Abstract
Introduction
One of the most important symptoms of Sjögren syndrome is xerostomia. The oral cavity deprived of saliva and its natural lubricative, protective and antibacterial properties is prone to a number of unfavourable consequences.
Aim
To present the most important lesions on the oral mucosa in primary and secondary Sjögren syndrome and in dry mouth syndrome.
Material and methods
The study group comprised 55 patients including 52 women and 3 men aged 20–72 years (average: 28.25 years).
Results
Basing on the accepted criteria, primary Sjögren syndrome was diagnosed in 22 (40%) patients, secondary Sjögren syndrome in 18 (32.7%) patients, and dry mouth syndrome in 15 (27.27%) patients. The physical examination and the examination of the mouth were performed and history was elicited from every patient.
Conclusions
The most common pathologies appearing on the oral mucosa in primary and secondary Sjögren syndrome are angular cheilitis, cheilitis, increased lip dryness as well as non-specific ulcerations, aphthae and aphthoid conditions.
Keywords: Sjögren syndrome, dry mouth syndrome
Introduction
Sjögren syndrome is a systemic autoimmune disease of unknown aetiology affecting 0.5–1% of the population. Its dominant manifestations are marked weakness, xerostomia, xerophthalmia, and arthritis. According to the criteria unified in 2002, its diagnosis may be made basing on the presence of at least 3 out of 4 groups of symptoms and signs given below (ophthalmic manifestations, oral manifestations, objective dry eye manifestations, and objective salivary gland injuries) as well as one histopathological or laboratory manifestation (group 5 and 6) (Table 1).
Table 1.
Diagnostic criteria of primary Sjögren syndrome
| 1. Ophthalmic manifestations (at least one symptom) | – Dryness of the eyes persisting for more than 3 months – Recurrent sandy-gritty eye irritation – The need to use artificial tears for more than 3 months |
| 2. Oral manifestations (at least one symptom) | – Everyday mouth dryness for more than 3 months – Recurrent swelling of the salivary glands – The need to sip fluids with swallowing |
| 3. Objective features of dry eye (at least one symptom) | – Schirmer test < 5 mm in 5 min – Rose Bengal dye > 4 |
| 4. Objective manifestations of salivary gland injuries (at least one symptom) | – Salivary gland scintigraphy – Sialography – Salivary secretion at rest < 1.5 ml in 15 min |
| 5. Histopathological features | – Lacrimal gland biopsy > 1 – Small salivary gland biopsy > 1 (more than 50 lymphocytes/4 mm2 glandular tissue) |
| 6. Laboratory tests | Anti-60-Kda Ro (SS-A) |
Sjögren syndrome occurs nine times more often in women than men. It is characterised by two relapses: one between 20 and 30 year of life and the other in the middle of the 5th decade of life. It may show as a primary disease without any other accompanying symptoms or as a secondary disease appearing simultaneously with other autoimmune diseases such as rheumatoid arthritis, lupus erythematosus, dermatomyelitis, scleroderma, and primary cirrhosis of the liver. Sjögren syndrome also shows a tendency towards fibromyalgia, migraines, hypothyroidism, Raynaud symptom, and lymphomas [1–12]. One of the most bothering symptoms of the syndrome is xerostomia. The oral cavity deprived of saliva and its natural lubricative, protective and antibacterial properties is prone to a number of unfavourable consequences. They may include exacerbation of diseases affecting hard dental tissues and periodontium as well as predisposition to opportunistic infections and pathologies of the oral mucosa.
Aim
The aim of the paper is to present the most important lesions on the oral mucosa in primary and secondary Sjögren syndrome and in dry mouth.
Material and methods
The study group comprised 55 patients including 52 wo-men and 3 men aged 20–72 years (average: 28.25 years). Basing on the accepted criteria, primary Sjögren syndrome was diagnosed in 22 (40%) patients, secondary Sjögren syndrome in 18 (32.7%) patients, and dry mouth syndrome in 15 (27.27%) patients. Duration of the disease ranged from 6 months to 27 years (average: 3.5 years). There were 6 cigarette smokers among the patients (10.9%).
Consent to conduct the research was obtained from the Bioethics Committee based at the Medical University in Poznan (consent number 211/13). The physical examination and the examination of the mouth were performed and history was elicited from every patient.
Results
The most frequent complaints as obtained from history were: dryness of the throat and nasal mucosa, dryness of conjunctivae, sandy-gritty eye irritation, dryness of the mouth in the morning and at night, frequent need to sip water, lip dryness, exfoliation, fissuring, predisposition to aphtae, ulcers, mouth sores, burning sensation in the mouth, sensation of regurgitation of stomach contents to the mouth.
The studied patients most often reported sensation of dryness in the mouth, getting worse at night and in the morning, the need to moisten the mouth with water many times a day. Oral dryness was accompanied by dryness of the throat and nasal mucosa. Sensation of dryness in the initial part of the alimentary tract made swallowing and bolus formation difficult. The patients also complained of distorted taste, dyspepsia and stomach contents regurgitation to the mouth. Half of the patients presented with dryness of the oral mucosa. It was dry, with no elasticity, easily adhering to fingers or a dental mirror. In only 2 (9.1%) female patients marked dryness was accompanied by bright red colour of the mucosa. Otherwise, the mucosa was properly coloured. Mucosal dryness was most evident buccally, least evident palatally and on the inner labial aspect. Additionally, some patients complained of burning sensation in the mouth. The second most frequent sign was angular cheilitis, cheilitis, including exfoliative cheilitis. The lips were fissured, exfoliative, bleeding easily. The patients reported the need to frequently moisten and lubricate the lips. In 5 female patients, non-specific ulceration or oral aphtae were observed. The majority of patients reported a tendency towards such lesions and complained of their chronic and long-lasting treatment. Ulcerations were chiefly present buccally and on the inner lip aspect. Denture and other removable appliance wearers reported an increased predisposition towards abrasions and sores under a denture base, though they did not have problems with denture retention. Geographic tongue was present in 3 (11.1%) female patients in 2, the tongue was bright red and fissured. One (4.5%) female patient reported non-specific symptoms of paresis affecting the inferior alveolar and mental nerves. In 2 (9.1%) female patients with primary Sjögren syndrome, enlargement of the salivary glands was noted. In one case these were the parotid glands, in the other – the submandibular ones (Table 2, Figures 1 and 2).
Table 2.
Oral mucosa lesions in patients with primary and secondary Sjögren syndrome and dry mouth
| Clinical signs | Primary Sjögren syndrome (22 patients, 40%) |
Secondary Sjögren syndrome (18 patients, 32.7%) | Dry mouth (15 patients, 27.3%) |
|---|---|---|---|
| Angular cheilitis | 4 (18.2%) | 4 (22.2%) | 1 (6.7%) |
| Exfoliative cheilitis | 4 (18.2%) | 3 (16.6%) | – |
| Non-specific ulcerations | 2 (9.1%) | 4 (22.2%) | 1 (6.7%) |
| Small aphtae | 3 (13.6%) | 2 (11.1%) | 1 (6.7%) |
| Sutton's aphtae | 1 (4.5%) | – | – |
| Geographic tongue | – | 2 (11.1%) | 1 (6.7%) |
| Fissured tongue | 1 (4.5%) | – | – |
| Enlargement of salivary glands | 2 (9.1%) | – | – |
| Paresthesias | 1 (4.5%) | – | – |
| Lip dryness | 14 (63.6%) | 9 (50%) | 3 (20%) |
| Generalised stomatitis | 1 (4.5%) | – | – |
| Throat and nasal mucosa dryness | 7 (31.88%) | 9 (50%) | 3 (20%) |
| Generalised reddening and dryness of oral mucosa | 2 (9.1%) | – | – |
| Paleness of oral mucosa | – | 2 (11.1%) | – |
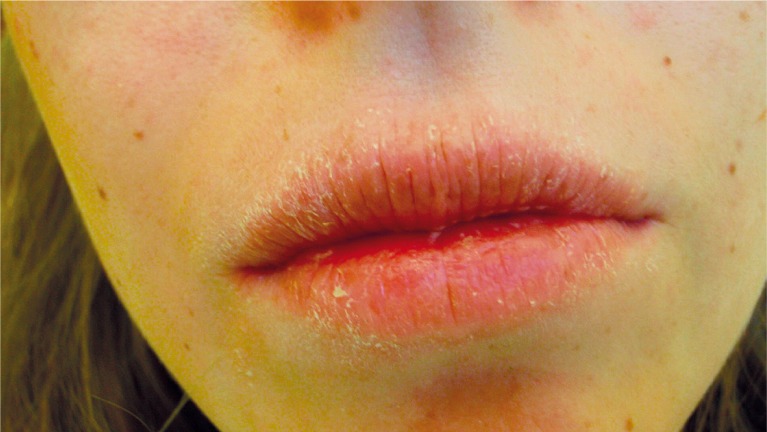
Figure 1.
Patient, 22 years. Primary Sjögren syndrome. Cheilitis
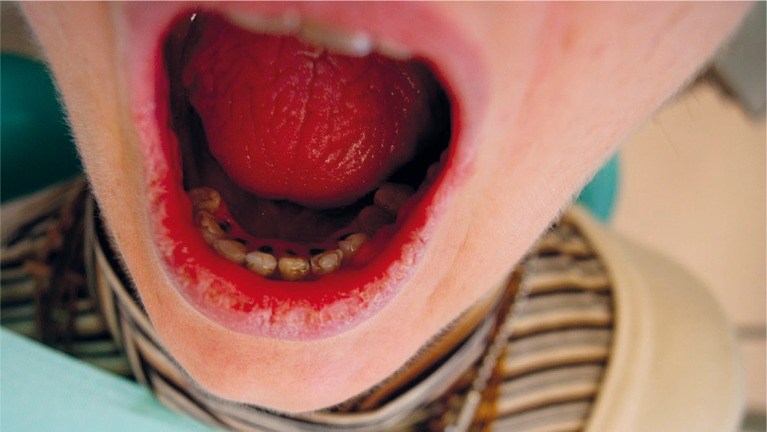
Figure 2.
Patient. Primary Sjögren syndrome. Cheilitis and glossitis. Stomatitis. Cervical caries
Discussion
Oral manifestations following xerostomia appear in more than half of the patients affected by primary Sjögren syndrome and are diagnosed the earliest. Exacerbation of xerostomia is directly proportional to the disease duration. The patients suffering from the disease for a shorter time have a higher level of stimulated saliva than those with a longer disease duration [13]. The oral cavity deprived of saliva with its lubricative, protective and antibacterial properties is exposed to a number of unfavourable factors.
With time, lactoferrin, β2-microglobulin, potassium and cystatin C concentration in saliva is growing while amylase and carbonic anhydrase concentration is dropping [14]. Decreased secretion of saliva, the loss of its buffer properties and lower concentration of saliva proteins such as histamine, mucin, IgA, proteins rich in proline and statherin increase the risk of opportunistic infections, mainly fungal infections by Candida albicans (C. albicans). Oral candidiasis may be asymptomatic or may show as fissured tongue, rhomboid mid-tongue, non-specific ulcerations, prosthetic stomatopathies, or generalised candidiasis. It most often takes the form of chronic candidiasis, less often of pseudodiphtheritic candidiasis. In Yan et al. studies, candidiasis was diagnosed in 87% of patients with primary Sjögren syndrome. In 42%, it was of a mixed character. Apart from C. albicans, other species were isolated, too, namely C. tropicalis, C. glabrata, and C. parapsilosis [15]. Candida albicans infection accompanies angular cheilitis and exfoliative cheilitis very often observed in Sjögren syndrome patients. Angular cheilitis may be due to fungal infection but may also be caused by staphylococcal infection or anaemia. Factors favouring angular cheilitis are immunosuppression, tobacco smoking, endocrine disorders, pharmacotherapy, old age, wearing removable prosthetic appliances, and prosthetic stomatopathies [16].
Exfoliative cheilitis is also present in leukemias and HIV infections. For a dermatologist, it is of crucial importance to elicit the history thoroughly and to connect patient's complaints such as sensation of dryness in the mouth, the need to sip water frequently, cracking and shedding of the lips with the lesions observed on the physical examination which are due to xerostomia.
In case dryness in the mouth is noted, it is indispensable to do mycological and bacteriological tests. Although candidiasis may be asymptomatic, it is more likely to appear when wearing removal prostheses with tongue discolourations, generalised reddening of the oral cavity, and angular stomatitis (splitting at the corners of the mouth). Antifungal prevention is performed with the application of chlorhexidine rinses and antifungal drugs presented as gels, suspensions and chewable tablets.
The sufferers should avoid alcohol consumption, tobacco smoking and alcohol-based mouth rinses which could promote xerostomia. Some antihistaminic, hypotensive, antidepressant drugs decrease saliva secretion and promote mouth dryness; it requires intensified symptomatic treatment or modification of pharmacological therapy used so far to minimise its negative effect on saliva secretion. In treatment of xerostomia, sialagogues especially anticholinergic: pilocarpine, cevimeline and bromhexine drugs are recommended. Efficacy of pilocarpine (5 mg three times a day) and cevimeline (30 mg three times a day) in secretion of saliva is similar but cevimeline has less side-effects particularly in long-lasting therapy [17, 18].
Apart from a decrease in salivary production, another factor predisposing to opportunistic infections is a higher concentration of microorganisms of the Lactobacillus acidophilus, Streptococcus mutans and Candida albicans species. Similarly, a high concentration of these microorganisms accompanies other causes of xerostomia. More cariogenic and acid-resistant plaque together with a disturbed process of mouth self-clearing favours the development of caries especially cervical caries (Figure 2) and periodontal diseases. A higher concentration of bacterial strains of Fusobacterium nucleatum and Prevotella intermedia, recognised as main periodontal diseases pathogens, are not observed in patients with Sjögren syndrome. Numerous independent studies have not confirmed any relationship between Sjögren syndrome and an increased likelihood of periodontal diseases [19, 20]. On the other hand such indices as probing depth (PD), gingival index (GI), plaque index (PI), clinical attachment level (CAL), bleeding on probing (BOP) are higher in Sjögren patients. Sjögren syndrome seemed to negatively affect the periodontal condition because gingival inflammation is more evident in the individuals with Sjögren syndrome particularly those with secondary Sjögren syndrome [21]. According to Scardina, periodontal disease can be caused by capillary alterations especially in gingival microcirculation [22]. A likely effect of anti-inflammatory, immunosuppressive and glucocorticoid drugs on an inflammatory response of periodontal tissues must also be considered. In our study, the patients did not complain of periodontal problems, what is reflected in the majority of publications (Table 2). Other frequent complaints are angular cheilitis, simple cheilitis (Figures 1 and 2), exfoliative cheilitis, lip cracking and dryness (Table 2). Apart from symptomatic treatment and minimising the dryness, application of lip sunscreen is of major importance.
In simple cheilitis dominant manifestations are bad lips exfoliation and cracking, their proneness to bleeding, periodic swelling and burning (Figure 1). The lesions are mostly limited to lip vermillion, less often labial mucosa or the facial skin around the vermillion is affected. Protective ointments containing lanoline, vitamin A or clobetasol provide an effective treatment. In exfoliative cheilitis thick brown keratin plaques are formed, too. Skin redness over the lip vermillion and swelling are more often observed. In the treatment of exfoliative cheilitis, protective ointments containing lactic acid or ointments and preparations with tacrolimus or steroids are applied [23]. Lowered buffering saliva properties and frequently concurrent gastro-oesophageal reflux lead to erosion and pathological teeth abrasion and may also promote formation of lesions on the mucosa. Many patients gave the history of frequent aphthae and non-specific ulcerations. They may be related to concurrent diseases, not necessarily to Sjögren syndrome. Lupus erythematosus predisposes to non-specific oral mucosa lesions and to lichen planus. Typical lupus lesions are whitish, round or discoid plaques with erythema inside and marked keratinisation around. Systemic scleroderma promotes oral mucosa fibromatosis and its paleness due to its improper vascularisation (Table 2). Anaemia and a low level of ferritin may, in turn, predispose to frequent aphthae and aphthoid lesions formation. Frequently in Sjögren syndrome, an enlargement or swelling of salivary glands may be observed, resembling lymphoma or an acute inflammation. In our studies we observed cases of enlargement and swelling of submandibular and parotid glands (Table 2). Lymphomas are not determined. Primary Sjögren syndrome is an autoimmune disease associated with an increased risk of lymphoma. Lymphomas complicating primary Sjögren syndrome are mostly low-grade B cell non-Hodgkin lymphomas. Lymphomas often develop in organs where primary Sjögren syndrome is active such as the salivary gland [24]. The increased cancer risk in the patients is mainly due to high inflammatory activity and severity of the disease rather than the immunosuppressive therapy [25]. In Sjögren patients B type lymphomas appear 44 times more often than in the unaffected population and in majority of cases suggest the disease progression [26]. One patient with primary Sjögren syndrome suffered from paraesthesia of the mental nerve (Table 2). In patients with Sjögren syndrome neurological manifestations may occur such as peripheral neuropathy and other forms of neuropathies including sensory ataxia, painful sensory neuropathy without sensory ataxia, multiple mononeuropathy, multiple cranial neuropathy, autonomic neuropathy, radiculoneuropathy and intra- and extraoral paresthesias, facial hypaesthesia and trigeminal nerve neuropathy [27]. Screening for Sjögren syndrome should be systematically performed in cases of acute or chronic myelopathy, axonal neuropathy or cranial nerve involvement [28].
Conclusions
The most common pathologies appearing on the oral mucosa in primary and secondary Sjögren syndrome are angular cheilitis, cheilitis, increased lip dryness as well as non-specific ulcerations, aphthae and aphthoid conditions. The crucial issue in suspected Sjögren syndrome is to perform mycological and bacteriological diagnostics, treat mycoses and other opportunistic infections, and implement antifungal prophylaxis.
Patients with increased oral dryness are advised to apply artificial saliva or anticholinergic drugs. In exfoliative cheilitis sunscreen protection is necessary to be applied on the lips together with oiling, lubricating and soothing preparations. Regular basic laboratory tests such as whole blood test, blood film, iron and ferritin levels are required, too.
Conflict of interest
The authors declare no conflict of interest
References
- 1.Carr AJ, Ng WF, Figueiredo F, et al. Sjögren's syndrome – an update for dental practitioners. Br Dental J. 2012;7:353–57. doi: 10.1038/sj.bdj.2012.890. [DOI] [PubMed] [Google Scholar]
- 2.Fox RI, Liu AY. Sjögren's syndrome in dermatology. Clin Dermatol. 2006;24:393–413. doi: 10.1016/j.clindermatol.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Fox RI. Sjögren's syndrome. Lancet. 2005;366:321–31. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 4.Steinfeld S, Simonart T. New approaches to the treatment of Sjögren's syndrome: soon beyond symptomatic relief? Dermatology. 2003;207:6–9. doi: 10.1159/000070933. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez S, Sung H, Sepulveda D, et al. Oral manifestations and their treatment in Sjögren's syndrome. Oral Dis. 2014;20:153–61. doi: 10.1111/odi.12105. [DOI] [PubMed] [Google Scholar]
- 6.Mays J, Sarmadi M, Moutsopoulos NM. Oral manifestations of systemic autoimmune and inflammatory diseases: diagnosis and clinical management. J Evid Base Dent Pract. 2012;12:265–82. doi: 10.1016/S1532-3382(12)70051-9. [DOI] [PubMed] [Google Scholar]
- 7.Hernanández-Molina G, Ávila-Casado C, Cárdenas-Velázquez F, et al. Similarities and differences between primary and secondary Sjögren's syndrome. J Reumatol. 2009;37:800–8. doi: 10.3899/jrheum.090866. [DOI] [PubMed] [Google Scholar]
- 8.Mavragani CP, Moutsopoulos HM. Conventional therapy of Sjögren's syndrome. Clinic Rev Allerg Immunol. 2007;32:284–91. doi: 10.1007/s12016-007-8008-3. [DOI] [PubMed] [Google Scholar]
- 9.Soy M, Piskin S. Cutaneous findings in patients with primary Sjögren's syndrome. Clin Rheumatol. 2007;26:1350–0. doi: 10.1007/s10067-006-0374-3. [DOI] [PubMed] [Google Scholar]
- 10.Isaksen K, Jonsson R, Omdal R. Anti-CD20 treatment in primary Sjögren's syndrome. J Immunol. 2008;68:554–64. doi: 10.1111/j.1365-3083.2008.02185.x. [DOI] [PubMed] [Google Scholar]
- 11.Marqaix-Muńoz M, Bagán JV, Poveda R, et al. Sjögren syndrome of the oral cavity. Review and update. Med Oral Patol Oral Cir Bucal. 2009;14:325–30. [PubMed] [Google Scholar]
- 12.Gilboe IM, Kvien TK, Uhliq T, Husby G. Sicca symptoms and secondary Sjögren's syndrome in systemic lupus erythematosus: comparison with rheumatoid arthritis and correlation with disease variables. Ann Rheum Dis. 2001;60:1103–9. doi: 10.1136/ard.60.12.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pijpl J, Kalk WW, Bootsman H, et al. Progression of salivary gland dysfunction in patients with Sjögren syndrome. Ann Rheum Dis. 2007;66:107–12. doi: 10.1136/ard.2006.052647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathews SA, Kurien BT, Scofield RH. Oral manifestation of Sjögren syndrome. J Dent Res. 2008;87:308–18. doi: 10.1177/154405910808700411. [DOI] [PubMed] [Google Scholar]
- 15.Yan Z, Young AL, Hua H, Xu Y. Multiple oral Candida infections in patients with Sjögren syndrome – prevalence and clinical and drug susceptibility profiles. J Rheumatol. 2011;38:2428–31. doi: 10.3899/jrheum.100819. [DOI] [PubMed] [Google Scholar]
- 16.Sharon V, Fazel N. Oral candidiasis and angular cheilitis. Dermatol Ther. 2010;23:230–41. doi: 10.1111/j.1529-8019.2010.01320.x. [DOI] [PubMed] [Google Scholar]
- 17.Noaiseh G, Baker JF, Vivino FB. Comparison of the discontinuation rates and side-effect profiles of pilocarpine and cevimeline for xerostomia in primary Sjögren syndrome. Clin Exp Rheumatol. 2014;32:575–7. [PubMed] [Google Scholar]
- 18.Brimhall J, Jhaveri MA, Yepes JF. Efficacy of cevimeline vs pilocarpine in secretion of saliva: a pilot study. Spec Care Dentist. 2013;33:123–7. doi: 10.1111/scd.12010. [DOI] [PubMed] [Google Scholar]
- 19.Schiødt M, Christensen LB, Petersen PE, Thorn II. Periodontal disease in primary Sjögren's syndrome. Oral Dis. 2001;7:106–8. [PubMed] [Google Scholar]
- 20.Kuru B, McCullough MJ, Yilmaz S, Porter SR. Clinical and microbiological studies of periodontal disease in Sjögren syndrome patients. J Clin Periodontol. 2002;29:92–102. doi: 10.1034/j.1600-051x.2002.290202.x. [DOI] [PubMed] [Google Scholar]
- 21.Antoniazzi RP, Miranda LA, Zanatta FB, et al. Periodontal conditions of individuals with Sjögren syndrome. J Periodontol. 2009;80:429–35. doi: 10.1902/jop.2009.080350. [DOI] [PubMed] [Google Scholar]
- 22.Scardina GA, Ruggieri A, Messina P. Periodontal disease and Sjögren syndrome: a possible correlation? Angiology. 2010;61:289–93. doi: 10.1177/0003319709344576. [DOI] [PubMed] [Google Scholar]
- 23.Connolly M, Kennedy C. Exfoliative cheilitis successfully treated with topical tacrolimus. Br J Dermatol. 2004;151:241–2. doi: 10.1111/j.1365-2133.2004.06043.x. [DOI] [PubMed] [Google Scholar]
- 24.Nocturne G, Mariette X. Sjögren syndrome-associated lymphomas: an update on pathogenesis and management. Br J Haematol. 2015;168:317–27. doi: 10.1111/bjh.13192. [DOI] [PubMed] [Google Scholar]
- 25.Boussios S, Pentheroudakis G, Somarakis G, et al. Cancer diagnosis in a cohort of patients with Sjögren's syndrome and rheumatoid arthritis: a single-center experience and review of the literature. Anticancer Res. 2014;34:6669–76. [PubMed] [Google Scholar]
- 26.Cornec D, Saraux A, Jousse-Joulin S, et al. The different diagnosis of dry eyes, dry mouth and parotidomegaly: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:278–87. doi: 10.1007/s12016-014-8431-1. [DOI] [PubMed] [Google Scholar]
- 27.Koike H, Sobue G. Sjögren syndrome-associated neuropathy. Brain Nerve. 2013;65:1333–42. [PubMed] [Google Scholar]
- 28.Teixeira F, Moreira I, Silva AM, et al. Neurological involvement in primary Sjögren syndrome. Acta Reumatol Port. 2013;38:29–36. [PubMed] [Google Scholar]