Abstract
Repeated exposure to psychostimulants induces locomotor sensitization and leads to persistent changes in the circuitry of the mesocorticolimbic dopamine (DA) system. G-protein-gated inwardly rectifying potassium (GIRK; also known as Kir3) channels mediate a slow IPSC and control the excitability of DA neurons. Repeated 5 d exposure to psychostimulants decreases the size of the GABAB receptor (GABABR)-activated GIRK currents (IBaclofen) in ventral tegmental area (VTA) DA neurons of mice, but the mechanism underlying this plasticity is poorly understood. Here, we show that methamphetamine-dependent attenuation of GABABR-GIRK currents in VTA DA neurons required activation of both D1R-like and D2R-like receptors. The methamphetamine-dependent decrease in GABABR-GIRK currents in VTA DA neurons did not depend on a mechanism of dephosphorylation of the GABAB R2 subunit found previously for other neurons in the reward pathway. Rather, the presence of the GIRK3 subunit appeared critical for the methamphetamine-dependent decrease of GABABR-GIRK current in VTA DA neurons. Together, these results highlight different regulatory mechanisms in the learning-evoked changes that occur in the VTA with repeated exposure to psychostimulants.
SIGNIFICANCE STATEMENT Exposure to addictive drugs such as psychostimulants produces persistent adaptations in inhibitory circuits within the mesolimbic dopamine system, suggesting that addictive behaviors are encoded by changes in the reward neural circuitry. One form of neuroadaptation that occurs with repeated exposure to psychostimulants is a decrease in slow inhibition, mediated by a GABAB receptor and a potassium channel. Here, we examine the subcellular mechanism that links psychostimulant exposure with changes in slow inhibition and reveal that one type of potassium channel subunit is important for mediating the effect of repeated psychostimulant exposure. Dissecting out the components of drug-dependent plasticity and uncovering novel protein targets in the reward circuit may lead to the development of new therapeutics for treating addiction.
Keywords: addiction, PDZ, plasticity, potassium channel, psychostimulants, reward
Introduction
Drug addiction is a psychiatric disorder characterized by continued use of drug despite severe adverse consequences (Robinson and Berridge, 2003; Koob and Volkow, 2010). Addiction is thought to involve, in part, long-term adaptations in the mesocorticolimbic dopamine (DA) circuits that occur in response to repeated drug use, promoting a risk of relapse after discontinuing use (Lüscher and Malenka, 2011). In rodents, repeated administration of many drugs of abuse produces locomotor sensitization, a progressive increase in the locomotor-stimulating effects of these drugs, a phenomenon that is context dependent and persists for months after the last drug exposure (Vanderschuren and Kalivas, 2000). Locomotor sensitization has been used as a behavioral read-out of an underlying neural sensitization in addiction circuits (Robinson and Berridge, 2003).
The development of locomotor sensitization requires increased activity in the mesocorticolimbic DA circuit, particularly in the ventral tegmental area (VTA) (Robinson and Berridge, 2003). Several studies have demonstrated that exposure to psychostimulants alters both excitatory and inhibitory inputs to VTA DA neurons (Lüscher and Malenka, 2011). For example, single or multiple exposure to cocaine enhances glutamatergic signaling in the VTA (Ungless et al., 2001; Saal et al., 2003; Chen et al., 2008). Multiple exposure to cocaine also reduces the amplitude of fast GABAA-evoked IPSCs on VTA DA neurons, promoting LTP induction (Liu et al., 2005) while enhancing IPCSs onto VTA GABA neurons (Bocklisch et al., 2013). Together, these changes promote an increase in activity of VTA DA neurons in drug-exposed animals. Another important pathway that controls the output of the VTA involves the G-protein-gated inwardly rectifying potassium (GIRK, also referred to as Kir3) channels. Activation of GIRK channels produces a slow IPSC that hyperpolarizes the neuron, thereby reducing excitability and inhibiting the firing of DA neurons (Lacey et al., 1988; Seutin et al., 1994; Beckstead et al., 2004; Cruz et al., 2004; Munoz and Slesinger, 2014). Stimulation of GABAB, DA D2-like or κ opioid receptors has been reported to activate GIRK channels in VTA DA neurons, wheres GABAB and μ opioid receptors couple to GIRK channels in VTA GABA neurons (Cameron et al., 1997; Margolis et al., 2003; Beckstead et al., 2004; Cruz et al., 2004; Kotecki et al., 2015).
Recent work with rodents has established that psychostimulant exposure alters slow inhibition mediated by GABAB receptors and GIRK channels. For example, acute exposure to a psychostimulant such as methamphetamine (METH) or cocaine reduces baclofen-activated GIRK currents (IBaclofen) in VTA GABA neurons (Padgett et al., 2012). Multiple injections of cocaine dampen IBaclofen in VTA DA neurons (Arora et al., 2011). Recently, Sharpe et al. (2015) reported that self-administration of METH also diminishes IBaclofen in VTA DA neurons, indicating that both contingent (i.e., self-administration) and noncontingent (i.e., experimenter injections) drug exposure leads to a reduction in GIRK signaling in the VTA. This attenuation in IBaclofen leads to changes in the excitability of VTA DA neurons and is hypothesized to contribute to circuit-level changes in DA signaling involved in addiction. Consistent with a role for GABAB receptors (GABABRs) in the reward pathway, systemic or intra-VTA administration of a GABABR agonist is sufficient to prevent the acquisition of locomotor sensitization and self-administration of psychostimulants (Shoaib et al., 1998; Brebner et al., 1999; Lhuillier et al., 2007; Backes and Hemby, 2008).
To develop new therapeutics for treating addiction, it is essential to dissect the components of drug-dependent plasticity in the brain. In VTA GABA neurons (Padgett et al., 2012) and in mPFC pyramidal neurons (Hearing et al., 2013), the reduction in IBaclofen can be explained by a decrease in the surface expression of GABABRs, a process that depends on dephosphorylation of a C-terminal serine (S783) in the GABABR2 subunit via a PP2A phosphatase (Padgett et al., 2012). Repeated injections of cocaine or self-administration of METH weaken GABABR-GIRK signaling in VTA DA neurons (Arora et al., 2011; Sharpe et al., 2015). The subcellular mechanism underlying the reduction in IBaclofen in VTA DA neurons with psychostimulants, however, is poorly understood. In the current study, we investigated whether dephosphorylation of the GABABR2-S783 subunit is involved in the METH-dependent reduction of IBaclofen in VTA DA neurons. We discovered that the GIRK3 subunit, and not the dephosphorylation of GABABR2-S783, promotes the METH-dependent decrease in IBaclofen.
Materials and Methods
Mouse husbandry.
The following mouse lines were used, Pitx3+/−-GFP (Zhao et al., 2004), GIRK3 knock-out (KO) (Torrecilla et al., 2002), GABABR2-S783A knock-in (KI) (Terunuma et al., 2014), TH-Cre (Friedman et al., 2014), and littermate controls. TH-Cre mice were injected with AAV DIO-YFP (Munoz and Slesinger, 2014) to label VTA DA neurons for the control experiment shown in Figure 2F. All mice either originated in C57BL/6 or were backcrossed multiple generations into the C57BL/6 background. Mice were housed together in groups of two to five and separated by sex. All procedures were performed in the light phase of the circadian cycle using Institutional Animal Care and Use Committee-approved protocols for animal handling at the Salk Institute and at the Icahn School of Medicine at Mount Sinai. Animals were housed under constant temperature and humidity on a 12 h light/dark cycle (light 6:00–18:00) with food and water available ad libitum. Tail biopsies were collected from animals at weaning (>postnatal day 21) and prepared for PCR genotyping, as described previously (Torrecilla et al., 2002; Zhao et al., 2004; Friedman et al., 2014; Terunuma et al., 2014).
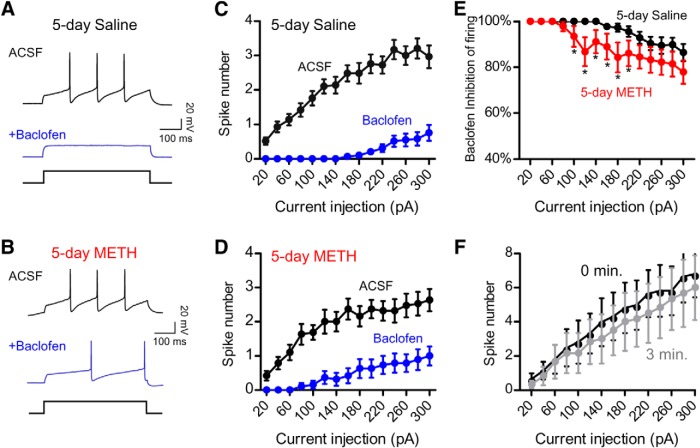
Figure 2.
Baclofen-induced inhibition of firing is reduced in VTA DA neurons after 5 d METH injections. A, B, Current-clamp recordings showing firing induced with a 200 pA current injection before (black trace) and after (blue trace) addition of 300 μm baclofen. Recordings are from a GFP+ VTA DA neuron in a Pitx3-GFP+/− mouse injected with 5 d saline (0.9%) (A) or 5 d METH (2 mg/kg) (B) in a novel environment. Input–output plots show the increase in spike number with increasing amplitude current injections for 5 d saline- (C) or 5 d METH (D)-injected mice before (black) and after baclofen (blue) exposure. E, Percentage baclofen-dependent inhibition of firing for VTA DA neurons from 5 d saline and 5 d METH groups (*p < 0.05 Student's unpaired one-tailed t test, n = 17–29). F, Input–output plot for VTA DA neuron firing in control mice (TH-Cre, n = 6) showing no decrement in spiking over 3 min of recording.
Drug administration.
Mice [postnatal day 30 (P30)–P50 male and female] were given intraperitoneal injections of METH (methamphetamine hydrochloride; Sigma-Aldrich) at 2 mg/kg in 0.9% saline or an equivalent volume of 0.9% saline. For antagonist experiments, the D1R antagonist SCH39166 (0.3 mg/kg, i.p.) or the D2R antagonist eticlopride (0.1 mg/kg, i.p.) was coinjected with METH (2 mg/kg). For home cage injections, animals were weighed, injected, and returned to the home cage. For novel environment experiments, animals were transported to a different room, weighed, injected, placed in new cage with cage mates for 1 h, and then returned to home cage. The novel environment consisted of a different plastic cage (Innovive) that contained soft bedding and brightly colored paper along the outside. All animals had full access to food and water during drug administration in either home or novel environment cages and injections took place during the light cycle between 10:00 and 16:00.
Electrophysiology.
Mice were killed with isoflurane and horizontal or coronal slices from midbrain (200 μm) were prepared in ice-cold artificial CSF (ACSF) containing the following (in mm): NaCl 119, KCl 2.5, MgCl2 1.3, CaCl2 2.5, NaH2PO4 1.0, NaHCO3 26.2, and glucose 11, pH 7.2, bubbled with 95% O2 and 5% CO2. Slices were equilibrated for ∼42 min at 33°C in ACSF supplemented with the following (in mm): l-ascorbic acid 0.4, Na pyruvate 2, myo-inositol 3, and then transferred to a recording chamber equipped with constant perfusion of ACSF (2 ml/min) (Cruz et al., 2004).
Neurons were visualized by infrared illumination on an Olympus fluorescent scope (BX51WI) and whole-cell patch-clamp recordings were made from neurons in the VTA, identified as the region medial to the medial terminal nucleus of the accessory optical tract (Cruz et al., 2004; Labouèbe et al., 2007; Munoz and Slesinger, 2014). DA neurons were identified by presence of a large hyperpolarization-activated current (Ih), large capacitance (30–50 pF), and slow spontaneous firing (1–3 Hz). In some experiments, DA neurons were identified by GFP expression in Pitx3+/−-GFP mice (Zhao et al., 2004), as indicated. No significant differences in firing, capacitance or cell size were observed in DA neurons from Pitx3+/−-GFP mice (Labouèbe et al., 2007). GABA neurons were identified by the absence of Ih, a small capacitance (<20 pF), and a fast spontaneous firing rate (5–10 Hz) (Cruz et al., 2004).
The internal solution contained the following (in mm): K-gluconate 140, NaCl 4, MgCl2 2, EGTA 1.1, HEPES 5, Na2ATP 2, Na creatine phosphate 5, and Na3GTP 0.6, pH 7.3 with KOH. Whole-cell voltage-clamp recordings were used to measure GIRK currents. For agonist-induced currents, changes in holding currents (Vm = −35 mV with junction potential −15.7 mV; Vhold = −50 mV) in response to bath application of a saturating dose of baclofen (300 μm) or quinpirole (30 μm) were measured every 10 s. Currents were amplified (Molecular Devices Axopatch 200B), filtered at 1 kHz, and digitized at 5 kHz (Molecular Devices Digidata 1320). Data were acquired and analyzed with Molecular Devices Clampex 9.0 software. Ih current was monitored through a series of hyperpolarizing 200 ms voltage steps to −120 mV. Series resistance (Rs) was monitored throughout the experiment and recordings were excluded from analysis if the Rs varied by >20%. To calculate spike number, the number of spikes elicited by a 500 ms current injection step was measured. Baclofen inhibition was calculated by measuring the spike number in the presence of baclofen divided by the spike number in the absence of baclofen plus the spike number in the presence of baclofen. GIRK basal currents were inhibited with bath-applied 1 mm BaCl2 in ACSF. For the GTPγS experiment, guanosine 5′-O-[gamma-thio]triphosphate (GTPγS; Sigma-Aldrich) at 0.1 μm was substituted for Na3GTP in the intracellular solution.
Statistical analysis.
Data were analyzed using Prism 5.0 software. All reported values are mean ± SEM. One or two-way ANOVA with Bonferroni post hoc test was used for multiple comparisons; Student's unpaired t test was used for groups of two, as indicated. Statistical significance was defined as p < 0.05.
Results
Context pairing enhances METH-dependent decrease in IBaclofen
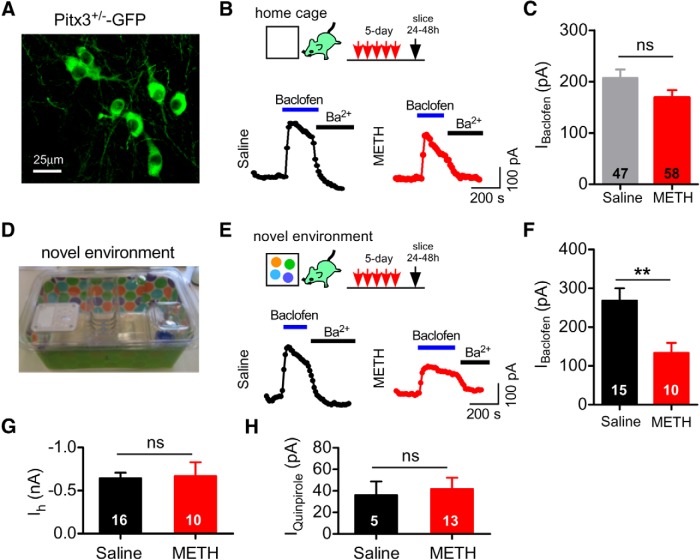
To investigate the mechanism underlying the psychostimulant-dependent decrease in IBaclofen in VTA DA neurons (Arora et al., 2011; Sharpe et al., 2015), we examined the effect of repeated METH injections on the maximally activated IBaclofen in VTA DA neurons. Male and female mice (P30–P50) were injected once daily with 2 mg/kg METH intraperitoneally for 5 d in the home cage and then brain slices containing the VTA were prepared 24–48 h after the fifth injection for whole-cell patch-clamp electrophysiology. DA neurons were identified based on the presence of an Ih current of ∼600 pA at −120 mV (Cruz et al., 2004; Labouèbe et al., 2007; Padgett et al., 2012). VTA DA neurons are heterogeneous, with the Ih-expressing VTA DA neurons primarily projecting to the NAc (Lammel et al., 2008). In addition to Ih, we used Pitx3+/−-GFP mice, in which GFP is expressed in DA neurons (Zhao et al., 2004), to further confirm the identification of VTA DA neurons (Labouèbe et al., 2007; Padgett et al., 2012; Bocklisch et al., 2013) (Fig. 1A). Bath application of a maximal concentration of (±)-baclofen (300 μm) (Cruz et al., 2004) elicited an outward current that peaked within ∼30 s and decayed with time, as described previously (Cruz et al., 2004). This outward current was largely absent in mice lacking GIRK2 subunits, indicating that it is GIRK dependent (Labouèbe et al., 2007). IBaclofen was inhibited by bath application of Ba2+(Fig. 1B), as expected for an inwardly rectifying K+ channel (Lüscher and Slesinger, 2010). Surprisingly, IBaclofen in VTA DA neurons of 5 d METH mice did not reach statistical significance from IBaclofen in 5 d saline mice (Fig. 1C, p > 0.05, Student's t test).
Figure 1.
Attenuation of IBaclofen in VTA DA neurons after 5 d METH injections in a novel environment. A, Live fluorescent image of GFP+ DA neurons in the VTA of a Pitx3+/−-GFP mouse. B, Examples of maximal (300 μm) IBaclofen recorded from VTA DA neurons 24–48 h after 5 d of intraperitoneal injections of either saline (0.9%) or METH (2 mg/kg in 0.9% saline) in the home cage. Application of 1 mm BaCl2 inhibits IBaclofen. C, Mean IBaclofen recorded 24–48 h after 5 d saline or 5 d METH injected in the home cage (ns, p > 0.05 Student's t test). D, Photograph of the novel cage environment with different bedding and brightly colored paper. E, Examples of IBaclofen recorded from VTA DA neurons 24–48 h after 5 d injections of saline or METH in a novel environment. F, Mean IBaclofen in VTA DA neurons 24–48 h after 5 d injections of METH or saline in a novel environment (**p < 0.01, Student's t test). G, No difference in mean Ih between 5 d saline- or METH-injected mice. H, No change in mean IQuinpirole (30 μm) with 5 d METH compared with 5 d saline in a novel environment.
Although sensitization of the locomotor-stimulant effects of psychostimulants can occur with repeated administration in the home cage, it is more robust when the drug is administered in a separate, novel environment (Badiani and Robinson, 2004). We therefore investigated whether altering the environment (i.e., context) for the METH injection affected the drug-dependent modulation of IBaclofen. To enhance the novelty cues, we used a new cage that was wrapped in brightly colored wallpaper and contained different flooring material (Fig. 1D). Mice were also injected in a room separate from the home cage. With this novel cage environment, 5 d METH administration triggered a significant decrease in IBaclofen in VTA DA neurons (Fig. 1E,F). Conversely, the amplitude of the Ih current was unaffected by METH administration (Fig. 1G).
In addition to GABABR, stimulation of DA D2-like receptors (D2Rs) in VTA DA neurons also activates GIRK channels (Beckstead et al., 2004; Cruz et al., 2004), providing a mechanism for auto-inhibition after DA release (Beckstead et al., 2004). Bath application of a maximal concentration of the D2R agonist quinpirole (30 μm) elicited an outward GIRK current, IQuinpirole (Cruz et al., 2004; Arora et al., 2011). In contrast to IBaclofen, IQuinpirole in the 5 d METH group was not statistically different from that in the 5 d saline treatment group (Fig. 1H).
We next investigated whether the decrease in IBaclofen was sufficient to alter the GABABR-dependent inhibition of firing. In current-clamp mode, a series of increasing amplitude current injections elicited one or more spikes, which were suppressed by exposure to baclofen (300 μm) (Fig. 2A,C). In saline-treated mice, VTA DA neurons typically required ∼180 pA of current injection to induce firing in the presence of baclofen (300 μm). In VTA DA neurons from METH-treated mice, however, smaller current injections (e.g., ∼80 pA) were sufficient to induce firing in the presence of baclofen (Fig. 2A,D), suggesting that there was less inhibition with baclofen. To examine this more closely, we calculated the percentage inhibition of firing with baclofen and determined that there was a significant decrease in baclofen-dependent inhibition of VTA DA neuron firing in 5 d METH-treated mice (Fig. 2E). For control, we examined the input–output relationship for VTA DA neurons in control mice (TH-Cre, n = 6) and found no decrement in spiking over 3 min of recording (Fig. 2F). Together, these results suggest that there is a reduction in GABABR-dependent inhibition of firing after 5 d of METH treatment.
A decrease in IBaclofen after 5 d of METH treatment could result from reduced surface expression of GIRK channels. To investigate this possibility, we interrogated indirectly the levels of GIRK channels on the plasma membrane by examining the effect of direct G-protein activation of GIRK channels. To accomplish this, we included in the intracellular solution the nonhydrolyzable GTP analog GTPγS (0.1 μm), which constitutively activates endogenous G-proteins in the absence of receptor activation (Logothetis et al., 1987). In these experiments, GTPγS activates G-proteins slowly upon formation of the whole-cell recording, with a rate of activation that takes minutes in the absence of receptor stimulation. In the 5 d saline injection control mice, GTPγS induced an outward current in VTA DA neurons that was subsequently inhibited by bath application of 1 mm Ba2+ (Ba2+-sensitive current; Fig. 3A). We measured maximal activation with GTPγS by subtracting the initial basal current from the peak GTPγS-activated current. In the 5 d METH group, the GTPγS-activated current in VTA DA neurons was significantly smaller than in the 5 d saline group (Fig. 3B). We also analyzed the amplitude of Ba2+-inhibited current following GTPγS-dependent activation and found that this current was also significantly reduced in the 5 d METH group. Together, these results suggest that 5 d of METH exposure decreases the expression of GIRK channels on the plasma membrane, similar to the effect of 5 d of cocaine treatment (Arora et al., 2011).
Figure 3.
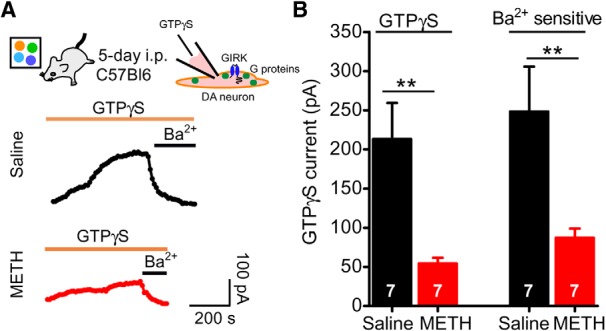
Effect of 5 d METH injections on the direct G-protein activation of GIRK channels in VTA DA neurons. A, Examples of outward currents induced by 100 μm GTPγS in the recording pipette from mice injected for 5 d saline or 5 d METH in a novel environment. Outward currents are inhibited with extracellular barium (Ba2+), consistent with G-protein activation of GIRK channels. B, Bar graph showing significantly smaller mean GTPγS-evoked and Ba2+-sensitive currents in 5 d METH-injected mice compared with 5 d saline (**p < 0.01 Student's unpaired t test).
METH-induced decrease in IBaclofen requires D1R-like and D2R-like DA receptor activation
Psychostimulants are powerful enhancers of DA signaling, elevating extracellular DA by blocking reuptake through the DA transporter or inducing the release of DA (Sulzer, 2011). DA stimulates two classes of DA receptors, D1R-like and D2R-like (Yager et al., 2015). Activation of both D1R-like and D2R-like receptors is involved in the locomotor and sensitization response to psychostimulants (Kalivas and Stewart, 1991; Xu et al., 1994; Kelly et al., 2008; Hikida et al., 2010). We therefore investigated the requirement of both D1R-like and D2R-like signaling in the METH-dependent attenuation of GABAB-GIRK currents in VTA DA neurons. In Pitx3+/−-GFP mice, coadministration of the D1R antagonist SCH39166 (0.3 mg/kg, i.p.) with METH (2 mg/kg) for 5 d in a novel environment completely abrogated the decrease in IBaclofen (Fig. 4A,B). Maximally activated IBaclofen was indistinguishable from saline-injected mice (Fig. 4B). Similarly, coadministration of the D2R-like antagonist eticlopride (0.1 mg/kg, i.p.) with METH (2 mg/kg) for 5 d also prevented the decrease in IBaclofen (Fig. 4B). These results indicate that METH-dependent attenuation of IBaclofen in VTA DA neurons requires activation of both D1- and D2-like receptors.
Figure 4.
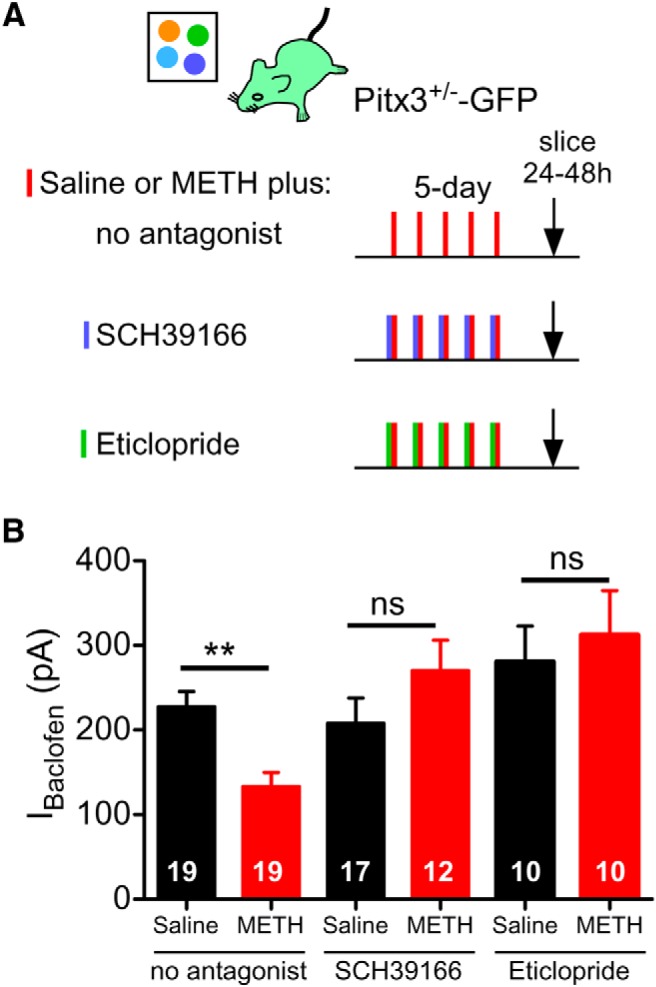
METH-induced decrease in IBaclofen requires activation of D1-like and D2-like receptors. A, Protocols for examining the effect of D1R-like or D2R-like receptor antagonists coinjected with METH or saline in Pitx3+/−-GFP mice. B, Mean IBaclofen (300 μm) is reduced in VTA DA neurons 24–48 h after 5 d injections of METH (2 mg/kg) compared with saline (0.9%). Coinjection of METH with the D1R-like antagonist SCH39166 (0.3 mg/kg) or METH with the D2R-like antagonist eticlopride (0.1 mg/kg) prevents METH-dependent decrease in IBaclofen (**p < 0.01 two-way ANOVA with Bonferroni post hoc test.).
Subcellular mechanism underlying METH-dependent attenuation of IBaclofen in VTA DA neurons
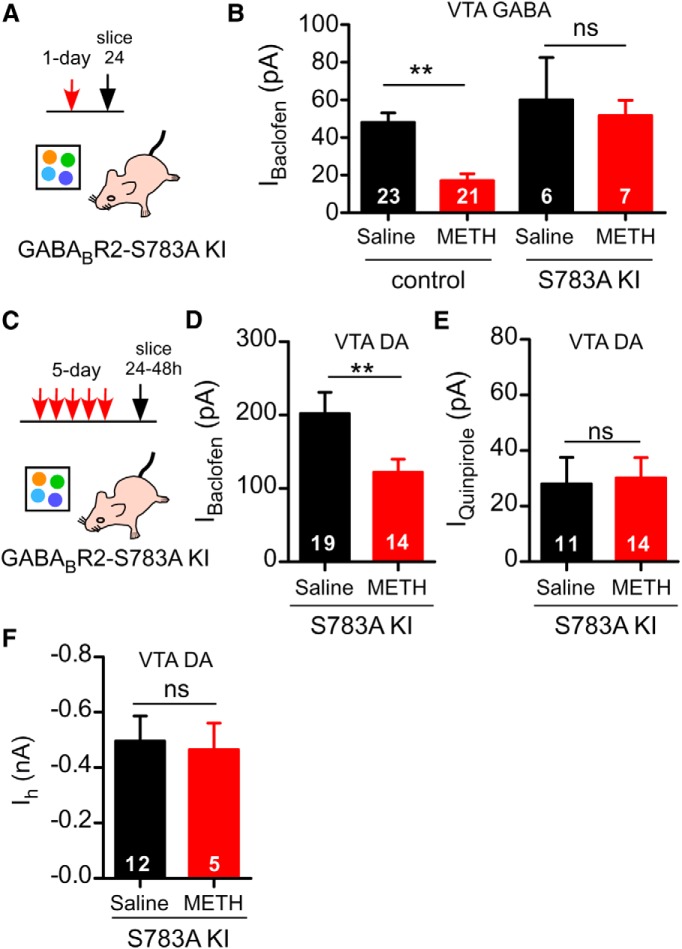
In VTA GABA neurons (Padgett et al., 2012) and mPFC pyramidal neurons (Hearing et al., 2013), psychostimulant treatment can lead to internalization of GABAB receptors through dephosphorylation of serine-783 (S783) on the GABABR2 subunit. Terunuma et al. (2014) demonstrated that neurons derived from a KI mouse engineered with the GABABR2-S783A mutant are insensitive to modulation by PP2A dephosphorylation. We used the GABABR2-S783A KI mouse to probe the mechanism underlying the METH-dependent decrease in IBaclofen in VTA DA neurons. For control, we first confirmed that GABABR2-S783A KI mice are resistant to the PP2A-dependent decrease in GABAB receptor surface expression. We examined the effect of a 1 d of METH treatment (Fig. 5A); that is, 24 h after a single 2 mg/kg injection, on IBaclofen in VTA GABA neurons. This attenuation of IBaclofen involves dephosphorylation of S783 on the GABAB R2 subunit (Padgett et al., 2012). GABA neurons were identified by the absence of Ih, a small capacitance (<20 pF) and a fast spontaneous firing rate (5–10 Hz) (Cruz et al., 2004). As expected, the 1 d METH treatment did not significantly decrease IBaclofen in VTA GABA neurons of GABABR2 S783A mice, in contrast to the decrease in littermate controls (Fig. 5B).
Figure 5.
Lack of role for dephosphorylation of GABABR2–S783 in METH-dependent decrease in IBaclofen in VTA DA neurons. A, Protocol for VTA GABA neurons. Single injection of METH in GABABR2–S783A KI mice. B, Mean IBaclofen (100 μm) in VTA GABA neurons is reduced 24 h after a single injection of METH (2 mg/kg, n = 21) compared with saline (0.9%, n = 23) (Padgett et al., 2012) in littermate controls. **p < 0.01 two-way ANOVA with Bonferroni post hoc test. In GABABR2–S783A KI mice, there was no decrement in IBaclofen after 1 d METH (n = 6 saline, n = 7 METH). C, Protocol for VTA DA neurons after 5 d injections of METH or saline in GABABR2–S783A KI mice. D, Mean IBaclofen (300 μm) is significantly reduced (24–48 h) in VTA DA neurons of GABABR2–S783A KI receiving 5 d METH (2 mg/kg, n = 14) compared with 5 d saline (0.9%, n = 19). **p < 0.05 unpaired t test. E, Mean IQuinpirole (30 μm) in VTA DA neurons from GABABR2–S783A KI mice with 5 d METH or 5 d saline treatments. F, Mean Ih in VTA DA neurons from GABABR2–S783A KI mice with 5 d METH or 5 d saline treatments.
We next investigated the effect of 5 d METH injections on IBaclofen in VTA DA neurons from GABABR2-S783A KI mice (Fig. 5C). Surprisingly, the 5 d METH treatment in a novel environment decreased IBaclofen in VTA DA neurons of GABABR2-S783A KI mice, similar to that in control Pitx3+/−-GFP mice (Figs. 1F, 5D). Similarly, IQuinpirole in GABABR2-S783A KI mice was not significantly different between the 5 d saline and the 5 d METH groups (Fig. 5E). Further, there was no significant difference in the Ih current of the 5 d METH or 5 d saline GABABR2-S783A KI mice (Fig. 5F). The finding that the METH treatment reduces IBaclofen in VTA DA neurons, but not in VTA GABA neurons of GABABR2-S783A KI mice, suggests that the regulation of GABAB receptors and GIRK channels in VTA DA neurons with psychostimulants involves a subcellular mechanism that is different from the PP2A mechanism in VTA GABA neurons.
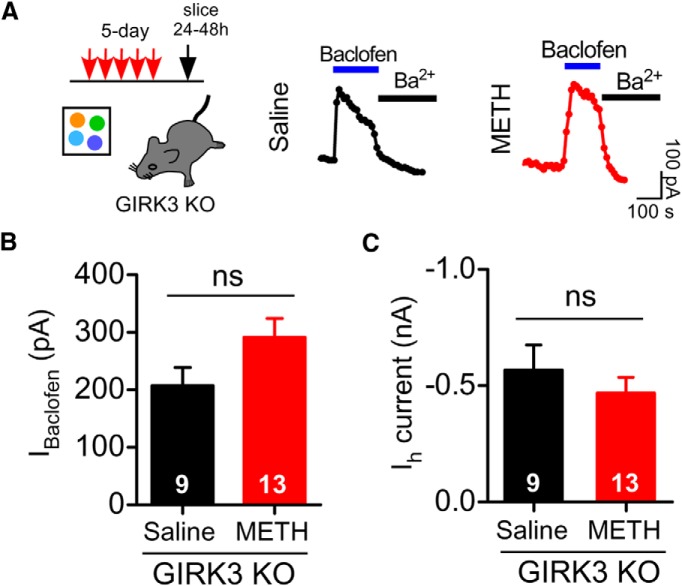
Because dephosphorylation of the GABABR did not appear to be involved, we next investigated the role of the GIRK3 subunit. In contrast to VTA GABA neurons that express GIRK1, GIRK2c, and GIRK3 subunits, VTA DA neurons express only GIRK2c and GIRK3 subunits (Cruz et al., 2004). GIRK2c/GIRK3 channels also have unique properties, including reduced Gβγ sensitivity (Jelacic et al., 2000), reduced coupling efficiency with GABABRs (Cruz et al., 2004), and the presence of a PDZ-binding motif that associates with SNX27 (Lunn et al., 2007; Munoz and Slesinger, 2014). In addition, the GIRK3 subunit regulates plasma membrane expression of GIRK channels in cell lines (Ma et al., 2002) and in neurons (Lalive et al., 2014; Munoz and Slesinger, 2014). To study the role of the GIRK3 subunit, we examined the effect of 5 d METH injections in mice lacking the GIRK3 subunit (i.e., GIRK3 KO mice) (Torrecilla et al., 2002; Morgan et al., 2003). In contrast to Pitx3+/−-GFP (Fig. 1F) or GABABR2-S783A KI (Fig. 5D) mice, 5 d METH injections in a novel environment did not attenuate IBaclofen in VTA DA neurons of GIRK3 KO mice (Fig. 6B). Ih in VTA DA neurons of GIRK3 KO mice was indistinguishable between 5 d saline and 5 d METH (Fig. 6C). Therefore, the GIRK3 subunit, but not dephosphorylation of S783 in GABABR2, appears to be important for the 5 d METH-dependent decease in IBaclofen.
Figure 6.
Requirement for the GIRK3 subunit in METH-dependent decrease in IBaclofen. A, Protocol shown for GIRK3 KO mice: 5 d injections of METH or saline. Examples of IBaclofen (300 μm) recorded from VTA DA neurons 24–48 h after 5 d intraperitoneal injections of saline (0.9%) or METH (2 mg/kg) in GIRK3 KO mice. B, No significant decrement in mean IBaclofen in VTA DA neurons from GIRK3 KO mice receiving 5 d METH injection (n = 13) compared with 5 d saline injection mice (n = 9) (ns, p > 0.05 unpaired t test). C, Ih in VTA DA neurons after 5 d METH or 5 d saline in GIRK3 KO mice.
Discussion
Repeated exposure to drugs of abuse induces changes in the neurocircuitry of the reward pathway that underlie addiction (Koob and Volkow, 2010; Lüscher and Malenka, 2011). In the current study, we demonstrate a significant attenuation of IBaclofen in VTA DA neurons 24–48 h after repeated 5 d METH injections. This decrease in IBaclofen was enhanced when METH injections were delivered in a novel environment. Similarly, Arora et al. (2011) found that 5 d of cocaine injections outside of the home cage also produced a smaller IBaclofen in VTA DA neurons (Arora et al., 2011). Consistent with a context-dependent effect, sensitization of the locomotor-stimulant effects of psychostimulants is more robust when the drug is administered in a novel environment away from the home cage (Badiani and Robinson, 2004). Further, high-novelty-seeking rodents acquire sensitization and self-administration more quickly to psychostimulants (Hooks et al., 1991; Blanchard et al., 2009). A direct VTA DA projection to the hippocampus is postulated to be important for regulating the long-term memory associated with novel events; these hippocampal pyramidal neurons project back to the VTA indirectly via the nucleus accumbens (NAc) and ventral pallidum (Lisman and Grace, 2005; Kafkas and Montaldi, 2015). Our results with the novel environment suggest that hippocampal-mediated inputs into the VTA could play an important role in modulating GABABR-GIRK signaling in VTA DA neurons.
Previous studies demonstrated that repeated exposure to cocaine (Arora et al., 2011) or self-administration of METH (Sharpe et al., 2015) reduces IBaclofen in VTA DA neurons, similar to our results with 5 d METH in a novel environment. However, the mechanism underlying the attenuation was not fully elucidated in those studies. In mPFC pyramidal neurons (Hearing et al., 2013) and in VTA GABA neurons (Padgett et al., 2012), cocaine and METH exposure decrease IBaclofen through dephosphorylation of S783 in the GABABR2 subunit. The phosphorylation and dephosphorylation of S783 controls the postendocytic sorting of the GABABR1/R2 heterodimer (Terunuma et al., 2010). In cortical and hippocampal neurons, NMDAR-dependent attenuation of GABABR signaling occurs through PP2a-dependent dephosphorylation of S783, which can be abrogated by mutating S783 to alanine (S783A) in the GABABR2 subunit (Terunuma et al., 2014). Consistent with that mechanism, we found that GABABR2-S783A KI mice were resistant to the 1 d METH-dependent decrease in IBaclofen in VTA GABA neurons. In VTA DA neurons of GABABR2-S783A KI mice, however, 5 d METH continued to decrease IBaclofen. Conversely, in mice lacking the GIRK3 subunit, 5 d METH did not trigger a decrease in IBaclofen, in contrast to the ∼50% decrease observed in METH-treated Pitx3+/−-GFP or GABABR2-S783A mice. These results suggest the subcellular mechanisms underlying the METH-dependent modulation of IBaclofen are different for VTA DA and VTA GABA neurons.
How could the GIRK3 subunit in VTA DA neurons mediate the METH-dependent decrease in IBaclofen? A simple explanation for a decrease in IBaclofen is a shift in the EC50 for baclofen activation. In fact, the EC50 for baclofen is shifted to lower concentrations in GIRK3 KO mice (Labouèbe et al., 2007). However, all measurements of IBaclofen in the current study were made with a saturating concentration of baclofen. Another possibility is that the GIRK3 subunit contributes to a change in trafficking of GIRK channels. GIRK3 possesses a strong lysosomal targeting sequence, for example, YWSI, and, when in complex with other GIRK subunits, GIRK3 reduces maximal currents (Ma et al., 2002). Therefore, trafficking of GIRK channels could be impaired in VTA DA neurons of GIRK3 KO mice, making them resistant to the METH-dependent decrease in IBaclofen. The reduction in GTPγS-stimulated currents in VTA DA neurons of 5 d METH-treated mice is consistent with a defect in GIRK trafficking. Further supporting a role for GIRK3, the C-terminal domain of GIRK3 was implicated in activity-dependent potentiation of IBaclofen in VTA DA neurons (Lalive et al., 2014). Recently, Sharpe et al. (2015) demonstrated that chelating intracellular Ca2+ in VTA DA neurons enabled the recovery of IBaclofen in rats self-administering METH. It will be interesting to see whether changes in intracellular Ca2+ somehow affect GIRK3-dependent trafficking after drug exposure, especially considering that activity-dependent potentiation of IBaclofen requires NMDAR activation (Lalive et al., 2014).
Both GIRK2c and GIRK3 subunits possess a C-terminal PDZ-binding motif, which has been shown to interact with the PDZ domain of sorting nexin 27 (Lunn et al., 2007). SNX27 is a cytoplasmic trafficking protein that is localized to early endosomes and regulates IBaclofen in VTA DA neurons via a PDZ domain interaction (Cullen, 2008; Munoz and Slesinger, 2014). Further, a splice variant of SNX27, SNX27b, is upregulated in rodents after a 5 d METH treatment (Kajii et al., 2003). Together, these studies suggest that psychostimulant exposure may promote an interaction of SNX27 with the GIRK3 subunit, leading to reduced surface expression in VTA DA neurons and a smaller IBaclofen. Future experiments will need to determine whether a decrease in GIRK channel surface expression arises from an increase in channel degradation or a decrease in recycling back to the membrane. Supporting a modulatory role for the GIRK3 subunit, mice lacking the GIRK3 exhibit altered alcohol binge drinking (Herman et al., 2015), exhibit less severe withdrawal from alcohol and other sedatives (Kozell et al., 2009), and self-administer less cocaine than littermate controls (Morgan et al., 2003).
Psychostimulants increase extracellular DA by blocking reuptake or enhancing DA release (Sulzer, 2011), leading to activation of both D1R-like and D2R-like receptors on distinct populations of medium spiny neurons in the dorsal striatum and NAc (Yager et al., 2015). We found that coinjection of an antagonist for either D1R-like (SCH39166) or D2R-like (eticlopride) DA receptors prevented the 5 d METH-induced decrease in IBaclofen in VTA DA neurons. Similar results were found with METH-dependent decrease in IBaclofen in VTA GABA neurons (Padgett et al., 2012). Arora et al. (2011) reported that an antagonist for D2R-like, but not D1R-like, activation prevented acute cocaine-dependent attenuation of IBaclofen in VTA DA neurons. These differences in the involvement of D1R-like versus D2R-like receptors could depend on the time course of treatment (i.e., acute vs 5 d). In addition, METH and cocaine differ in their pharmacokinetics in that the actions of METH last longer than those of cocaine (Sulzer, 2011). Activation of D1R-like receptors is required for the locomotor response to psychostimulants (Xu et al., 1994; Kelly et al., 2008; Yager et al., 2015). However, both D1R-like and D2R-like receptors act in concert to influence acquisition of METH sensitization (Kelly et al., 2008) and, when impaired, attenuate psychostimulant-dependent sensitization (Hikida et al., 2010). Additional studies are needed to determine how stimulation of D1R-like and D2R-like pathways induces a change in GIRK channel expression in VTA DA neurons.
In contrast to the decrease in IBaclofen, we did not observe a METH-induced decrease in IQuinpirole, suggesting differential regulation of D2R- and GABABR-coupled GIRK channels. One possibility is that GIRK2 homotetramers associate with D2Rs, whereas GIRK2c/GIRK3 heterotetramers associate with GABABRs. However, Lacey et al. (1988) proposed that stimulation of D2R and GABABR in substantia nigra DA neurons converges on the same population of GIRK channels. Further, in previous studies, repeated exposure to cocaine or METH attenuated both GABABR and D2R-activated GIRK currents (Arora et al., 2011; Sharpe et al., 2015). It will be important to determine whether these differences are due to the psychostimulant exposure conditions or heterogeneity of DA neurons in the VTA.
What impact does attenuation of IBaclofen in VTA DA neurons have on the reward pathway? A reduction of IBaclofen would be expected to increase the excitability of VTA DA neurons. For example, mice lacking the β3 GABAA subunit in DA neurons have reduced inhibitory GABAA currents, which increases DA release in the NAc and enhances acquisition of appetitive learning (Parker et al., 2011). Repeated cocaine exposure downregulates GABAAR signaling, leading to enhanced LTP of excitatory synapses (Liu et al., 2005). For GIRK channels, mice lacking SNX27 in VTA DA neurons have a smaller IBaclofen and an enhanced locomotor response to cocaine (Munoz and Slesinger, 2014), suggesting hyperexcitability of VTA DA neurons. In parallel with changes of inhibitory inputs into VTA DA neurons, psychostimulant exposure also affects excitatory synaptic transmission (Lüscher and Malenka, 2011). Single or multiple exposure to drugs of abuse induces neuroadaptations that prime for compulsive behavior (Lüscher and Malenka, 2011). For example, a single exposure to cocaine produces plasticity changes at excitatory synapses in VTA DA neurons (Ungless et al., 2001; Borgland et al., 2004). Repeated administration leads to potentiation in excitatory transmission and is sufficient to induce downstream plasticity in the NAc (Zhang et al., 1997; Borgland et al., 2004; Bellone and Lüscher, 2006). The psychostimulant-dependent attenuation of inhibition may synergize with enhancement of excitatory transmission, driving dopaminergic activity in the VTA and changing the motivation for drug-seeking behaviors (Lüscher and Malenka, 2011).
Footnotes
This work was supported by the National Institute on Drug Abuse (Grants DA025236 and DA037170 to P.A.S., Grant DA034696 to K.W., and Ruth L. Kirschstein National Research Service Award F31 DA029401 to M.B.M.), the Salk Institute Chapman Foundation (M.B.M.), and the National Institute on Alcohol Abuse and Alcoholism (Grant AA018734 to P.A.S. and Grant AA020913 to C.C.). We thank M. Li for providing Pitx3-GFP mice, members of the Slesinger laboratory for technical help, Max Kreifeldt in C. Contet's laboratory for breeding and genotyping GIRK3 mice, and Gilles Speter for providing novel environment plastic cages for our studies.
The authors declare no competing financial interests.
References
- Arora D, Hearing M, Haluk DM, Mirkovic K, Fajardo-Serrano A, Wessendorf MW, Watanabe M, Luján R, Wickman K. Acute cocaine exposure weakens GABA(B) receptor-dependent G-protein-gated inwardly rectifying K+ signaling in dopamine neurons of the ventral tegmental area. J Neurosci. 2011;31:12251–12257. doi: 10.1523/JNEUROSCI.0494-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backes EN, Hemby SE. Contribution of ventral tegmental GABA receptors to cocaine self-administration in rats. Neurochem Res. 2008;33:459–467. doi: 10.1007/s11064-007-9454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Robinson TE. Drug-induced neurobehavioral plasticity: the role of environmental context. Behav Pharmacol. 2004;15:327–339. doi: 10.1097/00008877-200409000-00004. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Blanchard MM, Mendelsohn D, Stamp JA. The HR/LR model: Further evidence as an animal model of sensation seeking. Neurosci Biobehav Rev. 2009;33:1145–1154. doi: 10.1016/j.neubiorev.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Bocklisch C, Pascoli V, Wong JC, House DR, Yvon C, de Roo M, Tan KR, Lüscher C. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science. 2013;341:1521–1525. doi: 10.1126/science.1237059. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brebner K, Froestl W, Andrews M, Phelan R, Roberts DC. The GABA(B) agonist CGP 44532 decreases cocaine self-administration in rats: demonstration using a progressive ratio and a discrete trials procedure. Neuropharmacology. 1999;38:1797–1804. doi: 10.1016/S0028-3908(99)00094-5. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Wessendorf MW, Williams JT. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience. 1997;77:155–166. doi: 10.1016/S0306-4522(96)00444-7. [DOI] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Lüscher C. Bi-directional effects of GABAB receptor agonists on the mesolimbic dopamine system. Nat Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- Cullen PJ. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol. 2008;9:574–582. doi: 10.1038/nrm2427. [DOI] [PubMed] [Google Scholar]
- Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, Li X, Dietz DM, Pan N, Vialou VF, Neve RL, Yue Z, Han MH. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344:313–319. doi: 10.1126/science.1249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing M, Kotecki L, Marron Fernandez de Velasco E, Fajardo-Serrano A, Chung HJ, Luján R, Wickman K. Repeated cocaine weakens GABA(B)-Girk signaling in layer 5/6 pyramidal neurons in the prelimbic cortex. Neuron. 2013;80:159–170. doi: 10.1016/j.neuron.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Sidhu H, Stouffer DG, Kreifeldt M, Le D, Cates-Gatto C, Munoz MB, Roberts AJ, Parsons LH, Roberto M, Wickman K, Slesinger PA, Contet C. GIRK3 gates activation of the mesolimbic dopaminergic pathway by ethanol. Proc Natl Acad Sci U S A. 2015;112:7091–7096. doi: 10.1073/pnas.1416146112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Individual differences in locomotor activity and sensitization. Pharmacol Biochem Behav. 1991;38:467–470. doi: 10.1016/0091-3057(91)90308-O. [DOI] [PubMed] [Google Scholar]
- Jelacic TM, Kennedy ME, Wickman K, Clapham DE. Functional and biochemical evidence for G-protein-gated inwardly rectifying K+ (GIRK) channels composed of GIRK2 and GIRK3. J Biol Chem. 2000;275:36211–36216. doi: 10.1074/jbc.M007087200. [DOI] [PubMed] [Google Scholar]
- Kafkas A, Montaldi D. Striatal and midbrain connectivity with the hippocampus selectively boosts memory for contextual novelty. Hippocampus. 2015;25:1262–1273. doi: 10.1002/hipo.22434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajii Y, Muraoka S, Hiraoka S, Fujiyama K, Umino A, Nishikawa T. A developmentally regulated and psychostimulant-inducible novel rat gene mrt1 encoding PDZ-PX proteins isolated in the neocortex. Mol Psychiatry. 2003;8:434–444. doi: 10.1038/sj.mp.4001258. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-U. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Low MJ, Rubinstein M, Phillips TJ. Role of dopamine D1-like receptors in methamphetamine locomotor responses of D2 receptor knockout mice. Genes Brain Behav. 2008;7:568–577. doi: 10.1111/j.1601-183X.2008.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotecki L, Hearing M, McCall NM, Marron Fernandez de Velasco E, Pravetoni M, Arora D, Victoria NC, Munoz MB, Xia Z, Slesinger PA, Weaver CD, Wickman K. GIRK channels modulate opioid-induced motor activity in a cell type- and subunit-dependent manner. J Neurosci. 2015;35:7131–7142. doi: 10.1523/JNEUROSCI.5051-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozell LB, Walter NA, Milner LC, Wickman K, Buck KJ. Mapping a barbiturate withdrawal locus to a 0.44 Mb interval and analysis of a novel null mutant identify a role for Kcnj9 (GIRK3) in withdrawal from pentobarbital, zolpidem, and ethanol. J Neurosci. 2009;29:11662–11673. doi: 10.1523/JNEUROSCI.1413-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouèbe G, Lomazzi M, Cruz HG, Creton C, Luján R, Li M, Yanagawa Y, Obata K, Watanabe M, Wickman K, Boyer SB, Slesinger PA, Lüscher C. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci. 2007;10:1559–1568. doi: 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. On the potassium conductance increase activated by GABAB and dopamine D2 receptor in rat substantia nigra neurones. J Physiol. 1988;401:437–453. doi: 10.1113/jphysiol.1988.sp017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalive AL, Munoz MB, Bellone C, Slesinger PA, Lüscher C, Tan KR. Firing modes of dopamine neurons drive bidirectional GIRK channel plasticity. J Neurosci. 2014;34:5107–5114. doi: 10.1523/JNEUROSCI.5203-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lhuillier L, Mombereau C, Cryan JF, Kaupmann K. GABA(B) receptor-positive modulation decreases selective molecular and behavioral effects of cocaine. Neuropsychopharmacology. 2007;32:388–398. doi: 10.1038/sj.npp.1301102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The βγ subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Lunn ML, Nassirpour R, Arrabit C, Tan J, McLeod I, Arias CM, Sawchenko PE, Yates JR, 3rd, Slesinger PA. A unique sorting nexin regulates trafficking of potassium channels via a PDZ domain interaction. Nat Neurosci. 2007;10:1249–1259. doi: 10.1038/nn1953. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Zerangue N, Raab-Graham K, Fried SR, Jan YN, Jan LY. Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and heart. Neuron. 2002;33:715–729. doi: 10.1016/S0896-6273(02)00614-1. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AD, Carroll ME, Loth AK, Stoffel M, Wickman K. Decreased cocaine self-administration in Kir3 potassium channel subunit knockout mice. Neuropsychopharmacology. 2003;28:932–938. doi: 10.1038/sj.npp.1300100. [DOI] [PubMed] [Google Scholar]
- Munoz MB, Slesinger PA. Sorting nexin 27 regulation of G protein-gated inwardly rectifying K+ channels attenuates in vivo cocaine response. Neuron. 2014;82:659–669. doi: 10.1016/j.neuron.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett CL, Lalive AL, Tan KR, Terunuma M, Munoz MB, Pangalos MN, Martínez-Hernández J, Watanabe M, Moss SJ, Luján R, Lüscher C, Slesinger PA. Methamphetamine-evoked depression of GABA(B) receptor signaling in GABA neurons of the VTA. Neuron. 2012;73:978–989. doi: 10.1016/j.neuron.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JG, Wanat MJ, Soden ME, Ahmad K, Zweifel LS, Bamford NS, Palmiter RD. Attenuating GABA(A) receptor signaling in dopamine neurons selectively enhances reward learning and alters risk preference in mice. J Neurosci. 2011;31:17103–17112. doi: 10.1523/JNEUROSCI.1715-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/S0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Seutin V, Johnson SW, North RA. Effect of dopamine and baclofen on N-methyl-D-aspartate-induced burst firing in rat ventral tegmental neurons. Neuroscience. 1994;58:201–206. doi: 10.1016/0306-4522(94)90167-8. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Varela E, Bettinger L, Beckstead MJ. Methamphetamine self-administration in mice decreases GIRK channel-mediated currents in midbrain dopamine neurons. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu073. pii:pyu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Swanner LS, Beyer CE, Goldberg SR, Schindler CW. The GABAB agonist baclofen modifies cocaine self-administration in rats. Behav Pharmacol. 1998;9:195–206. [PubMed] [Google Scholar]
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M, Vargas KJ, Wilkins ME, Ramírez OA, Jaureguiberry-Bravo M, Pangalos MN, Smart TG, Moss SJ, Couve A. Prolonged activation of NMDA receptors promotes dephosphorylation and alters postendocytic sorting of GABAB receptors. Proc Natl Acad Sci U S A. 2010;107:13918–13923. doi: 10.1073/pnas.1000853107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M, Revilla-Sanchez R, Quadros IM, Deng Q, Deeb TZ, Lumb M, Sicinski P, Haydon PG, Pangalos MN, Moss SJ. Postsynaptic GABAB receptor activity regulates excitatory neuronal architecture and spatial memory. J Neurosci. 2014;34:804–816. doi: 10.1523/JNEUROSCI.3320-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecilla M, Marker CL, Cintora SC, Stoffel M, Williams JT, Wickman K. G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci. 2002;22:4328–4334. doi: 10.1523/JNEUROSCI.22-11-04328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Xu M, Hu XT, Cooper DC, Moratalla R, Graybiel AM, White FJ, Tonegawa S. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994;79:945–955. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Yager LM, Garcia AF, Wunsch AM, Ferguson SM. The ins and outs of the striatum: Role in drug addiction. Neuroscience. 2015;301:529–541. doi: 10.1016/j.neuroscience.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ, Wolf ME. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. J Pharmacol Exp Ther. 1997;281:699–706. [PubMed] [Google Scholar]
- Zhao S, Maxwell S, Jimenez-Beristain A, Vives J, Kuehner E, Zhao J, O'Brien C, de Felipe C, Semina E, Li M. Generation of embryonic stem cells and transgenic mice expressing green fluorescence protein in midbrain dopaminergic neurons. Eur J Neurosci. 2004;19:1133–1140. doi: 10.1111/j.1460-9568.2004.03206.x. [DOI] [PubMed] [Google Scholar]