Abstract
Serotonin affects memory formation via modulating long-term potentiation (LTP) and depression (LTD). Accordingly, acute selective serotonin reuptake inhibitor (SSRI) administration enhanced LTP-like plasticity induced by transcranial direct current stimulation (tDCS) in humans. However, it usually takes some time for SSRI to reduce clinical symptoms such as anxiety, negative mood, and related symptoms of depression and anxiety disorders. This might be related to an at least partially different effect of chronic serotonergic enhancement on plasticity, as compared with single-dose medication. Here we explored the impact of chronic application of the SSRI citalopram (CIT) on plasticity induced by tDCS in healthy humans in a partially double-blinded, placebo (PLC)-controlled, randomized crossover study. Furthermore, we explored the dependency of plasticity induction from the glutamatergic system via N-methyl-D-aspartate receptor antagonism. Twelve healthy subjects received PLC medication, combined with anodal or cathodal tDCS of the primary motor cortex. Afterwards, the same subjects took CIT (20 mg/day) consecutively for 35 days. During this period, four additional interventions were performed (CIT and PLC medication with anodal/cathodal tDCS, CIT and dextromethorphan (150 mg) with anodal/cathodal tDCS). Plasticity was monitored by motor-evoked potential amplitudes elicited by transcranial magnetic stimulation. Chronic application of CIT increased and prolonged the LTP-like plasticity induced by anodal tDCS for over 24 h, and converted cathodal tDCS-induced LTD-like plasticity into facilitation. These effects were abolished by dextromethorphan. Chronic serotonergic enhancement results in a strengthening of LTP-like glutamatergic plasticity, which might partially explain the therapeutic impact of SSRIs in depression and other neuropsychiatric diseases.
INTRODUCTION
Serotonin (or 5-HT), one of the most important neuromodulators in the central nervous system, is related to learning and memory formation in animals and humans (Bert et al, 2008; Jacobs and Formal, 1997). It is also an important agent in depression. One important foundation for its effects might be its impact on neuroplasticity (Gu, 2002). Animal experiments have shown that serotonin can affect long-term potentiation (LTP) and long-term depression (LTD) in slice preparations. The direction of the effects depends on receptor subtypes, dosage of respective drugs, duration of 5-HT receptor activation, and site of action (Kemp and Manahan-Vaughan, 2005; Kojic et al, 1997; Mori et al, 2001). Both LTP-enhancing and -abolishing effects were described in different studies (Kojic et al, 1997; Park et al, 2012). Furthermore, application of 5-HT agonists blocks LTD or even converts it into LTP, whereas 5-HT antagonists enhance LTD expression (Kemp and Manahan-Vaughan, 2005). Concerning human studies, it was shown that selective serotonin reuptake inhibitor (SSRI) enhance LTP-like plasticity of late visual-evoked potentials in healthy subjects, whereas LTD-like plasticity was converted into facilitation (Normann et al, 2007). Similar effects were observed for motor cortex plasticity (Batsikadze et al, 2013; Nitsche et al, 2009). Moreover, several studies have demonstrated that serotonin enhancers can improve learning and memory formation, and motor functions in healthy individuals, as well as functional outcome in depression and stroke patients (Acler et al, 2009; Brunoni et al, 2013), ie, functional effects that involve neuroplasticity. These results confirm that serotonin is involved in brain plasticity, but the specific effects are complex.
The effects of acute serotonin enhancement on motor cortical plasticity induced by transcranial direct current stimulation (tDCS) and paired associative stimulation (PAS) were explored recently in healthy humans. These noninvasive brain stimulation tools induce prolonged cortical excitability changes (Nitsche et al, 2003b; Nitsche and Paulus, 2000, 2001; Stephan et al, 2000; Ziemann et al, 2008). tDCS induces non-focal plasticity via the primary mechanism of tonic subthreshold modulation of resting membrane potentials (Nitsche et al, 2007, 2008). Cathodal tDCS results in neural hyperpolarization and anodal tDCS elicits neural depolarization. For motor cortex stimulation, anodal tDCS enhances, whereas cathodal tDCS diminishes cortical excitability (Nitsche et al, 2003a; Nitsche and Paulus, 2000, 2001). In contrast, PAS induces focal and synapse-specific plasticity of the respective target neurons, via combined activation of the motor cortex and peripheral afferents. PAS shares some features with spike timing-dependent plasticity. The direction of plasticity depends on the synchronous or asynchronous activation of the target neurons (Stephan et al, 2000). Both stimulation protocols induce LTP-/LTD-like plasticity of the glutamatergic system, which is N-methyl-D-aspartate (NMDA) receptor- and calcium-dependent (Liebetanz et al, 2002; Nitsche et al, 2003b; Stephan et al, 2000). In a foregoing study, a single dose of the SSRI citalopram (CIT) enhanced both the amplitude and duration of the after-effects of anodal tDCS until the same evening of stimulation, and it reversed the excitability diminution seen after cathodal tDCS into facilitation (Nitsche et al, 2009). Likewise for PAS, acute application of CIT enhanced LTP-like PAS-induced after-effects and abolished LTD-like PAS-induced after-effects (Batsikadze et al, 2013). These results show a prominent impact of serotonin on plasticity in humans.
For clinical implications in psychiatric diseases, pathologically altered plasticity, especially compromised LTP, recently came into the focus of attention as a potentially important pathophysiological mechanism in major depression. Distress disrupts neuroplasticity, whereas antidepressant treatment, including serotonin enhancement via SSRI, produces opposing effects in animal models (Henn and Vollmayr, 2004; Holderbach et al, 2007). Accordingly, plasticity is disturbed in patients with depression, but restituted by SSRI application (Normann et al, 2007). Thus, SSRI treatment may exert a therapeutic effect via modulation of brain plasticity. Although an delayed clinical impact of antidepressant medication is usually assumed, these might act faster than previously thought. New data suggest that the maximum improvement of clinical symptoms occurs during the first 2 weeks after initiation of antidepressant medication (Stassen and Angst, 2012). However, a single dosage of SSRI has usually no prominent clinical effects. Thus, it can be speculated that the physiological effects of acute and chronic serotonin enhancement differ in a similar manner. However, the effect of chronic administration of SSRIs on neuroplasticity in humans has not yet been explored. We hypothesized that chronic application of the SSRI CIT would enlarge the neuroplastic excitability enhancement, which is induced by anodal tDCS, whereas cathodal tDCS-induced inhibitory plasticity should be converted into excitation, in accordance with the results obtained by acute administration of CIT. Based on the superior clinical effects induced by repeated administration of SSRI, we hypothesized that chronic application should enhance LTP-like plasticity, ie, induce a larger and/or longer-lasting excitability enhancement compared with the acute medication condition. We additionally combined SSRI administration with an NMDA receptor antagonist to demonstrate that in accordance with its neuromodulatory effects, serotonin enhancement does not induce plasticity itself, but modulates plasticity of the glutamatergic system. Accordingly we hypothesized that the NMDA receptor antagonist would prevent plasticity induction via tDCS also in the presence of SSRI.
MATERIALS AND METHODS
Subjects
Twelve right-handed healthy subjects participated in the experiment (five men and seven women, aged 27.5±4.01 years). Inclusion criteria were age between 18 and 50 years, no history of chronic or acute neurological, psychiatric, or medical diseases, no family history of epilepsy, no present pregnancy, no cardiac pacemaker, no previous surgery involving implants in the head (cochlear implants, aneurysm clips, brain electrodes), and absent acute or chronic medication or drug intake, including nicotine. All participants gave written informed consent. The study was approved by the ethics committee of the University of Göttingen, and conforms to the Declaration of Helsinki.
Transcranial Direct Current Stimulation
We used a battery-driven constant current stimulator (NeuroConn GmbH, Ilmenau, Germany) with a maximum output of 4.5 mA. Two saline-soaked surface sponge electrodes (35 m2) were applied to deliver the current. One electrode was positioned over the motor cortex representation area of the right abductor digiti minimi muscle (ADM), and the other electrode was located above the right orbit. tDCS was administered with a current strength of 1 mA for 13 min (anodal tDCS) or 9 min (cathodal tDCS). These stimulation protocols induce prolonged excitability changes in the human motor cortex: anodal stimulation increases and cathodal stimulation decreases cortical excitability for ~1 h after stimulation (Nitsche et al, 2003a; Nitsche and Paulus, 2001).
Pharmacological Interventions
Two hours before the start of each experimental session, 20 mg CIT with placebo (PLC), 20 mg CIT with 150 mg dextromethorphan (DMO), or equivalent PLC medication only were administered orally to the subjects. The maximum plasma level is achieved ~2 h after oral intake of these substances, and the respective doses are sufficient to elicit prominent effects in the central nervous system (Bezchilbnyk-Butler et al, 2000). The chosen dosage of CIT is identical with that applied in our foregoing experiments (Batsikadze et al, 2013; Nitsche et al, 2009). The chosen dosage of DMO abolished tDCS-induced plasticity in other experiments (Liebetanz et al, 2002; Nitsche et al, 2003b, 2004).
Monitoring of Motor Cortical Excitability
Cortical excitability was monitored by peak-to-peak amplitudes of motor-evoked potentials (MEPs) induced by transcranial magnetic stimulation (TMS) of the motor cortical representation of the right ADM. Single-pulse TMS was conducted by a Magstim 200 magnetic stimulator (Magstim Company, Whiteland, Dyfed, United Kingdom) connected with a figure-of-eight magnetic coil (diameter of one winding=70 mm, peak magnetic coil=2.2 T). The coil was held tangentially to the skull, with the handle pointing backwards and laterally at an angle of 45° to the mid-sagittal plane. Electromyographic recording was obtained from the right ADM with Ag-AgCl electrodes attached in a belly-tendon montage. Signals were filtered (2 Hz to 2 kHz), amplified, and then stored on computer via a Power 1401 data acquisition interface (Cambridge Electronic Design, Cambridge, United Kingdom). TMS intensity was adjusted to elicit baseline MEPs of averaged 1 mV peak-to-peak MEP amplitude and was kept constant for the post-stimulation assessment unless adjusted (see below).
Experimental Procedures
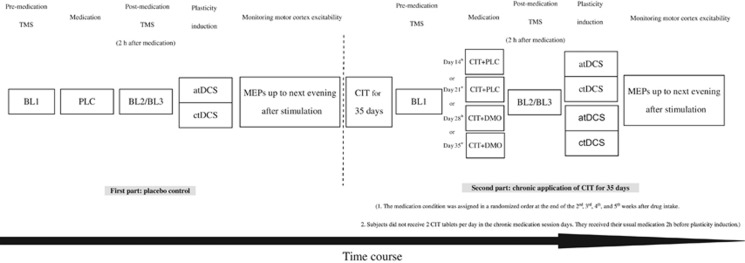
The experiment was conducted in a partially blinded (subjects were blinded for all stimulation and medication conditions, and the experimenter was blinded for the medication conditions in the second part), complete crossover, and PLC-controlled design. Each volunteer participated in all experimental sessions (six sessions per subject). The experimental sessions were carried out in randomized order and separated by 1 week to avoid cumulative drug or tDCS effects. A specific sequence of experimental sessions was randomly assigned to each subject, which differed for all participants. The study was separated in two parts. For the first part, subjects received PLC medication combined with cathodal or anodal tDCS before chronic CIT medication was started. In the second part, participants received CIT (20 mg/day) consecutively for 35 days. During this period, the other four sessions (CIT and PLC with anodal/cathodal tDCS, CIT and dextromethorphan with anodal/cathodal tDCS) were conducted in randomized order at the end of the second, third, fourth, and fifth week after the start of chronic drug intake. At the day of the respective stimulation session, the participants were seated in a comfortable chair with head and arm rest. TMS was applied over the left motor cortical representational area of the right ADM where it produced consistently the largest MEPs in the resting muscle (optimal site). The intensity of the TMS stimulus was adjusted to elicit MEPs with a peak-to-peak amplitude of on average 1 mV (baseline 1). Two hours after intake of the medication (CIT (the usual once-daily dosage) plus dextromethorphan or PLC), a second baseline was recorded to monitor the possible influence of the drug on cortical excitability (baseline 2) and make the experimental design comparable to that of Nitsche et al (2009). The TMS intensity was adjusted to result in baseline MEP amplitudes of 1 mV when necessary (baseline 3). Afterwards, tDCS was performed. Immediately after tDCS, 25 MEPs were recorded at the time points of 0, 5, 10, 15, 20, 25, 30, 60, 90, and 120 min, and then again the same evening (SE: between 6 pm and 7 pm), next morning (NM: between 9 am and 10 am), next noon (NN: between 12 am and 1 pm), and next evening (NE: between 6 pm and 7 pm; Figure 1).
Figure 1.
Experimental course of the present study. The study was conducted in two parts. For the first part, subjects received placebo medication (PLC) with cathodal or anodal tDCS. For the second part, the same subjects took 20 mg CIT consecutively for 35 days. During this period, the other four sessions were conducted (CIT and PLC with anodal/cathodal tDCS, CIT and 150 mg dextromethorphan (DMO) with anodal/cathodal tDCS). For each session, first transcranial magnetic stimulation (TMS) was applied over the left motor cortical representation area of the right abductor digiti minimi muscle (ADM) with an intensity to elicit motor-evoked potentials (MEPs) with a peak-to-peak amplitude of on average 1 mV (BL1). Two hours after intake of the medication, a second baseline (BL2) was determined to control for a possible influence of the drug on cortical excitability and adjusted if necessary (BL3). Afterwards, transcranial direct current stimulation (tDCS) was applied and MEPs were recorded immediately after stimulation. Further TMS measurements were conducted in the evening of the same day (SE), next morning (NM), next noon (NN), and next evening (NE).
Data Analysis and Statistics
Individual MEP amplitude means were calculated for each time bin, including baseline 1, 2, and 3 and post-stimulation time points, separately for each stimulation/medication combination. The post-intervention MEPs were normalized and are given as ratios of the third baseline.
A repeated-measure analysis of variance (ANOVA) for the time bins up to the next evening measurement after tDCS was performed with the within-subject factors time course (time bins up to the next evening after stimulation), drug condition (PLC, CIT with PLC, CIT with DMO), stimulation type (anodal and cathodal tDCS), and the dependent variable MEP. The Mauchly test of sphericity was conducted, and the Greenhouse-Geissser correction was applied when necessary. In case of significant results of the ANOVA, post hoc comparisons were performed using Student's t-tests (paired samples, two-tailed, p<0.05, not corrected for multiple comparisons) to determine whether the MEP amplitudes before and after tDCS differed in each intervention condition and whether those differences depend on drug condition. Furthermore, we performed an ANCOVA for the chronic medication condition with order as co-variate to rule out systematic effects of order of conditions on the results. To explore whether medication modified baseline MEPs, additional tests (Student's t-tests, p<0.05) for comparison of baseline 1 and 2 were performed. To explore if baseline MEP, and the TMS intensity needed to obtain baseline MEP (percentage of maximal stimulator output) differed between tDCS and medication conditions, the respective baseline values were compared via Student's t-tests.
RESULTS
All subjects tolerated tDCS and medication without adverse events. No subjects dropped out due to side effects of medication or tDCS. The average baseline MEP values and percentage of maximal stimulator output did not significantly differ between groups (P>0.05, Student's paired, two-tailed t-test). The peak–to-peak amplitude of the baseline MEPs was not significantly affected by medication between first and second baseline values (P>0.05, Student's paired, two-tailed t-test; Table 1).
Table 1. MEP Amplitudes and Stimulation Intensity Before and After Citalopram Administration.
| Stimulation | TMS parameter | Medication condition | Baseline 1 | Baseline 2 | Baseline 3 | p value |
|---|---|---|---|---|---|---|
| Anodal tDCS | MEP | PLC | 0.97±0.1 | 0.98±0.01 | 0.99±0.01 | 0.728 |
| CIT+PLC | 1.09±0.05 | 1.00±0.05 | 0.98±0.01 | 0.103 | ||
| CIT+DMO | 1.03±0.07 | 1.00±0.09 | 1.03±0.09 | 0.266 | ||
| % MSO | PLC | 52±8.09 | 52±8.09 | 53.38±9.18 | 0.166 | |
| CIT+PLC | 53.23±9.18 | 53.23±9.18 | 53.38±9.18 | 0.485 | ||
| CIT+DMO | 53.75±8.93 | 53.75±8.93 | 54.08±9.25 | 0.305 | ||
| Cathodal tDCS | MEP | PLC | 0.98±0.1 | 0.97±0.06 | 0.99±0.05 | 0.786 |
| CIT+PLC | 1±0.06 | 0.94±0.18 | 0.98±0.04 | 0.266 | ||
| CIT+DMO | 0.98±0.08 | 0.99±0.05 | 1.00±0.14 | 0.815 | ||
| % MSO | PLC | 54.25±8.77 | 54.25±8.77 | 54.33±9.06 | 0.795 | |
| CIT+PLC | 53.08±9.28 | 53.08±9.28 | 53.75±9.29 | 0.166 | ||
| CIT+DMO | 54±9.28 | 54±9.28 | 54.42±9.17 | 0.096 |
Shown are the mean MEP amplitudes±SD and stimulation intensity (percentage of maximum stimulator output, %MSO) mean±SD of baseline 1, 2, and 3. The intensity of TMS was determined to elicit MEPs with peak-to-peak amplitude of ~1 mV (baseline1). A second baseline (baseline 2) was recorded 2 h after medication intake to determine the effect of the drug on cortical excitability and adjusted if necessary (baseline 3). Student's t-tests revealed no significant differences between conditions (P>0.05).
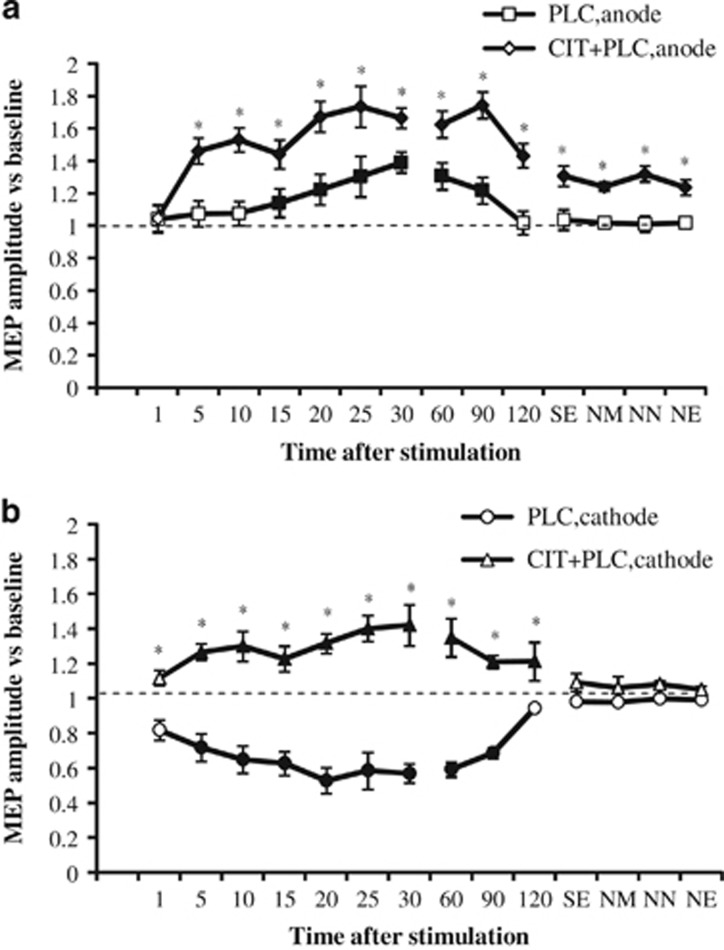
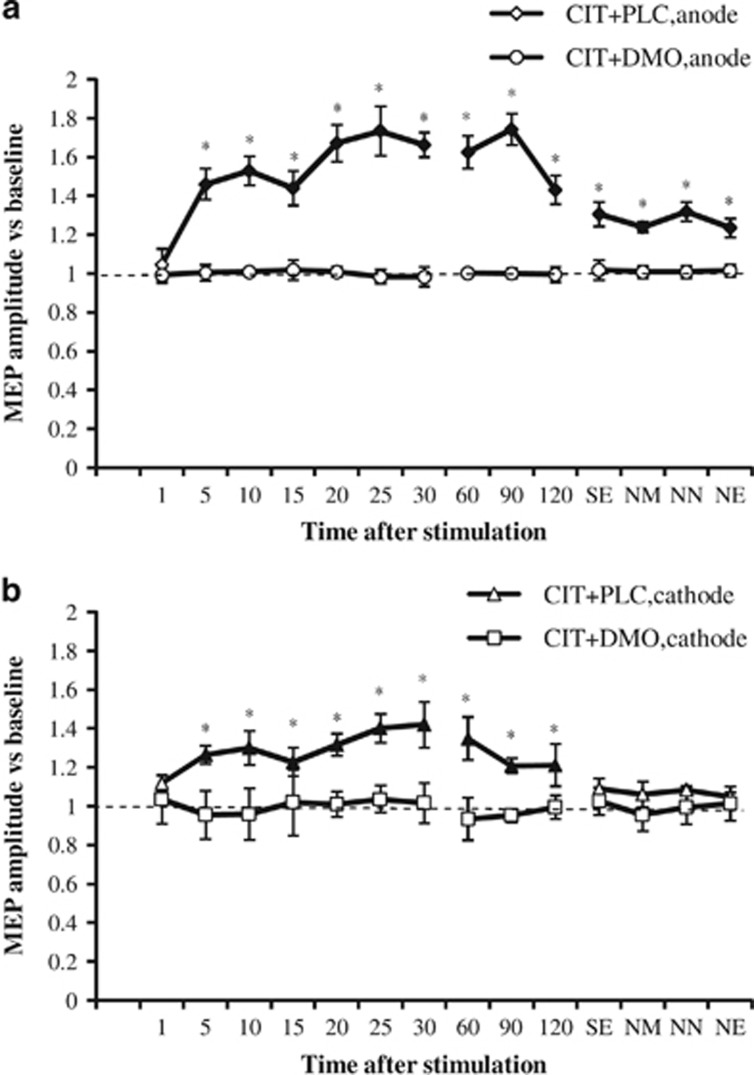
The ANOVA revealed significant main effects of drug and time, drug × stimulation, drug × time, stimulation × time, and drug × stimulation × time interactions (Table 2). This is due to the following pattern of results: in the PLC condition, anodal and cathodal tDCS significantly increased or decreased cortical excitability, respectively, until 90 min after the end of stimulation. Interestingly, the excitability-enhancing effects of anodal tDCS developed with a delay of 15 min after the end of tDCS, and also the excitability-diminishing effects of cathodal stimulation became significant not immediately, but 5 min after stimulation. Chronic application of CIT enlarged the MEP amplitudes significantly vs PLC medication and extended the duration of the facilitation induced by anodal tDCS until the evening of the day after tDCS, and thus for more than 24 h, whereas it turned cathodal tDCS-induced LTD-like into LTP-like plasticity (Figure 2). The latter effects, however, vanished 120 min after tDCS. When combined with the NMDA receptor antagonist DMO, the after-effects generated by anodal and cathodal tDCS were absent (Figure 3). In this case, post-tDCS MEPs did not differ from baseline values, but did significantly differ from MEP amplitudes after cathodal and anodal tDCS combined with PLC medication.
Table 2. Results of the Repeated Measures ANOVA and ANCOVA.
|
ANOVA |
ANCOVA |
|||||
|---|---|---|---|---|---|---|
| Factor | d.f. | F | P1 | d.f. | F | p |
| Drug | 2 | 23.894 | <0.001a | 1 | 156.69 | <0.001a |
| tDCS | 1 | 1.709 | 0.218 | 1 | 8.973 | 0.003a |
| Time course | 14 | 4.372 | <0.001a | 14 | 5.572 | <0.001a |
| Drug × tDCS | 2 | 40.447 | <0.001a | 1 | 42.781 | <0.001a |
| Drug × time | 28 | 3.703 | <0.001a | 14 | 0.558 | 0.987 |
| tDCS × time | 14 | 3.467 | <0.001a | 14 | 3.315 | <0.001a |
| Drug × tDCS × time | 28 | 11.467 | <0.001a | 14 | 0.953 | 0.502 |
| Order | 3 | 2.561 | 0.11 | |||
Abbreviation: d.f., degrees of freedom.
Significant results at p<0.05.
Figure 2.
Impact of chronic serotonin enhancement on transcranial direct current stimulation (tDCS)-induced motor cortex plasticity. Shown are baseline-standardized MEP amplitudes after plasticity induction by anodal/cathodal tDCS under placebo (PLC) or citalopram with placebo (CIT+PLC) conditions up to the next evening of the post-stimulation day. (a) In the placebo medication condition (square), anodal tDCS induced a significant excitability enhancement for up to 90 min after stimulation. Citalopram (diamond) enhanced and prolonged these excitability enhancements until next evening. (b) In the placebo medication condition (circle), cortical excitability was significantly reduced after cathodal tDCS for 90 min, whereas citalopram (triangle) converted the inhibitory effect into facilitation. Error bars indicate SEM. Filled symbols indicate significant differences of post-stimulation MEP amplitudes from respective baseline values; asterisks indicate significant differences between the drug and placebo medication conditions at the same time points (Student's t-test, two-tailed, paired samples, p<0.05). NE, next evening; NM, next morning; NN, next noon; SE, same evening.
Figure 3.
NMDA receptor block abolishes serotonin-dependent tDCS-induced plasticity enhancements. Shown are the mean±SEM MEP amplitudes versus baseline across time following plasticity induction by anodal or cathodal tDCS for CIT with PLC (CIT+PLC) and CIT with DMO (CIT+DMO) conditions. (a) DMO (circle) eliminated after-effects following anodal tDCS under CIT (diamonds). (b) DMO (square) likewise abolished the after-effects following cathodal tDCS under citalopram (triangle). Error bars indicate SEM. Filled symbols indicate significant differences of post-stimulation MEP amplitudes from respective baseline values; asterisks indicate significant differences between two conditions at the same time points (Student's t-test, two tailed, paired samples, p<0.05). NE, next evening; NM, next morning; NN, next noon; SE, same evening.
The ANCOVA conducted for the chronic medication conditions did not result in a significant effect of order (p=0.11; Table 2).
DISCUSSION
The results of the present study show that chronic application of SSRI in healthy humans increased and extended the duration of the facilitation induced by anodal tDCS for more than 24 h after intervention, whereas it turned cathodal tDCS-induced inhibition into facilitation. These findings support recent concepts that the impact on neural plasticity is relevant for therapeutic effects of serotonergic enhancement (Holderbach et al, 2007; Pittenger and Duman, 2007). The LTP-like effects of anodal tDCS lasted longer than 24 h after intervention. This duration exceeds that induced by single dose SSRI application in a foregoing study relevantly. Although direct comparability between study results is limited because of different participant groups, this might be a hint for better effects of chronic SSRI application on plasticity, which might—given the relevance of compromised LTP for depression—at least partially explain a need for repeated application of SSRI to exert antidepressant effects. DMO, a NMDA-antagonist, prevented both anodal and cathodal tDCS-induced after-effects, indicating that chronic SSRI effects are NMDA-receptor dependent, and are compatible with a neuromodulatory, but not plasticity-driving function of serotonin.
Our results extend previous data obtained in humans. Acute application of SSRI resulted in enhancement and prolongation of anodal tDCS-induced LTP-like plasticity and conversion of cathodal tDCS-induced LTD- into LTP-like plasticity (Nitsche et al, 2009). Furthermore, a single dose of SSRI strengthened excitatory PAS-induced, whereas it abolished inhibitory PAS-induced after-effects (Batsikadze et al, 2013). Likewise, application of SSRI increased facilitatory plasticity of late visual-evoked potentials and converted inhibitory plasticity into facilitation in healthy individuals (Normann et al, 2007). Thus, the impact of SSRI on plasticity is not restricted to single cortical areas, and seems not to be as qualitatively different between plasticity induction protocols, as it is the case for other neuromodulators like dopamine (Kuo et al, 2008; Monte-Silva et al, 2010; Thirugnanasambandam et al, 2011).
Our results also comply with some of the animal studies. Repeated application of SSRI can enhance LTP (Kojic et al, 1997; Mori et al, 2001). In addition, chronic application of the SSRI fluvoxamine prevented stress-induced facilitation of LTD and increased LTP both in stressed and non-stressed animals (Holderbach et al, 2007). Activation of 5-HT receptors was also shown to block LTD or further convert LTD induction into LTP (Kemp and Manahan-Vaughan, 2005; Normann and Clark, 2005). However, serotonin enhancement or activation of serotonergic receptors via specific agonists resulted in divergent effects in other studies. In some studies, serotonin or 5-HT receptor activation reduced or abolished LTP (Huang and Kandel, 2007; Kojima et al, 2003). This might be explained by the activation of different 5-HT receptor subtypes, the concentration of 5-HT agonists and antagonists, and different brain sites of action (Mori et al, 2001; Staubli and Otaky, 1994). To explore the reasons for these inconsistent results and to learn more about the impact of serotonin on neuroplasticity in humans, future studies should consider specific serotonergic receptor subtypes, apply different 5-HT-receptor agonists and antagonists, and explore the impact of different dosages, which might be a relevant factor for the strength and direction of effects, as seen for other neuromodulators, which have nonlinear effects on plasticity (Frensnoza et al, 2014; Monte-Silva et al, 2010).
Mechanistically, the impact of CIT on tDCS-generated plasticity can be explained by the impact of serotonin on calcium influx through NMDA receptors and voltage-gated calcium channels (Normann and Clark, 2005; Reiser et al, 1989). CIT reduces membrane potassium conductance and hereby enhances neuronal depolarization, which will lead to enhanced calcium influx via the above-mentioned channels (Panicker et al, 1991). Increased postsynaptic intracellular calcium concentration is an important signal for the induction of long-term synaptic plasticity (Criti and Malenka, 2008; Gu, 2002). High enhancement of intracellular calcium induces LTP, whereas low enhancement results in LTD (Lisman, 2001). As the after-effects of tDCS are NMDA receptor- and calcium-dependent (Liebetanz et al, 2002; Nitsche et al, 2003b), serotonergic enhancement might have strengthened the excitability enhancement induced by anodal tDCS and the conversion of LTD-like to LTP-like plasticity in case of cathodal tDCS via an enhancement of calcium influx. This assumption is supported by the fact that block of NMDA receptors, which reduces calcium influx, prevented plasticity induction via tDCS under CIT. Other mechanisms, such as reduction of serotonin autoreceptor density by CIT, or indirect effects by its modulatory effect on other transmitters and neuromodulators, cannot be ruled out, however, at present (Consolo et al, 1994; Wood and Wren, 2008). Which specific serotonin receptors are involved in these mechanisms is unclear. 5-HT2 and 5-HT4 receptors might be candidates. The 5-HT2 receptor stimulates intracellular calcium release, whereas the 5-HT4 receptor modulates calcium conductance, which induces LTP as well as depotentiation (Kulla and Manahan-Vaughan, 2002; Reiser et al, 1989). Concerning to the after-effects of tDCS, activation of the 5-HT2 receptor facilitates the induction of NMDA receptor-dependent LTP in the rat visual cortex (Kojic et al, 1997), and 5-HT4 receptor activation leads to expression on LTD in hippocampal slices (Kemp and Manahan-Vaughan, 2005). Given the LTP-like plasticity-enhancing and LTD-like plasticity-abolishing effects of CIT in our study, the 5-HT2 receptor might be the more probable candidate.
In the present study, we aimed to investigate the effects of chronic serotonin enhancement on neuroplasticity in the human motor cortex. In summary, our results show that the SSRI CIT enhances and prolongs facilitatory plasticity, and converts inhibitory plasticity into facilitation. These findings add important information to our understanding of the mechanisms of consolidation of neuroplasticity in the human cortex. The modulatory action of SSRIs could also explain their positive effects on learning and memory in humans (Savaskan et al, 2008). In stroke and depression, reduced facilitatory and enhanced inhibitory plasticity have been described (Foy et al, 1987; Schaechter, 2004). SSRIs might reduce clinical symptoms in these diseases by antagonizing this imbalance.
tDCS is increasingly applied for treatment of neuropsychiatric diseases (Kuo et al, 2014), and in many cases it is used to induce LTP-like plasticity for therapeutic effects. Given the strengthening effect of CIT on the after-effects of tDCS, combining tDCS with SSRI might be a promising venue to enhance its clinical impact. In accordance, it was shown recently that combined tDCS and SSRI treatment had a superior impact on major depression, compared both of the interventions alone or PLC treatment (Brunoni et al, 2013). Likewise, SSRIs might be suited to strengthen tDCS effects in other diseases such as Alzheimer's disease, Parkinson's disease, or motor rehabilitation after stroke. Future studies should explore these possibilities and include functional outcomes to assess the agonistic effects of brain stimulation and SSRI therapy. Beyond tDCS, a variety of stimulation methods with different plasticity mechanisms can be used to evaluate the effects of SSRI on plasticity in humans. For example, PAS shares some characteristics with spike timing-dependent plasticity, which is assumed to be closely related to learning processes, which might be relevant for depression, especially with regard to therapeutic aspects (Stephan et al, 2000; Ziemann et al, 2008). Furthermore, plasticity induction of the dorsolateral prefrontal cortex with this stimulation protocol could be relevant for our understanding of the pathophysiological foundation of this disease, because this cortical area is closer related to respective symptoms of the disease as compared with the primary motor cortex, which was explored in the present study (Rajji et al, 2013).
Some limitations of the present study should be taken into consideration. First, we performed the PLC medication tDCS interventions before the start of long-term SSRI medication, thus the study was conducted in a partially blinded design, as described above. However, we did not tell subjects if they received real or PLC medication in the acute and chronic medication condition, furthermore we did not tell them about the respective tDCS condition. As participants described no side effects of medication, and tDCS polarity is not discernable, we assume that subjects were blinded. Second, we did not compare acute and chronic effects of serotonin enhancement in identical participants to restrict the number of sessions per participant. Consequently, comparability of effects between groups is limited. Nevertheless, taking into account the very similar duration of the after-effects under PLC-medication in both studies, comparability should be given at least to a certain degree. Third, participants had not the exact identical duration of CIT intake in the respective chronic medication conditions (medication duration between 14 and 35 days). To rule out systematic effects of order of conditions on the results, we conducted an ANCOVA for the chronic medication condition with order as co-variate. As the results show no significant impact of order, we think that pooling of data is justified. Fourth, this study was conducted in healthy young humans, and the primary motor cortex served as model for plasticity. One-to-one transferability of the results to participants with other characteristics (eg, patients with major depression) cannot be taken for granted, because basal state of brain activity and excitability will differ between groups, which might impact on tDCS effects. Moreover, it is currently unclear to what degree motor cortex plasticity results are transferable to other areas, which are more relevant for depression, such as the dorsolateral prefrontal cortex. However, as also motor cortex plasticity is reduced in major depression, but recovers along with reduction of depression symptoms (Player et al, 2014), M1 plasticity is assumed to be a feasible, although not ideal model for plasticity in major depression.
To our knowledge, this is the first pharmacological tDCS study evaluating effects of chronic pharmacological treatment on neuroplasticity in healthy participants. This paradigm can be used for other substances in future as well, in which different effects of acute and chronic medication are assumed, eg, for benzodiazepines. Our findings indicate that CIT shifts tDCS-induced plasticity into a facilitatory direction. This impact of serotonin on plasticity may be a relevant neurophysiological foundation of the effects of SSRIs in depressed patients. The results also suggest that modulation of brain plasticity via long-term SSRI application might be a promising pathway to treat patients with neurological deficits or psychiatric diseases who suffer from compromised plasticity.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
WP is a member of Advisory Boards of GSK, UCB, Desitin. MAN is a member of the Advisory Board of Neuroelectrics. H-IK is supported by the government scholarship, Republic of China (Taiwan). This study was supported by the BMBF-project, “Netzwerk psychische Erkrankungen”, grant 01EE1403C. M-FK, H-IK, AJ, and GB received no financial support or compensation from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Acler M, Robol E, Fiaschi A, Manganotti P (2009). A double blind placebo RCT to investigate the effcts of serotonergic modulation on brain excitability and motor recovery in stroke patients. J Neurol 256: 1152–1158. [DOI] [PubMed] [Google Scholar]
- Batsikadze G, Paulus W, Kuo M, Nitsche M (2013). Effect of serotonin on paired associative stimulation-induced plasticity in the human motor cortex. Neuropsychopharmacology 38: 2260–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bert B, Fink H, Rothe J, Walstab J, Bonisch H (2008). Learning and momory in 5-HT(1A)-receptor mutant mice. Behav Brain Res 195: 78–85. [DOI] [PubMed] [Google Scholar]
- Bezchilbnyk-Butler K, Aleksic I, Kennedy S (2000). Citalopram- a review pf pharmachological and clinical effects. J Psychiatry Neurosci 25: 241–254. [PMC free article] [PubMed] [Google Scholar]
- Brunoni A, Valiengo L, Baccaro A, Zanao T, Oliveira Jd, Goulart A (2013). The sertraline vs electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry 70: 1–9. [DOI] [PubMed] [Google Scholar]
- Consolo S, Arnaboldi S, Giogi S, Russi G, Ladinsky H (1994). 5-HT4 receptor stimulation facilitates acetylcholine release in rat frontal cortex. Neuroreport 5: 1230–1232. [DOI] [PubMed] [Google Scholar]
- Criti A, Malenka R (2008). Synaptic plasticity: multiple foms, functions, and mechanisms. Neuropsychopharmachology 33: 18–41. [DOI] [PubMed] [Google Scholar]
- Foy M, Stanton M, Levine S, Thompason R (1987). Behavioral stress impairs long-term potentiation in rodent hippocampus. Behav Neural Biol 48: 138–149. [DOI] [PubMed] [Google Scholar]
- Frensnoza S, Paulus W, Nitsche MA, Kuo MF (2014). Nonlinear dose-dependent impact of D1 receptor activation on motor cortex plasticity in humans. J Neurosci 34: 2744–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q (2002). Neuromodulatory transmitter systems in the cortex and their role cortical plasticity. Neuroscience 111: 815–835. [DOI] [PubMed] [Google Scholar]
- Henn FA, Vollmayr B (2004). Basic pathophysiological mechanisms in depression: what are they and how might they affect the course of the illness? Pharmacopsychiatry 37 (Suppl 2): S152–S156. [DOI] [PubMed] [Google Scholar]
- Holderbach R, Clark K, Moreu J, Bischofberger J, Norman C (2007). Enhanced long-term synaptic depression in an animal model of depression. Biol Psychiatry 62: 373–380. [DOI] [PubMed] [Google Scholar]
- Huang Y, Kandel E (2007). 5-Hydroxytryptamine induces a protein kinase a mitogen-dependent protein kinase-mediated and macromolecular synthesis-dependent late phase of long-term potentiation in the amagdala. J Neurosci 27: 3111–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Formal C (1997). Serotonin and motor activity. Curr Opin Neurobiol 7: 820–825. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D (2005). The 5-hydroxytryptamine4 receptor exhibits frequnecy-dependent properties in synaptic plasticity and behavioral metaplasticity in the hippocampal CA1 region in vivo. Cereb Cortex 15: 1037–1043. [DOI] [PubMed] [Google Scholar]
- Kojic L, Gu Q, Douglas R, Cynader M (1997). Serotonin facilitates synaptic plasticity in kitten visual cortex: an in vitro study. Brain Res DEv Brain Res 101: 299–304. [DOI] [PubMed] [Google Scholar]
- Kojima T, Matsumoyo M, Yogashi H, Tachibana K, Kemmotsu O, Yoshioka M (2003). Fluvoxamine suppress the long-term potentiation in the hippocampal CA1 field of anesthetized rats: an effect medisted via 5-HT 1A receptors. Brain Res 959: 165–168. [DOI] [PubMed] [Google Scholar]
- Kulla A, Manahan-Vaughan D (2002). Mosulation by serotonin 5-HT(4) receptors of long-term potentiation and depotentiation in the dentate gyrus of freely mobing rats. Cereb Cortex 12: 150–162. [DOI] [PubMed] [Google Scholar]
- Kuo MF, Paulus W, Nitsche MA (2008). Boosting focally-induced brain plasticity by dopamine. Cereb Cortex 18: 648–651. [DOI] [PubMed] [Google Scholar]
- Kuo MF, Paulus W, Nitsche MA (2014). Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage 15: 948–960. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche M, Tergau F, Paulus W (2002). Pharmacological approach to synaptic and membrane mechanisms of DC-induced neuroplasticity in man. Brain 125: 2238–2247. [DOI] [PubMed] [Google Scholar]
- Lisman J (2001). Three Ca2+ levels affect plasticity differently: the LTP zone, the LTD zone and no man's land. J Physiol 532: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte-Silva K, Liebetanz D, Grundey J, Paulus W, Nitsche M (2010). Dosage-dependent non-linear effect of L-dopa on human motor cortex plasticity. J Physiol 588: 3415–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Togashi H, Kojima T, Matsumoto M, Ohaishi S, Ueno K et al (2001). Different effects of anxiolytic agents, diazeoam and 5-HT(1A) agonist tandospirone, on hippocampal long-term potentiation in vivo. Pharmacol Biochem Behav 69: 367–372. [DOI] [PubMed] [Google Scholar]
- Nitsche M, Grundey J, Liebetanz D, Lang N, Tergau F, Paulus W (2004). Catecholaminergic consolidation of motor cortical neuroplasticity in humans. Cereb Cortex 14: 1240–1245. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A et al (2008). Transcranial direct surrent stimulation: state of art 2008. Brain Stimul 1: 206–223. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebtanz D, Lang N et al (2003. b). Pharmacological modulation of cortical excitability shifts induced by transcrnial DC stimulation. J Physiol 533: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Klein C, Tergau F, Rothwell J, Paulus W (2003. a). Level of action of cathodal DC polarization induced inhibition of the human motor cortex. Clin Neurophysiol 144: 600–604. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Kuo M, Karrasch R, Warden B, Liebtanz D, Paulus W (2009). Serotonin affects transcrnial direct current (tDCS)-induced neuroplasticity in humans. Biol Psychiatry 66: 503–508. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527: 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57: 1899–1901. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Roth A, Kuo NF, Fischer AK, Liebetanz D, Lang N (2007). Timing-dependent modulation of associative plasticity by general network excitability in the human motor cortex. J Neurosci 27: 3807–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann C, Clark K (2005). Selective modulation of Ca(2+) influx pathways by 5-HT regulates synaptic long-term plasticity in the hippocampus. Brain Res 1037: 187–193. [DOI] [PubMed] [Google Scholar]
- Normann C, Schmitz D, Furmaier A, Doing C, Bach M (2007). Long-term platsicity of visually evoked potentials in humans is altered in major depression. Biol Psychiatry 62: 373–380. [DOI] [PubMed] [Google Scholar]
- Panicker MM, Parker I, Miledi R (1991). Receptors of the serotonin 1C subtype expressed from cloned DNA mediate the closing of K+ membrane channels encoded by brain mRNA. Proc Natl Acad Sci USA 88: 2560–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Jang H, Cho K, Kim M, Yoon S, Rhie D (2012). Developmental switch of the serotonergic role in the induction of synaptic long-term potentiarion in the rat visual cortex. Korean J Physiol Pharmacol 16: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Duman R (2007). Stress, depression and neuroplasticity: a convergence of mechanisms. Neuropharmachology 33: 88–109. [DOI] [PubMed] [Google Scholar]
- Player MJ, Taylor JL, Weikert CS, Alonzo A, Sachdev PS, Martin D et al (2014). Increase in PAS-induced neuroplasticity after a treatment course of transcranial direct current stimulation for depression. J Affect Disord 167: 140–147. [DOI] [PubMed] [Google Scholar]
- Rajji TK, Sun Y, Zomorrodi-Moghaddam R, Farzan1 F, Blumberger DM, Benoit Mulsant BH et al (2013). PAS-induced potentiation of cortical-evoked activity in the dorsolateral prefrontal cortex. Neuropsychopharmacology 38: 2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser G, Donie F, Binmoller FJ (1989). Serotonin regulates cytosolic Ca2+ activity and membrane potential in a neuronal and in a glial cell line via 5-HT3 and 5-HT2 receptors by different mechanisms. J Cell Sci 93: 545–555. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Muller S, Boehringer A, Schulz A, Schaechinger H (2008). Antidepressive therapy with escitalopram improves mood, cognitive sumptoms, and identity memory for angry faces in elderly depressed patients. Int J Neuropsychopharmacol 11: 381–388. [DOI] [PubMed] [Google Scholar]
- Schaechter J (2004). Motor rehabilitation and brain plasticity after hemiparetic stroke. Prog Neurobiol 73: 61–72. [DOI] [PubMed] [Google Scholar]
- Stassen HH, Angst J (2012). Delayed onset of action of antidepressant. CNS Drugs 9: 177–184. [Google Scholar]
- Staubli U, Otaky N (1994). Serotonin controls the magnitude of LTP induced by theta bursts via action on NMDApreceptor-mediated responses. Brain Res 643: 10–16. [DOI] [PubMed] [Google Scholar]
- Stephan K, Kunesch E, Cohen LG, Benecke R, Classen J (2000). Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 123: 572–584. [DOI] [PubMed] [Google Scholar]
- Thirugnanasambandam N, Grundey J, Paulus W, Nitsche MA (2011). Dose-dependent nonlinear effect of L-DOPA on paired associative stimulation-induced neuroplasticity in humans. J Neurosci 31: 5294–5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MD, Wren PB (2008). Serotonin-dopamine interactions: omplications for the design of novel therapeitic agents for psychiatric disorders. Prog Brain Res 172: 213–230. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Nitsche M, Pascual-Leone A, Byblow W, Berardelli A et al (2008). Consesus: motor cortex plasticity protocols. Brain Stimul 1: 164–182. [DOI] [PubMed] [Google Scholar]