Abstract
Nanogels are being explored as drug delivery agents for targeting cancer due to their easy tailoring properties and ability to efficiently encapsulate therapeutics of diverse nature through simple mechanisms. Nanogels are proficiently internalized by the target cells, avoid accumulating in nontarget tissues thereby lower the therapeutic dosage and minimize harmful side effects. However, there is an urgent need for relevant clinical data from nanogels so as to allow translation of the nanogel concept into a viable therapeutic application for the treatment of cancer. This review highlights some of the recent progress in nanogels as a carrier in the field of nanomedicine for the treatment of cancer. The present review critically analyzes the use of extracellular pH targeting for nanogels, siRNA delivery, PEGylated nanogels, multi-responsive nanogels and intracellular delivery of nanogels for improved therapy of cancer.
Keywords: Nanogel carriers, siRNA, PEGylated nanogels, Intracellular delivery, Tumor extracellular pH targeting, Stimuli-responsive nanogels
1. Introduction
There has been considerable work on hydrogels, which are described as hydrophilic three dimensional polymer networks that are able to take up large amounts of water or physiological fluid, while maintaining their internal network structure (Peppas et al., 2000, Bromberg, 2005, Raemdonck et al., 2009a). In the last decade there has been increasing interest in hydrogels confined to nanoscopic dimensions (nanogels).
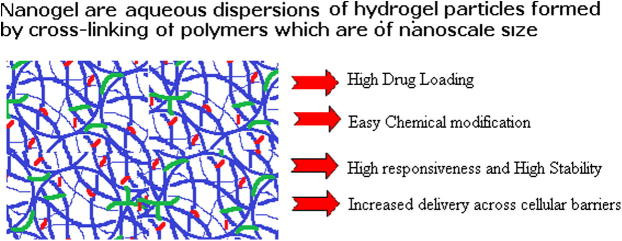
Nanogels can be designed to facilitate the encapsulation of diverse classes of bioactive compounds and following applications of nanogels show their utility as a potential nanomedicine carrier:
-
1.
Upon intravenous injection, nanogels can reach the areas which are not easily accessed by hydrogels.
-
2.
Nanogels are ideal candidates for intracellular delivery and can be safely delivered into the cytoplasm of the cell (Toita et al., 2011).
-
3.
Nanogel dispersions have a larger surface area which is important for in vivo applications.
-
4.
Nanogels have sizable drug loading capacity, low buoyant density and high dispersion stability in aqueous media.
-
5.
Nanogels enhance the efficacy of therapeutic nucleoside analogs (Vinogradov et al., 2005).
-
6.
Nanogels can encapsulate delicate compounds with low or high molecular weights and can significantly prolong their activity in biological environments (Bae et al., 2008).
-
7.
Weakly cross-linked polyelectrolyte nanogels can incorporate biomacromolecules of the opposite charge. Whereas; biomacromolecules are not able to accommodate in hydrogels due to the effects of excluded volume and cross-linking density (Kabanov and Vinogradov, 2009).
-
8.
Nanogels can be chemically modified to incorporate various ligands for targeted drug delivery, triggered drug release or preparation of composite materials (Vinogradov et al., 2002).
-
9.
Nanogels can be used for efficient delivery of biopharmaceuticals in cells as well as for increasing drug delivery across cellular barriers (Park et al., 2010).
-
10.
The nanoscale dimension of nanogels makes them to respond rapidly to environmental changes such as pH and temperature.
Nanogels show promise as a suitable nanomedicine carrier as compared to other nanoparticles especially in terms of drug loading (Fig. 1). Nanogels can be prepared or synthesized even in the absence of the drug to be loaded as drug loading in nanogels can be efficiently done later on when the nanogels are swollen and equilibrated in water or biological fluid. Drug loading occurs spontaneously in nanogels. As compared to other conventional nanoparticles, nanogels allow much higher drug loading (up to 50% of weight). Moreover the methods of preparation of nanogels are simpler and do not involve the use of mechanical energy or organic solvents (Vinogradov et al., 2004). Hence the loaded drug or therapeutic is not exposed to any vigorous condition during preparation. After administration the nanogels safely carry the payload, move within the cells and release the contents in the desired place in vivo.
Figure 1.
3D structure of nanogels.
Two of the approaches most commonly used for preparation of nanogels are physical self-assembly of interactive polymers and chemical cross-linking of preformed polymers (Oh et al., 2007). The physical self-assembly of polymers involves controlled aggregation of hydrophilic polymers capable of bonding with each other. Physical cross-linking in nanogel formation occurs via non-covalent attractive forces, such as hydrophilic–hydrophilic, hydrophobic–hydrophobic, ionic interactions and/or hydrogen bonding (Yallapu et al., 2011). The formation of nanogels occurs within a few minutes after suitable association of amphiphilic block copolymers and complexation of oppositely charged polymeric chains.
The preparation of nanogels is conducted in mild conditions and in aqueous media. According to Kabanov and Vinogradov (2009) “Self-associating hydrophilic polymers allow encapsulation of biomacromolecules and are useful for preparation of protein-loaded nanogels”. Chemical crosslinking is a method for creating nanogel with large pore sizes (Vinogradov, 2006). Crosslinks have to be present in a hydrogel in order to prevent dissolution of the hydrophilic polymer chains in aqueous media. Labile bonds are frequently introduced into hydrogels to make them biodegradable (Raemdonck et al., 2009a, Raemdonck et al., 2009b). Degradable bonds such as ester, carbonate, amide, anhydride, phosphazene and phosphate esters, are inserted either in cross-linkers or in polymeric chains for allowing degradation of the nanogel network.
Although the hydrophilic nature of nanogels may offer limitation for encapsulation of hydrophobic drugs; suitable engineering of the polymer structure allows high encapsulation of poorly soluble anticancer drugs (Soni and Yadav, 2014). Poorly soluble anticancer drugs can also be incorporated into nanogels for improving their solubility and stability thereby increasing their chances of cellular uptake than the free drug. In a study by Li et al. two poorly soluble anticancer drugs, paclitaxel (PTX) and 10-hydroxycamptothecin were loaded in nanogels by shell cross-linking of Pluronic F127 micelles (Li et al., 2011). The nanogels had a smooth and distinct spherical shape with the drug homogenously dispersed in the polymer matrix.
Nanogels hold promise to be a versatile drug delivery carrier for utilization in different therapeutics, as already discussed in many review articles (Lee et al., 2008, Liechty and Peppas, 2012, Chacko et al., 2012, Gonçalves et al., 2010). This expert review will specifically focus on the recent developments in the use of nanogels for tumor-targeting.
2. Nanogels for loading siRNA
A small interfering RNA (siRNA) is a class of double-stranded RNA molecules consisting of 21–23 nucleotides, involved in inhibition of protein synthesis encoded by the messenger RNAs (mRNA) (Dykxhoorn et al., 2006). The siRNA mediates posttranscriptional gene silencing of a specific target protein by disrupting mRNA when introduced into cells (Oliveira et al., 2006). They show promise to be used for any disease-causing gene as well as for targeting any cell or tissue (Reischl and Zimmer, 2009). siRNA as a gene regulating tool has a tremendous therapeutic potential in the areas of cancer treatment.
However, the clinical application of siRNA is hindered by its poor stability (Cun et al., 2010), degradation by endogenous enzymes (Singha et al., 2011), low cellular uptake efficiency, low endosomal escape efficiency (Xie et al., 2006) and short half-life in blood (Morrissey et al., 2005). Also the naked siRNA is unable to penetrate cellular membranes due to its large size and high negative charge. Such obstacles restrict the delivery of siRNA in vivo and require a suitable delivery carrier. Among different carriers, nanogels show promise as a novel transport medium for siRNA.
Dickerson et al. suggest targeted delivery of siRNAs by nanogels may be a promising strategy to increase the efficacy of chemotherapy drugs for the treatment of cancer (Dickerson et al., 2010).
It is difficult to load siRNA into nanogel carrier with high encapsulation efficiency as it easily leaks from the carrier due to its hydrophilic character. To increase siRNA loading efficiency it is complexed with cationic excipients to enhance the affinity between the siRNA and the particle matrix. Mimi et al. used polyethyleneimine (PEI) nanogels as an effective siRNA carrier. The negative charge of siRNA allows it to form a strong electrostatic interaction with the positively charged polyethyleneimine (Mimi et al., 2012). The consequential polyionic complexes also protect siRNA against enzymatic degradation. Other negatively charged complexing agents used are di oleyltrim ethyl ammonium propane and polyamines.
Chitosan has been shown to be useful as a carrier for improving the cellular uptake of naked siRNA both in vitro and in vivo via different administration routes (Mao et al., 2010). Chitosan is also useful for preventing the rapid degradation of siRNA in vivo.
Lee et al. explored the potential possibility of Hyaluronic acid as a biocompatible and biodegradable nanogel for delivery of siRNA. These nanogels crosslinked with disulfide linkages showed target-specific intracellular delivery of siRNA to HA-specific CD44 receptor over-expressing cancer cells (Lee et al., 2007).
Cationic dextran hydroxyethyl methacrylate (dex-HEMA) based nanogels are promising carriers for siRNA delivery since they can be loaded efficiently with siRNA, taken up by cells in vitro and were able to deliver intact siRNA into the cytosol of cells (Raemdonck et al., 2009b). Raemdonck et al. used the photopolymerization method to load siRNA into dextran nanogels (dex-HEMA-co-TMAEMA) using UV induced emulsion (Raemdonck et al., 2010). These nanogels were used as a siRNA depot from which siRNA released at the desired time to prolong the gene silencing effect. It was reported that in this way the siRNA dose was more efficiently deployed. Photochemical internalization was used as a trigger to induce endosomal escape of siRNA through the use of amphiphilic photosensitizers.
Naeye et al. PEGylate cationic dex-HEMA nanogels by covalent attachment of NHS-PEG to the reactive amine groups of the nanogels with an aim to deliver siRNA in vivo (Naeye et al., 2010). It was shown that dex-(HE)-MA-co-AEMA-co-TMAEMA nanogels retained their high loading efficiency of siRNA after PEGylation. The diffusion of the negatively charged siRNA molecules inside the gels occurred very slowly and that the siRNA was trapped by the cationic charges in the nanogels. Also, siRNA-loaded PEGylated dex-(HE)MA-co-AEMA-co-TMAEMA nanogels were able to successfully down regulate EGFP without causing severe toxicity in a HuH-7 EGFP cell line.
3. PEGylated nanogels
PEGylation refers to the modification of a particle surface by covalently grafting, entrapping or adsorbing polyethylene glycol (PEG). PEGylated nanoparticles are long circulating making the drug available for a prolonged period of time (Yadav et al., 2011a). PEGylation of nanogels not only improves their circulation time but also delivers their drug load into tumors following intravenous injection.
PEGylated nanogels are also prepared by chemically cross-linking poly(2-N,N-(diethylamino)ethyl methacrylate) (PEAMA) gel cores surrounded by PEG palisade layers. The PEGylated nanogels showed high stability under extremely dilute and high salt conditions, in contrast to self-assembled nanocarriers. Moreover, the nanogels showed pH-dependent swelling/deswelling transitions across the pKa of the PEAMA gel core around pH 7.0. Such nanogels swell under acidic conditions and they deswell under alkaline conditions.
Shimoda et al. chemically crosslinked nanogels with polyethylene glycol (PEG) derivatives to overcome the instability of physically crosslinked nanogel in vivo (Shimoda et al., 2012). Polysaccharide–PEG hybrid nanogels (CHPOA–PEGSH) were crosslinked by both covalent ester bonds and physical interactions by the reaction of a thiol-modified poly(ethylene glycol) (PEGSH) with acryloyl-modified cholesterol-bearing pullulan (CHPOA). The formulations were injected intravenously in mice to study their blood clearance. CHP nanogels were eliminated from the blood within 6 h, whereas the CHPOA–PEGSH nanogels had a significantly longer circulation time: approximately 40–50% of the nanoparticles remained in circulation 6 h following injection and, after 24 h, 20–30% of the nanoparticles remained in the blood. The half-life of CHPOA–PEGSH nanoparticles was about 15-fold greater than that of CHP nanogels indicating long circulation behavior of PEGylated nanogels.
4. Intracellular delivery of nanogels
Intracellular drug delivery refers to the delivery of therapeutic agents to specific compartments or organelles within the cell (Yadav et al., 2011b). This targeted intracellular drug delivery results in higher bioavailability of a therapeutic agent at its site of action, increases the pharmacologic effect and reduces the side effects of the drug (Panyam and Labhasetwar, 2004).
Studies such as intracellular delivery, cytotoxicity, cellular uptake hold promise for cancer chemotherapeutics. Nanogels due to their small size offer intracellular delivery of therapeutic molecules with respect to cellular uptake via endocytosis and the enhanced permeation and retention (EPR) effect (Ayame et al., 2008).
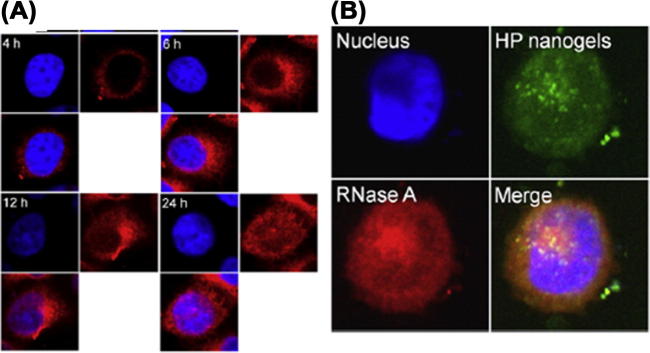
Choi et al. (2010) developed a novel self-assembled heparin-Pluronic nanogel incorporating RNase A for intracellular delivery of proteins. Their investigation showed that the nanogel was more efficiently internalized into HeLa cells and even localized to the nucleus (Fig. 2). The uptake mechanism reported was via caveolae/lipid-raft-mediated endocytosis. The nucleus penetration of heparin-based nanogels exhibited significant cytotoxicity that was dependent on the concentration of RNase A.
Figure 2.
Intracellular uptake of HP nanogels: (A) Confocal image of HeLa cells incubated with HP nanogels with Alexa 488-labeled RNase A (red), (B) Locolization of RNase A (red), Nucleus is stained in blue (Ref. Choi et al., 2010).
In a study by Murphy et al. (2011) drug-loaded nanogels showed enhanced potency when compared to free drug after exposure to M21 cells. There was an important finding that the EC50 values of the cells exposed for 20 min with the nanogels were comparable with the cells exposed to the free drug for 72 h. Oh et al. (2010) prepared a pH-responsive self-organized nanogel loaded with DOX and evaluated their cytotoxicity against A2780 cell lines.
Huang et al. (2009) used PF127 to produce amphiphilic nanocarriers for doxorubicin (DOX). In order to stabilize the nanocarriers, the hydroxyl groups on both termini of PF127 were acrylated and reacted with methacrylated chondroitin sulfate (CSMA) to form CS-PF127.
The better cellular internalization of FA-CS-PF127 into the FR overexpressing KB cells was evidenced by CLSM and flow cytometry. Flow cytometry analysis was applied to check the targeting efficiency of the FA-modified nanogel (FA-CS-PF127) and to investigate the cellular uptake in FR-positive KB cells. It was shown that FA-CS-PF127 nanogels entered into KB cells efficiently and this FA targeting moiety was responsible for the better internalization into KB cells.
Li et al. (2011) prepared paclitaxel (PTX) loaded nanogel using shell cross-linking of Pluronic F127 micelles with polyethylenimine (PEI) (F127/PEI nanogel). The cytotoxicity of PTX-loaded F127/PEI nanogel was investigated using HEPG-2 cells. The IC50 value data suggested that PTX-loaded nanogel displayed higher cytotoxicity compared with that of the free drug. The empty folate modified F127/PEI nanogel did not show significant toxicity in the whole concentration range compared with the free PTX. The folate-modified PTX-loaded nanogel demonstrated a significantly superior cytotoxicity as it has a much lower IC50 value. Folate-modified PTX-loaded nanogel was uptaken efficiently into HEPG- 2 cells than the non-modified F127/PEI nanogel due to the interaction between the folate on the nanogel surface and the folate receptors on the HEPG-2 cell surface. This interaction ensured that more drugs were pumped into the tumor cells to give a better anti-cancer effect.
5. Nanogel for tumor extracellular pH Targeting
Differences in pH between healthy tissue and tumor tissue can be exploited as an internal stimulus for triggered drug release (Oerlemans et al., 2010). Due to the high rate of aerobic and anaerobic glycolysis in the cancer cells, the pH of tumor tissue is slightly lower (pH 6.8) than the healthy tissue (pH 7.4).
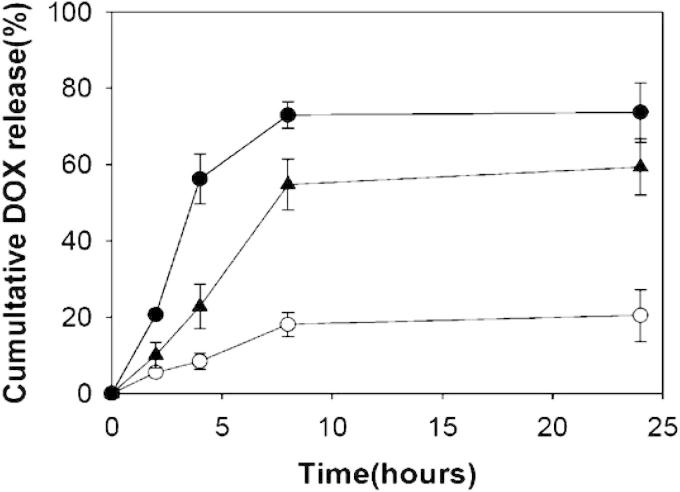
Oh et al. (2010) utilized glycol chitosan (GCS) to prepare a novel pH-responsive drug-carrying system that recognized tumor pH. The pH sensitivity was provided by grafting 3-diethylaminopropyl isothiocyanate (DEAP) to GCS. The pKb of DEAP ranges from 7.0 to 7.3 which is similar to tumor pH. The GCS-g-DEAP conjugate was used to prepare pH-responsive self-organized nanogel loaded with doxorubicin (DOX). The release of DOX was enhanced at pH 6.8 compared to that at normal pH 7.4, signifying higher concentration of DOX at cancer sites with an acidic pH (pH 6.8) (Fig. 3A). Such nanogel would therefore maximize the therapeutic activity of the drug for treatment of in vivo cancers. It was suggested that DOX loaded GCS-g-DEAP nanogels could be successfully used for targeting cancer-associated acidic pH (i.e., pH 6.8) and may also be utilized for triggering release at endosomal pH (i.e., pH 6.0).
Figure 3a.
pH-dependent DOX release from DOX-loaded GCS-g-DEAP nanogels at pH 7.4 (O), pH 6.8 (▴), and pH 6.0 (●) for 0–24 h of incubation (Ref. Oh et al., 2010).
Na et al. fabricated DOX loaded self-organized nanogels composed of hydrophobized pullulan (PUL)-Nα-Boc-l-histidine (bHis) conjugates (Na et al., 2007). Nanogels showed tumor specific release of DOX which increased significantly with reductions in pH.
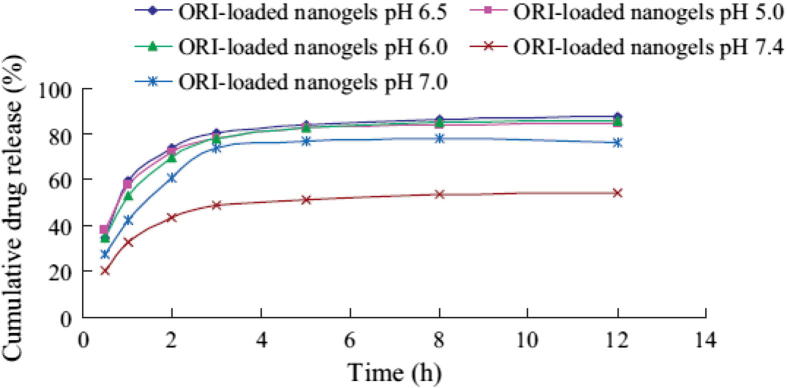
Tumoral acidic extracellular pH targeting was exploited for oridonin (ORI), a hydrophobic anticancer drug in Chinese traditional medicine (Duan et al., 2011). A pH-responsive chitosan-g-poly(N-isopropylacrylamide) (CS-g-PNIPAm) based nanogel delivery system was developed by a self-assembly method. ORI-loaded nanogels exhibited a pH-triggered fast drug release under a slightly acidic condition. The drug release was slow at pH 7.4 while it accelerated at lower pH of 6.5 and 6.0 (Fig. 3B). ORI-loaded nanogels showed a higher cellular cytotoxicity relative to the ORI solution at the same pH. The anticancer cytotoxicity of ORI-loaded nanogels against HepG2 cells was significantly increased at pH 6.5 compared to that at pH 7.4. Moreover, the IC50 value for the ORI-loaded nanogels was lower (8.86 μg/ml) at pH 6.5 as compared to pH 7.4 (13.19 μg/ml) indicating a pH-dependent effect. This was attributed to the pH sensitive rapid drug release of drug at lower pH.
Figure 3b.
pH-dependent ORI release from ORI- loaded nanogels at different pH (Ref. Duan et al., 2011).
6. Stimuli-responsive nanogels
Nanogels exhibiting a phase transition in response to change in external conditions such as pH, ionic strength, temperature or electric currents are known as “stimuli-responsive” nanogels. Nanogels swell due to solvent penetration into free spaces and this swelling behavior is influenced by external triggers, such as changes in environmental pH, ionic strength or temperature. Nanogels show much faster responsiveness as compared to the conventional hydrogels. Multi-stimuli responsive nanogels are more effective in targeted therapy for cancer as compared to single responsive nanogels (Morinloto et al., 2008, Rijcken et al., 2007).
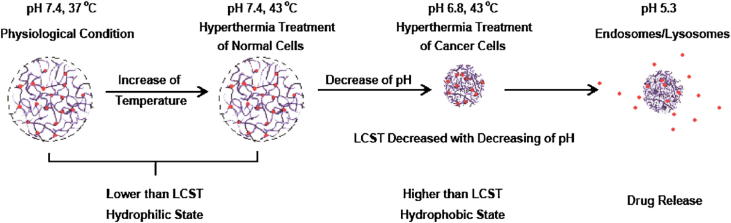
Among multi-stimuli responsive drug vehicles, dual temperature-/pH-stimuli responsive carriers find wider application in cancer therapy because in cancer there are changes in the temperature and pH of body tissue and these two signals could be regulated easily by external triggers (Zhang et al., 2007). Xiong et al. (2011) prepared dual temperature-/pH-sensitive poly(N-isopropylacrylamide-co-acrylic acid) nanogels (PNA), and conjugated DOX onto PNA nanogels via acid-cleavable bonds (DOX–PNA). The PNA nanogels were hydrophilic under physiological condition, but underwent a dual temperature/pH induced phase transition upon heating through its LCST, which was affected by the pH value (Fig. 4). This difference in pH between tumor and normal tissues allowed cancer cellular internalization under region hyperthermia treatments. The drug loaded nanogel cleaved upon mildly acidic triggering in the endosome of tumor cells to release DOX. It was shown that such dual temperature-/pH-sensitive DOX–PNA nanogels would have better tumor targeted delivery than common temperature sensitive materials.
Figure 4.
Tumor extracellular pH targeting by nanogels (Ref. Xiong et al. (2011)).
Qiao et al. prepared a new type of triply responsive nanogels by mini emulsion radical copolymerization of monomethyl oligo(ethylene glycol) acrylate and ortho ester-containing acrylic monomer, 2-(5,5-dimethyl-1,3-dioxan-2-yloxy) ethyl acrylate, with bis(2-acryloyloxyethyl) disulfide as a crosslinker (Qiao et al., 2011). The thermo/pH/redox responsive behavior of the nanogels is depending on the composition and crosslinking of the polymer. These nanogels were able to encapsulate the hydrophobic drug paclitaxel (PTX), had good stability at pH 7.4 and showed potential cytotoxicity to tumor cells.
Chen et al. have recently reported an injectable dual-responsive micellar nanogel system for controlled delivery of PTX in cancer therapy (Chen et al., 2013). The micellar nanogel improved the solubility and stability of PTX. The dual-responsive micellar nanogel showed sol–gel transition at 37 °C. The in vitro PTX release showed that nanogel could release about 70% for 70 h under pH 5.0 while about 10% release at pH 7.4 and pH 9.0. The dual-responsive micellar nanogel suppressed tumor growth and showed potential as a dual-responsive drug delivery system for cancer therapy.
Kim et al. loaded doxorubicin in polypeptide-based nanogels with hydrophobic moieties in the cross-linked ionic cores (Kim et al., 2013). The nanogels had high drug loading capacity of 30 w/w% and were of considerably small size of 70 nm in diameter. The nanogels were found to be enzymatically degradable leading to accelerated drug release under simulated lysosomal acidic pH. The doxorubicin loaded nanogels showed improved antitumor activity compared to a free doxorubicin in an ovarian tumor xenograft mouse model signifying the use of such biodegradable nanogels as attractive carriers for delivery of chemotherapeutics. Doxorubicin was also loaded in chitin nanogels having pH sensitive controlled release (Jayakumar et al., 2012). The drug release studies showed that doxorubicin release was more in acidic pH compared to neutral pH. In the first hour 32% doxorubicin was released, which was similar in acidic and neutral pH. But after 24 h 60% of the drug was released under acidic condition, whereas only about 40% was released in the neutral environment. This difference in release was attributed to higher swelling of nanogel in acidic pH. Yet again in another study doxorubicin was loaded in dual stimuli-responsive hollow nanogel spheres for pH dependent intracellular delivery (Chiang et al., 2012). The nanogels exhibited a pH-controlled drug release profile in an aqueous solution at 37 or 4 °C. The cumulative drug release performed at pH 5.0 over a period of 3 h (50%) was much higher than that (20%) at pH 7.4. The delivery system demonstrated promise in intracellular drug release for transport within acidic endosomal or lysosomal compartments.
7. Expert opinion and conclusion
Recent years have witnessed an extraordinary expansion in drug delivery research in the area of cancer. There is an increasing assurance that nanotechnology applied to medicine will bring significant advances in the diagnosis and treatment of cancer. When most of the chemotherapeutics fail to show effect clinically in the treatment of cancer due to their toxic side effects, nanogels as nanomedicine yield more effective therapies.
Nanogels are being explored as drug delivery agents for targeting cancer due to their easy tailoring properties and ability to efficiently encapsulate therapeutics of diverse nature through simple mechanisms. Nanogels are proficiently internalized by the target cells, avoid accumulating in non-target tissues thereby lower the therapeutic dosage and minimize harmful side effects.
Although, the last decade has witnessed intensive research in the nanogel formulations some factors are slowing the process of industrial production of the same. These factors include inefficient translation of in vitro properties to in vivo efficacy, toxicity concerns, immunogenicity, pharmacokinetics of in vivo models, biodistribution and regulatory issues. There is an urgent need for relevant clinical data from nanogels so as to allow translation of the nanogel concept into a viable therapeutic application for treatment of cancer. Nanogels as a drug delivery carrier would improve the efficacy of cancer chemotherapy and benefit of the cancer patients.
Acknowledgements
Dr. K.S. Yadav thanks AICTE, New Delhi, for the award of Research Promotion Scheme project (Ref No. 8023/RID/RPS-71/POLICY III(PVT)/2011-12).
Footnotes
Peer review under responsibility of King Saud University.
References
- Ayame H., Morimoto N., Akioshi K. Self-assembled cationic nanogels for intracellular protein delivery. Bioconjug. Chem. 2008;19:882–890. doi: 10.1021/bc700422s. [DOI] [PubMed] [Google Scholar]
- Bae K.H., Mok H., Park T.G. Synthesis, characterization, and intracellular delivery of reducible heparin nanogels for apoptotic cell death. Biomaterials. 2008;29:3376–3383. doi: 10.1016/j.biomaterials.2008.04.035. [DOI] [PubMed] [Google Scholar]
- Bromberg L. Intelligent hydrogels for the oral delivery of chemotherapeutics. Expert Opin. Drug Deliv. 2005;2(6):1003–1013. doi: 10.1517/17425247.2.6.1003. [DOI] [PubMed] [Google Scholar]
- Chacko R.T., Ventura J., Zhuang J., Thayumanavan S. Polymer nanogels: a versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 2012;64(9):836–851. doi: 10.1016/j.addr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Yu H., Sun K., Liu W., Wang H. Dual thermoresponsive and pH-responsive self-assembled micellar nanogel for anticancer drug delivery. Drug Deliv. 2013 doi: 10.3109/10717544.2013.838717. (posted online on October 9, 2013) [DOI] [PubMed] [Google Scholar]
- Chiang W.H., Ho V.T., Huang W.C., Huang Y.F., Chern C.S., Chiu H.C. Dual stimuli-responsive polymeric hollow nanogels designed as carriers for intracellular triggered drug release. Langmuir. 2012;28(42):15056–15064. doi: 10.1021/la302903v. [DOI] [PubMed] [Google Scholar]
- Choi J.H., Jang J.Y., Joung Y.K., Kwon M.H., Park K.D. Intracellular delivery and anti-cancer effect of self-assembled heparin-Pluronic nanogels with RNase A. J. Control. Release. 2010;147:420–427. doi: 10.1016/j.jconrel.2010.07.118. [DOI] [PubMed] [Google Scholar]
- Cun D., Foged C., Yang M., Frøkjær S., Nielsen H.M. Preparation and characterization of poly (dl-lactide-co-glycolide) nanoparticles for siRNA delivery. Int. J. Pharm. 2010;390(1):70–75. doi: 10.1016/j.ijpharm.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Dickerson E.B., Blackburn W.H., Smith M.H., Kapa L.B., Lyon L.A., McDonald J.F. Chemosensitization of cancer cells by siRNA using targeted nanogel delivery. BMC Cancer. 2010;10:10. doi: 10.1186/1471-2407-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C., Zhang D., Wang F., Zheng D., Jia L., Feng F., Liu Y., Wang Y., Tian K., Wang F., Zhang Q. Chitosan-g-poly(N-isopropylacrylamide) based nanogels for tumor extracellular targeting. Int. J. Pharm. 2011;409:252–259. doi: 10.1016/j.ijpharm.2011.02.050. [DOI] [PubMed] [Google Scholar]
- Dykxhoorn D.M., Palliser D., Lieberman J. The silent treatment: siRNAs as small molecule drugs. Gene Ther. 2006;13:541–552. doi: 10.1038/sj.gt.3302703. [DOI] [PubMed] [Google Scholar]
- Gonçalves C., Pereira P., Gama M. Self-assembled hydrogel nanoparticles for drug delivery applications. Materials. 2010;3(2):1420–1460. [Google Scholar]
- Huang S.J., Sun S.L., Feng T.H., Sung K.H., Lui W.L., Wang L.F. Folate-mediated chondroitin sulfate-Pluronic® 127 nanogels as a drug carrier. Eur. J. Pharm. Sci. 2009;38:64–73. doi: 10.1016/j.ejps.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Jayakumar R., Nair A., Rejinold N.S., Maya S., Nair S.V. Doxorubicin-loaded pH-responsive chitin nanogels for drug delivery to cancer cells. Carbohydr. Polym. 2012;87(3):2352–2356. [Google Scholar]
- Kabanov A.V., Vinogradov S.V. Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew. Chem. Int. Ed. 2009;48:5418–5429. doi: 10.1002/anie.200900441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.O., Oberoi H.S., Desale S., Kabanov A.V., Bronich T.K. Polypeptide nanogels with hydrophobic moieties in the cross-linked ionic cores: synthesis, characterization and implications for anticancer drug delivery. J. Drug Target. 2013 doi: 10.3109/1061186X.2013.831421. (posted online on September 2, 2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Mok H., Lee S., Oh Y.K., Park T.G. Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. J. Control. Release. 2007;119:245–252. doi: 10.1016/j.jconrel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Lee E.S., Gao Z., Bae Y.H. Recent progress in tumor pH targeting nanotechnology. J. Control Release. 2008;132:164–170. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Wang J., Yang X., Li L. Novel nanogels as drug delivery systems for poorly soluble anticancer drugs. Colloids Surf. B. 2011;83:237–244. doi: 10.1016/j.colsurfb.2010.11.027. [DOI] [PubMed] [Google Scholar]
- Liechty W.B., Peppas N.A. Expert opinion: responsive polymer nanoparticles in cancer therapy. Eur. J. Pharm. Biopharm. 2012;80:241–246. doi: 10.1016/j.ejpb.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S., Sun W., Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010;62:12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Mimi H., Ho K.M., Siu Y.S., Wu A., Li P. Polyethyleneimine-based core-shell nanogels: a promising Sirna carrier for argininosuccinate synthetase mRNA knockdown in HeLa cells. J. Control. Release. 2012;158:123–130. doi: 10.1016/j.jconrel.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Morinloto N., Qiu X.P., Winnik F.M., Akiyoshi K. Dual stimuli-responsive nanogels by self-assembly of polysaccharides lightly grafted with thiol-terminated poly(Nisopropylacrylamide) chains. Macromolecules. 2008;41:5985–5987. [Google Scholar]
- Morrissey D.V., Blanchard K., Shaw L., Jensen K., Lockridge J.A., Dickinson B., McSwiggen J.A., Vargeese C., Bowman K., Shaffer C.S., Polisky B.A., Zinnen S. Activity of stabilized short interfering RNA in a mouse model of hepatitis B virus replication. Hepatology. 2005;41:1349–1356. doi: 10.1002/hep.20702. [DOI] [PubMed] [Google Scholar]
- Murphy E.A., Majeti B.K., Mukthavaram R., Acevedo L.M., Barnes L.A., Cheresh D.A. Targeted nanogels: a versatile platform for drug delivery to tumors. Mol. Cancer Ther. 2011;10(6):972–982. doi: 10.1158/1535-7163.MCT-10-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na K., Lee E.S., Bae Y.H. Self-organized nanogels responding to tumor extracellular ph: pH-dependent drug release and in vitro cytotoxicity against MCF-7 cells. Bioconjug. Chem. 2007;18:1568–1574. doi: 10.1021/bc070052e. [DOI] [PubMed] [Google Scholar]
- Naeye B., Raemdonck K., Remaut K., Sproat B., Demeester J., De Smedt S.C. PEGylation of biodegradable dextran nanogels for siRNA delivery. Eur. J. Pharm. Sci. 2010;40:342–351. doi: 10.1016/j.ejps.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Oerlemans C., Bult W., Bos M., Storm G., Nijsen J.F.W., Hennink W.E. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm. Res. 2010;27(12):2569–2589. doi: 10.1007/s11095-010-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J.K., Siegwart D.J., Lee H.I., Sherwood G., Peteanu L., Hollinger J.O., Kataoka K., Matyjaszewski K. Biodegradable nanogels prepared by atom transfer radical polymerization as potential drug delivery carriers: synthesis, biodegradation, in vitro release, and bioconjugation. J. Am. Chem. Soc. 2007;129:5939–5945. doi: 10.1021/ja069150l. [DOI] [PubMed] [Google Scholar]
- Oh N.M., Oh K.T., Baik H.J., Lee B.R., Lee A.H., Youn Y.S., Lee E.S. A self-organized 3-diethylaminopropyl-bearing glycol chitosan nanogel for tumor acidic pH targeting: In vitro evaluation. Coll. Surf. B Biointerfaces. 2010;78:120–126. doi: 10.1016/j.colsurfb.2010.02.023. [DOI] [PubMed] [Google Scholar]
- Oliveira S., Storm G., Schiffelers R. Targeted delivery of siRNA. J. Biomed. Biotechnol. 2006:1–9. doi: 10.1155/JBB/2006/63675. Article ID: 63675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyam J., Labhasetwar V. Targeting intracellular targets. Curr. Drug Deliv. 2004;1(3):235–247. doi: 10.2174/1567201043334768. [DOI] [PubMed] [Google Scholar]
- Park W., Park S.J., Na K. Potential of self-organizing nanogel with acetylated chondroitin sulfate as an anti-cancer drug carrier. Colloids Surf. B Biointerfaces. 2010;79:501–508. doi: 10.1016/j.colsurfb.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Peppas N.A., Bures P., Leobandung W., Ichikawa H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000;50(1):27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- Qiao Z.Y., Zhang R., Du F.S., Liang D.H., Li Z.C. Multi-responsive nanogels containing motifs of ortho ester, oligo(ethylene glycol) and disulfide linkage as carriers of hydrophobic anti-cancer drugs. J. Control. Release. 2011;152:57–66. doi: 10.1016/j.jconrel.2011.02.029. [DOI] [PubMed] [Google Scholar]
- Raemdonck K., Demeester J., De Smedt S. Advanced nanogel engineering for drug delivery. Soft Matter. 2009;5:707–715. [Google Scholar]
- Raemdonck K., Naeye B., Buyens K., Vandenbroucke R.E., Hogset A., Demeester J., De Smedt S.C. Biodegradable dextran nanogels for RNA interference: focusing on endosomal escape and intracellular siRNA delivery. Adv. Funct. Mater. 2009;19:1406–1415. [Google Scholar]
- Raemdonck K., Naeye B., Høgset A., Demeester J., De Smed S.C. Prolonged gene silencing by combining siRNA nanogels and photochemical internalization. J. Control. Release. 2010;145:281–288. doi: 10.1016/j.jconrel.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Reischl D., Zimmer A. Drug delivery of siRNA therapeutics: potentials and limits of nanosystems. Nanomedicine. 2009;5:8–20. doi: 10.1016/j.nano.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Rijcken C.J., Soga O., Hennink W.E., Van Nostrum C.F. Triggered destabilisation of polymeric micelles and vesicles by changing polymers polarity: an attractive tool for drug delivery. J. Control Release. 2007;120:131–148. doi: 10.1016/j.jconrel.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Shimoda A., Sawada S., Kano A., Maruyama A., Moquin A., Winnik F.M., Akiyoshi K. Dual crosslinked hydrogel nanoparticles by nanogel bottom-up method for sustained-release delivery. Colloids Surf. B: Biointerfaces. 2012;99:38–44. doi: 10.1016/j.colsurfb.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Singha K., Namgung R., Kim W.J. Polymers in small-interfering RNA delivery. Nucleic Acid Ther. 2011;21(3):133–147. doi: 10.1089/nat.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni G., Yadav K.S. High encapsulation efficiency of poloxamer-based injectable thermoresponsive hydrogels of etoposide. Pharm. Dev. Tech. 2014;19(6):651–661. doi: 10.3109/10837450.2013.819014. [DOI] [PubMed] [Google Scholar]
- Toita S., Sawada S., Akiyoshi K. Polysaccharide nanogel gene delivery system with endosome-escaping function: Co-delivery of plasmid DNA and phospholipase A2. J. Control. Release. 2011;155:54–59. doi: 10.1016/j.jconrel.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Vinogradov S.V. Colloidal microgels in drug delivery applications. Curr. Pharm. Des. 2006;12(36):4703–4712. doi: 10.2174/138161206779026254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S.V., Bronich T.K., Kabanov A.V. Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv. Drug Deliv. Rev. 2002;54:135–147. doi: 10.1016/s0169-409x(01)00245-9. [DOI] [PubMed] [Google Scholar]
- Vinogradov S.V., Batrakova E.V., Kabanov A.V. Nanogels for oligonucleotide delivery to the brain. Bioconjug. Chem. 2004;15:50–60. doi: 10.1021/bc034164r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S.V., Zeman A.D., Batrakova E.V., Kabanov A.V. Polyplex nanogel formulations for drug delivery of cytotoxic nucleoside analogs. J. Control. Release. 2005;107:143–157. doi: 10.1016/j.jconrel.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F.Y., Woodle M.C., Lu P.Y. Harnessing in vivo siRNA delivery for drug discovery and therapeutic development. Drug Discov. Today. 2006;11:67–73. doi: 10.1016/S1359-6446(05)03668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W., Wang W., Wang Y., Zhao Y., Chen H., Xu H., Yang X. Dual temperature/pH-sensitive drug delivery of poly(N-isopropylacrylamide-co-acrylic acid) nanogels conjugated with doxorubicin for potential application in tumor hyperthermia therapy. Coll. Surf B: Biointerfaces. 2011;84:447–453. doi: 10.1016/j.colsurfb.2011.01.040. [DOI] [PubMed] [Google Scholar]
- Yadav K.S., Jacob S., Sachdeva G., Chuttani K., Mishra A.K., Sawant K.K. Long circulating PEGylated PLGA nanoparticles of cytarabine for targeting leukemia. J. Microencapsulation. 2011;28(8):729–742. doi: 10.3109/02652048.2011.615949. [DOI] [PubMed] [Google Scholar]
- Yadav K.S., Jacob S., Sachdeva G., Sawant K.K. Intracellular delivery of etoposide loaded biodegradable nanoparticles: cytotoxicity and cellular uptake studies. J. Nanosci. Nanotechnol. 2011;11:6657–6667. doi: 10.1166/jnn.2011.4225. [DOI] [PubMed] [Google Scholar]
- Yallapu M.M., Jaggi M., Chauhan S.C. Design and engineering of nanogels for cancer treatment. Drug Discovery Today. 2011;2011(16):457–463. doi: 10.1016/j.drudis.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Guo R., Yang M., Jiang X., Liu B. Thermo and pH dual responsive nanoparticles for anti cancer drug delivery. Adv. Mater. 2007;19:2988–2992. [Google Scholar]