Abstract
Purpose
There is no consensus for parametrial boost technic while both transvaginal and transperineal approaches are discussed. A prototype was developed consisting of a perineal template, allowing transperineal needle insertion. This study analyzed acute toxicity of concomitant cervical and transperineal parametrial high-dose-rate brachytherapy (HDRB) boost for locally advanced cervical cancer.
Material and methods
From 01.2011 to 12.2014, 33 patients (pts) presenting a locally advanced cervical cancer with parametrial invasion were treated. After the first course of external beam radiation therapy with cisplatinum, HDRB was performed combining endocavitary and interstitial technique for cervical and parametrial disease. Post-operative delineation (CTV, bladder, rectum, sigmoid) and planification were based on CT-scan/MRI. HDRB was delivered in 3-5 fractions over 2-3 consecutive days. Acute toxicities occurring within 6 months after HDRB were retrospectively reviewed.
Results
Median age was 56.4 years (27-79). Clinical stages were: T2b = 23 pts (69.7%), T3a = 1 pt (3%), T3b = 6 pts (18.2%), and T4a = 3 pts (9.1%). Median HDRB prescribed dose was 21 Gy (21-27). Median CTVCT (16 pts) and HR-CTVMRI (17 pts) were 52.6 cc (28.5-74.3), 31.9 cc (17.1-58), respectively. Median EQD2αβ10 for D90CTV and D90HR-CTV were 82.9 Gy (78.2-96.5), 84.8 Gy (80.6-91.4), respectively. Median EQD2αβ3 (CT/MRI) for D2cc bladder, rectum and sigmoid were 75.5 Gy (66.6-90.9), 64.4 Gy (51.9-77.4), and 60.4 Gy (50.9-81.1), respectively. Median follow-up was 14 months (ranged 6-51). Among the 24 pts with MFU = 24 months, 2-year LRFS rate, RRFS, and OS were 86.8%, 88.8%, and 94.1%, respectively. The rates of acute genitourinary and gastrointestinal toxicities were 36% (G1 dysuria = 8 pts, G2 infection = 2 pts, G3 infection = 2 pts), and 27% (G1 diarrhea = 9 pts), respectively. One patient presented vaginal bleeding at the time of applicator withdrawal (G3-blood transfusion); no bleeding was observed due to the parametrial implant.
Conclusions
Concomitant cervical and transperineal parametrial HDRB boost for locally advanced cervical cancer appears feasible and safe with no specific acute toxicity compare to cervical HDRB alone. Longer follow-up and larger patient cohort will be needed.
Keywords: boost, brachytherapy, cervical cancer, parametrial invasion, radiotherapy
Purpose
Cervical cancer is the fourth most common cancer in women in the world, with an estimated 528,000 new cases and 266,000 deaths in 2012 [1]. Surgery is the standard treatment for early stage cervical cancer (≤ T1b1). For locally advanced tumors, gold-standard treatment consists in a weekly platin-based chemotherapy combined with external beam radiation therapy (EBRT) followed by brachytherapy (BT) boost [2].
Brachytherapy remains a key component for locally advanced stage cervical cancer. Indeed, Han et al. published the results of a US brachytherapy survey showing that the use of BT decreased significantly from 83% in 1988 to 58% in 2009 (p < 0.001) [3]. The authors have shown that during the same period, BT was independently associated with significantly higher disease-specific survival (HR 0.64, 95% CI: 0.57-0.71) and overall survival (HR 0.66, 95% CI: 0.60-0.74), and should be implemented in all feasible cases [3]. However, due to important technological improvements available with high-tech EBRT, some authors proposed to use new EBRT techniques in place of BT. Gill et al. reported that compared to BT boost using intensity modulated radiation therapy or stereotactic body radiation therapy, resulted in significantly lower OS (HR 1.86, 95% CI: 1.35-2.55, p < 0.01), confirming that BT boost was a critical component of locally advanced cervix carcinoma treatment [4].
Different applicators, such as ovoid or ring tandems, molds, and vaginal cylinders with intra-uterine tubes are commonly used. In most cases, a typical pear-shaped isodose results from the intra-vaginal and intra-uterine sources but in case of larger tumors and especially with parametrial involvement, dose distribution is often non-optimal. In order to improve parametrial coverage, some applicators combining intracavitary (IC) and interstitial (IS) approach were proposed without increasing the dose to the organs at risk (OAR): Vienna applicator: ring applicator plus 6 to 9 interstitial needles around intrauterine tandem (Nucletron, an Elekta company, Elekta AB, Stockholm, Sweden) [5, 6], Utrecht applicator: tandem ovoids + 10 needles (Nucletron, an Elekta company, Elekta AB, Stockholm, Sweden) [7], and Nice Gynecologic Applicator: NGA – uterine tandem + vaginal cylinder + 8 needles within the cervix (Nucletron, an Elekta company, Elekta AB, Stockholm, Sweden) [8].
Currently, there is no consensus for parametrial boost technic while both transvaginal and transperineal approaches are discussed. Recently, for locally advanced tumors with parametrial involvement, interstitial BT parametrial boost using trans-vaginal approach was shown to be dosimetrically superior to EBRT parametrial boost in terms of target volume coverage and OAR sparing [9]. In order to improve the parametrium coverage, we developed a prototype consisting of a dedicated perineal template fixed to the NGA allowing a transperineal needle insertion.
This study aimed to assess feasibility, reproducibility, and acute toxicity of concomitant cervical and transperineal parametrial high dose rate brachytherapy (HDRB) boost for locally advanced cervix carcinomas.
Material and methods
Patient features
From January 2011 to December 2014, 33 patients (pts) presenting a histology proven locally advanced cervix carcinoma with parametrial invasion were retrospectively analyzed regarding dosimetric data, acute toxicities, and early clinical outcomes. All patients underwent parametrial HDRB boost. A local ethics committee initially approved this therapeutic approach.
All patients had a clinical exam and follow-up performed by trained physicians. Patients underwent cervical, vaginal, and rectal examination. Computed tomography scan (CT), pelvic magnetic resonance imaging (MRI), and positron emission tomography/computed tomography (PET) were performed. Para-aortic lymph node dissection was performed at the discretion of the physician in order to improve cancer staging and EBRT target volume definition. Tumors were staged using UICC-TNM classification [10].
Treatment features
After the first course of EBRT with concomitant platin-based chemotherapy, a single implantation of HDRB was performed combining endocavitary and interstitial technique for cervical disease, and transperineal interstitial approach for parametrial extension.
Concomitant radio-chemotherapy
The first part of the treatment consisted of EBRT with concurrent chemotherapy. EBRT delivered a total dose of 45 to 46 Gy (ICRU point) in 25 or 23 fractions, based on a 3-dimensional conformal technique with or without modulated intensity using > 10 MV X-photons. Gross tumor volume (GTV) was determined clinically and radiologically for the primary tumor and any pathological nodes. The clinical target volume (CTV) included the GTV and surrounding subclinical disease. CTV involved the uterus, parametrial tissue, upper vagina (or whole vagina for T3a disease), and broad and utero-sacral ligaments. All pelvic-lymph nodal stations were included in the CTV with a recommended 7 mm margin around the blood vessels. Planning target volume (PTV) included the CTV plus margin to account for internal organ movements, setup, and delivery uncertainties. Concomitant chemotherapy mainly consisted in weekly platin-based chemotherapy using the following protocols: weekly cisplatin 40 mg/m2, weekly carboplatin 100 mg/m2, weekly cisplatin 40 mg/m2 + gemcitabin 125 mg/m2, monthly cisplatin 50-75 mg/m2 for 1 day + 5-fluorouracile 1000 mg/m2 from day 2 to day 5, weeks 1 and 5.
Brachytherapy
Brachytherapy was planned after the completion of radio-chemotherapy (RCT) with a time interval between the last RCT session and brachytherapy of less than 21 days. The single brachytherapy implantation was performed under general anesthesia starting by a gynecologic examination in order to evaluate the clinical response obtained after RCT.
Endocavitary and interstitial procedure for central disease
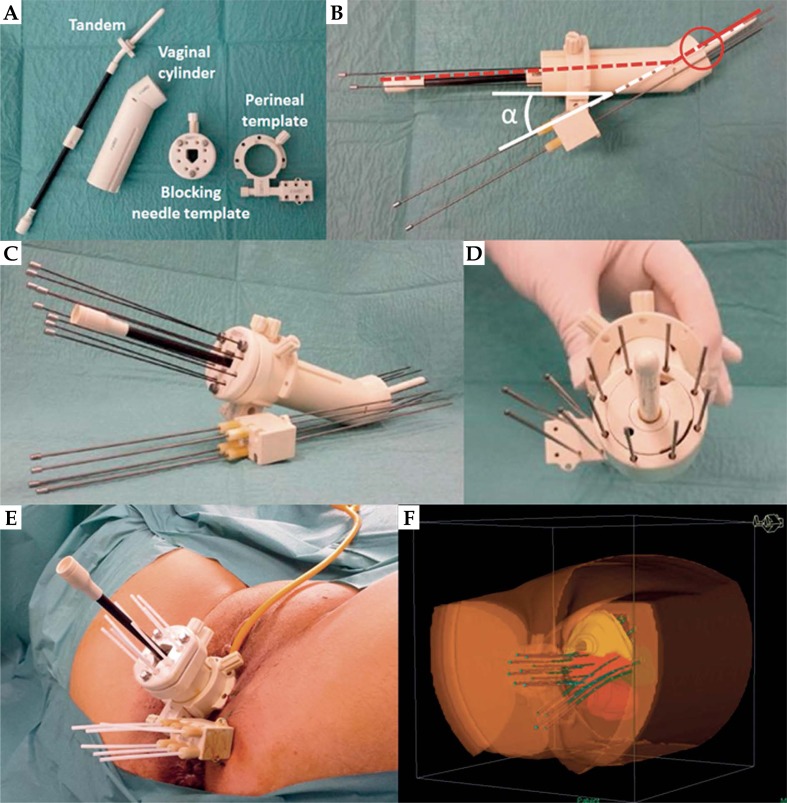
The endocavitary and interstitial procedure for central disease was already described [8]. Briefly, uterine tandem was introduced in the uterus cavity; it is then preceded to the placement of a vaginal cylinder including eight equidistant channels allowing the placement of eight plastic needles (240 mm Sharp Needles™; Elekta company, Elekta AB, Stockholm, Sweden) using flexible chucks, within the cervical tissue (40 mm depth). Vaginal cylinder was sutured to the skin through a skin suture template and a blocking needle template fixed the needles into the vaginal cylinder (Figure 1A).
Fig. 1.
Overview of the applicator allowing concomitant endocavitary and interstitial implant for cervical and parametrial disease. A) Different pieces of the applicator before assembling. B) Assembled applicator showing the oblique orientation (α angle) of the perineal template allowing the upper-inner parametrial needle to cross the uterus tandem at the distal part of the vaginal cylinder. C) Overview of the assembled applicator. D) Frontal proximal view of the assembled applicator. E) Post-operative view of the applicator showing vaginal and parametrial needles. F) Post-operative 3D reconstruction
Interstitial procedure for parametrial disease
The perineal template punched by a total of 6 holes was fixed to the vaginal cylinder before its introduction into the vaginal cavity. Due to the ischium, this perineal template has to be placed under the bone structure but has also to be obliquely oriented allowing the upper-inner parametrial needle to cross the uterus tandem at the distal part of the vaginal cylinder (Figures 1B, 1C, 1D). Once the perineal template was fixed on the skin perineum, parametrial transperineal 240 mm needles (Sharp Needles™; Elekta Company, Elekta AB, Stockholm, Sweden) were placed into the parametrium through the perineal template, using rigid chucks. Number of needles (2 to 6) was defined according to the pre-treatment target volume and clinical examination performed during HDRB (Figure 1E).
After recovery, post-implant planning CT scan (until 12.2013), then MRI (after 01.2014) was performed for treatment planning purposes. CTV (CT scan planning), high-risk CTV (HR-CTV), and intermediate-risk (IR-CTV) (MRI planning) as well as OARs (bladder, rectum, and sigmoid) were delineated according to the Gyn GEC-ESTRO recommendations [11] (Figure 1F).
HDRB dose was delivered in respect to the current Gyn. GEC-ESTRO dose constraint rules recommending EQD2αβ10 for D90HR-CTV > 80 Gy and EQD2αβ3 for D2ccbladder < 90 Gy, and for D2ccrectum/sigmoid < 75 Gy. Brachytherapy dose was delivered in 3 to 5 fractions over 2 to 3 consecutive days. Total dose and dose per fraction changed also according to the year of treatment. Between 2011 and 2012, the total delivered dose was 25 Gy in 5 fractions over 3 days, while 1 fraction of 7 Gy was delivered the day of implantation and 2 fractions of 4.5 Gy twice daily (at least 6 hours apart) for two days (EQD2αβ10 = 32 Gy/EQD2αβ3 = 41 Gy). Since May 2013, in order to make brachytherapy less uncomfortable, the number of fractions was reduced from 5 to 3 with a total dose of 21 Gy in 3 fractions over 2 days with 1 fraction of 7 Gy delivered the day of implantation, and 2 fractions of 7 Gy (at least 6 hours apart) the day after (EQD2αβ10 = 30 Gy/EQD2αβ3 = 42 Gy). Total dose and dose per fraction could also vary according to the clinical response observed at the time of HDRB.
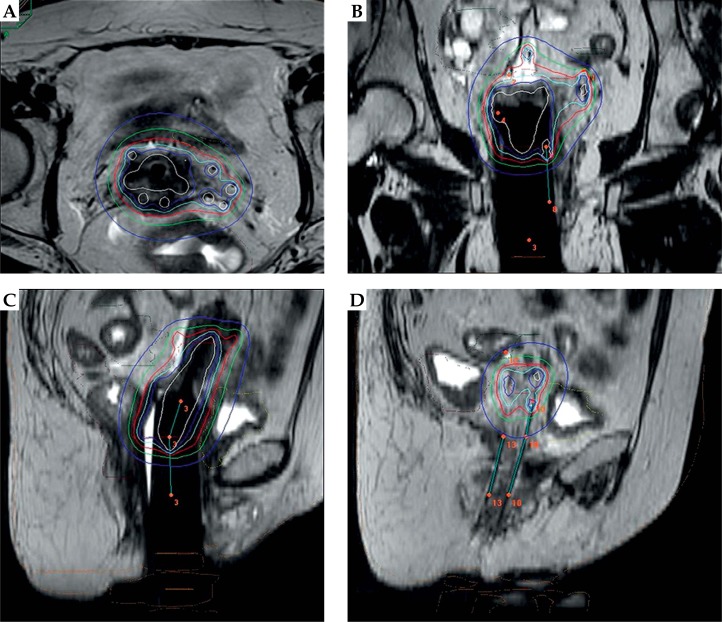
Dose volume adaptation was manually achieved using graphical optimization (OncentraBrachy, Elekta Company, Elekta AB, Stockholm, Sweden) by dwell location and time variation (Figure 2). Patient stayed in supine position during all the hospitalization in a non-shielded room receiving specific care such as massages and anti-thrombotic therapy. The patient was transferred to her bed to the brachytherapy bunker for each fraction. After the last brachytherapy session and analgesic pre-medication, applicator and needles were removed, paying attention to the risk of vaginal and perineal bleeding. The patient left the hospital the day after in the absence of early complications.
Fig. 2.
Post-operative MRI-planning dose distribution analysis: A) transversal view, B) frontal view, C) mid-sagittal view, D) para-sagittal view
Follow-up and evaluation
Patients were followed-up at first and 6 months after HDRB completion; then every 6 months alternatively by the radiation oncologist and the gynecologic surgeon. Surveillance consisted of clinical examination while CT, MRI, and PET were used in case of suspicion of local, regional, or distant progression. RCT and HDRB were proposed as definitive treatment, while post-RCT hysterectomy was proposed only in case of persistent residual disease or local recurrence.
Acute toxicities occurring within the 6 months after HDRB were graded using the NCI-Common Toxicity Criteria version 4.0 (CTCV4.0) [12] based on the following items: urinary disorders (hematuria, increase in frequency, urgency, dysuria, nocturia, incontinence), gastro-intestinal disorders (diarrhea, proctitis), infections (urinary tract, kidney, uterine, and pelvic infections), hemorrhages (intra and post-operative, vaginal, retroperitoneal).
Statistical analysis
Quantitative data were represented as median, extremes, and standard deviation. Qualitative data were represented as percentage and extremes. For a subgroup of patients who had a median follow-up of 24 months, local-relapse free survival (LRFS), regional-relapse free survival (RRFS), and overall survival (OS) were calculated (Kaplan-Meier method – 24 and 36 months) with standard errors. The follow-up was calculated between the date of the first fraction of RCT, and the date of local recurrence and the date of last follow-up. Patients were censored at the moment of their death or their last follow-up.
Results
Patients and treatments
Patient and treatment characteristics are reported in Tables 1 and 2, respectively. Median age was 56.4 years (27-79). Histological type was squamous cell carcinoma, adenocarcinoma, and adenoid basal carcinoma for 29 pts (87.9%), 3 pts (9.1%), and 1 pt (3%), respectively. According to the UICC-TNM classification [10], patients were staged as: T2b (23 pts – 69.7%), T3a (1 pt – 3%), T3b (6 pts – 18.2%), and T4a (3 pts – 9.1%). Fifteen pts (45.5%) presented pelvic-lymph node involvement diagnosed after MRI (6 pts) and/or PET-scan (9 pts). Median initial tumor size defined on MRI was 45 mm (13-83). Five patients had bilateral parametrial extension. Thirty-two patients (97%) received concomitant chemotherapy: weekly cisplatin (27 pts), weekly carboplatin (3 pts), weekly cisplatin-gemcitabine (1 pt), and 5 fluorouracil-cisplatin (W1-W5, 1 pt). Median EBRT dose was 46 Gy (45.5-50.6). Median HDRB dose was 21 Gy (21-27) in 3 to 5 fractions with a median treatment time of 2 days. Median number of parametrial implanted needles was 4. Median overall treatment time was 54 days (44-72) and median delay between EBRT and HDRB was 17 days (5-47). Three patients underwent adjuvant hysterectomy after a median time interval [HDRB-surgery] of 59 days (49-70): 2 pts presented a complete pathological response and macroscopic residual disease was observed in 1 pt. Median follow-up for the whole cohort was 14 months (6-51) while 24 pts (72.7%) had a median follow-up of 24 months (8-51).
Table 1.
Patient features
| Data | N/Median | %/[Min-max] |
|---|---|---|
| Age (years) | 56.4 | [27-79] |
| Tumor stage | ||
| T2b | 23 | 69.7 |
| T3a | 1 | 3.0 |
| T3b | 6 | 18.2 |
| T4a | 3 | 9.1 |
| Parametrial involvement | ||
| Right | 10 | 30.3 |
| Left | 18 | 54.5 |
| Bilateral | 5 | 15.2 |
| Tumor size (mm) | 45 | [13-83] |
| Lymph node status | ||
| N– | 18 | 54.5 |
| N+ | 15 | 45.5 |
| Metastatic status | ||
| M– | 32 | 97.0 |
| M+ | 1 | 3.0 |
| Histological type | ||
| Epidermoid carcinoma | 29 | 87.9 |
| Well differentiated | 7 | 12.3 |
| Moderately differentiated | 9 | 15.8 |
| Poorly differentiated | 11 | 19.3 |
| Undifferentiated | 1 | 1.8 |
| Unknown | 1 | 1.8 |
| Adenocarcinoma | 3 | 5.3 |
| Other | 1 | 1.8 |
Table 2.
Treatment features
| Data | N/Median | %/[Min-max] |
|---|---|---|
| EBRT | 33 | 100 |
| Dose per fraction (Gy) | 2 | [1.8-2.2] |
| Total dose | 46 | [45.5-50.6] |
| Concomitant CT | 32 | 97.0 |
| Weekly cisplatin | 27 | 81.8 |
| Weekly cisplatin-gemcitabine | 1 | 3.0 |
| W1-W5 5FU-cisplatin | 1 | 3.0 |
| Weekly carboplatin | 3 | 9.1 |
| HDRB | 33 | 100 |
| Number of parametrial needles | 4 | [2-6] |
| 2 | 6 | 18.2 |
| 3 | 2 | 6.1 |
| 4 | 23 | 69.7 |
| 6 | 2 | 6.1 |
| Number of fractions | 3 | [3-5] |
| 5 | 11 | 33.3 |
| 3 | 22 | 66.7 |
| Total dose | 21 | [21-27] |
| 27 Gy | 1 | 3.0 |
| 25 Gy | 9 | 27.3 |
| 23 Gy | 1 | 3.0 |
| 21 Gy | 22 | 66.7 |
| Interval time [EBRT – HDRB] (days) | 17 | [5-47] |
| Overall treatment time (days) | 54 | [44-72] |
| Surgery | 3 | 9 |
| Time interval [HDRB – surgery] (days) | 59 | [49-70] |
| Pathologic response | ||
| No residual disease | 2 | 6 |
| Macroscopic residual disease | 1 | 3 |
5FU – 5-fluorouracile, EBRT – external beam radiation therapy, CT – chemotherapy, HDRB – high-dose-rate brachytherapy
Dosimetric data
Dosimetric data are reported in Table 3. Planification was performed on CT for 16 pts (48.5%) and on MRI for 17 pts (51.5%). Median CTV, HR-CTVMRI, and IR-CTVMRI were 52.6 cc (28.5-74.3), 31.9 cc (17.1-58.0), and 83.1 cc (53.8-129.0), respectively. EQD2αβ10 for D90CTV, D90HR-CTV and D90IR-CTV were 82.9 Gy (78.2-96.5), 84.8 Gy (80.6-91.4) and 70.5 Gy (59.9-78.3), respectively. Median V100%CT and median V100%MRI were 99.0% (95.3-100.0) and 98.6% (92.3-99.3), respectively.
Table 3.
Dosimetric data reported for the 16 patients with CT-planification and the 17 patients with MRI-planification
| Data | CT (n = 16) | MRI (n = 17) | |||||
|---|---|---|---|---|---|---|---|
| Median | Min-max | SD | Median | Min-max | SD | ||
| CTV | (cc) | 52.6 | 28.5-74.3 | 13.1 | – | – | – |
| D90CTV | (%) | 118.0 | 108.0-127.6 | 5 | – | – | – |
| (Gy) | 28.1 | 22.7-31.9 | 3 | – | – | – | |
| D90CTV EQD2αβ10 | (Gy) | 82.9 | 78.2-96.5 | 5.7 | – | – | – |
| HR-CTV | (cc) | – | – | – | 31.9 | 17.1-58.0 | 11.7 |
| D90HR-CTV | (%) | – | – | – | 122.4 | 107.9-135.9 | 7.2 |
| (Gy) | – | – | – | 25.7 | 22.7-28.5 | 1.5 | |
| EQD2αβ10 D90HR-CTV | (Gy) | – | – | – | 84.8 | 80.6-91.4 | 3.1 |
| IR-CTV | (cc) | – | – | – | 83.1 | 53.8-129 | 19.6 |
| D90IR-CTV | (%) | – | – | – | 88.0 | 67.8-106.0 | 10.9 |
| (Gy) | – | – | – | 18.3 | 12.6-22.3 | 3 | |
| EQD2αβ10 D90IR-CTV | (Gy) | – | – | – | 70.5 | 59.9-78.3 | 5.2 |
| V100 | (%) | 99.0 | 95.3-100 | 1.2 | 98.6 | 92.3-99.3 | 2.2 |
| V150 | (%) | 52.2 | 28.3-69.4 | 11.2 | 57.2 | 48.5-67.1 | 5.3 |
| V200 | (%) | 16.1 | 11.6-39.6 | 7.5 | 23.9 | 20.5-34.0 | 3.6 |
| Bladder | |||||||
| D0.1ccb | (%) | 100.8 | 84.8-125.1 | 10.2 | 92.8 | 82.3-103.7 | 6 |
| D1ccb | (%) | 88.6 | 72.0-106.5 | 8.8 | 85.0 | 76.7-90.5 | 4.5 |
| D2ccb | (%) | 83.8 | 65.0-100.4 | 9.5 | 79.6 | 71.6-85.0 | 4.5 |
| EQD2αβ3 D2ccb | (Gy) | 76.8 | 66.6-90.9 | 6 | 74.5 | 70.3-78.0 | 2.3 |
| Rectum | |||||||
| D0.1ccr | (%) | 86.5 | 29.6-104.0 | 21.1 | 82.2 | 52.1-90.7 | 11 |
| D1ccr | (%) | 72.6 | 21.3-85.3 | 18.4 | 68.4 | 42.3-79.1 | 10.9 |
| D2ccr | (%) | 65.1 | 19.0-77.8 | 15.1 | 60.7 | 38.1-75.1 | 10.5 |
| EQD2αβ3 D2ccr | (Gy) | 66.4 | 51.9-77.4 | 7.6 | 64.3 | 55.1-72.0 | 4.6 |
| Sigmoid | |||||||
| D0.1ccs | (%) | 78.8 | 61.1-129.6 | 23.9 | 75.2 | 35.1-93.8 | 17.9 |
| D1ccs | (%) | 70.0 | 50.2-106.0 | 17.6 | 56.5 | 29.2-81.5 | 15.6 |
| D2ccs | (%) | 60.9 | 46.0-97.8 | 17.2 | 52.4 | 24.5-73.7 | 14.4 |
| EQD2αβ3 D2ccs | (Gy) | 59.8 | 53.4-81.1 | 8.5 | 60.7 | 50.9-70.3 | 5.7 |
CT – planification based on CT-scan = 16 patients (pts); MRI – planification based on MRI = 17 pts; CTV – clinical target volume; HR-CTV – high-risk CTV; IR-CTV – intermediate-risk CTV; D90CTV – dose delivered to 90% of the CTV; D90HR-CTV – dose delivered to 90% of the HR-CTV; D90IR-CTV – dose delivered to 90% of the IR-CTV; EQD2αβ10 – equivalent dose at 2 Gy per fraction for αβ = 10 (tumor); EQD2αβ3 – equivalent dose at 2 Gy per fraction for αβ = 3 (normal tissues); V100 – volume receiving 100% of the prescribed dose; V150 – volume receiving 150% of the prescribed dose; V200 – volume receiving 200% of the prescribed dose; D0.1ccb – dose delivered to 0.1% of the bladder volume; D0.1ccr – dose delivered to 0.1% of the rectum volume; D0.1ccs – dose delivered to 0.1% of the sigmoid volume; D1ccb – dose delivered to 1% of the bladder volume; D1ccr – dose delivered to 1% of the rectum volume; D1ccs – dose delivered to 1% of the sigmoid volume; D2ccb – dose delivered to 2% of the bladder volume; D2ccr – dose delivered to 2% of the rectum volume; D2ccs – dose delivered to 2% of the sigmoid volume; SD – standard deviation
For OARs, D2cc EQD2αβ3 of the whole cohort for bladder, rectum, and sigmoid were 75.5 Gy (66.6-90.9), 64.4 Gy (51.9-77.4), and 60.4 Gy (50.9-81.1), respectively. D2cc EQD2αβ3 of CT-planning patients for bladder, rectum, and sigmoid were 76.8 Gy (66.6-90.9), 66.4 Gy (51.9-77.4), and 59.8 Gy (53.4-81.1), respectively. D2cc EQD2αβ3 of MRI-planning patients for bladder, rectum, and sigmoid were 74.5 Gy (70.3-78.0), 64.3 Gy (55.1-72.0), and 60.7 Gy (50.9-70.3), respectively.
Acute toxicities
Acute toxicities occurring within the 6 months after HDRB are reported in Table 4. Urinary and gastro-intestinal disorders occurred in 8 pts (100% G1) and 9 pts (100% G1), respectively. Infections were scored G2 for 2 pts (urinary) and G3 for 2 pts (urinary and endometritis). One patient presented a vaginal bleeding at the time of applicator withdrawal (G3 because of blood transfusion) but no bleeding was observed due to parametrial implant.
Table 4.
Acute toxicities occurring within the 6 months after HDRB using the CTCAE 4.0.; results expressed as a number of events and a percentage of patients. Total number of toxicities expressed as a number of patients in whom at least one toxicity scored G0, G1, G2, G3, or G4 occurred
| Toxicities | G0 | G1 | G2 | G3 | G4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| pts | % | pts | % | pts | % | pts | % | pts | % | |
| GU | 25 | 75.8 | 8 | 24.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| GI | 24 | 72.7 | 9 | 27.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bleeding | 32 | 97.0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 |
| Infectious | 29 | 87.9 | 0 | 0 | 2 | 6.1 | 2 | 6.1 | 0 | 0 |
| Total | 16 | 48.5 | 14 | 42.4 | 2 | 6.1 | 3 | 9.1 | 0 | 0 |
GU – genito-urinary toxicities, GI – gastro-intestinal toxicities
Survival analysis
Among 24 pts who had a median follow-up of 24 months, the staging was: T2b (17 pts), T3b (6 pts), and T4a (1 pt). For this sub-group, 2-year LRFS rate was 86.8% (±14.2) remaining stable at 3 years. Two-year RRFS and OS rates were 88.8% (± 15.4) and 94.1% (± 20.4), respectively; while at 3 years, those survival rates were 76.1 (± 27) and 85.6 (± 19.4), respectively.
Discussion
Currently, standard brachytherapy technique is based on pulsed dose rate (PDR) or HDR [13]. In case of parametrial involvement, the combination between endocavitary and interstitial brachytherapy is generating a lot of interest [14]. Indeed, increasing the “field” number makes dose distribution optimization easier and more accurate. However, the parametrial boost technique remains under discussion. Historically, parametrial boost was performed through EBRT, before or after brachytherapy increasing not only treatment protraction but also the risk of overlap of irradiated volumes between EBRT-boost and cervical brachytherapy. Besides those clinical considerations, Mohamed et al. compared parametrial boost dose distribution delivered either by EBRT or interstitial brachytherapy (ISBT) [9]. The authors confirmed that ISBT was superior to EBRT in terms of organ sparing (less normal tissue exposure to intermediate doses – V60) and target coverage (more conformal).
Currently, interstitial parametrial brachytherapy boost can be performed through a transvaginal or a transperineal technique. In this study, the results of a transperineal approach were reported.
Regarding dose distribution analysis, D2cc EQD2αβ3 of the bladder matched the GEC-ESTRO dose constrain recommendations (< 90 Gy), constraints were also respected for D2cc EQD2αβ3 of the rectum and sigmoid (< 75 Gy) [15]. However, considering more rigorous OAR dose constraints as discussed during the 5th EMBRACE Annual Meeting in Vienna (17th-18th January, 2014), bladder D2cc EQD2αβ3 was superior to 80 Gy for 3 patients (9%), rectum D2cc EQD2αβ3 was superior to 70 Gy for 6 pts (18%), and sigmoid D2cc EQD2αβ3 was superior to 75 Gy for 1 pt (3%).
Beside the transperineal approach described in this study, mainly Vienna and Aarhus teams presented a transvaginal technique. Berger et al. reported dosimetric and clinical outcome of 6 patients who underwent oblique needle implantation from the vagina using a modified Vienna applicator [16]. HR-CTVs of mean 50 cc were treated with mean D90 of 86 Gy. Based on 2 to 3 implants of PDR brachytherapy Lindegaard et al. described an oblique needle implantation from the vagina [14]. The authors obtained a mean D90HR-CTV of 87 Gy in tumors with a mean HR-CTV of 53 cc. More recently, Mohamed et al. reported the dosimetric results of a cohort of 23 patients who underwent 2 PDR implants (1 week apart) based on endocavitary and oblique needle implantation from the vagina [9]. The authors calculated D90HR-CTV > 84 Gy for all patients.
Transperineal interstitial approach has been criticized for bleeding risk due to vascular injury at the time of needle insertion. In the present study, one patient presented vaginal bleeding at the time of applicator withdrawal but no bleeding was observed due to parametrial implant. Indeed, at least six experimented brachytherapy teams analyzed the feasibility of transperineal brachytherapy technique for gynecological malignancies [17, 18, 19]. Among a total of 481 patients, no severe bleeding complications were reported. Bleeding risk remains a rare but potential event, which is strongly correlated to inter-individual heterogeneity in blood vessel architecture.
In this study, we reported the results of a single implant allowing delivering 3 to 5 fractions based on HDRB. According to our knowledge, this is the first analysis of such approach while combination between endocavitary and interstitial brachytherapy was mainly reported using PDR brachytherapy and always with at least 2 implants 1 week apart. Utrecht and Aarhus teams described their results. Jürgenliemk-Schulz et al. reported the results of 24 patients with a mean D90HR-CTV of 84 Gy [20]. Nomden et al. analyzed the dosimetric results of 20 patients treated with two pulsed dose rate applications combining endocavitary and interstitial, and noticed a mean D90 HR-CTV of 83.9 Gy [7]. More recently, Aarhus University published the results of 71 patients treated with 2 PDR implants with a mean D90HR-CTV of 94.5 Gy while mean EQD2 D2cc of bladder, rectum, and sigmoid were 68.5, 61.0, and 64.9 Gy, respectively [21]. Fokdal et al. reported only minor morbidity, which was resolved at a 3 month follow-up after 3 implants of endocavitary and interstitial PDR BT in 58 patients [22].
The American Brachytherapy Society recommends a cumulative delivered dose of approximately 80-90 Gy as a component of the definitive treatment of locally advanced cervix carcinoma while precise applicator placement remains necessary to maximize the probability of achieving local control without major side effects [23]. For HDRB, the recommended D90 EQD2αβ10 is 80 to 90 Gy, depending on tumor size at the time of brachytherapy [24, 25]. Dimopoulos et al. demonstrated that a local control superior to 90% can be achieved if the D90 EQD2αβ10 was at least 86 Gy [26]. Pötter et al. study's generated satisfying results in terms of local control after EBRT with or without chemotherapy followed by HDRB (4 × 7 Gy) with the objective of D90HR-CTV EQD2αβ10 > 85 Gy. In this study, brachytherapy was guided by clinical examination and MRI and the authors reported a 3-year local-control rate of 96% and 86% for stage IIB and IIIB, respectively [27]. In our study, median D90CTV EQD2αβ10 (CT-scan data) and median D90HR-CTV EQD2αβ10 (MRI data) ranged between 80 to 90 Gy (82.5 Gy and 84.8 Gy, respectively) in respect to the GEC-ESTRO dose constraint recommendations [15]. No patient received less than 80 Gy and we calculated a D90HR-CTV EQD2αβ10 > 84 Gy for 12 patients (71%) in the MRI-planning group. However, Tanderup et al. recently suggested that D90HR-CTV EQD2αβ10 > 90 Gy and > 85 Gy for small and large residual tumors, respectively, could improve local control rate by 3-4% [28]. In order to reach the dose levels suggested by Tanderup et al., it would be necessary to increase our prescribed dose up to 24 Gy in 3 fractions over 2 days. Computer simulation modeling revealed that this increase would allow reaching a D90HR-CTV EQD2αβ10 of 94.5 Gy (86.2-102.7) with an acceptable increase of D2cc EQD2αβ3 for OARs (data not shown).
However, those dose constraints derived from different prescription dose protocols, which do not take into account treatment protraction, which could significantly impact on the final biological effect. Here, we propose a single implant of HDRB for cervical and parametrial disease with 3 fractions over 2 consecutive days. Biological impact on target volume and OARs of 21 Gy delivered over 2 days (3 × 7 Gy) could be different than the same physical dose delivered during a longer treatment scheme (2 to 3 weeks) and 3 different implants.
Conclusions
Short follow-up, small cohort of patient, and retrospective approach are the principal limiting factors of this study. However, concomitant cervical and transperineal parametrial HDRB boost for locally advanced cervical cancer appears feasible and safe with no specific acute toxicity compare to cervix HDRB alone. More specifically, transperineal parametrial HDRB boost does not increase the risk of bleeding. This technique allows respecting dose constraints for HR-CTV and OARs. Longer follow-up and larger patient cohort will be necessary to confirm this approach.
Disclosure
Prof. Jean-Michel Hannoun-Levi declares association with ELEKTA, related to the patent No 8834339: Assembly for performing brachytherapy treatment of a tumour tissue in an animal body (http://www.google.com.au/patents/US8834339 [1]).
The other authors report no conflict of interest.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Lanciano RM, Martz K, Coia LR, et al. Tumor and treatment factors improving outcome in stage III-B cervix cancer. Int J Radiat Oncol Biol Phys. 1991;20:95–100. doi: 10.1016/0360-3016(91)90143-r. [DOI] [PubMed] [Google Scholar]
- 3.Han K, Milosevic M, Fyles A, et al. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;87:111–119. doi: 10.1016/j.ijrobp.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 4.Gill BS, Lin JF, Krivak TC, et al. National Cancer Data Base analysis of radiation therapy consolidation modality for cervical cancer: The impact of new technological advancements. Int J Radiat Oncol Biol Phys. 2014;90:1083–1090. doi: 10.1016/j.ijrobp.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Dimopoulos JC, Kirisits C, Petric P, et al. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: clinical feasibility and preliminary results. Int J Radiat Oncol Biol Phys. 2006;66:83–90. doi: 10.1016/j.ijrobp.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 6.Kirisits C, Lang S, Dimopoulos J, et al. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: design, application, treatment planning, and dosimetric results. Int J Radiat Oncol Biol Phys. 2006;65:624–630. doi: 10.1016/j.ijrobp.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Nomden CN, de Leeuw AA, Moerland MA, et al. Clinical use of the Utrecht applicator for combined intracavitary/interstitial brachytherapy treatment in locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2012;82:1424–1430. doi: 10.1016/j.ijrobp.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 8.Hannoun-Levi J-M, Chand-Fouche M-E, Gautier M, et al. Interstitial preoperative high-dose-rate brachytherapy for early stage cervical cancer: dose-volume histogram parameters, pathologic response and early clinical outcome. Brachytherapy. 2013;12:48–155. doi: 10.1016/j.brachy.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Mohamed S, Kallehauge J, Fokdal L, et al. Parametrial boosting in locally advanced cervical cancer: Combined intracavitary/interstitial brachytherapy vs. intracavitary brachytherapy plus external beam radiotherapy. Brachytherapy. 2015;14:23–28. doi: 10.1016/j.brachy.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Sobin L, Gospodarowicz M, Wittekind CE. TNM Classification of Malignant Tumors. 7th ed. Oxford: Wiley-Blackwell; 2009. [Google Scholar]
- 11.Haie-Meder C, Pötter R, Van Limbergen E, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74:235–245. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 12.CTCAE.4. National Cancer Institute Common Terminology Criteria for Adverse Events v4.0 NCI, NIH, DHHS; 2009. May 29, NIH publication # 09-7473. [Google Scholar]
- 13.Miglierini P, Malhaire J-P, Goasduff G, et al. Cervix cancer brachytherapy: high dose rate. Cancer Radiother. 2014;18:452–457. doi: 10.1016/j.canrad.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Lindegaard JC, Tanderup K. Counterpoint: Time to retire the parametrial boost. Brachytherapy. 2012;11:80–83. doi: 10.1016/j.brachy.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Pötter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy – 3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Berger D, Pötter R, Dimopoulos JCA, et al. New Vienna applicator design for distal parametrial disease in cervical cancer. Brachytherapy. 2010;9:S23–S102. [Google Scholar]
- 17.Martinez A, Cox RS, Edmundson GK. A multiple-site perineal applicator (MUPIT) for treatment of prostatic, anorectal, and gynecologic malignancies. Int J Radiat Oncol Biol Phys. 1984;10:297–305. doi: 10.1016/0360-3016(84)90016-6. [DOI] [PubMed] [Google Scholar]
- 18.Weitmann HD, Knocke TH, Waldhäusl C, et al. Ultrasound-guided interstitial brachytherapy in the treatment of advanced vaginal recurrences from cervical and endometrial carcinoma. Strahlenther Onkol. 2006;182:86–95. doi: 10.1007/s00066-006-1420-4. [DOI] [PubMed] [Google Scholar]
- 19.Mahantshetty U, Shrivastava S, Kalyani N, et al. Template-based high-dose-rate interstitial brachytherapy in gynecologic cancers: a single institutional experience. Brachytherapy. 2014;13:337–342. doi: 10.1016/j.brachy.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Jürgenliemk-Schulz IM, Tersteeg RJ, Roesink JM, et al. MRI-guided treatment-planning optimization in intracavitary or combined intracavitary/interstitial PDR brachytherapy using tandem ovoid applicators in locally advanced cervical cancer. Radiother Oncol. 2009;93:322–330. doi: 10.1016/j.radonc.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Mohamed S, Nielsen SK, Fokdal LU, et al. Feasibility of applying a single treatment plan for both fractions in PDR image guided brachytherapy in cervix cancer. Radiother Oncol. 2013;107:32–38. doi: 10.1016/j.radonc.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Fokdal L, Tanderup K, Hokland SB, et al. Clinical feasibility of combined intracavitary/interstitial brachytherapy in locally advanced cervical cancer employing MRI with a tandem/ring applicator in situ and virtual preplanning of the interstitial component. Radiother Oncol. 2013;107:63–68. doi: 10.1016/j.radonc.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Viswanathan AN, Thomadsen B. American Brachytherapy Society Cervical Cancer Recommendations Committee, American Brachytherapy Society. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part I: general principles. Brachytherapy. 2012;11:33–46. doi: 10.1016/j.brachy.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Viswanathan AN, Beriwal S, De Los Santos JF, et al. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part II: high-dose-rate brachytherapy. Brachytherapy. 2012;11:47–52. doi: 10.1016/j.brachy.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee LJ, Das IJ, Higgins SA, et al. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part III: low-dose-rate and pulsed-dose-rate brachytherapy. Brachytherapy. 2012;11:53–57. doi: 10.1016/j.brachy.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Dimopoulos JC, Pötter R, Lang S, et al. Dose-effect relationship for local control of cervical cancer by magnetic resonance image-guided brachytherapy. Radiother Oncol. 2009;93:311–315. doi: 10.1016/j.radonc.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Pötter R, Georg P, Dimopoulos JC, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol. 2011;100:116–123. doi: 10.1016/j.radonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanderup K, Fokdal L, Sturdza A. Dose and Volume Response for Local Control in Locally Advanced Cervical Cancer Treated With EBRT Combined With MRI-Guided Adaptive Brachytherapy. Int J Radiat Oncol Biol Phys. 2014;(Suppl.) Abstract S92. [Google Scholar]