Abstract
The scourge of multidrug-resistant bacterial infections necessitates the urgent development of novel antimicrobials to address this public health challenge. Drug repurposing is a proven strategy to discover new antimicrobial agents; given that these agents have undergone extensive toxicological and pharmacological analysis, repurposing is an effective method to reduce the time, cost and risk associated with traditional antibiotic innovation. In this study, the in vitro and in vivo antibacterial activities of an antirheumatic drug, auranofin, was investigated against multidrug-resistant Staphylococcus aureus. The results indicated that auranofin possesses potent antibacterial activity against all tested strains of S. aureus, including meticillin-resistant S. aureus (MRSA), vancomycin-intermediate S. aureus (VISA) and vancomycin-resistant S. aureus (VRSA), with minimum inhibitory concentrations (MICs) ranging from 0.0625 μg/mL to 0.125 μg/mL. In vivo, topical auranofin proved superior to conventional antimicrobials, including fusidic acid and mupirocin, in reducing the mean bacterial load in infected wounds in a murine model of MRSA skin infection. In addition to reducing the bacterial load, topical treatment of auranofin greatly reduced the production of inflammatory cytokines, including tumour necrosis factor-α (TNFα), interleukin-6 (IL-6), interleukin-1 beta (IL-1β) and monocyte chemoattractant protein-1 (MCP-1), in infected skin lesions. Moreover, auranofin significantly disrupted established in vitro biofilms of S. aureus and Staphylococcus epidermidis, more so than the traditional antimicrobials linezolid and vancomycin. Taken together, these results support that auranofin has potential to be repurposed as a topical antimicrobial agent for the treatment of staphylococcal skin and wound infections.
Keywords: Repurposing, Auranofin, Multidrug resistance, Topical antimicrobials, Inflammatory cytokines
1. Introduction
Staphylococcus aureus is the most frequently isolated pathogen from human skin infections and is the leading cause of nosocomial wound infections [1–4]. Virulence factors and toxins (such as α-haemolysin and Panton–Valentine leukocidin) secreted by drug-resistant strains of S. aureus allow this pathogen to evade the host immune system, leading to recurring/chronic infections, prolonged inflammation and delayed healing of infected wounds [3,4]. Furthermore, cutaneous staphylococcal skin infections can develop into invasive infections that ultimately result in septicaemia [5,6]. Recently, skin infections with biofilm-producing staphylococci have become an emerging clinical problem; treatment failure is occurring more frequently with the topical drugs of choice, including mupirocin and fusidic acid, indicating that new treatment options are urgently required [2,7,8]. The recent US Food and Drug Administration (FDA) approval of drugs such as tedizolid phosphate and dalbavancin to combat skin infections caused by Gram-positive pathogens [9,10] further highlights the pressing need for the identification of new antibacterials capable of treating cutaneous meticillin-resistant S. aureus (MRSA) infections.
Most current antibiotics were discovered by screening libraries of chemical compounds to find new lead `hits' that could be subsequently modified to enhance potency/physicochemical properties and to mitigate toxicity [11]. However, this process is a risky venture given the significant financial and time investment required by researchers and the limited success rate of translating these compounds to the clinical setting. An alternative approach to unearthing new antibacterials that has received more attention recently is evaluating the repository of approved drugs (or drugs that made it to clinical trials but failed to receive regulatory approval) in order to identify candidates that can be repurposed as antimicrobials [11]. Recently, we assembled and screened one-half of all commercially available drugs (ca. 2200 drugs) and small molecules used in human clinical trials [2,12] and identified three drugs (auranofin, ebselen and 5-fluoro-2'-deoxyuridine) [2,13,14] that exhibited potent antibacterial activity against important clinical pathogens. One of these drugs, auranofin, was found to inhibit the growth of clinical isolates of MRSA at submicrogram/mL concentrations in vitro.
Auranofin is an oral gold-containing drug initially approved for the treatment of rheumatoid arthritis [15]. Recent studies have demonstrated that auranofin also possesses potent antiparasitic [15] and antibacterial activities [16,17], including against MRSA and Streptococcus pneumoniae [16,18–20]. Recent studies by Harbut et al. [16] and Aguinagalde et al. [18] demonstrated that auranofin is efficacious in the treatment of invasive staphylococcal infections. However, the efficacy of auranofin for the treatment of cutaneous MRSA infections remains unexplored.
Building upon these recent reports, the present study investigated the in vitro antibacterial and antibiofilm activities of auranofin against multidrug-resistant clinical isolates of S. aureus and tested the efficacy of auranofin in a mouse model of MRSA skin infection. In addition, this study aimed to examine the immunomodulatory activity of auranofin in MRSA-infected skin lesions. The findings presented in this study lay the foundation for repurposing auranofin as a novel topical antibacterial agent for treatment of cutaneous MRSA infections in humans.
2. Materials and methods
2.1. Bacterial strains and reagents
The bacterial strains used in this study are presented in Table 1. Auranofin (Enzo Life Sciences, Farmingdale, NY), mupirocin (AppliChem, Maryland Heights, MO), clindamycin (Sigma-Aldrich, St Louis, MO), vancomycin hydrochloride (Gold Biotechnology, St Louis, MO), linezolid (Selleck Chemicals, Houston, TX), retapamulin (Oxchem Corporation, Irwindale, CA), crystal violet (Sigma-Aldrich), 95% ethanol (Fisher Scientific, Pittsburgh, PA), MTS assay reagent (Promega Corp., Madison, WI), dimethyl sulphoxide (DMSO) and fusidic acid (Sigma-Aldrich) were all purchased from commercial vendors. Mueller–Hinton broth was purchased from Sigma-Aldrich, and trypticase soy broth (TSB), trypticase soy agar (TSA) and mannitol salt agar (MSA) were purchased from Becton Dickinson & Co. (Cockeysville, MD).
Table 1.
Minimum inhibitory concentrations (MICs) of auranofin and control antibiotics against Staphylococcus aureus and Staphylococcus epidermidis
| Strain type | Strain ID | Source | Phenotypic properties | MIC (μg/mL) |
||
|---|---|---|---|---|---|---|
| Auranofin | Linezolid | Vancomycin | ||||
| Meticillin-sensitive S. aureus (MSSA) | ATCC 6538 | Quality control and biofilm-forming strain | 0.0625 | 2 | 1 | |
| Meticillin-resistant S. aureus (MRSA) | RN4220 | USA | Resistant to penicillin | 0.0625 | 2 | 1 |
| NRS72 | UK | 0.125 | 2 | 1 | ||
| NRS77 | UK | 0.0625 | 2 | 1 | ||
| NRS846 | – | 0.0625 | 2 | 1 | ||
| NRS860 | – | 0.125 | 2 | 1 | ||
| USA300 | USA (Mississippi) | Resistant to erythromycin, meticillin and tetracycline | 0.125 | 2 | 1 | |
| NRS194 | USA (North Dakota) | Resistant to meticillin | 0.0625 | 2 | 1 | |
| NRS108 | France | Resistant to gentamicin | 0.125 | 2 | 1 | |
| NRS119 (Linr) | USA (Massachusetts) | Resistant to linezolid | 0.0625 | >16 | 1 | |
| ATCC 43300 | USA (Kansas) | Resistant to meticillin | 0.0625 | 2 | 1 | |
| ATCC BAA-44 | Lisbon, Portugal | Multidrug-resistant strain | 0.0625 | 2 | 1 | |
| NRS70 | Japan | Resistant to erythromycin, clindamycin and spectinomycin | 0.0625 | 2 | 1 | |
| NRS71 | UK | Resistant to tetracycline and meticillin | 0.0625 | 2 | 1 | |
| NRS100 | USA | Resistant to tetracycline and meticillin | 0.0625 | 2 | 1 | |
| NRS123 | USA (North Dakota) | Resistant to tetracycline and meticillin | 0.0625 | 2 | 2 | |
| NRS107 | – | Resistant to meticillin and mupirocin | 0.0625 | 2 | 1 | |
| Vancomycin-intermediate S. aureus (VISA) | NRS1 | Japan | Resistant to aminoglycosides and tetracycline; glycopeptide-intermediate S. aureus | 0.0625 | 2 | 8 |
| NRS19 | USA (Illinois) | Glycopeptide-intermediate S. aureus | 0.125 | 1 | 2 | |
| NRS37 | France | Glycopeptide-intermediate S. aureus | 0.125 | 1 | 4 | |
| Vancomycin-resistant S. aureus (VRSA) | VRS1 | USA | Resistant to vancomycin | 0.0625 | 1 | >16 |
| VRS2 | USA | Resistant to vancomycin, erythromycin and spectinomycin | 0.0625 | 1 | 8 | |
| VRS3a | USA | Resistant to vancomycin | 0.0625 | 2 | >16 | |
| VRS3b | USA | Resistant to vancomycin | 0.0625 | 2 | >16 | |
| VRS4 | USA | Resistant to vancomycin, erythromycin and spectinomycin | 0.0625 | 2 | >16 | |
| VRS5 | USA | Resistant to vancomycin | 0.0625 | 2 | >16 | |
| VRS6 | USA | Resistant to vancomycin | 0.125 | 2 | >16 | |
| VRS7 | USA | Resistant to vancomycin and β-lactams | 0.0625 | 2 | >16 | |
| VRS8 | USA | Resistant to vancomycin | 0.0625 | 2 | >16 | |
| VRS9 | USA | Resistant to vancomycin | 0.0625 | 2 | >16 | |
| VRS10 | USA | Resistant to vancomycin | 0.125 | 2 | >16 | |
| VRS11a | USA | Resistant to vancomycin | 0.0625 | 2 | >16 | |
| VRS11b | USA | Resistant to vancomycin | 0.0625 | 2 | >16 | |
| VRS12 | USA | Resistant to vancomycin | 0.125 | 2 | >16 | |
| VRS13 | USA | Resistant to vancomycin | 0.0625 | 2 | >16 | |
| S. epidermidis | NRS101 | USA | Prototype biofilm-producer; resistant to meticillin and gentamicin | 0.0625 | 2 | 1 |
2.2. Antibacterial assays
To examine the antibacterial activity of auranofin against S. aureus, the broth microdilution method was utilised to determine the minimum inhibitory concentration (MIC) of each drug (tested in triplicate) following the guidelines outlined by the Clinical and Laboratory Standards Institute (CLSI) [21]. Each drug was incubated with the respective strain of S. aureus for 16 h at 37 °C before the MIC was confirmed. The MIC was classified as the lowest concentration of each test agent where no bacterial growth was visible.
2.3. Mice infection
Eight-week-old female BALB/c mice (Harlan Laboratories, Indianapolis, IN) were used in this study. All animal procedures were approved by the Purdue University Animal Care and Use Committee (West Lafayette, IN). An in vivo murine MRSA skin infection study was conducted as described elsewhere [2,22–24]. Briefly, mice (five mice per group) received an intradermal injection (40 μL) of MRSA USA300 containing 1.65 × 108 CFU. Approximately 2 days later, an open wound/abscess formed at the site of injection. Five groups of mice were then treated topically with a suspension containing 2% fusidic acid, 2% mupirocin, or 0.5%, 1% or 2% auranofin in petroleum jelly. Another two groups were treated orally with 25 mg/kg of either linezolid or clindamycin. The control group was treated with petroleum jelly (vehicle). Mice were treated twice daily for 5 days. Then, 24 h after the last dose was administered, mice were humanely euthanised via CO2 asphyxiation. The region around the skin wound was slightly swabbed with 70% ethanol and the wound (1 cm2) was precisely excised, homogenised, serially diluted in phosphate-buffered saline (PBS) and then transferred to MSA plates. Plates were incubated at 37 °C for 24 h prior to enumeration of MRSA.
2.4. Detection of cytokines from the MRSA murine skin infection experiment
Skin homogenates obtained from the murine skin infection experiment described above were centrifuged. The supernatant was collected and was used to quantify the levels of inflammatory cytokines, including tumour necrosis factor-a (TNFα), interleukin-6 (IL-6), interleukin-1 beta (IL-1β) and monocyte chemoattractant protein-1 (MCP-1). Duo-set ELISA Kits (R&D Systems, Inc., Minneapolis, MN) were used for cytokine detection according to the manufacturer's protocol.
2.5. Combination testing of auranofin with commercial antibiotics
The additive activity of auranofin with conventional topical antibiotics (mupirocin, fusidic acid and retapamulin) was evaluated as described previously [25,26]. Briefly, MRSA USA300 was incubated with auranofin, control antibiotics, or a combination of auranofin plus a control antibiotic at different concentrations for 16 h. Next, the optical density at 600 nm was measured using a spectrophotometer. The percent bacterial growth for each treatment regimen was calculated.
2.6. Biofilm assay
The ability of auranofin to disrupt adherent staphylococcal biofilm was analysed using the microtitre dish biofilm formation assay [2,27]. Staphylococcus aureus ATCC 6538 and Staphylococcus epidermidis ATCC 35984 were inoculated in TSB supplemented with 1% glucose and were transferred to the wells of a 96-well tissue culture treated plate (CELLTREAT Scientific, Shirley, MA). Bacteria were incubated at 37 °C for 24 h to allow the formation of an adherent biofilm. The medium was removed and the wells were carefully washed with PBS four times to remove planktonic bacteria. TSB was transferred to all wells of the 96-well plate prior to addition of auranofin and control antibiotics (linezolid and vancomycin). Drugs were added at the indicated concentrations and were incubated again at 37 °C for 24 h. Afterward, plates were washed by submerging in tap water. Biofilms were stained with 0.1% (w/v) crystal violet for 30 min at room temperature before subsequently being washed four times with water. Plates were air dried for 1 h prior to the addition of 95% ethanol to solubilise dye bound to the biofilm. The biofilm mass was quantified by measuring the optical density of wells at 595 nm using a microplate reader (BioTek Instruments Inc., Winooski, VT). Data are presented as the mean percent biofilm mass reduction of each test agent (tested in triplicate) in relation to untreated wells.
2.7. Effect of auranofin and conventional antibiotics on planktonic persister cells
The effect of auranofin and conventional antibiotics (linezolid, retapamulin and vancomycin) on S. aureus planktonic persister cells that demonstrated tolerance to ciprofloxacin was investigated as described previously [28]. Briefly, an overnight culture of MRSA USA300 (1 × 1010 CFU) was incubated with 10 μg/mL ciprofloxacin (80× MIC; MIC for ciprofloxacin was 0.125 μg/mL) at 37 °C for 6 h. Bacteria were then centrifuged and test agents (auranofin, linezolid, retapamulin, vancomycin and ciprofloxacin) were added at a concentration of 100× MIC. The MICs of retapamulin and ciprofloxacin against MRSA USA300 were 0.5 μg/mL and 0.125 μg/mL, respectively. Bacteria were incubated with test agents at 37 °C for 48 h. Samples were collected after 0, 2, 4, 6, 24 and 48 h, were diluted in PBS and were transferred to TSA plates. Plates were incubated at 37 °C for 24 h before viable CFU for each treatment group were determined.
2.8. Toxicity assay
Human keratinocyte (HaCaT) cells were seeded at a density of 40 000 cells per well in a 96-well tissue culture plate and the MTS assay was performed. Auranofin at a concentration ranging from 0 μg/mL to 16 μg/mL was added to appropriate wells and the cells were incubated for 24 h. Finally, the cells were washed with PBS and the MTS assay reagent 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H tetrazolium) was added. After 4 h incubation at 37 °C, absorbance was measured at 490 nm using an ELISA microplate reader (Molecular Devices, Sunnyvale, CA). The results are expressed as percent cell viability of auranofin-treated cells in comparison with cells treated with DMSO.
2.9. Statistical analyses
Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA). P-values were calculated using the Student's t-test or Kaplan–Meier (log-rank) survival test, as indicated. P-values of ≤0.05 were deemed significant.
3. Results and discussion
3.1. In vitro antibacterial activity of auranofin
The antimicrobial activity of auranofin was assessed against a panel of clinically relevant strains of multidrug-resistant S. aureus (Table 1). Auranofin inhibited the growth of all tested strains, including those resistant to conventional antimicrobials such as meticillin and vancomycin. The MICs of auranofin required to inhibit 50% (MIC50) and 90% (MIC90) of MRSA, vancomycin-resistant S. aureus (VRSA) and meticillin-sensitive S. aureus (MSSA) strains were found to be 0.0625 μg/mL and 0.125 μg/mL, respectively. With regard to vancomycin-intermediate S. aureus (VISA), both the MIC50 and MIC90 values were found to be 0.125 μg/mL. The MICs determined for auranofin correlate with the results reported in other studies [18–20]. Interestingly, the antibacterial activity of auranofin (MIC ranged from 0.0625–0.125 μg/mL) against MSSA and MRSA is more potent than the antibiotics vancomycin (MIC of 1 μg/mL) and linezolid (MIC ranged from 2 μg/mL to >16 μg/mL). Auranofin managed to retain its antibacterial activity against MRSA strains that were resistant to several antibiotic classes including glycopeptides, oxazolidinones, tetracycline, β-lactams, macrolides and aminoglycosides, suggesting that cross-resistance between these particular antibiotics and auranofin is unlikely to occur.
3.2. Auranofin is superior to conventional antibiotics in reducing the bacterial load in a mouse model of MRSA skin infection
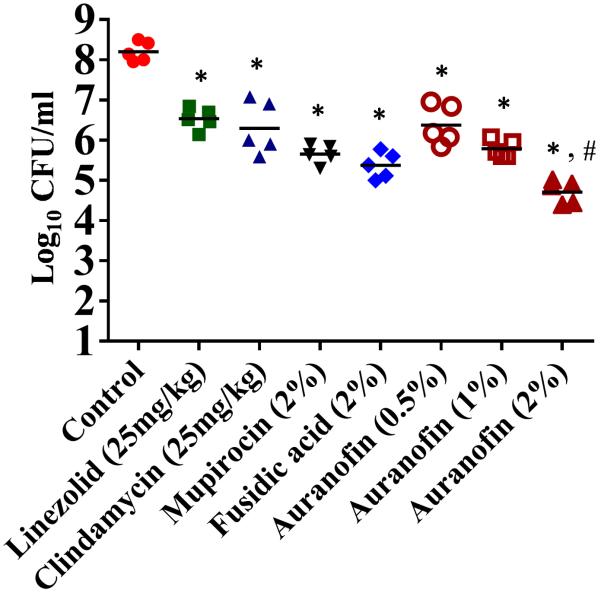
Confirmation of the potent in vitro anti-MRSA activity of auranofin led us to next investigate the efficacy of this drug in treating MRSA skin infections. Staphylococcus aureus, in particular MRSA, is a leading cause of skin infections in humans globally; of particular concern is MRSA USA300, which has been linked to the majority of skin and soft-tissue infections in the USA [1]. To assess the potential use of auranofin as a topical antimicrobial agent in vivo, mice were infected intradermally with MRSA USA300 to allow the formation of an open skin wound. A significant reduction in the mean bacterial load was observed for each treatment condition compared with the control group receiving the vehicle (petroleum jelly) alone (P ≤ 0.05) (Fig. 1). Mice treated with 2% auranofin produced the largest reduction in MRSA CFU (3.64 ± 0.14 log10), followed by 2% fusidic acid (2.83 ± 0.16 log10), 2% mupirocin (2.63 ± 0.14 log10), 1% auranofin (2.51 ± 0.11 log10), 25 mg/kg clindamycin (1.90 ± 0.24 log10), 0.5% auranofin (1.88 ± 0.18 log10) and 25 mg/kg linezolid (1.77 ±0.11 log10) (Fig. 1). Topical application of 2% auranofin produced a more significant reduction (P ≤ 0.05) in the mean bacterial load compared with treatment with drugs of choice, including 2% mupirocin and 2% fusidic acid. Thus, auranofin shows promise for use as a topical antimicrobial and, in this study, was superior to conventional antimicrobials commonly used to treat MRSA skin infections.
Fig. 1.
Efficacy of treatment of meticillin-resistant Staphylococcus aureus (MRSA) murine skin lesions with auranofin 0.5%, 1% and 2%, linezolid and clindamycin (25 mg/kg), mupirocin (2%), fusidic acid (2%) and petroleum jelly (control). Statistical analysis was performed by the two-tailed Student's t-test. *,# P-values of ≤0.05 were considered significant; auranofin was compared both with controls (*) and with antibiotics (#).
3.3. Auranofin reduces inflammatory cytokines induced by MRSA skin infection
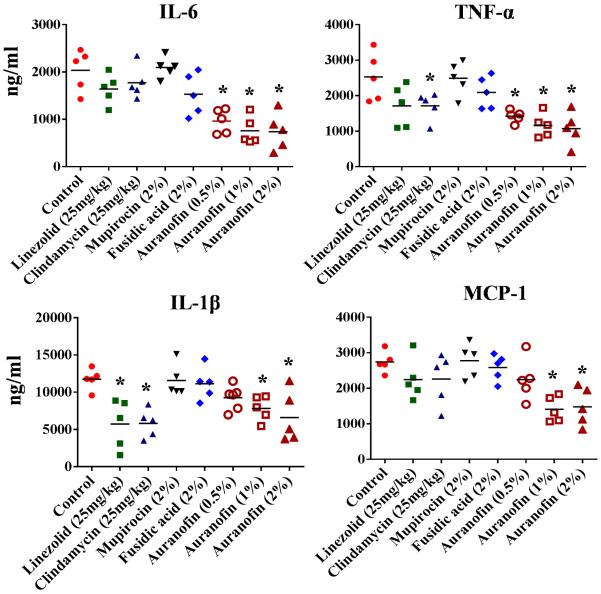
Exotoxins including α-haemolysin, leukocidins and toxic shock syndrome toxin (TSST-1) secreted by S. aureus during infection induce a strong inflammatory cascade reaction [3,4]. This cascade is thought to play a greater role in the severity of S. aureus skin infections than the size of the bacterial burden and can lead to an infection persisting for a longer time period [3]. Therefore, we investigated the immunomodulatory activity of auranofin in a topical application against MRSA skin infection. Supernatants collected from the wounds of mice infected with MRSA USA300 were used to detect the levels of inflammatory cytokines such as TNFα, IL-6, IL-1β and MCP-1. Wounds treated with either a 1% or 2% ointment of auranofin significantly reduced all inflammatory cytokines tested (IL-6, IL-1β, TNFα and MCP-1) (Fig. 2). Auranofin at 0.5% also significantly reduced IL-6 and TNFα. Mice administered an oral dose of clindamycin reduced IL-1β and TNFα, whereas oral treatment of mice with linezolid reduced only IL-1β. Thus, it appears that auranofin has more potent anti-inflammatory activity owing to the reduction in the presence of several pro-inflammatory cytokines compared with the conventional antimicrobials tested (linezolid, clindamycin, mupirocin and fusidic acid). The results garnered from this study suggest that the anti-inflammatory properties of auranofin warrant further investigation in the treatment of chronic wounds caused by S. aureus [4,29,30].
Fig. 2.
Effect of auranofin on inflammatory cytokines in meticillin-resistant Staphylococcus aureus (MRSA) skin lesions with auranofin 0.5%, 1% and 2%, linezolid and clindamycin (25 mg/kg), mupirocin (2%), fusidic acid (2%) and petroleum jelly (control). Statistical analysis was performed by the two-tailed Student's t-test. * P-values of ≤0.05 were considered significant. IL-6, interleukin-6; TNFα, tumour necrosis factor-α; IL-1β; interleukin-1 beta; MCP-1, monocyte chemoattractant protein-1.
3.4. Combination therapy of auranofin with topical antimicrobials
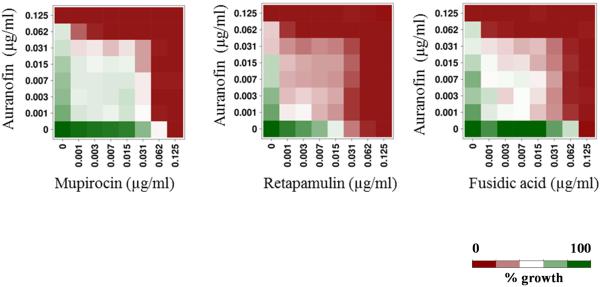
With the rapid emergence of MRSA strains resistant to topical antimicrobials of choice, including mupirocin and fusidic acid, combination therapy using multiple antibacterials is being explored [8,31,32]. Therefore, we assessed the activity of auranofin against MRSA USA300 in the presence of topical antimicrobials such as mupirocin, retapamulin and fusidic acid. Auranofin, in combination with all three tested topical antibiotics, exhibited additive activity (fractional inhibitory concentration index ranged from 0.5 to 1) in inhibiting MRSA growth (Fig. 3). This suggests that auranofin can be potentially combined with traditional topical antimicrobials such as mupirocin, retapamulin and fusidic acid for the treatment of staphylococcal skin infections, although further in vivo studies are needed to confirm this point.
Fig. 3.
Synergistic activity of auranofin in combination with three topical antimicrobials (mupirocin, retapamulin and fusidic acid).
3.5. Auranofin kills planktonic persister cells and reduces preformed biofilms
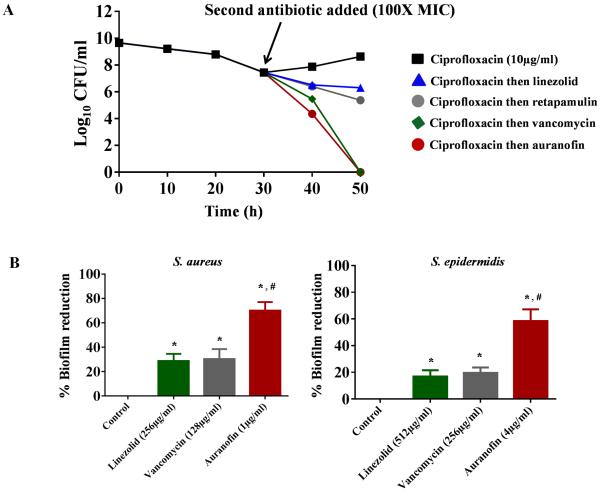
Treatment of bacterial infections with current antimicrobials is often challenging due to the inability of conventional antibiotics to target and disrupt adherent bacterial biofilms [33]. These problematic infections can become chronic when specialised dormant cells called planktonic persisters (that are normally sensitive to antibiotics) become encased within these biofilms, thus protecting them from exposure to and eradication by antibiotics [28]. To assess the ability of auranofin to mitigate the impact of staphylococcal biofilms, we first investigated the effect of auranofin on persister cells. When treated with ciprofloxacin, MRSA USA300 in exponential growth phase produces a biphasic killing pattern that results in surviving planktonic persister cells (Fig. 4A). Subsequent addition of conventional antimicrobials such as linezolid and retapamulin had minimal impact in reducing the number of planktonic persisters. However, treatment with auranofin resulted in complete eradication of planktonic persister cells after 48 h, a result that is comparable with vancomycin (Fig. 4A).
Fig. 4.
Activity of auranofin against planktonic persister cells and established biofilms of Staphylococcus aureus and Staphylococcus epidermidis. (A) Effect of auranofin on planktonic S. aureus persister cells. (B) Effect of auranofin and control antibiotics (vancomycin and linezolid) on established biofilms of S. aureus and S. epidermidis. Statistical analysis was performed using the two-tailed Student's t-test. *,# P-values of ≤0.05 were considered significant; auranofin was compared both with controls (*) and with antibiotics (#). MIC, minimum inhibitory concentration.
The ability of auranofin to kill S. aureus planktonic persisters led us to next assess the impact of auranofin on disrupting preformed staphylococcal biofilms. Auranofin at 1 μg/mL significantly reduced S. aureus biofilm mass by >60%; in contrast, even at high concentrations neither linezolid (256 μg/mL) nor vancomycin (128 μg/mL) were able to reduce biofilm mass by >30% (Fig. 4B). Similarly, auranofin at 4 μg/mL was more effective at reducing S. epidermidis biofilm mass (60% reduction observed) compared both with linezolid (512 μg/mL) and vancomycin (256 μg/mL), which reduced biofilm mass by only 20% (Fig. 4B). These results demonstrate that auranofin is capable of killing S. aureus persister cells and reducing adherent staphylococcal biofilms. This lays the foundation for further analysis using auranofin as a novel treatment option both for chronic and biofilm-related staphylococcal infections.
3.6. In vitro cytotoxicity study
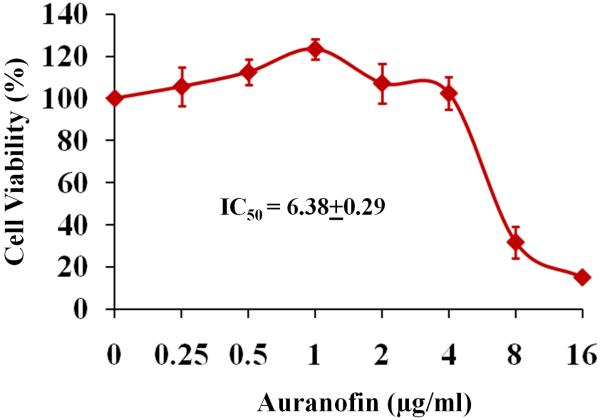
The toxicity of auranofin to HaCaT cells was investigated using the MTS assay. The results indicated that the concentration of auranofin required to inhibit 50% (IC50) of HaCaT cell growth was 6.38 ± 0.29 μg/mL (Fig. 5). This value is nearly 100 times larger than the MIC50 value for auranofin against MRSA. In addition, auranofin is currently approved for the long-term treatment of rheumatoid arthritis and patients have been taking the drug daily (6 mg/day) for more than 5 years, a much longer course of treatment than is traditionally prescribed for antibiotics (1–2 weeks) [34]. Thus, toxicity with auranofin should not be a significant impediment to repurposing this drug as a novel antibacterial agent for the treatment of cutaneous MRSA infections.
Fig. 5.
Cytotoxicity assay in human keratinocyte (HaCaT) cells. IC50, concentration of auranofin required to inhibit 50% of HaCaT cell growth.
In summary, the present study demonstrates that auranofin, an antirheumatic drug, also possesses potent in vitro antistaphylococcal activity against multidrug-resistant S. aureus. The in vitro results for auranofin were confirmed in a murine MRSA skin infection model, which demonstrated that auranofin is superior to conventional antimicrobials (mupirocin and fusidic acid) in reducing the bacterial burden in infected wounds. In addition to decreasing the bacterial load, auranofin exhibits potent anti-inflammatory activity, reducing the presence of four key cytokines (IL-6, IL-1β, TNFα and MCP-1) known to increase the morbidity associated with skin infections. Furthermore, the ability of auranofin to disrupt adherent staphylococcal biofilms and to kill persister cells, combined with its excellent safety profile, collectively support the notion that auranofin is a good candidate for repurposing as a topical antimicrobial for the treatment of staphylococcal skin infections.
Highlights.
Auranofin is superior to conventional antimicrobials (mupirocin and fusidic acid) in reducing the bacterial load in a mouse model of MRSA skin infection.
Auranofin reduces inflammatory cytokines in MRSA-infected skin lesions.
Auranofin exhibits potent antibiofilm activity.
Acknowledgment
The authors would like to thank the Network of Antimicrobial Resistance in Staphylococcus aureus (NARSA) program, supported under National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIAID/NIH) contract # HHSN272200700055C, for providing MRSA strains used in this study.
Funding: Research reported in this publication was supported by the NIAID/NIH [Award no. R56AI114861].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: None declared.
Ethical approval: All animal procedures were approved by the Purdue University Animal Care and Use Committee (PACUC) (West Lafayette, IN) [protocol no. 1207000676].
References
- [1].Stryjewski ME, Chambers HF. Skin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46(Suppl 5):S368–77. doi: 10.1086/533593. [DOI] [PubMed] [Google Scholar]
- [2].Thangamani S, Younis W, Seleem MN. Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections. Sci Rep. 2015;5:11596. doi: 10.1038/srep11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Montgomery CP, Daniels MD, Zhao F, Spellberg B, Chong AS, Daum RS. Local inflammation exacerbates the severity of Staphylococcus aureus skin infection. PLoS One. 2013;8:e69508. doi: 10.1371/journal.pone.0069508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fournier B, Philpott DJ. Recognition of Staphylococcus aureus by the innate immune system. Clin Microbiol Rev. 2005;18:521–40. doi: 10.1128/CMR.18.3.521-540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].del Rio A, Cervera C, Moreno A, Moreillon P, Miro JM. Patients at risk of complications of Staphylococcus aureus bloodstream infection. Clin Infect Dis. 2009;48(Suppl 4):S246–53. doi: 10.1086/598187. [DOI] [PubMed] [Google Scholar]
- [6].Keynan Y, Rubinstein E. Staphylococcus aureus bacteremia, risk factors, complications, and management. Crit Care Clin. 2013;29:547–62. doi: 10.1016/j.ccc.2013.03.008. [DOI] [PubMed] [Google Scholar]
- [7].McNeil JC, Hulten KG, Kaplan SL, Mason EO. Mupirocin resistance in Staphylococcus aureus causing recurrent skin and soft tissue infections in children. Antimicrob Agents Chemother. 2011;55:2431–3. doi: 10.1128/AAC.01587-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Farrell DJ, Castanheira M, Chopra I. Characterization of global patterns and the genetics of fusidic acid resistance. Clin Infect Dis. 2011;52(Suppl 7):S487–92. doi: 10.1093/cid/cir164. [DOI] [PubMed] [Google Scholar]
- [9].Corey GR, Jiang H, Moeck G. Dalbavancin or oritavancin for skin infections. N Engl J Med. 2014;371:1162–3. doi: 10.1056/NEJMc1407925. [DOI] [PubMed] [Google Scholar]
- [10].Shorr AF, Lodise TP, Corey GR, De Anda C, Fang E, Das AF, et al. Analysis of the phase 3 ESTABLISH trials of tedizolid versus linezolid in acute bacterial skin and skin structure infections. Antimicrob Agents Chemother. 2015;59:864–71. doi: 10.1128/AAC.03688-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Thangamani S, Mohammad H, Younis W, Seleem MN. Drug repurposing for the treatment of staphylococcal infections. Curr Pharm Des. 2015;21:2089–100. doi: 10.2174/1381612821666150310104416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chong CR, Sullivan DJ., Jr New uses for old drugs. Nature. 2007;448:645–6. doi: 10.1038/448645a. [DOI] [PubMed] [Google Scholar]
- [13].Thangamani S, Younis W, Seleem MN. Repurposing clinical molecule ebselen to combat drug resistant pathogens. PLoS One. 2015;10:e0133877. doi: 10.1371/journal.pone.0133877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Younis W, Thangamani S, Seleem MN. Repurposing non-antimicrobial drugs and clinical molecules to treat bacterial infections. Curr Pharm Des. 2015;21:4106–11. doi: 10.2174/1381612821666150506154434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Debnath A, Parsonage D, Andrade RM, He C, Cobo ER, Hirata K, et al. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat Med. 2012;18:956–60. doi: 10.1038/nm.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Harbut MB, Vilcheze C, Luo XZ, Hensler ME, Guo H, Yang BY, et al. Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc Natl Acad Sci U S A. 2015;112:4453–8. doi: 10.1073/pnas.1504022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jackson-Rosario S, Cowart D, Myers A, Tarrien R, Levine RL, Scott RA, et al. Auranofin disrupts selenium metabolism in Clostridium difficile by forming a stable Au-Se adduct. J Biol Inorg Chem. 2009;14:507–19. doi: 10.1007/s00775-009-0466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Aguinagalde L, Díez-Martínez R, Yuste J, Royo I, Gil C, Lasa Í , et al. Auranofin efficacy against MDR Streptococcus pneumoniae and Staphylococcus aureus infections. J Antimicrob Chemother. 2015;70:2608–17. doi: 10.1093/jac/dkv163. [DOI] [PubMed] [Google Scholar]
- [19].Cassetta MI, Marzo T, Fallani S, Novelli A, Messori L. Drug repositioning: auranofin as a prospective antimicrobial agent for the treatment of severe staphylococcal infections. Biometals. 2014;27:787–91. doi: 10.1007/s10534-014-9743-6. [DOI] [PubMed] [Google Scholar]
- [20].Hokai Y, Jurkowicz B, Fernández-Gallardo J, Zakirkhodjaev N, Sanaú M, Muth TR, et al. Auranofin and related heterometallic gold(I)-thiolates as potent inhibitors of methicillin-resistant Staphylococcus aureus bacterial strains. J Inorg Biochem. 2014;138:81–8. doi: 10.1016/j.jinorgbio.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Clinical and Laboratory Standards Institute . Document M07-A9. CLSI; Wayne, PA: 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—ninth edition. [Google Scholar]
- [22].Mohamed MF, Seleem MN. Efficacy of short novel antimicrobial and anti-inflammatory peptides in a mouse model of methicillin-resistant Staphylococcus aureus (MRSA) skin infection. Drug Des Devel Ther. 2014;8:1979–83. doi: 10.2147/DDDT.S72129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thangamani S, Mohammad H, Abushahba MF, Hamed MI, Sobreira TJ, Hedrick VE, et al. Exploring simvastatin, an antihyperlipidemic drug, as a potential topical antibacterial agent. Sci Rep. 2015;5:16407. doi: 10.1038/srep16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Thangamani S, Younis W, Seleem MN. Repurposing celecoxib as a topical antimicrobial agent. Front Microbiol. 2015;6:750. doi: 10.3389/fmicb.2015.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Meletiadis J, Pournaras S, Roilides E, Walsh TJ. Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro–in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrob Agents Chemother. 2010;54:602–9. doi: 10.1128/AAC.00999-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].King AM, Reid-Yu SA, Wang W, King DT, De Pascale G, Strynadka NC, et al. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature. 2014;510:503–6. doi: 10.1038/nature13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mohammad H, Mayhoub AS, Cushman M, Seleem MN. Anti-biofilm activity and synergism of novel thiazole compounds with glycopeptide antibiotics against multidrug-resistant staphylococci. J Antibiot (Tokyo) 2015;68:259–66. doi: 10.1038/ja.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Conlon BP, Nakayasu ES, Fleck LE, LaFleur MD, Isabella VM, Coleman K, et al. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature. 2013;503:365–70. doi: 10.1038/nature12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jialal I, Miguelino E, Griffen SC, Devaraj S. Concomitant reduction of low-density lipoprotein-cholesterol and biomarkers of inflammation with low-dose simvastatin therapy in patients with type 1 diabetes. J Clin Endocrinol Metab. 2007;92:3136–40. doi: 10.1210/jc.2007-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13:465–76. doi: 10.1038/nrd4275. [DOI] [PubMed] [Google Scholar]
- [31].Hu Y, Coates AR. Enhancement by novel anti-methicillin-resistant Staphylococcus aureus compound HT61 of the activity of neomycin, gentamicin, mupirocin and chlorhexidine: in vitro and in vivo studies. J Antimicrob Chemother. 2013;68:374–84. doi: 10.1093/jac/dks384. [DOI] [PubMed] [Google Scholar]
- [32].Huang L, Dai T, Xuan Y, Tegos GP, Hamblin MR. Synergistic combination of chitosan acetate with nanoparticle silver as a topical antimicrobial: efficacy against bacterial burn infections. Antimicrob Agents Chemother. 2011;55:3432–8. doi: 10.1128/AAC.01803-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mah TF, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–9. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- [34].Egsmose C, Lund B, Borg G, Pettersson H, Berg E, Brodin U, et al. Patients with rheumatoid arthritis benefit from early 2nd line therapy: 5 year followup of a prospective double blind placebo controlled study. J Rheumatol. 1995;22:2208–13. [PubMed] [Google Scholar]