form of colitis (Fig. 6E-H). In comparison, no overt colitic phenotypes were observed in KO/CoH-WT mice.
FIGURE 6. ApoE-KO mice harbors a colitogenic microbiota that is transmissible to IL-10KO mice.
Four-week-old female IL-10KO mice (n=4) and eight-week-old female ApoE-KO mice (n=4) were cohoused for 4-6 weeks. At the end of six weeks (or when mice developed rectal prolapse), IL-10KO mice were euthanized and the following colitis parameters were analyzed: (A) gross colon, (B) body weight changes, (C) spleen weight, (D) colon weight, (E) colonic MPO activity, (F) serum KC, (G) serum Lcn2 and (H) fecal Lcn2. Arrows indicate shrunken cecum and rectal prolapse. Results presented as mean ± SEM. *p< 0.05.
Discussion
In recent years, many studies have begun to support the notion that host-microbiotal metabolism and inflammatory diseases are tightly linked (40-44). Among those that are extensively studied, the anti-atherogenic ApoE has emerged as having dual roles in regulating cholesterol homeostasis and mediating anti-inflammatory properties (16-20). Herein, we report that colonic ApoE expression was significantly upregulated in various murine models of chemical-, aberrant immune activation- and spontaneously-induced colitis, suggesting that ApoE may play a role during gut inflammation. Further, ApoE-KO mice are susceptible to develop acute or chronic colitis when IL-10 signaling was neutralized by administering a single or multiple doses of αIL-10R, respectively. Remarkably, the development of colitis is microbiota-dependent as antibiotics treatment significantly protected ApoE-KO mice against colitis.
Even though the etiology of human IBDs are not completely understood, several studies on murine models of colitis suggest the involvement of the gut microbiota. We have previously demonstrated that gut inflammation in TLR5-KO mice is microbiota-dependent due to their inability to manage the gut microbiota (29, 45-47). In the present study, we observed an unprecedented role of ApoE in the regulation of the gut microbiota. By performing fecal bacterial 16S rRNA sequencing, we found that ApoE-KO mice harbor an elevated gut microbiotal burden and an altered composition with a notably higher relative abundance of Bacteroidetes. The increase in the members of the gram negative Bacteroidetes phyla have been suggested to correlate with the risk of colitis (48-50). Additionally, the elevated gut bacterial load reflects an increased pool of bacterial ligands, toxins or metabolic products that could potentially translocate across the mucosal barrier, especially during gut inflammation when gut permeability is often compromised. Indeed, we detected elevated serum immunoreactivity against flagellin and LPS in αIL-10R-induced colitic ApoE-KO mice, confirming increased translocation of bacteria and/or their products that may further drive the disease. Further analysis revealed that the microbiotal dysbiosis in ApoE-KO mice could be, at least in part, due to impaired production of host antimicrobial peptides Ang4, RegIIIγ and Lcn2 (37-39), which may be associated with the loss of colonic ApoE expression. Interestingly, the sharing of microbiota between cohoused ApoE-KO and IL-10KO mice accelerated the induction of chronic inflammation in IL-10KO mice, confirming that ApoE-KO mice harbor a transmissible colitogenic microbiota. Nevertheless, we cannot rule out the possibility that exacerbated colitis in cohoused IL-10KO mice could be due to elevated microbiotal load in ApoE-KO mice, rather than altered microbiota composition.
Our current knowledge on human IBD have advanced significantly due to the development of various murine models of colitis over the past decades. One such model is the IL-10KO mice which develop spontaneous colitis that are representative of human IBD (26). As such, many studies have generated IL-10 deficient mice on the background of their gene of interest [i.e. double knockout (DKO) mice] as a standard approach to study the role of target genes/proteins in gut inflammation (17, 46, 51-53). However, this approach is often complicated with many logistical problems including the tediousness in generating DKO, developmental defects, and the inability to maintain the colony due to spontaneous IL-10 deficiency-exacerbated rectal prolapse (17, 26, 46, 51). To circumvent these problems, we therefore employed the use of αIL-10R that neutralizes IL-10 signaling as an alternative approach to study IL-10 deficiency-induced colitis in ApoE-KO mice. The αIL-10R treatment consistently induce a uniform 100% induction of colitis in genetically-susceptible mice, allowing better control on the onset and progression of disease compared to the conventional IL-10KO mice model (29, 30). The specificity of αIL-10R-induced colitis is well-noted as not all genetically-modified mice develop disease after treatment. As shown in this study, lipogenic enzyme, SCD1 deficient mice were resistant to αIL-10R-induced colitis, in addition to other mice strains (i.e. TLR4-KO, MyD88-KO and IL-1R-KO mice) that we have shown previously (29, 54).
The susceptibility of ApoE-KO mice to IL-10 neutralization-induced colitis strongly suggests a possible cross-talk between ApoE and IL-10 signaling in the gut. In one study, IL-10 deficiency was shown to disrupt the balance between Th1/Th2 cytokines levels and accelerate the development of atherosclerosis in ApoE-KO mice (17). Incidentally, both ApoE and IL-10 have been independently demonstrated to promote the establishment of alternatively activated anti-inflammatory M2 macrophage while inactivating the pro-inflammatory M1 macrophage (18, 55). It is possible that both ApoE and IL-10 may have similar and overlapping functions that are not limited to atherosclerosis, but also apply to other inflammatory processes as well. Although current studies on colonic ApoE expression are limited, we speculate that colonic macrophages may be one of the major producers of ApoE in the gut. Hence, the loss of ApoE and IL-10 in the gut could potentially skew macrophage polarization and the gut microenvironment towards more pro-inflammatory and potentiate colitis.
The polymorphisms in the ApoE gene have been recently reported to affect the susceptibility to IBD. In humans, three alleles (ε2, ε3, and ε4) of the ApoE gene have been identified to encode three major isoforms of the ApoE proteins (13). Due to the available combinations of these alleles, any given individual can be genotyped as either ε2/2, ε2/3, ε2/4, ε3/3, ε3/4 or ε4/4. In one epidemiological study, IBD patients were reported to display a higher frequency for the ε4 allele, suggesting that ApoE polymorphism could be a risk factor for IBD (56). This finding was supported by another study in which the prevalence of ε2 and ε4 alleles were shown to be significantly higher among IBD patients (57). Further studies are required to investigate the functional differences among the ApoE isoforms and on how these can be exploited as potential therapeutic targets in IBD.
The translational significance of ApoE has been recognized as potential therapeutics primarily against atherosclerosis. In particular, there had been a longstanding interest in studying the beneficial effects of synthetic ApoE mimetic peptides that are functionally similar to the native protein, despite the differences in size and structure (58). Several ApoE mimetic peptides were designed and have been tested to be bioactive in binding lipoprotein and clearing cholesterol from circulation (59, 60). The anti-inflammatory properties of ApoE mimetic peptides were also demonstrated in murine models of multiple sclerosis (61). In a study by Singh et al. (62), the group further demonstrated the protective efficacy of ApoE mimetic peptide COG112 in inhibiting inflammatory responses in murine models of Citrobacter rodentium-induced and DSS-induced colitis. In other studies with mice, the ApoE mimetic peptide COG133 was shown to be equally protective against 5-fluorouracil-induced intestinal mucositis (63) and mitigating the inflammatory response to LPS challenge (64). In this regard, our present findings further highlights the importance of ApoE in maintaining gut homeostasis and that perhaps ApoE mimetic peptides would present a potential therapeutics to correct ApoE deficiency-associated microbiotal dysbiosis that predisposes colitis.
In summary, our study highlights a novel interplay between ApoE and IL-10 in maintaining gut-microbiota homeostasis and that such cross-talk modulate a critical inflammatory axis in IBD and possibly other inflammatory disorders. Due to the association between ApoE polymorphism and the susceptibility to IBD, ApoE poses as a potential therapeutic target in IBD. It is possible to further design/engineer effective ApoE mimetic peptides as an adjunct therapy that could be utilized to correct microbiotal dysbiosis and to treat human IBD.
Acknowledgements
This work was supported by grants from the National Institutes of Health (NIH) R01 (DK097865) and PSU Dean’s Schultz endowment, College of Health and Human Development Seed grant to M.V.-K. B.S.Y. is supported by NIH T32 (T32AI074551). We thank Alyssa Grube for technical assistance.
Abbreviations
- ApoE
apolipoprotein E
- IL-10R
interleukin-10 receptor
- mAb
monoclonal antibody
- Lcn2
lipocalin 2
- MPO
myeloperoxidase
Footnotes
Conflicts of Interest
The authors have declared that no conflict of interest exists.
References
- 1.Kamada N, Seo SU, Chen GY, et al. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 2.Devkota S, Wang Y, Musch MW, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nature medicine. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 5.Ordas I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet. 2012;380:1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 6.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Loftus EV., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 8.Reinecker HC, Steffen M, Witthoeft T, et al. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens C, Walz G, Singaram C, et al. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig Dis Sci. 1992;37:818–826. doi: 10.1007/BF01300378. [DOI] [PubMed] [Google Scholar]
- 10.Sandborn WJ, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis. 1999;5:119–133. doi: 10.1097/00054725-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Clark M, Colombel JF, Feagan BC, et al. American gastroenterological association consensus development conference on the use of biologics in the treatment of inflammatory bowel disease, June 21-23, 2006. Gastroenterology. 2007;133:312–339. doi: 10.1053/j.gastro.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Ferrante M, Vermeire S, Fidder H, et al. Long-term outcome after infliximab for refractory ulcerative colitis. J Crohns Colitis. 2008;2:219–225. doi: 10.1016/j.crohns.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Horejsi B, Ceska R. Apolipoproteins and atherosclerosis. Apolipoprotein E and apolipoprotein(a) as candidate genes of premature development of atherosclerosis. Physiol Res. 2000;49(Suppl 1):S63–69. [PubMed] [Google Scholar]
- 14.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 15.Lin CT, Xu YF, Wu JY, et al. Immunoreactive apolipoprotein E is a widely distributed cellular protein. Immunohistochemical localization of apolipoprotein E in baboon tissues. J Clin Invest. 1986;78:947–958. doi: 10.1172/JCI112685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly ME, Clay MA, Mistry MJ, et al. Apolipoprotein E inhibition of proliferation of mitogen-activated T lymphocytes: production of interleukin 2 with reduced biological activity. Cell Immunol. 1994;159:124–139. doi: 10.1006/cimm.1994.1302. [DOI] [PubMed] [Google Scholar]
- 17.Caligiuri G, Rudling M, Ollivier V, et al. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9:10–17. [PMC free article] [PubMed] [Google Scholar]
- 18.Baitsch D, Bock HH, Engel T, et al. Apolipoprotein E induces antiinflammatory phenotype in macrophages. Arterioscler Thromb Vasc Biol. 2011;31:1160–1168. doi: 10.1161/ATVBAHA.111.222745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali K, Middleton M, Pure E, et al. Apolipoprotein E suppresses the type I inflammatory response in vivo. Circ Res. 2005;97:922–927. doi: 10.1161/01.RES.0000187467.67684.43. [DOI] [PubMed] [Google Scholar]
- 20.Ouyang Q, Huang Z, Lin H, et al. Apolipoprotein E deficiency and highfat diet cooperate to trigger lipidosis and inflammation in the lung via the toll-like receptor 4 pathway. Mol Med Rep. 2015;12:2589–2597. doi: 10.3892/mmr.2015.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhry A, Samstein RM, Treuting P, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 23.Shouval DS, Biswas A, Goettel JA, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40:706–719. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore KW, de Waal Malefyt R, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 25.Hoshi N, Schenten D, Nish SA, et al. MyD88 signalling in colonic mononuclear phagocytes drives colitis in IL-10-deficient mice. Nat Commun. 2012;3:1120. doi: 10.1038/ncomms2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn R, Lohler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 27.Chassaing B, Aitken JD, Malleshappa M, et al. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:25. doi: 10.1002/0471142735.im1525s104. Unit 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheiffele F, Fuss IJ. Induction of TNBS colitis in mice. Curr Protoc Immunol. 2002:19. doi: 10.1002/0471142735.im1519s49. Chapter 15:Unit 15. [DOI] [PubMed] [Google Scholar]
- 29.Carvalho FA, Nalbantoglu I, Ortega-Fernandez S, et al. Interleukin-1beta (IL-1beta) promotes susceptibility of Toll-like receptor 5 (TLR5) deficient mice to colitis. Gut. 2012;61:373–384. doi: 10.1136/gut.2011.240556. [DOI] [PubMed] [Google Scholar]
- 30.Singh V, Yeoh BS, Carvalho F, et al. Proneness of TLR5 deficient mice to develop colitis is microbiota dependent. Gut Microbes. 2015;6:279–283. doi: 10.1080/19490976.2015.1060390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh V, Yeoh BS, Xiao X, et al. Interplay between enterobactin, myeloperoxidase and lipocalin 2 regulates E. coli survival in the inflamed gut. Nat Commun. 2015;6:7113. doi: 10.1038/ncomms8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chassaing B, Srinivasan G, Delgado MA, et al. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One. 2012;7:e44328. doi: 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziegler TR, Luo M, Estivariz CF, et al. Detectable serum flagellin and lipopolysaccharide and upregulated anti-flagellin and lipopolysaccharide immunoglobulins in human short bowel syndrome. Am J Physiol Regul Integr Comp Physiol. 2008;294:R402–410. doi: 10.1152/ajpregu.00650.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katakura K, Lee J, Rachmilewitz D, et al. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodson L, Fielding BA. Stearoyl-CoA desaturase: rogue or innocent bystander? Prog Lipid Res. 2013;52:15–42. doi: 10.1016/j.plipres.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hooper LV, Stappenbeck TS, Hong CV, et al. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4:269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 38.Vaishnava S, Yamamoto M, Severson KM, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borregaard N, Cowland JB. Neutrophil gelatinase-associated lipocalin, a siderophore-binding eukaryotic protein. Biometals. 2006;19:211–215. doi: 10.1007/s10534-005-3251-7. [DOI] [PubMed] [Google Scholar]
- 40.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 41.Becker SA, McClave SA. Lipid profiles in Crohn's disease patients with and without ileal resection. Am J Gastroenterol. 1996;91:2452. [PubMed] [Google Scholar]
- 42.Levy E, Rizwan Y, Thibault L, et al. Altered lipid profile, lipoprotein composition, and oxidant and antioxidant status in pediatric Crohn disease. Am J Clin Nutr. 2000;71:807–815. doi: 10.1093/ajcn/71.3.807. [DOI] [PubMed] [Google Scholar]
- 43.Ripolles Piquer B, Nazih H, Bourreille A, et al. Altered lipid, apolipoprotein, and lipoprotein profiles in inflammatory bowel disease: consequences on the cholesterol efflux capacity of serum using Fu5AH cell system. Metabolism. 2006;55:980–988. doi: 10.1016/j.metabol.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Sappati Biyyani RS, Putka BS, Mullen KD. Dyslipidemia and lipoprotein profiles in patients with inflammatory bowel disease. J Clin Lipidol. 2010;4:478–482. doi: 10.1016/j.jacl.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 45.Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vijay-Kumar M, Sanders CJ, Taylor RT, et al. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carvalho FA, Koren O, Goodrich JK, et al. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe. 2012;12:139–152. doi: 10.1016/j.chom.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bloom SM, Bijanki VN, Nava GM, et al. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. 2011;9:390–403. doi: 10.1016/j.chom.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lucke K, Miehlke S, Jacobs E, et al. Prevalence of Bacteroides and Prevotella spp. in ulcerative colitis. J Med Microbiol. 2006;55:617–624. doi: 10.1099/jmm.0.46198-0. [DOI] [PubMed] [Google Scholar]
- 50.Sokol H, Seksik P, Furet JP, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 51.Hale LP, Greer PK. A novel murine model of inflammatory bowel disease and inflammation-associated colon cancer with ulcerative colitis-like features. PLoS One. 2012;7:e41797. doi: 10.1371/journal.pone.0041797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Froicu M, Zhu Y, Cantorna MT. Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology. 2006;117:310–318. doi: 10.1111/j.1365-2567.2005.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matharu KS, Mizoguchi E, Cotoner CA, et al. Toll-like receptor 4-mediated regulation of spontaneous Helicobacter-dependent colitis in IL-10-deficient mice. Gastroenterology. 2009;137:1380–1390. doi: 10.1053/j.gastro.2009.07.004. e1381-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carvalho FA, Nalbantoglu I, Aitken JD, et al. Cytosolic flagellin receptor NLRC4 protects mice against mucosal and systemic challenges. Mucosal Immunol. 2012;5:288–298. doi: 10.1038/mi.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li K, Wang B, Sui H, et al. Polymorphisms of the macrophage inflammatory protein 1 alpha and ApoE genes are associated with ulcerative colitis. International journal of colorectal disease. 2009;24:13–17. doi: 10.1007/s00384-008-0575-0. [DOI] [PubMed] [Google Scholar]
- 57.Al-Meghaiseeb ES, Al-Otaibi MM, Al-Robayan A, et al. Genetic association of apolipoprotein E polymorphisms with inflammatory bowel disease. World journal of gastroenterology : WJG. 2015;21:897–904. doi: 10.3748/wjg.v21.i3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White CR, Garber DW, Anantharamaiah GM. Anti-inflammatory and cholesterol-reducing properties of apolipoprotein mimetics: a review. J Lipid Res. 2014;55:2007–2021. doi: 10.1194/jlr.R051367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharifov OF, Nayyar G, Garber DW, et al. Apolipoprotein E mimetics and cholesterol-lowering properties. Am J Cardiovasc Drugs. 2011;11:371–381. doi: 10.2165/11594190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 60.Dyer CA, Cistola DP, Parry GC, et al. Structural features of synthetic peptides of apolipoprotein E that bind the LDL receptor. J Lipid Res. 1995;36:80–88. [PubMed] [Google Scholar]
- 61.Li FQ, Sempowski GD, McKenna SE, et al. Apolipoprotein E-derived peptides ameliorate clinical disability and inflammatory infiltrates into the spinal cord in a murine model of multiple sclerosis. J Pharmacol Exp Ther. 2006;318:956–965. doi: 10.1124/jpet.106.103671. [DOI] [PubMed] [Google Scholar]
- 62.Singh K, Chaturvedi R, Barry DP, et al. The apolipoprotein E-mimetic peptide COG112 inhibits NF-kappaB signaling, proinflammatory cytokine expression, and disease activity in murine models of colitis. J Biol Chem. 2011;286:3839–3850. doi: 10.1074/jbc.M110.176719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azevedo OG, Oliveira RA, Oliveira BC, et al. Apolipoprotein E COG 133 mimetic peptide improves 5-fluorouracil-induced intestinal mucositis. BMC Gastroenterol. 2012;12:35. doi: 10.1186/1471-230X-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lynch JR, Tang W, Wang H, et al. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem. 2003;278:48529–48533. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]