Abstract
Cannabis is the most commonly used illicit drug worldwide, and use is typically initiated during adolescence. The endocannabinoid system has an important role in formation of the nervous system, from very early development through adolescence. Cannabis exposure during this vulnerable period might lead to neurobiological changes that affect adult brain functions and increase the risk of cannabis use disorder. The aim of this study was to investigate whether exposure to Δ9-tetrahydrocannabinol (THC) in adolescent rats might enhance reinforcing effects of cannabinoids in adulthood. Male adolescent rats were treated with increasing doses of THC (or its vehicle) twice/day for 11 consecutive days (PND 45–55). When the animals reached adulthood, they were tested by allowing them to intravenously self-administer the cannabinoid CB1-receptor agonist WIN55,212-2. In a separate set of animals given the same THC (or vehicle) treatment regimen, electrophysiological and neurochemical experiments were performed to assess possible modifications of the mesolimbic dopaminergic system, which is critically involved in cannabinoid-induced reward. Behavioral data showed that acquisition of WIN55,212-2 self-administration was enhanced in THC-exposed rats relative to vehicle-exposed controls. Neurophysiological data showed that THC-exposed rats displayed a reduced capacity for WIN55,212-2 to stimulate firing of dopamine neurons in the ventral tegmental area and to increase dopamine levels in the nucleus accumbens shell. These findings—that early, passive exposure to THC can produce lasting alterations of the reward system of the brain and subsequently increase cannabinoid self-administration in adulthood—suggest a mechanism by which adolescent cannabis exposure could increase the risk of subsequent cannabis dependence in humans.
Introduction
Worldwide, cannabis remains the most widely used illicit drug (UNODC, 2014). Initiation of cannabis use typically occurs during adolescence, a critical phase of brain development characterized by progressive and specific neuroplastic modifications that determine the morphology and functionality of the brain (ie, synaptic plasticity, neuronal cell proliferation, migration, and differentiation) (Rice and Barone, 2000). The endocannabinoid system via CB1 receptors (CB1Rs) has an important role during this period as it is involved in neuromaturation and synaptic pruning as well as in the maintenance and survival of differentiated neural cells (Galve-Roperh et al, 2009; Viveros et al, 2012). Expression of CB1Rs increases from early stages of brain development and reaches maximal levels during adolescence, after which levels remain stable or decrease into adulthood (Rodriguez de Fonseca et al, 1993; McLaughlin et al, 1994; Belue et al, 1995). CB1Rs are present at high densities in brain areas important for executive functioning, reward, and memory processing (Mackie, 2005; Burns et al, 2007). The adolescent brain is particularly sensitive to perturbations, and consequently exposure to cannabis might affect development of the endocannabinoid system and induce neurobiological changes that affect adult brain function. These long-lasting changes might contribute to negative outcomes, such as problematic patterns of use of cannabis and other illicit drugs (Copeland and Swift, 2009; Chadwick et al, 2013; Hurd et al, 2014).
In fact, both clinical and epidemiological evidence suggest that cannabis use during adolescence is linked to increased risk for subsequent use of addictive drugs such as heroin and cocaine (ie, the so-called ‘gateway hypothesis' Kandel, 1975; Fergusson et al, 2006; Fergusson and Boden, 2008) and also to increased risk of developing cannabis use disorder (Gray, 2013). For example, people who initiate cannabis use during adolescence have greater risk of developing cannabis dependence later in life, as compared with those who start cannabis use as adults (Coffey et al, 2003; Fergusson et al, 2003; Chen et al, 2005, 2009). Animal models of drug abuse provide an objective and controlled means of studying such gateway effects by exposing animals to Δ9-tetrahydrocannabinol (THC, the main psychoactive component of cannabis) or to synthetic cannabinoids during adolescence and then offering them other drugs for self-administration in adulthood (Rubino et al, 2012). Using such procedures, THC exposure during adolescence has been shown to increase morphine and heroin self-administration by rats in adulthood (Biscaia et al, 2008; Ellgren et al, 2007). This effect is associated with alterations of the endogenous opioid system in limbic-related neuronal populations known to mediate reward behavior (Ellgren et al, 2007; Tomasiewicz et al, 2012). Moreover, chronic THC exposure during adolescence augments vulnerability to stress-induced relapse to heroin seeking (Stopponi et al, 2013) and the sensitivity to morphine conditioning in the place preference paradigm in adult rats (Morel et al, 2009). In contrast with these consistent findings of enhanced opioid self-administration after cannabinoid exposure, conflicting results have been found with self-administration of psychostimulant drugs. Ellgren et al (2004) found that pretreatment with THC or the synthetic cannabinoid CB1R agonist WIN 55,212-2 during early adolescence did not change the dopaminergic or behavioral responses to amphetamine in either adolescence or adulthood. Chronic administration of a different synthetic CB1R agonist, CP 55,940, during adolescence increased cocaine self-administration in adult female rats but produced no effects in males (Higuera-Matas et al, 2008). Exposure to cannabinoids during adolescence enhanced the acquisition and reinstatement of 3,4-methylenedioxymetamphetamine hydrochloride-induced conditioned place preference in mice (Rodríguez-Arias et al, 2010).
To the best of our knowledge, no animal study has assessed the possibility that exposure to THC during adolescence increases susceptibility to cannabinoid self-administration in adulthood. Thus, in the present study, independent groups of adolescent rats were exposed to either THC or an equivalent volume of vehicle for 11 days. Then, when they had reached adulthood, they were allowed to intravenously (i.v.) self-administer the synthetic CB1R agonist WIN 55,212-2 using the same self-administration protocol as in our previous studies (Fattore et al, 2001, 2007). It is well established that the endocannabinoid system is involved in the regulation of reward-related processes and that cannabinoids can activate mesolimbic dopamine circuitry by enhancing the activity of dopamine (DA) neurons in the ventral tegmental area (VTA) (French et al, 1997; Gessa et al, 1998), resulting in increased release of DA from nerve terminals in the shell of the nucleus accumbens (NAc shell) (Tanda et al, 1997). Therefore, to assess possible modifications in the mesolimbic dopaminergic system function in rats exposed to THC in adolescence, we analyzed: (1) the electrophysiological effects of WIN 55,212-2 administration on the activity of VTA DA neurons in anesthetized rats; and (2) the capacity of WIN 55,212-2 to induce elevation in DA levels in the NAc shell in freely moving rats. Moreover, because cannabis use during adolescence confers an increased risk of mental health problems, and because preclinical studies can be useful in confirming and studying neurocognitive alterations (Schneider and Koch, 2003; Rubino et al, 2008; Chadwick et al, 2013), we characterized the neurobehavioral profile of adult rats that had been exposed to THC in adolescence; this characterization included tests of spontaneous locomotor activity, elevated plus maze behavior (a model of anxiety), prepulse inhibition of the acoustic startle reflex (a test of sensorimotor gating, which is often abnormal in humans with schizophrenia or other psychiatric disorders), and sucrose preference (a test for depression-like anhedonia) (Rubino et al, 2008; Rubino and Parolaro, 2014).
Material and methods
Animals
Male Lister-Hooded rats (PND 38, Harlan-Nossan, Milan, Italy) were housed (5 per cage) in a climate-controlled animal room (21±2 °C temperature; 60% humidity) under a reversed 12-h light/dark cycle (lights on 0700 hours) with standard rat chow and water ad libitum. All experiments were approved by the local Animal Care Committee and carried out in strict accordance with the E.C. Regulations for Animal Use in Research (CEE No. 86/609). All efforts were made to minimize animal suffering and reduce the number of animals used.
Drugs
THC (RTI International, Research Triangle Park, NC, 1 g/5 ml in ethanol solution), was dissolved in a vehicle containing 2% Tween 80, 2% ethanol, and saline and injected intraperitoneally (i.p.) in a volume of 1 ml/kg of body weight. For self-administration, WIN55,212-2 (R-[2,3-dihydro-5-methyl-3 [(morpholinyl)methyl]-pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)-methanone mesylate), (WIN, Tocris, Bristol, UK) was first dissolved in one drop of Tween 80 and then diluted in heparinized (1%) sterile saline solution and made available at a concentration of 12.5 μg/100 μl. For electrophysiology and microdialysis experiments, WIN was first dissolved in one drop of Tween 80 and then diluted in sterile saline solution and injected i.v. in a volume of 1 ml/kg of body weight.
Treatment
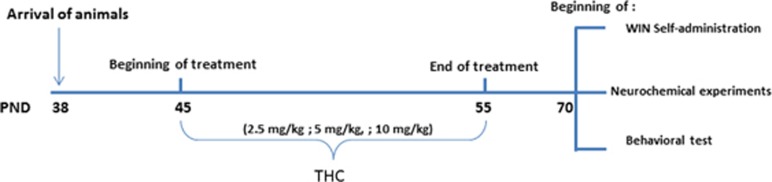
Rats were acclimated for 1 week before starting treatment with THC or vehicle at PND 45, in the mid-adolescence period (Schneider, 2013). Increasing doses of THC (2.5 mg/kg, PND 45–47; 5 mg/kg, PND 48–51; 10 mg/kg, PND 52–55) or vehicle were given twice/day for 11 consecutive days. Theses doses of THC were chosen according to the literature (Rubino et al, 2008). Body weight and food intake were monitored for the entire period of treatment. Once animals reached adulthood (70 PND), experiments were started (Figure 1).
Figure 1.
Time schedule of THC exposure and behavioral testing following during adulthood.
Food Intake and Body Weight
At the beginning of treatment, rats were divided into two groups matched for body weight and food intake (n=10 rats per group). These two parameters were monitored throughout the entire treatment. As animals were housed five per cage, chow amounts consumed per animal per day were averaged by dividing the amount per cage by five.
Intravenous WIN Self-Administration
Apparatus and procedure were the same as described previously (Fattore et al, 2001, 2007). Under isoflurane 2% (Virbac, Italy) anesthesia, rats (PND 70) were surgically implanted with a silastic catheter in the right jugular vein. After surgery, each rat was given subcutaneous antibiotic treatment (0.1 ml Baytril, Bayer) and given 7 days to recover in an individual cage. Then, food intake was limited to 20 g/day, and animals were trained to press a lever for a response-contingent infusion of WIN under a one-response (FR1) schedule of reinforcement during 2-h daily sessions. WIN self-administration was performed in 12 operant chambers (29.5 × 32.5 × 23.5 cm3), each encased in a sound- and light-attenuating cubicle equipped with a ventilation fan (Med Associates, USA). The house light was illuminated to signal the start of the session. Depression of one lever, defined as the ‘active' lever, resulted in: (i) extinction of the house light and illumination of the stimulus light, which remained on for 5 s; (ii) retraction of both levers; and (iii) activation of the infusion pump for 5 s, which delivered a 12.5 μg/kg dose of WIN in a volume of 0.1 ml. There was a 15-s time-out after each drug infusion, after which the two levers were re-extended into the chamber, the stimulus light went out, and the house light was illuminated. Depressions of the other lever (defined as ‘inactive') were recorded but had no programmed consequences. Assignment of the active (drug-paired) and inactive (no drug-paired) levers to the left and right sides was counterbalanced and remained constant for each subject throughout all phases of the study. Rats met the acquisition criterion when the ratio of active to inactive responses over three consecutive days was >2 : 1.
Electrophysiology
Experiments were performed on animals that were only tested in the electrophysiology experiment. These rats were divided into vehicle- and THC-exposed groups and tested on PND 70 (n=6 rats per group). For all electrophysiology experiments, extracellular single unit recordings were obtained from DA cells located within the lateral portion of the posterior VTA, a subregion which contains the majority of DA neurons projecting to the NAc lateral shell (Lammel et al, 2014). Briefly, rats were anesthetized with urethane (1.3 g/kg, i.p.), and their femoral vein was cannulated for i.v. administration of drugs. Animals were placed in the stereotaxic apparatus (Kopf, Tujunga, CA, USA) with their body temperature maintained at 37±1 °C by a heating pad. Unit activity of putative DA neurons located in the VTA (6.0 mm posterior from bregma, 0.4–0.6 mm lateral from midline, V 7.0–8.0 mm from the cortical surface) (Paxinos and Watson 1998) was recorded extracellularly with glass micropipettes filled with 2% pontamine sky blue dissolved in 0.5 M sodium acetate (impedance 2–5 MΩ). Single unit activity was filtered (bandpass 500–5000 Hz), and individual spikes were isolated by means of a window discriminator (Neurolog Instruments, Digitimer, UK) and digitally recorded and sampled by a PC with Spike2 software and CED 1401 interface (Cambridge Electronic Design, Cambridge, UK). VTA putative DA neurons were isolated and identified according to already published criteria (Lecca et al, 2012; reviewed in Ungless and Grace, 2012): firing rate ⩽10 Hz, action potential duration ⩾2.5 ms, and inhibitory responses to hindpaw pinching. After 5 min of stable baseline activity, WIN was administered i.v. at exponentially increasing doses (125–500 μg/kg) every 2 min. Only one cell was recorded per rat. At the end of each recording session, direct current (10 mA for 15 min) was passed through the recording electrode to eject Pontamine sky blue. Rats were deeply anesthetized with urethane and decapitated. Brains were then removed and fixed in 8% w/v paraformaldehyde in PBS. The position of electrodes was microscopically identified on serial sections (60 μm) stained with Neutral Red.
In vivo Microdialysis
Experiments were performed in a room dimly illuminated by a red lamp starting on PND 70. These rats were only tested in the microdialysis experiment and were divided into vehicle- and THC-exposed groups (n=5 rats per group). During the same surgery session, rats were implanted with i.v. silastic catheters into the external jugular vein and microdialysis probes aimed at the NAc shell as described previously under anesthesia with Equithesin (5 ml/kg i.p.), (Fadda et al, 2006; Justinova et al, 2013). NAC shell coordinates in mm relative to bregma were: AP, +1.6; ML, ±1.1; and DV, −7.9; with the mouth bar set to −3.3 flat skull (Paxinos and Watson, 1998). Dialysate samples were collected every 20 min and analyzed by high-performance liquid chromatography coupled to electrochemical detection. Rats were treated with WIN (300 μg/kg i.v.) only after DA values were stable (<10% variability) for at least three consecutive samples. This drug dose was selected on the basis of the daily amount of WIN typically self-administered by male Lister Hooded rats under the same experimental conditions (Fattore et al, 2007; Justinova et al, 2013). Importantly, this dose of WIN was also shown to significantly increase DA release in the shell part of the NAc of rats (Tanda et al, 1997). Probe location in the NAc shell was determined histologically after each experiment.
Behavioral Tests
Animals that were only used in the behavioral-test experiments were divided into vehicle- and THC-exposed rats. Testing started on PND 70, with a recovery period of 4 days between each testing condition in the experiment (n=8 rats per group).
Locomotor activity
Rats were individually tested for locomotor activity using the Digiscan Animal Activity Analyzer (Omnitech Electronics, USA) in a dark room dimly illuminated by a red lamp as previously described (Spano et al, 2013). During a session of 60 min, the following behavioral parameters were measured: distance travelled (cm), and time (s) spent in the center zone (<1 cm from wall).
Elevated plus maze
The elevated plus maze test was carried out as described previously (Rubino et al, 2008). The apparatus consisted of two opposite open arms (50 × 10 cm2) and two enclosed arms (50 × 10 × 40 cm3) extended from a common central platform (10 × 10 cm2) The test was performed in a room dimly illuminated by a red lamp. Rats were acclimatized to the experimental room for 30 min and then were placed in the central platform of the apparatus and video recorded for 5 min (Ugo Basile, Any-maze). Percentage of time spent in open arms and percentage of entries in the open arms were measured.
Prepulse inhibition (PPI)
The general procedure was carried out as described previously (Spano et al, 2010). The startle reflex system consisted of four standard cages each placed inside a sound-attenuated and ventilated chamber (Med Associated, USA). Startle cages were non-restrictive Plexiglas cylinders (diameter 9 cm) mounted on a piezoelectric accelerometer platform connected to an analog-digital converter. Background noise and acoustic bursts were conveyed through two speakers placed in proximity to the startle cages so that the sound intensities did not differ by >1 dB between cages. On the test day, each rat was placed in the experimental cage for a 5-min acclimatization period with a 70-dB white noise background; the white noise was continued for the remainder of the session. Animals were then tested on three consecutive trial blocks. The first and the third blocks consisted of 5 pulse-alone trials of 40 ms at 115 dB, while the second block (test block) was a pseudorandom sequence of 50 trials, including 12 pulse-alone trials, 30 pulse trials preceded by 74, 78, or 82 dB prepulses (10 for each level of prepulse loudness), and 8 no-stimulus trials (where the only background noise was delivered). The percentage of (%) PPI was calculated based only on the values relative to the second block and using the following formula: 100−((mean startle amplitude for prepulse+pulse trials/mean startle amplitude for pulse-alone trials) × 100).
Sucrose preference test
The general procedure was carried out as described previously (Rubino et al, 2008). Subjects were housed singly for the 3 days of test. Rats were given two bottles, one of sucrose (2%) and one of tap water. The position of the bottles was changed every 24 h to control for potential preference for drinking location, and the amount of sucrose and water consumed was evaluated. Fluid consumption (g) was measured by weighing the bottles before and after each test session. The sucrose preference index was calculated as the percentage of sucrose solution ingested relative to the total amount of liquid consumed: Sucrose intake (g)/(Sucrose intake (g)+Water intake (g)) × 100 (Amchova et al, 2014).
Statistical Analysis
All results are presented as means±SEM. Data from body weight and food intake were analyzed by two-way analysis of variance (ANOVA) for repeated measures, with drug treatment (vehicle and THC) and day as between-groups factors, and day as a repeated factor. For self-administration experiments, the cumulative number of responses on both the active and inactive levers over the 2 h was measured. The difference between the two groups was analyzed by two-way ANOVA for repeated measures, with drug treatment (vehicle and THC) and session as between-groups factors and session as a repeated factor. Within each group, the difference between active and inactive lever responding was analyzed by two-way ANOVA for repeated measures, with lever and session as between-groups factors and session as a repeated factor. For electrophysiology experiments, drug-induced changes in spontaneous firing rate were calculated by averaging the effects for the 2 min following drug administration and normalized to the predrug baseline. Data within each group were analyzed by one-way ANOVA for repeated measures. Difference between the two groups was analyzed by two-way ANOVA for repeated measures, with drug treatment (vehicle and THC) and doses as between-groups factors and dose as a repeated factor. For microdialysis experiments, basal DA values were calculated as the mean of the three consecutive samples and results are expressed as a percentage of basal DA values. Data within each group were analyzed by one-way ANOVA for repeated measures. Difference between the two groups was analyzed by two-way ANOVA for repeated measures, with drug treatment (vehicle and THC) and time as between-groups factors and time as a repeated factor. Behavioral experiments were analyzed with two-way ANOVA (with the two factors being drug treatment and prepulses for PPI and drug treatment and day for the sucrose preference data) or Student's t-test when only two conditions were compared. Post hoc multiple comparisons were performed by Dunnett's test or by Bonferroni's test. In all cases, differences with a P<0.05 were considered significant.
Results
Body Weight and Food Intake
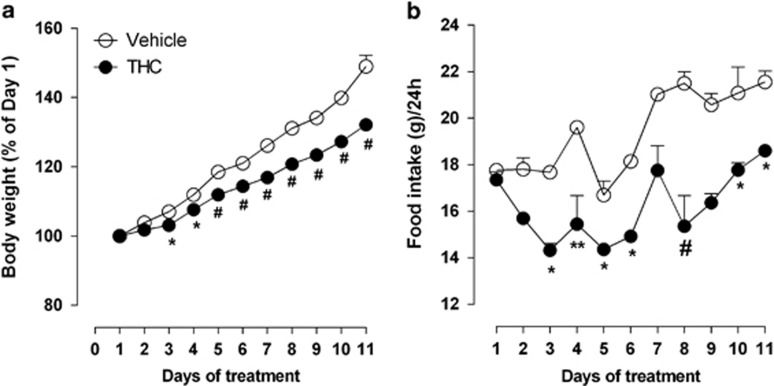
As shown in Figure 2a, we found a significant difference in the percentage of body weight gain between THC- and vehicle-exposed rats. The weight gain of the rats treated with THC was lower than that of rats exposed to vehicle (two-way ANOVA significant main effect of drug treatment × days interaction: F(10,180)=24.87, P<0.0001). Post hoc analysis showed that the significant difference in body weight started from the third day of treatment (Days 3 and 4 P<0.05; from Days 5–11 P< 0.001). Moreover, the effect on weight gain found in THC-exposed rats was accompanied by a concomitant reduction of food intake as compared with vehicle-exposed rats (Figure 2b; two-way ANOVA significant main effect of treatment × days interaction: F(10,20)= 3.07, P<0.0152; post-hoc analysis (Days 3, 5, 6, 10, and 11 P<0.05; Day 4 P<0.01; Day 8 P<0.001)). However, these differences in body weight were restricted to the period of THC exposure. When the rats reached adulthood, no significant differences were found between controls and drug-treated rats (PND 70: vehicle-exposed rats 322.2±4.6 vs THC-exposed rats 318.3±6.8).
Figure 2.
Effect of chronic treatment with THC (n=10) or its vehicle (n=10) on body weight (a) and food intake (b) during THC exposure (PND 45–55). Each point represents the mean±SEM. Two-way ANOVA: *P<0.05, **P<0.01 and #P<0.001 vs vehicle-exposed rats.
Effects of Adolescent THC Treatment on Acquisition and Maintenance of WIN Self-Administration in Adulthood
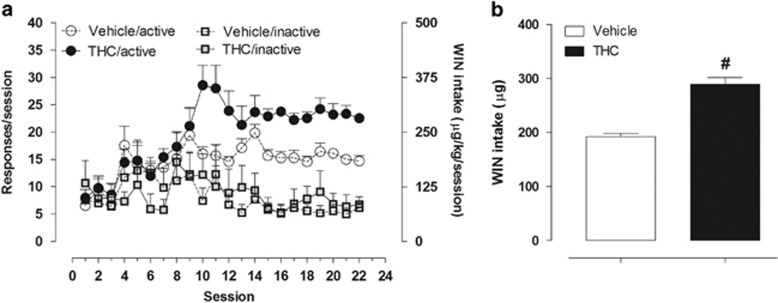
Acquisition of WIN (12.5 μg/100 μl) self-administration behavior was studied over 22 sessions in rats that had been exposed to THC or vehicle during adolescence (Figure 3a). In line with previous studies (Fattore et al, 2001, Spano et al, 2013), in the first few days of training there was no clear discrimination between the active and inactive levers in either group. However, a significant increase in responding on the active lever developed over subsequent sessions in both vehicle- and THC-treated rats, whereas responding on the inactive lever remained at low levels. Rats in the THC-exposed group acquired the self-administration response significantly faster (t(14)=2.695, P=0.018), meeting the acquisition criterion in 12.4±0.7 days compared with 15.29±0.4. Two-way ANOVA revealed a significant lever × session interaction in both groups (vehicle-exposed rats F(21,252)=1.65, P=0.0400; THC-exposed rats F(21,336)=4.65, P<0.0001). The THC-exposed rats had a significantly higher pressing rate on the active lever compared with vehicle-exposed rats (two-way ANOVA significant main effect of drug treatment × session interaction: F(21,294)=2.37, P=0.0008). In accordance with this, the total amount of WIN consumed by THC-exposed rats during the maintenance phase (ie, once animals stabilized their drug intake) was significantly higher than that consumed by vehicle-exposed rats. More specifically, during the last week of training, the mean total amount of WIN self-administered by vehicle- and THC-exposed rats was, respectively, 192.4±5.7 and 288.7±12.73 μg/kg (Student's unpaired t-test: t(14)=6.261, P<0.0001; Figure 3b). On the last day of training, response rates were 50% higher in THC- than in vehicle-exposed rats.
Figure 3.
WIN self-administration on a fixed ratio 1 (FR1) schedule. (a) Data points represent the average number of total responses produced by WIN on the active or inactive levers during daily 2-h sessions in vehicle- (n=7) and THC-exposed rats (n=9). (b) Mean WIN intake over the last seven training sessions in vehicle- (n=7) and THC-exposed rats (n=9) during WIN self-administration. Student's unpaired t-test: #P<0.0001 vs vehicle-treated rats.
Effect of Adolescent THC Exposure on WIN-Induced Stimulation of VTA DA Neuronal Activity in Adulthood
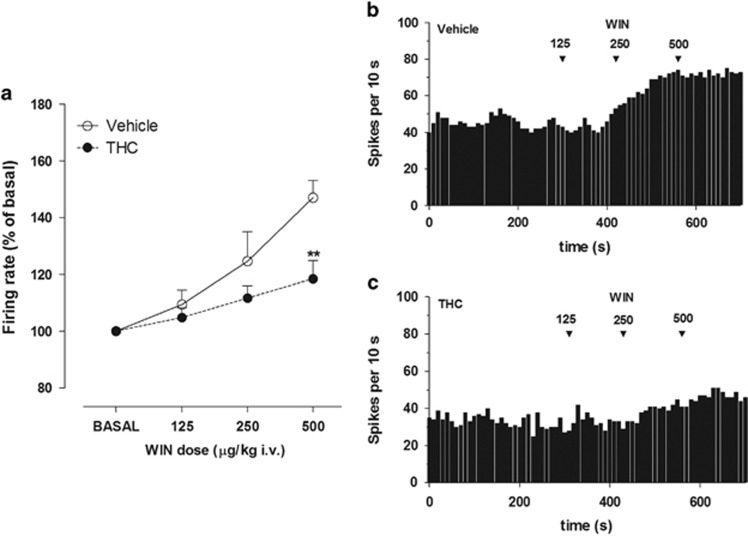
Adolescent THC administration did not affect the spontaneous basal activity of DA neurons, with mean (±SEM) firing rates of 3.53±0.42 and 2.86±0.7 Hz in vehicle- and THC-exposed rats, respectively (unpaired Student's t-test: t(9)=0.638, P=0.43). However, THC exposure during adolescence clearly altered midbrain DA neuronal response to cannabinoids in adulthood. I.v. injection of WIN (125–500 μg/kg, cumulative doses) dose dependently increased the discharge activity of VTA DA neurons in both vehicle-exposed and THC-exposed rats but had a significantly smaller effect in THC-exposed rats. Specifically, maximal firing was increased to 147.64±6.12% of the predrug baseline at WIN 500 μg/kg (one-way ANOVA F(3,16)=9.8, P=0.0007) in vehicle-exposed rats and to 118.4±6.5% of baseline in THC-exposed rats (one-way ANOVA F(3,20)=3.538, P=0.033) (Figure 4a), but the maximum rate was significantly lower in THC-exposed rats compared with controls (two-way ANOVA, treatment × dose interaction: F(3,27)=4.18, P=0.0149, with post hoc analysis revealing a significant difference between the two groups at the dose of 500 μg/kg (P<0.01)). Representative firing rate histograms of VTA DA neurons recorded from a vehicle- and THC-exposed rats are shown in Figure 4b and c, respectively.
Figure 4.
(a) Dose–response curves displaying the effect of cumulative doses of WIN on the firing rate of VTA DA neurons recorded from vehicle- (n=5) and THC- exposed rats (n=6). Results are presented as mean±SEM of firing rate expressed as a percentage of baseline levels. Two-way ANOVA: **P<0.01 vs vehicle-exposed rats. Exemplificative firing rate histograms of DA neurons recorded from (b) a vehicle- and (c) a THC-exposed rat show frequency increases after systemic administration of WIN. Arrows indicate the time of injection. Numbers above arrows indicate the dosages expressed in μg/kg i.v.
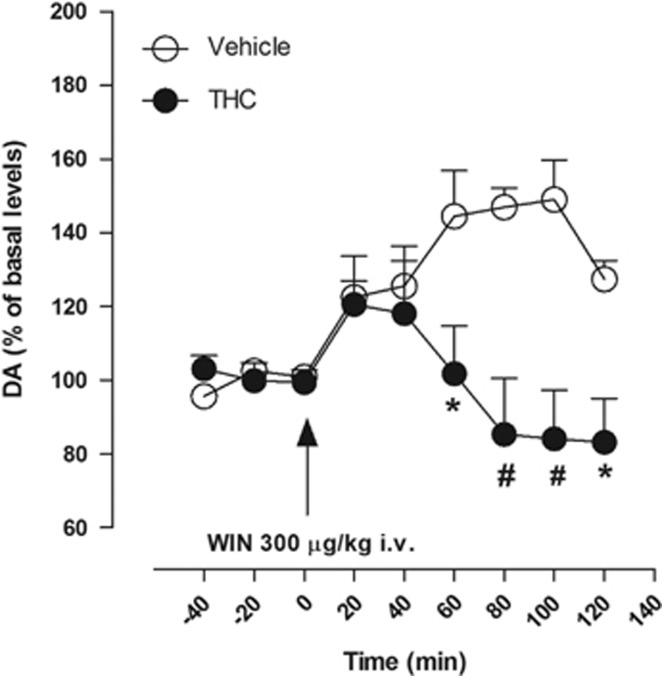
Effects of Adolescent THC Treatment on WIN-Induced Elevation in DA Levels in the NAc Shell in Adulthood
Basal extracellular values of DA in the NAc shell did not differ significantly between the two groups (38.89±3.8 and 41.45±4.74 fmol/50 μl in vehicle- and THC-exposed rats, respectively; unpaired Student's t-test: t(8)=0.2541, P=0.8058). At a dose that mimics the mean daily amount of drug typically self-administered by trained rats (300 μg/kg i.v.), acute i.v. injection of WIN significantly increased extracellular levels of DA in the NAc shell of vehicle-exposed rats to approximately 50% higher of basal levels (one-way ANOVA for repeated measures F(8,32)=6.647, P<0.0001; Figure 5). In contrast, in THC-exposed rats, WIN did not significantly increase extracellular levels of DA (one-way ANOVA for repeated measures F(8,32)=2.164 P=0.0581). Comparing the two experimental groups, two-way ANOVA revealed a significant treatment × time interaction (F(8,72)=4.66, P=0.0001). Post hoc analysis revealed a significant difference between the two groups from the sixtieth minute after injection (P<0.05 and P<0.001); although both groups showed a slight increase during the first 40 min after the injection, this was not significantly different from baseline for either group.
Figure 5.
Effect of an intravenous administration of WIN 300 μg/kg on DA release in the NAc shell of vehicle- (n=5) and THC-exposed rats (n=5). Results are means, with vertical bars representing SEM, of DA levels in 20-min dialysate samples, expressed as a percentage of basal values. Arrows represent the time of injection of WIN. Two-way ANOVA: *P<0.05 and #P<0.001 vs vehicle-exposed rats.
Effects of Adolescent THC Treatment in Behavioral Tests
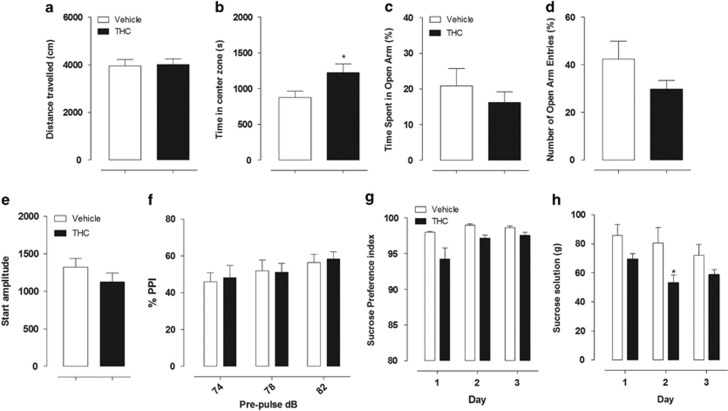
Spontaneous locomotor activity
There was no significant difference between the THC- and vehicle-exposed groups on distance traveled during the session (unpaired Student's t-test: t(14)=0.1767, P=0.7702; Figure 6a). However, THC-exposed rats spent more time in the center zone compared with vehicle-exposed rats, an effect that might indicate lower levels of anxiety in the THC-exposed rats (unpaired Student's t-test: t(14)=2.340, P=0.0346; Figure 6b).
Figure 6.
Effect of adolescent treatment with THC (n=8) or its vehicle (n=8) on behavioral test after THC exposure in adult rats. Locomotor activity: (a) distance travelled, expressed in cm and (b) amount of time spent in the center zone during the 60-min session, as a measure of anxiety. Student's unpaired t-test:*P<0.05 vs vehicle-exposed rats. Elevated plus maze: (c) percentage of time spent in open arms and (d) percentage of open arm entries. There were no significant differences between the vehicle- and THC-exposed rats. PPI: (e) startle amplitude and (f) PPI at prepulse intensities of 74, 78, and 82 dB. Data represent the mean±SEM. There were no significant differences between the vehicle- and THC-exposed rats for both measures. Sucrose preference test: (g) sucrose preference index and (h) amount of sucrose solution ingested expressed in grams. Two-way ANOVA: *P<0.05 vs vehicle-exposed rats. All data represent the mean±SEM.
Elevated plus maze
The results obtained in the elevated plus maze are presented in Figure 6c and d. No significant group differences were found on the percentage of time or entries into the open in THC-exposed rats when compared with vehicle-exposed rats (unpaired Student's t-test: t(14)=0.8201, P=0.7694 and t(14)=1.521, P=0.3077, respectively).
Prepulse inhibition
Vehicle- and THC-exposed rats did not show differences in acoustic-startle response amplitude (unpaired Student's t-test: t(14)=1.179, P=0.2582; Figure 6e). Moreover, we also found no significant differences between the two groups when analyzing the inhibition (PPI) of startle response at the three different prepulse intensities (two-way ANOVA treatment × prepulse interaction F(2,28)=0.16, P=0.8575; Figure 6f).
Sucrose preference test
Vehicle- and THC-exposed rats did not show any significant group differences in sucrose preference during the 3 days of testing (two-way ANOVA treatment × day interaction F(2,28)=1.77, P=0.1897), and both groups had about the same sucrose preference index (Figure 6g). However, the groups did differ in the amount of sucrose solution ingested (expressed in grams), with vehicle-exposed rats consuming significantly more sucrose solution than THC-exposed rats (Figure 6h); two-way ANOVA revealed a significant main effect of treatment (F(1,28)=5.34 P=0.0366), and post hoc analysis revealed a significant difference between the two groups on day 2 (P<0.05).
Discussion
The present study evaluated whether adolescent THC exposure might produce long-lasting effects that alter subsequent addiction-related responses to cannabinoids in adult rats. The first finding of this study was that chronic treatment with THC in adolescent rats caused a reduction in weight gain associated with a decrease in food intake compared with vehicle-exposed rats. Although it is generally accepted that stimulation of CB1Rs typically increases food intake, opposite results similar to ours have also been reported. Indeed, previous studies (Rubino et al, 2008; Stopponi et al, 2013) have also shown that food intake is decreased during THC exposure using the same regimen of THC treatment that we used (ie, 2.5 mg/kg, PND 45–47; 5 mg/kg, PND 48–51; 10 mg/kg, PND 52–55). Keeley et al (2015) also found that chronic treatment with THC (5 mg/kg for 14 days) in adolescent rats led to a significant reduction of food intake and body weight. Possible explanations for this decreased food intake include the use of relatively high doses of THC and possible differences in the sensitivity of CB1R between adolescent and adult rats.
Using a rodent model of cannabinoid reinforcement in which rats i.v. self-administer WIN 55,212–2, we confirmed our earlier demonstrations that this synthetic cannabinoid has a clear reinforcing effect in rats (Fattore et al, 2001, 2007) and extended these findings to demonstrate that adolescent exposure to THC significantly increases WIN self-administration in adulthood. Indeed, THC-exposed rats acquired cannabinoid self-administration more rapidly than controls, and they showed higher rates of cannabinoid consumption when self-administration behavior reached asymptote. These findings suggest that rats exposed to THC in adolescence are fully susceptible to the reinforcing effects of cannabinoid agonists in adulthood and that they require higher levels of drug to reach a satiation-like effect.
It is well documented that a history of cannabinoid exposure in adolescent animals can enhance sensitivity to other drugs of abuse (Rubino et al, 2012). For example, adolescent THC exposure increases opiate self-administration and increases vulnerability to stress-induced relapse to heroin seeking in adult rats, and these effects are associated with alterations in limbic opioid neuronal populations (Ellgren et al, 2007; Stopponi et al, 2013). Exposure to escalating doses of THC during adolescence also induced an increase in sensitivity to morphine conditioning in the place preference paradigm (Morel et al, 2009). Moreover, an enhancement of cocaine self-administration was found after adolescent chronic administration of CP 55,940 in adult female rats (Higuera-Matas et al, 2008). All these findings together are in line with epidemiological evidence that prior cannabis use among adolescents could be linked to increased risk for subsequent use of illicit drugs such as heroin or cocaine as well as to increased risk of cannabis use disorder (Kandel 1975; Fergusson et al, 2006; Fergusson and Boden, 2008; Grey, 2013).
The reasons why adolescent THC exposure might increase sensitivity to drugs of abuse are still not clear. The results of the present study suggest that enhanced WIN self-administration might be due to modifications at the level of the mesolimbic dopaminergic system. As with other drugs of abuse, cannabinoids' reinforcing effects are related to activation of the mesolimbic dopaminergic system (Solinas et al, 2008). Specifically, cannabinoids activate mesolimbic DA circuitry by enhancing the firing of DA neurons in the VTA and by preferentially increasing the release of DA from nerve terminals in the NAc Shell (French et al, 1997; Tanda et al, 1997; Gessa et al, 1998). Moreover, in our recent study, we demonstrated that the mesolimbic dopaminergic system is significantly activated during voluntary cannabinoid intake in experienced subjects (Fadda et al, 2006). In fact, in Lister-Hooded rats trained to self-administer WIN, DA content appreciably increased with respect to basal values during cannabinoid intake.
Our present neurophysiological data show that, as previously reported (Gessa et al, 1998; Pistis et al, 2004; Melis et al, 2013), acute administration of escalating doses of WIN (125–500 μg/kg, i.v.) induces a dose-dependent increase in DA neuron firing rate in vehicle-treated rats. However, the increases in DA neuron firing induced by the high dose of WIN (500 μg/kg) are significantly attenuated in THC-exposed rats compared with vehicle-exposed controls. Furthermore, in the present study vehicle-exposed rats showed significant increases in DA levels in the NAc shell when they received passive i.v. administration of WIN at a dose very similar to the daily amount (300 μg/kg) typically self-administered by rats (Justinova et al, 2013), but this same WIN administration did not significantly enhance DA levels in the same brain region of THC-exposed rats.
These neurochemical results suggest that the increase of WIN intake in our adolescent THC-exposed rats might actually be due to a decrease in sensitivity to the reinforcing effects of WIN, which is compensated for by higher consumption of the drug. This increased propensity to self-administer cannabinoids seems to be associated with decreased reactivity of DA neurons to pharmacological stimuli. In agreement, Pistis et al (2004) reported that, in cannabinoid-exposed adolescent rats, VTA DA neurons were significantly less responsive to the stimulating action of acute WIN, evaluated 2 weeks after last exposure. Moreover, the same authors found that passive cannabinoid administration during adolescence also affected the response of VTA DA neurons to morphine, with the opioid having little effect on firing rate in exposed rats but a stimulating action in controls (Pistis et al, 2004). These findings could provide a basis for effects observed in clinical studies, such as lower DA release following amphetamine administration in participants with earlier age of onset of cannabis use compared with participants who started smoking cannabis later in life (Urban et al, 2012).
All these data together indicate that the adolescent mesolimbic dopaminergic system might be highly sensitive to cannabinoid exposure. It is generally accepted that the endocannabinoid system is an important constituent of neuronal substrates involved in brain reinforcement/reward processes and that endocannabinoids can modulate excitatory and inhibitory inputs that control DA neurons of the mesolimbic system. Within adolescent ontogeny, fluctuations in endocannabinoid levels have been found in brain areas central to reward, and chronic intermittent THC exposure during adolescence has been shown to induce alterations of those levels in discrete areas, most evidently in the NAc, that may contribute to the disturbance of the neuronal activity of the DA neurons (Ellgren et al, 2008). Moreover, young recreational users show morphological abnormalities in the brain, including increases in gray matter volume in the NAc and amygdala, two brain regions implicated in drug addiction (Gilman et al, 2014).
It is well established that cannabinoid administration can rapidly induce tolerance to behavioral and biochemical effects and induce a downregulation of CB1Rs in cannabinoid-tolerant rats (Rubino et al, 1997; Romero et al, 1998; Breivogel et al, 1999). The same THC-exposure regimen that we used was found to produce lasting changes in CB1Rs' expression and desensitization following adolescent THC exposure in adult rats, although the effect was stronger in female than in male rats (Rubino et al, 2008). Thus it is possible that the behavioral and biochemical differences observed in our study between vehicle- and THC-exposed rats could be due to the onset of tolerance to the rewarding effects of WIN or to other effects of WIN, such as suppression of operant responding. If the obtained results are indeed due to tolerance, it is important to recognize that the effect of this tolerance was not to prevent WIN from having a rewarding effect on behavior but rather to accelerate acquisition of the self-administration response and to increase the asymptotic level of intake.
It should also be recognized that Pistis et al (2004) showed that DA neurons became significantly less responsive to the stimulating action of WIN even when chronic treatment was performed in adult animals. For this reason, it cannot be excluded that repeated THC exposure in adults might also alter cannabinoid self-administration. Further studies evaluating this possibility are needed. However, it has been shown that chronic exposure of adolescent rats to THC produces more long-term behavioral effects than chronic treatment of adult rats (Stiglick and Kalant 1983; Schneider and Koch 2003; Quinn et al, 2008). In agreement, epidemiological evidence shows that people who initiate cannabis use during adolescence have greater risk of developing cannabis dependence later in life, as compared with those who start cannabis use when adults (Coffey et al, 2003; Fergusson et al, 2003; Chen et al, 2005). Although our study was not designed to determine whether adolescent rats are affected differently from adult rats, it clearly shows that exposure in adolescence can have robust effects on behavioral and neural reactions to cannabinoids in adulthood. It could be valuable to determine the effects of initial exposure in adulthood, but epidemiologically, adolescent exposure is more prevalent and represents a more pressing concern.
Cannabis use during adolescence is also associated with morphological alterations in regions related to motivational, emotional, and affective processing; for example, gray matter volume is reduced in the medial temporal cortex, insula, and orbitofrontal cortex (Battistella et al, 2014). To screen for possible effects of adolescent THC exposure on such processes in the present study, we conducted a series of behavioral tests to assess the neurobehavioral profile of our animals. As previously demonstrated (Rubino et al, 2008), spontaneous locomotor activity in the open field and anxiety responses in the elevated plus maze were not significantly modified by the adolescent THC-exposure protocol used here; however, in contrast with the findings of Rubino et al (2008), THC-exposed rats in the present study did show signs of decreased anxiety in the open-field test (ie, decreased thigmotaxis). Together, these findings suggest that the group differences in the self-administration experiment were not due to changes in levels of general locomotor activity or anxiety due to THC exposure. The sucrose preference test was run to evaluate anhedonia, the reduced responsiveness to pleasurable stimuli that is the main symptom of depression; no difference were found between the groups in the sucrose preference index. This finding is in apparent contrast with previous reports by Rubino et al (2008) who found a significant reduction in preference in Sprague-Dawley rats. The contrasting findings between studies might be due to differences related to the animal strains used, as significant variances have been reported between rat strains in the ability of cannabinoids to affect different behaviors (Deiana et al, 2007; Cadoni et al, 2013). Also, it is important to note that despite the two groups in our experiments having the same sucrose preference index, the THC-treated rats consumed less sucrose solution than controls in all the 3 days of test, consistent with their lower food intake during cannabinoid exposure. The mesolimbic dopaminergic system, which was found to be altered by THC exposure in the present study, has an important role in driving hedonic feeding (Meye and Adan, 2014), and it is well recognized that palatable food, similar to drugs of abuse, increases DA release in the NAc shell (Bassareo and Di Chiara, 1999). However, it is unclear whether the decreased feeding and fluid intake in the present study, without a change in the relative preference for sucrose over tap water, can be attributed to changes in VTA and NAc DA signaling. Finally, our THC-treated rats did not exhibit sensorimotor gating impairments, suggesting that THC exposure did not induce a schizophrenia-like state.
In conclusion, our findings show that adolescent THC exposure enhances the acquisition of cannabinoid self-administration and increases the asymptotic level of cannabinoid intake. If analogous effects occur in humans, the increased levels of cannabis use might lead to higher risk of adverse effects on physical and mental health. This increased intake might represent compensation for a reduction of rewarding effects, owing to decreased neurotransmission of cells projecting from the VTA to the NAc shell.
Funding and disclosure
This study was supported by funds from Compagnia di San Paolo (2008), by the Department of Biomedical Sciences Project (2013), University of Cagliari, and by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, Baltimore, MD, USA. The authors declare no conflict of interest.
References
- Amchova P, Kucerova J, Giugliano V, Babinska Z, Zanda MT, Scherma M et al (2014). Enhanced self-administration of the CB1 receptor agonist WIN55,212-2 in olfactory bulbectomized rats: evaluation of possible serotonergic and dopaminergic underlying mechanisms. Front Pharmacol 5: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G (1999). Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci 11: 4389–4397. [DOI] [PubMed] [Google Scholar]
- Battistella G, Fornari E, Annoni JM, Chtioui H, Dao K, Fabritius M et al (2014). Long-Term effects of cannabis on brain structure. Neuropsychopharmacology 39: 2041–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belue RC, Howlett AC, Westlake TM, Hutchings DE (1995). The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicol Teratol 17: 25–30. [DOI] [PubMed] [Google Scholar]
- Biscaia M, Fernandez B, Higuera-Matas A, Miguens M, Viveros MP, Garcia-Lecumberri C et al (2008). Sex-dependent effects of periadolescent exposure to the cannabinoid agonist CP-55,940 on morphine self-administration behaviour and the endogenous opioid system. Neuropharmacology 54: 863–873. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ (1999). Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem 73: 2447–2259. [DOI] [PubMed] [Google Scholar]
- Burns HD, Van LK, Sanabria-Bohorquez S, Hamill TG, Bormans G, Eng WS et al (2007). [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc Natl Acad Sci USA 104: 9800–9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Simola N, Espa E, Fenu S, Di Chiara G (2013). Strain dependence of adolescent Cannabis influence on heroin reward and mesolimbic dopamine transmission in adult Lewis and Fischer 344 rats. Addict Biol 20: 132–142. [DOI] [PubMed] [Google Scholar]
- Chadwick B, Miller ML, Hurd YL (2013). Cannabis use during adolescent development: susceptibility to psychiatric illness. Front Psychiatry 4: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, O'Brien MS, Anthony JC (2005). Who becomes cannabis dependent soon after onset of use? Epidemiological evidence from the United States: 2000-2001. Drug Alcohol Depend 79: 11–22. [DOI] [PubMed] [Google Scholar]
- Chen CY, Storr CL, Anthony JC (2009). Early-onset drug use and risk for drug dependence problems. Addict Behav 34: 319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey C, Carlin JB, Lynskey M, Li N, Patton GC (2003). Adolescent precursors of cannabis dependence: findings from the Victorian Adolescent Health Cohort Study. Br J Psychiatry 182: 330–336. [DOI] [PubMed] [Google Scholar]
- Copeland J, Swift W (2009). Cannabis use disorder: epidemiology and management. Int Rev Psychiatry 21: 96–103. [DOI] [PubMed] [Google Scholar]
- Deiana S, Fattore L, Spano MS, Cossu G, Porcu E, Fadda P et al (2007). Strain and schedule-dependent differences in the acquisition, maintenance and extinction of intravenous cannabinoid self-administration in rats. Neuropharmacology 52: 646–654. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH et al (2008). Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur Neuropsychopharmacol 18: 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgren M, Hurd YL, Franck J (2004). Amphetamine effects on dopamine levels and behavior following cannabinoid exposure during adolescence. Eur J Pharmacol 497: 205–213. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Spano SM, Hurd YL (2007). Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology 32: 607–615. [DOI] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Spano MS, Salis P, Melis V, Fattore L et al (2006). Cannabinoid self-administration increases dopamine release in the nucleus accumbens. Neuroreport 17: 1629–1632. [DOI] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta CM, Fratta W (2001). Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology 156: 410–416. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W (2007). Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol 152: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM (2008). Cannabis use and later life outcomes. Addiction 103: 969–976. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ (2006). Cannabis use and other illicit drug use: testing the cannabis gateway hypothesis. Addiction 101: 556–566. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT, Madden PA (2003). Early reactions to cannabis predict later dependence. Arch Gen Psychiatry 60: 1033–1039. [DOI] [PubMed] [Google Scholar]
- French ED, Dillon K, Wu X (1997). Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport 8: 649–652. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Palazuelos J, Aguado T, Guzmán M (2009). The endocannabinoid system and the regulation of neural development: potential implications in psychiatric disorders. Eur Arch Psychiatry Clin Neurosci 259: 371–382. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Melis M, Muntoni AL, Diana M (1998). Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol 341: 39–44. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Kuster JK, Lee S, Lee MJ, Kim BW, Makris N et al (2014). Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci 34: 5529–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM (2013). New developments in understanding and treating adolescent marijuana dependence. Adolesc Psychiatry 3: 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuera-Matas A, Soto-Montenegro ML, del Olmo N, Miguens M, Torres I, Vaquero JJ et al (2008). Augmented acquisition of cocaine self-administration and altered brain glucose metabolism in adult female but not male rats exposed to a cannabinoid agonist during adolescence. Neuropsychopharmacology 33: 806–813. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Michaelides M, Miller ML, Jutras-Aswad D (2014). Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology 76: 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Mascia P, Wu HQ, Secci ME, Redhi GH, Panlilio LV et al (2013). Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid. Nat Neurosci 16: 1652–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel D (1975). Stages in adolescent involvement in drug use. Science 190: 912–914. [DOI] [PubMed] [Google Scholar]
- Keeley RJ, Trow J, McDonald RJ (2015). Strain and sex differences in puberty onset and the effects of THC administration on weight gain and brain volumes. Neuroscience 305: 328–342. [DOI] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC (2014). Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M (2012). Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology 37: 1164–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K (2005). Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol 168: 299–325. [DOI] [PubMed] [Google Scholar]
- McLaughlin CR, Martin BR, Compton DR, Abood ME (1994). Cannabinoid receptors in developing rats: detection of mRNA and receptor binding. Drug Alcohol Depend 36: 27–31. [DOI] [PubMed] [Google Scholar]
- Melis M, De Felice M, Lecca S, Fattore L, Pistis M (2013). Sex-specific tonic 2-arachidonoylglycerol signaling at inhibitory inputs onto dopamine neurons of Lister Hooded rats. Front Integr Neurosci 7: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meye FJ, Adan RA (2014). Feelings about food: the ventral tegmental area in food reward and emotional eating. Trends Pharmacol Sci 35: 31–40. [DOI] [PubMed] [Google Scholar]
- Morel LJ, Giros B, Dauge V (2009). Adolescent exposure to chronic delta-9- tetrahydrocannabinol blocks opiate dependence in maternally deprived rats. Neuropsychopharmacology 34: 2469–2476. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998) The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego, CA, USA. [Google Scholar]
- Pistis M, Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL (2004). Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biol Psychiatry 56: 86–94. [DOI] [PubMed] [Google Scholar]
- Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N et al (2008). Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology 33: 1113–1126. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S Jr (2000). Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108: 511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernández-Ruiz JJ (1993). Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport 4: 135–138. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Arias M, Manzanedo C, Roger-Sánchez C, Do Couto BR, Aguilar MA, Miñarro J (2010). Effect of adolescent exposure to WIN 55212-2 on the acquisition and reinstatement of MDMA-induced conditioned place preference. Prog Neuropsychopharmacol Biol Psychiatry 34: 166–171. [DOI] [PubMed] [Google Scholar]
- Romero J, Berrendero F, García-Gil L, Ramos JA, Fernández-Ruiz JJ (1998). Cannabinoid receptor and WIN-55,212-2-stimulated [35S]GTP gamma S binding and cannabinoid receptor mRNA levels in the basal ganglia and the cerebellum of adult male rats chronically exposed to delta 9-tetrahydrocannabinol. J Mol Neurosci 11: 109–119. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D (2014). Cannabis abuse in adolescence and the risk of psychosis: a brief review of the preclinical evidence. Prog Neuropsychopharmacol Biol Psychiatry 52: 41–44. [DOI] [PubMed] [Google Scholar]
- Rubino T, Patrini G, Parenti M, Massi P, Parolaro D (1997). Chronic treatment with a synthetic cannabinoid CP-55,940 alters G-protein expression in the rat central nervous system. Brain Res Mol Brain Res 44: 191–197. [PubMed] [Google Scholar]
- Rubino T, Vigano' D, Realini N, Guidali C, Braida D, Capurro V et al (2008). Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology 33: 2760–2771. [DOI] [PubMed] [Google Scholar]
- Rubino T, Zamberletti E, Parolaro D (2012). Adolescent exposure to cannabis as a risk factor for psychiatric disorders. J Psychopharmacol 26: 177–188. [DOI] [PubMed] [Google Scholar]
- Schneider M (2013). Adolescence as a vulnerable period to alter rodent behavior. Cell Tissue Res 354: 99–106. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M (2003). Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology 28: 1760–1769. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR, Piomelli D (2008). The endocannabinoid system in brain reward processes. Br J Pharmacol 154: 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano MS, Fadda P, Frau R, Fattore L, Fratta W (2010). Cannabinoid self-administration attenuates PCP-induced schizophrenia-like symptoms in adult rats. Eur Neuropsychopharmacol 20: 25–36. [DOI] [PubMed] [Google Scholar]
- Spano MS, Fattore L, Cadeddu F, Fratta W, Fadda P (2013). Chronic cannabinoid exposure reduces phencyclidine-induced schizophrenia-like positive symptoms in adult rats. Psychopharmacology 225: 531–542. [DOI] [PubMed] [Google Scholar]
- Stiglick A, Kalant H (1983). Behavioral effects of prolonged administration of delta 9-tetrahydrocannabinol in the rat. Psychopharmacology 80: 325–330. [DOI] [PubMed] [Google Scholar]
- Stopponi S, Soverchia L, Ubaldi M, Cippitelli A, Serpelloni G, Ciccocioppo R (2013). Chronic THC during adolescence increases the vulnerability to stress-induced relapse to heroin seeking in adult rats. Eur Neuropsychopharmacol 24: 1037–1045. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G (1997). Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276: 2048–2050. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz HC, Jacobs MM, Wilkinson MB, Wilson SP, Nestler EJ, Hurd YL (2012). Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biol Psychiatry 72: 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Grace AA (2012). Are you or aren't you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci 35: 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC (2014). United Nations Office on Drugs and Crime: World Drug Report 2014.
- Urban NB, Slifstein M, Thompson JL, Xu X, Girgis RR, Raheja S et al (2012). Dopamine release in chronic cannabis users: a [11c]raclopride positron emission tomography study. Biol Psychiatry 71: 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Suarez J, Llorente-Berzal A, López-Gallardo M, de Fonseca FR (2012). The endocannabinoid system in critical neurodevelopmental periods: sex differences and neuropsychiatric implications. J Psychopharmacol 26: 164–176. [DOI] [PubMed] [Google Scholar]