Abstract
Background
Dosing algorithms for warfarin incorporate clinical and genetic factors, but human intervention to overrule algorithm-based dosing may occasionally be required. The frequency and reasons for varying from algorithmic warfarin management have not been well studied.
Methods
We analyzed a prospective cohort of 1015 participants from the Clarification of Optimal Anticoagulation through Genetics trial who were randomized to either pharmacogenetic- or clinically-guided warfarin dosing algorithms. Clinicians and participants were blinded to dose but not international normalized ratio (INR) during the first 28 days. If an issue arose that raised concern for clinicians but might not be adequately accounted for by the protocol, then clinicians contacted the unblinded medical monitor who could approve exceptions if clinically justified. All granted exceptions were logged and categorized. We analyzed the relationships between dosing exceptions and both baseline characteristics and the outcome of percentage of time in the therapeutic INR range during the first 4 weeks.
Results
Sixteen percent of participants required at least one exception to the protocol-defined warfarin dose (15% in the genotype arm and 17% in the clinical arm). Ninety percent of dose exceptions occurred after the first 5 days of dosing. The only baseline characteristic associated with dose exceptions was congestive heart failure (odds ratio 2.12, 95% confidence interval, 1.49-3.02, P <.001). Neither study arm nor genotype was associated with dose exceptions.
Conclusion
Despite rigorous algorithms, human intervention is frequently employed in the early management of warfarin dosing. Congestive heart failure at baseline appears to predict early exceptions to standardized protocol management.
Keywords: Anticoagulants, Congestive heart failure, Pharmacogenomics, Warfarin
Warfarin is one of the most commonly prescribed medications but is difficult to manage because of substantial variability in dose requirements within and across individuals. Despite the advent of newer oral anticoagulants for atrial fibrillation and deep venous thrombosis, warfarin continues to be widely used for these and many other clinical indications. While some clinicians use an empiric approach to adjust the dose of warfarin, there are computer-assisted algorithms that have been shown to improve time in the therapeutic international normalized ratio (INR) range compared with empiric dosing.1,2 Widely available algorithms for choosing the initial dose of warfarin incorporate clinical factors including: age, race, body surface area, smoking status, history of diabetes, history of stroke, deep vein thrombosis or pulmonary embolism as the primary indication for warfarin therapy, target INR, and major interacting medications (ie, amiodarone or fluvastatin).3,4 When available, the addition of pharmacogenetic data, including genotypes for cytochrome P-450 family 2 subfamily C polypeptide 9 enzyme (CYP2C9) and vitamin K epoxide reductase complex 1 (VKORC1), appeared to further improve warfarin dose prediction in some models,4 but not in a randomized clinical trial.5
The key components of dosing algorithms cannot account for every circumstance affecting each individual, and human intervention to overrule algorithm-based dosing may occasionally be required.2 The frequency and reasons for varying from algorithm-based warfarin management have not been well studied.
The Clarification of Optimal Anticoagulation through Genetics (COAG) trial5 was a randomized clinical trial that aimed to determine if initiation of warfarin therapy using algorithms based on genotype and clinical information (ie, pharmacogenetic-guided dosing) improved the time in the INR range compared with algorithms based on clinical information alone (ie, clinically-guided dosing). The trial found no significant difference between study arms, but provided a rare opportunity to study the applicability of warfarin dosing algorithms. During the first 28 days after enrollment in COAG, the actual dose of warfarin was blinded to both clinicians and patients, but was directed by a series of standardized computerized algorithms. Clinicians were aware of INRs. If an issue arose that raised concern for clinicians but might not be adequately accounted for by the algorithm, then clinicians contacted an unblinded COAG medical monitor who could approve exceptions to the protocol algorithm if clinically justified.
In order for these algorithms to be relied upon in clinical practice, providers should know before their use if there are specific patients or circumstances in which they might fail and how often, and if the addition of genetic data limits the need for these exceptions. We hypothesized that the baseline characteristics that would predict which patients require exceptions to algorithm-based dosing would be other medical comorbidities or indications for warfarin therapy not included in current algorithms, and location of the patient (inpatient vs outpatient) on the day of enrollment. If confirmed, these findings could lead to refinements of existing algorithms that would improve warfarin dosing in the future. Moreover, those predicted to require frequent overruling of the standard algorithms might be better served with an alternative anticoagulant.
Methods
This study was a secondary but prespecified analysis of data collected during the COAG trial. The design, rationale, and primary results of the COAG trial were previously reported.6,7 Briefly, we randomly assigned 1015 patients at 18 clinical centers in the US to initiate warfarin therapy using either a pharmacogenetic-guided or a clinically guided dosing strategy, applied during the first 5 days of therapy. The genetic variants included in the pharmacogenetic algorithms were CYP2C9 and VKORC1. For each dosing strategy, a dose-initiation algorithm was used during the first 3 days of therapy,3 and a dose-revision algorithm was used on day 4, day 5, or both.4 Randomization was stratified by self-reported race (African American vs non-African American) and study site. The trial was approved by the institutional review board at each participating site.
All study participants and clinicians were blinded to the intervention and the dose of warfarin by the use of blinded encapsulated warfarin tablets during the first 4 weeks of therapy. During the first 3 days, INRs were not required. If an INR was obtained during that time, algorithmic dose adjustments were made without any information available to clinicians about the magnitude of those adjustments. The first INR mandated by the protocol was on day 4 or 5 and again provided no information to clinicians about the revised dose. The frequency of subsequent INR testing was guided by protocol for the first 28 days. During that period, clinicians were aware of the percent change in warfarin dose but not the actual dose itself. The primary outcome of COAG was the percentage of time in therapeutic INR range (PTTR) during the initial 4 weeks, using a standard linear interpolation method between successive INR values.8
For the present analysis, the primary outcome of interest was an exception to the dosing algorithm during the first 4 weeks of therapy due to clinical issues or concerns, as defined by the medical monitor. If the medical monitors granted an exception, then their nonalgorithmic warfarin dose was provided to the study pharmacist but not to the clinical team. The medical monitor maintained a log of every dose exception decision. Every discrepancy between the calculated and dispensed dose was categorized as one of the following: interacting medication or concurrent illness, nutritional status, adherence issue or participant error, bleeding, invasive procedure, too many adjustments due to overly frequent (usually daily) INR, clinician concern due to persistently low INR, clinician concern due to persistently high INR, adjustment after prior zero dose, and conflicting or nonstudy INR. An adjustment after a prior zero dose could not be calculated by the algorithm because a percentage change to zero is still zero. If the prior zero dose was a result of bleeding or invasive procedure, it was initially categorized according to those specific reasons for exception. If the prior zero dose was a result of excessively high INR or other reason for temporary cessation of warfarin, then it was categorized as adjustment after zero dose. Each of these was considered a true exception to the dosing algorithm, as human intervention was required. Data entry and minor rounding discrepancies were not considered exceptions to the dosing algorithm.
Statistical Methods
Exceptions to the protocol-specified doses algorithm were summarized overall and by self-reported race (African American or non-African American), both as a proportion of all doses dispensed and as a proportion of participants. Fisher's exact tests and Wilcoxon rank-sum tests compared characteristics at randomization between participants with and without any exception to the dosing algorithms to which they were randomized. Any characteristic with P <.2 was included in a multivariable logistic regression model to estimate the characteristic's association with the odds of an exception, adjusted for age, sex, and prespecified study design variables (study intervention and variables used to stratify randomization: self-reported race and clinical center). In secondary analyses, we determined whether these associations differed by study intervention (clinically guided or pharmacogenetic-guided initiation) using interaction terms. Linear regression models estimated the difference in mean PTTR between participants with and without any exception to the dosing algorithm. An initial model adjusted for design variables; characteristics identified above were also included in the model. All statistical tests were 2-sided. All analyses were conducted using R 3.0.1 (R Development Core Team, Vienna, Austria).
Results
A total of 1015 participants were randomized in the COAG trial; 161 (16%) required at least one dose exception during the first 4 weeks of therapy (127 had one and 34 had more than one dose exception). There was no difference between study arms (15% pharmacogenetic and 17% clinical arms, P = .55).
Among the 8969 blinded warfarin doses during the first 4 weeks, 207 (2%) were dose exceptions. Of these, 10% occurred during the first 5 days, during which the dose initiation and dose revision algorithms were employed and differed according to study arm, and 90% occurred between days 6 and 28, during which the dose was titrated according to an algorithm based solely on INR. The frequency and reasons for exceptions to the dosing algorithms are summarized in Table 1. The most common reasons were dose adjustments after a dose of zero (which was the result of previously excessively high INRs or other interruptions of therapy) or other clinician concerns due to repeatedly high INRs. The absolute value of the difference between the algorithm-prescribed dose and the dose exception was a mean of 1.7 ± 2.0 mg daily and median 0.7 (interquartile range 0.4-2.2) mg daily, and 33% of the dose exceptions were lower than the algorithm-defined dose.
Table 1. Frequency and Reasons for Exceptions to the Dosing Algorithms.
| Dose Requests | Participants | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| All (8969) | African American (2409) | Non-African American (6559) | All (1015) | African American (275) | Non-African American (740) | |
| Any exception | 207 (2) | 70 (3) | 137 (2) | 161 (16) | 54 (20) | 107 (14) |
| Reason for exception* | ||||||
| Interacting medication or concurrent illness | 20 (<1) | 6 (<1) | 14 (<1) | 17 (2) | 6 (2) | 11 (1) |
| Nutritional status | 17 (<1) | 3 (<1) | 14 (<1) | 14 (1) | 3 (1) | 11 (1) |
| Adherence issue or participant error | 32 (<1) | 15 (1) | 17 (<1) | 29 (3) | 14 (5) | 15 (2) |
| Bleeding | 6 (<1) | 1 (<1) | 5 (<1) | 6 (1) | 1 (<1) | 5 (1) |
| Invasive procedure | 19 (<1) | 6 (<1) | 13 (<1) | 14 (1) | 3 (1) | 11 (1) |
| Too frequent INR | 11 (<1) | 6 (<1) | 5 (<1) | 11 (1) | 6 (2) | 5 (1) |
| Clinician concern due to persistently low INR | 14 (<1) | 2 (<1) | 12 (<1) | 14 (1) | 2 (1) | 12 (2) |
| Clinician concern due to persistently high INR | 40 (<1) | 14 (1) | 26 (<1) | 33 (3) | 12 (4) | 21 (3) |
| Adjustment after prior zero dose | 45 (<1) | 16 (<1) | 29 (<1) | 37 (4) | 12 (4) | 25 (3) |
| Conflicting or nonstudy INR | 3 (<1) | 1 (<1) | 2 (<1) | 3 (<1) | 1 (<1) | 2 (<1) |
Summaries presented as n (% of dose requests) and n (% of participants) overall and by self-reported race.
INR = international normalized ratio.
An exception could have multiple reasons.
The characteristics of the patients according to their need for dose exceptions are summarized in Table 2. Participants requiring exceptions were more likely to have a history of congestive heart failure (20% vs 11%) than those without exceptions (P = .004). Dose exceptions also appeared to be more frequent in African Americans, participants with hypertension, and those taking amiodarone, although none of these differences were statistically significant in unadjusted analyses.
Table 2. Participant Characteristics at Randomization, Stratified by Subsequent Need for Any Exception to the Dosing Algorithm*.
| Exception Required (n = 161) | Exception Not Required (n = 849) | P Value | |
|---|---|---|---|
| Intervention | |||
| Pharmacogenetic-guided dosing | 78 (48) | 436 (51) | .55 |
| Demographic characteristics | |||
| Age, years, median† | 58 (44, 70) | 58 (47, 69) | .73 |
| Male sex | 78 (48) | 437 (51) | .49 |
| African American race† | 54 (34) | 220 (26) | .053 |
| Hispanic ethnicity | 9 (6) | 55 (6) | .86 |
| Education | .17 | ||
| Did not complete high school | 21 (13) | 75 (9) | |
| High school degree only | 39 (24) | 224 (26) | |
| Postsecondary education | 86 (53) | 510 (60) | |
| Did not respond | 15 (9) | 40 (5) | |
| Current smoker† | 24 (15) | 120 (14) | .81 |
| Body surface area, m2, median† | 1.98 (1.83, 2.18) | 2.03 (1.84, 2.22) | .21 |
| Warfarin and other therapies | |||
| Inpatient initiation | 91 (57) | 433 (51) | .23 |
| Indication for warfarin therapy | .30 | ||
| DVT or PE only | 90 (56) | 498 (59) | |
| Atrial fibrillation/flutter only | 32 (20) | 189 (22) | |
| Other indication only | 17 (11) | 90 (11) | |
| Multiple indications | 20 (12) | 66 (8) | |
| No indication given | 2 (1) | 6 (1) | |
| DVT or PE as primary indication † | 98 (61) | 522 (61) | .93 |
| Expected duration of warfarin therapy | .70 | ||
| 1 month | 9 (6) | 57 (7) | |
| 1-3 months | 8 (5) | 56 (7) | |
| >3 months | 144 (89) | 736 (87) | |
| Prior warfarin use | 13 (8) | 71 (9) | >.99 |
| Current amiodarone use† | 7 (4) | 16 (2) | .077 |
| Current fluvastatin use† | 1 (1) | 2 (<1) | .41 |
| Current heparin use | 93 (58) | 464 (55) | .49 |
| Medical history | |||
| Congestive heart failure | 32 (20) | 94 (11) | .004 |
| Deep vein thrombosis | 47 (31) | 246 (30) | .92 |
| Diabetes† | 42 (26) | 196 (23) | .42 |
| Hypertension | 96 (62) | 443 (54) | .077 |
| Myocardial infarction | 20 (13) | 74 (9) | .14 |
| Pulmonary embolism | 38 (25) | 175 (22) | .40 |
| Stroke† | 10 (6) | 58 (7) | .87 |
| Genetic variants | |||
| CYP2C9*2† | .97 | ||
| No variants | 135 (84) | 697 (82) | |
| Heterozygous | 25 (16) | 137 (16) | |
| Homozygous | 1 (1) | 10 (1) | |
| Withdrew before genotyping | 0 (0) | 5 (1) | |
| CYP2C9*3† | .26 | ||
| No variants | 142 (88) | 776 (91) | |
| Heterozygous | 19 (12) | 67 (8) | |
| Homozygous | 0 (0) | 1 (<1) | |
| Withdrew before genotyping | 0 (0) | 5 (1) | |
| VKORC1 (VKORC1 3673G>A) | .45 | ||
| No variants (GG) | 75 (47) | 408 (48) | |
| Heterozygous (AG or GA) | 62 (39) | 340 (40) | |
| Homozygous (AA) | 24 (15) | 96 (11) | |
| Withdrew before genotyping | 0 (0) | 5 (1) | |
DVT = deep vein thrombosis; PE = pulmonary embolism.
Summaries presented as n (%) unless otherwise indicated as median (25th, 75th percentile). P values obtained from Fisher's exact tests or Wilcoxon rank-sum tests.
Variable used in pharmacogenetic or clinical dose-initiation or dose-refinement algorithm.
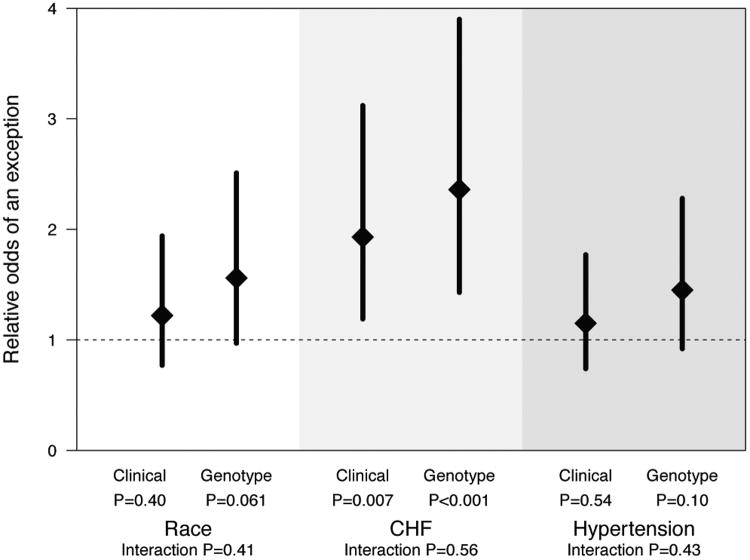
In multivariable analyses adjusted for age, sex, race, intervention, and clinical center, only congestive heart failure was found to be associated with the odds of any dose exception (odds ratio 2.12; 95% confidence interval, 1.49-3.02, P <.001) (Table 3). This finding was consistent in both the clinically guided or pharmacogenetic-guided dosing groups (Figure). Amiodarone use was too rare to include in the model.
Table 3. Adjusted Odds Ratio Comparing the Odds of an Exception between Groups.
| Adjusted Odds Ratio (95% CI)* | P Value | |
|---|---|---|
| Race (African American vs Non) | 1.37 (0.96-1.97) | .084 |
| History of CHF (Yes vs No)† | 2.12 (1.49-3.02) | <.001 |
| History of hypertension (Yes vs No) | 1.29 (0.93-1.79) | .13 |
CHF = congestive heart failure; CI = confidence interval.
Relative odds of an exception between groups (eg, African American vs non-African American race), estimated from a multivariable logistic regression model, adjusted for age, sex, intervention, and clinical center.
Among participants with congestive heart failure, there were 39 exceptions (19% of all 207 exceptions). Reasons for the exceptions were: interacting medication or concurrent illness (n = 3); nutritional status (n = 1); adherence issue or participant error (n = 6); bleeding (n = 2); invasive procedure (n = 6); too frequent international normalized ratio (INR) (n = 3); clinical concern due to persistently low INR (n = 3); clinical concern due to persistently high INR (n = 7); adjustment after prior zero dose (n = 6); conflicting or nonstudy INR (n = 2).
Figure.
Relative odds of an exception stratified by intervention (ie, clinically guided or pharmacogenetic-guided initiation). Diamonds represent estimated odds ratios comparing the odds of an exception between groups (eg, African American vs non-African American race), estimated from multivariable logistic regression models with group-by-intervention interaction terms and adjusted for age, sex, and clinical center. Models for congestive heart failure (CHF) and hypertension additionally adjusted for race. Error bars represent 95% confidence intervals. P values evaluate whether odds ratios are equal to 1. Interaction P values evaluate equality in odds ratios between interventions.
The 126 participants with congestive heart failure accounted for 12% of the overall COAG population but required 39 (19%) of all 207 exceptions. Reasons for the exceptions among these participants were: interacting medication or concurrent illness (n = 3); nutritional status (n = 1); adherence issue or participant error (n = 6); bleeding (n = 2); invasive procedure (n = 6); too frequent INR (n = 3); clinical concern due to persistently low INR (n = 3); clinical concern due to persistently high INR (n = 7); adjustment after prior zero dose (n = 6); conflicting or nonstudy INR (n = 2).
The impact of dose exceptions on PTTR during the first 28 days was tested in a series of multivariable models. Participants requiring a dose exception had markedly lower PTTR (adjusted mean difference 12.6%, P <.001) after adjustment for race, study intervention, and clinical center, as well as after additional adjustment for congestive heart failure and hypertension.
Discussion
Numerous patient-level and system-level factors impact the use of warfarin in clinical practice.3,4,9 Only a selection of these have been incorporated into standardized dose prediction algorithms, namely age, race, body size, diabetes, current smoking, indication for anticoagulation (limited to deep venous thrombosis/pulmonary embolism or stroke), and concomitant use of 2 specific interacting medications (amiodarone or fluvastatin). However, no model for predicting warfarin dosing has been able to account for more than about 50% of the interindividual variability in PTTR,4,10 suggesting that dose titration will often rely on other unknown clinical, environmental, or genetic factors. Further, the extent of human oversight needed for the application of these algorithms has not been studied, yet the clinical judgment imposed by such oversight likely considers a variety of overt and implicit factors that may have been overlooked in the development of the algorithms. The COAG trial provided an opportunity to evaluate the basis for these decisions because clinicians were blinded to dose and had to get approval for dosing exceptions. We found that only 2% of all dose requests during the first 4 weeks of therapy required an exception to the standardized algorithm. However, this corresponded to about 1 in 6 patients, indicating the need for close clinical monitoring during this critical time period rather than blind reliance on existing algorithms. Further, the need for an exception was associated with lower PTTR, suggesting that these exceptions might be potent markers of early warfarin management failure, although it is also possible that the need for dose exceptions simply identified a group of patients who were already not well controlled on the drug (eg, due to poor adherence or other reasons).
The sole clinical factor at baseline associated with the need for dose exceptions was congestive heart failure. Congestive heart failure has not been established by itself to be an indication for warfarin,11,12 but is a common comorbid condition among patients with atrial fibrillation, cardiomyopathy, recent myocardial infarction, and other cardiac disorders that may require anticoagulation. Further, heart failure is a dynamic process, with concomitant renal and hepatic dysfunction, and often requires adjustment of several medications. These factors may contribute to the high risk of warfarin dosing failure, and these patients may derive greater benefit from other anticoagulant strategies. However, subjects with both systolic heart failure and atrial fibrillation did not have larger reductions in the risk of stroke and systemic embolism in trials of apixaban,13 rivaroxaban,14 or dabigatran15 compared with warfarin.
Interestingly, neither genotype nor study arm were associated with the need for dose exceptions. Although dosing algorithms that incorporate genotype information may better predict the final warfarin maintenance dose (particularly among non-African Americans), these algorithms did not improve PTTR in this study. The premise that the use of genetics for dose prediction at initiation of warfarin leads to a period of better INR control, including fewer dose exceptions, in the ensuing month is not supported by these data.
There are several limitations to this analysis. In the COAG trial, clinicians were blinded to dose and may have acted differently than in routine clinical practice, which may affect the generalizability of our findings. Dose exceptions were requested based on the concern of the local clinician and approved by the medical monitor, but it is possible that the algorithm-prescribed dose could have performed better without clinician intervention or better than the dose chosen by the medical monitor.2 We believe this is unlikely because the joint opinion of both clinicians was that the algorithm dose did not fully account for other clinical factors, but it is also possible that other dose changes could have been more effective at achieving the target INR. We also did not record requests for dose exceptions that were not granted by the unblinded medical monitor, but in practice some of those may have led to additional human intervention. Congestive heart failure was categorized only as present or absent, without detailed information about the cause or severity. Similarly, assessments of hepatic function were not performed, though patients with severe liver disease at baseline were excluded from COAG. We also performed multiple comparisons that could have yielded spurious results. Finally, as noted above, we cannot exclude a reversal of cause and effect to explain the association between dose exceptions and PTTR.
In conclusion, we found that a substantial fraction of patients treated with warfarin required manual overrides of a standardized dosing algorithm during the first 4 weeks of therapy. We further identified an association between congestive heart failure and the need for human oversight and intervention in the management of warfarin dosing.
Clinical Significance.
We found that a substantial fraction of patients (16%) treated with warfarin required manual overrides of a standardized dosing algorithm during the first 4 weeks of therapy.
We further identified an association between congestive heart failure and the need for human oversight and intervention in the management of warfarin dosing.
As heart failure remains one of the leading disorders for which warfarin is still prescribed in this era of newer anticoagulants, this finding suggests an ongoing and unmet need for improved therapy in this population.
Acknowledgments
Funding: National Heart Lung and Blood Institute, National Institutes of Health (contract HHSN-268200800003C). Bristol-Myers Squibb donated Coumadin (warfarin). GenMark Diagnostics and AutoGenomics loaned genotyping platforms to the clinical centers.
Footnotes
Conflict of Interest: No other potential conflicts of interest relevant to this article were reported.
References
- 1.Poller L, Keown M, Ibrahim S, et al. An international multicenter randomized study of computer-assisted oral anticoagulant dosage vs. medical staff dosage. J Thromb Haemost. 2008;6(6):935–943. doi: 10.1111/j.1538-7836.2008.02959.x. [DOI] [PubMed] [Google Scholar]
- 2.Grzymala-Lubanski B, Själander S, Renlund H, Svensson PJ, Själander A. Computer aided warfarin dosing in the Swedish national quality registry AuriculA – algorithmic suggestions are performing better than manually changed doses. Thromb Res. 2013;131(2):130–134. doi: 10.1016/j.thromres.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Gage BF, Eby C, Johnson JA, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther. 2008;84(3):326–331. doi: 10.1038/clpt.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenzini P, Wadelius M, Kimmel S, et al. Integration of genetic, clinical, and INR data to refine warfarin dosing. Clin Pharmacol Ther. 2010;87(5):572–578. doi: 10.1038/clpt.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimmel SE, French B, Kasner SE, et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med. 2013;369(24):2283–2293. doi: 10.1056/NEJMoa1310669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French B, Joo J, Geller NL, et al. Statistical design of personalized medicine interventions: the Clarification of Optimal Anticoagulation through Genetics (COAG) trial. Trials. 2010;11:108. doi: 10.1186/1745-6215-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimmel SE, French B, Anderson JL, et al. Rationale and design of the Clarification of Optimal Anticoagulation through Genetics trial. Am Heart J. 2013;166(3):435–441. doi: 10.1016/j.ahj.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–239. [PubMed] [Google Scholar]
- 9.Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 10.Johnson JA, Gong L, Whirl-Carrillo M, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther. 2011;90(4):625–629. doi: 10.1038/clpt.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massie BM, Collins JF, Ammon SE, et al. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation. 2009;119(12):1616–1624. doi: 10.1161/CIRCULATIONAHA.108.801753. [DOI] [PubMed] [Google Scholar]
- 12.Homma S, Thompson JL, Pullicino PM, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366(20):1859–1869. doi: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 14.van Diepen S, Hellkamp AS, Patel MR, et al. Efficacy and safety of rivaroxaban in patients with heart failure and nonvalvular atrial fibrillation: insights from ROCKET AF. Circ Heart Fail. 2013;6(4):740–747. doi: 10.1161/CIRCHEARTFAILURE.113.000212. [DOI] [PubMed] [Google Scholar]
- 15.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]