Abstract
Bone marrow derived mesenchymal progenitor cells (MPCs) play an important role in bone homeostasis. Age-related changes occur in bone resulting in a decrease in bone density and a relative increase in adipocity. Although in vitro studies suggest the existence of an age-related lineage switch between osteogenic and adipogenic fates, stem cell and microenvironmental contributions to this process have not been elucidated in vivo. In order to study the effects of MPC and microenvironmental aging on functional engraftment and lineage switching, transplantation studies were performed under non-myeloablative conditions in old recipients, with donor MPCs derived from young and old green fluorescent protein (GFP) transgenic mice. Robust engraftment by young MPCs or their progeny was observed in the marrow, bone-lining region and in the matrix of young recipients; however, significantly lower engraftment was seen at the same sites in old recipients transplanted with old MPCs. Differentiation of transplanted MPCs strongly favored adipogenesis over osteogenesis in old recipients irrespective of MPC donor age, suggesting that microenvironmental alterations that occur with in vivo aging are predominately responsible for MPC lineage switching. These data indicate that aging alters bone-fat reciprocity and differentiation of mesenchymal progenitors toward an adipogenic fate.
Keywords: aging, osteoporosis, mesenchymal stem cells, engraftment, bone, fat
1. Introduction
Aging leads to osteopenia-related bone fragility and eventually osteoporosis with high risk of fracture. There is also an increase in bone marrow fat with aging [1–3]. Not only is this increase in fat associated with an overall decrease in bone mass, implying a decrease in osteoblasts and osteocytes, but also there is histomorphometric evidence suggesting that such changes occur at sites of bone loss [4]. Moreover, similar increases in fat content are also observed in conditions where bone loss is due to reasons other than aging, including immobilization[5], exposure to zero gravity[6, 7], ovariectomy[8], or by administration of pharmacological doses of glucocorticoids [9, 10]. Such an inverse relationship between adipocyte and osteoblast/osteocyte content in bone indicates the possibility of lineage switching with age.
It is now well established that both adipocytes and osteoblasts arise from marrow mesenchymal progenitor cells (MPCs) [11, 12]. Increases in MPCs committed towards the adipocyte lineage and decreases in those committed towards the osteoblast lineage with age could possibly explain their reciprocal representation in aging bone [13]. This hypothesis is supported by in vitro experiments on bone marrow-derived MPCs showing that factors which induce adipocyte differentiation inhibit osteoblast differentiation [14, 15], while those that induce osteoblast differentiation inhibit adipogenesis [16, 17]. Several factors and signaling pathways have been implicated in the control of MPC differentiation into osteoblastic and adipocytic cells, including TAZ (transcriptional coactivator with PDZ-binding motif) [18], PPARg2 [19], ΔFosB [20], canonical Wnt-β-catenin and non-canonical Wnt signaling pathways [21]. The effects of the BMPs on adipogenesis are context and cell type-specific [22–25]. Pre-osteoblastic and pre-adipocytic cells exist in bone marrow stroma and have been used to identify secreted factors that exert regulatory effects on osteoblastogenesis and adipogenesis, including secreted frizzled-related protein 1 (sFRP-1) and delta-like1 (preadipocyte factor 1) (Dlk1/Pref-1) [26].
Enhanced adipocyte differentiation from MPCs may lead to reduction in the number of stem cells available for osteoblast differentiation and subsequent bone formation [27]. This hypothesis is supported by experimental evidence in MSC cultures [14, 27, 28]. Studies in older mice [13] and in the senescence-accelerated mouse model (SAMP-6) [29] demonstrated relatively greater adipogenesis and less osteoblastogenesis in murine MPC cultures. Conversely, Justesen et al. 2002 showed that the adipocyte-forming capacity of human MPCs does not change with donor age [30]. However, sera from elderly donors inhibited osteoblast differentiation [31] and enhanced adipogenesis of MPC [32], suggesting that age-related changes in the bone microenvironment may play a role in directing MSC differentiation into osteoblasts and adipocytes. The decline in mineral apposition rate and mean wall thickness with increasing age as well as the associated increased bone marrow adipose tissue volume [33, 34] are hallmarks of the complex relationship between reciprocal osteoblast-adipocyte differentiation and MPC aging in vitro and in vivo and has been reviewed in detail [35, 36].
In the present study we use MPCs transplanted under non-myeloablative conditions in order to address biologically important questions regarding the effects of intrinsic and micro-environmental aging on functional engraftment and differentiation of these cells in bone.
2. Materials & Methods
2.1 Chemicals and tissue culture reagents
Rabbit anti-GFP polyclonal antibody and rabbit anti-mouse FABP4 polyclonal antibody were obtained from Abcam, Cambridge, MA. Goat anti-rabbit Alexa® 647, goat-anti-rabbit Marina® blue, mouse anti-Sca1 F(ab’)2 Alexa® 647, rat anti-mouse CD45, goat anti-rat Alexa® 555, goat anti-mouse Alexa® 555, Sytox® green, BrdU (bromodeoxyuridine), mouse anti-BrdU, DAPI (4’,6-diamidino-2-phenylindole), α-MEM (minimum essential medium), FBS (fetal bovine serum), Aqua Live/Dead stain, TrypLE™ xpress and PBS were procured from Life Technologies (Grand Island, NY). Dexamethasone, beta-glycerophosphate, ascorbic acid, 3-isobutyl-1-methylxanthine, rosiglitazone, oil-red ‘O’, and DMSO (dimethyl sulfoxide) were obtained from Sigma-Aldrich (St Louis, MO). Alizarin red was obtained from Ricca Chemical Company (Arlington, TX). Immunocal™ was purchased from Decal Chemical Corporation (Tallman, NY). Tween-20 was purchased from Biorad Laboratories (Hercules, CA), Paraformaldehyde was purchased from Electron Microscopy Sciences (Hatfield, PA) whereas Flouromount G and glass cover-slips (12 mm) were obtained from Fisher Scientific (Pittsburg, PA) and cultureware from Corning (Corning, NY).
2.2 Animals
C57BL/6-Tg (CAG-EGFP)1Osb/J female mice (JAX Labs, Bar Harbor, Maine) were used as the source of GFP positive bone marrow cells for all experiments. The recipients were C57BL/6 wild type males. All experiments involving animals were approved by The University of Pennsylvania Institutional Animal Care and Use Committee (IACUC).
2.3 Mesenchymal progenitor cell (MPC) isolation and culture
Long bones (femurs and tibiae) obtained from GFP positive animals were de-crowned by removing the epiphyseal regions and flushed with α-MEM containing 10 % FBS and antibiotic/antimycotic solution (Invitrogen, Grand Island, NY), using a 23 G needle. Bone marrow cells were collected by centrifugation (300 × g, 5 min) and plated in 100 mm tissue culture dishes. After 3 days, the supernatant was removed and medium was replenished in the original culture. Media was changed every 3 days until a confluent culture was obtained. Cells were passaged using TrypLE Express treatment for 2 min and plated into fresh plates (1 × 104 cells/cm2). At each passage cells were counted and values for the cumulative population doubling level (CPDL) were calculated as described [37]. Cultures were defined as being at the end of their proliferative lifespan when they were unable to complete one population doubling during a 2-week period.
2.4 Osteoblast differentiation
Briefly, 1×104 MPCs/cm2 were placed in 6 well plates. Cells were maintained in medium consisting of α-MEM with 10% FBS and antibiotic/antimycotic solution until confluency. Osteogenic induction was given with media consisting of α-MEM, 10% FBS, 50 µg/ml L-ascorbic acid-2-phosphate, 2 mM L-glutamine, 10−7 M dexamethasone, and 10 mM β-glycerophosphate. Media was replaced every 3 days for 2–3 weeks or until the appearance of mineralization. Cells were fixed for 15 minutes with 3.7% formaldehyde at room temperature and mineralization was determined by staining with Alizarin red S solution by standard procedures and visualized using a Nikon Eclipse TS100 (Nikon Instruments Inc. Milville, NY) microscope. Images were captured using a Nikon (Digital Sight DS-U2) camera and Nikon NIS Elements software (version F3.0).
2.5 Adipogenic Differentiation
Briefly, 1×104 MPCs/cm2 were plated/well in 6 well plates in DMEM supplemented with 10% FBS and antibiotic/antimycotic solution until confluency. Media was then replaced with DMEM containing 500 mM 3-isobutyl-1-methylxanthine, 1 mM dexamethasone, and 1 mM rosiglitazone. After 2–3 weeks, cells were fixed with 4% paraformaldehyde, washed with 60% isopropanol for 5 minutes, and stained with Oil Red O (0.25% wt/vol) for 10 minutes. After staining, cells were washed several times with H2O. Stained cells were visualized using a Nikon Eclipse TS100 (Nikon Instruments Inc. Milville, NY) microscope. Images were captured using a Nikon (Digital Sight DS-U2) camera and Nikon NIS Elements software (version F3.0).
2.6 BrdU labeling Index
Cells were plated on 12 mm cover-slips placed in a 24-well plate (1 × 104 cells/well) and cultured for 12 h to allow adherence to the surface. This was followed by pulsing with BrdU (10 µM final conc.) for 24 h. The cover-slips were then washed with PBS (0.1M) (×2) and fixed with 4% paraformaldehyde. After fixation, cells were incubated with 1N HCl for 10 min on ice followed by 10 min with 2N HCl at room temperature (RT) and then for 20 min with 2N HCl at 37°C. The reaction was neutralized with borate buffer and cover-slips were washed with PBS-1% triton x-100 (×3). The samples were then blocked with a solution containing 0.1M PBS-1% triton x-100 and 5 % normal goat serum (NGS) for 1 h, followed by incubation with mouse anti-BrdU antibody (1:200 dilution) for 18 h. The samples were then washed with PBS-1% triton x-100 (×3) followed by incubation with goat anti-mouse Alexa® 555 antibody (1:500 dilution) for 1 hr. Cell nuclei were stained with DAPI and the cover-slips were mounted with flouromount G. One thousand DAPI+ cells were scored per sample and the BrdU labeling index was calculated as [(number of Alexa® 555 positive cells/total number of cells) × 100].
2.7 Flow Cytometry
Cells were analyzed for CD45 and Sca-1 expression using flow cytometry. Briefly, 0.5 × 106 cells/sample were washed with FACS buffer (PBS + 2% FBS, ×2) and blocked with anti-mouse Fc receptor IgG (1:10 dilution) for 10 min on ice. After two washes with PBS, cells were incubated with goat anti-mouse Sca1 Alexa® 647 (1:50 dilution) and rat anti-mouse CD45 IgG (1:50 dilution) for 30 min. After 2 washes with FACS buffer, samples were incubated with goat anti-rat Alexa® 555 (1:50 dilution). Samples were analyzed using Becton Dickinson FACS Canto A running DiVa software. A total of 10,000 events for each sample were analyzed. Cell viability was assessed by negative staining using the aqua Live/Dead reagent. Offline analysis was performed by Flow Jo analytical software (Treestar, Ashland, OR) and cells were gated to exclude dead cells, doublets, and higher order cell aggregates.
2.8 Transplantation
MPCs obtained from GFP positive females were transplanted into wild type males intravenously (2×106 cells/animal). The donors/recipients were either 2 months (young) or 24 months (old) of age. Following transplantation, the animals were kept under standard conditions with monitoring for loss of weight. The recipients were euthanized 8 weeks post-transplantation. Four animals were used in each transplant group.
2.9 Histology
Mouse hind limbs were excised, cleaned of soft tissue, and fixed in 3.7% formaldehyde for 72 hours. Decalcification was performed using immunocal™ for 3 days according to the manufacturer’s instructions. Isolated bone tissue was dehydrated in graded alcohols (70 to 100%), cleared in xylene and embedded in paraffin by standard methods. Paraffin blocks were cut into 5 µm or 7 µm sections for histomorphometry or immunohistochemistry, respectively.
For histomorphometry, femur sections were deparaffinized in xylene and assessed for static parameters of bone formation. The femur sections were then stained with hemotoxylin and eosin (H&E). Osteoblast number (Ob N/BS, /mm), adipocyte number (Adipocyte N/Total Area, /mm2) and adipocyte Area (Adipocyte Area/Total Area, %) were identified morphologically and quantified as previously described [38, 39]. Selected regions of interest ROIs (100µm distal to the growth plate and 50µm in from the endosteal cortical bone) were visualized using a Nikon Eclipse 90i microscope. Image capture was performed using NIS Elements Imaging Software 3.10 Sp2 and a Nikon DS-Fi1 camera using Nikon 4×/0.2 Plan Apo, Nikon 20×/0.75 Plan Apo objective and 40×/0.95 Plan Apo objectives. Photoshop and Image J were used for image analysis as previously described [39].
2.10 Immunohistochemistry
For GFP staining, the sections were de-paraffinized, dehydrated and then re-hydrated, followed by permeabilization using Tween® 20 (0.2 % in distilled water) and blocking for 1 h with 10% NGS. The samples were then incubated with rabbit anti-GFP antibody (1:1000 dilution) for 16 h at 4°C. Following washes with PBS containing tween® 20, samples were incubated with goat anti-rabbit Alexa® 647 antibody (1:1000 dilution) for 1 h. Samples were then stained with 4’,6-Diamidino-2-Phenylindole (DAPI; 300 nM, 5 min) as the nuclear stain. Samples were viewed under a Nikon 90i eclipse inverted microscope and images were captured using Photometrics Cool Snap camera and NIS elements Ar version 3.2 software. Emission times were standardized to negative control slides (no primary antibody). For FABP4 staining of adipocytes, samples were treated in a similar manner as for GFP except for using Sytox® Green (1:15000) as the nuclear stain and goat anti-rabbit Marina® Blue (1:100) as the secondary antibody. Rabbit anti-FABP4 was used at 1:40 dilution. At least 2000 cells per cell type per transplant group were scored under high power magnification (×400).
2.11 Statistics
Differences among transplanted groups were evaluated using one-way ANOVA. All statistics were performed using GraphPad Prism 4.0 software (San Diego, Ca). Differences were considered statistically significant at a p-value of < 0.05, and all data is represented as mean + SEM.
3.0 Results
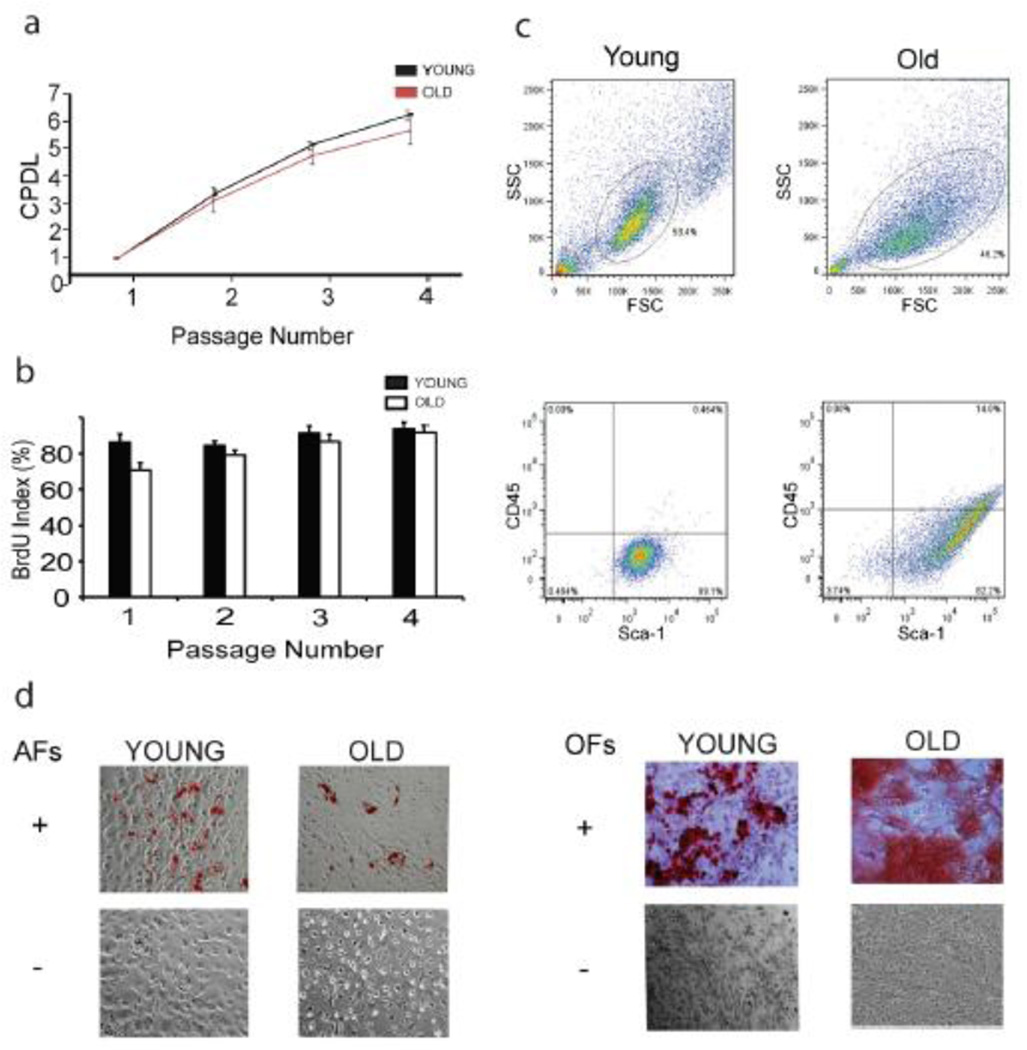
MPCs were not propagated beyond the fourth passage in order to minimize the effects of in vitro replicative senescence. Proliferation parameters were measured at every passage and the average CPDL at the time of transplantation was 6.3 for all cultures. At the time of transplantation, the age of MPC cultures derived from young and old animals represented less than 40 percent of their in vitro lifespan completed, based on retrospective calculation using the number of cumulative doublings at the end of their proliferative life in culture. The change in population doubling at the fourth passage was 1.76 ± 0.66 (figure 1a), and the BrdU index (reflecting the number of cells capable of DNA synthesis) was nearly 85 percent (figure 1b). Based on these parameters, there was no significant difference in proliferation between cells derived from young and old donors prior to MPC transplantation.
Figure 1.
Donor MPCs from young and old animals display similar characteristics of proliferation and differentiation at early passage in vitro. (a) Growth curves through the fourth in vitro passage; (b) BrdU labeling index; (c) Sca-1+ CD45- MPCs by flow cytometry; (d) Differentiation of MPCs into adipocytes and osteoblasts. CPDL, cumulative population doubling level; AF, adipogenic factors; OF, osteogenic factors
In both young and old donors, MPCs were essentially negative for CD45 expression and positive for Sca-1 expression (figure 1c). Importantly, the isolated cells demonstrated a strong potential to differentiate into osteoblast and adipocyte lineages irrespective of their origin from young or old donors (figure 1d). Thus, we defined MPCs as adherent, CD45 negative, Sca-1 positive cells with the ability to differentiate into osteoblasts and adipocytes.
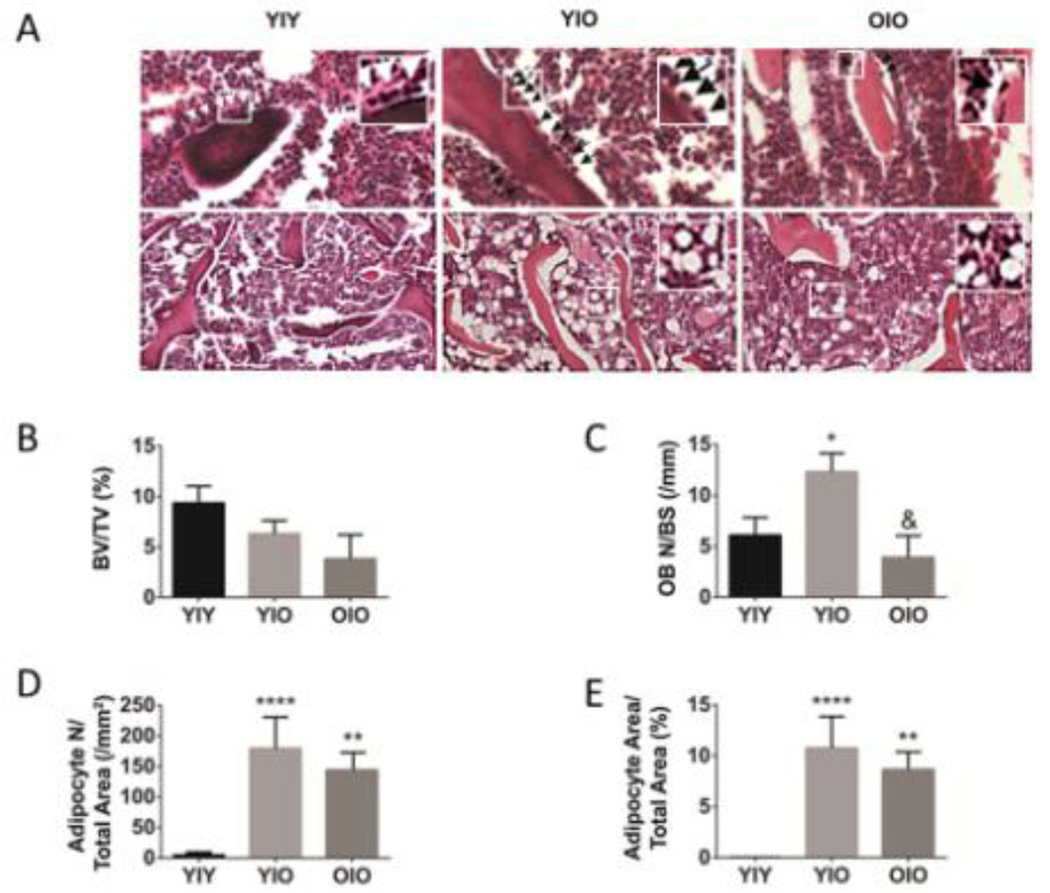
Figure 2 shows that transplantation of young MPCs into old recipients increases both osteoblast (figure 2A and 2C) and adipocyte numbers (figure 2A and 2D). There is a non-statistically significant trend toward increased bone volume in the young donor MPC into old recipient transplantation group (Y|O) compared to the old donor MPC into old recipient group (O|O) [figure 2B]. However, total adipocyte area is dramatically and significantly increased in both the Y|O and O|O transplant groups (figure 2E).
Figure 2.
Transplantation of young MPCs into old recipients increases osteoblast and adipocyte numbers. (A) Representative sections of the metaphyseal distal femur from Y|Y, Y|O, and O|O mice, stained with H&E, and showing osteoblasts (upper panels, arrows) or adipocytes (lower panels). (B) Bone volume (BV/TV, %). Higher magnification insets show the greater numbers of bone-lining osteoblasts in the Y|O transplantation group (compared to the O|O group) and more adipocytes in the Y|O and O|O groups compared to the Y|Y group. (C) Osteoblast number per bone surface (OB N/BS, /mm). (D,E) Adiposity increased in old femurs from recipients transplanted with young or old MSCs. (D) Adipocyte number (Adipocyte N/Total Area, /mm2). (E) Adipocyte Area (Adipocyte Area/Total Area, %). Data represent means + s.e.m. Statisical significance is *p<0.05, **p<0.01, and ****p<0.0001 compared to YIY group. Statisical significance is &p<0.05, compared to Y|O transplant group.
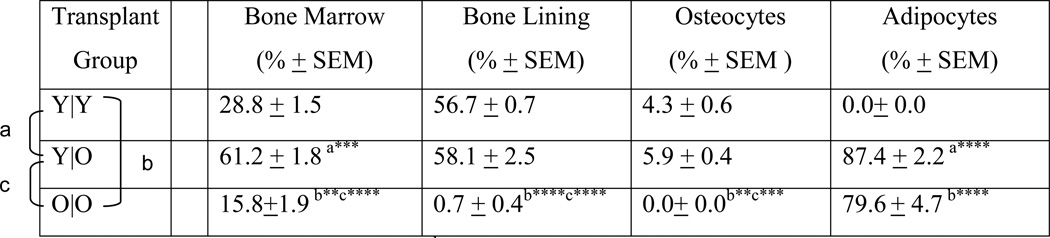
Tracing studies after transplantation confirmed that young donor MPCs showed high functional engraftment in the bone marrow compartment (28.8 ± 1.5 %) and in the bone-lining region (56.7 ± 0.7 %) of young recipients (Table 1, figure 3). The engrafted cells differentiated further into osteocytes as was observed by 4.3 ± 0.6 % of osteocytes staining positively for GFP in this group (Table 1, figure 3).
Table 1.
Distribution of engrafted and differentiated MPCs in recipient animals among transplantation groups.
Comparison between Y|Y and Y|O
Comparison between Y|Y and O|O
Comparison between Y|O and O|O.
p< 0.01;
p<0.001;
p<0.0001;
Y, Young; O, Old; The first letter in young and old transplant pairings indicates the donor cell; the second letter indicates the recipient animal.
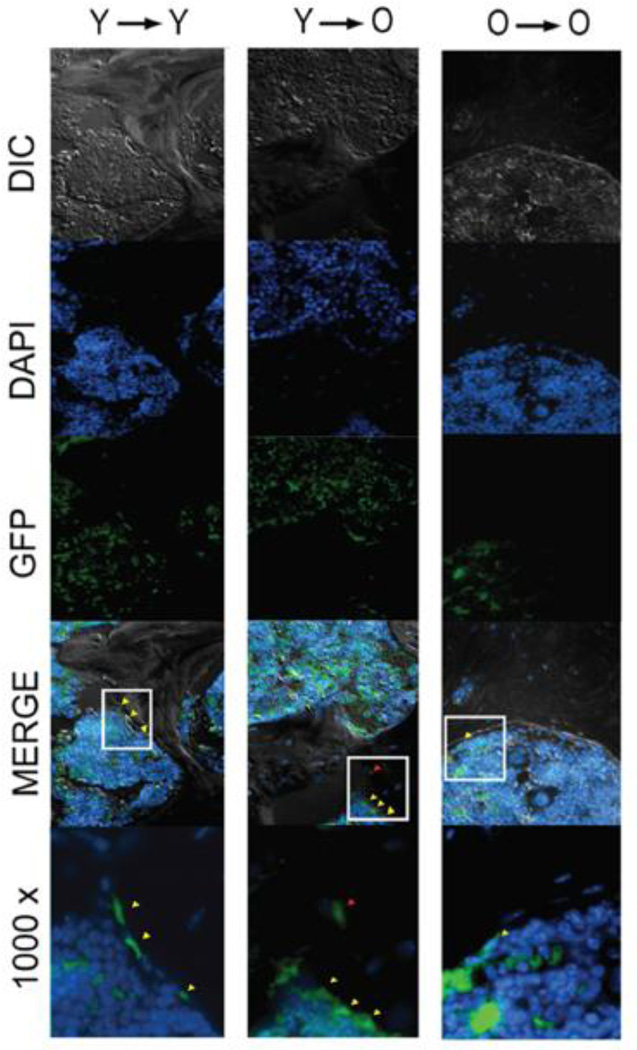
Figure 3.
MPC osteogenic differentiation with aging is a cell autonomous process superimposed upon microenvironmental influences. Y, Young; O, Old; →, indicates the transplant pairings as donor cells → transplant recipient; Yellow arrowheads, bone-lining cells; Red arrowheads, osteocytes.
GFP tracing studies after transplantation also confirmed that functional engraftment was observed in old recipients receiving MPCs from young animals; in fact, young donor MPCs showed highest engraftment into the marrow of old recipients (Table 1). Noticeably, the differentiation of young (donor) MPCs into osteoblasts was not adversely affected by recipient age, as high numbers of GFP positive cells were observed in old recipients as well (Table 1; figure 3). Old donor-derived MPCs, however, showed a significantly decreased ability to engraft into old recipients as compared to young donors (Table 1). Thus MPC osteogenic differentiation with aging is a cell autonomous process superimposed upon microenvironmental influences.
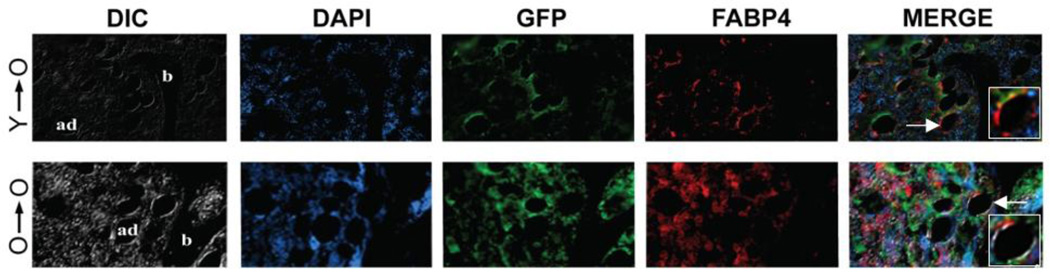
Marrow fat is increased in old age and is thought to be an effect of lineage switching of progenitor cells present in bone. In our transplantation experiments we used FABP4 to identify adipocytes and pre-adipocytes, and we found that GFP-positive MPCs derived from both young and old donors differentiated into fat cells in vivo, but only in old recipients (figure 4; Table 1). Young recipients did not show any presence of GFP positive adipocytes. However, 87.4% of adipocytes in young donor MPC/old recipient transplants and 79.6% of old donor MPC/old recipient transplants were GFP positive. Thus, adipogenic differentiation is strongly dependent upon microenvironment-related aging processes.
Figure 4.
Engrafted MPCs differentiate into adipocytes only in old recipients and independent of donor MPC age. Higher magnification insets illustrate co-staining of both GFP (green) and FABP4 (red) in panels showing the merged images. Note that in the processing of tissue, sections were deparaffinized in xylene, which causes lipid extraction of adipocytes and limits immunostaining to the cellular periphery. Ad, adipocyte; b, bone
4.0 Discussion
In the present study we investigated the role of aging in engraftment of MPCs under non-myeloablative conditions. MPCs have a strong potential for usage in transplantation protocols, not only as immunosupressors but also as pluripotent progenitors capable of differentiating into a variety of cell types. Though MPCs are widely studied in vitro, little is known about the influence of the micro-environment on their ability to differentiate in vivo. Here, we studied the relative contributions of intrinsic cellular aging and micro-environmental aging on MPC engraftment and differentiation into osteoblasts and adipocytes.
Non-myeloablative transplantation is a preferred way of transplantation in order to minimize the deleterious side-effects of radiation or other myeloablative protocols. In addition, radiation is also known to adversely affect bone strength by causing cell death among the osteoblast lineage [40, 41]. Bone loss following radiotherapy is thought to be a result of physiological changes that occur to both vasculature and bone cells [42–45]. We therefore used a non-myeloablative approach in our experiments.
We found that MPCs from young but not old donors exhibited high functional engraftment in old recipients. Engraftment of MPCs, thus, seemed to predominately be a cell autonomous process. This may be attributed to age-related changes in MPCs prior to isolation for transplantation, and perhaps a reflection of in vivo cell senescence. Replicative senescence is a major limitation of bone marrow derived mesenchymal cell cultures. Human MPCs undergo morphological changes with continued expansion in culture leading to proliferation arrest [46]. Similar results have been reported in mouse cultures [47]. In our experiments we did observe in vitro senescence in MPCs but only at passages well beyond those obtained prior to transplantation.
Several studies have examined the effect of donor age on the in vitro differentiation potential of MPCs. MPCs cultured from younger, older and osteoporotic patients are reported to maintain osteoblast differentiation potential at early passage in culture [48]. Similarly, Muraglia et al. [49] tested the osteogenic, chondrogenic and adipogenic differentiation capacity of MPCs in human donors of various ages and found that the number of tripotent clones did not change with age in culture at early passage. We used early passage cells from young and old mice and found no observable differences in either osteogenic or adipogenic potential.
There are reports which provide indirect evidence of age-related changes in osteoblast differentiation of MPCs. Stenderup et al. demonstrated that MPCs derived from both young and old human donors were able to form similar amounts of mineralized matrix in vitro and normal lamellar bone in vivo [50]. The implication of these results is that age-related decline in bone formation can be attributed to decreased MPC number and not function. In contrast, Mendes et al. demonstrated that when human MPCs from 53 donors of various ages were seeded on calcium phosphate scaffolds and implanted under the skin of nude mice, the ability of cultures to form bone in vivo declined at as function of age with 67% of the cultures able to form bone when derived from donors between 41–50 years of age, 50% when derived from donors 51–70 years of age and less than 46% when derived from donors beyond 70 years of age [51]. Studies on ectopic bone formation using demineralized bone matrix powder from donors of different ages have reported an age-related decrease in bone formation [52–54].
Taken together, our results show direct evidence of intrinsic cellular aging to be responsible for decreased osteoblast differentiation in vivo. This direct evidence is provided by a comparison of Y|O versus O|O transplantation groups with respect to engraftment of MPCs that differentiate into bone-lining osteoblasts and osteocytes (Table 1). In this comparison the recipients (old animals) are the same and only the age of the donor MPCs varies. Robust engraftment occurred by MPCs that subsequently differentiated into bone-lining osteoblasts and osteocytes of the bony matrix in young recipients; however, significantly lower engraftment was seen at the same sites in old recipients transplanted with old MPCs. These results are supported by other indirect or circumstantial evidence for intrinsic MPC aging, such as decreased in vitro osteogenic capacity of MPCs derived from older individuals, and decline in mineral apposition rate and mean wall thickness with aging.
MPCs can differentiate into osteoblasts and other lineages including adipocytes. We found in vivo adipocyte differentiation to be directly related to recipient age. Old recipients seem to promote adipocyte differentiation preferentially, thus suggesting an environment-mediated process with aging. In mouse models of accelerated senescence, including telomere-based and other models, decreased bone formation has been reported to be associated with enhanced adipogenesis [29, 55]. However, in this study ex vivo cultures from the animals were used to establish the propensity of MPCs to differentiate into adipocytes.
There is growing evidence that a reciprocal relationship exists between osteogenic and adipogenic differentiation which may explain the increased adipocyte and decreased osteoblast formation that occurs with aging and osteoporosis. An inverse relationship exists between osteoblast and adipocyte differentiation that likely is regulated at the level of a common mesenchymal precursor [56–58]. Grossly, in bone there is a decrease in the number of osteoblasts and an increase in the number of marrow adipoctyes with age [59, 60]. Activation of adipogenic and suppression of osteogenic programs occur in marrow MPCs with advancing age [60] and may in part be regulated by estrogen [31].
Although circumstantial evidence exists that supports this reciprocal relationship, direct evidence has until now been lacking. In the current study, we show in vivo evidence for lineage switching, thus providing a biological basis for the testing and potential use of already existing compounds shown to inhibit adipogenesis and promote osteogenesis. For example, naturally-occurring oxysterols have been shown to be pro-osteogenic and anti-adipogenic, effects likely mediated through hedgehog-dependent mechanism(s) that inhibit PPAR-gamma expression [61–63]. Estrogen, reduced atherogenic diets, protection against lipoprotein and lipid oxidation products, and other potential PPAR-gamma modulators/antagonists may have similar effects on the balance between marrow osteogenesis and adipogenesis [58].
Reports based on in vitro expansion of cells have led to the hypothesis that with aging MPCs acquire a lineage switch in favor of adipogenesis over osteogenesis [14, 28]. Some studies, however, suggested either no difference or decreased in vitro differentiation of MPCs from old animals towards the adipogenic lineage as compared to younger animals [64, 65]. In our in vivo studies, an old bone environment promoted adipogenic differentiation, thus forcing young transplanted MPCs to differentiate into adipocytes. This is consistent with in vitro studies involving the use of sera from elderly donors reported to be selectively inhibitory to osteoblast but not adipocyte differentiation of MPCs in vitro [31].
Non-myeloablative transplantation protocols in humans are emerging as a possible method of choice. The pluripotency of MPCs makes them promising candidates for transplantation regimens. Our results indicate that older recipients would particularly benefit from non-myeloablative transplantation of MPCs as a possible treatment for various bone disorders, especially given that engraftment would likely be high in older individuals. This approach might necessarily be accompanied by the administration of compounds that inhibit adipogenesis.
Highlights.
In order to study the effects of mesenchymal progenitor cells (MPCs) and microenvironmental aging on functional engraftment and lineage switching, transplantation studies were performed under non-myeloablative conditions in old recipients, with donor MPCs derived from young and old green fluorescent protein (GFP) transgenic mice.
Robust engraftment by young MPCs or their progeny was observed in the marrow, bone-lining region and in the matrix of young recipients; however, significantly lower engraftment was seen at the same sites in old recipients transplanted with old MPCs.
Differentiation of transplanted MPCs strongly favored adipogenesis over osteogenesis in old recipients irrespective of MPC donor age, suggesting that microenvironmental alterations that occur with in vivo aging are predominately responsible for MPC lineage switching.
These data indicate that aging alters bone-fat reciprocity and differentiation of mesenchymal progenitors toward an adipogenic fate.
Acknowledgments
This work was supported by the National Institutes of Health/ National Institute on Aging grant R01AG028873 (R.J.P) and the Glenn Foundation/American Federation for Aging Research Postdoctoral Fellowship for Translational Research on Aging (T.A.B.). Author contributions are as follows. L.S.: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; T.A.B.: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; E.R.: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript, J-H.K.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; final approval of manuscript; Q.C.: provision of study materials, final approval of manuscript; F.B.J.: provision of study materials, data analysis and interpretation, manuscript writing, final approval of manuscript; R.J.P.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All authors state that they have no conflicts of interest.
References
- 1.Burkhardt R, Kettner G, Bohm W, Schmidmeier M, Schlag R, Frisch B, Mallmann B, Eisenmenger W, Gilg T. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone. 1987;8:157–164. doi: 10.1016/8756-3282(87)90015-9. [DOI] [PubMed] [Google Scholar]
- 2.Rozman C, Reverter JC, Feliu E, Berga L, Rozman M, Climent C. Variations of fat tissue fraction in abnormal human bone marrow depend both on size and number of adipocytes: a stereologic study. Blood. 1990;76:892–895. [PubMed] [Google Scholar]
- 3.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 4.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–154. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Minaire P, Neunier P, Edouard C, Bernard J, Courpron P, Bourret J. Quantitative histological data on disuse osteoporosis: comparison with biological data. Calcif Tissue Res. 1974;17:57–73. doi: 10.1007/BF02547214. [DOI] [PubMed] [Google Scholar]
- 6.Wronski TJ, Morey-Holton E, Jee WS. Skeletal alterations in rats during space flight. Adv Space Res. 1981;1:135–140. doi: 10.1016/0273-1177(81)90254-4. [DOI] [PubMed] [Google Scholar]
- 7.Zayfaron M, Gathings WE, McDonald JM. Modeled microgravity inhibit osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology. 2004;145:2421–2432. doi: 10.1210/en.2003-1156. [DOI] [PubMed] [Google Scholar]
- 8.Wronski TJ, Walsh CC, Ignaszewski LA. Histologic evidence for osteopenia and increased bone turnover in ovariectomized rats. Bone. 1986;7:119–123. doi: 10.1016/8756-3282(86)90683-6. [DOI] [PubMed] [Google Scholar]
- 9.Wang GJ, Sweet DE, Reger SI, Thompson RC. Fat-cell changes as a mechanism of avascular necrosis of the femoral head in cortisone-treated rabbits. J Bone Joint Surg Am. 1977;59:729–735. [PubMed] [Google Scholar]
- 10.Forsen L, Meyer HE, Midthjell K, Edna TH. Diabetes mellitus and the incidence of hip fracture: results from the Nord-Trondelag Health Survey. Diabetologia. 1999;42:920–925. doi: 10.1007/s001250051248. [DOI] [PubMed] [Google Scholar]
- 11.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 13.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102(Pt 2):341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- 15.Falconi D, Oizumi K, Aubin JE. Leukemia inhibitory factor influences the fate choice of mesenchymal progenitor cells. Stem Cells. 2007;25:305–312. doi: 10.1634/stemcells.2006-0417. [DOI] [PubMed] [Google Scholar]
- 16.Gimble JM, Morgan C, Kelly K, Wu X, Dandapani V, Wang CS, Rosen V. Bone morphogenetic proteins inhibit adipocyte differentiation by bone marrow stromal cells. J Cell Biochem. 1995;58:393–402. doi: 10.1002/jcb.240580312. [DOI] [PubMed] [Google Scholar]
- 17.Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- 18.Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, Hopkins N, Yaffe MB. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 19.Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabatakos G, Sims NA, Chen J, Aoki K, Kelz MB, Amling M, Bouali Y, Mukhopadhyay K, Ford K, Nestler EJ, Baron R. Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat Med. 2000;6:985–990. doi: 10.1038/79683. [DOI] [PubMed] [Google Scholar]
- 21.Taipaleenmaki H, Abdallah BM, AlDahmash A, Saamanen AM, Kassem M. Wnt signalling mediates the cross-talk between bone marrow derived pre-adipocytic and pre-osteoblastic cell populations. Exp Cell Res. 2011;317:745–756. doi: 10.1016/j.yexcr.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Bowers RR, Lane MD. A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle. 2007;6:385–389. doi: 10.4161/cc.6.4.3804. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Song TJ, Li X, Hu L, He Q, Liu M, Lane MD, Tang QQ. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A. 2009;106:12670–12675. doi: 10.1073/pnas.0906266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X, Chen J, Bi Y, He BC, Park JK, Jiang W, Tang Y, Huang J, Su Y, Zhu GH, He Y, Yin H, Hu Z, Wang Y, Chen L, Zuo GW, Pan X, Shen J, Vokes T, Reid RR, Haydon RC, Luu HH, He TC. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2009;18:545–559. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neumann K, Endres M, Ringe J, Flath B, Manz R, Haupl T, Sittinger M, Kaps C. BMP7 promotes adipogenic but not osteo-/chondrogenic differentiation of adult human bone marrow-derived stem cells in high-density micro-mass culture. J Cell Biochem. 2007;102:626–637. doi: 10.1002/jcb.21319. [DOI] [PubMed] [Google Scholar]
- 26.Abdallah BM, Kassem M. New factors controlling the balance between osteoblastogenesis and adipogenesis. Bone. 2012;50:540–545. doi: 10.1016/j.bone.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 27.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–124. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett JH, Joyner CJ, Triffitt JT, Owen ME. Adipocytic cells cultured from marrow have osteogenic potential. J Cell Sci. 1991;99(Pt 1):131–139. doi: 10.1242/jcs.99.1.131. [DOI] [PubMed] [Google Scholar]
- 29.Kajkenova O, Lecka-Czernik B, Gubrij I, Hauser SP, Takahashi K, Parfitt AM, Jilka RL, Manolagas SC, Lipschitz DA. Increased adipogenesis and myelopoiesis in the bone marrow of SAMP6, a murine model of defective osteoblastogenesis and low turnover osteopenia. J Bone Miner Res. 1997;12:1772–1779. doi: 10.1359/jbmr.1997.12.11.1772. [DOI] [PubMed] [Google Scholar]
- 30.Justesen J, Stenderup K, Eriksen EF, Kassem M. Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcif Tissue Int. 2002;71:36–44. doi: 10.1007/s00223-001-2059-x. [DOI] [PubMed] [Google Scholar]
- 31.Abdallah BM, Haack-Sorensen M, Fink T, Kassem M. Inhibition of osteoblast differentiation but not adipocyte differentiation of mesenchymal stem cells by sera obtained from aged females. Bone. 2006;39:181–188. doi: 10.1016/j.bone.2005.12.082. [DOI] [PubMed] [Google Scholar]
- 32.Stringer B, Waddington R, Houghton A, Stone M, Russell G, Foster G. Serum from postmenopausal women directs differentiation of human clonal osteoprogenitor cells from an osteoblastic toward an adipocytic phenotype. Calcif Tissue Int. 2007;80:233–243. doi: 10.1007/s00223-007-9016-2. [DOI] [PubMed] [Google Scholar]
- 33.Compston J. Mechanisms of bone loss and gain in untreated and treated osteoporosis. Endocrine. 2002;17:21–27. doi: 10.1385/ENDO:17:1:21. [DOI] [PubMed] [Google Scholar]
- 34.Parfitt AM, Villanueva AR, Foldes J, Rao DS. Relations between histologic indices of bone formation: implications for the pathogenesis of spinal osteoporosis. J Bone Miner Res. 1995;10:466–473. doi: 10.1002/jbmr.5650100319. [DOI] [PubMed] [Google Scholar]
- 35.Kassem M, Marie PJ. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell. 2011;10:191–197. doi: 10.1111/j.1474-9726.2011.00669.x. [DOI] [PubMed] [Google Scholar]
- 36.Marie PJ, Kassem M. Extrinsic mechanisms involved in age-related defective bone formation. J Clin Endocrinol Metab. 2011;96:600–609. doi: 10.1210/jc.2010-2113. [DOI] [PubMed] [Google Scholar]
- 37.Cristofalo VJ, Charpentier R. Cellular senescence: cell proliferation and its control. Adv Pathobiol. 1980;7:100–114. [PubMed] [Google Scholar]
- 38.Elbaz A, Rivas D, Duque G. Effect of estrogens on bone marrow adipogenesis and Sirt1 in aging C57BL/6J mice. Biogerontology. 2009;10:747–755. doi: 10.1007/s10522-009-9221-7. [DOI] [PubMed] [Google Scholar]
- 39.Egan KP, Brennan TA, Pignolo RJ. Bone histomorphometry using free and commonly available software. Histopathology. 2012;61:1168–1173. doi: 10.1111/j.1365-2559.2012.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu AL, Greven KM, Maruyama Y. Radiation osteitis and insufficiency fractures after pelvic irradiation for gynecologic malignancies. Am J Clin Oncol. 1994;17:248–254. doi: 10.1097/00000421-199406000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis) N Engl J Med. 1992;326:1473–1479. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- 42.Bliss P, Parsons CA, Blake PR. Incidence and possible aetiological factors in the development of pelvic insufficiency fractures following radical radiotherapy. Br J Radiol. 1996;69:548–554. doi: 10.1259/0007-1285-69-822-548. [DOI] [PubMed] [Google Scholar]
- 43.Ergun H, Howland WJ. Postradiation atrophy of mature bone. CRC Crit Rev Diagn Imaging. 1980;12:225–243. [PubMed] [Google Scholar]
- 44.Gal TJ, Munoz-Antonia T, Muro-Cacho CA, Klotch DW. Radiation effects on osteoblasts in vitro: a potential role in osteoradionecrosis. Arch Otolaryngol Head Neck Surg. 2000;126:1124–1128. doi: 10.1001/archotol.126.9.1124. [DOI] [PubMed] [Google Scholar]
- 45.Hopewell JW. Radiation-therapy effects on bone density. Med Pediatr Oncol. 2003;41:208–211. doi: 10.1002/mpo.10338. [DOI] [PubMed] [Google Scholar]
- 46.Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3:e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, Baggett LS, Mikos AG, Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Justesen J, Stenderup K, Kassem MS. [Mesenchymal stem cells. Potential use in cell and gene therapy of bone loss caused by aging and osteoporosis] Ugeskr Laeger. 2001;163:5491–5495. [PubMed] [Google Scholar]
- 49.Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113(Pt 7):1161–1616. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- 50.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Mendes SC, Tibbe JM, Veenhof M, Bakker K, Both S, Platenburg PP, Oner FC, de Bruijn JD, van Blitterswijk CA. Bone tissue-engineered implants using human bone marrow stromal cells: effect of culture conditions and donor age. Tissue Eng. 2002;8:911–920. doi: 10.1089/107632702320934010. [DOI] [PubMed] [Google Scholar]
- 52.Hosny M, Sharawy M. Osteoinduction in young and old rats using demineralized bone powder allografts. J Oral Maxillofac Surg. 1985;43:925–931. doi: 10.1016/0278-2391(85)90004-7. [DOI] [PubMed] [Google Scholar]
- 53.Irving JT, LeBolt SA, Schneider EL. Ectopic bone formation and aging. Clin Orthop Relat Res. 1981:249–253. [PubMed] [Google Scholar]
- 54.Nishimoto SK, Chang CH, Gendler E, Stryker WF, Nimni ME. The effect of aging on bone formation in rats: biochemical and histological evidence for decreased bone formation capacity. Calcif Tissue Int. 1985;37:617–624. doi: 10.1007/BF02554919. [DOI] [PubMed] [Google Scholar]
- 55.Brennan TA, Egan KP, Lindborg CM, Chen Q, Sweetwyne MT, Hankenson KD, Xie SX, Johnson FB, Pignolo RJ. Mouse models of telomere dysfunction phenocopy skeletal changes found in human age-related osteoporosis. Disease Models & Mechanisms. 2014;7:583–592. doi: 10.1242/dmm.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bergman RJ, Gazit D, Kahn AJ, Gruber H, McDougall S, Hahn TJ. Age-related changes in osteogenic stem cells in mice. J Bone Miner Res. 1996;11:568–577. doi: 10.1002/jbmr.5650110504. [DOI] [PubMed] [Google Scholar]
- 57.Parhami F, Jackson SM, Tintut Y, Le V, Balucan JP, Territo M, Demer LL. Atherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cells. J Bone Miner Res. 1999;14:2067–2078. doi: 10.1359/jbmr.1999.14.12.2067. [DOI] [PubMed] [Google Scholar]
- 58.Pei L, Tontonoz P. Fat’s loss is bone’s gain. J Clin Invest. 2004;113:805–806. doi: 10.1172/JCI21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19:117–125. doi: 10.1016/S0736-0266(00)00010-3. [DOI] [PubMed] [Google Scholar]
- 60.Nishida S, Endo N, Yamagiwa H, Tanizawa T, Takahashi HE. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab. 1999;17:171–177. doi: 10.1007/s007740050081. [DOI] [PubMed] [Google Scholar]
- 61.Fujieda M, Kiriu M, Mizuochi S, Hagiya K, Kaneki H, Ide H. Formation of mineralized bone nodules by rat calvarial osteoblasts decreases with donor age due to a reduction in signaling through EP(1) subtype of prostaglandin E(2) receptor. J Cell Biochem. 1999;75:215–225. doi: 10.1002/(sici)1097-4644(19991101)75:2<215::aid-jcb4>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]
- 62.Kveiborg M, Rattan SI, Clark BF, Eriksen EF, Kassem M. Treatment with 1,25-dihydroxyvitamin D3 reduces impairment of human osteoblast functions during cellular aging in culture. J Cell Physiol. 2001;186:298–306. doi: 10.1002/1097-4652(200002)186:2<298::AID-JCP1030>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 63.Lee WY, Cho SW, Oh ES, Oh KW, Lee JM, Yoon KH, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim CC. The effect of bone marrow transplantation on the osteoblastic differentiation of human bone marrow stromal cells. J Clin Endocrinol Metab. 2002;87:329–335. doi: 10.1210/jcem.87.1.8135. [DOI] [PubMed] [Google Scholar]
- 64.Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 65.Bellantuono I, Keith WN. Stem cell ageing: does it happen and can we intervene? Expert Rev Mol Med. 2007;9:1–20. doi: 10.1017/S146239940700049X. [DOI] [PubMed] [Google Scholar]