Abstract
Aims
It is known that bladder exposure to noxious stimuli elicits nerve growth factor (NGF) expression with region wise differences. Here, we investigated the effect of bladder distension (cystometry) and bladder wall injection of NGF antisense oligonucleotide (ODN) together as well as separately on spontaneous (constitutive) expression of NGF and its cognate p75 neurotrophin receptor (p75NTR).
Method
Under isoflurane anesthesia, either 15µg of protamine sulfate (vehicle) alone or complexed with 1.5µg of NGF antisense or scrambled ODN was injected (10µL) at 4 sites in bladder wall of 24 adult female Sprague-Dawley rats and 6 rats were left untreated (n=30). Under urethane anesthesia, cystometry (CMG) was performed in treated and control rats. Fluorescent ODN and NGF/p75NTR expression was localized in harvested tissue.
Key findings
Complexation of ODN with protamine was essential for the retention of ODN in bladder tissue as the uncomplexed ODN was untraceable after injection. Bladder distension from CMG raised the expression of NGF and p75NTR relative to CMG naïve rats. The groups treated with vehicle, scrambled and antisense ODN were indistinct with regard to CMG parameters, but the intense immunoreactivity of NGF and p75NTR seen in the vehicle and scrambled ODN groups was reduced following treatment with NGF antisense.
Significance
The constitutive expression of NGF and p75NTR is responsive to bladder distension and administration of NGF antisense. Complexation with protamine reduces the clearance of ODN and demonstrates the potential of ODN nanoparticles as an option for reducing the inducible NGF expression in OAB patients following intradetrusor injection.
Keywords: NGF, p75NTR, constitutive, reflex voiding, Protamine
Introduction
NGF belongs to a class of target tissue-derived secreted proteins known as neurotrophins, which are important for the establishment of neuronal connections and the regulation of synaptic structures (Vizzard, 2000). NGF influences the neuronal development, function, and response to injury in bladder (Vizzard, 2000). Like other neurotrophins, NGF is produced as a preproprotein of approximately 250 amino acids, which undergo a series of cleavage and folding events to form the mature protein with molecular weight of ~13 kDa (Yoshimura et al., 2006).
Increased levels of neurotrophic factors including NGF have been detected in the bladder and in the dorsal root ganglion after spinal cord injury (SCI) (Vizzard, 2000) and after bladder outlet obstruction (Ha et al., 2011). Several studies have shown that chronic administration of exogenous NGF intravesically into the bladder and intrathecally into the spinal cord can induce bladder hyperactivity and increase the firing frequency of dissociated bladder afferent neurons (Yoshimura et al., 2006, Yoshimura and de Groat, 1999). Furthermore, bladder hyperactivity induced by noxious stimuli or SCI was suppressed by both systemic and local antibody mediated neutralization of NGF (Guerios et al., 2008, Jaggar et al., 1999, Seki et al., 2004). Thus, NGF is established as a chemical mediator of pathology-induced changes in C-fiber afferent nerve excitability which can result in bladder hyperactivity (Yoshimura and de Groat, 1999).
It is known that bladder exposure to noxious stimuli elicits much higher increase in NGF expression in urothelium, (Schnegelsberg et al., 2010, Zhang and Qiao, 2012) compared to detrusor (Zhang and Qiao, 2012). NGF immunofluorescence measurement in cold cup bladder biopsy of OAB and interstitial cystitis patients demonstrated that NGF expression in urothelium is acutely sensitive to bladder diseases(Lowe et al., 1997). But, studies on cultured human and rat smooth muscle cells demonstrated that bladder smooth muscles are the primary source of NGF expression (Clemow et al., 2000, Persson et al., 1997) which is responsive to cyclic stretch (Persson et al., 1995). Physiological increase in NGF expression with bladder stretching was also noted in healthy subjects (Liu and Kuo, 2009).
The cellular response to NGF is mediated by two structurally unrelated cognate receptors, TrkA and p75 neurotrophin receptor (p75NTR), which form homo or hetero dimers in response to NGF binding (Jahed and Kawaja, 2005). The p75NTR is expressed in many different bladder cell types and binds to neurotrophins including NGF with low affinity for mediating a variety of cellular functions by activating distinct signaling pathways (Jahed and Kawaja, 2005). The p75NTR is known to exhibit constitutive, ligand-independent activity (Vilar et al., 2009). Earlier studies indicated region wise differences in the expression of p75NTR in response to noxious stimuli (Girard et al., 2011, Wakabayashi et al., 1996), akin to NGF
In the present study, we examined the region wise differences in constitutive expression of NGF and p75NTR in rat bladder following cystometry in absence of any exposure to noxious stimuli and after bladder wall injection of nanoparticles containing oligonucleotide (ODN). We investigated whether spontaneous NGF expression is responsive to mechanical stretch and reflex bladder activity induced by cystometry. Anionic charge of phosphorothioate-modified ODN is known to hinder cellular uptake and therefore we analyzed the retention of native and complexed ODN within bladder.
Methods
Reagents
The 18mer phosphorothioated antisense oligonucleotide (ODN) with the sequence 5'GCCCGAGACGCCTCCCGA 3' was directed against unique sequence in exon 3 of rat NGF mRNA and were custom made by Integrated DNA technologies (San Diego, CA). Experiments involving bladder localization used ODN with a 5' tag of TYE™ 563. Similar length scrambled sequence 5'ACGACCTCGCGACCGGCC3' was designed using web-based tool https://www.genscript.com/ssl-bin/app/scramble. TYE 563 is a bright red fluorescent dye that emits in the red spectral range of 563 nm and absorbs at 557nm. Lyophilized fluorescent ODN 1.5µg was dispersed in 10µL of nuclease free water, which was then complexed with 15 µg of protamine sulfate at room temperature for 30 min. Dose of ODN for bladder wall injection was selected based on previous in vitro experiments conducted on urothelium cell line (data not shown).
Animals
All experiments were performed on 30 female Sprague-Dawley rats (225–250G; Harlan, Indianapolis, Ind), which were carried out in accordance with the National Institutes of Health's “Guide for the Care and Use of Laboratory Animals.” Special efforts were made to minimize the number of animals used and their suffering. The experimental protocol was approved by the Institutional Animal Care and Use Committee of University of Pittsburgh.
Animal Surgery and Physiology Experiments
Bladder Localization of ODN
Six rats split into two groups (n=3) were used for this experiment. Under isoflurane anesthesia, rats were given bladder wall injections of either 1.5µg of fluorescent ODN (n=3 rats; native ODN group) or 1.5µg of fluorescent ODN complexed with 15 µg of protamine sulfate in 10µL of nuclease free deionized water (n=3 rats; ODN + protamine group). ODN and protamine complex was prepared by incubation of both reagents for 30min at room temperature prior to bladder wall injection. A total of 10 µl of native ODN or ODN complexed with protamine was injected into 4 separate sites (2.5µL per site X 4 sites) with a 30-gauge needle. The bladder dome was grasped with a forceps, and the needle was inserted tangentially into the anterior, posterior and lateral walls to perform these injections. Rats were monitored regularly for any adverse behavior for upto 48h after injection, including food and water intake and ambulatory behavior. Bladders were harvested at the time of sacrifice and cryosectioned into 10µm thick sections. Sections were counterstained with fluorescent nuclear dye, 4′,6-diamidino-2-phenylindol (DAPI) and mounted with an antifade agent. Images were taken with a fluorescence microscope (BX51, Olympus America Inc, Center Valley, PA,) and digitized using software Magnafire 2.1C. Bladder sections were scanned sequentially for TYE 563 fluorophore viewed in 550–615 nm channel and DAPI counterstain viewed in 418–473 nm channel.
Cystometry (CMG)
Adult female Sprague-Dawley rats were split into four groups of 6 rats each. One group was left untreated to act as a control for NGF expression in absence of bladder wall injection. Other three groups received bladder wall injection at four sites with a total volume of 10µL of different treatments and 48h later, transurethral open cystometry under urethane anesthesia (1g/kg, s.c) was performed on three treated groups along with 3 rats from control group. Under isoflurane anesthesia, the three treated groups received either bladder wall injection of 15 µg protamine sulfate (n=6; vehicle group) or 1.5µg NGF antisense ODN complexed with 15 µg protamine sulfate (n=6) or 1.5µg scrambled ODN complexed with 15 µg protamine sulfate (n=6)(10µL). 48h later, rats from control and treated groups were placed under urethane anesthesia, and saline was infused via transurethral catheter at 0.04ml/min for 4h to elicit repetitive voiding via a polyethylene catheter (PE-50) connected to a pressure transducer and to a syringe pump by a three-way stopcock for recording intravesical pressure. Cyclic stretch of bladder over a 4h period during CMG is expected to increase NGF expression (Clemow et al., 2000, Persson et al., 1997). The intravesical pressure was recorded by a data-acquisition software LabChart 7 version 7.3.7 (sampling rate 400 Hz; ADInstruments, USA). Their body temperature was maintained in the physiologic range using a heating lamp. The intercontractile interval (ICI) was determined as the time between 2 continuing contraction cycles. Bladders harvested from control rats were used for NGF ELISA and groups receiving bladder wall injection were used in immunofluorescence experiments.
Immunofluorescence
Bladders were cryopreserved at the end of cystometry and cut into 10µm thick cryosections. Sections were fixed in chilled acetone at 4°C for 15 min followed by washing with PBST (PBS + 0.4% Triton X-100). Sections were further incubated in PBST containing 5% normal Donkey serum (Jackson Immunoresearch) for 30–40 min at room temperature to block nonspecific-binding. For the simultaneous demonstration of ligand (NGF) and receptor (p75NTR) expression, antibodies against NGF (dilution 1:50, polyclonal goat, Santa Cruz Biotechnology, CA) and p75NTR (1:250, polyclonal rabbit, Abcam, USA) raised in different animals were applied as a cocktail. Sections were incubated with primary antibodies in PBST containing 1% donkey serum for 16–18 h at 4°C. After incubation with the primary antibodies, sections were washed thrice in PBST for 15 min each at room temperature. Rabbit primary antibodies were visualized using secondary donkey anti goat Alexa Fluor 594 or donkey anti rabbit Alexa Flour 488 (1:200, Abcam) applied for 2h at room temperature in PBST containing 1% donkey serum. Sections were again washed thrice in PBST for 15 min at room temperature mounted with DAPI Fluoromount-G mounting medium (Southern Biotech). Immunofluorescence was detected by florescent microscope (Olympus Bx51) equipped with software Magnafire 2.1C at 10X magnification. Double-labelled tissue was scanned sequentially for three different fluorophores, using three different channels: two fluorophores (A594 and A488) and DAPI nuclear stain. NGF-Alexa Fluor 594 stain is seen in 550–615 nm channel and p75NTR-Alexa Flour 488 stain is viewed in 450–500 nm channel. The DAPI counterstain was viewed in 418– 473 nm channel and exposure time for each stain was same in all sections. The specificity of the antibodies (Kashyap et al., 2013) was explored by omission of the primary antibody in negative controls in our earlier reports.
Bladder Tissue NGF Levels
A portion of the bladder tissue from animals in the control group that did not undergo bladder wall injection were harvested and tissues homogenized in RIPA lysis buffer system (Santa Cruz Biotechnology Inc., USA) in the presence of 1mM Na3VO4, 2mM PMSF and 10µL/mL protease inhibitor cocktail (Sigma). Protein estimation was done using Pierce BCA protein Assay kit (Thermo Scientific, USA). Bladder tissue lysates were stored at −20°C until assay. The samples were assayed in triplicate in an antigen capture ELISA Emax Immuno- Assay System (Promega, Madison, WI) according to the manufacturer’s instructions. ELISA plates were read at 450 nm on an Elx800 microplate reader (Bio-Tek Instruments, Winooski, VT). Tissue NGF values were normalized against the protein concentrations of each sample and expressed as picograms per microgram protein.
Statistics
The results are presented as means ± the standard error of the mean (SEM). The level of statistical significance between the mean values of different sub groups of control group were analyzed by student’s t test and differences between bladder injected groups were analyzed by One way ANOVA, followed by Dunnett`s post hoc test. In all statistical tests, the minimum criterion chosen to discard the null hypothesis was set at p < 0.05.
Results
Bladder Localization of ODN
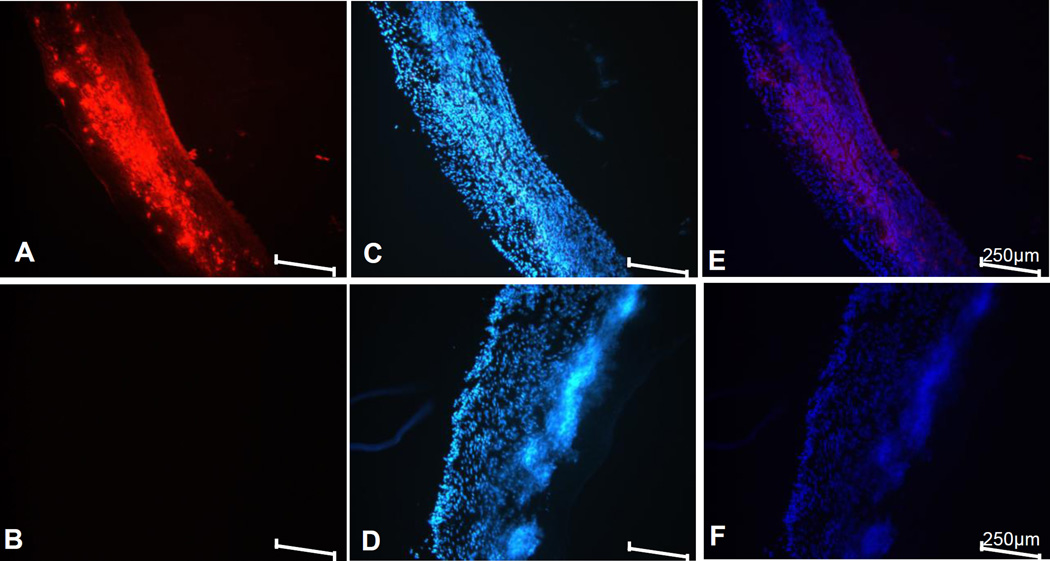
Bladder tissue from two groups were harvested 48h after bladder wall injection and then cryo-sectioned to assess the cellular internalization of ODN under fluorescence microscope. The presence of ODN in bladder was localized by the fluorescence emitted from TYE 563 tagged to ODN (Fig. 1A). Corresponding DAPI counterstain for nucleus localization in respective sections of two groups is shown in Fig. 1C and Fig. 1D. Rat bladder injected with ODN carrying fluorescent tag without complexation with protamine sulfate failed to show any bright red fluorescence (Fig. 1B). Disappearance of the fluorescent ODN (Fig. 1B), 48h after bladder wall injection indicates that protamine sulfate is essential for the retention of ODN within the detrusor smooth cells (SM) of the bladder and for reducing the clearance of ODN from bladder.
Fig. 1. Bladder Localization of Injected ODN.
Fluorescence images of bladder sections harvested from rat group injected with ODN carrying a 5' tag of TYE™ 563 complexed with protamine sulfate (panel A) and rat group injected with ODN carrying a 5' tag of TYE™ 563 without protamine sulfate (panel B). The bright red fluorescence in panel A and the absence of red fluorescence in panel B illustrates the benefit of ODN nanoparticles formed by protamine complexation for successful uptake and retention within detrusor. Corresponding DAPI stained images are shown in panel C and D, respectively, where detrusor smooth muscle is indicated by SM and urothelium is indicated by U. Panel E and F shows the merged image of both channels. Scale bar is 250µm in all panels.
Effect of Cystometry (CMG) on Constitutive Expression of NGF and p75NTR
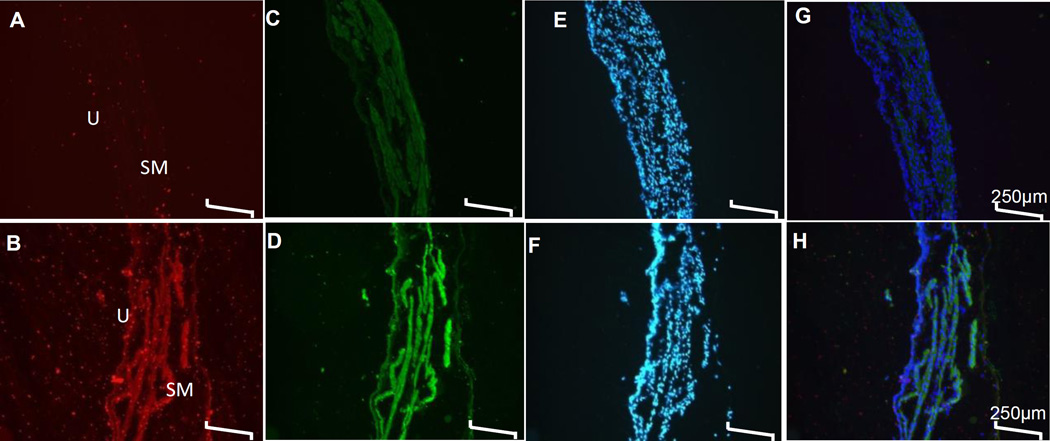
Rats that did not undergo bladder distension showed weak immunofluorescence of NGF in urothelium and in detrusor regions of bladder (Fig. 2A). However, NGF immunoreactivity was increased in urothelium (U) and smooth muscle (SM) regions of control group following bladder distension (Fig. 2B). Double- immunofluorescence with antibodies binding to NGF and the p75NTR showed that bladder distension (Fig. 2D) evoked a parallel increase in the intensity of the ligand NGF and its low affinity receptor p75NTR. The co-localized expression of NGF and p75NTR was visible in sections harvested without CMG (Fig. 2C) and after CMG (Fig. 2D).The tissue sections from different subgroups of control group did not differ with respect to DAPI stain (Fig. 2E–F). Quantitatively, NGF expression of 270.7 ± 45.27 pg/mg of protein in whole bladder tissue harvested post CMG was significantly higher compared to 41.72 ± 8.75 pg/mg of protein measured in bladder tissue harvested from untreated control rats that were neither exposed to bladder distension nor to bladder wall injection (p<0.05, unpaired student’s t test, n=3). The constitutively expressed NGF in untreated control group was upregulated relative to the control group that did not undergo bladder distention as a consequence of CMG.
Fig. 2. Effect of CMG on Constitutive NGF and p75NTR Expression.
Double immunofluorescence of NGF (red) and p75NTR (green) is shown in the respective cross-sections of the bladder wall of control group. The staining for NGF (panel A) and p75NTR (panel C) was relatively weaker in the bladder section harvested without performing the CMG. In contrast, both NGF (Panel B) and p75NTR (Panel D) was increased in urothelium and smooth muscle (SM) regions of bladder sections harvested post-CMG. Panel E–F shows the DAPI (blue) counterstain for the nucleus of tissue sections shown in panel A–D, while Panel G–H show the merged images. Scale bar is 250µm in all panels.
Effect of Treatment on CMG
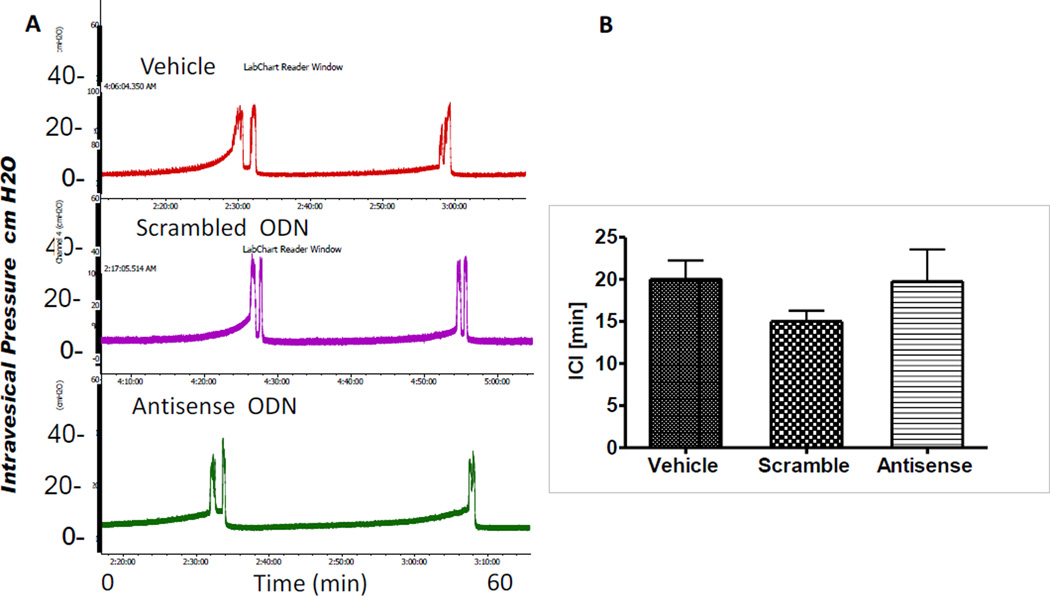
Transurethral open CMG under saline infusion is not considered to activate any inflammatory pathways and cause inducible NGF expression. Representative tracings from each group are shown in Fig. 3A. Group wise CMG data, especially ICI showed no significant difference across groups (n=6; p>0.05; One-way ANOVA; Fig. 3B). Other CMG parameters remained unchanged (data not shown).
Fig. 3. Effect of Treatment on CMG.
Panel A: Representative CMG of rats 48h after bladder wall injection of protamine sulfate 15µg (top tracing), 1.5 µg scramble ODN complexed with 15µg protamine sulfate (middle trace) or 1.5 µg NGF antisense ODN complexed with 15µg protamine ( bottom trace) were indistinct. Panel B: Group wise analysis of ICI between groups showed no significant difference across groups (One way ANOVA; p>0.05; n=6).
Effect of Treatment on Constitutive NGF and p75NTR Expression
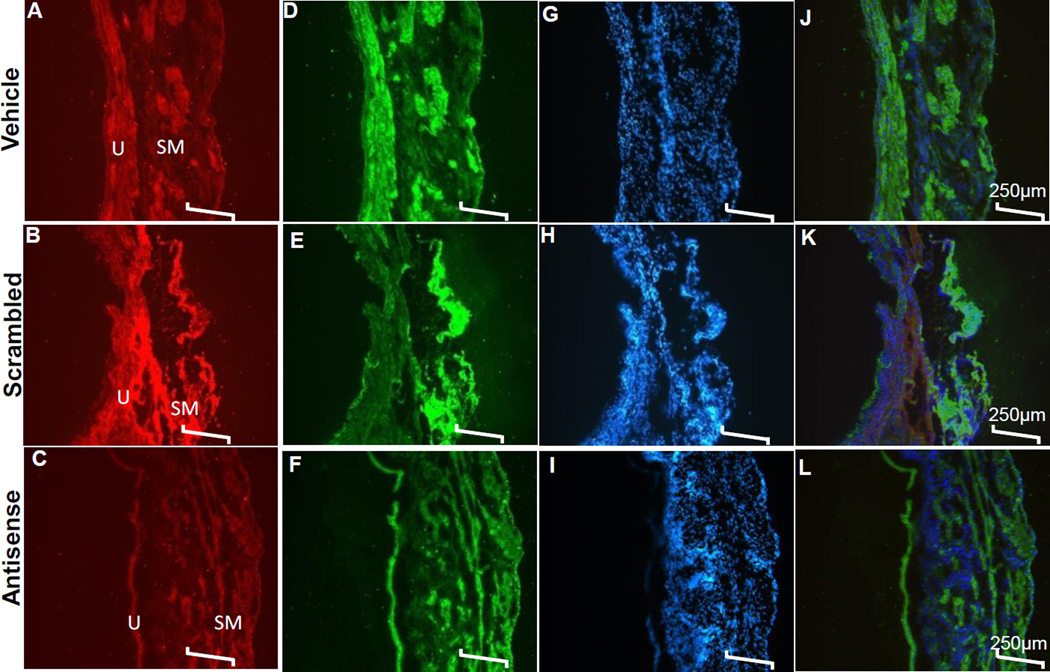
Bladder tissues from the three bladder injected groups were harvested at the end of CMG for double immunofluorescence (Fig. 4). Intense immunoreactivity for NGF is visible in urothelium (U) and smooth muscle (SM) regions of bladder sections harvested from vehicle (Fig. 4A) and scrambled ODN (Fig. 4B) treated groups and signal is consistent with the increased NGF expression following CMG as shown in Fig. 2B. In contrast, antisense ODN targeting NGF substantially reduced the immunoreactivity for NGF (Fig. 4C) that was seen increased in other groups (Fig. 4A&B) due to cyclic stretching induced by CMG. The p75NTR expression in different treatment groups mirrored the intensity of NGF expression with highest expression in vehicle treated (Fig. 4D) followed by scrambled (Fig. 4E) and the NGF antisense (Fig. 4F) treated groups. Bladder wall injection of NGF antisense reduced the expression of p75NTR to greater extent in smooth muscles than in urothelium. Images in bottom panels of (Fig. 4G,H and I) show corresponding DAPI stain for sections shown in respective panels above. DAPI staining in all sections look indistinguishable from each other.
Fig. 4. Effect of Treatment on NGF and p75NTR Expression Induced by CMG.
Double immunofluorescence of NGF (red) and p75NTR (green) is shown in the respective cross-sections of the bladder wall of the three treated groups. Bladder of vehicle (panel A) and scrambled ODN (panel B) treated groups showed intense red fluorescence representing the strong NGF expression induced by CMG is visible in urothelium (U) and smooth muscle (SM) regions of bladder sections. In contrast, NGF antisense ODN substantially reduced the immunoreactivity for NGF in SM regions (panel C) relative to U regions. The immunoreactivity for p75NTR (panel C–D) in the same sections mirrored the findings for constitutive NGF expression in respective groups (panel A–C) indicating that changes in NGF and p75NTR expression occur in parallel. Images of the DAPI counterstain taken in the 418–473 nm channels are shown below in panel (G–I) corresponding to respective images shown in Panel A–F. Panel J–L shows the merged images of all three stains. Scale bar is 250µm in all panels.
Discussion
Here, we demonstrated that cyclic stretching of bladder during cystometry upregulates the spontaneous NGF expression in bladder. We further showed that bladder wall injection of NGF antisense complexed with protamine reduces the immunofluorescence of NGF in detrusor relative to urothelium. However, antisense mediated reduction of constitutive NGF expression was not accompanied by any cystometric changes. Overall, our findings support the existence of NGF expression in two separate states, one that occurs in the background in absence of noxious stimuli is called constitutive and the other state that exists when noxious stimuli amplifies the NGF expression is called inducible phase (Persson et al., 1997, Tanner et al., 2000).
Published research has established that inducible NGF expression affects the expression of its cognate receptors, the p75NTR and Trk-A in urothelium (Murray et al., 2004). Studies involving ectopic NGF expression in urothelium implicated region wise differences in the expression (Girard et al., 2011) of its receptors. Interestingly, region wise differences in the expression of the ligand (NGF) was also reported by several groups following exposure to noxious stimuli (Zhang and Qiao, 2012), where the NGF expression in urothelium (Zhang and Qiao, 2012) was found to be higher than in detrusor. The expression of the two cognate receptors remained unchanged in urothelium, but the expression of p75NTR in detrusor was drastically increased following NGF overexpression in urothelium induced by genetic means (Girard et al., 2011, Wakabayashi et al., 1996). Therefore, it was relevant to investigate the constitutive expression of NGF on the p75NTR expression in bladder in absence of any noxious stimuli.
The constitutive expression of NGF in bladder is demonstrated to be quantitatively lower than the levels measured in response to noxious stimuli such as cyclophosphamide (Vizzard, 2000), lipopolysaccharide (Saban et al., 2001) or acetic acid (Kashyap et al., 2013). Tissue NGF levels in control group, which was only exposed to bladder distension, but not to bladder wall injection were measured by ELISA. In order to isolate the effect of treatment from the procedure of bladder wall injection, the NGF levels in the experimental groups were measured by tissue immunofluorescence. It is known that reflex voiding during CMG involves cyclic stretching and contraction of bladder and cyclic stretch was previously demonstrated to increase the NGF expression of cultured detrusor smooth muscle cells (Clemow et al., 2000, Persson et al., 1997).
NGF expression is considered to play an important role in normal bladder sensation as bladder stretching leading to urge to void in healthy subjects was accompanied with physiological increase in NGF expression (Liu and Kuo, 2009). Creatinine corrected NGF levels in urine were correlated with bladder volume at urge sensation in the controls but pathologic elevation of NGF levels in OAB patients at lower bladder capacity precluded the significant association of NGF expression with urgency sensation at full bladder capacity (Liu and Kuo, 2009). Bladder stretching in rodents is expected to be higher with the increased bladder capacity under anesthesia (Masuda et al., 2007). Therefore, cyclic stretching and contraction of bladder during CMG was expected to increase the expression of NGF and its receptor.
We found that CMG induced increase in NGF expression was found to be constitutive in nature and its reduction by NGF antisense did not alter the CMG outcomes. It is likely that the role of constitutive NGF expression in reflex voiding is redundant to the purinergic signaling and other autocoids such as prostaglandins. The p75NTR is an obligate signaling receptor, which can affect voiding frequency (Klinger and Vizzard, 2008) by affecting afferent neuron sensitization and interaction with phosphodiesterase isoforms (Sachs and Akassoglou, 2007) and Rho GTPase pathway (Sachs et al., 2007) in smooth muscle. These reports taken together with our findings imply that the parallel increase in the constitutive expression of both NGF and p75NTR noted here is a homeostatic response of the bladder to the CMG procedure.
A previous report on diabetic cystopathy found that changes NGF and p75NTR expression occur in parallel (Tong and Cheng, 2007) and we observed that bladder wall injection of NGF antisense reduced the immunofluorescence of p75NTR in detrusor to a greater extent than in urothelium. Double immunofluorescence of NGF and p75NTR in scrambled group indicated that changes in p75NTR expression in urothelium were not in parallel with NGF expression. Observed changes in p75NTR expression of scrambled group may be linked to the insignificant changes in cystometry of scrambled group. It is also likely that p75NTR expression in scrambled group may be unrelated to the changes in NGF expression as unlike TrkA, which is specific for NGF, p75NTR binds non-selectively to all neurotrophins with low affinity (Jahed and Kawaja, 2005). The p75NTR is also known to form disulfide-linked dimers at the cell surface independently of ligand binding (Vilar et al., 2009).
Our previous study (Kashyap et al., 2013) demonstrated that bladder instillation of NGF antisense can directly suppress the NGF expression of urothelium in response to noxious stimuli. Urothelium responds to bladder distension and noxious stimuli by producing several diffusible mediators, including NGF (Ochodnicky et al., 2013) and antisense mediated reduction of inducible NGF expression in the urothelium was able to alter cystometric outcomes (Kashyap et al., 2013). The CMG findings shown here imply that in contrast to the NGF expression in detrusor, the NGF expressed in urothelium is not redundant and appears to be the key driver of bladder overactivity. This however needs to be tested in urothelial specific NGF knockout transgenic mice.
Complexation of anionic ODN with cationic protamine sulfate is considered to form nanoparticles after brief incubation at room temperature (Lochmann et al., 2005). Binary complex of ODN and protamine at mass ratio used in our experiments have positive surface potentials (Junghans et al., 2001) due to excess of protamine. Based on cell culture experiments, protamine sulfate was used to facilitate the intracellular uptake of injected ODN. Interaction of cationic amino acid residues of protamine with the anionic residues of glycosaminoglycan expressed on the cell surfaces is considered important for adsorption on cell membranes and internalization. Protamine also possesses several amino acid sequences resembling that of a nuclear localization signal (Sorgi et al., 1997) capable of facilitating ODN delivery into the nucleus and thereby reduce the systemic clearance of injected ODN from bladder.
Our findings indicates that protamine sulfate is essential for the intracellular retention of the red fluorescent ODN in bladder and therefore increased clearance of ODN not bound to the protamine sulfate cannot be ruled out. Based on earlier reports, an energy dependent uptake mechanism based on endocytosis is likely for the cellular uptake of nanoparticles formed by protamine+ODN complex(Barthel et al., 1993). Protamine injected in micrograms is not demonstrated to be toxic as it is thousand fold lower than the dose of milligram range previously shown to injure (Shioyama et al., 2008) the bladder of animals.
Inhibition of NGF synthesis may be more efficacious and feasible approach than inhibition of NGF action on its receptors. Reduced NGF immunofluorescence in detrusor following bladder wall injection of NGF antisense corroborates with the reported decrease in NGF after intradetrusor injection of botulinum toxin in OAB patients (Giannantoni et al., 2013). Bladder wall injection of NGF antisense is effective at 22 fold lower dose of ODN compared to intravesical instillation (Kashyap et al., 2013), which may prove to be cost effective when ODN is available in limited supply. Effect of intravesical NGF antisense on detrusor NGF expression was inconclusive in our earlier studies (Kashyap et al., 2013) and here, we demonstrated the effect of NGF antisense on the constitutive NGF expression in detrusor by direct bladder wall injection.
The lack of any effect on cystometric parameters from reduced constitutive NGF expression can be easily reconciled with the fact that voiding disorders are typically associated with inducible NGF expression and not with constitutive NGF expression. Therefore NGF antisense will be preferred for gene silencing of inducible NGF expression. Present study substantiates the therapeutic potential of the NGF antisense (Tyagi et al., 2014) for treating voiding disorders and support intradetrusor injection of NGF antisense for treating OAB refractory to conventional treatments.
Conclusions
Overall, these findings suggest that spontaneous expression of NGF and p75NTR in bladder is raised by CMG and the complexation of ODN with protamine reduces the clearance of injected ODN. The role of constitutive NGF expression in the maintenance of voiding frequency is likely to be redundant. Findings support the intradetrusor injection of NGF antisense for gene silencing of inducible NGF expression as a potential treatment option for voiding disorders.
Acknowledgments
This work was partly supported by funding from NIH (DK088836 and DK093424)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barthel F, Remy JS, Loeffler JP, Behr JP. Gene transfer optimization with lipospermine-coated DNA. DNA and cell biology. 1993;12:553–560. doi: 10.1089/dna.1993.12.553. [DOI] [PubMed] [Google Scholar]
- Clemow DB, Steers WD, Tuttle JB. Stretch-activated signaling of nerve growth factor secretion in bladder and vascular smooth muscle cells from hypertensive and hyperactive rats. J Cell Physiol. 2000;183:289–300. doi: 10.1002/(SICI)1097-4652(200006)183:3<289::AID-JCP1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Giannantoni A, Conte A, Farfariello V, Proietti S, Vianello A, Nardicchi V, et al. Onabotulinumtoxin-A intradetrusorial injections modulate bladder expression of NGF, TrkA, p75 and TRPV1 in patients with detrusor overactivity. Pharmacological research : the official journal of the Italian Pharmacological Society. 2013;68:118–124. doi: 10.1016/j.phrs.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Girard BM, Malley SE, Vizzard MA. Neurotrophin/receptor expression in urinary bladder of mice with overexpression of NGF in urothelium. Am J Physiol Renal Physiol. 2011;300:F345–F355. doi: 10.1152/ajprenal.00515.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R111–R122. doi: 10.1152/ajpregu.00728.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha US, Park EY, Kim JC. Effect of botulinum toxin on expression of nerve growth factor and transient receptor potential vanilloid 1 in urothelium and detrusor muscle of rats with bladder outlet obstruction-induced detrusor overactivity. Urology. 2011;78:721, e1–e6. doi: 10.1016/j.urology.2011.03.070. [DOI] [PubMed] [Google Scholar]
- Jaggar SI, Scott HC, Rice AS. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth. 1999;83:442–448. doi: 10.1093/bja/83.3.442. [DOI] [PubMed] [Google Scholar]
- Jahed A, Kawaja MD. The influences of p75 neurotrophin receptor and brain-derived neurotrophic factor in the sympathetic innervation of target tissues during murine postnatal development. Autonomic neuroscience : basic & clinical. 2005;118:32–42. doi: 10.1016/j.autneu.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Junghans M, Kreuter J, Zimmer A. Phosphodiester and phosphorothioate oligonucleotide condensation and preparation of antisense nanoparticles. Biochim Biophys Acta. 2001;1544:177–188. doi: 10.1016/s0167-4838(00)00219-3. [DOI] [PubMed] [Google Scholar]
- Kashyap M, Kawamorita N, Tyagi V, Sugino Y, Chancellor M, Yoshimura N, et al. Down-regulation of nerve growth factor expression in the bladder by antisense oligonucleotides as new treatment for overactive bladder. J Urol. 2013;190:757–764. doi: 10.1016/j.juro.2013.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger MB, Vizzard MA. Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2008;295:F1778–F1789. doi: 10.1152/ajprenal.90501.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HT, Kuo HC. Urinary nerve growth factor levels are elevated in patients with overactive bladder and do not significantly increase with bladder distention. Neurourology and urodynamics. 2009;28:78–81. doi: 10.1002/nau.20599. [DOI] [PubMed] [Google Scholar]
- Lochmann D, Weyermann J, Georgens C, Prassl R, Zimmer A. Albumin-protamine-oligonucleotide nanoparticles as a new antisense delivery system. Part 1: physicochemical characterization. Eur J Pharm Biopharm. 2005;59:419–429. doi: 10.1016/j.ejpb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol. 1997;79:572–577. doi: 10.1046/j.1464-410x.1997.00097.x. [DOI] [PubMed] [Google Scholar]
- Masuda H, Ogawa T, Kihara K, Chancellor MB, de Groat WC, Yoshimura N. Effects of anaesthesia on the nitrergic pathway during the micturition reflex in rats. BJU Int. 2007;100:175–180. doi: 10.1111/j.1464-410X.2007.06872.x. [DOI] [PubMed] [Google Scholar]
- Murray E, Malley SE, Qiao LY, Hu VY, Vizzard MA. Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic Ganglia and bladder. J Urol. 2004;172:2434–2439. doi: 10.1097/01.ju.0000143549.29867.4e. [DOI] [PubMed] [Google Scholar]
- Ochodnicky P, Michel MB, Butter JJ, Seth J, Panicker JN, Michel MC. Bradykinin modulates spontaneous nerve growth factor production and stretch-induced ATP release in human urothelium. Pharmacol Res. 2013;70:147–154. doi: 10.1016/j.phrs.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Persson K, Sando JJ, Tuttle JB, Steers WD. Protein kinase C in cyclic stretch-induced nerve growth factor production by urinary tract smooth muscle cells. Am J Physiol. 1995;269:C1018–C1024. doi: 10.1152/ajpcell.1995.269.4.C1018. [DOI] [PubMed] [Google Scholar]
- Persson K, Steers WD, Tuttle JB. Regulation of nerve growth factor secretion in smooth muscle cells cultured from rat bladder body, base and urethra. J Urol. 1997;157:2000–2006. [PubMed] [Google Scholar]
- Saban MR, Hellmich H, Nguyen NB, Winston J, Hammond TG, Saban R. Time course of LPS-induced gene expression in a mouse model of genitourinary inflammation. Physiol Genomics. 2001;5:147–160. doi: 10.1152/physiolgenomics.2001.5.3.147. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Akassoglou K. Regulation of cAMP by the p75 neurotrophin receptor: insight into drug design of selective phosphodiesterase inhibitors. Biochem Soc Trans. 2007;35:1273–1277. doi: 10.1042/BST0351273. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Baillie GS, McCall JR, Passino MA, Schachtrup C, Wallace DA, et al. p75 neurotrophin receptor regulates tissue fibrosis through inhibition of plasminogen activation via a PDE4/cAMP/PKA pathway. J Cell Biol. 2007;177:1119–1132. doi: 10.1083/jcb.200701040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, et al. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol. 2010;298:R534–R547. doi: 10.1152/ajpregu.00367.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S, Sasaki K, Igawa Y, Nishizawa O, Chancellor MB, De Groat WC, et al. Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J Urol. 2004;171:478–482. doi: 10.1097/01.ju.0000088340.26588.74. [DOI] [PubMed] [Google Scholar]
- Shioyama R, Aoki Y, Ito H, Matsuta Y, Nagase K, Oyama N, et al. Long-lasting breaches in the bladder epithelium lead to storage dysfunction with increase in bladder PGE2 levels in the rat. Am J Physiol Regul Integr Comp Physiol. 2008;295:R714–R718. doi: 10.1152/ajpregu.00788.2007. [DOI] [PubMed] [Google Scholar]
- Sorgi FL, Bhattacharya S, Huang L. Protamine sulfate enhances lipid-mediated gene transfer. Gene Ther. 1997;4:961–968. doi: 10.1038/sj.gt.3300484. [DOI] [PubMed] [Google Scholar]
- Tanner R, Chambers P, Khadra MH, Gillespie JI. The production of nerve growth factor by human bladder smooth muscle cells in vivo and in vitro. BJU Int. 2000;85:1115–1119. doi: 10.1046/j.1464-410x.2000.00562.x. [DOI] [PubMed] [Google Scholar]
- Tong YC, Cheng JT. Aldose reductase inhibitor ONO-2235 restores the alterations of bladder nerve growth factor and neurotrophin receptor p75 genetic expression in streptozotocin induced diabetic rats. J Urol. 2007;178:2203–2207. doi: 10.1016/j.juro.2007.06.048. [DOI] [PubMed] [Google Scholar]
- Tyagi P, Kashyap M, Chancellor M, Yoshimura N. Investigation Into Constitutive Expression Of Nerve Growth Factor In Bladder By Bladder Wall Injection Of NGF Antisense. J Urology. 2014;191:882. [Google Scholar]
- Vilar M, Charalampopoulos I, Kenchappa RS, Reversi A, Klos-Applequist JM, Karaca E, et al. Ligand-independent signaling by disulfide-crosslinked dimers of the p75 neurotrophin receptor. J Cell Sci. 2009;122:3351–3357. doi: 10.1242/jcs.055061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000;161:273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Maeda T, Kwok YN. Increase of p75 immunoreactivity in rat urinary bladder following inflammation. Neuroreport. 1996;7:1141–1144. doi: 10.1097/00001756-199604260-00008. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, et al. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci. 2006;26:10847–10855. doi: 10.1523/JNEUROSCI.3023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QL, Qiao LY. Regulation of IGF-1 but not TGF-beta1 by NGF in the smooth muscle of the inflamed urinary bladder. Regul Pept. 2012;177:73–78. doi: 10.1016/j.regpep.2012.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]