Summary
Objective
To examine whether panoramic radiograph-determined mandibular cortical thickness correlated with quantitative computed tomography-derived bone mineral density (BMD) in survivors of childhood acute lymphoblastic leukemia (ALL).
Methods
We identified patients treated for ALL at St. Jude Children’s Research Hospital, seen in the After Completion of Therapy (ACT) Clinic between January of 2006 and January of 2014 who had QCT-derived BMD and panoramic radiographs obtained within 1 month of each other. Panoramic radiographs were independently scored by a pediatric radiologist, two pediatric dentists and a general dentist using the Klemmeti technique. We used the Spearman’s rank correlation test and the multivariate regression model to investigate the effect of evaluator experience on results.
Results
The study cohort comprised 181 patients with 320 paired studies: 112 (62%) male, 112 (71%) were white. Median age at ALL diagnosis was 6.4 (range, 0 – 18.8) years. Median age at study was 11.9 (range, 3.3 to 29.4) years. The median average BMD was 154.6 (range, 0.73 – 256) mg/cc; median QCT Z-score (age and gender-adjusted) was −0.875 (range, −5.04 to 3.2). We found very weak association between panoramic radiograph score and both QCT-BMD average (p =0.53) and QCT Z-score (p = 0.39). Results were not influenced by level of reader experience.
Conclusions
The Klemetti technique of estimating BMD does not predict BMD deficits in children and young adult survivors of ALL, regardless of reviewer expertise. Alternative methods are needed whereby dental healthcare providers can identify and refer patients at risk of BMD deficits for detailed assessment and intervention.
Introduction
Children treated for acute lymphoblastic leukemia (ALL), the most common type of cancer affecting children, are at increased risk for developing bone mineral density deficits compared with the healthy population1. As survival rates now exceed 90%.2 long-term complications are becoming more prevalent within the growing survivor population. Treatment with chemotherapy and radiation contribute to an increased risk of low BMD, in survivors of childhood ALL3–6. A decrease in BMD may be associated with an increased risk of fracture at an unusually young age in children who have undergone treatment for ALL.2 The increased risk of fracture in turn may result in long-term morbidity. Identifying pediatric patients with BMD deficits allows for implementing early interventions to improve or ameliorate BMD when growth and skeletal maturation are most active and when such interventions may be most beneficial.
Mandibular alveolar bone undergoes aging processes that are similar to other bones in the body.7, 8 As bone ages, trabeculae thin and the bone becomes demineralized, while the inferior mandibular cortex becomes more porous and focally thin.9–11 Panoramic radiographs are widely used in preventive dental evaluations and could serve as a means of identifying children with low BMD who warrant referral for BMD assessment.9, 12–14
Thus, we sought to determine whether BMD changes could be detected using routine panoramic radiographs in long-term survivors of childhood ALL by scoring the mandibular cortical thickness according to a published method validated in adults and determining whether or not Klemetti scores correlated with lumbar spine BMD in these patients as determined by QCT.15 We also examined whether reviewer experience influenced the scoring.
Materials and Methods
Study Population
We identified patients treated at St. Jude Children’s Research Hospital for ALL who were seen between January of 2006 and January of 2014 and who had QCT-derived BMD and digital panoramic radiographs obtained within 1 month of each other. Institutional Review Board approved this project and data was managed in accordance with the Health Insurance Portability and Accountability Act of 2006 (HIPAA). We captured patient demographics and treatment-related details for all included patients.
BMD Measurements
QCT-BMD of the lumbar spine (LS) vertebral trabecular BMD is a well-established method for estimating BMD. QCT-BMD was determined with a General Electric Lightspeed Ultra 8 slice scanner from 2006 to 2007 and subsequently using a General electric VCT 64 slice (Waukesha, WI) and Mindways QCT calibration phantoms and Mindways QCT-PRO software (Mindways Software, Inc., Austin, TX), as previously reported.16–19 Typically, bone mineral content was determined of the first two lumbar vertebral bodies (L1, L2). If one of these vertebral bodies was deformed or fractured, an alternate vertebral body from T11 to L4 was chosen. The average BMD of the two vertebrae was used for analysis. Age and gender specific BMD Z-score were provided by the manufacturer.1, 3
Panoramic Radiographs
Digital panoramic radiographs were obtained using Siemens Orthophos 3C (Sirona Dental Systems, Charlotte, NC) from 2006–2007 and Sirona Orthophos XG Plus (Bensheim, Germany) from 2007 to present. Images were reviewed in one of two venues, both having dimmed ambient lighting: the Dental Clinic office using a HPL 2245W monitor with 1680 × 1050 resolution, or in Diagnostic Imaging using 541mm PACs monitor with 2048 × 1536 landscape display resolution.
Panoramic radiographs (Orthophos XG Plus, Sirona Dental Systems, 4835 Sirona Drive, Suite 100, Charlotte, NC 28273) were obtained using 68 kV and 8 mA for extremely small patients. For larger and very large patients, those who were wheelchair-bound or had very broad shoulders, higher parameters were utilized (71 kV and 15 mA or 77 kV and 14 mA).
Evaluators
Radiographic evaluation was completed by four examiners who each have varying years of experience interpreting panoramic radiographs: a pediatric dentist with 14 years of experience, pediatric dental resident with two years of experience, a pediatric radiologist with 23 years of experience and a general dentist with 42 years of experience.
Scoring
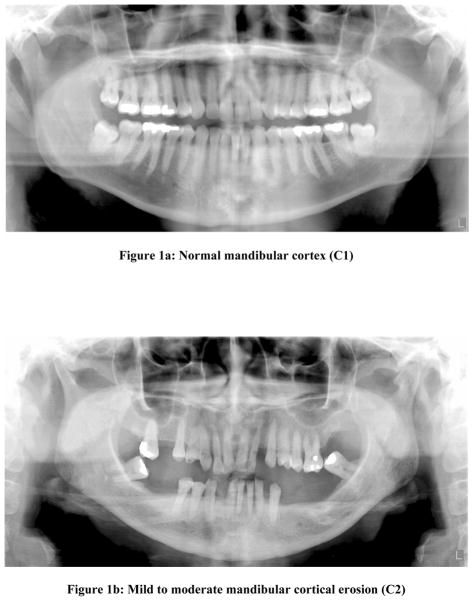
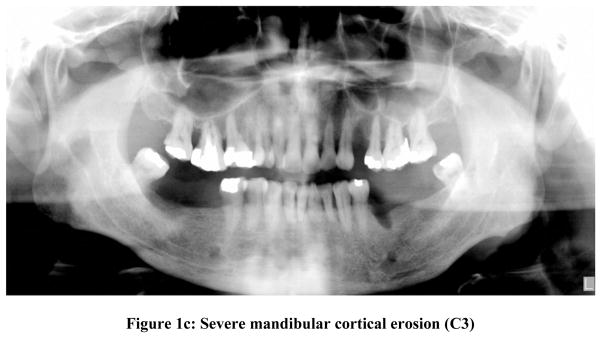
To standardize scoring of panoramic radiographs, evaluators were trained in classifying the Mandibular Cortical Index (MCI) described by Klemetti et al (1997)15. This technique was chosen for the study because it is a well-documented technique available to classify radiographic changes in the mandibular cortex and due to the simplicity of its use. In short, the appearance of the lower border cortex of the mandible distal to the mental foramen on a three-point scale (figure 1) was evaluated as follows:
Figure 1.
Panoramic radiographs demonstrating the appearance of mandibular cortex for each of the panoramic radiograph scores.
Figure 1a: Normal mandibular cortex (C1)
Figure 1b: Mild to moderate mandibular cortical erosion (C2)
Figure 1c: Severe mandibular cortical erosion (C3)
C1- Normal mandibular cortex: the endosteal margin of the cortex was even and sharp on both sides (Fig 1a)
C2- Mild to moderate mandibular cortical erosion: the endosteal margin showed lacunar resorption forming one to three layers on one or both sides of the mandible (Fig 1b)
C3- Severe erosion of the mandibular cortex: the cortical layer formed heavy endosteal cortical residues and was clearly porous (Fig 1c)
Statistical Methods
Association between the average panoramic radiograph evaluation scores and the QCT measurements (QCT average and QCT Z-score) were evaluated using both the correlation coefficient test (Spearman’s rank correlation test) and the multivariate regression model adjusted for the patient demographics. Each evaluator independently scored each observation. Inasmuch as the QCT manufacturer database provided Z-scores that were age and sex matched but not race-matched, we performed statistical analysis of correlations using the entire patient population and also restricted to the white subset.
Results
Study cohort
Between January of 2006 and January of 2014, 181 long-term survivors of ALL were seen in the ACT clinic and had 320 paired QCT measurements and panoramic radiographic evaluations obtained within 1 month of each other. Sixty-four of the181 (35.4%) patients had more than one observation. The maximum number of observations per patient was six (range, 1 – 6). Of the 181 patients: 112 (62%) were males. 129 (71%) were White, 28 (15%) were African Americans, and 24 (13%) were of mixed or other races. Age at diagnosis ranged from 0 to 18.8 (mean = 7.9; median = 6.4) years. Age at the time of study (treated as 320 paired studies) ranged from 3.3 to 39.4 (mean = 12.8; median = 11.9) years.
Of the 320 QCT measurements, the median QCT average value was 154.6 mg/cc (range, 0.73 to 256 mg/cc) while the median QCT Z-score (age and gender adjusted) was −0.875 (range, −5.04 to 3.2). In particular, 65 of 320 (20.3%) observed QCT Z-scores fell more than 2SD below the mean; 78 (24.4%) QCT Z-scores were between −2SD and −1SD.
Comparing the panoramic radiograph coding amongst all four reviewers revealed that agreement in coding ranged from 63 to 78%. Evaluators differed by one level of severity in coding cases in 5 to 36% of cases; coding differences of two levels did not exceed 1%.
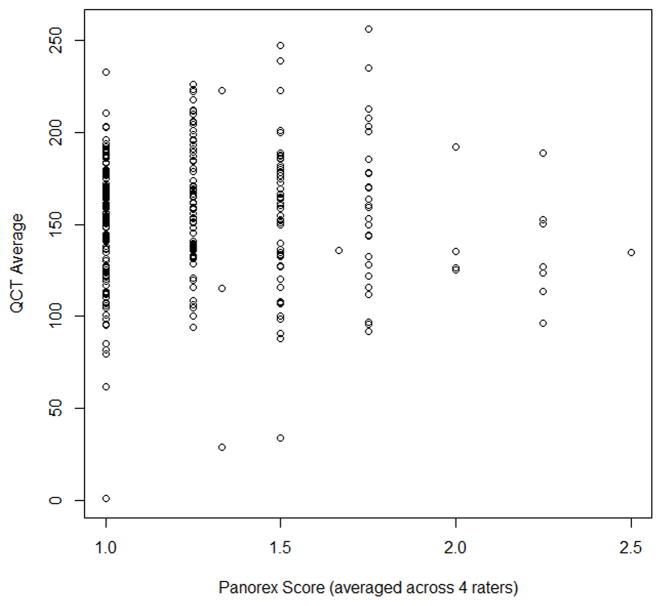
Kappa statistics were calculated between all pairs of raters and the disagreement were weighted equally. The Kappa statistics with equal weight ranges from 0.16 to 0.29.The association between the Klemetti technique with QCT measurements was not statistically significantly whether or not the analysis was restricted to only the white population. We examined the association between QCT average and QCT Z-score and the corresponding Klemetti score treated as numerical numbers 1, 2, 3 representing C1, C2, C3 and then averaged the scores of the four raters. The Spearman’s rank correlation coefficient between QCT average and panoramic radiograph score was very weak at 0.035 (p-value=0.53). The Spearman’s correlation coefficient between QCT Z-score and panoramic radiograph score was weak at 0.048 (p-value=0.39). From the scatter plot (Figure 2, we found no statistically significant association between panoramic radiograph scores and QCT measurements. Thus, the Klemetti technique does not predict BMD in the study population.
Figure 2.
Scatter plot comparing panoramic radiograph score and QCT
Discussion
This study demonstrated that the Klemetti technique used to show changes in the mandibular cortical bone did not predict BMD in survivors of childhood acute lymphoblastic leukemia. The detection of bone changes in the mandible that can occur after cancer therapy in this population would allow the dental provider to refer patients for medical care of this important complication.
There are currently more than 600, 000 survivors of childhood ALL in U.S. alone [Howlander N NA, Krapcho M …. SEER Cancer Statistics Review, 1975–2011, NCI, Bethesda, MD, http://seer.cancer.gov/csr/1975-2011/, based on November 2013 SEER data submission, posted to SEER website April 2014] who are at risk for decreased BMD related to their primary disease, treatment, lifestyle, and genetic predisposition. 1, 3, 18, 20 Early identification of patients at risk provides the opportunity to implement interventions prior to skeletal maturation, during a time when the impact of treatment may be most effective. 16, 21
Since dentists are frontline members of the healthcare team, they offer a means to identify pediatric patients at risk for BMD deficits would enhance patient health. Panoramic radiographs are a routine part of the dental evaluation and could serve as a screening tool to assess BMD as has been shown in adults.15, 22 Existing literature lacks studies addressing the possible correlation between alterations in mandibular cortical thickness, BMD and association with development of chronic physiological complications resulting from cancer or its therapy among childhood survivors of ALL. Thus, we examined whether mandibular cortex thickness as shown on dental panoramic radiographs correlated with QCT examinations in a cohort of pediatric patients treated for ALL by using a technique validated in adults.
Oral healthcare providers can be more involved in identifying changes in BMD in an effort to initiate treatment early enough to prevent osteoporosis and other bone-related complications. If bone changes present in panoramic radiographs could be comparable to changes in QCT exams, the dentist could play an important role in the early identification of patients at risk for bone complications and serve as a source for early patient referral for management. It has been shown that dental students can be trained to screen panoramic radiographs and identify changes suggestive of osteoporosis.23 Thus, trained dentists could play an important role in screening for such changes and in educating cancer survivors and parents about the importance of leading a bone-healthy lifestyle by increasing weight-bearing exercise, and having adequate nutritional intake of calcium and vitamin D. Proper referrals of patients to address endocrinopathies can also serve as a direct method of prevention and treatment for BMD.24
Corticosteroids, a backbone of most ALL therapeutic regimens, inhibit osteoblast activity, increase bone resorption, interfere with growth hormone/insulin-like growth factor 1 axis, reduces muscle strength, and disturbs calcium balance at the level of the gut and kidney; these inhibitory activities of corticosteroids result in a decrease in BMD.12–14 Treatment-related growth hormone deficiency and hypogonadism may also lead to BMD deficit in ALL survivors.25 High doses (>0.5mg/kg) of corticosteroids directly induce apoptosis of osteoblasts and mature osteocytes, and indirectly increase the marrow fat content, which leads to fat embolization and vascular compression.26, 27
The results of our study must be interpreted within the context of both strengths and limitations. The results were generated by rigorous statistical analysis. Despite these strengths, the results may not be applicable to a general population of healthy children and young adults. In addition, a degree of bias could have been introduced in assessing the correlation between QCT-derived BMD and panoramic radiograph scoring by those patients who had more than a single set of paired studies. However, this potential bias may be less influential in analyzing our aim of assessing inter-reader variability. Finally, the Klemetti technique was described for adults and we have found, it is not applicable to children and young adults.
In summary, dentists may serve as proactive healthcare providers regarding children and young adults at risk for BMD deficits such as those who have been treated for leukemia during youth. A panoramic radiograph, standard in dental practices, could serve as a means of identifying such patients and thereby provide for timely referral for dedicated assessment of BMD. Development of a standardized method of classifying mandibular bone density in young patients is warranted, as the Klemetti technique is invalid.
Based on literature review, we found no available techniques that are specific for the pediatric population to evaluate bony changes of the jaw. We selected the Klemetti technique because it is simple and easy to use, though it has only been validated in adults. A potential explanation for why this technique did not correlate with BMD in children is because the pediatric jaw is in the early stages of maturation and the radiographic techniques used to detect BMD changes could not capture subtle nuances in BMD that can be better seen in adults with mature bones.
A limitation of this study is that all of the patients included had been treated for ALL and therefore may not be representative of the general pediatric population. Future investigations may include choosing a population of healthy patients as a comparison group.
Why this paper is important to pediatric dentists.
This paper identifies a deficit where oral healthcare providers could enhance overall health in pediatric patients treated for leukemia.
This paper demonstrates that the Klemetti technique is not a predictor of BMD in this population, and points out the need to study other techniques whereby pediatric patients at risk for BMD deficits can be identified and referred for timely intervention.
Acknowledgments
Supported in part by Grant CA 21765 from the National Institutes of Health, a Center of Excellence grant from the State of Tennessee, and the American Lebanese Syrian Associated Charities (ALSAC).
References
- 1.Kaste SC, Jones-Wallace D, Rose SR, et al. Bone mineral decrements in survivors of childhood acute lymphoblastic leukemia: frequency of occurrence and risk factors for their development. Leukemia. 2001;15(5):728–34. doi: 10.1038/sj.leu.2402078. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–41. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaste SC, Rai SN, Fleming K, et al. Changes in bone mineral density in survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2006;46(1):77–87. doi: 10.1002/pbc.20553. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson SA, Halton JM, Bradley C, Wu B, Barr RD. Bone and mineral abnormalities in childhood acute lymphoblastic leukemia: influence of disease, drugs and nutrition. Int J Cancer Suppl. 1998;11:35–9. [PubMed] [Google Scholar]
- 5.Boot AM, van den Heuvel-Eibrink MM, Hahlen K, Krenning EP, de Muinck Keizer-Schrama SM. Bone mineral density in children with acute lymphoblastic leukaemia. Eur J Cancer. 1999;35(12):1693–7. doi: 10.1016/s0959-8049(99)00143-4. [DOI] [PubMed] [Google Scholar]
- 6.Crofton PM, Ahmed SF, Wade JC, et al. Bone turnover and growth during and after continuing chemotherapy in children with acute lymphoblastic leukemia. Pediatr Res. 2000;48(4):490–6. doi: 10.1203/00006450-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Chavassieux P, Seeman E, Delmas PD. Insights into material and structural basis of bone fragility from diseases associated with fractures: how determinants of the biomechanical properties of bone are compromised by disease. Endocr Rev. 2007;28(2):151–64. doi: 10.1210/er.2006-0029. [DOI] [PubMed] [Google Scholar]
- 8.Jonasson G, Billhult A. Mandibular bone structure, bone mineral density, and clinical variables as fracture predictors: a 15-year follow-up of female patients in a dental clinic. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(3):362–8. doi: 10.1016/j.oooo.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Jonasson G, Sundh V, Hakeberg M, et al. Mandibular bone changes in 24 years and skeletal fracture prediction. Clin Oral Investig. 2013;17(2):565–72. doi: 10.1007/s00784-012-0745-x. [DOI] [PubMed] [Google Scholar]
- 10.von Wowern N. In vivo measurement of bone mineral content of mandibles by dual-photon absorptiometry. Scand J Dent Res. 1985;93(2):162–8. doi: 10.1111/j.1600-0722.1985.tb01326.x. [DOI] [PubMed] [Google Scholar]
- 11.Seeman E, Delmas PD. Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354(21):2250–61. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 12.Mattano L. The skeletal remains: porosis and necrosis of bone in the marrow transplantation setting. Pediatr Transplant. 2003;7 (Suppl 3):71–5. doi: 10.1034/j.1399-3046.7.s3.11.x. [DOI] [PubMed] [Google Scholar]
- 13.Hochberg Z. Mechanisms of steroid impairment of growth. Horm Res. 2002;58 (Suppl 1):33–8. doi: 10.1159/000064764. [DOI] [PubMed] [Google Scholar]
- 14.Leonard MB. Assessment of bone health in children and adolescents with cancer: promises and pitfalls of current techniques. Med Pediatr Oncol. 2003;41(3):198–207. doi: 10.1002/mpo.10337. [DOI] [PubMed] [Google Scholar]
- 15.Klemetti E, Kolmakow S. Morphology of the mandibular cortex on panoramic radiographs as an indicator of bone quality. Dentomaxillofac Radiol. 1997;26(1):22–5. doi: 10.1038/sj.dmfr.4600203. [DOI] [PubMed] [Google Scholar]
- 16.Wasilewski-Masker K, Kaste SC, Hudson MM, et al. Bone mineral density deficits in survivors of childhood cancer: long-term follow-up guidelines and review of the literature. Pediatrics. 2008;121(3):e705–13. doi: 10.1542/peds.2007-1396. [DOI] [PubMed] [Google Scholar]
- 17.Pfeilschifter J, Diel IJ. Osteoporosis due to cancer treatment: pathogenesis and management. J Clin Oncol. 2000;18(7):1570–93. doi: 10.1200/JCO.2000.18.7.1570. [DOI] [PubMed] [Google Scholar]
- 18.Kaste SC, Tong X, Hendrick JM, et al. QCT versus DXA in 320 survivors of childhood cancer: association of BMD with fracture history. Pediatr Blood Cancer. 2006;47(7):936–43. doi: 10.1002/pbc.20854. [DOI] [PubMed] [Google Scholar]
- 19.Cann CE. Quantitative CT for determination of bone mineral density: a review. Radiology. 1988;166(2):509–22. doi: 10.1148/radiology.166.2.3275985. [DOI] [PubMed] [Google Scholar]
- 20.Barr RD, Simpson T, Webber CE, et al. Osteopenia in children surviving brain tumours. Eur J Cancer. 1998;34(6):873–7. doi: 10.1016/s0959-8049(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 21.Gilsanz V. Bone density in children: a review of the available techniques and indications. Eur J Radiol. 1998;26(2):177–82. doi: 10.1016/s0720-048x(97)00093-4. [DOI] [PubMed] [Google Scholar]
- 22.Taguchi A. Triage screening for osteoporosis in dental clinics using panoramic radiographs. Oral Dis. 2010;16(4):316–27. doi: 10.1111/j.1601-0825.2009.01615.x. [DOI] [PubMed] [Google Scholar]
- 23.Shintaku WH, Enciso R, Covington JS, Migliorati CA. Can dental students be taught to use dental radiographs for osteoporosis screening? J Dent Educ. 2013;77(5):598–603. [PubMed] [Google Scholar]
- 24.Nathan PC, Wasilewski-Masker K, Janzen LA. Long-term outcomes in survivors of childhood acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23(5):1065–82. vi–vii. doi: 10.1016/j.hoc.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Kang MJ, Lim JS. Bone mineral density deficits in childhood cancer survivors: Pathophysiology, prevalence, screening, and management. Korean J Pediatr. 2013;56(2):60–7. doi: 10.3345/kjp.2013.56.2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landier W, Wallace WH, Hudson MM. Long-term follow-up of pediatric cancer survivors: education, surveillance, and screening. Pediatr Blood Cancer. 2006;46(2):149–58. doi: 10.1002/pbc.20612. [DOI] [PubMed] [Google Scholar]
- 27.Lafforgue P. Pathophysiology and natural history of avascular necrosis of bone. Joint Bone Spine. 2006;73(5):500–7. doi: 10.1016/j.jbspin.2006.01.025. [DOI] [PubMed] [Google Scholar]