Abstract
Background and Objectives
In the proximal tubule, basic drugs are transported from the renal cells to the tubule lumen through the concerted action of the H+/organic cation antiporters, multidrug and toxin extrusion 1 (MATE1) and 2K (MATE2K). Dual inhibitors of the MATE transporters have been shown to have a clinically relevant effect on the pharmacokinetics of concomitantly administered basic drugs. However, the clinical impact of selective renal organic cation transport inhibition on the pharmacokinetics and pharmacodynamics of basic drugs, such as metformin, is unknown. This study sought to identify a selective MATE2K inhibitor in vitro and to determine its clinical impact on the pharmacokinetics and pharmacodynamics of metformin in healthy subjects.
Methods
A strategic cell-based screen of 71 U.S. Food and Drug Administration (FDA)-approved medications was conducted to identify selective inhibitors of renal organic cation transporters that are capable of inhibiting at clinically relevant concentrations. From this screen, nizatidine was identified and predicted to be a clinically potent and selective inhibitor of MATE2K-mediated transport. The effect of nizatidine on the pharmacokinetics and pharmacodynamics of metformin was evaluated in 12 healthy volunteers in an open-label, randomized, two-phase crossover drug-drug interaction (DDI) study.
Results
In healthy volunteers, the MATE2K-selective inhibitor, nizatidine, significantly increased the apparent volume of distribution, half-life and hypoglycemic activity of metformin. However, despite achieving unbound maximum concentrations greater than the in vitro inhibition potency (IC50) of MATE2K-mediated transport, nizatidine did not affect the renal clearance or net secretory clearance of metformin.
Conclusion
This study demonstrates that a selective inhibition of MATE2K by nizatidine, affected the apparent volume of distribution, tissue levels and peripheral effects of metformin. However, nizatidine did not alter systemic concentrations or the renal clearance of metformin, suggesting that specific MATE2K inhibition may not be sufficient to cause renal DDIs with basic drugs.
1 Introduction
In the proximal tubule of the kidney, basic drugs are transported from the blood to the lumen of the kidney by organic cation transporter 2 (OCT2) and are eliminated to the urine by the concerted action of the H+/organic cation antiporters, multidrug and toxin extrusion 1 (MATE1) and 2K (MATE2K). Broadly selective inhibitors of multiple organic cation transporters (e.g., cimetidine for OCT2/MATE1/MATE2K, pyrimethamine for MATE1/MATE2K) have been shown to have a clinical impact on the pharmacokinetics of concomitantly administered organic cations (e.g., metformin, procainamide, ranitidine) through reduction in their renal clearance [1–4]. However, the clinical impact of selective inhibition of a single organic cation transporter on the pharmacokinetics and pharmacodynamics of basic drugs is unknown.
MATE2K is believed to be an important renal transporter for many drugs. In comparison to MATE1, which is expressed in multiple tissues (e.g., kidney, liver, muscle), MATE2K is predominately expressed in the kidney [5], and at equivalent or higher levels than MATE1 (S.W. Yee, A. Chhibber, D.L. Kroetz and K.M. Giacomini, unpublished data). MATE2K also specifically transports some drugs (e.g., oxaliplatin), which do not appear to be substrates of MATE1 [6, 7]. Studies from our laboratory have shown that a common MATE2K promoter variant (g.-130G>A, rs12943590) is associated with poor response to the biguanide, metformin in type 2 diabetic subjects [8, 9]. Taken together, these data suggest that MATE2K is important for the renal elimination of many basic drugs including metformin.
As transporter-mediated drug-drug interactions (DDIs) occur in clinical situations and have an impact on pharmacokinetics and drug safety, regulatory agencies in the United States (U.S.) and European Union have issued guidances that recommend using in vitro transporter studies to inform the decision of when to conduct a clinical DDI study. The U.S. Food and Drug Administration (FDA) recommends that a clinical investigation of a transporter-mediated drug interaction should be conducted when the Ifu/IC50 ratio (maximum plasma concentration [Cmax] of the inhibitor that is not bound to plasma proteins [Cmax,u] divided by the concentration associated with half the maximum inhibition in an in vitro assay) of the new molecular entity is ≥ 0.1 [10]. The European Medicines Agency (EMA) guidance is more stringent with a clinical study initiation cut-off ≥ 0.02 [11]. Although the current guidances focus primarily on the uptake transporters in the kidney (OCT2 and organic anion transporters 1 and 3 [OAT1 and OAT3]), the EMA and a recent publication from the International Transporter Consortium (ITC) recommend extending these guidelines to include MATE-mediated drug interactions [12].
The current decision trees within the FDA and EMA guidances focus on evaluating DDIs of individual transporters (e.g., OCT2, OAT1), rather than a transporter family (e.g., OATs, OCTs, MATEs). With the exception of cimetidine and pyrimethamine, few drugs have been identified as clinically potent inhibitors of renal organic ion transporters, and no drugs are selective inhibitors of individual renal organic cation transporters. In addition, because the inhibitors that have been identified are either promiscuous and inhibit multiple transporter families (e.g. quinidine) or are more potent inhibitors of organic cation transporter 1 (OCT1), an organic cation transporter that is highly expressed in the liver (e.g. procainamide) [13–15], distinguishing the impact of renal organic cation transporters on the victim drug’s pharmacokinetics has been difficult.
In this study, a strategic screen was implemented to identify probe inhibitors of renal organic cation transport that are selective and can inhibit transport at clinically relevant concentrations. Through in vitro assays we identified the histamine 2 antagonist, nizatidine, as a selective inhibitor of MATE2K-mediated transport with an Ifu/IC50 ratio ≥ 0.1. To determine the clinical impact of MATE2K-selective inhibition on the pharmacokinetics and pharmacodynamics of a victim organic cation, an open-label, randomized, two-phase crossover DDI study was conducted using metformin as the victim drug and nizatidine as the perpetrator. Metformin is eliminated unchanged by renal mechanisms and while the liver is important for the pharmacologic activity of metformin, biliary excretion of metformin is negligible [16, 17]. The hypotheses of this clinical DDI study were (i) the co-administration of metformin and nizatidine will reduce the renal clearance (CLR) of metformin, increase metformin concentration in the kidney and potentially lead to increased plasma concentrations and (ii) the interaction will enhance the hypoglycemic activity of metformin.
2 Methods
2.1 Physicochemical properties and clinical concentration of test inhibitors
Predicted charge at pH 7.4 was calculated using MarvinView Version 5.3.6 (ChemAxon, http://www.chemaxon.com). Cmax values of test inhibitors in human subjects and percent protein binding in human plasma were obtained from literature sources (Supplemental Table 1; [18, 19]). Cmax,u values were calculated by Cmax,u = Cmax • fu, where fu is the unbound fraction in human plasma.
2.2 In vitro identification of renal transporter inhibitors
2.2.1 Cell lines
Flp-In human embryonic kidney (HEK293-Flp-In) cells stably expressing human OCT1 (HEK-OCT1), OCT2 (HEK-OCT2), OCT3 (HEK-OCT3), MATE1 (HEK-MATE1), MATE2K (HEK-MATE2K) and the pcDNA5/FRT empty vector (HEK-EV) were previously established in our laboratory [8, 20–26]. Madin–Darby canine kidney type II (MDCK-II) cells stably expressing human PMAT (MDCK-PMAT) and the pcDNA3.1(+) vector (MDCK-EV) were also established previously [27].
2.2.2 Cellular Uptake Study
Cells were suspended in cell culture medium, seeded on poly-D-lysine-coated 48-well plates (Greiner Bio-One, Monroe, NC) and grown to ~90% confluency (~48 hours post seeding). Immediately prior to uptake, HEK-EV, HEK-OCT1, HEK-OCT2 and HEK-OCT3 cells were preincubated for 20 min with Hank’s balanced salt solution (HBSS). HEK-EV, HEK-MATE1 and HEK-MATE2K cells were preincubated with HBSS plus 30 mM NH4Cl for 20 mins. MDCK-EV and MDCK-PMAT cells were preincubated with Krebs-Ringer-Henseleit (KRH) Buffer for 20 min. Preincubation media was removed and uptake was initiated with the addition of uptake buffer (HBSS [HEK cell lines] or KRH [MDCK cell lines] containing [14C]-metformin [10 μM] with and without a test inhibitor at its 10x Cmax,u) at 37°C for a period of time for which linear uptake of metformin was observed (2–5 min). At the end of the uptake, cells were washed twice with ice-cold buffer (HBSS [HEK cell lines] or KRH [MDCK cell lines]) and lysed with 0.1 N NaOH/0.1% SDS. Intracellular radioactivity was determined by liquid scintillation counting and normalized per well of protein content as measured by bicinchoninic acid protein assay (Thermo Scientific, Rockford, IL). Each test condition was conducted in triplicate. Compounds that were selective inhibitors of the renal organic cation transporters at their 10x Cmax,u were subjected to experimental IC50 determination. Studies were conducted exactly as described above using increasing concentrations of the inhibitor (ranging from 0–40x Cmax,u of the inhibitor for a total of 8 concentrations).
2.2.3 Determination of IC50 Values
After adjusting for protein quantity and subtracting non-specific transport of metformin (measured from empty vector cells), residual values were normalized to the rate of uptake in the absence of the inhibitor (set at 100%). Dose response curves and IC50 values were obtained using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA). Briefly, inhibitor concentrations were transformed to log scale and dose-response inhibition curves were fitted with the following equation:
where bottom is the plateau of maximum inhibition observed.
Absolute IC50 values were calculated by interpolation of the fitted curve.
2.3 Clinical Drug-Drug Interaction Study in Healthy Volunteers
2.3.1 Study Design
This was an open-label, randomized, two-treatment crossover study conducted in healthy subjects (n=12) at the University of California, San Francisco (UCSF) Clinical and Translational Science Institute Clinical Resources Services, San Francisco General Hospital (SFGH) Clinical site. To be eligible for the study, subjects had to provide written informed consent, be between the ages of 18 and 45 years, be in good health (as evidenced by medical histories, physical examination and routine clinical laboratory evaluations), not be on any medications other than oral contraceptives and not have any known allergies to iodine. Once enrolled, volunteers were directed to follow a controlled carbohydrate diet (200–250 g/day) 3 days prior to each inpatient visit. Subjects were admitted to the clinical facility the night before the first dose and remained on site for the duration of the study (36 hours). After an overnight fast (10 hours), study participants received either (i) an 850 mg oral dose of metformin (Glucophage®) or (ii) a simultaneous 850 mg oral dose of metformin and a 600 mg oral dose of nizatidine (Axid®). Each study participant received both treatments separated by a minimum of 7 days. A 3-hour oral glucose tolerance test (OGTT, 75 g) was conducted 2 hours after metformin dosing (with or without nizatidine). Baseline OGTT’s, without metformin, were obtained from the same subjects in a previous study [9]. Standardized meals were provided during inpatient visits after completion of the OGTT. Following the metformin dose, subjects were asked to drink 8 oz of water every 2 hours to maintain urine flow and pH. At 10 hours after metformin dosing, 1500 mg of iohexol (Omnipaque®) was administered by slow IV push over 5 minutes. Frequent timed blood samples were collected up to 24 hours post dosing to determine plasma metformin and nizatidine concentrations. Additional blood samples were collected 10 to 14 hours post metformin dosing to determine clearance of iohexol (a measure of glomerular filtration rate). For metformin pharmacodynamics, frequent blood samples were collected 0 to 180 minutes after glucose administration. An additional blood sample was collected at 12 hours after the second dose of metformin to determine serum creatinine concentrations. Urine samples were collected 0–2, 2–4, 4–8, 8–12 and 12–24 hours after metformin dosing to calculate metformin and creatinine CLR.
2.3.2 Bioanalytical Methods
Metformin concentrations in plasma and urine were assayed by a validated liquid chromatography-tandem mass spectrometry method [28]. Nizatidine plasma concentrations were measured using the transitions m/z 332.29 to 58.08. Both the intra-day and inter-day coefficients of analysis variation were <5%. Iohexol concentrations in plasma were measured by University of Minnesota Physicians Outreach Labs (Minneapolis, MN). Lactate and glucose concentrations in plasma were analyzed by ARUP laboratories (Salt Lake City, UT). Creatinine concentrations in plasma and urine were measured by the clinical laboratories of SFGH using standard methodologies.
2.3.3 Data Analysis
2.3.3.1 Clinical Pharmacokinetics
The concentration-time curves of metformin and nizatidine were plotted using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA). The pharmacokinetic parameters of metformin, nizatidine and iohexol were determined by non-compartmental analysis (WinNonlin 4.1, Pharsight Corporation, Mountain View, CA).
2.3.3.2 Statistical Analysis
Using previously reported metformin pharmacokinetic data in healthy volunteers [29], a sample size of 12 was needed to detect a 30% difference in metformin’s renal clearance between the two treatments with >80% power. Data are presented as mean ± SD unless indicated otherwise. Paired nonparametric Student’s t-tests were used to analyze the differences in metformin pharmacokinetic and pharmacodynamic parameters using GraphPad Prism 4.0. A statistically significant result was defined when p < 0.05.
3 Results
3.1 Identification of Drugs That Selectively Inhibit Renal Organic Cation Transporters at Clinically Relevant Concentrations
FDA-approved medications (n=71, Supplemental Table 1) were selected to be screened as potential inhibitors of MATE1, MATE2K and OCT2, if they met one or more of the following criteria: (i) Cmax in human subjects was greater than 0.1 μM, (ii) fu was greater than 0.10 and (iii) predicted to have a positive or neutral charge at physiological pH (pH 7.4). These criteria were used to maximize the chance of identifying a clinically potent inhibitor of renal organic cation transporters. The selected drugs (Supplemental Table 1) span across various therapeutic classes (e.g., antibiotic, antiulcer, antiarrhythmic, antiemetic). Of the 71 drugs, which were screened at different concentrations reflecting their clinical concentrations, 4 (naloxone, quinine, procainamide and praziquantel) were inhibitors of OCT2, 10 (trimethoprim, cimetidine, ranitidine, moxifloxacin, chlorphenesin, quinine, clofazimine, abacavir, norfloxacin and ondansetron) were inhibitors of MATE1 and 11 (trimethoprim, chlorphenesin, cimetidine, ranitidine, moxifloxacin, norfloxacin, procainamide, ondansetron, famciclovir, nizatidine and quinine) were inhibitors of MATE2K, with ≥ 50% inhibition of [14C]-metformin uptake at 10x Cmax,u (Supplemental Figure 1a–c).
Based on the OCT2, MATE1 and MATE2K screening results, inhibitors were grouped as follows: (i) OCT2-selective (naloxone and praziquantel), (ii) MATE1-selective (abacavir and clofazimine), (iii) MATE2K-selective (famciclovir and nizatidine), (iv) MATE1/MATE2K dual inhibitors (chlorphenesin, cimetidine, moxifloxacin, norfloxacin, ondansetron, ranitidine and trimethoprim) and (v) OCT2/apical inhibitors (procainamide and quinine). These compounds (n=15) were then assessed for their potential to inhibit OCT1 and OCT3, which are not thought to play a significant role in renal drug elimination. Of the 15 compounds, moxifloxacin, norfloxacin, ondansetron, ranitidine and nizatidine demonstrated minimal inhibition of OCT1- or OCT3-mediated metformin uptake at their 10x Cmax,u (metformin uptake was >50%) and were therefore designated as selective inhibitors of the renal organic cation transporters (Supplemental Figure 2a–e).
Follow-up IC50 studies of moxifloxacin, norfloxacin, ondansetron, ranitidine and nizatidine were conducted in cell lines expressing each of the known metformin transporters (OCT1, OCT2, OCT3, MATE1, MATE2K and PMAT) (Figure 1a–e, Table 1). Of note, moxifloxacin, norfloxacin and ondansetron, were identified as potential clinical inhibitors of both MATE1 (Cmax,u/IC50 is 0.74, 0.20 and 0.62, respectively) and MATE2K (Cmax,u/IC50 is 3.16, 0.84 and 0.39, respectively), but not of any other metformin transporters tested. In addition, nizatidine was identified as a potent and selective inhibitor of MATE2K (Cmax,u/IC50=0.37), but not of any other metformin transporters tested. Although ranitidine was a potent inhibitor of MATE1 and MATE2K-mediated transport, it did not demonstrate selective inhibition of the two MATE transporters. In all cases, the Hill coefficient was greater than one, suggesting positive cooperativity where more than one binding site is involved in the inhibition. However, additional kinetic analyses are warranted to confirm this observation.
Figure 1. Inhibitory potency of various drugs on metformin uptake in cell lines expressing organic cation transporters.
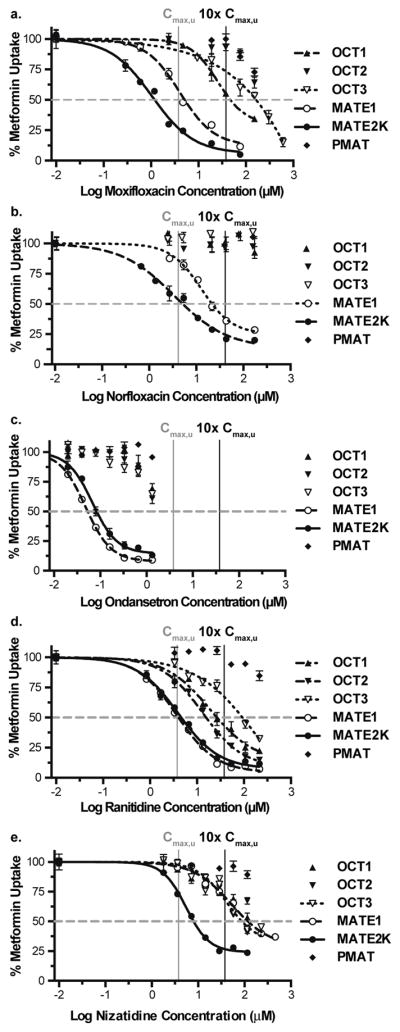
Shown are five compounds that potently inhibited MATE2K in the screening studies at or below therapeutically relevant unbound concentrations. Increasing concentrations of (a) moxifloxacin, (b) norfloxacin, (c) ondansetron, (d) ranitidine and (e) nizatidine were analyzed for their ability to inhibit metformin uptake in cells stably overexpressing OCT1, OCT2, OCT3, MATE1, MATE2K and PMAT. IC50 values were determined for drugs/cell lines that display ≥50% inhibition of metformin uptake (horizontal red line). The vertical grey and black lines are drawn at 1x and 10x the inhibitor’s Cmax,u, respectively. Values are presented as mean ± SEM (n = 3).
Table 1.
In vitro potency of putative clinical inhibitors in cells expressing OCT1, OCT2, OCT3, MATE1, MATE2K and PMAT.
| Drug | Parameter | OCT1 | OCT2 | OCT3 | MATE1 | MATE2K | PMAT |
|---|---|---|---|---|---|---|---|
| moxifloxacin | IC50 (μM) | 47.0 | >151 | >151 | 5.12 | 1.19 | >151 |
| Cmax/IC50 | 0.13 | <0.04 | <0.04 | 1.22 | 5.22 | <0.04 | |
| Cmax,u/IC50 | 0.08 | <0.02 | <0.02 | 0.74 | 3.16 | <0.02 | |
| norfloxacin | IC50 (μM) | >160 | >160 | >160 | 19.9 | 4.74 | >160 |
| Cmax/IC50 | <0.03 | <0.03 | <0.03 | 0.24 | 0.99 | <0.03 | |
| Cmax,u/IC50 | <0.02 | <0.02 | <0.02 | 0.20 | 0.84 | <0.02 | |
| ondansetron | IC50 (μM) | >1.32 | >1.32 | >1.32 | 0.05 | 0.08 | >1.32 |
| Cmax/IC50 | <0.10 | <0.10 | <0.10 | 2.47 | 1.57 | <0.10 | |
| Cmax,u/IC50 | <0.02 | <0.02 | <0.02 | 0.62 | 0.39 | <0.02 | |
| ranitidine | IC50 (μM) | 26.8 | 15.7 | 93.5 | 4.04 | 4.63 | >214 |
| Cmax/IC50 | 0.24 | 0.40 | 0.07 | 1.56 | 1.36 | <0.03 | |
| Cmax,u/IC50 | 0.20 | 0.34 | 0.06 | 1.33 | 1.16 | <0.02 | |
| nizatidine | IC50 (μM) | >115 | >115 | 41.1 | 52.7 | 7.81 | >115 |
| Cmax/IC50 | <0.04 | <0.04 | 0.11 | 0.08 | 0.56 | <0.04 | |
| Cmax,u/IC50 | <0.02 | <0.02 | 0.07 | 0.05 | 0.37 | <0.02 |
Cmax and calculated Cmax,u were obtained from literature values (see Supplemental Table 1). Cmax/IC50 and Cmax,u/IC50 values that exceed the 0.1 cut-off are bolded.
IC50, concentration at half the maximum inhibition of active transport; Cmax, maximum plasma concentration; Cmax,u, maximum plasma concentration that is not bound to plasma proteins; OCT, organic cation transporter; MATE, multidrug and toxin extrusion transporter; PMAT, plasma membrane monoamine transporter.
3.2 Determination of the Clinical Impact of MATE2K Selective Inhibition on the Pharmacokinetics and Response Of Metformin in Healthy Volunteers
An open-label, randomized, two-phase crossover DDI study was conducted in healthy volunteers (n=12) to determine the effect of MATE2K-selective inhibition by nizatidine on the exposure and response of metformin. All subjects completed the study and no adverse events were reported. The demographic and baseline characteristics of the healthy volunteers are shown in Table 2.
Table 2.
Demographic and baseline characteristics of healthy volunteers who participated in the clinical drug-drug interaction study between nizatidine and metformin
| Characteristic | N = 12 |
|---|---|
|
| |
| Age (years) | |
| Mean (range) | 27 (23 – 33) |
|
| |
| Sex, n (%) | |
| Male | 6 (50.0) |
| Female | 6 (50.0) |
|
| |
| Ethnicity, n (%) | |
| African American | 6 (50.0) |
| Caucasian | 3 (25.0) |
| Asian American | 3 (25.0) |
|
| |
| Weight (kg) | |
| Mean (range) | 74 (49 – 101) |
|
| |
| Height (cm) | |
| Mean (range) | 176 (160 – 192) |
|
| |
| BMI (kg/m2) | |
| Mean (range) | 23.9 (18.1 – 28.6) |
|
| |
| eGFR (mL/min) | |
| Mean (range) | 114 (89 – 143) |
BMI, body mass index; eGFR, estimation of glomerular filtration rate as assessed by MDRD (Modification of Diet in Renal Disease) at screening using the African American correction factor when relevant.
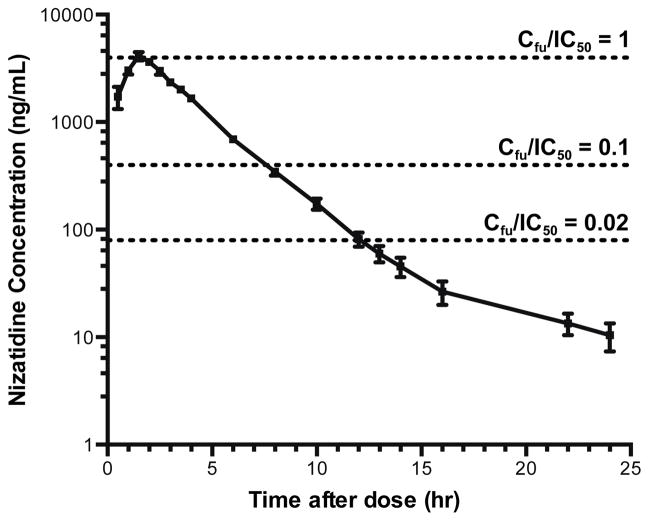
To maximize the potential for a clinical impact of MATE2K inhibition by nizatidine, a 600 mg oral dose of nizatidine (the maximum recommended daily dose) was administered to healthy volunteers. To verify that this dose of nizatidine achieved sufficient concentrations predicted to alter MATE2K activity, the pharmacokinetics of nizatidine was determined (Figure 2, Supplemental Table 2). Nizatidine reached a Cmax of 4.2 ± 0.3 μg/mL (12.7 μM) and with 35% protein binding [19], its calculated Cmax,u was 2.7 ± 0.2 μg/mL (8.2 μM). This value is greater than the in vitro MATE2K inhibition potency (IC50=7.8 μM) and above the recommended cut-off for conducting a clinical DDI study (Cmax,u/IC50=1.1) [11, 12].
Figure 2. Mean nizatidine plasma concentrations following administration of a single oral dose to 12 healthy volunteers.
Nizatidine plasma concentrations were determined after a 600 mg single oral dose in combination with metformin. The dotted horizontal lines are drawn at Cfu/IC50 ratios of 1, 0.1 (FDA cut-off) and 0.02 (EMA cut-off). Data represent mean ± SEM.
Cfu, unbound concentration in plasma; IC50, concentration at half the maximum inhibition of active transport; FDA, Food and Drug Administration; EMA, European Medicines Agency
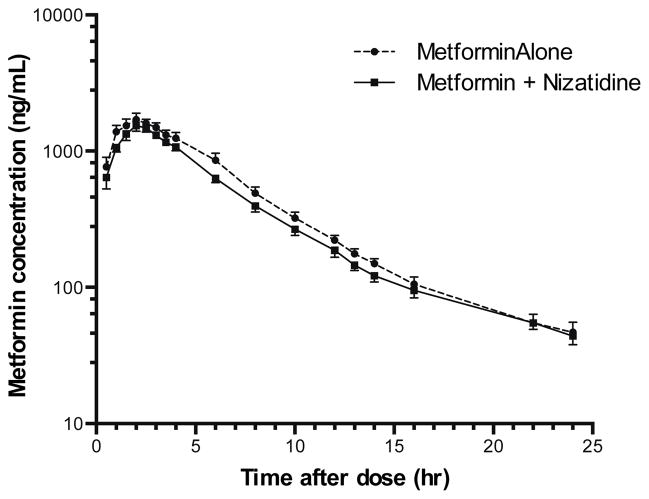
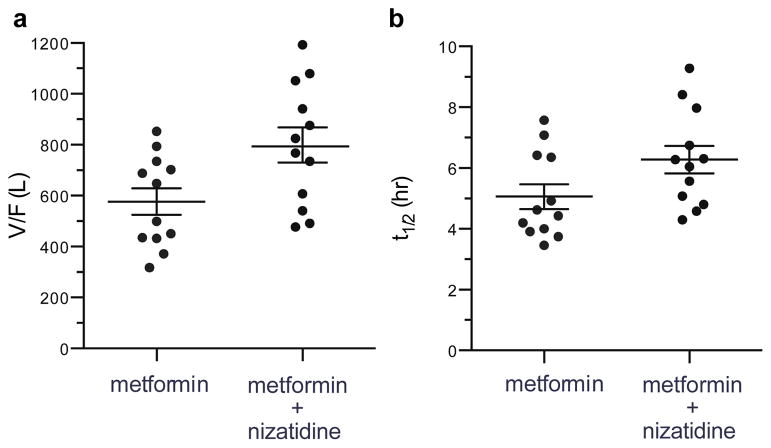
Plasma concentration-time profiles for metformin were similar following administration of metformin alone or with nizatidine (Figure 3). When nizatidine was co-administered, the V/F (apparent volume of distribution) and t1/2 (half-life) of metformin increased slightly, but significantly, by 38% and 24%, respectively (p<0.05, Table 3, Figure 4). Co-administration of nizatidine had no significant effect on metformin tmax (time to the maximal plasma concentration), Cmax, AUC0–24 (area under the concentration-time curve from 0–24 h time point), AUCinf (AUC from 0 to infinity), and CL/F (apparent oral clearance) (Figure 3, Table 3). Importantly, no effects of nizatidine on the CLR or CLSR (net renal clearance by secretion) of metformin were observed in the 11 subjects with complete urine collection.
Figure 3. Mean metformin plasma concentration-time curves after administration of metformin alone or with nizatidine to 12 healthy volunteers.
Metformin plasma concentrations were determined after a single oral dose (850 mg) alone (blue) or in combination with a single oral dose (600 mg) of nizatidine (red). Data represent mean ± SEM.
Table 3.
Summary of the pharmacokinetic parameters of metformin in healthy volunteers with and without nizatidine co-administration.
| Metformin Alone | Metformin + Nizatidine | p-value | |
|---|---|---|---|
| tmax (hr) | 2.46±0.66 | 2.13±0.38 | 0.09 |
| Cmax (μg/mL) | 1.81±0.60 | 1.68±0.55 | 0.43 |
| AUC0–24 (μg.hr/mL) | 11.0±3.1 | 9.6±2.1 | 0.20 |
| AUCinf (μg.hr/mL) | 11.4±3.3 | 10.0±2.4 | 0.25 |
| V/F (L) | 577±179 | 799±239 | 0.01 |
| CL/F (mL/min) | 1360±440 | 1480±330 | 0.46 |
| t1/2 (hr) | 5.1±1.4 | 6.3±1.6 | 0.05 |
| a Amount in urine0–24 (mg) | 437±86 | 376±128 | 0.10 |
| a CLR (mL/min) | 736±204 | 670±133 | 0.18 |
| CLIHX (mL/min) | 118±19 | 110±13 | 0.09 |
| a CLCR (mL/min) | 156±40 | 147±26 | 0.28 |
| a CLSR (mL/min) | 615±201 | 558±134 | 0.22 |
Due to incomplete urine collection from one subject, the amount in urine, CLR, CLCR, CLSR were calculated from 11 subjects. Data is presented as mean ± SD.
tmax, time to the maximal plasma concentration; Cmax, maximal plasma concentration; AUC0–24, area under the concentration-time curve from 0–24 hr time point; AUCinf, area under the concentration-time curve from 0 to infinity; V/F, apparent volume of distribution; CL/F, apparent oral clearance; t1/2, plasma terminal elimination half-life; CLR, renal clearance; CLIHX, iohexol clearance, CLCR, creatinine clearance; CLSR, net renal clearance by secretion (metformin CLR less CLIHX for each individual subject)
Figure 4. Volume of distribution (a) and half-life (b) alterations between treatment groups.
Symbols, horizontal line and brackets represent individual data points, mean value of all subjects and standard error of the mean, respectively.
V/F, apparent volume of distribution; t1/2, plasma terminal elimination half-life.
A rare, but serious adverse effect associated with metformin is lactic acidosis [30], where plasma lactate levels exceed 5 mM. There was no significant difference in plasma lactate concentrations between treatment arms (data not shown) and observed maximum concentrations were below the 5 mM toxicity threshold in both treatment groups (metformin alone, 1.7 ± 0.1 mM; metformin with nizatidine, 1.6 ± 0.1 mM).
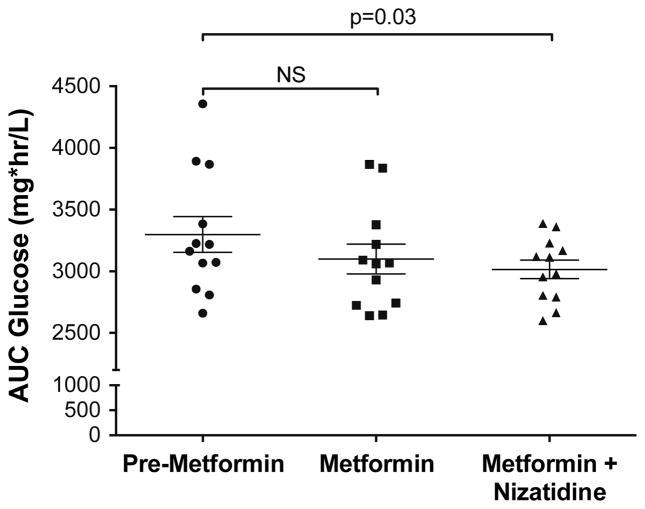
The glucose-lowering effect of metformin was determined in healthy subjects by administering a 3-hour OGTT (Supplemental Figure 3). We observed similar glucose AUC values after the OGTT between pre-metformin and metformin alone treatment periods (pre-metformin, 3298 ± 145 mg hr/L; metformin alone, 3100 ± 121 mg hr/L; p>0.1; Figure 5). However, after metformin was co-administered with nizatidine, there was a significantly lower glucose AUC (greater response) compared to pre-metformin values (pre-metformin, 3298 ± 145 mg hr/L; metformin with nizatidine, 3015 ± 74 mg hr/L; p=0.03; Figure 5).
Figure 5. The effect of nizatidine on plasma glucose concentrations after metformin treatment.
The area under the plasma glucose concentration-time curve (AUC glucose) was calculated during the oral glucose tolerance test. Symbols, horizontal line and brackets represent individual data points, mean value of all subjects and standard error of the mean, respectively. NS, not significant.
4 Discussion
Historically, DDIs were thought to occur primarily by interactions with drug-metabolizing enzymes. Current evidence suggests a role for drug transporters in mediating clinical DDIs [31, 32], however transporter-mediated DDIs have been less well characterized. As noted previously, few inhibitors of renal organic cation transporters have been identified and, of these inhibitors, none has been shown to selectively inhibit a single transporter involved in the renal elimination of basic drugs. Further, previous clinical investigations of renal organic cation transporter-mediated DDIs have focused on drugs that are predicted to alter the activity of more than one transporter [1–4, 33–36]. Thus, there is a poor understanding of the mechanisms involved in the renal clearance of organic cations in general, and of metformin in particular. Through a strategic in vitro screen, nizatidine was identified as a clinically potent and selective inhibitor of MATE2K mediated uptake of metformin. Thus, nizatidine represents a useful tool to understand the clinical significance of MATE2K in the renal elimination of metformin. In this study, nizatidine increased the apparent volume of distribution and hypoglycemic activity of metformin. However, despite achieving unbound maximum concentrations that exceeded the in vitro inhibition potency of MATE2K-mediated transport, nizatidine did not alter the renal clearance or net secretory clearance of metformin.
In our in vitro screen, several drugs appeared to stimulate metformin uptake in OCT2, MATE1 and MATE2K-transfected cells. This enhancement of in vitro activity has also been observed for a variety of solute carrier and ATP-binding cassette transporters including OAT1, MRP2, OATP1B1/1B3, and OCT2 [37–41]. Previous reports have observed the dependence of OCT2-mediated transport on inside-negative membrane potential [42, 43]. Thus, alterations in membrane potential by test compounds may explain an enhancement in metformin uptake. In our data set, the enhancement in metformin uptake was more common in MATE-transfected cell lines, which are particularly affected by alterations in pH as they transport organic cations in exchange for a proton [5, 44, 45]. Therefore, it is possible that the test compounds could have altered the pH gradient, leading to an enhancement in metformin uptake by MATE-transfected cells. Other possibilities include positive cooperativity of compounds with the transporters as well as effects of compounds on potential difference across intracellular organelles (e.g., mitochondria).
Several reasons may be proposed to explain the lack of effect of nizatidine on metformin CLR. First, previous DDI studies examining the effect of perpetrator drugs on metformin CLR have used non-selective inhibitors of MATE1 and MATE2K. For example, in a previous clinical DDI study, there was a significant reduction in the CLR and CLSR of metformin after co-administration of a MATE1/MATE2K dual inhibitor, pyrimethamine [2]. However, in the current study, we did not observe a significant reduction in the CLR or CLSR of metformin when co-administered with nizatidine, a MATE2K selective inhibitor. Compared to pyrimethamine, the half-life of nizatidine is much shorter (pyrimethamine t1/2, ~4 days [19]; nizatidine t1/2, ~3 hours). It is probable that the duration of inhibition is an important determinant of alterations in CLR, which is generally estimated over a 24-hour period. However, we also examined the fractional CLR and CLSR (e.g. 0–2 hour, 2–4 hour, 0–4 hour) of metformin, and found no differences between treatment arms (data not shown). Thus, the selective inhibition of MATE2K may be insufficient to have a measurable effect on the CLR and CLSR of metformin.
A second reason for the lack of reduction in metformin renal clearance by nizatidine is that metformin may cross the luminal membrane of the proximal tubule cell primarily by MATE1, and that MATE2K has a limited role. However, studies demonstrating an association between genetic variants of MATE2K and metformin CLR suggest that MATE2K does play a role in the renal elimination of metformin [8, 9, 46]. It is also possible that the in vitro methods used in this study incorrectly predicted the concentrations of nizatidine required to inhibit MATE2K in vivo. The IC50 for nizatidine on MATE2K-mediated transport was determined in vitro using a proton-driven influx of metformin. However, in vivo MATE2K functions as an efflux pump. A previous study examining MATE1 transport of MPP+ (1-methyl-4-phenylpyridinium) demonstrated that there are symmetrical interactions of H+ with inward-facing and outward-facing MATE1 [47]. This has not been confirmed for MATE2K-mediated transport of metformin. However, it should be noted that for in vitro studies of both pyrimethamine and cimetidine, MATE1/2K dual inhibitors of metformin transport, influx methods were used to predict IC50 values [2, 15].
Other possibilities are that concentrations of nizatidine in the renal tubule or tubule cells were not sufficient to inhibit the transporter. However, nizatidine had no effect on fractional CLR of metformin at earlier times, when nizatidine concentrations were higher. Notably, metformin concentrations were also higher at these earlier times; thus, nizatidine concentrations may not have been sufficient to reduce metformin CLR. Though speculative, it is also possible that nizatidine inhibited transporters involved in metformin reabsorption that counteracted its effects on CLSR. Because nizatidine is actively secreted in the kidney, its concentrations in the proximal tubule and proximal tubule cells should be higher than its plasma unbound concentrations [48].
These results suggest that further studies are needed to inform current guidelines provided by the EMA [11]. The EMA suggests that Ifu/IC50 ≥0.02, should trigger consideration of a clinical DDI study. However, our data suggest that very selective inhibitors may not be sufficient to cause measurable DDIs with organic cation transporters in the kidney, or that threshold ratios between Ifu and IC50 for selective inhibitors should be higher to trigger consideration of a clinical study. More promiscuous inhibitors such as pyrimethamine and cimetidine clearly result in clinical DDIs and current ratios to trigger consideration of a clinical study, though conservative, are acceptable for these non-selective inhibitors. However, our data with nizatidine suggest that a MATE2K selective inhibitor, which achieved concentrations above its IC50, clearly did not result in a clinical DDI, and that different threshold ratios for triggering consideration of a clinical DDI may be warranted.
In this study, we observed a significant increase in metformin V/F and a related increase in metformin t1/2. These results are consistent with previous reports that inhibitors of renal transporters have a direct effect on the victim drug’s volume of distribution [49]. As MATE2K is highly expressed in the kidney, an increase in metformin V/F suggests that metformin may accumulate in the renal cell when its MATE2K-mediated efflux is blocked by nizatidine co-administration.
Interestingly, while we did not see any alterations in systemic levels of metformin, the hypoglycemic activity (as determined by glucose AUC) was enhanced above pre-metformin values only when nizatidine was co-administered. Although not determined in the study, nizatidine is not known to alter glucose levels [50]. While the kidney is mostly known for its role in the reabsorption of glucose from the filtrate, it also has a significant impact on glucose uptake from blood (20% of total uptake in the post-absorptive state) [51], gluconeogenesis (20–25% of total glucose release in the post-absorptive state) [52–54] and glucose utilization (post-absorptive, 5–10%; post-prandial, 10–15% of total) [55, 56]. In the liver, metformin is known to enhance glucose uptake, decrease glucose production and increase glucose utilization (for review see [57]). Consistent with the increase in metformin V/F, our data suggest that nizatidine increases metformin levels in the kidney where it is then able to enhance glucose uptake and utilization. Our data demonstrating that inhibition of MATE2K increases response to metformin in healthy subjects are consistent with previous studies from our laboratory demonstrating a reduced response to metformin in type 2 diabetic patients who are carriers of an allele of MATE2K that is associated with increased transcription rates of the transporter [8, 9]. The concept that increasing metformin levels in the liver or kidney through inhibition of MATEs enhances its pharmacologic effects needs further study. Similar concepts have been shown for renal toxicity to platinums [58], i.e., inhibition of MATEs enhances platinum accumulation in the kidney resulting in increased nephrotoxicity [6, 7]. Further, mice with Mate1 deficiency exhibit increased metformin-induced lactic acidosis [59].
This study raises questions about extrapolating in vitro inhibition IC50 data to clinical DDI studies, and suggests that further refinement of current FDA and EMA guidelines are needed. Those refinements may include recommendations related to measurements of intracellular drug levels, relative half-life of the inhibitor compared with the substrate, and selectivity of the inhibitor for renal transporters. Clearly, future studies are needed to define best practices and standardize in vitro methodologies that accurately predict clinical DDIs and to understand the effects of selective versus nonselective inhibitors on renal drug elimination.
5 Conclusions
In healthy volunteers, a MATE2K-selective inhibitor, nizatidine, increased the apparent volume of distribution and hypoglycemic activity of metformin. However, despite achieving unbound maximum concentrations that were greater than the in vitro inhibition potency of MATE2K-mediated transport, nizatidine did not change metformin’s renal clearance or net secretory clearance. This study highlights that current guidelines, which rely on in vitro predictions to inform the decision to conduct transporter-mediated clinical DDI studies, need refinement [10–12]. Further, this study suggests that physiologically based pharmacokinetic models to predict intracellular and intra-tubular concentrations of drugs should be developed and used in combination with in vitro data to predict DDIs in the kidney. Finally, this study supports previous studies highlighting that inhibition of efflux transporters will have important effects on the apparent volume of distribution, tissue levels and peripheral effects of victim drugs [49].
Supplementary Material
Key Points.
Nizatidine was identified as a selective in vitro inhibitor of MATE2K-mediated transport of metformin at clinically relevant concentrations.
In healthy subjects, nizatidine increased the apparent volume of distribution and hypoglycemic activity of metformin. However, despite achieving unbound maximum concentrations that exceeded the in vitro inhibition potency of MATE2K-mediated transport, nizatidine did not alter the renal clearance or net secretory clearance of metformin.
The data from this study demonstrates that very selective inhibitors may not be sufficient to cause measurable DDIs with organic cation transporters in the kidney. In addition, this study challenges current health authority guidelines that rely on in vitro predictions to inform the decision to conduct transporter-mediated clinical DDI studies.
Acknowledgments
We thank Jennifer Hibma for the assistance in preparing the clinical study protocol. We thank Hector Vizoso and all the staff at GCRC at SFGH for their professionalism and enthusiastic assistance in our clinical study. We thank Yong Huang at the UCSF Drugs Services Unit for access to their bioanalytical facilities and Chav Doherty from UCSF SFGH Clinical Laboratory for excellent service.
Funding This project was supported by National Institutes of Health/National Center for Research Resources (NIH/NCRR) University of California, San Francisco (UCSF)-CTSI grant UL1 RR024131 and NIH grant GM61390. The authors are solely responsible for the contents and do not necessarily represent the official views of the NIH. This work was also made possible in part by core services provided by the General Clinical Research Center (GCRC) at San Francisco General Hospital (SFGH), funded by the National Center for Research Resources (NIH MO1-RR00083-44). The authors also acknowledge the following funding sources for S.L.S. (Allergan), E.C.C. and K.M.M. (NIH Training Grant T32 GM007175, FDA-CDER/ORISE).
Footnotes
Conflicts of Interest The authors have no conflicts of interest that might be relevant to the content of this manuscript
7 Compliance with Ethical Standards
Ethical Approval The Committee on Human Research at UCSF (Institutional Review Board [IRB] 11-06968) approved the clinical DDI study, and all subjects were recruited directly from the Study of Pharmacogenetics in Ethnically Diverse Populations (IRB 10-03167). Informed consent was obtained from all study participants.
References
- 1.Somogyi A, Stockley C, Keal J, Rolan P, Bochner F. Reduction of metformin renal tubular secretion by cimetidine in man. Br J Clin Pharmacol. 1987;23(5):545–51. doi: 10.1111/j.1365-2125.1987.tb03090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusuhara H, Ito S, Kumagai Y, Jiang M, Shiroshita T, Moriyama Y, et al. Effects of a MATE protein inhibitor, pyrimethamine, on the renal elimination of metformin at oral microdose and at therapeutic dose in healthy subjects. Clinical pharmacology and therapeutics. 2011 Jun;89(6):837–44. doi: 10.1038/clpt.2011.36. [DOI] [PubMed] [Google Scholar]
- 3.Somogyi A, McLean A, Heinzow B. Cimetidine-procainamide pharmacokinetic interaction in man: evidence of competition for tubular secretion of basic drugs. Eur J Clin Pharmacol. 1983;25(3):339–45. doi: 10.1007/BF01037945. [DOI] [PubMed] [Google Scholar]
- 4.van Crugten J, Bochner F, Keal J, Somogyi A. Selectivity of the cimetidine-induced alterations in the renal handling of organic substrates in humans. Studies with anionic, cationic and zwitterionic drugs. J Pharmacol Exp Ther. 1986 Feb;236(2):481–7. [PubMed] [Google Scholar]
- 5.Masuda S, Terada T, Yonezawa A, Tanihara Y, Kishimoto K, Katsura T, et al. Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. Journal of the American Society of Nephrology: JASN. 2006 Aug;17(8):2127–35. doi: 10.1681/ASN.2006030205. [DOI] [PubMed] [Google Scholar]
- 6.Yokoo S, Yonezawa A, Masuda S, Fukatsu A, Katsura T, Inui K. Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochemical pharmacology. 2007 Aug 1;74(3):477–87. doi: 10.1016/j.bcp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Yonezawa A, Masuda S, Yokoo S, Katsura T, Inui K. Cisplatin and oxaliplatin, but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1-3 and multidrug and toxin extrusion family) J Pharmacol Exp Ther. 2006 Nov;319(2):879–86. doi: 10.1124/jpet.106.110346. [DOI] [PubMed] [Google Scholar]
- 8.Choi JH, Yee SW, Ramirez AH, Morrissey KM, Jang GH, Joski PJ, et al. A common 5′-UTR variant in MATE2-K is associated with poor response to metformin. Clinical pharmacology and therapeutics. 2011 Nov;90(5):674–84. doi: 10.1038/clpt.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stocker SL, Morrissey KM, Yee SW, Castro RA, Xu L, Dahlin A, et al. The effect of novel promoter variants in MATE1 and MATE2 on the pharmacokinetics and pharmacodynamics of metformin. Clinical pharmacology and therapeutics. 2013 Feb;93(2):186–94. doi: 10.1038/clpt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. [Accessed May 2013];Guidance for Industry (Draft): Drug Interaction Studies - Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations. [cited; Available from: < http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm064982.htm>.
- 11. [Accessed May 2013];European Medicines Agency’s Guideline on the Investigation of Drug Interactions. [cited; Available from: < http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129606.pdf.>.
- 12.Hillgren KM, Keppler D, Zur AA, Giacomini KM, Stieger B, Cass CE, et al. Emerging transporters of clinical importance: an update from the International Transporter Consortium. Clinical pharmacology and therapeutics. 2013 Jul;94(1):52–63. doi: 10.1038/clpt.2013.74. [DOI] [PubMed] [Google Scholar]
- 13.Bourdet DL, Pritchard JB, Thakker DR. Differential substrate and inhibitory activities of ranitidine and famotidine toward human organic cation transporter 1 (hOCT1; SLC22A1), hOCT2 (SLC22A2), and hOCT3 (SLC22A3) J Pharmacol Exp Ther. 2005 Dec;315(3):1288–97. doi: 10.1124/jpet.105.091223. [DOI] [PubMed] [Google Scholar]
- 14.Minematsu T, Iwai M, Umehara K, Usui T, Kamimura H. Characterization of human organic cation transporter 1 (OCT1/SLC22A1)- and OCT2 (SLC22A2)-mediated transport of 1-(2-methoxyethyl)-2-methyl-4,9-dioxo-3-(pyrazin-2-ylmethyl)- 4,9-dihydro-1H-naphtho[2,3-d]imidazolium bromide (YM155 monobromide), a novel small molecule survivin suppressant. Drug Metab Dispos. 2010 Jan;38(1):1–4. doi: 10.1124/dmd.109.028142. [DOI] [PubMed] [Google Scholar]
- 15.Tsuda M, Terada T, Ueba M, Sato T, Masuda S, Katsura T, et al. Involvement of human multidrug and toxin extrusion 1 in the drug interaction between cimetidine and metformin in renal epithelial cells. J Pharmacol Exp Ther. 2009 Apr;329(1):185–91. doi: 10.1124/jpet.108.147918. [DOI] [PubMed] [Google Scholar]
- 16.Ito S, Kusuhara H, Kuroiwa Y, Wu C, Moriyama Y, Inoue K, et al. Potent and specific inhibition of mMate1-mediated efflux of type I organic cations in the liver and kidney by pyrimethamine. J Pharmacol Exp Ther. 2010 Apr;333(1):341–50. doi: 10.1124/jpet.109.163642. [DOI] [PubMed] [Google Scholar]
- 17.Tucker GT, Casey C, Phillips PJ, Connor H, Ward JD, Woods HF. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol. 1981 Aug;12(2):235–46. doi: 10.1111/j.1365-2125.1981.tb01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman LS, Brunton LL, Chabner B, Knollmann BC. Goodman & Gilman’s pharmacological basis of therapeutics. 12. New York: McGraw-Hill; 2011. [Google Scholar]
- 19.Moffat AC, Osselton MD, Widdop B. Clarke’s analysis of drugs and poisons: in pharmaceuticals, body fluids and postmortem material. 3. London; Chicago: Pharmaceutical Press; 2004. [Google Scholar]
- 20.Zhang S, Lovejoy KS, Shima JE, Lagpacan LL, Shu Y, Lapuk A, et al. Organic cation transporters are determinants of oxaliplatin cytotoxicity. Cancer research. 2006 Sep 1;66(17):8847–57. doi: 10.1158/0008-5472.CAN-06-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Zhang S, Sorani M, Giacomini KM. Transport of paraquat by human organic cation transporters and multidrug and toxic compound extrusion family. J Pharmacol Exp Ther. 2007 Aug;322(2):695–700. doi: 10.1124/jpet.107.123554. [DOI] [PubMed] [Google Scholar]
- 22.Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA, et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. The Journal of clinical investigation. 2007 May;117(5):1422–31. doi: 10.1172/JCI30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.More SS, Li S, Yee SW, Chen L, Xu Z, Jablons DM, et al. Organic cation transporters modulate the uptake and cytotoxicity of picoplatin, a third-generation platinum analogue. Molecular cancer therapeutics. 2010 Apr;9(4):1058–69. doi: 10.1158/1535-7163.MCT-09-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kido Y, Matsson P, Giacomini KM. Profiling of a prescription drug library for potential renal drug-drug interactions mediated by the organic cation transporter 2. Journal of medicinal chemistry. 2011 Jul 14;54(13):4548–58. doi: 10.1021/jm2001629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minematsu T, Giacomini KM. Interactions of tyrosine kinase inhibitors with organic cation transporters and multidrug and toxic compound extrusion proteins. Molecular cancer therapeutics. 2011 Mar;10(3):531–9. doi: 10.1158/1535-7163.MCT-10-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, et al. Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. Journal of medicinal chemistry. 2013 Feb 14;56(3):781–95. doi: 10.1021/jm301302s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou M, Xia L, Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos. 2007 Oct;35(10):1956–62. doi: 10.1124/dmd.107.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shu Y, Brown C, Castro RA, Shi RJ, Lin ET, Owen RP, et al. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clinical pharmacology and therapeutics. 2008 Feb;83(2):273–80. doi: 10.1038/sj.clpt.6100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Li S, Brown C, Cheatham S, Castro RA, Leabman MK, et al. Effect of genetic variation in the organic cation transporter 2 on the renal elimination of metformin. Pharmacogenet Genomics. 2009 Jul;19(7):497–504. doi: 10.1097/FPC.0b013e32832cc7e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howlett HC, Bailey CJ. A risk-benefit assessment of metformin in type 2 diabetes mellitus. Drug safety: an international journal of medical toxicology and drug experience. 1999 Jun;20(6):489–503. doi: 10.2165/00002018-199920060-00003. [DOI] [PubMed] [Google Scholar]
- 31.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010 Mar;9(3):215–36. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau YY, Huang Y, Frassetto L, Benet LZ. effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clinical pharmacology and therapeutics. 2007 Feb;81(2):194–204. doi: 10.1038/sj.clpt.6100038. [DOI] [PubMed] [Google Scholar]
- 33.Abel S, Nichols DJ, Brearley CJ, Eve MD. Effect of cimetidine and ranitidine on pharmacokinetics and pharmacodynamics of a single dose of dofetilide. Br J Clin Pharmacol. 2000 Jan;49(1):64–71. doi: 10.1046/j.1365-2125.2000.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng B, Obach RS, Burstein AH, Clark DJ, de Morais SM, Faessel HM. Effect of human renal cationic transporter inhibition on the pharmacokinetics of varenicline, a new therapy for smoking cessation: an in vitro-in vivo study. Clinical pharmacology and therapeutics. 2008 Apr;83(4):567–76. doi: 10.1038/sj.clpt.6100405. [DOI] [PubMed] [Google Scholar]
- 35.Shiga T, Hashiguchi M, Urae A, Kasanuki H, Rikihisa T. Effect of cimetidine and probenecid on pilsicainide renal clearance in humans. Clinical pharmacology and therapeutics. 2000 Mar;67(3):222–8. doi: 10.1067/mcp.2000.104018. [DOI] [PubMed] [Google Scholar]
- 36.Somogyi AA, Bochner F, Sallustio BC. Stereoselective inhibition of pindolol renal clearance by cimetidine in humans. Clinical pharmacology and therapeutics. 1992;51(4):379–87. doi: 10.1038/clpt.1992.37. [DOI] [PubMed] [Google Scholar]
- 37.Pedersen JM, Matsson P, Bergstrom CA, Norinder U, Hoogstraate J, Artursson P. Prediction and identification of drug interactions with the human ATP-binding cassette transporter multidrug-resistance associated protein 2 (MRP2; ABCC2) Journal of medicinal chemistry. 2008 Jun 12;51(11):3275–87. doi: 10.1021/jm7015683. [DOI] [PubMed] [Google Scholar]
- 38.Vanwert AL, Srimaroeng C, Sweet DH. Organic anion transporter 3 (oat3/slc22a8) interacts with carboxyfluoroquinolones, and deletion increases systemic exposure to ciprofloxacin. Molecular pharmacology. 2008 Jul;74(1):122–31. doi: 10.1124/mol.107.042853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kindla J, Muller F, Mieth M, Fromm MF, Konig J. Influence of non-steroidal anti-inflammatory drugs on organic anion transporting polypeptide (OATP) 1B1- and OATP1B3-mediated drug transport. Drug Metab Dispos. 2011 Jun;39(6):1047–53. doi: 10.1124/dmd.110.037622. [DOI] [PubMed] [Google Scholar]
- 40.Karlgren M, Vildhede A, Norinder U, Wisniewski JR, Kimoto E, Lai Y, et al. Classification of inhibitors of hepatic organic anion transporting polypeptides (OATPs): influence of protein expression on drug-drug interactions. Journal of medicinal chemistry. 2012 May 24;55(10):4740–63. doi: 10.1021/jm300212s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulgaonkar A, Venitz J, Grundemann D, Sweet DH. Human organic cation transporters 1 (SLC22A1), 2 (SLC22A2), and 3 (SLC22A3) as disposition pathways for fluoroquinolone antimicrobials. Antimicrobial agents and chemotherapy. 2013 Jun;57(6):2705–11. doi: 10.1128/AAC.02289-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busch AE, Karbach U, Miska D, Gorboulev V, Akhoundova A, Volk C, et al. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Molecular pharmacology. 1998 Aug;54(2):342–52. doi: 10.1124/mol.54.2.342. [DOI] [PubMed] [Google Scholar]
- 43.Dudley AJ, Bleasby K, Brown CD. The organic cation transporter OCT2 mediates the uptake of beta-adrenoceptor antagonists across the apical membrane of renal LLC-PK1 cell monolayers. British journal of pharmacology. 2000 Sep;131(1):71–9. doi: 10.1038/sj.bjp.0703518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terada T, Masuda S, Asaka J, Tsuda M, Katsura T, Inui K. Molecular cloning, functional characterization and tissue distribution of rat H+/organic cation antiporter MATE1. Pharmaceutical research. 2006 Aug;23(8):1696–701. doi: 10.1007/s11095-006-9016-3. [DOI] [PubMed] [Google Scholar]
- 45.Tsuda M, Terada T, Asaka J, Ueba M, Katsura T, Inui K. Oppositely directed H+ gradient functions as a driving force of rat H+/organic cation antiporter MATE1. American journal of physiology Renal physiology. 2007 Feb;292(2):F593–8. doi: 10.1152/ajprenal.00312.2006. [DOI] [PubMed] [Google Scholar]
- 46.Chung JY, Cho SK, Kim TH, Kim KH, Jang GH, Kim CO, et al. Functional characterization of MATE2-K genetic variants and their effects on metformin pharmacokinetics. Pharmacogenet Genomics. 2013 Jul;23(7):365–73. doi: 10.1097/FPC.0b013e3283622037. [DOI] [PubMed] [Google Scholar]
- 47.Dangprapai Y, Wright SH. Interaction of H+ with the extracellular and intracellular aspects of hMATE1. American journal of physiology Renal physiology. 2011 Sep;301(3):F520–8. doi: 10.1152/ajprenal.00075.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Callaghan JT, Bergstrom RF, Rubin A, Chernish S, Crabtree R, Knadler MP, et al. A pharmacokinetic profile of nizatidine in man. Scandinavian journal of gastroenterology Supplement. 1987;136:9–17. doi: 10.3109/00365528709094480. [DOI] [PubMed] [Google Scholar]
- 49.Grover A, Benet LZ. Effects of drug transporters on volume of distribution. The AAPS journal. 2009 Jun;11(2):250–61. doi: 10.1208/s12248-009-9102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.FDA. [Accessed July 2015];Highlights of prescribing information: nizatidine (Axid) < http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/21494s001lbl.pdf>.
- 51.Stumvoll M, Chintalapudi U, Perriello G, Welle S, Gutierrez O, Gerich J. Uptake and release of glucose by the human kidney. Postabsorptive rates and responses to epinephrine. The Journal of clinical investigation. 1995 Nov;96(5):2528–33. doi: 10.1172/JCI118314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Contributions of gluconeogenesis to glucose production in the fasted state. The Journal of clinical investigation. 1996 Jul 15;98(2):378–85. doi: 10.1172/JCI118803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stumvoll M, Meyer C, Mitrakou A, Nadkarni V, Gerich JE. Renal glucose production and utilization: new aspects in humans. Diabetologia. 1997 Jul;40(7):749–57. doi: 10.1007/s001250050745. [DOI] [PubMed] [Google Scholar]
- 54.Gerich JE. Physiology of glucose homeostasis. Diabetes, obesity & metabolism. 2000 Dec;2(6):345–50. doi: 10.1046/j.1463-1326.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 55.Gerich JE. Control of glycaemia. Bailliere’s clinical endocrinology and metabolism. 1993 Jul;7(3):551–86. doi: 10.1016/s0950-351x(05)80207-1. [DOI] [PubMed] [Google Scholar]
- 56.Woerle HJ, Meyer C, Dostou JM, Gosmanov NR, Islam N, Popa E, et al. Pathways for glucose disposal after meal ingestion in humans. American journal of physiology Endocrinology and metabolism. 2003 Apr;284(4):E716–25. doi: 10.1152/ajpendo.00365.2002. [DOI] [PubMed] [Google Scholar]
- 57.Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012 Nov;22(11):820–7. doi: 10.1097/FPC.0b013e3283559b22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer treatment reviews. 2007 Feb;33(1):9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toyama K, Yonezawa A, Masuda S, Osawa R, Hosokawa M, Fujimoto S, et al. Loss of multidrug and toxin extrusion 1 (MATE1) is associated with metformin-induced lactic acidosis. British journal of pharmacology. 2012 Jun;166(3):1183–91. doi: 10.1111/j.1476-5381.2012.01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.