Abstract
Hepatitis C virus (HCV) infection is a significant cause of morbidity and mortality worldwide. The magnitude of the HCV burden has previously been the subject of debate, as representative data tend to exclude high-risk populations, including institutionalized persons. The purpose of this systematic review and meta-analysis was to estimate the prevalence of HCV infection among older adults in long-term care (LTC) and assess factors associated with the prevalence of HCVin this setting. The Preferred Reporting Items for Systematic Review and Meta-Analyses checklist was used as the methodological guide. Two reviewers independently assessed the study quality using a validated modified quality assessment tool. Six articles met inclusion criteria; the majority were cross-sectional studies (83.3 %) designed to estimate HCV infection prevalence rates and identify associated risk factors. HCV prevalence ranged from 1.4 to 11.8 %. A pooled HCV infection prevalence of 3.3 % (95 % confidence interval: 1.5–7.2 %) was estimated based on 1920 LTC residents with substantial heterogeneity noted (Q=51.1, p<0.001; I2=90.2). Three of six studies reported statistically significant factors associated with an increased risk for HCV infection, including older age, female gender, history of blood transfusions, short duration of LTC residence, and hepatitis B virus positivity. This study reports a higher prevalence of HCV infection among older adults in LTC settings compared to community-dwelling older adults; however, accurate estimation of prevalence is limited by heterogeneity between and within studies, variation in sampling and recruitment methodologies, and absence of the HCV-RNA test to confirm active infection.
Keywords: Hepatitis C virus, Older adults, Long-term care, Aging
Introduction
Hepatitis C virus (HCV) is the most common cause of blood-borne infection in the USA and worldwide [1•]. The magnitude of the HCV burden has previously been the subject of debate, as representative data are unavailable from many countries, and progress for improving data reporting has been minimal; therefore, the number of individuals chronically infected and the burden of disease are not well-established [2]. However, recent data suggest approximately 170 million individuals are positive for HCV antibodies (anti-HCV) worldwide, with particularly high rates in Latin America, Central and East Asia, and the Middle East [3, 4•]. Unfortunately, this number may markedly underestimate the true prevalence because of the long delay between acute infection and manifestation of clinical disease and because most surveys do not include institutionalized persons, populations known to have a high prevalence of HCV infection [5, 6••].
Individuals with chronic HCV infection have a higher overall morbidity and mortality compared to those not infected [2, 7]. Specifically, chronic infection can lead to hepatic fibrosis, cirrhosis and hepatocellular carcinoma [2, 6••] and is the leading cause of hepatic failure necessitating liver transplantation in the US [5, 6••, 8]. Moreover, the complications and conditions associated with chronic infection may not decline over the next decade because most individuals remain undiagnosed, do not receive the needed care (e.g., medical monitoring), and/or are not evaluated for treatment [6••, 9]. This is especially apparent among elders since most acquired HCV prior to identification of the virus and availability of screening tests [6••, 9].
HCV infection has been documented as the most frequent cause of viral hepatitis in older adults [10]. HCV infection prevalence increases with advanced age (75 % of anti-HCV positive individuals are older than 65 years), yet research on acquisition, risk factors and complications is limited among elders [11, 12]. Infection may pose an even greater threat to institutionalized elders because certain characteristics specific to this population may increase complications associated with infection and complicate treatment options [13–16]. Elderly residents in long-term care (LTC) are often frail, report multiple comorbidities, require frequent medical procedures, and may experience a declining immune response for combating infections acquired in old age [14–16]. An active HCV infection can spontaneously clear with a strong immune system, yet vulnerable elders who experience immune senescence and suffer from multiple comorbidities may not be able to clear the infection or respond and/or benefit from antiviral therapy and the risk for complications increases [9, 10, 15, 17]. One study reported that when HCV infection is acquired at an old age, it advances more rapidly to severe liver disease and cirrhosis compared with younger populations [10]. Finally, there is continued debate regarding the efficacy and safety of treating an active infection in elders [18, 19]. Treatment can be quite toxic, time-consuming, and expensive.
Outbreak reports of nosocomial infection with viral hepatitis are well described and in recent years considerable improvements have been made to reduce disease transmission [20–22]. However, reports of viral hepatitis outbreaks in healthcare settings due to lapses in infection control practices persist and are increasing, particularly in institutional settings which tend to have less oversight and fewer resources for infection control [23]. Although these reports are frequently cited to describe the incidence of HCV infection in LTC, [20–22] they are of limited value for assessing the overall burden of HCV infection in this setting. To our knowledge, there has been no systematic review published to estimate the prevalence of HCV infection among elders in LTC settings or to assess factors associated with the prevalence of HCV infection among LTC residents. Moreover, there are currently no pooled data available estimating the overall prevalence of HCV infection in this setting. To address this, we conducted a systematic review and meta-analysis to synthesize the body of published studies and to estimate HCV infection prevalence in LTC.
Methods
Search Strategy
Using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA, http://www.prisma-statement.org/) statement as the methodological guide, we systematically searched for potentially relevant original papers using three electronic databases: Ovid MEDLINE, PubMed, and Scopus. Keywords and subject headings used to identify relevant articles included: (hepatitis C) AND (long term care OR nursing home* OR skilled nursing facilit* OR home* for the aged) AND (prevalen* OR inciden*). In addition to the primary search, reference lists of research and review articles were also examined for relevant citations. The search terms and search strategy for this review were developed and finalized with the assistance of library information specialists.
Selection Criteria
Studies met the following eligibility criteria: (1) published in English in a peer-reviewed journal, (2) conducted in a LTC setting (LTC facility, nursing homes, skilled nursing facilities, and homes for the aged), (3) included elderly adults (aged 65 years and older) in the sampled population, (4) primary outcome of HCV infection, and (5) used a quantitative or a mixed-methods study design. There were no time limitations on the search; articles included were published through October 31, 2015. Reference lists of reviews were used to identify additional studies; studies meeting eligibility criteria were included. Excluded articles include case reports, commentaries, editorials, outbreak studies, and interventions.
Titles and abstracts were screened for relevance independently by two reviewers (KA and EL) in two stages. First, titles and abstracts were screened to exclude duplications and non-relevant articles. KA and EL independently screened titles and abstracts and reviewed and confirmed eligibility. Second, full-text articles deemed relevant after initial abstract screening were retrieved. Studies that met inclusion criteria were printed and reviewed. Inconsistencies between the two reviewers in eligibility assessments were discussed and adjudicated by consensus.
Outcome Measure
HCV infection was defined as infection detected by presence of HCV antibodies (anti-HCV). Risk factors for HCV infection identified in studies were included if they achieved statistical significance in multivariate regression models.
Assessment of Methodological Quality
Two reviewers independently assessed the quality of studies using a modified version of the STROBE checklist [24]. STROBE lists 22 criteria to evaluate the components of observational studies. The underlying rationale for STROBE is to enhance understanding and interpretation of observational studies and stimulate comparability of different reports [25]. Similar to other published reviews using the STROBE checklist, the original version was modified slightly [26]. Specifically, the modified checklist for this review assessed a study on a 7-point scale. Each article was reviewed to determine whether: (1) setting, location, relevant dates, exposure, and follow-up and data collection were adequately described; (2) study design was appropriate for obtaining prevalence; (3) outcome, exposure, and covariates were clearly defined and diagnostic criteria for HCV infection was specified; (4) sources of data and details of assessment methods were provided; (5) potential sources of bias were addressed; (6) participant numbers at each stage of study were reported; and (7) the number of outcome events were reported. The modified STROBE checklist scores were grouped into three rating categories: excellent (6–7), fair (4–5), and poor (<4).
Inter-rater reliability was established using a two-step process. First, all studies were independently scored by each reviewer using the modified STROBE checklist. Upon completion of this process, a weighted kappa score was calculated (using the 3-category ordinal outcome rating variable: excellent, fair, and poor) to establish inter-rater agreement. Second, comparing independently scored ratings, those with any score differences were discussed and resolved and a new quality score was established.
Statistical Analysis
Studies reporting HCV infection prevalence were eligible for inclusion in the meta-analysis. Data were extracted as frequency and sample size and standardized effect sizes were computed. A pooled prevalence of HCV infection was estimated using a random effects meta-analysis model. Heterogeneity was assessed using Cochran Q and I2 statistics with results presented as a forest plot. Sensitivity analyses were conducted to examine the effect of potential sources of heterogeneity across studies [27]: study quality, geographic location, facility type, performance of HCV confirmation assay, and year of study publication. To assess the potential for publication bias to have influenced meta-analysis findings, we performed Rosenthal’s classic fail-safe N test to estimate the number of missing studies that would need to be added to the meta-analysis for the pooled effect to no longer be statistically significant [28]. Data were analyzed using Comprehensive Meta-Analysis (CMA) statistical software (Biostat, Inc.).
Results
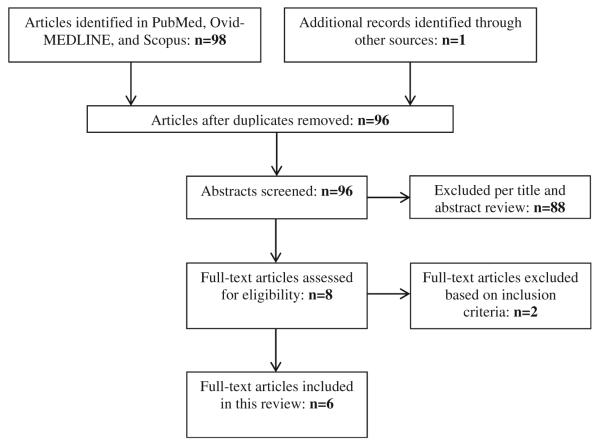
The electronic database search yielded 98 potential articles; one additional article was identified by hand searching reference lists of review articles. After excluding three duplicates, 96 publications were screened for eligibility. Of these, 88 articles were excluded based on title screening and abstract review (Fig. 1). The majority of excluded studies did not include older adults or older adults residing in LTC. As a result, six full-text articles were included.
Fig. 1.
Results of search strategy and selection procedure for a systematic review of quantitative studies on hepatitis C virus infection and long-term care settings
Characteristics of Studies
Characteristics of studies are presented in Table 1. Three studies were conducted in Europe, [29–31] and one each in the USA [14], Canada [32], and Iran [33]. All studies except one [14] were cross-sectional studies in which the primary objective was to assess HCV infection prevalence among institutionalized older adults, and the primary outcome was the presence of anti-HCV. One study was a prospective cohort study, reported similar objectives and assessed the same outcome [14]. Institutional settings included an LTC facility [32], nursing home [14, 29, 31, 33], and home for the aged [30]. Mean age of residents ranged from 58 to 84 years. Overall, the majority of studies (N = 4; 66.7 %) were single site settings with samples ranging from 288 to 508 participants. The majority of studies included a non-selective sample of residents (N = 4, 66.7 %) [29–31]. One study included three facilities and recruited 199 participants [14]; a second study included two facilities and recruited 227 participants [31]. In only one study were all residents in the LTC facility eligible to participate, unless an individual refused and/or was not present at the time baseline interviews and blood samples were conducted [14]. Participation rate for this study was 60.5 % [14]. Only one study described specific exclusion or inclusion criteria, including severe cognitive disorder [31].
Table 1.
Characteristics of publications included in a systematic review of hepatitis C virus and long-term care settings
| Source, (year), and location | Objective | Study design | Sample | Outcome measure |
Prevalence % (N) | Risk factorsa | Mean quality scoreb |
|---|---|---|---|---|---|---|---|
| Baldo et al. (2000) [29] Italy |
To evaluate the HCV prevalence in two groups of elderly people: nursing home and community-dwelling |
Cross-sectional | NH: 288 residents; mean age 84 from one NH Community: 208 subjects; mean age 73 from northeast Italy |
Anti-HCV | NH: 11.8 % (34/288) Community: 11.1 % (23/208) |
Age*, gender*, surgery, blood transfusion, dental therapy, household members with hepatitis, anti-HBs, and/or anti-HBc positivity* |
6 |
| Chien et al. (1999) [14] USA |
To assess the prevalence of current or previous infection with viral hepatitis in an older NH population |
Prospective cohort | 199 residents; mean age 79 from 3 NHs |
Anti-HCV | 4.5 % (9/199) | Age, ethnicity, history of blood transfusion*, end stage renal disease, manual labor, previous surgeries, and injection drug use |
4 |
| Floreani et al. (1992) [30] Italy |
To evaluate the prevalence of anti-HCV in a population of institutionalized older adults and to study the clinical features of anti-HCV positive subjects |
Cross-sectional | 315 residents; mean age 80 from one HFTA |
Anti-HCV | 2.2 % (7/315) | Blood transfusions, major surgery, length of institutionalization, and HBV serum markers |
3 |
| Mansour-Ghanaei et al. (2007) [33] Iran |
To determine the frequency of HBV and HCV serological makers in residents of a Guilan NH |
Cross-sectional | 383 residents, mean age 58 from one NH |
Anti-HCVAb | 2.3 % (9/383) | Gender, short duration of residency in NH*, transfusion history, mental retardation, physically handicap, and history of surgery |
5 |
| Maral et al. (2009) [31] Turkey |
The determine the seroprevalence of HBV and HCV in the elderly residing in two NHs |
Cross-sectional | 227 residents, mean age 76 from two NHs |
Anti-HCV IgG | 2.5 % (6/227) | Age, sex, and duration in NH | 4 |
| Simor et al. (1992) [32] Canada |
To determine the prevalence of HBsAg, anti-HCV, and anti-HIV among residents of a LTC facility |
Cross-sectional | 508 residents, mean age 83 from one LTC facility |
Anti-HCV | 1.4 % (7/508) | Non | 6 |
Data on each risk-factor for each study was systematically determined and statistically tested for significance regarding anti-HCV positivity; significant risk factors identified in multivariate analysis are marked with an asterisk
Average quality score between the two reviewers using the modified tool; Score ranges from 0-7
Anti-HCV HCV antibody, HBV hepatitis B virus, HBsAg hepatitis B surface antigen, HBc anti-hepatitis B core antigen, HBs anti-hepatitis B surface antigen, HCV hepatitis C virus, HFTA home for the aged, HIV human immunodeficiency virus, LTC long-term care, NH nursing home
One study compared two groups of older adults: nursing home residents and a non-institutionalized sample from the surrounding community [29]. The authors compared prevalence rates in the institutionalized versus community-dwelling elders. There were no significant differences reported between groups.
Prevalence of HCV Infection and Risk Factors
HCV infection was diagnosed by an anti-HCV test. All but one study [31] confirmed the presence of anti-HCV with an additional test. The prevalence of HCV infection varied considerably. Overall, the majority of studies reported a prevalence rate between 2.0 and 4.5 % [14, 30, 31, 33]. One study reported an 11.8 % HCV infection prevalence rate [29] whereas another study reported 1.4 % [32].
Five of the six studies identified specific factors previously shown to be associated with an increased HCV infection risk, including age, gender, race/ethnicity, history of blood transfusions, and major surgeries. Duration of residence in LTC was systematically determined and statistically tested for significance regarding anti-HCV positivity in three studies [30, 31, 33]. All studies simultaneously measured anti-hepatitis B surface antigen and/or anti-hepatitis B core antigen assessed whether HBV positivity was associated with an increased HCV infection risk.
Of the five, three reported statistically significant but differing results using regression models. Only one study identified specific demographic characteristics (age and gender) as significant risk factors; this same study identified HBV positivity to be associated with HCV infection [29]. The second study found blood transfusion history correlated significantly with anti-HCV positivity [14]. The last study reported a significant reverse relationship between the length of residency and positive anti-HCV test [33]. Specifically, anti-HCV positive residents reported shorter duration of residence than anti-HCV negative residents. This suggests HCV was more frequently acquired in the community rather than the nursing home since newcomers were more likely to be positive for HCV infection than LTC residents.
Methodological Quality of Studies
The methodological quality of the available evidence varied, and none of the included studies fulfilled all criteria. The majority of studies were appraised as fair to high quality. One received a score of 3, indicating poor quality. A frequently observed weakness among studies was the method in which the setting was described, including location, relevant dates, exposure, follow-up, and data collection methods. Only two of the six studies explicitly defined recruitment methods [14, 31].
The weighted kappa score was calculated (using the 3-category ordinal outcome rating variable: excellent, fair, poor) to establish inter-rater agreement. The score calculated was 0.422, indicating fair to good agreement between reviewers.
Quantitative Synthesis
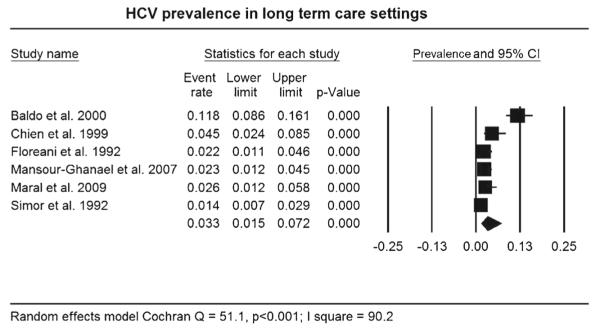
Figure 2 represents data from 1920 older adults residing in LTC settings with data regarding HCV infection status. Using a random effects model, the pooled HCV infection prevalence was 3.3 % (95 % confidence interval (CI): 1.5–7.2 %). Heterogeneity between and within studies was greater than expected by chance alone (Q = 51.1, p < 0.001) with the majority of the variability attributed to differences between studies (I2 = 90.2). Results of the sensitivity analysis report no significant differences in HCV infection prevalence noted by study quality, geographic location, facility type, use of HCV confirmation assay, and study publication year. Regarding potential publication bias, the result of Rosenthal’s classic fail-safe N test indicates that an additional 958 missing studies would be required to dramatically alter the pooled prevalence finding.
Fig. 2.
Forest plot of HCV infection prevalence in older adults in long-term care settings
Note: Squares represent effect sizes of individual studies with extended lines denoting 95% confidence intervals (CI). Sizes of squares indicate the weight of each study based on sample size using a random effects analysis. The diamond represents the estimated pooled prevalence.
Discussion
We conducted a systematic review and meta-analysis to estimate HCV infection prevalence in LTC settings and assess factors associated with HCV infection prevalence. A pooled estimate of 3.3 % (95 % CI: 1.5–7.2 %) HCV infection prevalence rate indicates a higher risk for infection among elders in LTC settings compared to the community. US studies report HCV infection prevalence rates between 0.9–1.0 % among non-institutionalized elders; however, these rates were reported with US data and may not represent background population rates from other countries [1•, 2]. While the definitions used for HCV infection were consistent across studies, outcome assessments were not standardized, and studies lacked information on data collection and recruitment methods. Moreover, studies varied considerably in terms of geographic location, facility type, sample size, and risk factor analysis. Nevertheless, some critical insights and meaningful patterns have emerged.
First, it is unclear if individuals were previously infected or were exposed to the virus in the LTC setting. In the studies reviewed, the anti-HCV test was used to confirm anti-HCV positivity; unfortunately, the anti-HCV test does not confirm an active infection. The HCV-RNA test detects the presence of HCV circulating in the blood and is among the most sensitive tests available because it is able to differentiate between past and current infection [12]. This is a key distinction to make in LTC settings as it can potentially have very important implications, including therapy recommendations. The goal of therapy for chronic HCV is eradication of the virus; however, in aged patients, the antiviral effect and tolerability to treatment require that treatment decisions be tailored on the basis of the severity of liver disease and presence of comorbidities [18, 19, 34]. Moreover, therapy is contraindicated for a subset of elders with poorly controlled chronic conditions, such as diabetes [35, 36]. Future studies in this setting should consider the HCV-RNA test to confirm an active infection, extend current antiviral therapies to institutionalized older adult populations, and develop effective antiviral protocols based on these studies.
Second, an important limitation in understanding differential susceptibility to HCV exposure in institutionalized older adults can be attributed to the biologic complexities of multiple comorbidities and its effect on HCV infection severity and duration. It has been hypothesized that older adults with multiple comorbidities experience accelerated disease progression and severity, including severe liver disease and cirrhosis, compared to those without comorbidities [17]. Decreased immune response, interaction with medications, and overall poor health may play a role in inhibiting response to treatment [9]. It is surprising the studies included in this review did not test the relationship between HCV infection and presence of comorbidities, given the high prevalence of chronic conditions among institutionalized elders. Future studies must evaluate HCV infection risk and consequences of treatment and progression of disease among those report multiple comorbidities.
Finally, an established body of information regarding best infection control practices exists for the acute hospital setting; however, preventive measures designed for acute hospitals may not apply to the LTC setting [13]. Besides setting and host-related factors, which make LTC residents more vulnerable to certain infectious disease or infectious diseases more easily transmissible, other differences between LTC settings and acute-care settings should be taken into account [13, 37]. In the last two decades, an increasing number of national and regional guidelines, surveillance, and infection control activities in LTC have been developed with a common emphasis on the importance of adherence to universal infection control precautions, including hand hygiene and safe injection practices [37]. The high prevalence rate of HCVand other blood-borne pathogens in LTC settings compared to the community, the potential increased acuity of patients being placed in LTC, and the growing older adult population should be strong motivators to enforce universal precautions to prevent nosocomial transmissions in this setting. Future studies should identify and evaluate facility-level characteristics, including infection control procedures, effective in preventing HCV in LTC.
Limitations
This systematic review has important limitations. First, the paucity of published data in LTC settings limits the ability to accurately estimate the overall burden in this setting. Attempts were made to be comprehensive in the search strategy, but because the study question and selection criteria had a narrow focus, this may have resulted in exclusion of additional studies. Second, studies were included only if they were published in English in peer-reviewed literature. Although we did not limit the search to specific regions, only two studies were conducted in North America. Third, the majority of the studies consistently excluded key methodological information, including duration of residence in LTC and recruitment methodology. Duration of LTC residence has been shown in previous studies to increase the risk for HCV infection, as individuals in this setting are constantly exposed to multiple risk factors during their stay [30, 31, 33]. Recruitment methods (e.g., sample population) were consistently missing across studies. These details are vital as they may indicate a study focused on a high-risk group, such as those with a history of blood transfusions or major surgeries, thereby explaining a high prevalence rate. Finally, the majority of studies had convenience samples and not all residents residing at the facility during recruitment were screened for inclusion.
Conclusion
Accurate estimation HCV infection among institutionalized elders is an increasingly important area of research, given the rise of older adults with prior exposure to HCV before routine screening was available. Ultimately, identification of potential factors likely to influence HCV infection risk in LTC is useful in understanding differential susceptibility between populations. Future research needs to establish a comprehensive understanding of accurate and consistent methodology for enhancing methodological clarity using clearly defined recruitment methods and include the HCV-RNA test to detect an active infection.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Kimberly J. Alvarez, Arlene Smaldone, and Elaine L. Larson declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by the author.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1 •.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. The high prevalence of global HCV infection highlights the urgency to identify and reduce the burden of complications and co-morbidities associated with infection.
- 2.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17(2):107–15. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 3.Holtzman D. [Accessed 25 Nov 2014];Hepatitis C [On-line] http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-3-infectious-diseases-related-to-travel/hepatitis-c.
- 4 •.Mohd Hanafiah K, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–42. doi: 10.1002/hep.26141. The development of primary prevention methods are vital to reduce chronic diseases associated with infection and improve survival, especially among older adults.
- 5.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340(10):745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 6 ••.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61(RR-4):1–32. In the United States, new hepatitis C virus infection therapies have been shown to be effective in reducing disease progression and offering a virologic cure. Therefore, it is important to identify at risk cohorts (adults 65 years and older) to offer targeted testing, education, and care to increase quality of life.
- 7.Omland LH, Jepsen P, Krarup H, et al. Increased mortality among persons infected with hepatitis C virus. Clin Gastroenterol Hepatol. 2011;9(1):71–8. doi: 10.1016/j.cgh.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 8.El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160(21):3227–30. doi: 10.1001/archinte.160.21.3227. [DOI] [PubMed] [Google Scholar]
- 9.Davis GL, Alter MJ, El-Serag H, et al. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513–21. 521, e511–516. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 10.Marcus EL, Tur-Kaspa R. Viral hepatitis in older adults. J Am Geriatr Soc. 1997;45(6):755–63. doi: 10.1111/j.1532-5415.1997.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 11.Mindikoglu AL, Miller RR. Hepatitis C in the elderly: epidemiology, natural history, and treatment. Clin Gastroenterol Hepatol. 2009;7(2):128–34. doi: 10.1016/j.cgh.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention [Accessed 17 Nov 2014];Know more hepatitis [On-line] Available at: http://www.cdc.gov/knowmorehepatitis/
- 13.Mathei C, Niclaes L, Suetens C, et al. Infections in residents of nursing homes. Infect Dis Clin N Am. 2007;21(3):761–72. doi: 10.1016/j.idc.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Chien NT, Dundoo G, Horani MH, et al. Seroprevalence of viral hepatitis in an older nursing home population. J Am Geriatr Soc. 1999;47(9):1110–3. doi: 10.1111/j.1532-5415.1999.tb05236.x. [DOI] [PubMed] [Google Scholar]
- 15.Marcus EL, Dahoudi N, Tur-Kaspa R. Hepatitis C virus infection among elderly patients in a geriatric hospital. Arch Gerontol Geriatr. 1994;19(3):213–21. doi: 10.1016/0167-4943(94)00562-1. [DOI] [PubMed] [Google Scholar]
- 16.Perz JF, Grytdal S, Beck S, et al. Case-control study of hepatitis B and hepatitis C in older adults: do healthcare exposures contribute to burden of new infections? Hepatology. 2013;57(3):917–24. doi: 10.1002/hep.25688. [DOI] [PubMed] [Google Scholar]
- 17.Floreani A. Hepatitis C: should antiviral therapy be offered to elderly patients? Nat Rev Gastroenterol Hepatol. 2009;6(9):503–4. doi: 10.1038/nrgastro.2009.138. [DOI] [PubMed] [Google Scholar]
- 18.Oze T, Hiramatsu N, Yakushijin T, et al. Indications and limitations for aged patients with chronic hepatitis C in pegylated interferon alfa-2b plus ribavirin combination therapy. J Hepatol. 2011;54(4):604–11. doi: 10.1016/j.jhep.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 19.Antonucci G, Longo MA, Angeletti C, et al. The effect of age on response to therapy with peginterferon alpha plus ribavirin in a cohort of patients with chronic HCV hepatitis including subjects older than 65 yr. Am J Gastroenterol. 2007;102(7):1383–91. doi: 10.1111/j.1572-0241.2007.01201.x. [DOI] [PubMed] [Google Scholar]
- 20.Fischer GE, Schaefer MK, Labus BJ, et al. Hepatitis C virus infections from unsafe injection practices at an endoscopy clinic in Las Vegas, Nevada, 2007–2008. Clin Infect Dis. 2010;51(3):267–73. doi: 10.1086/653937. [DOI] [PubMed] [Google Scholar]
- 21.Schaefer MK, Jhung M, Dahl M, et al. Infection control assessment of ambulatory surgical centers. JAMA. 2010;303(22):2273–9. doi: 10.1001/jama.2010.744. [DOI] [PubMed] [Google Scholar]
- 22.Gutelius B, Perz JF, Parker MM, et al. Multiple clusters of hepatitis virus infections associated with anesthesia for outpatient endoscopy procedures. Gastroenterology. 2010;139(1):163–70. doi: 10.1053/j.gastro.2010.03.053. [DOI] [PubMed] [Google Scholar]
- 23.Perz JF, Thompson ND, Schaefer MK, et al. US outbreak investigations highlight the need for safe injection practices and basic infection control. Clin Liver Dis. 2010;14(1):137–51. doi: 10.1016/j.cld.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147(8):W163–94. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 26.Yaphe S, Bozinoff N, Kyle R, et al. Incidence of acute hepatitis C virus infection among men who have sex with men with and without HIV infection: a systematic review. Sex Transm Infect. 2012;88(7):558–64. doi: 10.1136/sextrans-2012-050566. [DOI] [PubMed] [Google Scholar]
- 27.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 28.Rothstein HR, Borenstein M. Publication bias in meta-analysis: prevention, assessment, and adjustments. John Wiley & Sons, Ltd; West Sussex: 2005. [Google Scholar]
- 29.Baldo V, Floreani A, Menegon T, et al. Prevalence of antibodies against hepatitis C virus in the elderly: a seroepidemiological study in a nursing home and in an open population. The Collaborative Group. Gerontology. 2000;46(4):194–8. doi: 10.1159/000022159. [DOI] [PubMed] [Google Scholar]
- 30.Floreani A, Bertin T, Soffiati G, et al. Anti-hepatitis C virus in the elderly: a seroepidemiological study in a home for the aged. Gerontology. 1992;38(4):214–6. doi: 10.1159/000213330. [DOI] [PubMed] [Google Scholar]
- 31.Maral I, Dogruman-Al F, Bakar C, et al. Hepatitis B virus and hepatitis C virus seroprevalence in the elderly living in nursing homes. J Investig Med. 2009;57(6):717–9. doi: 10.2310/JIM.0b013e3181ab8cab. [DOI] [PubMed] [Google Scholar]
- 32.Simor AE, Gordon M, Bishai FR. Prevalence of hepatitis B surface antigen, hepatitis C antibody, and HIV-1 antibody among residents of a long-term-care facility. J Am Geriatr Soc. 1992;40(3):218–20. doi: 10.1111/j.1532-5415.1992.tb02071.x. [DOI] [PubMed] [Google Scholar]
- 33.Mansour-Ghanaei F, Fallah MS, Jafarshad R, et al. Seroprevalence of hepatitis B and C among residents of Guilan nursing home. Hepat Mon. 2007;7(3):139–41. [Google Scholar]
- 34.Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368(20):1907–17. doi: 10.1056/NEJMra1213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strader DB, Wright T, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39(4):1147–71. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 37.Nicolle LE. Preventing infections in non-hospital settings: long-term care. Emerg Infect Dis. 2001;7(2):205–7. doi: 10.3201/eid0702.010210. [DOI] [PMC free article] [PubMed] [Google Scholar]