Abstract
Tissue engineering presents a strategy to overcome the limitations of current tissue healing methods. Scaffolds, cells, external growth factors and mechanical input are combined in an effort to obtain constructs with properties that mimic native tissues. However, engineered constructs developed using similar culture environments can have very different matrix composition and biomechanical properties. Accordingly, a non-destructive technique to assess constructs during development such that appropriate compositional endpoints can be defined is desirable. Near infrared spectroscopy (NIRS) analysis is a modality being investigated to address the challenges associated with current evaluation techniques, which includes non-destructive compositional assessment. In the present study, cartilage tissue constructs were grown using chondrocytes seeded onto polyglycolic acid (PGA) scaffolds in similar environments in three separate tissue culture experiments and monitored using NIRS. Multivariate partial least squares (PLS) analysis models of NIR spectra were calculated and used to predict tissue composition, with biochemical assay information used as the reference data. Results showed that for combined data from all tissue culture experiments, PLS models were able to assess composition with significant correlations to reference values, including engineered cartilage water (at 5200 cm−1, R = 0.68, p = 0.03), proteoglycan (at 4310 cm−1, R = 0.82, p = 0.007), and collagen (at 4610 cm−1, R = 0.84, p = 0.005). In addition, degradation of PGA was monitored using specific NIRS frequencies. These results demonstrate that NIR spectroscopy combined with multivariate analysis provides a non-destructive modality to assess engineered cartilage, which could provide information to determine the optimal time for tissue harvest for clinical applications.
Keywords: Cartilage, Tissue engineering, Near infrared spectroscopy, Multivariate data analysis, Collagen, Proteoglycan
INTRODUCTION
Osteoarthritis (OA) is a musculoskeletal joint disease that affects an estimated 12% of the United States population.34 It is characterized by articular cartilage degeneration, advanced stages of which may lead to total loss of joint function and the need for arthroplasty. The limited capacity of articular cartilage to repair and regenerate, due to the lack of blood supply and limited migration of chondrocytes to the defect zone, has resulted in extensive research into the techniques of cartilage development or repair of defective cartilage.37 Current clinical methods for the replacement or repair of damaged cartilage include microfracture, autograft and allograft transplants and autologous chondrocyte implantation (ACI) procedures, each of which have limitations, for example, limited tissue generation, limited tissue availability for transplant/graft, tissue rejection by the host body, and fibrocartilage development.15,20,41 Disappointment with the long term outcomes of these procedures has stimulated the development of functional engineered replacement tissues through tissue engineering techniques.19,37
Studies focused on development of functional engineered cartilage replacement tissues typically involve the loading of a degradable scaffold with chondrocytes or stem cells and growth of the tissue for varied durations.17,19 These scaffolds may be constructed from natural or synthetic polymers, for example, collagen, polyglycolic acid (PGA) or hydrogels.12,16,23 The scaffold acts as a substrate for cell adhesion and provides a boundary for the retention of cells. In addition, growth factors and/or mechanical stimulation may be applied to the scaffold/cell system to improve cell differentiation, growth characteristics, and tissue type.11,23,38,54 Cell membrane receptors play a major role in signalling effects that determine extracellular matrix component deposition. In particular, integrin and sugar receptors bind to culture media molecules (e.g. proteins and sugar molecules in the fetal bovine serum, FBS.), which trigger downstream signalling events,55,60 and lead to matrix component synthesis. It has been shown that transforming growth factor beta (TGF-β, present in FBS) is a strong stimulator of proteoglycan and type II collagen synthesis in chondrocytes. Type I insulin like growth factor (IGF-1, also present in FBS) is also considered an essential factor to stimulate proteoglycan synthesis.21,22 Ascorbic acid is typically added to chondrocyte culture media to enhance type II collagen content via increased cell proliferation and protein synthesis. It also maintains the chondrogenic properties of the cells and inhibits cell differentiation to other cell types.33,52
It has been shown that optimal cartilage function depends on its chemical and mechanical properties and culture duration.9,19 Unfortunately, engineered cartilage constructs that are developed using similar materials and cells in the same culture environment demonstrate different physical and biological characteristics,12,16 and use of non-optimal constructs for clinical applications can lead to failure and tissue disintegration.43 Therefore, continuous monitoring of developing engineered cartilage is required to understand the influence of each input, and to determine when a cartilage mimetic construct is ready for implantation. Most characterization methods, e.g. histology and biochemical measurements of hydroxyproline (collagen) and sGAG (proteoglycan), are destructive, and cannot be used to characterize a specific construct during longitudinal development. Although non-destructive assessment of mechanical properties of cartilage is possible using strains as low as 20%,35 the majority of mechanical testing protocols for cartilage involve irreversible tissue deformation, and are not conducive to being performed in a sterile tissue environment. Non-destructive methods based on magnetic resonance imaging (MRI),31 optical imaging,18 ultrasound imaging,64 and X-ray imaging,42 are possible alternatives. Many MRI studies have successfully imaged matrix content in tissues,48 including in engineered constructs.31,49 However, optimal methods are still in development, and may not be widely accessible for many tissue engineering labs, due to the high cost of the equipment. Contrast-enhanced micro CT is another option for non-destructive assessment of cartilage PG content.57 However, this technique requires the addition of an external contrast agent, which would not be compatible with developing engineered constructs. Further, the use of ionizing radiation (e.g. X-ray imaging), still raises concerns about damage to the tissue sample.3
In the search for a non-destructive minimally invasive technique, infrared-based methods have been developed over the last 15–20 years. Infrared spectroscopy is based on absorbance of infrared light by tissue functional groups at specific vibrational frequencies,5 and thus, no external contrast is required. Different infrared wavelength (frequency) regions, the mid infrared (MIR) and near infrared (NIR), can be used to evaluate tissue composition.1,5,8,32 In addition, spectroscopic modalities, including imaging spectroscopy29,32,63 and fiber optic spectroscopy,1,2,36 have been developed for analysis of harvested or intact samples. Fiber optic MIR spectroscopy is a non-destructive technique that can be used to assess tissue composition and has been established as a method for monitoring cartilage degradation.27,63 The MIR spectroscopic technique is hampered, however, by limited penetration depth; non-destructive MIR spectroscopic measurements are only able to probe at most 2–10 μm through a sample 26 making it an inherently surface-biased technique. Articular cartilage is not a homogenous system and previous studies have shown heterogeneous composition within engineered constructs, including chondrocyte-PGA scaffold constructs.7 It is clearly desirable to have a non-destructive technique that probes the entire thickness of the construct, and NIR spectroscopy potentially fulfils these monitoring requirements.
NIR spectroscopy is based on higher energy photons than MIR and hence has a greater penetration depth.2,6,45,47 However, spectral signals observed using NIR spectroscopy are not as specific as those observed using MIR spectroscopy, and analysis of NIR spectroscopic data typically requires more complex methods. Previous MIR studies have successfully characterized cartilage using univariate or bivariate methods, e.g. intensity of proteoglycan peak or the ratio of the intensity of the proteoglycan peak to the intensity of the protein amide I peak.29,63 Analytical NIR spectroscopy however, typically requires the utilization of multivariate methods, e.g. principal component analysis (PCA) or partial least squares (PLS) regression. Previous studies in our lab and other research groups conducted on native and engineered cartilage have shown correlations between NIR spectroscopic parameters and cartilage properties.4,39 However, previous models for engineered tissues have been made using a single set of tissue engineering samples developed together in a single round of experiments. Ideally, engineered cartilage tissues would be grown in multiple tissue culture experiments, a PLS model derived, and that model utilized to predict composition of all samples from multiple experiments. This would confirm that multivariate analysis of NIR spectra can be applicable to measure cartilage tissue properties from different tissue engineering experiments performed with similar, but not identical, conditions. Presented here is an assessment of NIR spectroscopy for the non-destructive monitoring of development of chondrocyte-PGA scaffolds constructs over culture periods of three separate experiments in similar environments, with a single PLS model developed to predict chemical composition of the engineered tissues. It is hypothesized that a quantitative multivariate model based on NIR spectra can be developed to predict the composition of engineered cartilage constructs, independent of their time in culture. The outcome, when validated with gold standard techniques, presents a non-destructive modality for analysis of developing engineered tissues.
MATERIALS AND METHODS
Engineered Cartilage Constructs
Cartilage constructs were grown in three separate experiments using a protocol based on Vunjak-Novakovic et al.61 Articular cartilage was harvested from 2 to 14 days old bovine stifle joints (Research 87, Boylston, MA), with two to three knees required for each experiment. Tissues were digested in a spinner flask containing 250 U/mL type II collagenase (Worthington Biochemical, Lakewood, NJ), 99 ml DMEM (Life Technologies, Grand Island, NY), 1 ml penicillin/streptomycin (10,000 U/mL penicillin, 10,000 μg/ml streptomycin, (Life Technologies), and 0.2 ml fungizone (250 μg/ml amphotericin B and 205 μg/ml sodium deoxycholate, Life Technologies). Spinner flasks were incubated at 37 °C and 5% CO2 for 12 h. Chondrocytes were isolated from the digest solution by filtering through 70 μm sterile nylon filter and centrifugation at 1000×g. The cell pellet was collected, reconstituted in 1× phosphate buffered saline (Life Technologies), and cells counted using a hemocytometer.
PGA scaffolds (Biomedical Structures LLC, Warwick, RI) were cut out from non-woven felt at 2 mm thickness and 5 mm diameter and mounted on 14 gauge syringe needles fixed in a 250 ml spinner flask (Bellco Glass, Vineland, NJ). The spinner flask was filled with 200 ml of DMEM based media containing 9% v/v fetal bovine serum (Hyclone labs, Inc., Logan, UT), 2 mM glutamine, penicillin (88,000 U/L), streptomycin (88 mg/L), 440 μg/L amphotericin B, 360 μg/ L sodium deoxycholate, 44 mg/L gentamicin, 150 μM L-ascorbic acid-2-phosphate, 1× non-essential amino acids (MEM Non-Essential Amino Acids Solution, Life Technologies), and 350 μM of L-proline (all from Life Technologies or Sigma Aldrich, St. Loius, MO). Chondrocytes were loaded onto PGA scaffolds (n = 18, 18 and 24 for cultures 1, 2 and 3, respectively); this was done by immersing the PGA scaffolds in a chondrocyte media solution with a concentration of approximately 20 million cells per scaffold and incubating for 72 h (37 °C, 5% CO2). Seeded scaffolds were then removed from the spinner flasks and placed in glass bottomed 6-well tissue culture plates (one scaffold per well) (day 0). Glass-bottom well plates (In Vitro Scientific, Sunnyvale, CA) were used due to the absence of absorbance in the NIR spectral region. The same culture media used in cell loading was added to each well, 5 ml, and scaffolds were incubated at 37 °C and 5% CO2. Culture media was replaced every 2–3 days. Cartilage constructs were harvested on day 7 (1 week), 14 (2 weeks), and 21 (3 weeks) for Experiment 1, day 21 (3 weeks) and 42 (6 weeks) for Experiment 2, and day 21 (3 weeks) and 42 (6 weeks) for Experiment 3. The total number of samples for each harvest day across all three cultures was; Day 7: n = 6, Day 14: n = 6, Day 21: n = 24, and Day 42: n = 24. There are fewer samples for days 7 and 14 as these timepoints were added later in the study. Nevertheless, appropriate statistical analyses were performed to account for sample size variations. Samples were weighed at each harvest day (wet weight), and thickness measured with a calliper. Constructs were then soaked in protease inhibitor (Sigma Aldrich) and frozen at −20 °C before further biochemical analysis.
Near-Infrared Spectroscopy
Diffuse reflectance NIR spectra were collected during each media change when the construct was not in the culture media (to minimize external water absorbance) using a Remspec (Charlton, MA) NIR probe coupled to a matrix-F spectrometer (Bruker Optics, Billerica, MA) using OPUS software v.5.5 (Bruker Optik GmbH, Ettlingen, Germany) (Fig. 1). Background spectra of air were collected using a mirror and sample spectra were collected by positioning the probe 2 mm above the cartilage construct with the well plate placed on a mirrored surface. Each sample spectrum collected was the sum of 128 co-added scans across the spectral range of 10,000–4000 cm−1 with a spectral resolution of 8 cm−1, and was ratioed to a background spectrum. Three NIR spectra were collected from each construct on each day. NIR spectral data were also collected from a segment of 3 mm thick bovine cartilage for comparison to the engineered constructs.
FIGURE 1.

NIR probe data collection from engineered constructs.
NIR Data Pre-processing and Multivariate Data Analysis (PLS)
NIR spectra were analyzed using Unscrambler X (CAMO Software, Oslo, Norway). Three spectra per construct were collected and averaged prior to data processing. Spectra were pre-processed with an extended multiplicative scatter correction (EMSC),51 followed by area normalization and second derivative processing with a 41 and 83 point Savitzky–Golay smoothing window50 for water content analysis, and proteoglycan (PG) and collagen content analysis, respectively. Second derivative peak heights at 5200 cm−1 were used to monitor the combined bound and free water content of each construct.46 Based on previous literature indicating that the NIR absorbances at 4610 and 4310 cm−1 reflect collagen and chondroitin sulfate (proteoglyclan, PG), respectively,47 second derivative peak heights at these wavenumbers were used as a measure of these matrix components. The second derivative peak height at 4200 cm−1, an absorbance unique to PGA, was used to assess PGA degradation.56 To assess the reproducibility of the triplicate NIR measurements for individual samples, a coefficient of variation (CV) was calculated for the second derivative peak heights related to water and collagen content at 5200 and 4600 cm−1 respectively. The relevant peak heights were assessed for three spectra collected from an individual construct at 15 time points from Day 1 to Day 42. The CV was calculated as:
| (1) |
Pre-processed NIR spectra from all three tissue culture experiments were used to calculate a PLS model in the 8000–4000 cm−1 range (optimized based on investigation of several spectral ranges) using leave one out cross validation.13 Chemical composition information obtained from biochemical assays and gravimetric measurements were used as the reference content for PG, collagen, and water in the PLS models. The PLS analysis approach finds linear combinations of the predictors (factors) to predict the response values. The number of factors for each model was determined by examining loading weights and comparison of the root mean square error of calibration (RMSEC) and cross validation (RMSECV).13 The quality of the model was evaluated based on the root mean square error of calibration (RMSEC) or cross validation (RMSECV) (as a percentage of the range of data), and the R2 of actual vs. predicted values.
| (2) |
Biochemical Analyses
After gravimetric measurement of wet weight, samples were lyophilized for 24 h and weighed to obtain dry weight. Water percentage was calculated as the difference between the wet and dry weights expressed as a percentage of the wet weight. Lyophilized cartilage constructs were then digested with 1 mg/mL proteinase K (Sigma Aldrich) in tris–HCl buffer (50 mM tris–HCl and 1 mM CaCl2, pH 8) at 55 °C and the digests were stored at −20 °C (construct digest solutions).
Sulfated glycosaminoglycan (sGAG) content (reflecting PG content) was determined using a dimethylmethylene blue (DMMB) assay.14 Briefly, 1,9-DMMB (pH 3) (Sigma Aldrich) was added to the digest solution at 10–1 ratio and absorption recorded at 525 nm using a Tecan plate reader (Tecan Systems Inc., San Jose, CA). A series of chondroitin sulfate (Sigma Aldrich) solutions with known concentrations were used to plot a standard curve (absorbance vs. concentration) and the concentration of sGAG in the samples was calculated using the standard curve equation.
Hydroxyproline content (reflecting collagen content) was determined using a chloramine-T based assay.30 Construct digest solutions were hydrolysed using 6 M HCl (1:2 ratio) at 110 °C for 18 h. Then, hydrosylate samples were neutralised with 6 M NaOH, decolorized using activated carbon, and subsequently treated with chloramine-T (3:2:5 1-propanol:water:citrate buffer, 15 mM chloramine-T) and Erlich’s (47:37:16 1-propanol:water:perchloric acid, 160 mM p-dimethylaminobenzaldehyde) reagent. A series of cis-4-Hydroxy-D-proline (Sigma Aldrich) solutions with different concentrations were used to calculate a standard curve. Absorbance at 550 nm was measured using a Tecan plate reader. The concentration of hydroxyproline in the samples was calculated using the standard curve equation. Hydroxyproline content was converted to collagen content using a factor of 10.62 Biochemical assay measurement of sGAG, as a representative of PG concentration in the engineered cartilage, is referred to as PG content of constructs throughout this work. Collagen and PG content obtained from biochemical assays were normalized to construct wet weight.
Statistical Analysis
SigmaPlot 12.0 (Sysstat Software, San Jose, CA) was used to calculate average values and standard deviations, and for statistical analysis. Significant differences among average values were analyzed using two way Analysis of Variance (ANOVA) for comparisons among time and culture, and using one way ANOVA for comparisons among time only. Post-hoc comparisons were performed with significance set at p < 0.05. A Pearson correlation was performed to assess correlation of measured and predicted values from the PLS models, and between NIR-determined values and biochemical parameters.
RESULTS
Engineered Constructs
Cartilage construct thickness more than doubled to ~4.5 mm after one week of growth, and then increased to ~5 mm by 3 weeks (Fig. 2). Cartilage constructs were significantly thicker compared to initial PGA scaffolds at all timepoints. A visible color change from white (pre-culture) to red (3 weeks) to light pink (6 weeks) was seen in cartilage constructs during their growth (Fig. 2), likely attributable to the relative amount of nutrient-rich media present in the constructs.
FIGURE 2.
(a) Cartilage constructs were significantly thicker compared to initial PGA scaffolds for all timepoints (*significantly different compared to pre-culture). (b) Cartilage matrix formation within the scaffold mesh changes the size, morphology and color of constructs over time.
Gravimetric Water and Biochemical Assays
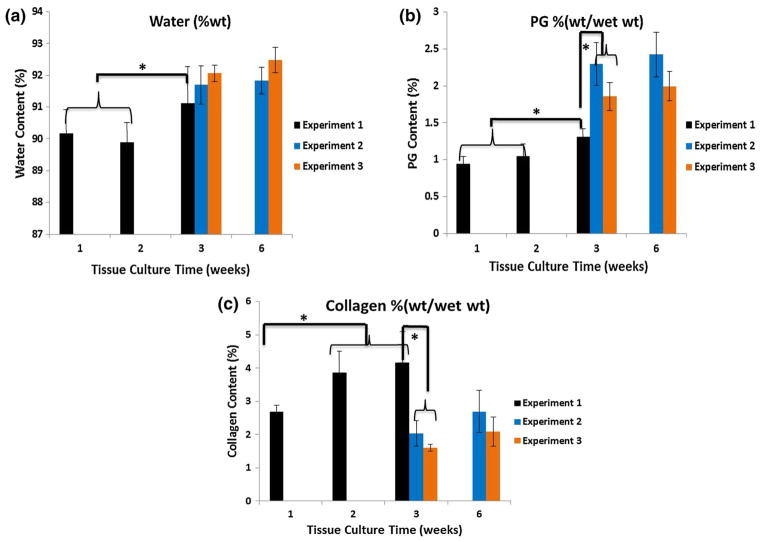
The culture duration and the specific culture experiment impacted water, PG, and collagen content (Fig. 3). The average PG and water content generally increased over time, but collagen increased over time only in Experiment 1, from 1 to 3 weeks. There was no significant increase in matrix parameters between 3 and 6 weeks for Experiments 2 and 3, indicating the matrix content was maximized by the 3 week timepoint. However, within each experiment, for a separate timepoint, the construct composition exhibited a range of values, as reflected in the standard deviations (Fig. 3).
FIGURE 3.
Water (a), sGAG (b), and collagen (c) content assessed using gravimetric measurements and biochemical assays for constructs in culture Experiments 1, 2, and 3. Construct composition varied with culture duration and with the specific experiment, although all constructs were grown using the same conditions. *Significant difference among values at different timepoints in a specific experiment, or between different experiments at the same timepoint (p < 0.05).
NIR Spectroscopy
Representative NIR spectra obtained from Experiment 2 constructs are shown in Fig. 4. A NIR spectrum of bovine native cartilage is also shown for comparison. Similar to native cartilage, the NIR spectra of tissue engineered cartilage constructs are dominated by peaks associated with water molecule vibrations at approximately 7150 and 5200 cm−1 (Fig. 4a), and band broadening is observed with construct growth over time. Second derivative spectra are used to visualize the specific peaks that underlie the broad bands in the raw NIR spectra (Fig. 4b). Except for the PGA peak at ~4200 cm−1, which is not present in the native cartilage, the overall contour and peak positions of the engineered constructs and native cartilage spectra are similar. The CV of the triplicate NIRS measurements for individual constructs was fairly narrow, ranging from 0.36% to 3.48% (average = 1.76%) for the 5200 cm−1 water measurement, and from 0.59% to 4.90% (average = 3.24%) for the 4610 cm−1 collagen measurement.
FIGURE 4.
NIR raw spectra (a) and second derivative spectra (b) of engineered cartilage constructs from Experiment 2 as a function of time showing the water absorbance dominance and peak broadening. A spectrum from native cartilage of similar thickness is included for comparison. Spectral details are resolved using second derivative spectra, and peaks reflecting specific matrix and water components are noted with dashed lines. Peaks at 4200, 4310, and 4610 cm−1 reflect PGA, PG and collagen respectively, while the peaks at 5200 and 7150 cm−1 arise from water.
The average intensity of the water peak measured at 5200 cm−1 changes with construct growth (Fig. 5a), reflecting changes in water content. Except for the samples in Experiment 1 grown for 1 and 2 weeks, the NIR-determined water content generally increased with cartilage development. Over time, cartilage matrix components replaced PGA, a biodegradable scaffold, and the NIR-determined PGA content decreased. Similar to the biochemical measurements, there were no significant NIR-determined increases in matrix parameters between 3 and 6 weeks, indicating the matrix content was maximized by the 3 week timepoint. The exception to this was NIR-determined PG content, which did increase significantly from 3 to 6 weeks (Figs. 5b–5d). Accordingly, significant correlations were found between the NIR-measured parameters and gravimetric/biochemical assessments of water and collagen (R = 0.62 and p = 0.04, R = 0.79 and p = 0.01, respectively), and a nearly significant correlation with PG content (R = 0.46, p = 0.06).
FIGURE 5.
The second derivative peak heights at 4200, 4310, 4610, 5200 cm−1 were used to reflect the NIR-derived concentration of water (a), PG (b), collagen (c), and PGA (d). A similar pattern of increase in water, PG, and collagen, and decrease in PGA, is observed among different experiments. However, the actual experimental values of specific components differ. *Significant difference among values at different timepoints in a specific experiment, or between different experiments at the same timepoint (p < 0.05).
PLS Model and Matrix Component Prediction
A summary of the PLS models is shown in Table 1. The models were calculated based on the NIR spectra as variables (X) and the matrix component content (water, collagen, PG) as the response (Y). The concentrations predicted by the PLS models were plotted vs. the actual measured values from the biochemical assays and water content measurements (Fig. 6). The errors for the calibration and validation of the models for individual components fell within the 6–9% range, and resulted in significant correlations to the experimental measured values of water content (R = 0.68, p = 0.03), PG (R = 0.82, p = 0.007), and collagen (R = 0.84, p = 0.005).
TABLE 1.
Results of PLS models in the spectral range 8000–4000 cm−1 for prediction of engineered cartilage composition from three combined experiments
| Component | Number of factors | Relative error of calibration (%) | R2 | Relative error of validation (%) | R2 |
|---|---|---|---|---|---|
| Water | 4 | 7 | 0.74 | 8 | 0.57 |
| sGAG (PG) (wt%/wet wt) | 5 | 6 | 0.79 | 8 | 0.66 |
| Collagen (wt%/wet wt) | 8 | 6 | 0.80 | 9 | 0.58 |
FIGURE 6.
Engineered construct composition predicted using PLS models vs. experimental values obtained from gravimetric and biochemical analysis, with regression lines. Significant correlations (R values shown, all p < 0.05) were found between the predicted and experimental values for all parameters.
DISCUSSION
The data presented here are in accord with the results of several other studies where it has been shown that engineered cartilage tissues developed using the same protocol do not grow uniformly, and can exhibit a range of compositional properties.12,16,40 This could be particularly challenging to address during two stage clinical cartilage repair procedures, such as autologous chondrocyte implantation (ACI), or matrix-induced ACI (MACI). For these procedures, patient cartilage is harvested, cells isolated, proliferated and seeded with growth factors and/or on a scaffold, typically for a predetermined amount of time prior to implantation.58 The optimal repair technique has not yet been established, and it is likely that in addition to finding the optimal material, identification of the ideal time for growth and implantation of an individual construct is a key factor in augmenting tissue repair. This motivates development of non-destructive analytical techniques, such as NIR spectroscopy, for in situ evaluation of construct properties during growth.
Based on biochemical measurements, PG and collagen content of engineered constructs developed in three separate experiments showed significant differences in the experimental values among samples grown for the same amount of time in different experiments, and variation in content among tissues grown in the same experiment. While it has been shown that external stimulation, such as mechanical force, will increase matrix component production by chondrocytes, here, as in previous studies with this PGA scaffold, mechanical stimulation was not required for matrix production.61 There were two approaches to NIR spectral assessments of composition, evaluation of specific frequencies associated with water and matrix components, and calculation of multivariate analysis models, where many frequencies are evaluated simultaneously. Using peak heights at specific frequencies, similar, but not identical results, compared to the biochemical results, were achieved (Figs. 3 and 5). The standard deviations of the measurements for the two techniques were generally similar, and the values correlated significantly, except for PG content. The NIR spectral data indicated that the Experiment 1 PG values were greater than those for Experiment 2 or 3, while the biochemistry data indicated that the Experiment 1 PG values were lower compared to the other two. There are several possibilities for the discrepancy in these measurements, but the fact that the biochemistry is an sGAG measurement, while the NIR measurement was developed based on chondroitin sulfate,47 is likely a contributor. In addition, evaluation of just one frequency from NIR spectra is generally not as reliable as multivariate analyses, which is the standard in industrial and research applications.13 The multivariate analysis PLS models presented in the current studies resulted in strong correlations with collagen and PG content, and with a lower, although still significant, correlation between measured and predicted water content. The lower correlation with water is likely attributable to the presence of media on the surface of constructs, as constructs needed to be kept moist while NIR spectral data were collected.
One limitation of using PLS methods is that many samples are required to construct the models initially. Ideally, the models would be constructed using one set of spectral data, and another data set would be used for independent prediction. In data sets such as those in the current study, where there are not enough spectra to separate out an independent prediction set, the cross-validation method is well-accepted for model development.13 To translate the NIR methodology to widespread use for non-destructive assessment of a specific type of cartilage construct, data from several more experiments would be collected, and a larger database of spectra, ideally from greater than 50 samples from each timepoint,10 would be collected. This technology could also be applied to development of multivariate analysis models of other engineered tissue types where extracellular matrix is deposited and a scaffold is remodelled, such as in skin, bone, and cardiovascular constructs, with collection of NIR data from the appropriate number of samples.
Currently, there are no non-destructive techniques in use for evaluation of engineered cartilage construct matrix prior to implantation. However, one current approach for optimization of engineered cartilage is found in ChondroCelect (Tigenix, Leuven, Belgium), a cell-based therapy approved for use in Europe that uses a gene expression score to assess construct development.53,59 This procedure, termed ‘‘characterized chondrocyte implantation (CCI)’’, involves expansion of chondrocytes based on expression of a gene marker profile predictive of the capacity to form hyaline cartilage, a process which aims to optimize individual batches of cells harvested from a patient. Although results from early repair were encouraging, at five years post-treatment, the clinical outcomes for CCI and MF were comparable. Thus, enhancement of an aspect of construct quality in vitro, in this case cells specifically selected for gene markers related to hyaline cartilage, did not necessarily translate into improved clinical outcome.
Although information on water and matrix composition, and on scaffold degradation, can be evaluated with NIR spectral data, information on cell type is not currently available. Previous work has shown that dedifferentiation of chondrocytes occurs in the PGA culture environment used here, and can result in formation of fibrous tissue around the construct perimeter, primarily composed of type I collagen, in addition to type II collagen-rich hyaline cartilage.61 The NIR fiber optic probe modality has not been investigated for its sensitivity to collagen type, and at this point, evaluation of NIR spectral data does not yield insight into specific compositional variations at the cell or collagen-type level. However, recent studies have shown sensitivity to collagen type with mid infrared spectroscopy, in both imaging29 and fiber optic28 modes. As mid infrared spectral data arises from surface evaluation only, this technique is not optimal for investigation of heterogeneous full-depth engineered constructs or repair tissue.44 Numerous studies have shown that repair of cartilage defects proceeds with the occurrence of fibrocartilage rich in type I collagen along with type II rich hyaline cartilage, and to date, clinical imaging modalities have not optimized differentiation of these two tissue types.29 If future investigations demonstrate the sensitivity of NIR spectral data to collagen types, this would be valuable for assessment of developing constructs in the lab, as well as for in vivo clinical evaluations.
In spite of the promising results presented, there are limitations to the use of the NIR technique. While the models developed provide compositional information on engineered constructs, further analysis is needed to determine whether the NIR-determined parameters correlate with mechanical properties of the constructs. An additional limitation is that even though NIR probe spectroscopy provides compositional information through the full depth of the tissue, it cannot be used in a confocal mode. Thus, three dimensional, layer by layer analysis of tissues is not possible. It is conceivable, however, that the NIR technique could be combined with other imaging methods to increase the understanding of engineered construct development. As an example, Gurjarpadhye et al. developed an optical coherence tomographic (OCT) technique, a catheter-based non-destructive method for assessment of engineered vascular grafts.24,25 A similar approach could be developed in combination with NIR, where OCT is used to obtain a three dimensional view of the tissues, and NIR spectra extracted from different regions, guided by OCT images.
The possibility of one modality that can be used in vitro during laboratory experiments of developing tissues to assess composition, as well as after the tissues are implanted clinically, is very appealing. Together, the data presented here strongly support the continued development of near infrared spectral analysis as a non-destructive modality for engineered construct assessment.
Acknowledgments
This work was supported in part by NIH R01AR056145 and by the National Institute on Aging, National Institutes of Health, Intramural Research Program
Footnotes
Associate Editor Dan Elson oversaw the review of this article.
CONFLICT OF INTEREST
The authors of this manuscript report no conflict of interests related to the work presented here.
References
- 1.Afara I, Prasadam I, Crawford R, Xiao Y, Oloyede A. Non-destructive evaluation of articular cartilage defects using near-infrared (NIR) spectroscopy in osteoarthritic rat models and its direct relation to Mankin score. Osteoarthr Cartil. 2012;20:1367–1373. doi: 10.1016/j.joca.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Afara I, Singh S, Oloyede A. Application of near infrared (NIR) spectroscopy for determining the thickness of articular cartilage. Med Eng Phys. 2013;35:88–95. doi: 10.1016/j.medengphy.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Appel AA, Anastasio MA, Larson JC, Brey EM. Imaging challenges in biomaterials and tissue engineering. Biomaterials. 2013;34:6615–6630. doi: 10.1016/j.biomaterials.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baykal D, Irrechukwu O, Lin PC, Fritton K, Spencer RG, Pleshko N. Nondestructive assessment of engineered cartilage constructs using near-infrared spectroscopy. Appl Spectrosc. 2010;64:1160–1166. doi: 10.1366/000370210792973604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boskey A, Camacho NP. FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials. 2007;28:2465–2478. doi: 10.1016/j.biomaterials.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown CP, Jayadev C, Glyn-Jones S, Carr AJ, Murray DW, Price AJ, Gill HS. Characterization of early stage cartilage degradation using diffuse reflectance near infrared spectroscopy. Phys Med Biol. 2011;56:2299–2307. doi: 10.1088/0031-9155/56/7/024. [DOI] [PubMed] [Google Scholar]
- 7.Bursac PM, Freed LE, Biron RJ, Vunjak-Novakovic G. Mass transfer studies of tissue engineered cartilage. Tissue Eng. 1996;2:141–150. doi: 10.1089/ten.1996.2.141. [DOI] [PubMed] [Google Scholar]
- 8.Camacho NP, Carroll P, Raggio CL. Fourier transform infrared imaging spectroscopy (FT-IRIS) of mineralization in bisphosphonate-treated oim/oim mice. Calcif Tissue Int. 2003;72:604–609. doi: 10.1007/s00223-002-1038-1. [DOI] [PubMed] [Google Scholar]
- 9.Danisovic L, Varga I, Zamborsky R, Bohmer D. The tissue engineering of articular cartilage: cells, scaffolds and stimulating factors. Exp Biol Med (Maywood) 2012;237:10–17. doi: 10.1258/ebm.2011.011229. [DOI] [PubMed] [Google Scholar]
- 10.Delwiche SR, Reeves JB., 3rd A graphical method to evaluate spectral preprocessing in multivariate regression calibrations: example with Savitzky-Golay filters and partial least squares regression. Appl Spectrosc. 2010;64:73–82. doi: 10.1366/000370210790572007. [DOI] [PubMed] [Google Scholar]
- 11.Elder BD, Athanasiou KA. Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue Eng Part B. 2009;15:43–53. doi: 10.1089/ten.teb.2008.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erickson IE, Huang AH, Chung C, Li RT, Burdick JA, Mauck RL. Differential maturation and structure-function relationships in mesenchymal stem cell-and chondrocyte-seeded hydrogels. Tissue Eng Part A. 2009;15:1041–1052. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esbensen KH. Multivariate Data Analysis-in Practice: An Introduction to Multivariate Data Analysis and Experimental Design. Woodbridge: CAMO Software; 2010. [Google Scholar]
- 14.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 15.Farr J, Yao JQ. Chondral defect repair with particulated juvenile cartilage allograft. Cartilage. 2011;2:346–353. doi: 10.1177/1947603511405838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher MB, Henning EA, Soegaard NB, Dodge GR, Steinberg DR, Mauck RL. Maximizing cartilage formation and integration via a trajectory-based tissue engineering approach. Biomaterials. 2014;35:2140–2148. doi: 10.1016/j.biomaterials.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freed LE, Engelmayr GC, Jr, Borenstein JT, Moutos FT, Guilak F. Advanced material strategies for tissue engineering scaffolds. Adv Mater. 2009;21:3410–3418. doi: 10.1002/adma.200900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgakoudi I, Rice WL, Hronik-Tupaj M, Kaplan DL. Optical spectroscopy and imaging for the noninvasive evaluation of engineered tissues. Tissue Eng Part B. 2008;14:321–340. doi: 10.1089/ten.teb.2008.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Getgood A, Brooks R, Fortier L, Rushton N. Articular cartilage tissue engineering: today’s research, tomorrow’s practice? J Bone Joint Surg. 2009;91:565–576. doi: 10.1302/0301-620X.91B5.21832. [DOI] [PubMed] [Google Scholar]
- 20.Gill TJ, Asnis PD, Berkson EM. The treatment of articular cartilage defects using the microfracture technique. J Orthop Sports Phys Ther. 2006;36:728–738. doi: 10.2519/jospt.2006.2444. [DOI] [PubMed] [Google Scholar]
- 21.Goldring MB. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract Res Clin Rheumatol. 2006;20:1003–1025. doi: 10.1016/j.berh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Grimaud E, Heymann D, Redini F. Recent advances in TGF-beta effects on chondrocyte metabolism. Potential therapeutic roles of TGF-beta in cartilage disorders. Cytokine Growth Factor Rev. 2002;13:241–257. doi: 10.1016/s1359-6101(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 23.Grunder T, Gaissmaier C, Fritz J, Stoop R, Hortschansky P, Mollenhauer J, Aicher WK. Bone morphogenetic protein (BMP)-2 enhances the expression of type II collagen and aggrecan in chondrocytes embedded in alginate beads. Osteoarthr Cartil. 2004;12:559–567. doi: 10.1016/j.joca.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Gurjarpadhye AA, DeWitt MR, Xu Y, Wang G, Rylander MN, Rylander CG. Dynamic assessment of the endothelialization of tissue-engineered blood vessels using an optical coherence tomography catheter-based fluorescence imaging system. Tissue Eng Part C. 2015;21:758–766. doi: 10.1089/ten.tec.2014.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurjarpadhye AA, Whited BM, Sampson A, Niu G, Sharma KS, Vogt WC, Wang G, Xu Y, Soker S, Rylander MN, Rylander CG. Imaging and characterization of bioengineered blood vessels within a bioreactor using free-space and catheter-based OCT. Lasers Surg Med. 2013;45:391–400. doi: 10.1002/lsm.22147. [DOI] [PubMed] [Google Scholar]
- 26.Hanh BD, Neubert RH, Wartewig S, Christ A, Hentzsch C. Drug penetration as studied by noninvasive methods: fourier transform infrared-attenuated total reflection, fourier transform infrared, and ultraviolet photoacoustic spectroscopy. J Pharm Sci. 2000;89:1106–1113. doi: 10.1002/1520-6017(200009)89:9<1106::aid-jps2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 27.Hanifi A, Bi XH, Yang X, Kavukcuoglu B, Lin PC, DiCarlo E, Spencer RG, Bostrom MPG, Pleshko N. Infrared fiber optic probe evaluation of degenerative cartilage correlates to histological grading. Am J Sports Med. 2012;40:2853–2861. doi: 10.1177/0363546512462009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanifi A, McCarthy H, Roberts S, Pleshko N. Fourier transform infrared imaging and infrared fiber optic probe spectroscopy identify collagen type in connective tissues. PLoS ONE. 2012;8:e64822. doi: 10.1371/journal.pone.0064822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanifi A, Richardson JB, Kuiper JH, Roberts S, Pleshko N. Clinical outcome of autologous chondrocyte implantation is correlated with infrared spectroscopic imaging-derived parameters. Osteoarthr Cartil. 2013;20:988–996. doi: 10.1016/j.joca.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoemann CD, Sun J, Chrzanowski V, Buschmann MD. A multivalent assay to detect glycosaminoglycan, protein, collagen, RNA, and DNA content in milligram samples of cartilage or hydrogel-based repair cartilage. Anal Biochem. 2002;300:1–10. doi: 10.1006/abio.2001.5436. [DOI] [PubMed] [Google Scholar]
- 31.Irrechukwu ON, Lin PC, Fritton K, Doty S, Pleshko N, Spencer RG. Magnetic resonance studies of macromolecular content in engineered cartilage treated with pulsed low-intensity ultrasound. Tissue Eng Part A. 2011;17:407–415. doi: 10.1089/ten.tea.2010.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim M, Bi X, Horton WE, Spencer RG, Camacho NP. Fourier transform infrared imaging spectroscopic analysis of tissue engineered cartilage: histologic and biochemical correlations. J Biomed Opt. 2005;10:031105. doi: 10.1117/1.1922329. [DOI] [PubMed] [Google Scholar]
- 33.Kim G, Okumura M, Bosnakovski D, Ishiguro T, Park CH, Kadosawa T, Fujinaga T. Effects of ascorbic acid on proliferation and biological properties of bovine chondrocytes in alginate beads. Jpn J Vet Res. 2003;51:83–94. [PubMed] [Google Scholar]
- 34.Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, Heyse SP, Hirsch R, Hochberg MC, Hunder GG, Liang MH, Pillemer SR, Steen VD, Wolfe F. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 35.Li LP, Buschmann MD, Shirazi-Adl A. Strain-rate dependent stiffness of articular cartilage in unconfined compression. J Biomech Eng. 2003;125:161–168. doi: 10.1115/1.1560142. [DOI] [PubMed] [Google Scholar]
- 36.Li G, Thomson M, Dicarlo E, Yang X, Nestor B, Bostrom MP, Camacho NP. A chemometric analysis for evaluation of early-stage cartilage degradation by infrared fiber-optic probe spectroscopy. Appl Spectrosc. 2005;59:1527–1533. doi: 10.1366/000370205775142593. [DOI] [PubMed] [Google Scholar]
- 37.Mahmoudifar N, Doran PM. Chondrogenesis and cartilage tissue engineering: the longer road to technology development. Trends Biotechnol. 2012;30:166–176. doi: 10.1016/j.tibtech.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Makris EA, Responte DJ, Paschos NK, Hu JC, Athanasiou KA. Developing functional musculoskeletal tissues through hypoxia and lysyl oxidase-induced collagen cross-linking. Proc Natl Acad Sci USA. 2013;111:E4832–E4841. doi: 10.1073/pnas.1414271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGoverin CM, Lewis K, Yang X, Bostrom MPG, Pleshko N. The contribution of bone and cartilage to the near-infrared spectrum of osteochondral tissue. Appl Spectrosc. 2014;68:1168–1175. doi: 10.1366/13-07327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer EG, Buckley CT, Steward AJ, Kelly DJ. The effect of cyclic hydrostatic pressure on the functional development of cartilaginous tissues engineered using bone marrow derived mesenchymal stem cells. J Mech Behav Biomed Mater. 2011;4:1257–1265. doi: 10.1016/j.jmbbm.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, Wickiewicz TL, Marx RG. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87:1911–1920. doi: 10.2106/JBJS.D.02846. [DOI] [PubMed] [Google Scholar]
- 42.Muller B, Beckmann F, Huser M, Maspero F, Szekely G, Ruffieux K, Thurner P, Wintermantel E. Nondestructive three-dimensional evaluation of a polymer sponge by microtomography using synchrotron radiation. Biomol Eng. 2002;19:73–78. doi: 10.1016/s1389-0344(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 43.Obradovic B, Martin I, Padera RF, Treppo S, Freed LE, Vunjak-Novakovic G. Integration of engineered cartilage. J Orthop Res. 2001;19:1089–1097. doi: 10.1016/S0736-0266(01)00030-4. [DOI] [PubMed] [Google Scholar]
- 44.O’Brien MP, Penmatsa M, Palukuru U, West P, Yang X, Bostrom MP, Freeman T, Pleshko N. Monitoring the progression of spontaneous articular cartilage healing with infrared spectroscopy. Cartilage. 2015;6:174–184. doi: 10.1177/1947603515572874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Padalkar MV, Pleshko N. Wavelength-dependent penetration depth of near infrared radiation into cartilage. Analyst. 2015;140:2093–2100. doi: 10.1039/c4an01987c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Padalkar MV, Spencer RG, Pleshko N. Near infrared spectroscopic evaluation of water in hyaline cartilage. Ann Biomed Eng. 2013;41:2426–2436. doi: 10.1007/s10439-013-0844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palukuru UP, McGoverin CM, Pleshko N. Assessment of hyaline cartilage matrix composition using near infrared spectroscopy. Matrix Biol. 2014;38:3–11. doi: 10.1016/j.matbio.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Potter HG, Black BR, Chong le R. New techniques in articular cartilage imaging. Clin Sports Med. 2009;28:77–94. doi: 10.1016/j.csm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Reiter DA, Irrechukwu O, Lin PC, Moghadam S, Von Thaer S, Pleshko N, Spencer RG. Improved MR-based characterization of engineered cartilage using multi-exponential T2 relaxation and multivariate analysis. NMR Biomed. 2012;25:476–488. doi: 10.1002/nbm.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rieppo L, Saarakkala S, Narhi T, Helminen HJ, Jurvelin JS, Rieppo J. Application of second derivative spectroscopy for increasing molecular specificity of Fourier transform infrared spectroscopic imaging of articular cartilage. Osteoarthr Cartil. 2012;20:451–459. doi: 10.1016/j.joca.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 51.Rinnan A, van den Berg F, Engelsen SB. Review of the most common pre-processing techniques for near-infrared spectra. Trac Trends Anal Chem. 2009;28:1201–1222. [Google Scholar]
- 52.Sandell LJ, Daniel JC. Effects of ascorbic acid on collagen mRNA levels in short term chondrocyte cultures. Connect Tissue Res. 1988;17:11–22. doi: 10.3109/03008208808992790. [DOI] [PubMed] [Google Scholar]
- 53.Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, Vandekerckhove B, Almqvist KF, Claes T, Handelberg F, Lagae K, van der Bauwhede J, Vandenneucker H, Yang KG, Jelic M, Verdonk R, Veulemans N, Bellemans J, Luyten FP. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235–246. doi: 10.1177/0363546507311095. [DOI] [PubMed] [Google Scholar]
- 54.Shahin K, Doran PM. Tissue engineering of cartilage using a mechanobioreactor exerting simultaneous mechanical shear and compression to simulate the rolling action of articular joints. Biotechnol Bioeng. 2011;109:1060–1073. doi: 10.1002/bit.24372. [DOI] [PubMed] [Google Scholar]
- 55.Shakibaei M, Csaki C, Mobasheri A. Diverse roles of integrin receptors in articular cartilage. Adv Anat Embryol Cell Biol. 2008;197:1–60. doi: 10.1007/978-3-540-78771-6. [DOI] [PubMed] [Google Scholar]
- 56.Shockley M, McGoverin C, Palukuru U, Glenn P, Spencer R, Pleshko N. Near infrared spectroscopy as a method for non-destructive monitoring of engineered cartilage growth. 39th Annual Northeast Bioengineering Conference; 2013; pp. 51–52. [Google Scholar]
- 57.Siebelt M, Groen HC, Koelewijn SJ, de Blois E, Sandker M, Waarsing JH, Muller C, van Osch GJ, de Jong M, Weinans H. Increased physical activity severely induces osteoarthritic changes in knee joints with papain induced sulfate-glycosaminoglycan depleted cartilage. Arthritis Res Ther. 2014;16:R32. doi: 10.1186/ar4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stein S, Strauss E, Bosco J., 3rd Advances in the surgical management of articular cartilage defects: autologous chondrocyte implantation techniques in the pipeline. Cartilage. 2013;4:12–19. doi: 10.1177/1947603512463226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vanlauwe J, Saris DB, Victor J, Almqvist KF, Bellemans J, Luyten FP. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med. 2011;39:2566–2574. doi: 10.1177/0363546511422220. [DOI] [PubMed] [Google Scholar]
- 60.Vinatier C, Bouffi C, Merceron C, Gordeladze J, Brondello JM, Jorgensen C, Weiss P, Guicheux J, Noel D. Cartilage tissue engineering: towards a biomaterial-assisted mesenchymal stem cell therapy. Curr Stem Cell Res Ther. 2009;4:318–329. doi: 10.2174/157488809789649205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vunjak-Novakovic G. Effects of mixing on the composition and morphology of tissue-engineered cartilage. AIChE. 1996;42:850–860. [Google Scholar]
- 62.Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130–138. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 63.West PA, Bostrom MP, Torzilli PA, Camacho NP. Fourier transform infrared spectral analysis of degenerative cartilage: an infrared fiber optic probe and imaging study. Appl Spectrosc. 2004;58:376–381. doi: 10.1366/000370204773580194. [DOI] [PubMed] [Google Scholar]
- 64.Xuan JW, Bygrave M, Jiang H, Valiyeva F, Dunmore-Buyze J, Holdsworth DW, Izawa JI, Bauman G, Moussa M, Winter SF, Greenberg NM, Chin JL, Drangova M, Fenster A, Lacefield JC. Functional neoangiogenesis imaging of genetically engineered mouse prostate cancer using three-dimensional power Doppler ultrasound. Cancer Res. 2007;67:2830–2839. doi: 10.1158/0008-5472.CAN-06-3944. [DOI] [PubMed] [Google Scholar]