Abstract
Alzheimer's disease (AD) is the leading cause of dementia among elderly population. AD is characterized by the accumulation of beta-amyloid (Aβ) peptides, which aggregate over time to form amyloid plaques in the brain. Reducing soluble Aβ levels and consequently amyloid plaques constitute an attractive therapeutic avenue to, at least, stabilize AD pathogenesis. The brain possesses several mechanisms involved in controlling cerebral Aβ levels, among which are the tissue-plasminogen activator (t-PA)/plasmin system and microglia. However, these mechanisms are impaired and ineffective in AD. Here we show that the systemic chronic administration of recombinant t-PA (Activase rt-PA) attenuates AD-related pathology in APPswe/PS1 transgenic mice by reducing cerebral Aβ levels and improving the cognitive function of treated mice. Interestingly, these effects do not appear to be mediated by rt-PA-induced plasmin and matrix metalloproteinases 2/9 activation. We observed that rt-PA essentially mediated a slight transient increase in the frequency of patrolling monocytes in the circulation and stimulated microglia in the brain to adopt a neuroprotective phenotype, both of which contribute to Aβ elimination. Our study unraveled a new role of rt-PA in maintaining the phagocytic capacity of microglia without exacerbating the inflammatory response and therefore might constitute an interesting approach to stimulate the key populations of cells involved in Aβ clearance from the brain.
Introduction
Amyloid deposits and neurofibrillary tangle formation are the core pathological hallmarks of Alzheimer's disease (AD; Selkoe, 2002). Clinically, AD manifests with early mild memory deficits that evolve with time to reach severe cognitive impairment and consequently the loss of executive functions (De Souza et al, 2009). Despite all efforts, no efficient treatment exists for AD. However, strategies targeting amyloid deposition seem promising.
The brain possesses several sophisticated mechanisms that tightly control beta-amyloid (Aβ) processing and clearance, such as transport across the blood–brain barrier (BBB) and degradation by microglia activity (Zlokovic, 2011; Rivest, 2009). Activated microglia adopt diverse phenotypes ranging from a ‘classical activation' (ie, pro-inflammatory) phenotype that exacerbates inflammation to an ‘alternative activation' (ie, anti-inflammatory) phenotype that helps in tissue repair (Saijo and Glass, 2011; Heneka et al, 2013). Although, resident microglia have been demonstrated to contribute to Aβ clearance (Rivest, 2009), this clearance is extremely slow and ineffective in the AD brain (Hickman et al, 2008). Besides microglia, monocytes have important roles in AD (Michaud et al, 2013; Simard et al, 2006). Monocytes are mononuclear phagocytic cells and, in rodents, are regrouped into two main subsets based on chemokine receptors and Ly6C expression level, the ‘pro-inflammatory' subset (Ly6Chigh), which is actively recruited to inflamed tissues and contributes in inflammatory responses, and the ‘anti-inflammatory', or patrolling, subset (Ly6Clow), which patrols the lumen of blood vessel and promote tissue repair (Geissmann et al, 2003). In parallel, our group has recently demonstrated that the patrolling monocyte subset contributes to cerebral Aβ clearance (Michaud et al, 2013). Importantly, the expansion and the phagocytic capacity of monocytes decrease with age and in AD patients (Naert and Rivest, 2012).
Tissue-plasminogen activator (t-PA) is a serine protease that converts plasminogen into plasmin, an enzyme involved in fibrin degradation, which has been reported to be also involved in Aβ microaggregate degradation (Melchor et al, 2003). In parallel, t-PA has been proposed to act as an anti-inflammatory cytokine, independently from its protease activity (Stringer, 2000). Interestingly, similar to microglia spatial localization, t-PA expression and activity have been reported to localize around Aβ plaques in the brain of a mouse model of AD, suggesting its implication in Aβ processing (Melchor et al, 2003). Unfortunately, over time, the t-PA/plasmin system gets inefficacious in degrading Aβ microaggregates (Melchor et al, 2003). Importantly, the depletion of endogenous t-PA in Tg2576 mice has been reported to increase cerebral Aβ accumulation, and consequently worsening AD-related pathology in these mice, thus outlining the potential of this system as a therapeutic target for AD treatment (Oh et al, 2014).
In the present study, we investigated the therapeutic potential of Activase, a recombinant t-PA (rt-PA) that is approved by the Food and Drug Administration for ischemic stroke treatment, in modulating AD pathology. For this purpose, we used 4-month-old APPswe/PS1 mice that were intravenously treated once a week with relatively low doses of Activase r-tPA (5 mg/kg/week for 10 weeks). Here we report that rt-PA significantly delayed the progression of AD pathology, which was mediated by its anti-inflammatory characteristics. More precisely, rt-PA reduced soluble Aβ levels and Aβ plaque number and load, resulting in improved cognitive function without affecting BBB function and integrity. Interestingly, rt-PA treatment stimulated microglial protective characteristics via low-density lipoprotein receptor-related protein 1 (LRP1), which resulted in enhancing the coverage of Aβ plaques by microglia, and increasing the internalization of Aβ microaggregates by these cells. Moreover, rt-PA specifically increased the subset of patrolling monocytes, which are involved in the clearance of vascular Aβ. Taken together, our study demonstrates that rt-PA may constitute a novel approach to treat AD by enhancing the reparative phenotype of microglia and monocytes.
Materials and methods
This section is described in detail in Supplementary Materials and Methods.
Animal Experiments
Experiments were performed according to the Canadian Council on Animal Care guidelines, as administered by the Laval University Animal Welfare Committee. Animal experiments and Activase r-tPA treatment regimen are detailed in Supplementary Material and Methods.
Generation of Chimeric Mice
APPswe/PS1 chimeric mice were generated by transplanting bone marrow-derived cells of GFP+/− mice in myeloablated APPswe/PS mice, as described previously (Lampron et al, 2013).
Aβ Plaques, Microglia Coverage and Aβ Internalization by Microglia Quantification
Aβ plaques were stained with an anti-human Aβ monoclonal antibody (6E10) and microglia were stained with an anti-Iba1 polyclonal antibody as described previously (Simard et al, 2006). Aβ plaque number and load, microglia coverage, and Aβ internalization by microglia were assessed in the brain using a stereological apparatus as described (Boissonneault et al, 2009).
Human t-PA and Soluble Aβ1–42/Aβ1–40 ELISA
Brain levels of rt-PA were assessed by using the specific human t-PA Platinum ELISA kit (eBioscience Inc., San Diego, CA, USA). Brain levels of soluble Aβ1–42 and Aβ1–40 were quantified by using the Human Amyloid β42 and Human Amyloid β40 Brain ELISA kits (Millipore, Billerica, MA, USA). The experimental procedure for human t-PA, Aβ1–42, and Aβ1–40 detection was performed according to the manufacturer's instructions.
Brain Microvessel Isolation
Brain capillaries were isolated on dextran gradient as described previously (ElAli and Hermann, 2010), with slight modification. The quality of isolated microvessels was checked with a bright-field microscopy (Supplementary Figure 1a).
Isolation and Analysis of Microglia by Flow Cytometry
Mice were killed as described previously. Brains were removed and homogenized in 1 ml ice-cold Dulbecco's PBS without calcium (Ca2+)/magnesium (Mg2+). Homogenized brain samples were used to isolate microglia by using a modified Percoll gradient protocol.
Flow Cytometry
Flow cytometry analysis was used to determine the population of monocytes in the circulation. Facial vein blood was collected in EDTA-coated vials (Sarstedt, Newton, NC, USA). Flow cytometry analysis was performed as described (Lampron et al, 2013).
Behavior Analysis
The T-water maze paradigm was used to assess the right-left discrimination learning of mice, in the weeks following the last injection (Filali and Lalonde, 2009).
In Vitro Experiments
The immortalized murine microglial cell line (BV2) was used to investigate the role of rt-PA in modulating microglia function in vitro. The experiments conducted with BV-2 cells are described in Supplementary Material and Methods.
Statistics
Results are expressed as mean±standard error of the mean (SEM). For behavior analysis, results are expressed as median±range. All statistical analyses were performed using GraphPad Prism Version 6 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Activase rt-PA Regimen does not Affect BBB Integrity and Function
It has been shown that rt-PA crosses the BBB without altering its function (Benchenane et al, 2005). We first tested the effects of Activase rt-PA regimen on BBB physical integrity in APPswe/PS1 mice. rt-PA did not compromise BBB tightness after chronic weekly injections of 5 mg/kg of rt-PA for a period of 10 weeks, which was translated by the absence of albumin (Supplementary Figure 1b) and IgG (Supplementary Figure 1c) extravasation within brain parenchyma. To fully address the effects of rt-PA on the BBB, the expression levels of proteins involved in BBB physical integrity and function in wild-type mice were investigated 3 and 24 h after injection. Interestingly, rt-PA did not change the expression levels of the tight junction protein, Occludin (Supplementary Figure 2a and b) and Claudin 5 (Supplementary Figure 2c and d). In addition, rt-PA did not change the expression levels of ABCB1 (Supplementary Figure 2e and f), a transporter involved in brain detoxification (ElAli and Hermann, 2012) and possibly involved in Aβ elimination across the BBB (Cirrito et al, 2005). In parallel, rt-PA did not change LRP1 protein levels, a t-PA receptor that is also involved in the clearance of cerebral Aβ across the BBB (Benchenane et al, 2005; Deane et al, 2009; Supplementary Figure 3a and b), and receptor for advanced glycation endproducts (RAGE) that is involved in peripheral Aβ entry into the brain via the BBB (Donahue et al, 2006; Supplementary Figure 3c and d). Systemically administered rt-PA reached the brain in a dose-dependent manner 3 h after injection, which was 306±30.46 pg/ml for a dose of 5 mg/kg, and 719.8±38.61 pg/ml for a dose of 10 mg/kg (positive control). These results clearly demonstrate that Activase rt-PA reached the brain without inducing the breakdown of the BBB, and did not change the expression levels of some key proteins involved in Aβ transport across the BBB.
Activase rt-PA Slows the Progression of AD-Like Pathology and Behavioral Deficits in APPswe/PS1
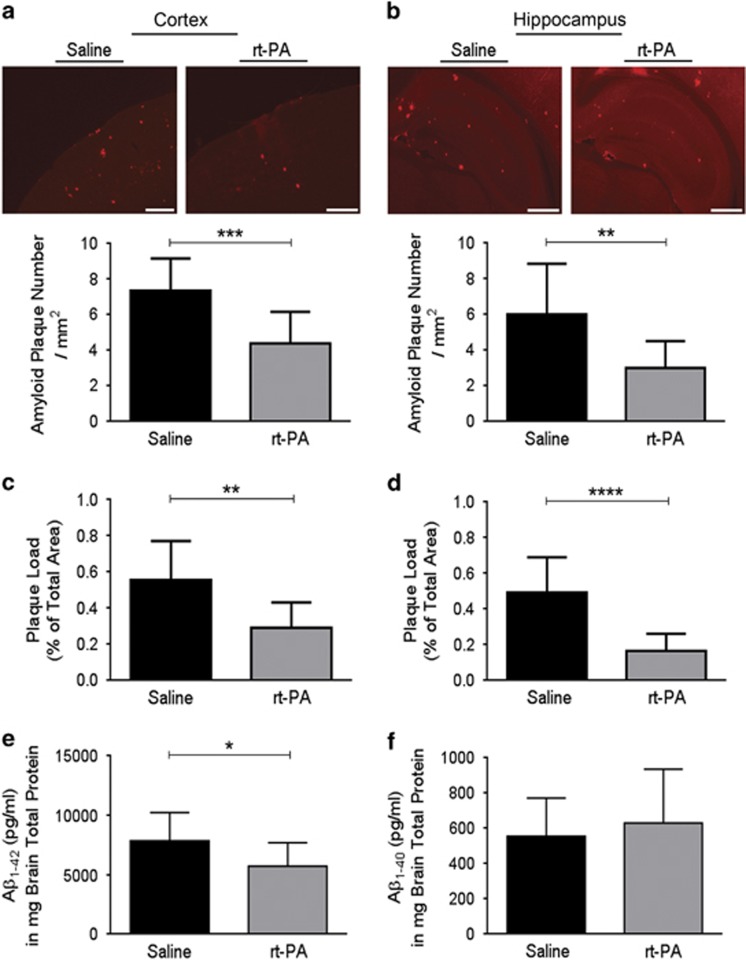
It has been proposed that the endogenous t-PA system is involved in cerebral Aβ processing. Therefore, the impact of Activase rt-PA regimen on plaque number and load was investigated. Interestingly, rt-PA systemic administration significantly reduced Aβ plaque number and load in the cortex (t=4.360, d.f.=24, p<0.001, and t=3.643, d.f.=24, p<0.01, respectively; Figure 1a and c) and hippocampus (t=3.309, d.f.=24, p<0.01, and t=5.148, d.f.=24, p<0.0001, respectively; Figure 1b and d). Moreover, the toxicity of soluble Aβ in the brain of AD patients (Lue et al, 1999) and mouse models of AD (Cheng et al, 2007) has been clearly demonstrated. Interestingly, rt-PA significantly reduced soluble Aβ1–42 levels (t=2.115, d.f.=17, p<0.05; Figure 1e) without affecting the levels of soluble Aβ1–40 (Figure 1f). To investigate the physiological relevance of rt-PA-induced Aβ clearance on mouse cognition, we used a T-water maze behavioral paradigm that assesses the right–left discrimination learning (Figure 2). No intergroup difference was seen during the acquisition phase of the T-water maze behavioral analysis (Kruskal–Wallis=0.78, p>0.05). The three groups of mouse took a similar number of trials before reaching criterion performance (Figure 2a). However, during the reversal phase, the analysis of the trials to criterion revealed a significant effect (Kruskal–Wallis=9.52, p<0.01; Figure 2b). Moreover, the Mann–Whitney multiples comparisons revealed that rt-PA-treated APPswe/PS1 mice and wild-type mice have both lower trials to criterion than saline-treated APPswe/PS1 mice (p<0.05 and p<0.01, respectively; Figure 2b). During the reversal phase, the three groups of mice did not exhibit any difference in swimming velocities (data not shown) indicating that motor function of tested mice was normal. Importantly, when animals were classified according to their cognitive performance (Figure 2c), the number of mice exhibiting severe cognitive deficit (SD) was lower in rt-PA-treated APPswe/PS1 mice and wild-type mice compared with saline-treated APPswe/PS1 mice (χ2 test, p<0.05; Figure 2c). In addition, the number of mice exhibiting no deficit (ND) was higher in rt-PA-treated APPswe/PS1 mice and wild-type mice compared with saline-treated APPswe/PS1 mice (χ2 test, p<0.05; Figure 2c). These results reveal that rt-PA-treated APPswe/PS1 and wild-type mice were largely similar in performance and exhibiting similar right–left discrimination learning profiles.
Figure 1.
Activase rt-PA administration reduces Aβ aggregates and soluble Aβ1–42 levels in the brain. Immunofluorescence staining (a–d) and ELISA (e and f) analyses examining the deposition of Aβ aggregates and Aβ1–42/Aβ1–40 soluble levels in the brain of APPswe/PS1 mice, 10 weeks after Activase rt-PA (5 mg/kg) weekly systemic administration. The 6E10 immunofluorescence staining shows (a) a decrease in Aβ plaque number in the cortex and the (b) hippocampus of treated animals. Moreover, 6E10 immunofluorescence staining shows (c) a reduction in Aβ plaque load in the cortex and the (d) hippocampus of treated animals. Finally, ELISA analysis shows (e) reduced levels of soluble Aβ1–42 and (f) unchanged levels of soluble Aβ1–40. Data are means±SEM (n=12–14 animals per group for immunofluorescence staining, and 9–10 animals per group for ELISA). Three sections representing the rostral, middle, and caudal levels of the hippocampus and overlaying cortex per animal's brain were used for immunofluorescence staining. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared with saline-treated group (standard two-tailed unpaired t-tests). Images were acquired with a × 4 objective. Scale bar=100 μm.
Figure 2.
Activase rt-PA administration improves APPswe/PS1 mouse cognitive functions. T-water maze behavioral test was used to examine spatial learning and memory (a and b) and to classify the cognitive performance of mice as a function of treatment (c). Activase rt-PA treatment (a) does not change the number of trials to reach criterion in the acquisition phase of the test, but (b) significantly enhances the cognitive functions of APPswe/PS1 mice as shown by their lower number of trials to reach the criterion in the reversal phase of the test. In addition, (c) rt-PA-treated APPswe/PS1 mice and wild-type (WT) mice exhibit similar cognitive deficit, which was less severe compared with saline-treated APPswe/PS1 mice. Data are median±range (n=13–16 animals per group). Each point represents an animal and the horizontal bars are the mean for each group. ND, no cognitive deficit; MD, mild cognitive deficit; SD, severe cognitive deficit. (a and b) *p<0.05/**p<0.01 compared with saline-treated group (Mann–Whitney U-test); (c) *p<0.05 compared with saline-treated group (χ2 two-sided test).
rt-PA-Dependent Plasmin and Matrix Metalloproteinase 2/9 (MMP2/9) Activation does not Contribute to Aβ Clearance
To shed light on the mechanisms that might be involved in the protective effects of rt-PA, we first investigated the implication of rt-PA in converting plasminogen into plasmin, an enzyme involved in Aβ degradation (Melchor et al, 2003). Moreover, t-PA can activate MMP2/9 (Adibhatla and Hatcher, 2008), two enzymes involved in vascular remodeling (Zhao et al, 2006) and Aβ degradation (Yan et al, 2006; Hernandez-Guillamon et al, 2010). The low dose Activase rt-PA regimen used in this study did not modulate the basal enzymatic activity of both plasmin (Supplementary Figure 4a) and MMP2/9 (Supplementary Figure 4c) in the brain and the microvasculature of APPswe/PS1-treated mice. To verify whether these unexpected results were due to the chronic systemic rt-PA administration in APPswe/PS1 mice and/or the presence of Aβ in the brain of these mice, we tested the effect of an acute bolus of rt-PA in wild-type mice. Similarly, the single systemic administration of rt-PA did not modulate the basal enzymatic activities of both plasmin (Supplementary Figure 4b) and MMP2/9 (Supplementary Figure 4d) in the brain and the microvasculature of wild-type mice. These results indicate that the mechanism underlying rt-PA-induced cerebral Aβ reduction and cognitive enhancement are probably independent of the rt-PA-induced plasmin/ MMP2/9 activation.
Activase rt-PA Modulates Monocyte Population Phenotypes in a Transient Manner
We next investigated the anti-inflammatory effects of rt-PA by analyzing its possible role in modulating monocyte populations. The chronic administration of rt-PA did not alter total monocyte frequency in the blood of APPswe/PS1 mice 24 h following last injection (Supplementary Figure 5a), but it induced a shift in monocyte phenotypes by reducing the frequency of Ly6Chigh inflammatory subset (t=2.785, d.f.=18, p<0.05; Supplementary Figure 5b and c) and by slightly increasing that of Ly6Clow patrolling subset (t=2.041, d.f.=18, p=0.0562; Supplementary Figure 5b and d). However, the acute administration of rt-PA significantly increased total monocyte frequency in APPswe/PS1 mice, 3 h following injection (t=2.397, d.f.=13, p<0.05), without altering the distribution of monocyte subpopulation (Supplementary Figure 6). To clearly address the direct effect of rt-PA on the population of monocytes in an Aβ-free context, which has been shown to influence monocyte responses (Naert and Rivest, 2012), we used wild-type mice that were injected with rt-PA (Supplementary Figure 7). The injection of rt-PA did not modulated the total monocyte and Ly6Chigh subset frequencies at 3 h (Supplementary Figure 7a and c) or 24 h (Supplementary Figure 7b and d) in wild-type animals, although it significantly increased Ly6Clow subset frequency at 3 h (t=2.329, d.f.=10, p<0.05; Supplementary Figure 7e), but not at 24 h (Supplementary Figure 7f). These results suggest that rt-PA induced a slight and transient phenotypic switch from Ly6Chigh inflammatory to Ly6Clow patrolling monocytes.
The Effects of Activase rt-PA on Resident Microglia
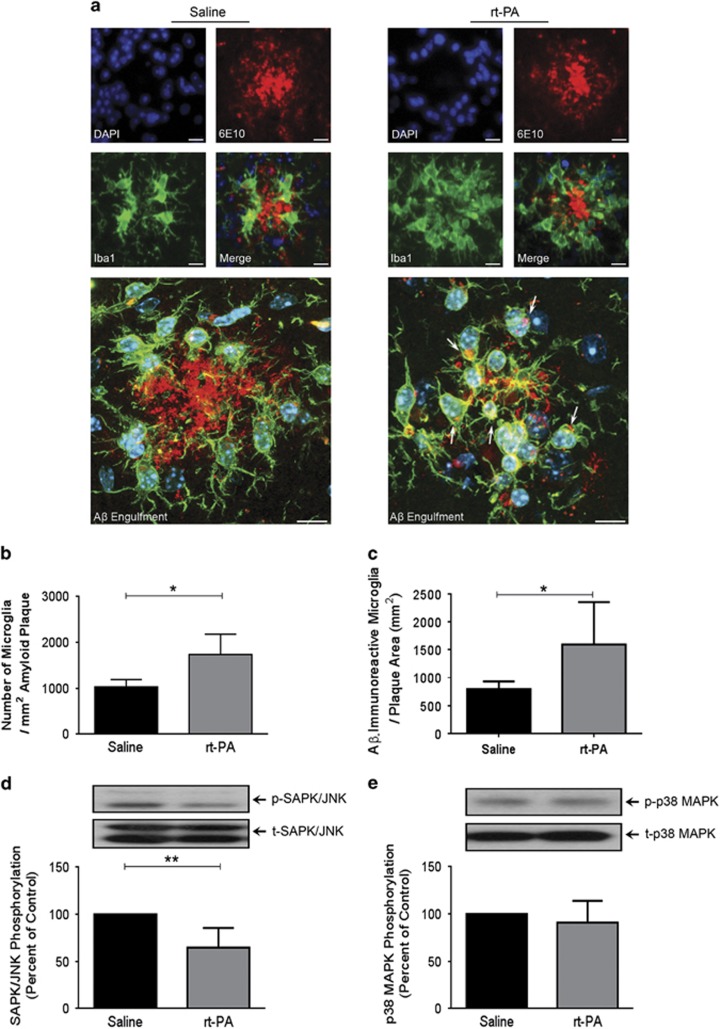
Endogenous t-PA has been shown to modulate microglia activity in vivo (Rogove et al, 1999). Therefore, we next investigated the effects of rt-PA administration on microglia behavior in the brain of APPswe/PS1. Chronic rt-PA systemic administration increased the number of resident microglia surrounding Aβ plaques (t=2.998, d.f.=6, p<0.05; Figure 3a and b) as well as the number of Aβ-immunoreactive resident microglia surrounding Aβ plaques (t=2.544, d.f.=10, p<0.05; Figure 3a and c), which translates Aβ internalization (ie, phagocytosis) in vivo by these cells. Interestingly, this phenomenon was associated with a significant global decrease in the activation (ie, phosphorylation) of the stress kinases SAPK/JNK (t=3.771, d.f.=8, p<0.01; Figure 3d), without affecting p38 MAPK activation (ie, phosphorylation; Figure 3e). Moreover, the enhanced microglial coverage of Aβ plaques was not due to an increased infiltration rate of circulating monocytes and their subsequent differentiation into mature microglia, as we did not detect any CD45high (blood-derived leukocytes)/Iba1 (differentiated microglia) double-positive staining (ie, blood-derived macrophages) within the cells surrounding Aβ plaques (Supplementary Figure 8a). In addition, we did not detect any changes in the frequency of CD11bhigh/CD45high (blood-derived macrophages) in the brain of APPswe/PS1 mice 3 and 24 h following a single rt-PA administration (Supplementary Figure 8b). Finally, these results were confirmed when we did not detect any GFP-positive cells in the brain of chimeric APPswe/PS1 mice treated with rt-PA (Supplementary Figure 8c). Moreover, chronic rt-PA administration did not alter the NF-κB signaling pathway activity that is involved in microglial pro-inflammatory activation, which was unraveled by the unchanged gene transcript expression levels of IκBα (Laflamme et al, 1999; Supplementary Figure 9). These data suggest that rt-PA might modulate resident microglia activity by enhancing their mobilization toward Aβ plaques and by decreasing the stress associated to inflammation, thus probably increasing their capacity to internalize Aβ present in their surrounding microenvironment.
Figure 3.
Chronic Activase rt-PA administration increases the number of resident microglia-associated to Aβ plaques and reduces the activation of stress-induced pathways. Immunofluorescence staining (a–c) and western blot (d and e) analyses examining microglia association to Aβ plaques, Aβ internalization by microglia, and the activation of stress-related kinases in the brain of APPswe/PS1 mice, 10 weeks after chronic systemic Activase rt-PA administration. Triple 6E10/ Iba1/DAPI immunofluorescence staining shows (a and b) an increased number of microglia (Iba1; green/DAPI; blue) surrounding Aβ plaques (6E10; red) and (a and c) an increased number of Aβ-immunoreactive resident microglia (microglia internalizing Aβ). Moreover, Activase rt-PA treatment (d) decreases the phosphorylation levels of SAPK/JNK (e) without affecting the phosphorylation levels of p38 MAPK. Optical densities were corrected with β-actin levels. Data are means±SEM (n=4–6 animals per group). Four sections representing the rostral, middle, and caudal levels of the hippocampus and overlaying cortex per animal's brain were used for immunofluorescence staining. *p<0.05, **p<0.01 compared with saline-treated group (standard two-tailed unpaired t-tests). Laser scan confocal images were acquired with a × 60 objective. Arrows indicate internalized Aβ microaggregates. Scale bar=10 μm.
Activase rt-PA Enhances Microglial Mobility and Acts as a Chemoattractant Molecule in a LRP1-Dependent Manner
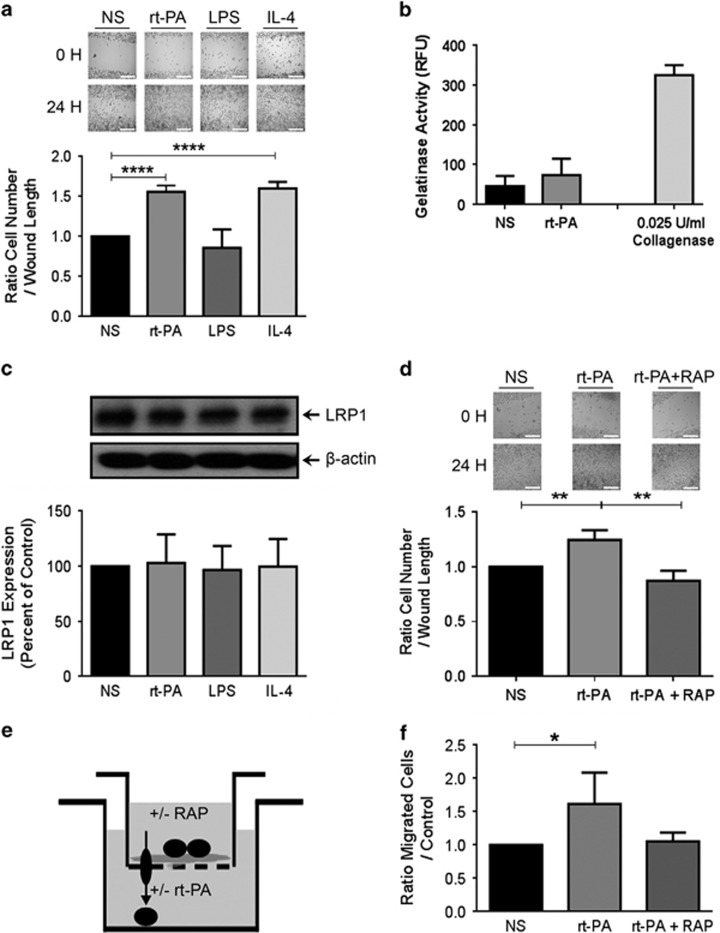
To fully address and decipher the effect of Activase rt-PA on microglia, we performed a series of experiments using microglial BV2 cell line. The strategy consisted on comparing BV2 cell stimulation with rt-PA along with two other molecules that are lipopolysaccharide (LPS) and interleukin 4 (IL-4), known to trigger the activation of these cells toward a pro-inflammatory phenotype (LPS) or anti-inflammatory one (IL-4). rt-PA (0.1 nM) and IL-4 (5 ng/ml) exposure enhanced microglial cell mobility in a similar manner (t=13.94, d.f.=6, p<0.0001, and t=14.69, d.f.=6, p<0.0001, respectively; Figure 4a), whereas they failed to modulate MMP2/9 activity (Figure 4b) and LRP1 expression, a receptor for t-PA (Figure 4c). However, rt-PA-induced microglial cell mobility was LRP1 dependent, because LRP1 inhibition by RAP essentially abolished rt-PA-induced cell mobility (t=5.097, d.f.=4, p<0.01; Figure 4d). We then verified the possible role of rt-PA as a chemoattractant molecule that triggers microglial cell mobility and migration using a two chambers Transwell experimental setting (Figure 4e) and found that rt-PA acted as a chemoattractant molecule that mobilized microglial cells to the lower chamber that contains the molecule (t=2.651, d.f.=6, p<0.05; Figure 4f). This role was mediated by microglial LRP1, as its inhibition by recombinant RAP prevented these effects (Figure 4f). Taken together, these results underlie a key chemoattractant role of rt-PA on microglial cells via LRP1.
Figure 4.
Activase rt-PA modulates microglia function in vitro. Cell migration assay (a and d), gelatinase activity assay (b), western blot (c) and chemotaxis assay (e and f) analyses examining the behavior of BV2 microglial cells after stimulation with Activase rt-PA (0,1 nM), LPS (2 μg/ml), IL-4 (5 ng/ml), and RAP (200 nM). Cell migration assay shows (a) an enhanced migration of microglial cells 24 h after stimulation with rt-PA or IL-4 compared with control or LPS exposure. Gelatin activity assay shows that (b) Activase rt-PA does not induce MMP2/9 activation in any conditions. Western blot analysis confirms (c) the unchanged expression levels of LRP1. Cell migration assay (d) shows that rt-PA-induced mobility is LRP1 dependent, as LRP1 inhibition with RAP, decreases cell migration. The two-chamber chemotaxis assay (e) provided evidence that rt-PA induces microglial mobilization toward a gradient of rt-PA present in the lower chamber. Microglia cells (f) are mobilized by Activase rt-PA in a LPR1-dependent manner 3 h after stimulation. Optical densities were corrected with β-actin levels. Data are means±SEM (n=3–4 independent experiments). *p<0.05, **p<0.01, ****p<0.0001 compared with control group (standard two-tailed unpaired t-tests). Dark spheres represent BV2 cells. The descending arrow illustrates cell mobilization from the upper chamber toward the lower chamber. Scale bar=250 μm.
Activase rt-PA Dampers Intracellular Stress in Microglia
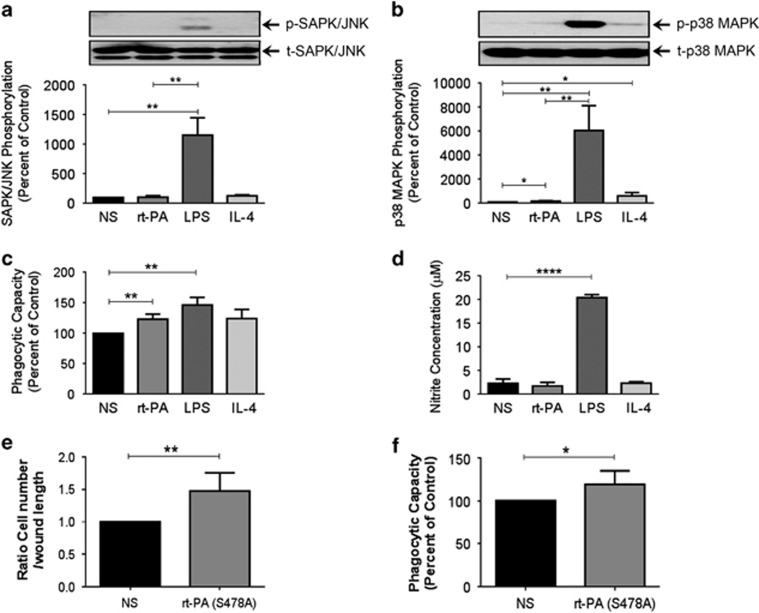
The effects of Activase r-tPA on the regulation of SAPK/JNK and p38 MAPK signaling pathways were investigated in BV2 microglial cells. In contrast to LPS (2 μg/ml), IL-4 and rt-PA failed to induce SAPK/JNK phosphorylation (Figure 5a), but they slightly increased p38 MAPK phosphorylation (t=3.270, d.f.=4, p<0.05; Figure 5b). These effects of rt-PA were in contrast with the effect of LPS that causes a robust p38 MAPK signaling induction, suggesting that rt-PA does not trigger a pro-inflammatory phenotype in BV2 microglial cells.
Figure 5.
Activase rt-PA decreases microglial intracellular stress and preserves their phagocytic capacity independently of its proteolytic activity in vitro. Western blot (a and b), phagocytosis assay (c and f), Griess assay (d), cell migration assay (e) analyses examining BV2 microglial cell intracellular stress responses, mobilization, and phagocytic capacity after stimulation with Activase rt-PA (0.1 nM), the mutated form of rt-PA (S478A; 0.1 nM), LPS (1 μg/ml), and IL-4 (5 ng/ml). Western blot analysis shows that (a) rt-PA and IL-4 do not increase the phosphorylation of SAPK/JNK, which was strongly increased in the presence of LPS. The endotoxin also caused higher phosphorylation levels (b) of p38 MAPK than rt-PA and IL-4, whereas the phagocytic capacity of microglial cells (c) remained similar between rt-PA and LPS. This response to LPS was associated with nitrite production and release by microglial cells, which was absent (d) in the presence of rt-PA. Cell migration assay shows (e) an enhanced migration of microglial cells 24 h after stimulation with the mutated form of rt-PA that is deprived of any enzymatic activity rt-PA (S478A). Finally, the phagocytosis assay (e) shows that rt-PA (S478A) still enhances phagocytic capacity of microglial cells. Data are means±SEM (n=3–5 independent experiments). *p<0.05, **p<0.01, ****p<0.0001 compared with control group (standard two-tailed unpaired t-tests).
The Effects of Activase rt-PA on the Phagocytic Capacity and Oxidative Stress Cascade in Microglia
Nitrite production and release by activated microglia are involved in microglial-derived oxidative stress (Kaushaland and Schlichter, 2008). Interestingly, rt-PA and LPS potently enhanced microglial cell phagocytic capacity 1 h after stimulation (t=5.037, d.f.=4, p<0.01, and t=6.374, d.f.=4, p<0.01, respectively; Figure 5c). However, in contrast to LPS stimulation (t=28.29, d.f.=4, p<0.0001), rt-PA stimulation did not trigger nitrite production and release by activated microglial cells (Figure 5d). Oligomeric HiLyte Fluor 488-labeled human Aβ42 internalization was confirmed by confocal microscopy following rt-PA stimulation (Supplementary Figure 10). These results suggest that rt-PA was able to enhance and preserve microglial cell phagocytic capacity without triggering microglial-derived harmful oxidative stress.
The Effects of Activase rt-PA on the Mobility and the Phagocytic Capacity of Microglia are Independent of its Proteolytic Activity
To verify whether the observed effects of rt-PA are not associated to its enzymatic activity, we stimulated BV2 microglial cells with a full-length mutated form of rt-PA that is deprived of any enzymatic activity, rt-PA (S478A), in which alanine has been substituted for the active-site serine. Interestingly, rt-PA (S478A) still potently enhanced cell mobility (t=3.806, d.f.=8, p<0.01; Figure 5e) and phagocytic capacity (t=2.731, d.f.=8, p<0.01; Figure 5f) of BV2 cells. These results suggest that rt-PA acted essentially as a cytokine/chemoattractant molecule that modulated microglial cell activity, which did not require its proteolytic activity.
Discussion
This study unravels a novel role of Activase rt-PA in counteracting the progression of AD-like pathology in APPswe/PS1 mice. These effects include Aβ clearance together with a slight increase in the frequency of patrolling monocytes and a preserved phagocytic capacity of resident microglia. It is noteworthy to mention that the Activase rt-PA regimen used in this study did not induce BBB breakdown, which was evaluated by albumin and IgG extravasation and the expression of the tight junction proteins Occludin and Claudin 5. Moreover, Activase rt-PA regimen did not alter BBB function, which was evaluated by the expression of several transporters and receptors involved in Aβ transport, such as ABCB1 (ElAli et al, 2013; Cirrito et al, 2005), LRP1 (Deane et al, 2009), and RAGE (Donahue et al, 2006). This study also shows that the chronic systemic administration of Activase rt-PA decreased Aβ plaque number and load, and the levels of soluble Aβ1–42 in the brain of APPswe/PS1. This specific reduction of Aβ1–42 is in line with a recent study, which reported that the specific modulation of microglial cell phagocytic capacity has more profound effects on Aβ1–42 levels compared with Aβ1–40 (Griciuc et al, 2013). Such differential reduction might be reflected by the fact that Aβ plaques contain higher levels of Aβ1–42 (Kowa et al, 2012). Importantly, the right–left discrimination learning in the T-water maze of rt-PA-treated APPswe/PS1 mice was significantly improved, which has been shown to be impaired in AD (Middei et al, 2004; Rouleau et al, 1992).
The t-PA/plasmin system has been shown to be involved in Aβ degradation either directly through the enzymatic activity of plasmin (Melchor et al, 2003) or indirectly through the enzymatic activity of t-PA-induced MMP2/9 activation (Yan et al, 2006; Hernandez-Guillamon et al, 2010). However, the low doses of Activase rt-PA used in this study did not significantly induce the activation of plasmin and MMP2/9. Our study indicates that the effects of rt-PA on cerebral Aβ load and memory seem to be independent of plasmin/MMP2/9 activation.
The relevance of t-PA system in AD has been outlined in some studies, suggesting it as a potential therapeutic target (Melchor et al, 2003; Oh et al, 2014). However, all these experimental studies have investigated endogenous t-PA with a focus of its well-characterized proteolytic function. In our study, the effects of Activase rt-PA do not appear to be mediated via plasmin/MMP2/9 activation. Therefore, it is conceivable to propose that cerebral Aβ reduction following systemic rt-PA administration is rather due to a dynamic and synergistic interaction between blood circulation and the brain. Importantly, t-PA has been shown to act as an anti-inflammatory cytokine, independently from its proteolytic activity (Stringer, 2000). Therefore, Activase rt-PA effects on circulating monocytes were first investigated. Consistent with its anti-inflammatory characteristics, rt-PA transiently decreased the frequency of pro-inflammatory monocyte subset and induced a slight increase in the frequency of patrolling monocyte subset in blood circulation. Interestingly, our group has demonstrated, by using a novel two-photon intravital imaging approach in the brain of live APPswe/PS1 mice, that the patrolling monocyte subset actively contributes in clearing Aβ microaggregate from brain vasculature, and their depletion increased the overall load of cerebral Aβ and worsened the cognitive function of these mice (Michaud et al, 2013). Taken together, these results suggest that the increased frequency of the patrolling monocyte subset, although modestly and transiently, partly contribute in rt-PA-induced Aβ clearance from the brain.
Although the role of neuronal and microglial-derived endogenous t-PA in modulating microglial cell function in different contexts has been described (Rogove et al, 1999; Siao et al, 2003), little is known about the role of exogenously administered rt-PA, which rather imitates vascular-derived t-PA production, on resident microglial cells in AD. Therefore, the effects of Activase rt-PA on brain resident microglia were also investigated. Activase rt-PA chronic administration significantly increased the number of resident microglia covering Aβ plaques, which translates an enhanced mobility and mobilization of these cells toward Aβ aggregates. Interestingly, this increased coverage was accompanied by an enhanced internalization of Aβ microaggregate into microglia, thus suggesting an enhance clearance of Aβ by these cells. In parallel, Activase rt-PA chronic administration significantly decreased the phosphorylation levels of SAPK/JNK in the brain of treated mice. SAPK/JNK is activated by a variety of environmental stresses, including Aβ (Tare et al, 2011). As such, these results outline a global reduction in the environmental stresses in the brain of APPswe/PS1 mice following rt-PA treatment. In parallel, NF-κB signaling pathway has been shown to mediate the pro-inflammatory actions of microglia (O'Neill and Kaltschmidt, 1997). Importantly, Activase rt-PA did not modulate IκBα gene transcript expression, which is an adaptor protein involved in controlling NF-κB signaling pathway, and widely used as an indicator of NF-κB activity (Laflamme et al, 1999). These results indicate that Activase rt-PA chronic systemic administration modulated resident microglia function, resulting in an enhanced clearance of Aβ, which was accompanied by a reduction in the stress signals in the brain.
The molecular mechanisms involved in Activase rt-PA effects on microglial cells were next investigated in vitro by using the BV2 microglial cell line, a valid substitute for primary microglial cell culture (Henn et al, 2009). Activase rt-PA stimulation enhanced microglial cell mobility and invasion in a similar manner as IL-4 but in contrast to LPS. This effect was dependent on the interaction between rt-PA and its receptor expressed on microglial cells, LRP1, as the inhibition of the latter with LRP1 inhibitor, RAP, essentially abolished the effects of rt-PA. Moreover, this invasion did not depend on the enzymatic activity of MMP2/9. The diffuse nature of endogenous t-PA and exogenously administered rt-PA within the brain parenchyma (Siao et al, 2003), prompted us to test its chemoattractant properties in vitro. Indeed, by using the two chambers Transwell experiment, we showed that Activase rt-PA triggered microglia cell mobilization into the chamber containing the molecule, which was LRP1-dependent, as microglial cell incubation in the upper chamber with RAP totally abolished the chemoattractant characteristics of rt-PA. In parallel, microglial cell stimulation with rt-PA and IL-4 did not induce SAPK/JNK phosphorylation, a kinase involved in mediating the pro-inflammatory actions of microglia (Waetzig et al, 2005), whereas LPS potently induced it. Moreover, rt-PA and IL-4 slightly increased p38 MAPK phosphorylation, another kinase that is associated to microglial stress (Kakimura et al, 2002), whereas LPS induced a robust increase in p38 MAPK phosphorylation. Importantly, Activase rt-PA stimulation enhanced and preserved the phagocytic capacity of microglial cells, without mediating nitrite production that is involved in microglial-derived harmful oxidative stress (Kaushal and Schlichter, 2008). For instance, it has been shown that the pronounced production of nitrites by activated microglial cells exacerbates the pro-inflammatory microenvironment that occurs in AD, thus contributing to the decreased efficiency of resident microglia to clear Aβ (Heneka et al, 2013; Jantzen et al, 2002). As such, these results clearly demonstrate that rt-PA enhanced the phagocytic capacity of microglia without generating nitrite production.
Taken all together, our study provides new insights into a proteolytic-independent mechanism via which rt-PA stimulates the reparative function of microglia, thus enhancing cerebral Aβ clearance and consequently ameliorating the cognitive function of treated mice. Although our study suggests that Activase rt-PA might constitute a potential treatment for AD, the possible detrimental effects of this drug should be taken into consideration. As such, it would be interesting in the future to test the effect of similar molecules that have less detrimental effects, such as Desmoteplase, or to develop specific LRP1 agonists. Strategies aiming at modulating monocyte and microglia function are discussed in Supplementary Conclusion.
Funding and disclosure
This work was supported by the Canadian Institutes in Health Research (CIHR), Canadian Stroke Network, the Fonds de la Recherche du Québec—Nature et Technologie (FRQNT), NORAMPAC Alzheimer Foundation, and the Centre de Recherche en Endocrinologie Moléculaire et Oncologique et Génomique Humaine (CREMOG). The authors declare no conflict of interest.
Acknowledgments
We thank Mrs Nataly Laflamme, Mrs Marie-Michèle Plante, and Mr Paul Préfontaine for their technical support.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Adibhatla RM, Hatcher JF (2008). Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets 7: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Berezowski V, Fernández-Monreal M, Brillault J, Valable S, Dehouck MP et al (2005). Oxygen glucose deprivation switches the transport of tPA across the blood-brain barrier from an LRP-dependent to an increased LRP-independent process. Stroke 36: 1059–1064. [DOI] [PubMed] [Google Scholar]
- Boissonneault V, Filali M, Lessard M, Relton J, Wong G, Rivest S (2009). Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer's disease. Brain 132: 1078–1092. [DOI] [PubMed] [Google Scholar]
- Cheng IH, Scearce-Levie K, Legleiter J, Palop JJ, Gerstein H, Bien-Ly N et al (2007). Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem 282: 23818–23828. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB et al (2005). P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest 115: 3285–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza LC, Sarazin M, Goetz C, Dubois B (2009). Clinical investigations in primary care. Front Neurol Neurosci 24: 1–11. [DOI] [PubMed] [Google Scholar]
- Deane R, Bell RD, Sagare A, Zlokovic BV (2009). Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer's disease. CNS Neurol Disord Drug Targets 8: 6–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue JE, Flaherty SL, Johanson CE, Duncan JA 3rd, Silverberg GD, Miller MC et al (2006). RAGE, LRP-1, and amyloid-beta protein in Alzheimer's disease. Acta Neuropathol 112: 405–415. [DOI] [PubMed] [Google Scholar]
- ElAli A, Hermann DM (2010). Apolipoprotein E controls ATP-binding cassette transporters in the ischemic brain. Sci Signal 3: ra72. [DOI] [PubMed] [Google Scholar]
- ElAli A, Hermann DM (2012). Liver X receptor activation enhances blood-brain barrier integrity in the ischemic brain and increases the abundance of ATP-binding cassette transporters ABCB1 and ABCC1 on brain capillary cells. Brain Pathol 22: 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElAli A, Thériault P, Préfontaine P, Rivest S (2013). Mild chronic cerebral hypoperfusion induces neurovascular dysfunction, triggering peripheral beta-amyloid brain entry and aggregation. Acta Neuropathol Commun 1: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filali M, Lalonde R (2009). Age-related cognitive decline and nesting behavior in an APPswe/PS1 bigenic model of Alzheimer's disease. Brain Res 1292: 93–99. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR (2003). Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19: 71–82. [DOI] [PubMed] [Google Scholar]
- Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K et al (2013). Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 78: 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A et al (2013). NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 493: 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn A, Lund S, Hedtjärn M, Schrattenholz A, Pörzgen P, Leist M (2009). The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 26: 83–94. [DOI] [PubMed] [Google Scholar]
- Hernandez-Guillamon M, Mawhirt S, Fossati S, Blais S, Pares M, Penalba A et al (2010). Matrix metalloproteinase 2 (MMP-2) degrades soluble vasculotropic amyloid-beta E22Q and L34V mutants, delaying their toxicity for human brain microvascular endothelial cells. J Biol Chem 285: 27144–27158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Allison EK, El Khoury J (2008). Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci 28: 8354–8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen PT, Connor KE, DiCarlo G, Wenk GL, Wallace JL, Rojiani AM et al (2002). Microglial activation and beta-amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J Neurosci 22: 2246–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimura J, Kitamura Y, Takata K, Umeki M, Suzuki S, Shibagaki K et al (2002). Microglial activation and amyloid-beta clearance induced by exogenous heat-shock proteins. FASEB J 16: 601–603. [DOI] [PubMed] [Google Scholar]
- Kaushal V, Schlichter LC (2008). Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J Neurosci 28: 2221–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowa H, Sakakura T, Matsuura Y, Wakabayashi T, Mann DM, Duff K et al (2012). Mostly separate distributions of CLAC- versus Abeta40- or thioflavin S-reactivities in senile plaques reveal two distinct subpopulations of beta-amyloid deposits. Am J Pathol 165: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme N, Lacroix S, Rivest S (1999). An essential role of interleukin-1beta in mediating NF-kappaB activity and COX-2 transcription in cells of the blood-brain barrier in response to a systemic and localized inflammation but not during endotoxemia. J Neurosci 19: 10923–10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampron A, Pimentel-Coelho PM, Rivest S (2013). Migration of bone marrow-derived cells into the central nervous system in models of neurodegeneration. J Comp Neurol 521: 3863–3876. [DOI] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L et al (1999). Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol 155: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchor JP, Pawlak R, Strickland S (2003). The tissue plasminogen activator-plasminogen proteolytic cascade accelerates amyloid-beta (Abeta) degradation and inhibits Abeta-induced neurodegeneration. J Neurosci 23: 8867–8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud JP, Bellavance MA, Préfontaine P, Rivest S (2013). Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Rep 5: 646–653. [DOI] [PubMed] [Google Scholar]
- Middei S, Geracitano R, Caprioli A, Mercuri N, Ammassari-Teule M (2004). Preserved fronto-striatal plasticity and enhanced procedural learning in a transgenic mouse model of Alzheimer's disease overexpressing mutant hAPPswe. Learn Mem 11: 447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naert G, Rivest S (2012). Age-related changes in synaptic markers and monocyte subsets link the cognitive decline of APPSwe/PS1 mice. Front Cell Neurosci 6: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SB, Byun CJ, Yun JH, Jo DG, Carmeliet P, Koh JY et al (2014). Tissue plasminogen activator arrests Alzheimer's disease pathogenesis. Neurobiol Aging 35: 511–519. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Kaltschmidt C (1997). NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci 20: 252–258. [DOI] [PubMed] [Google Scholar]
- Rogove AD, Siao C, Keyt B, Strickland S, Tsirka SE (1999). Activation of microglia reveals a non-proteolytic cytokine function for tissue plasminogen activator in the central nervous system. J Cell Sci 112: 4007–4016. [DOI] [PubMed] [Google Scholar]
- Rouleau I, Salmon DP, Butters N, Kennedy C, McGuire K (1992). Quantitative and qualitative analyses of clock drawings in Alzheimer's and Huntington's disease. Brain Cogn 18: 70–87. [DOI] [PubMed] [Google Scholar]
- Rivest S (2009). Regulation of innate immune responses in the brain. Nat Rev Immunol 9: 429–439. [DOI] [PubMed] [Google Scholar]
- Saijo K, Glass CK (2011). Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 11: 775–787. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ (2002). Alzheimer's disease is a synaptic failure. Science 298: 789–791. [DOI] [PubMed] [Google Scholar]
- Siao CJ, Fernandez SR, Tsirka SE (2003). Cell type-specific roles for tissue plasminogen activator released by neurons or microglia after excitotoxic injury. J Neurosci 23: 3234–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S (2006). Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron 49: 489–502. [DOI] [PubMed] [Google Scholar]
- Stringer KA (2000). Tissue plasminogen activator inhibits reactive oxygen species production by macrophages. Pharmacotherapy 20: 375–379. [DOI] [PubMed] [Google Scholar]
- Tare M, Modi RM, Nainaparampil JJ, Puli OR, Bedi S, Fernandez-Funez P et al (2011). Activation of JNK signaling mediates amyloid-ß-dependent cell death. PLoS One 6: e24361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waetzig V, Czeloth K, Hidding U, Mielke K, Kanzow M, Brecht S et al (2005). c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia 50: 235–246. [DOI] [PubMed] [Google Scholar]
- Yan P, Hu X, Song H, Yin K, Bateman RJ, Cirrito JR et al (2006). Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. J Biol Chem 281: 24566–24574. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ et al (2006). Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med 12: 441–445. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV (2011). Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 12: 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.