Abstract
In an effort to reconstruct the early evolution of animal genes and proteins, there is an increasing focus on basal animal lineages such as sponges, cnidarians, ctenophores and placozoans. Among the basal animals, the starlet sea anemone Nematostella vectensis (phylum Cnidaria) has emerged as a leading laboratory model organism partly because it is well suited to experimental techniques for monitoring and manipulating gene expression. Here we describe protocols adapted for use in Nematostella to characterize the expression of RNAs by in situ hybridization using either chromogenic or fluorescence immunohistochemistry (~1 week), as well as to characterize protein expression by whole-mount immunofluorescence (~3 d). We also provide a protocol for labeling cnidocytes (~3 h), the phylum-specific sensory-effector cell type that performs a variety of functions in cnidarians, including the delivery of their venomous sting.
INTRODUCTION
The starlet sea anemone, Nematostella vectensis, is a small, geographically widespread estuarine cnidarian1-3 whose phylogenetic position and relatively conservative history of molecular evolution make it particularly useful for helping to reconstruct the early evolution of animal genes and proteins4. As a member of the phylum Cnidaria, Nematostella represents an ancient lineage of animal evolution that diverged from the stem triploblasts ~30–80 million years before the split between protostomes (such as insects) and deuterostomes (such as vertebrates)5. Bioinformatic analyses have identified genes and gene families in Nematostella that were previously suspected of being unique to vertebrates (because of their absence from the sequenced genomes of the fruitfly and soil nematode)4. Furthermore, Nematostella has more orthologs in common with humans than does the tunicate, Ciona intestinalis, despite the fact that tunicates and vertebrates are fellow members of the phylum Chordata4. These findings imply that the genome of Nematostella has evolved in a relatively conservative manner compared with Drosophila, Caenorhabditis or Ciona.
A basic requirement for elucidating gene function in any model organism is the ability to determine the spatiotemporal expression of RNAs and proteins. Here we describe protocols for detecting the expression of individual RNAs and proteins in the starlet sea anemone N. vectensis and a protocol for revealing the location of cnidocytes6.
RNA detection
Spatiotemporal gene expression patterns can be determined by detecting the region-specific expression of mRNA transcripts in fixed animals from different developmental stages. This is a robust protocol in Nematostella that has been used in numerous publications by our laboratories and others since 2003 (e.g., refs. 7,8). There are two methods for detecting antisense RNA probes that are complementary to mRNA transcripts (Fig. 1). The most common method is chromogenic detection of an alkaline phosphatase-conjugated antibody using a colorimetric reaction involving nitro-blue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP) or Fast Red9. By combining multiple chromogenic detection methods, one can detect distinct RNA molecules in the same animal9. However, it is difficult to identify coexpression in a single cell in Nematostella using double chromogenic detection because the colors will blend visually, and the darker BCIP will obscure the lighter Fast Red.
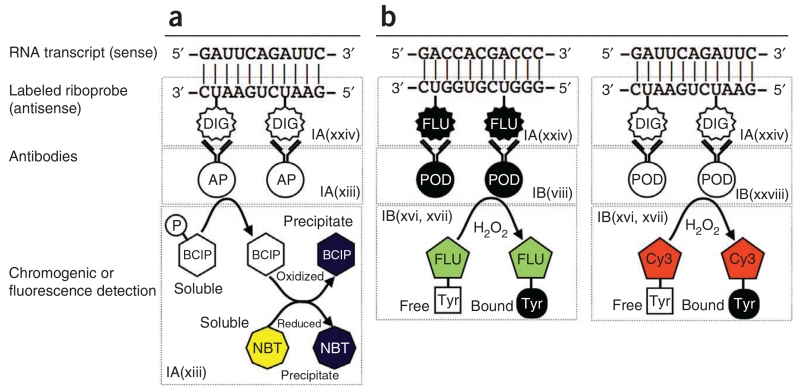
Figure 1.
Principal steps in chromogenic ISH and FISH. (a,b) Chromogenic ISH is shown in a, FISH in b. As described in the text, fluorescence is preferable for simultaneous detection of multiple RNA transcripts in the same cell. Both approaches use an antisense riboprobe in which some of the uridine residues are labeled with either digoxigenin (DIG) or fluorescein (FLU). Once the probe is bound to the target transcript, the digoxigenin or fluorescein molecules are then recognized by the appropriate antibodies. The antibodies are conjugated to either alkaline phosphatase (AP; in the case of chromogenic detection) or peroxidase (POD; in the case of fluorescence detection). In chromogenic detection, the presence of alkaline phosphatase is then visualized by BCIP and NBT. The alkaline phosphatase removes a phosphate group from BCIP, which is a colorless soluble compound. The BCIP then becomes oxidized in a reaction with NBT (a soluble yellow compound), which is simultaneously reduced. Both molecules precipitate out of the solution and assume a dark purple color. In fluorescence detection, numerous tyramide-conjugated fluorophores (e.g., Cy3 or fluorescein) aggregate in the vicinity of the bound RNA probe, as the tyramide is activated by the peroxidase (POD) bound to the antibody.
The protocol described here expands on prior in situ hybridization (ISH) methods in Nematostella that we have used in previous research papers7,9-12 by describing how fluorescent labeling of different antisense RNA probes can be used to simultaneously detect multiple distinct RNA molecules, even within a single cell. Because different fluorescent probes are detected at different wavelengths, there is no issue with visual blending as there is with use of multiple chromogenic probes. This basic approach has proven successful in a phylogenetically diverse range of animals13-15 including Nematostella (Fig. 2)16. Specifically, the protocol described here uses fluorescence immunohistochemistry rather than reflective fluorescence of chromogenic precipitates13, because the fluorescence detection is more reliable, it does not require specialized equipment (such as a confocal microscope) and it is not susceptible to signal masking caused by the crystals that form in chromogenic immunohistochemistry. It is important to note that fluorescence detection of RNA molecules is not as sensitive as chromogenic detection, so it may not be suitable for detecting low-level transcripts.
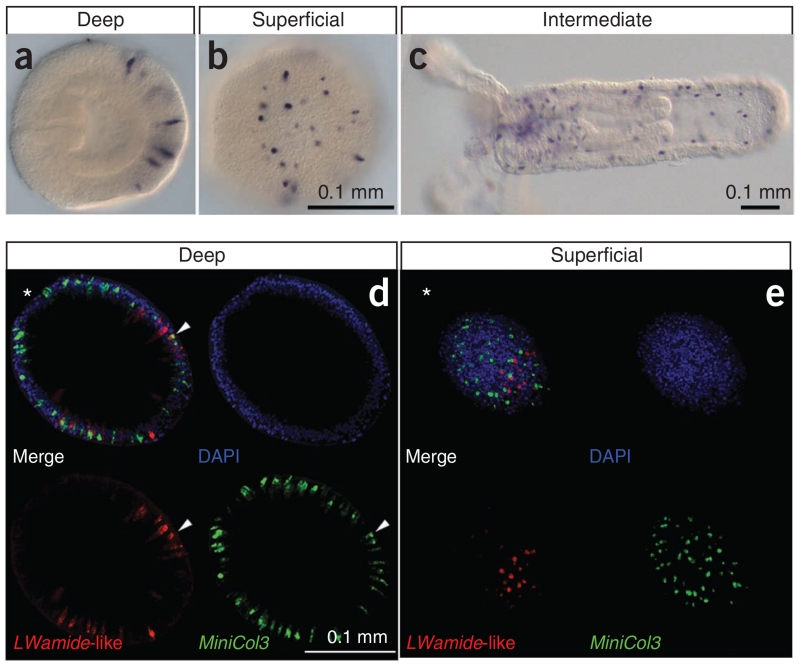
Figure 2.
Example of ISH in Nematostella. (a–c) An antisense RNA probe for a single LWamide-like gene was detected using NBT/BCIP. The transcript is expressed in scattered individual cells in the outer ectodermal layer of the animal, as seen in deep (a) and superficial (b) focal planes in the embryo and in an intermediate plane in the juvenile polyp (c). (d,e) Antisense RNA probes for the same LWamide-like gene and a minicollagen gene (MiniCol3) were simultaneously detected in a larval anemone using fluorescence. The animals were imaged using confocal microscopy. Shown are the merged and individual channels. Each image represents a 10-μm z-projection. These images reveal clearly that the two transcripts are expressed in largely nonoverlapping cell populations. In all images, oral is to the left or indicated by an asterisk.
Protein detection
Compared with the detection of RNA, the detection of proteins in or from Nematostella has been much less commonly reported. The spatiotemporal expression of proteins in Nematostella has been studied via immunohistochemistry, initially using cross-reactive antibodies developed against conserved peptides from other taxa17,18. More recently, antibodies have been developed against Nematostella proteins. Specifically, antisera against native Nematostella proteins, including Nv-NF-κB, Nv-IκB, 5HT serotonin receptor and minicollagen proteins (Nv-NCol-1, Nv-NCol-3 and Nv-NCol-4), have been used in indirect immunofluorescence staining of juvenile and adult anemones18-21. A technique called antigen retrieval, which breaks the protein cross-links formed during the process of tissue fixation, was found to lower the background and improve the consistency of indirect immunofluorescence staining of anemones at all stages of development (Fig. 3)19,22.
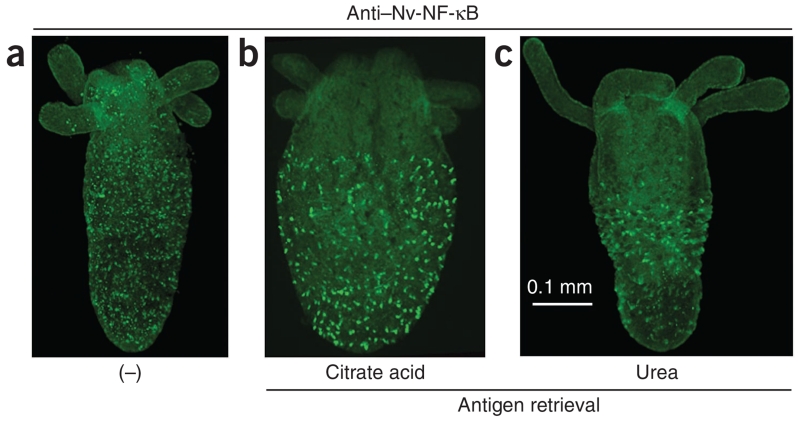
Figure 3.
Example of indirect immunofluorescence. Whole-mount indirect immunofluorescence was performed with Nv-NF-κB-specific antiserum on a 4-week-old Nematostella polyp. Nv-NF-κB was detected with FITC-conjugated secondary antiserum. (a) Without AR. (b) With AR using citrate buffer. (c) With AR using urea. All images were taken using a FluoView FV10i confocal microscope with approximately the same settings for FITC (green) detection.
Cnidocyte staining
Cnidocytes are a defining cell type unique to the phylum Cnidaria6. These cells are characterized by cnidae (or ‘cnidocysts’), which are large proteinaceous capsules connected to hollow threads6. Upon receiving the proper stimulation, the thread can be rapidly everted to deliver venom into potential prey or predators or to anchor the animal to a substrate, depending upon the class of cnidocyte involved. Cnidocytes are sensory-effector cells that can respond to a variety of chemical and mechanical stimuli, including input from neighboring cells23,24. Three different types of cnidocytes have been characterized in Nematostella, and they differ in size and morphology, as well as their prevalence in different body regions21.
The fluorescent cationic dyes DAPI and acridine orange (AO) were originally used to stain the poly-γ-glutamate of mature cnidocytes in the hydrozoa, Hydra vulgaris25. Detection of cnidocytes with DAPI (Fig. 4a) and AO (Fig. 4b) has since been adapted for use with Nematostella18,20,26. In addition, under calcium-free conditions, indirect immunofluorescence can be combined with a cnidocyte stain to visualize the expression of a protein relative to the location of the cnidocytes20. DAPI is best known for its ability to stain nuclei, but whereas DAPI stains nuclei blue, it stains cnidocytes green.
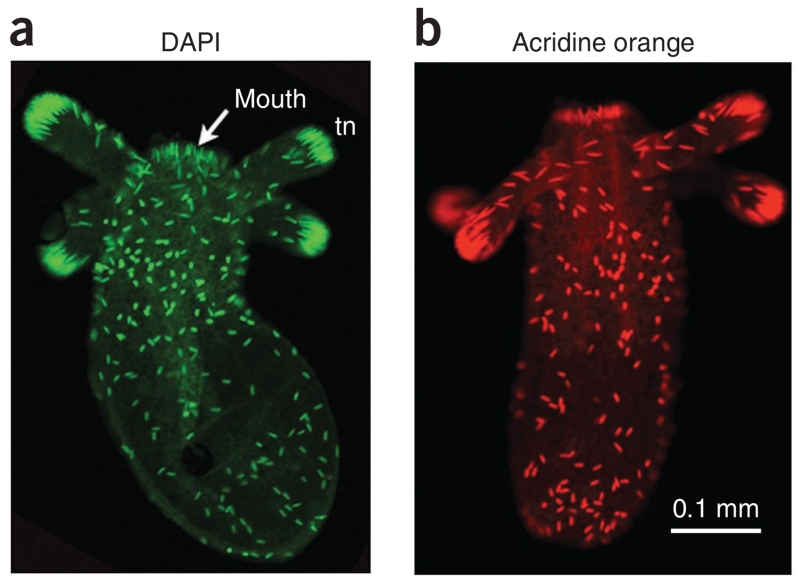
Figure 4.
Example of cnidocyte staining. Nematostella juvenile polyps were fixed and stained under calcium-free conditions. (a) DAPI staining of the poly-γ-glutamate of cnidocyte capsules was detected in a green (521 nm) emission channel. (b) AO also stains cnidocyte capsules, and its fluorescence was detected in a red (615 nm) emission channel. In both stains, cnidocytes are primarily detected in the ectoderm of the body column.
Experimental design
Controls for ISH
In Nematostella, ISH has been used to detect a variety of transcripts at different developmental stages, including embryos, larvae, four-tentacle polyps and adults undergoing regeneration and asexual fission10,12. At a given developmental stage, ~85–95% of individuals generally exhibit the same region-specific expression of transcripts. The well-characterized expression pattern of the Nv-forkhead transcript is robust and spatially discrete, and thus it can serve as a positive control for ISH experiments10,12,27,28. We additionally recommend generating a sense-strand RNA probe to serve as a control for background staining and to identify areas where the probe may become trapped (for example, in the lumen of a tentacle).
Probe design for ISH
To design a probe, one must first identify a transcript of interest in Nematostella and subsequently clone the transcript. These steps are aided by the availability of a sequenced genome and an extensive EST collection4. These genomic and transcriptomic resources can be interrogated using BLAST and other search modalities at two Nematostella-specific internet databases: StellaBase (http://www.stellabase.org) or the Joint Genome Institute’s Nematostella genome portal (http://genome.jgi-psf.org/Nemve1/Nemve1.home.html)4,32,33. BLAST searches of the nonredundant database at NCBI can also be taxonomically restricted to ‘Nematostella vectensis’ (NCBI taxid: 45351).
Validating antibody specificity for immunofluorescence
The spatial expression pattern of proteins can be determined by indirect immunofluorescence in Nematostella embryos, polyps and adults17-21. Before attempting to characterize the spatiotemporal expression of a protein using immunofluorescence, it is important to perform western blotting to demonstrate that the antibody is specific to the protein of interest and that the protein is expressed at a particular developmental stage. Western blotting of Nematostella proteins is described elsewhere29.
Optimizing antibody incubations for immunofluorescence
When performing indirect immunofluorescence, the dilution and duration of incubations with antisera must be experimentally optimized; for example, the optimal dilution of Nv-NF-κB–specific antiserum is 1:1,000, but this was determined after testing dilutions ranging from 1:50 to 1:10,000. Similarly, the optimal dilution of fluorophore-conjugated secondary antiserum may range from 1:80 to 1:20,000.To assist with optimization, we recommend transfecting vertebrate cells, such as chicken fibroblasts, with an expression vector for the protein of interest and performing indirect immunofluorescence with these cells19,20.
Controls for immunofluorescence
As a control for nonspecific staining, samples should be probed with either primary or secondary antiserum alone to ensure that staining is specific.
Antigen retrieval in immunofluorescence
Whenever possible, when performing indirect immunofluorescence, antigen retrieval (AR) is recommended in order to enhance signal detection22. Fixation of anemones with formaldehyde is necessary for maintaining tissue integrity, but the fixative cross-links proteins. AR is performed to break the cross-links that mask protein epitopes required for immunofluorescence staining. AR is recommended, although not always necessary, to obtain strong staining22. The two established AR methods are to incubate the anemones in near-boiling solutions of either urea or citrate acid buffer19,20. The urea treatment is preferred, as it better preserves tissue while still providing AR. The citrate acid buffer is similarly effective at AR, but it can damage tissue.
Cnidocyte staining
The protocol for cnidocyte staining is straight-forward but requires calcium-free conditions at every step. Mature cnidocytes are first detectable in 3-d-old larvae and number in the thousands in adults18. Staining of cnidocytes with DAPI (rather than AO) is recommended because DAPI fluoresces in blue and green emission channels, which allows for co-staining using red fluorophores.
Starting material
For all of these protocols, it is important to first establish a small laboratory colony of Nematostella to ensure sufficient fixed material from the desired developmental stage. The culture of Nematostella is described in an accompanying article30. Fixed animals from different developmental stages suitable for ISH, immunohistochemistry or cnidocyte detection can be prepared in advance and stored at −20 °C for 1 year.
MATERIALS
REAGENTS
Nematostella vectensis (collection of anemones and techniques for spawning and culturing adults, larvae and embryos are described elsewhere30)
For ISH
Acetic anhydride (Fisher Scientific, cat. no. A10-100)
Anti-digoxigenin-alkaline phosphatase (anti-Dig-AP; Roche Applied Science, cat. no. 11093274910)
Anti-digoxigenin peroxidase (anti-Dig-POD; Roche Applied Science, cat. no. 11207733910)
Anti-fluorescein peroxidase (anti-fluorescein-POD; Roche Applied Science, cat. no. 11426346910)
Appropriately labeled antisense probe, user-specific (Box 1)
Artificial seawater (ASW; Instant Ocean, cat. no. SS15-10)
BSA (Sigma-Aldrich, cat. no. A-7888)
Cy3-Mono NHS ester (Amersham Biosciences, cat. no. PA13101)
Cysteine (Merck, cat. no. 243005)
Diethyl pyrocarbonate (DEPC; Sigma-Aldrich, cat. no. D-5758)
Digoxigenin-11-UTP (Dig-UTP; Roche Applied Science, cat. no. 03359247910)
Fluorescein-12-UTP (fluorescein-UTP; Roche Applied Science, cat. no. 11427857910)
Fluorescein-Mono NHS ester (Pierce, cat. no. 46410)
Formaldehyde (37% (vol/vol); Fisher Scientific, cat. no. F79-500) ! CAUTION Formaldehyde is toxic if swallowed. It is hazardous if ingested, inhaled or if it comes into contact with the skin or eyes.
Formamide (Sigma-Aldrich, cat. no. F7508)! CAUTION Formamide is hazardous if it is ingested, inhaled or if it comes into contact with the skin or eyes.
Glutaraldehyde (25% (vol/vol) solution; Sigma-Aldrich, cat. no. G5882)! CAUTION Glutaraldehyde is hazardous if it is ingested, inhaled or comes into contact with the skin or eyes.
Glycine (Sigma-Aldrich, cat. no. G8898)
Heparin, sodium salt (Sigma-Aldrich, cat. no. H3393)! CAUTION This reagent is hazardous if it is ingested, inhaled if it or comes into contact with the eyes.
Hydrogen peroxide (30% (vol/vol); Sigma-Aldrich, cat. no 216763)
Imidazole (Sigma-Aldrich, cat. no. I5513)! CAUTION Imidazole is hazardous if it is ingested, inhaled or comes into contact with the skin or eyes.
In situ hybridization blocking reagent (Roche Applied Science, cat. no. 11096176001)
In vitro transcription kit (e.g., MEGAscript T7 Kit; Invitrogen, cat. no. AM1333M)
Maleic acid (Sigma-Aldrich, cat. no. M0375)! CAUTION This reagent is hazardous if it is ingested, inhaled or if it comes into contact with the skin or eyes.
Methanol (Sigma-Aldrich, cat. no. 179337)! CAUTION Methanol is poisonous. It is hazardous if ingested, inhaled or if it comes into contact with the skin or eyes. It is flammable both in the liquid and vapor states.
NaCl (Fisher Scientific, cat. no. BP-358-1)
N,N-dimethylformamide (DMF; Sigma-Aldrich, cat. no. 319937)! CAUTION This reagent is hazardous if it is ingested, inhaled or if it comes into contact with the skin or eyes.
Nuclease-free water (Life Technologies, cat. no. AM9938)
Paraformaldehyde (16% (vol/vol); Electron Microscopy Sciences, cat. no. 15710)! CAUTION Paraformaldehyde is toxic if swallowed. It is hazardous if ingested or inhaled or if it comes into contact with the skin or eyes.
PBS, 10× (Fisher Scientific, cat. no. BP661)
Proteinase K (20 mg ml−1; New England Biolabs, cat. no. P8102S)
SSC buffer (20×, pH 7.0; Fisher Scientific, cat. no. BP1325-1)
Salmon sperm DNA (SSDNA, 10 mg ml−1 SSDNA; Sigma-Aldrich, cat. no. D1626-250MG)
SDS (20% (wt/vol); Sigma-Aldrich, cat. no. L4522)! CAUTION SDS can cause skin, eye and respiratory irritation.
Sodium phosphate monobasic monohydrate (NaH2PO4·H2O; Sigma-Aldrich, cat. no. S9638)! CAUTION This reagent is hazardous if it is ingested, inhaled or if it comes into contact with the skin or eyes.
Sodium phosphate dibasic anhydrous (Na2HPO4; Sigma-Aldrich, cat. no. S9763)! CAUTION This reagent is hazardous if it is ingested, inhaled or if it comes into contact with the skin or eyes.
TEA (triethylamine; Sigma-Aldrich, cat. no. T0886)! CAUTION This reagent is hazardous if it is ingested, inhaled or if it comes into contact with the skin or eyes.
Triethanolamine (Sigma-Aldrich, cat. no. T58300)! CAUTION This reagent is hazardous if it is ingested, inhaled or if it comes into contact with the skin or eyes.
Tris-HCl (Fisher Scientific, cat. no. BP-152-5)
Triton X-100 (Sigma-Aldrich, cat. no. X100)
Tween-20 (20% (vol/vol); Fisher Scientific, cat. no. BP377-500)
Box 1. Generation of a labeled, single-stranded antisense RNA probe ● TIMING 1–2 weeks.
-
Design PCR primers to flank a 300- to 2,000-nt region in the transcript of interest (~1,000 nt is recommended).
▲ CRITICAL STEP When attempting to localize the spatiotemporal expression of a gene from a conserved gene family, we recommend that your probe contain portions of the 3′ UTR, which will generally guarantee a high degree of paralog specificity.
Clone the PCR product into a plasmid vector that contains RNA polymerase promoter sites (SP6, T3 and/or T7 sites). Alternatively, these promoter sites can be incorporated directly into your primers, so that the resulting linear PCR product can serve as a template for in vitro transcription34.
-
Use a commercially available in vitro transcription kit to generate a labeled, single-stranded antisense RNA probe. We prefer the MEGAscript kits available from Invitrogen.
▲ CRITICAL STEP If you are detecting a single transcript, incorporate digoxigenin-labeled UTP (Dig-UTP) into the RNA probe. If you intend to simultaneously detect two different transcripts, you will need to generate probes that incorporate two different labels. Typically, Dig-UTP and fluorescein-labeled UTP are used.
(Optional) Generate a sense-strand RNA probe for use as a negative control7. However, a sense-strand probe will not represent a true negative control in situations in which both strands of a given stretch of DNA are transcribed.
After precipitating the labeled RNA probe, resuspend the pellet in 30 μl of nuclease-free water.
Measure the optical density at 260 nm (OD260) with a spectrophotometer to determine the probe concentration.
Dilute the probe in ISH buffer to a final concentration of 50–100 ng ml−1.
For indirect immunofluorescence
ASW
BSA
Citric acid monohydrate (Fisher Scientific, cat. no. A110-3)
Coverslips (no. 1.5; VWR, cat. no. 48393-172)
Dry ice (pulverized)
MgCl2 (Sigma-Aldrich, cat. no. M-8266)
Nail polish (clear)
NaOH (Fisher Scientific, cat. no. 1310-73-2)
Normal goat serum (NGS)
Optimum cutting temperature compound (OCT, Sakura, cat. no. 4583)
PBS
Sodium citrate tribasic dehydrate (Fisher Scientific, cat. no. S377-3)
Sucrose (Fisher Scientific, cat. no. BP220-1)
Superfrost Plus microscope slides (Fisher Scientific, cat. no. 12-550-15)
Tris-buffered saline (TBS)
Triton X-100
Tween-20
Urea (Sigma-Aldrich, cat. no. U-0631)
For cnidocyte staining
DAPI (Sigma, cat. no. D-9542)
AO (Sigma, cat. no. A-6014)
ASW
EDTA (Fisher Scientific, cat. no. BP120-500)
Formaldehyde (37% (vol/vol); Fisher Scientific, cat. no. F79-500)! CAUTION Formaldehyde is toxic if swallowed. It is hazardous if ingested, inhaled or if it comes into contact with the skin or eyes.
Multi-well plates (e.g., 24-well plates)
Nail polish (clear)
NaCl
Tris-HCl
Vectashield HardSet mounting solution (Vector Labs, cat. no. H-1500)
EQUIPMENT
For ISH
Dissecting microscope
Fluorescent lamp
Hybridization oven
Microcentrifuge
Plastic container with airtight lid
Platform rocker
Polystyrene culture plates (24 or 48 wells)
Thermocycler
For indirect immunofluorescence
Cryostat (e.g., Jung Frigocut 2800)
Dry incubator
Fluorescence microscope (e.g., Olympus FluoView FV10i or equivalent)
Heat-resistant glassware (e.g., 30-ml Corex tubes and 500-ml beakers)
Microwave (e.g., 1450 watts)
Nutator
Orbital shaker
Parafilm
Plastic dishes for washing samples (e.g., a 12-well dish)
Pyrex dish (0.75–2.5 liter) with sealable lid
Specimen molds (Sakura, cat. no. 4566)
Thermometer
For staining of cnidocytes
Fluorescence microscope (e.g., Olympus FluoView FV10i)
Orbital shaker
REAGENT SETUP
ASW, 1/3-strength (1/3 ASW)
Combine 12 g of Instant Ocean with 900 ml dH2O. Mix thoroughly and adjust the volume to 1 liter with dH2O. The resulting seawater should have a salinity of ~12 parts per thousand (p.p.t.). Store it indefinitely at room temperature (20–22 °C).
Cysteine dejellying solution (3% (wt/vol) cysteine)
Dissolve 0.3 g of cysteine in 10 ml of 1/3 ASW. Adjust the pH to 7.4–7.6 with 5 N NaOH. ▲ CRITICAL Freshly prepare the solution every time before use.
DEPC-treated water or PBS
Add 1 ml of DEPC to 1 liter of deionized water or 1 liter of PBS. Cap and shake the solution vigorously and incubate it at 37 °C for at least 2 h. Autoclave the solution for 30 min and store it indefinitely at room temperature.
ISH fixative 1 (4% (vol/vol) paraformaldehyde, 0.3% (vol/vol) glutaraldehyde)
Add 2.5 ml of 16% (vol/vol) paraformaldehyde and 120 μl of 25% (vol/vol) glutaraldehyde to 7.38 ml of 1/3 ASW. Store the fixative at 4 °C for up to 1 month.
ISH fixative 2 (4% (vol/vol) paraformaldehyde)
Add 2.5 ml of 16% (vol/vol) paraformaldehyde to 7.5 ml of 1/3 ASW. Store the fixative at 4 °C for up to 1 month.
ISH buffer
Combine 20 ml of formamide, 10 ml of 20× SSC (pH. 4.5), 100 μl of 20 mg ml−1 heparin, 0.5 ml of 20% (vol/vol) Tween-20, 2 ml of 20% (wt/vol) SDS, 200 μl of 10 mg ml−1 SSDNA and 7.5 ml of DEPC-treated dH2O. Store the buffer at 4 °C for up to 1 month.
PBS, 10×
Add 2.56 g of NaH2PO4 and 11.94 g of Na2HPO4 to 800 ml of dH2O. Adjust the pH to 7.4 with 10 M NaOH or 12 N HCl. Add 102.2 g of NaCl. Bring the volume to 1 liter with dH2O. For ISH, treat the solution with DEPC as described above for DEPC water. Store the solution indefinitely at room temperature.
PBS-Triton X-100 (PTx) washing buffer (1× PBS, 0.2% (vol/vol) Triton X-100)
For ISH, combine 100 ml of 10× PBS and 10 ml of 20% (vol/vol) Triton X-100 to 890 ml DEPC-treated dH2O. For use in immunofluorescence staining, standard dH2O may be used instead of DEPC-treated dH2O. Store the buffer indefinitely at 4 °C. ▲ CRITICAL For immunofluorescence, the washing buffer PTx is preferred, because the Triton X-100 aids in tissue permeabilization. However, some commercial antibodies work better with PBS-Tween (PTw), which must be determined experimentally (see the Troubleshooting section).
PTw washing buffer (1× PBS, 0.05% (vol/vol) Tween-20)
For ISH, combine 100 ml 10× PBS, 898 ml of DEPC-treated dH2O and 2 ml 25% Tween-20. Store the buffer indefinitely at 4 °C. For use in immunofluorescence staining, standard dH2O may be used instead of DEPC-treated dH2O.
PBTI (1× PBS, 10 mM imidazole, 0.1% (wt/vol) BSA, 0.01% (vol/vol) Tween-20)
Add 100 ml of 10× PBS, 1 g of BSA and 100 ml of 100 mM imidazole to 800 ml of dH2O. Autoclave the solution and add 100 μl of Tween-20 when cooled. Store PBTI at 4 °C for up to 1 month.
TBST (120 mM Tris, 150 mM NaCl, 0.01% (vol/vol) Tween-20)
Add 100 ml of 1.2 M Tris (pH 7.5), 100 ml of 1.5 M NaCl and 100 μl of Tween-20 to 799.9 ml of dH2O. Store TBST indefinitely at room temperature.
Maleic acid buffer
Combine 6.91 g of maleic acid and 4.38 g of NaCl in ~300 ml dH2O. Adjust the pH to 7.5 with 10 M NaOH. Make up the volume to 500 ml with dH2O. Autoclave the buffer and store it indefinitely at room temperature. ▲ CRITICAL A large amount of NaOH is required to adjust the pH. Begin by adding NaOH pellets to approach the final pH; thereafter, add small volumes of 10 M NaOH solution to make the final adjustments.
ISH blocking buffer
Dissolve 50 g of ISH blocking reagent in maleic acid buffer. Bring the final volume to 500 ml with dH2O. Autoclave the buffer and store it in 10-ml aliquots for up to 6 months at −20 °C. Thawed aliquots can be stored for 1 week at 4 °C.
AP buffer (100 mM Tris (pH 9.5), 100 mM NaCl, 50 mM MgCl2, 0.5% (vol/vol) Tween-20)
Add 5 ml of 1 M Tris (pH 9.5), 2.5 ml of 2 M NaCl, 2.5 ml of 1 M MgCl2 and 250 μl of Tween-20 to 39.75 ml of dH2O. ▲ CRITICAL Always prepare fresh buffer on the day of use.
AP buffer without MgCl2
Follow the recipe for AP buffer but replace the MgCl2 with an equal volume of dH2O. ▲ CRITICAL Always prepare fresh buffer on the day of use.
TSA-Cy3
Prepare a 10 mg ml−1 stock of NHS ester in DMF by adding 1 mg of Cy3-NHS ester to 100 μl of DMF. Prepare DMF-TEA solution by combining 1 ml of DMF with 10 μl of TEA. Prepare a tyramide solution by combining 10 mg of tyramide and 1 ml of DMF-TEA. Combine 100 μl of Cy3-NHS in DMF with 33 μl of tyramide solution. Mix and incubate the solution in the dark at room temperature for 2 h. Add 1.2 ml of 100% ethanol. Divide the solution into single-use 500-μl volumes and store them in the dark at −20 °C for up to 6 months. ▲ CRITICAL This reaction is sensitive to moisture. The protocol above is adapted from Xenbase and references cited therein18-20. Note that tyramide-conjugated fluorophores are available for purchase from a number of companies. However, they are much more expensive and do not seem to work as robustly as the tyramide-conjugated fluorophores prepared in our hands. ▲ CRITICAL Allow the reagents to reach room temperature before opening to avoid condensation. Furthermore, you should be prepared to add DMF immediately to monoesters for this reason.
TSA-fluorescein
Prepare a 10 mg ml−1 stock of NHS ester in DMF by adding 100 mg of fluorescein-NHS ester to 10 ml of DMF. Prepare DMF-TEA solution by combining 4 ml of DMF and 40 μl of TEA. Make a tyramide solution by adding 40 mg of tyramide to 4 ml of DMF-TEA. To 10 ml of fluorescein-NHS ester in DMF, add 3.43 ml of tyramide solution. Mix and incubate the solution in the dark at room temperature for 2 h. Add 11.5 ml of 100% ethanol. Aliquot into single-use 500-μl volumes and store them for up to 6 months in the dark at −20 °C. ▲ CRITICAL This reaction is sensitive to moisture. The protocol above is adapted from Xenbase and references cited therein18-20. Note that tyramide-conjugated fluorophores are available for purchase from a number of companies. However, they are much more expensive and do not seem to work as robustly as the tyramide-conjugated fluorophores prepared in our hands. ▲ CRITICAL Allow the reagents to reach room temperature before opening to avoid condensation. Furthermore, you should be prepared to add DMF immediately to monoesters for this reason.
SSC (pH 4.5, 20×)
Add 175.3 g of NaCl and 88.2 g of sodium citrate to 800 ml dH2O. Adjust the pH to 4.5 with 12 N HCl. Adjust the volume to 1 liter with dH2O. Treat the solution with DEPC as described above and autoclave. Store the solution indefinitely at room temperature.
Immunofluorescence fixation solution (4.0% (vol/vol) formaldehyde)
Combine 892 μl of 12 p.p.t. 1/3 ASW and 108 μl of 37% (vol/vol) formaldehyde. Store the solution at 4 °C for up to 6 months.
Immunofluorescence blocking buffer (1× PBS, 5% (vol/vol) NGS, 1% (wt/vol) BSA, 0.2% (vol/vol) Triton X-100)
Combine 40 ml of dH2O, 5 ml of 10× PBS, 2.5 ml of NGS, 0.5 g of BSA and 0.1 ml of 10% (vol/vol) Triton X-100. Mix and bring the final volume to 50 ml with dH2O. Store the buffer indefinitely at −20 °C.
Urea (5% (wt/vol) urea)
Combine 5 g of urea with 100 ml of dH2O. ▲ CRITICAL Freshly prepare the solution before every use.
Citrate buffer (10 mM citric acid monohydrate, 10 mM sodium citrate tribasic dehydrate, 0.05% (vol/vol) Tween-20)
Combine 0.21 g of citric acid monohydrate, 0.29 g of sodium citrate tribasic dehydrate and 0.2 ml of 25% (vol/vol) Tween-20 with dH2O to bring the volume to 100 ml. Adjust the pH to 6.0 with 10 M NaOH. ▲ CRITICAL Freshly prepare the buffer before each use.
MgCl2 relaxant (7% (wt/vol) MgCl2)
Dissolve 7 g of MgCl2 in 100 ml of 1/3 ASW. Store the solution indefinitely at room temperature.
Sucrose gradient washes
To make 5% (wt/vol) sucrose, dissolve 2.5 g of sucrose in 50 ml dH2O. Proportionally increase the amount of sucrose for 10% (5 g), 15% (7.5 g), 20% (10 g), 25% (12.5 g) and 30% (15 g) washes (all wt/vol). ▲ CRITICAL Freshly prepare the solution before each use.
Cnidocyte fixation solution
Combine 872 μl of 12 p.p.t. ASW, 108 μl of 37% (vol/vol) formaldehyde and 20 μl of 0.5 M EDTA. Store the solution at 4 °C for up to 6 months.
Cnidocyte washing buffer (10 mM Tris-HCl, 10 mM NaCl, 10 mM EDTA)
Combine 400 ml of dH2O, 0.6 g of Tris-HCl, 0.29 g of NaCl and 1.86 g of EDTA. Adjust the pH to 7.6 with 10 M NaOH. Bring the volume to 500 ml with dH2O. Store the buffer indefinitely at room temperature.
DAPI staining solution
Add 49 μl of DAPI (1 mg ml−1) to 951 μl of cnidocyte washing buffer. ▲ CRITICAL Freshly prepare the solution before each use.
Acridine orange (AO) staining solution
Add 10 μl of AO (110 mM) to 990 μl of cnidocyte washing buffer. ▲ CRITICAL Freshly prepare the solution before each use.
PROCEDURE
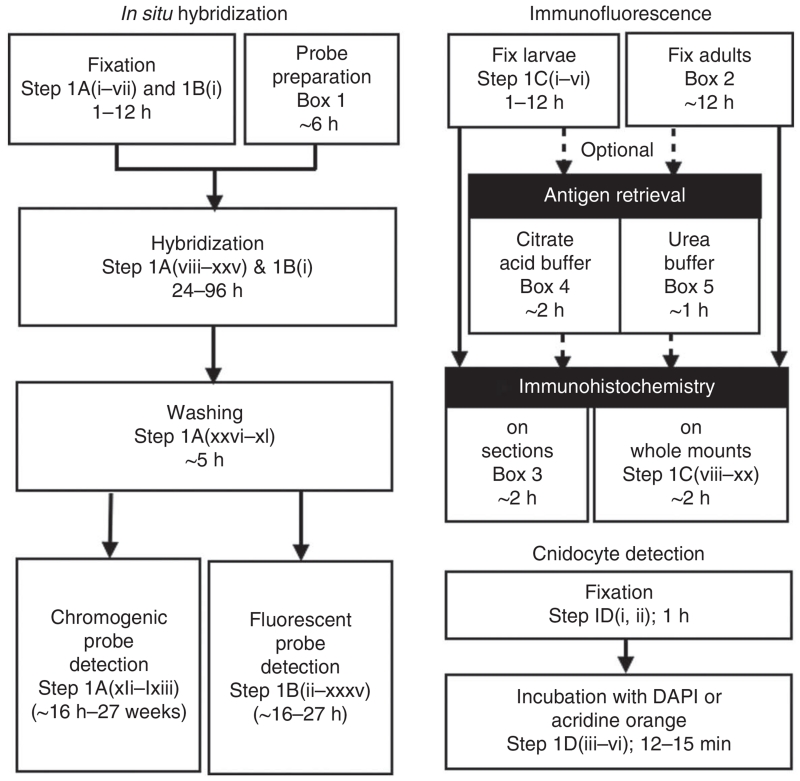
1| To detect RNA expression by ISH using chromogenic detection, follow option A. To detect RNA expression by ISH using fluorescence detection, follow option B. To detect protein by immunofluorescence staining, follow option C. To stain cnidocytes, follow option D. A flowchart summarizing the detection of RNA, protein and cnidocytes is presented in Figure 5.
-
(A)ISH using chromogenic detection ● TIMING 3–7 d
-
(i)Remove the egg jelly from early embryos by incubating the egg mass in ~10 volumes of 3% (wt/vol) cysteine in 1/3 ASW of pH 7.4–7.6 for 10–15 min at room temperature.
-
(ii)Place the animals to be fixed in 1 ml of 1/3 ASW in a single well of a 24-well tissue culture plate or in a 2-ml straight-walled screw-cap tube. Each well or 2-ml tube can hold one small adult polyp, 5–10 juvenile polyps or several dozen embryos or larvae.▲ CRITICAL STEP If you are planning to store the embryos in methanol, we recommend performing the fixations in 2-ml screw-cap tubes so that fixation, washing and storage can be completed in a single vessel, which will minimize the loss of material. The anemones can remain in these tubes for Step 1A(i-xiii) so that multiple juveniles, larvae or embryos undergo identical treatment before being dispensed to separate vessels for addition of individual probes.
-
(iii)Remove the 1/3 ASW and allow the planula larvae, juvenile polyps or adult polyps to relax in 7% (wt/vol) MgCl2 prepared in 1/3 ASW for 10 min at room temperature. It is necessary to relax the larvae and polyps because they will typically respond to being handled by substantially contracting the body column, and in the case of polyps, withdrawing their tentacles into the pharynx. If they are fixed in this contracted posture, it will be difficult to visualize the spatial expression of transcripts in the animal. There is no need to relax embryos.
-
(iv)Remove the 1/3 ASW and add 1 ml of ice-cold ISH fixative 1 to each well. Incubate the samples at room temperature for 15 min with rocking.
-
(v)Remove ISH fixative 1 and add 1 ml of ice-cold ISH fixative 2 to each well. Incubate the samples at 4 °C for 1 h with rocking.
-
(vi)Remove the fixative and wash 5 times with 1 ml of PTw. After each addition of PTw, allow the anemones to resettle to the bottom of the well before removing the wash.▲ CRITICAL STEP Where the protocol uses the term ‘wash’, remove as much of the previous solution as possible and replace it with the indicated volume of the solution being used in the wash step.
-
(vii)If you are storing fixed anemones, transition from PTw to 100% methanol is required. Wash with 1 ml of 30% methanol/70% PTw (vol/vol) for 5 min at room temperature on a rocker. Then wash with 1 ml of 60% methanol/40% PTw (vol/vol) for 5 min at room temperature on a rocker. Thereafter, add 1 ml of 100% methanol.■ PAUSE POINT Anemones can be stored indefinitely in 100% methanol at −20 °C in screw-top tubes.
-
(viii)Rehydrate the fixed embryos, larvae or adult polyps that are stored in methanol by washing for 5 min on a rocker at room temperature in 1 ml of 60% methanol/40% PTw (vol/vol).
-
(ix)Wash the samples with 1 ml of 30% methanol/70% PTw for 5 min at room temperature on a rocker.
-
(x)Wash four times with 1 ml of PTw for 5 min each at room temperature on a rocker.
-
(xi)Digest the samples with Proteinase K. For adult polyps, use 0.05 mg ml−1 Proteinase K in PTw for 20 min at room temperature without shaking. For embryos, larvae and newly settled polyps, use 0.01 mg ml−1 Proteinase K in PTw for 5 min at room temperature without shaking.
-
(xii)Wash the samples twice with 1 ml of 2 mg ml−1 glycine in PTw for 5 min each with gentle rocking at room temperature.▲ CRITICAL STEP Glycine is necessary to halt tissue digestion by Proteinase K.
-
(xiii)Wash the samples in 1 ml of 1% (vol/vol) triethanolamine in PTw for 5 min at room temperature on a rocker.
-
(xiv)Wash the samples in 1 ml of 1% (vol/vol) triethanolamine in PTw with 3 μl of acetic anhydride for 5 min at room temperature with gentle rocking. This treatment acetylates proteins, which is thought to minimize the background by preventing binding of the negatively charged RNA probe to positively charged amino groups on proteins.
-
(xv)Wash in 1 ml of 1% (vol/vol) triethanolamine in PTw with 6 μl of acetic anhydride for 5 min at room temperature on a rocker.
-
(xvi)Wash the samples twice in 1 ml of PTw for 5 min each at room temperature on a rocker.
-
(xvii)Remove the PTw wash and add 1 ml of 4% (vol/vol) paraformaldehyde in PTw. Postfix the samples for 1 h at room temperature on a rocker.
-
(xviii)Wash the samples five times in 1 ml of PTw for 5 min each at room temperature on a rocker.
-
(xix)Discard the PTw wash and add 1 ml of ISH buffer at room temperature. Incubate the mixture for 10 min at room temperature on a rocker.
-
(xx)Remove the hybridization buffer and add 1 ml of fresh ISH buffer. Incubate the samples for 1–24 h at selected hybridization temperature (typically 63 °C, but may vary depending on the probe).▲ CRITICAL STEP Although in most instances, a 1-h incubation is sufficient to yield a distinguishable expression pattern, a 24-h incubation is preferable, because it reduces the background.▲ CRITICAL STEP All subsequent washes at the hybridization temperature are performed in a hybridization oven. In addition, a small bowl of water is kept in the oven to prevent samples from drying out.
-
(xxi)Disperse the fixed animals in hybridization buffer into multiple wells of a 24- or 48-well plate, according to the number of probes to be assayed.▲ CRITICAL STEP Ensure that each anemone is entirely submerged in the hybridization buffer. Add additional buffer as necessary to prevent specimens from drying out.
-
(xxii)Prepare the probe solution by diluting labeled antisense RNA probe (Box 1) in ISH buffer to a final concentration of 1 ng μl−1. If you are performing ISH with two probes, both probes are hybridized simultaneously. Dilute the Dig-labeled probe to 1 ng μl−1 and the fluorescein-labeled probe to 1 ng μl−1 in the same hybridization buffer.▲ CRITICAL STEP Probe concentration can range from 0.1 to 10 ng μl−1, but a concentration of 1 ng μl−1 is typical. Additional concentrations can be tested depending on the ratio of signal-to-background for each probe.
-
(xxiii)Heat the probe solution to 90 °C for 5 min. Allow it to cool for 1 min at room temperature.
-
(xxiv)Remove the hybridization buffer and add 500 μl of working stock probe to each well. Place the plate in a plastic container with a moist paper towel and seal the container. Incubate the animals at 63 °C for 24–72 h.
-
(xxv)Remove and retain the probe solution.▲ CRITICAL STEP Probes can be reused multiple times. The ratio of signal-to-background is often greatly improved with repeated usage. Probe solution can be stored at −20 °C for up to 1 year
-
(xxvi)Wash the anemones with 500 μl of preheated (to the hybridization temperature) ISH buffer and then incubate them in this solution at hybridization temperature for 10 min.▲ CRITICAL STEP Here and for the subsequent steps using preheated buffers and washes, preheat the solutions to the temperature used for hybridization.
-
(xxvii)Wash the samples with 500 μl of preheated ISH buffer and then incubate them in this solution at hybridization temperature for 30 min.
-
(xxviii)Repeat Step 1A(xxvii).
-
(xxix)Wash the samples with 500 μl of preheated 75% ISH buffer/25% 2× SSC and then incubate them in this solution at hybridization temperature for 30 min.
-
(xxx)Wash the samples with 500 μl of preheated 50% ISH buffer/50% 2× SSC and then incubate them in this solution at hybridization temperature for 30 min.
-
(xxxi)Wash the samples with 500 μl of preheated 25% ISH buffer/75% 2× SSC and then incubate them in this solution at hybridization temperature for 30 min.
-
(xxxii)Wash the samples with 500 μl of preheated 2× SSC and then incubate them in this solution at hybridization temperature for 30 min.
-
(xxxiii)Wash the samples with 500 μl of preheated 0.02× SSC and then incubate them in this solution at hybridization temperature for 20 min.
-
(xxxiv)Repeat Step 1A(xxxiii).
-
(xxxv)Wash the samples with 500 μl of preheated 0.02× SSC and then incubate them in this solution at room temperature for 20 min on a rocker.
-
(xxxvi)Wash the samples with 500 μl of 75% 0.02× SSC/25% PTw at room temperature. Incubate them at room temperature for 10 min with rocking.
-
(xxxvii)Wash the samples with 500 μl of 50% 0.02× SSC/50% PTw at room temperature. Incubate them at room temperature for 10 min with rocking.
-
(xxxviii)Wash the samples with 500 μl of 25% 0.02× SSC/75% PTw at room temperature. Incubate at them room temperature for 10 min with rocking.
-
(xxxix)Wash the samples with 500 μl of PTw at room temperature. Incubate the animals at room temperature for 10 min with rocking.
-
(xl)Remove as much of the last wash as possible.
-
(xli)Add 1 ml of ISH blocking buffer. Incubate the animals at room temperature for 1 h or at 4 °C overnight.
-
(xlii)Remove the ISH blocking buffer and add 200–500 μl of 1:5,000 anti-Dig-AP antiserum diluted in ISH blocking buffer. Incubate the samples at 4 °C overnight with rocking.▲ CRITICAL STEP If more than one probe is being detected, both antibodies can be applied simultaneously as long as each probe is labeled with a distinct UTP (i.e., Dig-UTP for one probe and fluorescein-UTP for the other), and two different detection reactions will be used to visualize each probe.
-
(xliii)Remove the antibody solution.
-
(xliv)Wash the samples with 1 ml of PTx at room temperature. Allow embryos to settle.
-
(xlv)Repeat Step 1A(xliv).
-
(xlvi)Wash the samples with 1 ml of PTx. Incubate them for 20 min with rocking at room temperature.
-
(xlvii)Repeat Step 1A(xliv–xlvi) four times, for a total of 15 washes.
-
(xlviii)Remove all liquid from the last wash. Add 1 ml of AP buffer without MgCl2. Incubate the specimens for 10 min at room temperature with rocking.
-
(xlix)Wash the specimens with 1 ml of AP buffer. Incubate the animals for 10 min at room temperature with rocking.
-
(l)Repeat Step 1A(xlix).
-
(li)Prepare a fresh developing solution by diluting 3.3 μl each of NBT and BCIP in 1 ml of AP buffer. Keep the solution in the dark.
-
(lii)Remove the final AP wash. Add 500 μl of the NBT/BCIP solution from Step 1A(li) to each well.
-
(liii)Develop the specimens at room temperature with rocking. Check the development reaction every 5–10 min for the first 2 h and progressively less frequently after that.▲ CRITICAL STEP The ideal development time can vary from a few min to 2 weeks dependent on the signal-to-noise ratio for a given probe. This ratio can vary depending on the probe length, incorporation rate of labeled nucleotide, probe concentration and the expression level of the corresponding RNA. By monitoring the NBT/BCIP stain over a range of development times, cells that highly express the transcript can be identified7.▲ CRITICAL STEP The development reaction is light sensitive. Keep the specimens in the dark until development is completed.? TROUBLESHOOTING
-
(liv)Replace the developing solution every 2 h during the first day. For extended developing times, fresh developer can be added at the end of the day and the plate can be placed at 4 °C in the dark.▲ CRITICAL STEP It is imperative to change the developing solution frequently (4–5 times per d for the first couple of days; 2–3 times per d after the first couple of days) to avoid formation of NBT crystals on fixed tissue. Freshly prepare AP buffer for new developing solution each day.
-
(lv)Once probe development has proceeded to the desired level, stop the reaction by washing with 1 ml of PTx.
-
(lvi)Wash the specimens twice with 1 ml of PTx for 5 min each at room temperature with rocking. In order to reduce background staining, perform optional Step 1A(lvii-lx), otherwise proceed directly to Step 1A(lxi).
-
(lvii)Remove the last PTx wash. Add 500 μl of preheated ISH buffer.
-
(lviii)Place the specimens at hybridization temperature for 30–60 min. Monitor the embryos for the level of staining every 10 min. This step will dissolve some of the NBT/BCIP precipitate, which eliminates some background, but it will also dissolve genuine signal if the incubation continues for too long.
-
(lix)Add 500 μl of PTx to the hybridization buffer and embryos. Allow the embryos to settle. Remove all liquid.
-
(lx)Wash the specimens twice with 1 ml of PTx for 5 min each at room temperature with rocking.
-
(lxi)Wash the specimens twice with 1 ml of PTx for 30 min each at room temperature with rocking.
-
(lxii)Remove the PTx. Add 500 μl of 90% glycerol/1× PBS. Incubate the specimens at 4 °C overnight with rocking to clear the stains.
-
(lxiii)Once the specimens are settled and equilibrated in glycerol solution, mount and image them. For an example of a NBT/BCIP developed in situ (Fig. 2a–c).
-
(i)
- (B) ISH using fluorescence detection ● TIMING 3–7 d
-
(i)Carry out Step 1A(i–xxxix).
-
(ii)Remove the PTw. Add 1 ml of 3% (vol/vol) hydrogen peroxide (in sterile H2O). Incubate the anemones for 1 h in the dark at room temperature with rocking. This step quenches endogenous peroxidase activity, which could otherwise lead to high background as peroxidases react with the substrate solution (in Step 1B(xvii)), which contains hydrogen peroxide and the TSA-conjugated fluorophore.
-
(iii)Remove the hydrogen peroxide. Add 1 ml of PTw and incubate the specimens for 10 min at room temperature with rocking.
-
(iv)Wash the anemones three times in 1 ml of PTw for 10 min each at room temperature with rocking.
-
(v)Remove as much of the PTw wash as possible.
-
(vi)Add 1 ml ISH blocking buffer. Incubate the specimens at room temperature for 1 h or at 4 °C overnight.
-
(vii)For single fluorescence in situ analysis, dilute anti-Dig-POD or anti-fluorescein-POD antibodies (depending on which labeled nucleotide is contained in the probe) at a ratio of 1:1,000 in ISH blocking buffer. For double FISH, determine which probe will be detected first and dilute this POD-conjugated antibody in ISH blocking buffer.▲ CRITICAL STEP When detecting two different RNA probes, do not combine both antibodies in the same antibody solution. The order in which the antibodies are applied is not crucial. However, the fluorescein-specific antibody will detect both the fluorescein resulting from the detection reaction and the fluorescein-UTP of the probe, and this must be taken into account in the experimental design.
-
(viii)Remove the ISH blocking buffer and add 200–500 μl of antibody solution from Step 1B(vii). Incubate the specimens overnight at 4 °C with rocking.
-
(ix)Remove the antibody solution.
-
(x)Wash the specimens with 1 ml of PTx at room temperature. Allow the embryos to settle.
-
(xi)Repeat Step 1B(x).
-
(xii)Wash the specimens with 1 ml of PTx. Incubate them for 20 min with rocking at room temperature.
-
(xiii)Repeat Steps 1B(x–xii) four times, for a total of 15 washes.
-
(xiv)Wash the specimens twice for 5 min each in 500 μl of PBTI at room temperature with rocking.▲ CRITICAL STEP From this point onward, keep all reagents and reactions containing fluorophores in the dark at all times, even after detection is completed.
-
(xv)Dilute TSA-conjugated fluorophore in PBTI. If you are performing a double ISH, either of the fluorophores (Cy3 or fluorescein) can be used first.▲ CRITICAL STEP The range of dilution varies from 1:50 to 1:1,000. The ideal concentration must be empirically determined for each probe. Typically, a dilution of 1:500 is a good starting point.
-
(xvi)Remove as much liquid from the last wash as possible. Add 500 μl of TSA-conjugated fluorophore. Incubate the specimens for 10 min at room temperature with rocking.
-
(xvii)Add hydrogen peroxide to a final concentration of 0.001% (vol/vol). Incubate the specimens at room temperature for 45–60 min with rocking.
-
(xviii)(Optional) If desired, wash 3–5 times quickly with PBTI and assess the strength of the fluorescence signal on a fluorescence dissecting scope.
-
(xix)(Optional) To strengthen the signal, add fresh PBTI with fluorophore and 0.001% (vol/vol) hydrogen peroxide (in sterile H2O), and incubate the samples for 45–60 min at room temperature with rocking. This step can be repeated a maximum of three times.
-
(xx)If you are performing fluorescence detection of a single probe, proceed to Step 1B(xxix). If you are performing fluorescence detection of two probes, proceed to Step 1B(xxi).
-
(xxi)Wash the specimens three times with 500 μl of PBT for 10 min each at room temperature with rocking.
-
(xxii)Remove the last wash. Add 1 ml of 3% (vol/vol) hydrogen peroxide (in sterile H2O). Incubate the specimens for 1 h at room temperature with rocking to quench peroxidase activity.
-
(xxiii)Wash the specimens three times with 1 ml of PTx for 5 min each at room temperature with rocking.
-
(xxiv)Wash the specimens twice with 1 ml of PTx for 15 min each at room temperature with rocking.
-
(xxv)Wash the specimens with 1 ml of blocking solution for 10 min at room temperature with rocking.
-
(xxvi)Dilute antibody for remaining undetected probe (i.e., anti-Dig-POD if fluorescein probe was detected first) 1:1,000 in ISH blocking buffer.
-
(xxvii)Remove the blocking solution. Add 500 μl of antibody solution. Incubate the specimens overnight at 4 °C with rocking. Keep the specimens in the dark.
-
(xxviii)Repeat Step 1B(xvi-xix) to detect the second probe.▲ CRITICAL STEP Keep everything in the dark.
-
(xxix)Remove the final solution of TSA-conjugated fluorophore in PBTI.
-
(xxx)Wash the specimens three times with 500 μl of TBST. Each time, allow the embryos to settle and then remove the TBST.
-
(xxxi)Wash the samples with 500 μl of TBST for 30 min at room temperature with rocking.
-
(xxxii)(Optional) If desired, monitor development on a fluorescence microscope at this point. However, it can appear that there is no signal until fixed animals have been thoroughly washed.? TROUBLESHOOTING
-
(xxxiii)Wash the specimens in TBST for up to 2 d at 4 °C, replacing the solution at least four times per day.▲ CRITICAL STEP During this washing period, counterstains, such as propidium iodide, DAPI and Hoechst, can be applied.
-
(xxxiv)Mount the embryos by removing as much TBST as possible and adding 90% (vol/vol) glycerol in 1× PBS (or your preferred antifade reagent). Mounting using the Murray clear method is possible, although this may not be compatible with some counterstains31.
-
(xxxv)Image the animals using the appropriate fluorescent light source and filters.
-
(i)
- (C) Indirect immunofluorescence ● TIMING 2–4 d
-
(i)To fix embryos, transfer 10–100 individuals to a 1.5-ml microcentrifuge tube and wash once with 1/3 ASW. To fix juvenile or adult polyps, follow the protocol in Box 2 before continuing with the main PROCEDURE from Step 1C(vi).▲ CRITICAL STEP Do not centrifuge the embryos, which causes distension, but rather let them settle to the bottom. Put the 1.5-ml tube onto an orbital shaker (50 r.p.m.) and let them settle to the bottom over 5 min.? TROUBLESHOOTING
-
(ii)Remove the excess 1/3 ASW with a pipette, leaving the embryos at the bottom of the tube.
-
(iii)Add 1 ml of ice-cold immunofluorescence fixation solution to the 1.5-ml tube.
-
(iv)After 1 min, gently invert the tube to mix.
-
(v)Leave the tube on a nutator or shaker at 4 °C for at least 1 h, but preferably overnight.■ PAUSE POINT Embryos can be stored in the fixative solution for 1 month at 4 °C. For long-term storage (up to 1 year), wash the specimens three times with 1× TBS to remove the fixative and store them in 100% methanol at −20 °C.
-
(vi)For immunohistochemical analysis of whole mounts, continue with the main PROCEDURE (Step 1C(vii–xxi)). For immunohistochemical analysis of sectioned anemones, follow the protocol in Box 3.
-
(vii)Transfer the fixed anemones (embryos, juvenile polyps or adults) to a 12-well plate and wash them three times in PTx to remove fixative.
-
(viii)
▲ CRITICAL STEP If you are performing a co-stain for cnidocytes with DAPI or AO, supplement every buffer in Step 1C(ix–xvi) (including primary and secondary antisera) with EDTA to a final concentration of 10 mM.
-
(ix)Wash the anemones three times with PTx for 5 min each.
-
(x)Remove as much liquid as possible and add 500 μl of immunofluorescence blocking buffer to each well.
-
(xi)Place the plate on a shaker (~80 r.p.m.) at 4 °C to block it overnight.▲ CRITICAL STEP Unlike the sectioned tissue, whole anemones are thicker and require blocking overnight.■ PAUSE POINT Samples may be left to block at 4 °C for up to 3 d.
-
(xii)Remove the immunofluorescence blocking buffer.
-
(xiii)Add 300–500 μl of the primary antisera to each well and incubate the samples for the appropriate amount of time. Typically, whole-mount preparations of animals are incubated with primary antibody on a shaker/incubator at 37 °C and ~100 r.p.m. for 1.5 h.▲ CRITICAL STEP The dilutions for different primary and secondary antisera and the conditions used during the incubations are summarized in Table 1.
-
(xiv)Remove the primary antisera and wash the samples four times for 10 min each with PTx.
-
(xv)Remove the PTx and add 400 μl of secondary antisera.
-
(xvi)Remove the secondary antisera and wash the samples four times for 10 min each with PTx.▲ CRITICAL STEP The secondary antisera are susceptible to photobleaching when exposed to excess light. From this point onward, the protocol should be performed under low-light conditions.
-
(xvii)Transfer the anemones onto slides. Remove excess liquid and cover the anemones with 20 μl of the Vectashield HardSet mounting medium.
-
(xviii)Mount a coverslip and seal its edges with nail polish.▲ CRITICAL STEP Placing the coverslip directly on top of the anemones will flatten the animals and cause the tissue structure to be distorted. To avoid this problem, create small mounds with 5 μl of nail polish (~2 mm tall) that raise the profile of the slide. The coverslip will rest on these mounds and better preserve the samples. For confocal microscopy, use an appropriate coverslip or the images will be out of focus (e.g., use no. 1.5 glass coverslips).
-
(xix)Seal the edges of the coverslip with nail polish and allow it to harden for at least 1 h.
-
(xx)Image the slides within 2 d.? TROUBLESHOOTING
-
(i)
- (D) Staining of cnidocytes ● TIMING ~3 h
-
(i)Fix each individual anemone in 500 μl of ice-cold cnidocyte fixation solution for 1 h with gentle shaking at 4 °C in 1.5-ml microcentrifuge tubes.! CAUTION Work in a fume hood to avoid inhaling formaldehyde vapors.
-
(ii)Transfer each anemone to a single well of a multiwelled tissue culture plate (e.g. a 24-well plate).
-
(iii)Wash the anemones three times with cnidocyte washing buffer at ~80 r.p.m. on an orbital shaker (5 min per wash).
-
(iv)Remove the cnidocyte washing buffer and replace it with 500 μl of cnidocyte stain (either DAPI or AO).
-
(v)Cover the plate with aluminum foil (or darken the room) and shake the plate at a low speed (~50 r.p.m.). For DAPI, shake the plate for 12 min. For AO, shake the plate for 15 min.
-
(vi)Discard the stain and wash the specimens three times with cnidocyte washing buffer (5 min per wash with shaking).
-
(vii)If you are mounting on slides, use the Vectashield HardSet mounting solution. Seal the edges well with nail polish.
-
(viii)Image the slides immediately on a fluorescence microscope. Cnidocytes stained with DAPI fluoresce at a green wavelength (~515 nm). Cnidocytes stained with AO primarily fluoresce at a red wavelength (~615 nm).▲ CRITICAL STEP The stain is transient and dissipates after a few hours.? TROUBLESHOOTING
-
(i)
Figure 5.
Overview of the four protocol options described here. Steps 1A–C can be performed on Nematostella embryos, larvae or juvenile polyps. Cnidocytes cannot ordinarily be detected (Step 1D) in embryos or early larvae. A list of steps and timing for each element of each protocol option is provided.
Box 2. Fixation of adult anemones for indirect immunofluorescence ● TIMING 12–16 h.
-
Transfer 1–5 adult anemones to a small bowl containing 7% (wt/vol) MgCl2 made in ~12 p.p.t. ASW.
▲ CRITICAL STEP Allow the animals to fully relax in this medium for 10 min, or until their tentacles are fully extended and they lack a contractile response to tactile stimulation.
Remove the ASW/MgCl2 solution and submerge the animals in ~5 ml of immunofluorescence fixation solution for 10 min without mixing. At this point, transfer the anemones to a 1.5-ml microcentrifuge tube and add 500 μl of immunofluorescence fixation solution.
-
Invert the tube to mix, and then incubate the anemones overnight in the fixative at 4 °C with gentle shaking.
■ PAUSE POINT Adult anemones can be stored in the fixative solution for 1 month at 4 °C. For long-term storage (up to 1 year), wash them three times with 1× TBS to remove the fixative and store the animals in 100% methanol at −20 °C.
Box 3. Immunohistochemical analysis of sectioned animals ● TIMING 2–16 h.
Fix anemones as described for indirect immunofluorescence (Step 1C(i–v) of the main PROCEDURE).
-
Wash the fixed anemones three times at room temperature in PTx to remove fixative.
? TROUBLESHOOTING
-
Transfer the animals to a 15-ml tube containing 5 ml of 5% (wt/vol) sucrose in PBS. Rock the tube on a nutator for 20 min.
? TROUBLESHOOTING
-
Perform five washes in sucrose/PBS solution for 20 min each. Increase the concentration of sucrose in each successive wash in increments of 5% (i.e., 10, 15, 20, 25 and 30% wt/vol sucrose in PBS).
? TROUBLESHOOTING
-
After the final (30% wt/vol) sucrose wash, transfer the anemones onto a sheet of Parafilm, remove the remaining sucrose and cover the animals with OCT.
? TROUBLESHOOTING
-
Transfer the anemones to a sectioning mold filled with OCT and orient them vertically with the aboral end pointing toward the bottom of the mold. Transfer the mold to an ice bucket filled with crushed dry ice and freeze the mold for 2 h.
? TROUBLESHOOTING
■ PAUSE POINT After the mold is frozen, it can be stored at −20 °C indefinitely.
-
Immediately before sectioning, remove the frozen block from the mold, cryosection the samples into 12-μm-thick slices and mount the sections onto slides.
▲ CRITICAL STEP Use microscope slides that are coated with poly-l-lysine (e.g., Superfrost Plus slides) to ensure that the sections adhere to the slides.
■ PAUSE POINT Slides can be stored indefinitely at −20 °C.
Before immunohistochemical analysis, heat the slides in a dry incubator for 30 min at 60 °C.
Wash the slides once in PTx.
Wash the slides three times with PTx for 5 min each.
-
Block the samples overnight at 4 °C.
▲ CRITICAL STEP Take care not to allow the slides to dry out. Apply ~300 μl of immunofluorescence blocking buffer directly onto the sectioned tissue on each slide, and store the slides in a glass Pyrex dish with a sealable lid, along with a moistened paper towel that serves to maintain the humidity.
Remove the immunofluorescence blocking buffer by gently tapping the slides on a paper towel.
Apply 300 μl primary antiserum diluted in immunofluorescence blocking buffer to slides. Incubate the slides in the glass container under the appropriate conditions (temperature and duration of incubation need to be empirically determined). The dilutions for the primary antisera that we have used in our own research and the conditions used during the incubation are summarized in Table 1.
Wash the slides four times with PTx for 5 min each.
-
Tap slides dry and probe with secondary antisera. The dilutions for secondary antisera are summarized in Table 1.
▲ CRITICAL STEP The secondary antisera are susceptible to photobleaching when exposed to excess light. From this point forward, the protocol should be performed under low-light conditions.
Wash the slides four times with PTx for 5 min each.
Tap the slides dry and add 20 μl of Vectashield HardSet mounting medium.
-
Seal the edges of the slides with nail polish, allow the polish to set for 1 h and image the slides within 1 d.
■ PAUSE POINT Store the slides in a covered container at −20 °C while awaiting imaging.
Box 4. AR with urea ● TIMING 1 h.
For AR with urea, freshly prepare 5% (wt/vol) urea in dH2O and add to appropriate container. For treating sectioned animals mounted on slides, add ~200 ml of urea (or enough to submerge slides) to a 500-ml glass beaker. For whole polyps, substitute a 30-ml Corex test tube for the beaker and fill it with 10 ml of fresh urea.
Heat the urea solution in a microwave until it reaches 80 °C. Monitor the temperature closely.
Gently immerse the slides in the glass beaker (or transfer whole animal to the test tube).
-
Heat the samples submerged in urea on the microwave’s lowest setting (e.g., setting 1 of 10) for 5 min.
▲ CRITICAL STEP The urea solution should reach ~95 °C, but do not allow it to boil. Boiling damages the integrity of anemone tissue. Monitor the temperature closely with a thermometer.
Remove the vessel of urea from the microwave, and allow it to cool for 20 min at room temperature.
Remove the slides or whole anemones from the urea solution, and wash the animals three times in PTx.
Proceed with blocking (Step 1C(ix) of the main PROCEDURE).
Box 5. AR with citrate buffer ● TIMING 1.5 h.
-
Heat 200–300 ml of citrate buffer in a 500-ml glass beaker to near boiling (~98 °C) on a hot plate. The solution will become cloudy at higher temperatures. Monitor the temperature closely.
▲ CRITICAL STEP Citrate buffer should be made fresh for each use.
Gently submerge the slides or whole anemones into the hot citrate buffer.
Incubate the samples at ~98 °C for 30 min. Do not allow the solution to boil.
Allow the citrate buffer to cool at room temperature for 30 min.
Remove the slides or whole anemones and wash them three times with PTx.
Proceed with blocking (Step 1C(ix) of the main PROCEDURE).
TABLE 1.
Antisera used for immunofluorescence.
| Namea | Hostb | Dilution | Source | Blocking bufferc | Durationd |
|---|---|---|---|---|---|
| Primary antisera | |||||
| Nv-NF-κB | R | 1:100 | OpenBiosystems (custom) | TBS, 5% NGS, 1% BSA, 0.2% Tx | 1.5 h at RT |
| Nv-IκB | GP | 1:200 | OpenBiosystems (custom) | TBS, 5% NGS, 1% BSA, 0.2% Tx | 1.5 h at RT |
| Secondary antisera | |||||
| vFITC-α-R | G | 1:160 | Sigma, cat. no. F9887 | TBS, 5% NGS, 1% BSA, 0.2% Tx | 1.5 h at 37 °C |
| TR-α-R | G | 1:80 | Vector Labs, cat. no. TI-1000 | TBS, 5% NGS, 1% BSA, 0.2% Tx | 1.5 h at 37 °C |
| FITC-α-GP | G | 1:80 | ThermoFisher, cat. no. PA1-28,675 | TBS, 5% NGS, 1% BSA, 0.2% Tx | 1 h at 37 °C |
Antisera conjugations: fluorescein isothiocyanate, FITC; Texas Red, TR. Secondary antisera were created against antibodies from the indicated species (e.g., FITC-linked anti-guinea pig is listed as FITC-α-GP).
Antisera host animal: goat, G; guinea pig, GP; rabbit, R.
Blocking buffer was used to block membranes/tissue and to dilute primary antisera. Abbreviations: normal goat serum, NGS; Tris-buffered saline, TBS; Triton X-100, Tx. All percentages are measured in (vol/vol) except BSA (wt/vol).
Blocking was usually performed overnight at 4 °C on a shaker. Duration of incubation with primary antisera. Room temperature, RT.
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 2.
TABLE 2.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 1A(liii) | Weak or no signal | Degradation of NBT/BCIP | Use fresh NBT/BCIP in histochemical detection reaction |
| Transcript expression level below detection threshold |
For NBT/BCIP detection, if the background staining is low, extend the development time up to 2 weeks |
||
| 1A(liii), 1B(xxxii) |
Weak or no signal | Transcript expression level below detection threshold |
Use a longer probe, up to ~2.0 kb |
| If the background staining is low, increase the concentration of probe in the hybridization buffer |
|||
| RNA degradation | Use RNase-free conditions | ||
| Poor fixation | Repeat fixation with fresh reagents | ||
| Change fixative to 4% (vol/vol) formaldehyde in 1/3 ASW | |||
| Excessive background | Suboptimal fixation | Replace 1/3× ASW with 1× PTw in preparing the fixation solution |
|
| Probe needs to be preabsorbed | Preabsorb the probe on fixed animals for 1 h at hybridiza- tion temperature before use in ISH |
||
| Probe ‘trapped’ in the endodermal tissues or lumen of tentacles |
Use a labeled sense-strand riboprobe as a negative control to identify staining that is probably due to probe trapping |
||
| 1B(xxxii) | Weak or no signal | Transcript expression level below detection threshold |
For fluorescence detection, incorporate one additional round of antibody amplification. Use a POD-conjugated secondary against the POD-conjugated anti-Dig or anti-fluorescein. This increases the amount of peroxidase localized to your probe |
| For fluorescence detection, increase the concentration of the TSA-conjugated fluorophore |
|||
| Use confocal microscopy rather than epifluorescence to acquire images of the fluorescence signal |
|||
| 1C(i) | Distension of fixed embryos |
Orbital shaker rotating too rapidly | Reduce the speed of shaking. Never centrifuge embryos |
| 1C(xx) | High background staining |
Poor blocking of tissue | Be sure to use NGS and BSA in all blocking buffers. Use anti- gen retrieval to boost signal intensity |
| Detection of nonspecific punctate staining |
Antiserum is trapped by tissue | Use Triton X-100 in all washes. Aliquot antisera into 1.5-ml microcentrifuge tubes and centrifuge at 14,000g for 2 min at room temperature to remove insoluble portion of antiserum |
|
| 1D(viii) | No cnidocyte staining | Poor permeabilization of tissue | Determine if nuclei are DAPI stained. If nuclei are not stained, increase fixation time from 1 h to overnight at 4 °C |
| Abundance of Ca2+ ions | Ca2+ ions prevent cnidocyte staining. Ensure that all solutions are supplemented with 10 mM EDTA to chelate Ca2+ ions |
||
| Weak cnidocyte staining | Cnidocyte stain is transient | Reduce wash times after DAPI or AO staining. Image samples immediately after final wash |
|
|
Box 3, steps 2–5 |
Loss of ectoderm in cryosectioned animals |
Ectoderm sloughed off during sucrose/OCT wash |
Increase fixation time. Decrease the speed of orbital shaker |
|
Box 3, steps 6,19 |
Anemones are deformed when sections are mounted onto slides |
Anemones shrivel when in OCT for too long |
Reduce the amount of time that anemones are in OCT before the block is frozen. Never mount more than six anemones in a single block |
● TIMING
Steps 1A and 1B, ISH: not including the generation of the labeled riboprobe and the fixation of animals, the entire protocol takes ~1 week for most genes. However, weakly expressed genes require longer development times, prolonging the protocol
Box 1, generation of a labeled, single-stranded antisense RNA probe: ~6 h
Step 1A(i–vii), fixation of embryos, larvae and adult anemones: ~2 h
Step 1A(viii–xxv), in situ hybridization: 24–96 h
Step 1A(xxvi–xl), posthybridization washing: ~5 h
Step 1A(xli–lxiii), NBT/BCIP chromogenic detection (not including development): ~16–27 h (plus development of histochemical reaction: 5 min-2 weeks; clearing and mounting: ~12 h)
Step 1B(ii–xxxv), fluorescence detection: ~16–27 h
Step 1C(i–vi), fixation of embryos: ~1–12 h (Note that immunofluorescence staining of a single anemone can take 2–4 d depending on the protocol used. The full experiment can be divided into smaller steps that can each be accomplished in 1 d.)
Box 2, fixation of adults: ~12 h
Box 3, immunohistochemistry on sectioned animals: ~11–36 h
Box 4, AR with urea buffer: ~1 h
Box 5, AR with citrate buffer: ~2 h
Step 1C(viii–xx), immunohistochemistry on whole mounts: ~19 h
Step 1D, staining of cnidocytes: ~3 h; every step of the protocol must be performed consecutively and rapidly
ANTICIPATED RESULTS
In situ hybridization
In general, the staining patterns produced by ISH in Nematostella are slightly variable between individual animals for a given probe. However, 85–95% of embryos will display the same general pattern for each gene assayed. NBT/BCIP detection is the more sensitive of the two methods and should be used for weakly expressed genes. Both protocols allow expression patterns to be observed at single-cell resolution (Fig. 2). For strongly expressed genes, detection with NBT/BCIP is comparable to detection with fluorescence (Fig. 2). There are many areas for optimization of this ISH protocol (fixation, concentration of probes, developing time and so on.). However, we find that the protocol described here is sufficient to detect probes designed against most genes. One telltale sign of a successful experiment is a spatially discrete expression pattern that is consistent from individual to individual. In addition, the staining should be localized to cells, not to internal body cavities, such as the lumen of a tentacle. For example, the antisense LWamide probe labels a similar subset of scattered ectodermal cells in both embryos and juvenile polyps, and the staining is clearly localized to cells (Fig. 2). However, some genes are expressed widely and diffusely. For this reason, and because the protocol is lengthy and complicated, inclusion of a positive control with a robust and spatially discrete expression pattern is strongly recommended (e.g., Nv-forkhead28-31).
Indirect immunofluorescence
By following this protocol, successful immunofluorescence staining of whole mount anemones was performed for two primary antisera and three secondary antisera15,17. In all the cases, staining was greatly improved with the use of AR (Fig. 3). As with ISH, successful experiments will produce spatially discrete labeling that is clearly localized to cells and not to internal lumens within the body that can trap the probe.
Staining of cnidocytes
The use of either the DAPI (Fig. 4a) or AO (Fig. 4b) stain will produce highly specific fluorescent labeling of cnidocytes in the appropriate wavelength. Cnidocytes are 10–25 μm in length and are highly concentrated in the tips of the tentacles and scattered around the ectoderm of the body column in the juvenile polyp (Fig. 4).
ACKNOWLEDGMENTS
This research was supported by National Science Foundation grant no. MCB-0924749 to T.D.G. and J.R.F. and by the US National Institutes of Health (NIH) grant no. 1R21RR032121 to M.Q.M. F.S.W. was supported by a predoctoral grant from the Superfund Basic Research Program at Boston University (no. 5 P42 E507381) and by Warren-McLeod graduate fellowships in Marine Biology. M.J.L. was supported by a Ruth L. Kirschstein National Research Service Award (no. FHD0550002) from the NIH.
Footnotes
AUTHOR CONTRIBUTIONS F.S.W. optimized immunofluorescence and cnidocytelabeling protocols. M.J.L. optimized the in situ hybridization protocols. J.R.F., T.D.G. and M.Q.M. provided technical advice on protocol development. All authors participated in writing the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Hand C, Uhlinger K. The culture, sexual and asexual reproduction, and growth of the sea anemone Nematostella vectensis. Biol. Bull. 1992;182:169–176. doi: 10.2307/1542110. [DOI] [PubMed] [Google Scholar]
- 2.Hand C, Uhlinger K. The unique, widely distributed sea anemone, Nematostella vectensis Stephenson: a review, new facts, and questions. Estuaries. 1994;17:501–508. [Google Scholar]
- 3.Hand C, Uhlinger KR. Asexual reproduction by transverse fission and some anomalies in the sea anemone Nematostella vectensis. Invert. Biol. 1995;114:9–18. [Google Scholar]
- 4.Putnam NH, et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- 5.Peterson KJ, Cotton JA, Gehling JG, Pisani D. The Ediacaran emergence of bilaterians: congruence between the genetic and the geological fossil records. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:1435–1443. doi: 10.1098/rstb.2007.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fautin DG. Structural diversity, systematics, and evolution of cnidae. Toxicon. 2009;54:1054–1064. doi: 10.1016/j.toxicon.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Finnerty JR, Paulson D, Burton P, Pang K, Martindale MQ. Early evolution of a homeobox gene: the parahox gene Gsx in the Cnidaria and the Bilateria. Evol. Dev. 2003;5:331–345. doi: 10.1046/j.1525-142x.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 8.Scholz CB, Technau U. The ancestral role of Brachyury: expression of NemBra1 in the basal cnidarian Nematostella vectensis (Anthozoa) Dev. Genes Evol. 2003;212:563–570. doi: 10.1007/s00427-002-0272-x. [DOI] [PubMed] [Google Scholar]
- 9.Finnerty JR, Pang K, Burton P, Paulson D, Martindale MQ. Origins of bilateral symmetry: Hox and Dpp expression in a sea anemone. Science. 2004;304:1335–1337. doi: 10.1126/science.1091946. [DOI] [PubMed] [Google Scholar]
- 10.Martindale MQ, Pang K, Finnerty JR. Investigating the origins of triploblasty: ‘mesodermal’ gene expression in a diploblastic animal, the sea anemone Nematostella vectensis (phylum, Cnidaria; class, Anthozoa) Development. 2004;131:2463–2474. doi: 10.1242/dev.01119. [DOI] [PubMed] [Google Scholar]
- 11.Ryan JF, et al. Pre-bilaterian origins of the Hox cluster and the Hox code: evidence from the sea anemone, Nematostella vectensis. PLoS ONE. 2007;2:e153. doi: 10.1371/journal.pone.0000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton PM, Finnerty JR. Conserved and novel gene expression between regeneration and asexual fission in Nematostella vectensis. Dev. Genes Evol. 2009;219:79–87. doi: 10.1007/s00427-009-0271-2. [DOI] [PubMed] [Google Scholar]
- 13.Tessmar-Raible K, Steinmetz PR, Snyman H, Hassel M, Arendt D. Fluorescent two-color whole mount in situ hybridization in Platynereis dumerilii (Polychaeta, Annelida), an emerging marine molecular model for evolution and development. Biotechniques. 2005;39:460, 462, 464. doi: 10.2144/000112023. [DOI] [PubMed] [Google Scholar]
- 14.Kosman D, et al. Multiplex detection of RNA expression in Drosophila embryos. Science. 2004;305:846. doi: 10.1126/science.1099247. [DOI] [PubMed] [Google Scholar]
- 15.Ciruna B, Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev. Cell. 2001;1:37–49. doi: 10.1016/s1534-5807(01)00017-x. [DOI] [PubMed] [Google Scholar]
- 16.Layden MJ, Boekhout M, Martindale MQ. Nematostella vectensis achaete-scute homolog NvashA regulates embryonic ectodermal neurogenesis and represents an ancient component of the metazoan neural specification pathway. Development. 2012;139:1013–1022. doi: 10.1242/dev.073221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wikramanayake AH, et al. An ancient role for nuclear β-catenin in the evolution of axial polarity and germ layer segregation. Nature. 2003;426:446–450. doi: 10.1038/nature02113. [DOI] [PubMed] [Google Scholar]
- 18.Marlow HQ, Srivastava M, Matus DQ, Rokhsar D, Martindale MQ. Anatomy and development of the nervous system of Nematostella vectensis, an anthozoan cnidarian. Dev. Neurobiol. 2009;69:235–254. doi: 10.1002/dneu.20698. [DOI] [PubMed] [Google Scholar]
- 19.Wolenski FS, et al. Characterization of the core elements of the NF-κB signaling pathway of the sea anemone Nematostella vectensis. Mol. Cell Biol. 2011;31:1076–1087. doi: 10.1128/MCB.00927-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolenski FS, Bradham CA, Finnerty JR, Gilmore TD. NF-κB is required for the development of subset of cnidocytes in the body column of the sea anemoneNematostella vectensis. Dev. Biol. 2013;373:205–215. doi: 10.1016/j.ydbio.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Zenkert C, Takahashi T, Diesner MO, Özbek S. Morphological and molecular analysis of the Nematostella vectensis cnidom. PLoS ONE. 2011;6:e22725. doi: 10.1371/journal.pone.0022725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi SR, Chaiwun B, Young L, Cote RJ, Taylor CR. Antigen retrieval technique utilizing citrate buffer or urea solution for immunohistochemical demonstration of androgen receptor in formalin-fixed paraffin sections. J. Histochem. Cytochem. 1993;41:1599–1604. doi: 10.1177/41.11.7691930. [DOI] [PubMed] [Google Scholar]
- 23.Anderson PA, Bouchard C. The regulation of cnidocyte discharge. Toxicon. 2009;54:1046–1053. doi: 10.1016/j.toxicon.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Watson G, Mire P, Kinler K. Mechanosensitivity in the model sea anemone Nematostella vectensis. Mar. Biol. 2009;156:2129–2137. [Google Scholar]
- 25.Szczepanek S, Cikala M, David CN. Poly- -glutamate synthesis during formation of nematocyst capsules in Hydra. J. Cell Sci. 2002;115:745–751. doi: 10.1242/jcs.115.4.745. [DOI] [PubMed] [Google Scholar]
- 26.Marlow H, Roettinger E, Boekhout M, Martindale MQ. Functional roles of Notch signaling in the cnidarian Nematostella vectensis. Dev. Biol. 2012;362:295–308. doi: 10.1016/j.ydbio.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magie CR, Pang K, Martindale MQ. Genomic inventory and expression of Sox and Fox genes in the cnidarian Nematostella vectensis. Dev. Genes Evol. 2005;215:618–630. doi: 10.1007/s00427-005-0022-y. [DOI] [PubMed] [Google Scholar]
- 28.Fritzenwanker JH, Saina M, Technau U. Analysis of forkhead and snail expression reveals epithelial-mesenchymal transitions during embryonic and larval development of Nematostella vectensis. Dev. Biol. 2004;275:389–402. doi: 10.1016/j.ydbio.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Wolenski FS, Finnerty JR, Gilmore TD. Preparation of antiserum and detection of proteins by western blotting using the starlet sea anemone, Nematostella vectensis. Protocol Exchange. 2012 doi:10.1038/protex.2012.057. [Google Scholar]
- 30.Stefanik DS, Friedman L, Finnerty JR. Collecting, rearing, spawning, and inducing regeneration of the starlet sea anemone, Nematostella vectensis. Nat. Protoc. 2013;8:916–923. doi: 10.1038/nprot.2013.044. [DOI] [PubMed] [Google Scholar]
- 31.Magie CR, Daly M, Martindale MQ. Gastrulation in the cnidarian Nematostella vectensis occurs via invagination not ingression. Dev. Biol. 2007;305:483–497. doi: 10.1016/j.ydbio.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan JC, et al. StellaBase: the Nematostella vectensis Genomics Database. Nucl. Acids Res. 2006;34:D495–D499. doi: 10.1093/nar/gkj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan JC, Reitzel AM, Finnerty JR. Upgrades to StellaBase facilitate medical and genetic studies on the starlet sea anemone, Nematostella vectensis. Nucl. Acids Res. 2008;36:D607–D611. doi: 10.1093/nar/gkm941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urrutia R, McNiven MA, Kachar B. Synthesis of RNA probes by the direct in vitro transcription of PCR-generated DNA templates. J. Biochem. Biophys. Methods. 1993;26:113–120. doi: 10.1016/0165-022x(93)90041-l. [DOI] [PubMed] [Google Scholar]