Abstract
Objective
We investigated whether the Bereitschaftspotential (BP), an event related potential believed to reflect motor planning, would be modulated by language-related parameters prior to speech. We anticipated that articulatory complexity would produce effects on the BP distribution similar to those demonstrated for complex limb movements. We also hypothesized that lexical semantic operations would independently impact the BP.
Methods
Eighteen participants performed 3 speech tasks designed to differentiate lexical semantic and articulatory contributions to the BP. EEG epochs were time-locked to the earliest source of speech movement per trial. Lip movements were assessed using EMG recordings. Doppler imaging was used to determine the onset of tongue movement during speech, providing a means of identification and elimination of potential artifact.
Results
Compared to simple repetition, complex articulations produced an anterior shift in the maximum midline BP. Tasks requiring lexical search and selection augmented these effects and independently elicited a left lateralized asymmetry in the frontal distribution.
Conclusions
The findings indicate that the BP is significantly modulated by linguistic processing, suggesting that the premotor system might play a role in lexical access.
Significance
These novel findings support the notion that the motor systems may play a significant role in the formulation of language.
Keywords: Bereitschaftspotential, Lexical access, ERP, Language, Motor, Speech
1. Introduction
The most extensively studied electrophysiological index of voluntary movement, known as the Bereitschaftspotential (BP), was first characterized by Kornhuber and Deecke (1965). The BP is a slow, negative waveform which precedes electromyographic (EMG) activity associated with volitional movement, by approximately 1–1.5 s and has been most thoroughly studied in relation to finger and other distal limb movements.
The early portion of the limb BP (BP1), is symmetrically distributed across the central and parietal regions of the scalp with its maximum negativity at the scalps vertex. The later portion of the BP (BP2) begins approximately 500 ms prior to movement and is characterized by a relatively steeper slope and a slight lateralization over the central scalp region (Shibasaki and Hallett, 2006).
The BP has been shown to also precede other willed actions, such as eye blinks, swallowing, and orofacial movements. A number of researchers have investigated the topography and morphology of the BP preceding speech (Brooker and Donald, 1980; Deecke et al., 1986; Empson, 1982; Grabow and Elliott, 1974; McAdam and Whitaker, 1971; Morrell and Huntington, 1972; Szirtes and Vaughan, 1977; Wohlert, 1993). A comprehensive understanding of the conditions that elicit a speech BP and the factors that affect its amplitude and topographical distribution would be beneficial for many reasons; it would advance our understanding of the brain mechanisms that underlie normal speech production and help to elucidate the pathophysiology and treatment of neurological disorders which impair these mechanisms.
The results of the earlier investigations of the speech-related BP cited above, however, have been inconsistent, and the relationship of the BP to the planning, formulation, and cognitive/linguistic properties of speech has yet to be determined.
As with movements of the limbs, the speech BP appears to begin approximately 2 s prior to the onset of muscle activity and to have an early (BP1) medial frontocentral distribution (Deecke et al., 1986; Grözinger et al., 1975). However, the topographic distribution of the later (BP2) component has been more controversial and varied within the literature. Deecke et al. (1986) reported a left precentral and frontal asymmetry in the BP2 topography. While, Wohlert (1993) reported a consistently symmetrical distribution for both the early and later part of the speech BP. The issue of laterality has dominated the literature on the topic of speech BP and evidence from multiple studies has lent support to both the lateralized distribution found by Deecke (Empson, 1982; McAdam and Whitaker, 1971) and the symmetrical distribution reported by Wohlert (Brooker and Donald, 1980; Grabow and Elliott, 1974; Morrell and Huntington, 1972).
This lack of consensus may stem in part from several variations in the methodologies used by previous researchers. However, the influence of speech muscle artifact contamination seems to be a primary source of confusion and inconsistency.
Speech related electrophysiological data have been shown to be highly susceptible to electromyographic (EMG) artifact due to the close proximity of the articulators to scalp EEG recording sites (Szirtes and Vaughan, 1977). Studies that have examined the BP prior to limb movement generally used the onset of EMG from a muscle involved in the initial movements as the time-locking event. However, in the case of speech, since the source of the earliest movements was unclear, many early researchers (Brooker and Donald, 1980; Empson, 1982; Grabow and Elliott, 1974; McAdam and Whitaker, 1971; Morrell and Huntington, 1972; Szirtes and Vaughan, 1977) selected the onset of phonation rather than muscle activity as a time-locking event for averaging of the BP. Nevertheless, articulatory movements typically precede actual vocalization and may therefore introduce EMG artifact into the averaged BP signal. Later studies sought to avoid contamination by time-locking BP averaging to the onset of lip EMG. Yet speech involves multiple articulatory muscles and the initial movements associated with speech do not invariantly originate from the lips but can also involve movements of the glottis or the tongue.
The initial goal of this study was to establish a reliable means of measuring the speech BP. To do so, a novel approach was developed for the exclusion of artifacts: in addition to the acoustic signal, speech movement onset was simultaneously monitored from the lips, tongue and glottis, in order to identify on a trial by trial basis, the initial source of movement. Thus, for each individual trial, data averaging could be time-locked to the precise onset of articulatory movement, so that the BP would remain unobscured by any preceding articulation-related muscle artifact. The means by which each of these articulators was monitored and the method employed for determining their onset patterns is discussed in Section 2.
Once a reliable speech BP was obtained, the principal goal of the present study was to determine the ways in which the BP might be modulated by the articulatory and linguistic features of spoken language.
It is clear that articulatory complexity should be expected to affect the speech BP in some manor. The effects of motor complexity are well established for the BP preceding distal limb movement. Several authors have demonstrated, for example, that the BP preceding complex sequences of limb movements are generally higher in amplitude and of longer latency than those preceding more simplistic motor sequences (Cui et al., 2000; Kristeva, 1984; Schreiber et al., 1983; Simonetta et al., 1991). Though no direct comparisons between simple and complex speech utterances appear within the literature, some evidence suggests that motor complexity might also influence features of the speech-derived BP. For example, speech BP studies which utilized simple speech tasks, such as the repetition of a single, motorically non-complex utterance (Grabow and Elliott, 1974; Morrell and Huntington, 1972; Wohlert, 1993) tended to report lower BP amplitudes than those which required participants to perform varied, and thus somewhat more complex, speech utterances (Brooker and Donald, 1980; Deecke et al., 1986; Empson, 1982; McAdam and Whitaker, 1971).
Topographic differences in the speech BP distribution might also be anticipated between simple and complex utterances. Findings from functional neuroimaging studies (Picard and Strick, 1996) suggest differential roles of the pre-SMA, SMA and other midline brain structures in the planning and formulation of simple utterances and those which require complex articulatory movements.
Beyond articulatory complexity, there is also reason to expect that cognitive–linguistic operations may impact the features of the speech BP. It is known, for example, that cognitive factors such as attention (Kornhuber and Deecke, 1965) and motivation (McAdam and Seales, 1969) influence the features of the BP prior to limb movements. Some evidence suggests that such factors might also affect the speech BP. For example, lateralized speech BP distributions appear to have been more frequency reported by researchers who used tasks requiring the participants to make lexical decisions prior to speech (Deecke et al., 1986; McAdam and Whitaker, 1971), while symmetrical BP distributions were more often reported in studies that used simple word repetition tasks (Grabow and Elliott, 1974; Morrell and Huntington, 1972; Wohlert, 1993). Moreover, a growing body of literature suggests a functional interaction between language and motor systems at multiple levels (Corballis, 2003; Hauk et al., 2004; Kimura, 1993; Liberman and Whalen, 2000; Lieberman, 1985; Pulvermuller et al., 2001).
Considering this evidence, the present set of experiments was designed to characterize and disambiguate the effects of articulatory and cognitive complexity on the characteristic features of the speech-related BP. It was hypothesized that the BP would be independently affected by task related variations in both domains.
Variations in both articulatory complexity and lexical selection were expected to result in changes in the BP topography along the midline. It was hypothesized that tasks requiring simple, invariant articulatory output would produce their maximum BP amplitude over the midline vertex of the scalp. In contrast, tasks that necessitate variations in the spoken utterances, resulting in more complex articulatory patterns, were expected to produce an anterior shift in the midline BP maximum amplitude.
It was also predicted that in tasks requiring lexical selection prior to articulation, when words of equivalent phonological complexity had to first be selected from among competing items in the mental lexicon, there would be a further shift of the maximum BP amplitude along the anterior midline.
Moreover, it was hypothesized that tasks requiring online lexical selection would also result in left lateralized asymmetries in the BP distribution, while tasks requiring simple repetition or word reading, would yield more symmetrical BP distributions.
2. Methods
Eighteen healthy volunteers (8 female) between the ages of 18 and 40 ( = 27:5, SD = 5.86) participated in the study. All were right handed, as assessed through the Edinburgh inventory (Oldfield, 1971) ( = 94:4, SD = 9.2), with normal or corrected vision, and reported English as their native language. All participants were neurologically intact, and were not taking any psychoactive medications at the time of their participation in the study. Participants granted their informed written consent in accordance with the protocol approved by the NINDS/NIDCD Institutional Review Board.
Participants were seated in an electrostatically shielded chamber during task related EEG recording. All electrophysiological signals were recorded using 9 mm sintered silver silver-chloride electrodes. EEG was recorded with a 60 channel electrode cap, conforming to the extended 10–20 electrode placement system and referenced to physically linked ears. Data were continuously recorded in DC with 100 Hz low-pass filter using two 32-channel Synamp bio-amplifiers. Electrical impedance between the ground and all electrodes was maintained below 5 KΩ. Bipolar leads were placed above and lateral to the left eye, in order to measure the electro-oculogram. Additional bipolar electrodes were placed at the vermilion boarders of the upper and lower lips, overlying the orbicularis oris in order to measure electromyographic activity associated with the onset of lip movements during speech.
Tongue movement onset during speech was acquired through Doppler imaging of the oral cavity. An Acuson, model 128 XP sonograph with a C7 transducer was used to generate the Doppler signals. The transducer was placed between the neck and chin. Adjustments to the placement of the transducer were made so that a full lateral image of the base of the tongue could be viewed on the sonograph’s CRT monitor. Signal gain adjustments were made in order to ensure that any lateral or vertical movement of the tongue base produced detectable changes in the amplitude of the Doppler signal. Glottal movements during speech were indexed through throat surface recordings of the electroglottogram (EGG), an impedance based measure. The participants’ spoken responses were recorded to a Sony model ICD-MS1 digital audio recorder and to the hard-drive of a Compaq computer, with a Pentium III processor using the sound capture function of the Presentation software package (Neurobehavioral Systems, Inc.; http://nbs. neuro-bs.com).
The amplitudes of the Doppler, EGG, EMG and acoustic signals were attenuated by a factor of 500, using signal conditioning hardware, and acquired in synchrony with EEG, all at the sampling rate of 2000 Hz.
2.1. Experiment 1: assessment of multiple sources of articulatory movement
A preliminary experiment was conducted with 7 participants in order to determine the utility of the selected measures of articulatory movement. Participants performed verbal tasks while articulatory movements and acoustic signals were monitored in real time.
2.2. Experiment 2: comparison of the bp derived from speech and limb movements
Experiment 2 was conducted in order to compare the topographic distributions of the BP prior to speech and simple distal limb movements. Six participants performed right hand finger flexion and a simple speech task. Limb movement onset was determined using bipolar surface EMG recorded over the digitorum communis.
2.3. Experiment 3: impact of articulatory complexity and lexical access on the speech-related BP
Participants were seated facing a 34 cm LCD monitor. For each of the three tasks, trials began with a visual cue presented to the participant at a 5° visual angle from 1.5 m. Though initiation of speech was self-paced, participants were instructed to wait approximately 3–5 s following the cue and then to make crisp vocal response from a resting state in which the articulatory muscles were relaxed and the tongue rested on the floor of the mouth. Participants were trained to achieve this without counting and had ample practice sessions prior to recording. Approximately 100 trials were recorded per task for 18 participants.
2.3.1. Verbal fluency task (VF)
Eleven category names (mammals, fish, birds, vegetables, fruit, trees, beverages, US states, furniture, musical instruments, and tools) were orthographically presented to participants in random order. Interstimulus intervals (ISI) varied from 8 to 10 s between trials. Participants were instructed to wait 3–5 s and then respond by naming a member from the category. For example, appropriate responses to the cue “Mammal” might be “Bear” or “Rabbit”. Participants were encouraged to generate unique category members for each trial and not to name the same member more than once. Data were collected from 100 trials. A research assistant monitored and transcribed the participant’s verbal responses.
2.3.2. Word reading task (WR)
In order highlight the effects of semantic operations on the characteristics of the speech BP it was necessary to include a comparison task which was motorically equivalent to the VF task but did not require lexical search and selection. A word reading task was selected for this purpose. Words, which were transcribed from the participant’s responses during the VF task, were randomized and presented orthographically at a variable ISI between 8 and 10 s. Participants were instructed to wait 3–5 s and then to repeat aloud each presented word. Data were collected from 100 trials.
2.3.3. Simple speech task (SS)
The single word “pool” was presented repeatedly at the same variable ISI mentioned above. Participants were instructed to wait approximately 3–5 s and then to repeat the word “pool” aloud. Data were collected from 100 trials.
Task order was counterbalanced between participants, with the exception of the VF task, which by necessity always preceded word reading.
ERP averaging was time-locked to the onset of the earliest source of articulation related movement on trial by trial bases. Movement onset was determined using a program included in the BESA electrophysiological data analysis software package, which identified the time points at which specified voltage thresholds had been reached for each of the articulator movement channels. Movement data from the tongue and lips were rectified and smoothed by a low-pass filter with 40 Hz cut-off prior to the identification of lip EMG and tongue movement onset. Due to amplitude variations between participants, voltage thresholds for the lips and tongue were determined individually per participant. Participants’ lip and tongue data from each of the three conditions were visually inspected and 20 EMG and Doppler bursts which appeared to typify those recorded from the participant were arbitrarily selected. Amplitudes from the earliest peaks of each burst were then averaged in order to determine voltage threshold. From the 18 participants, the mean EMG and Doppler voltage thresholds were 14.30 μV, SD = 1.05 and 168.41 μV, SD = 24.15, respectively. A second inspection of the articulator movement data was performed following trigger placement in order to identify any anomalous, non-speech related markers. Raw EEG data were epoched into 6000 ms interval, beginning 3000 ms prior to, and ending 3000 ms following the onset of articulator movement. DC offset corrections were performed using the first 500 ms of each epoch as a baseline interval. Epochs were visually inspected for ocular and speech related muscle artifact. Those determined to contain muscle artifacts preceding speech onset were removed from the analysis. The remaining epochs were averaged in order to derive the BP.
An inspection of individual participant averages revealed BP morphological characteristics similar to the early, slow BP1 and the steeper BP2, which typically precede limb movements. The BP1 began approximately 2500 ms prior to movement onset and was followed by the BP2 at 2000 ms premovement, which was consistent with the latencies of the BP recorded prior to speech and complex movements from prior studies (Deecke et al., 1986; Kristeva, 1984; Wohlert, 1993). However, due to individual differences in the onsets of the BP1 and BP2 between participants the grand average (n = 18) demonstrated no morphological distinctions between the BP1 and BP2. Maximum amplitude variations between tasks were within the interval of −1000 ms and the onset of movement, thus the average amplitude within this interval was selected as the dependent measure.
Homologous electrode pairs were selected in order to assess the laterality and anteriority effects described in the hypotheses section. For midline comparisons, electrodes Cz and FCz were selected. Lateral pairs included F3–F4, FC1–FC2, FC3–FC4, and C5–C6. Averaged voltages of the BP from electrodes representing right hemisphere or posterior medial cortical regions were compared to BP voltages from their left homolog or in the case of the electrode representing the posterior medial cortex, the electrode to its immediate anterior. Mean amplitude values were obtained from ERP data recorded during each of the three tasks for statistical comparison.
2.4. Data analysis
The hypothesized effects of task on the lateral and medial BP distribution were assessed through comparisons of mean amplitudes between paired electrodes. Two factor repeated measures analyses of variance (ANOVA) were performed for each of the hypothesized regional differences in BP topography. Thus, four separate ANOVAs were conducted to test for hypothesized hemispheric asymmetries over the frontal, superior frontocentral, mid-frontocentral, and central regions and a 5th to test differences in midline topographic distribution. Factors included were task: 3 levels (verbal fluency, simple speech, and word repetition) × electrode: 2 levels (left and right or in the case of medial differences, anterior and posterior). Task and electrode were treated as repeated variables. Due to potential covariation between experimental conditions, introduced by the repeated measures design, the Huynh and Feldt Epsilon correction was applied to each calculated F-statistic. All tests were held to a family-wise α of .05. Planned comparisons were performed only when the F-test associated with a specific hypothesis produced an interaction significant at p ≤ .05. Hypotheses specific mean comparisons were performed using paired t-tests, with Bonferroni corrections in order to maintain the specified experiment-wise type I error rate.
3. Results
3.1. Experiment 1: assessment of multiple sources of articulatory movement
Experiment 1 was conducted in order to assess the utility of several measures of articulatory movement. Table 1 contains the mean onset latencies of the articulatory movements and the acoustic signal. For speech utterances beginning with various phonemes, movements of the lips and tongue always preceded vocal fold adduction and the acoustic signal. Articulation began with lip movement on 60% and with tongue movement on 40% of the trials (see Fig. 1). It was therefore deemed unnecessary to measure vocal fold adduction in Experiment 3.
Table 1.
Mean articulator movement onset latencies.
| Articulator | Onset (s) | SE |
|---|---|---|
| Lip-EMG | 4.99 | 0.73 |
| Tongue-Doppler | 4.95 | 1.02 |
| Glottis-EGG | 5.19 | 0.69 |
| Acoustic signal-microphone | 5.31 | 0.73 |
Note. Mean onset latencies following visual cues for 7 participants (4 performed a semantic verbal fluency task and 3 named objects presented as line drawings).
Fig. 1.
Synchronous recordings of articulation related movement from the tongue, lip, and phonogram during single word utterance. (A) For the word “Piranha” lip movement begins 220 ms prior to the onset of tongue movement and 600 ms prior to the phonation. (B) For the word “Dog” tongue movement begins 335 ms prior to the onset of lip movement and 417 ms prior to the phonation.
3.2. Experiment 2: comparison of the bp derived from speech and limb movements
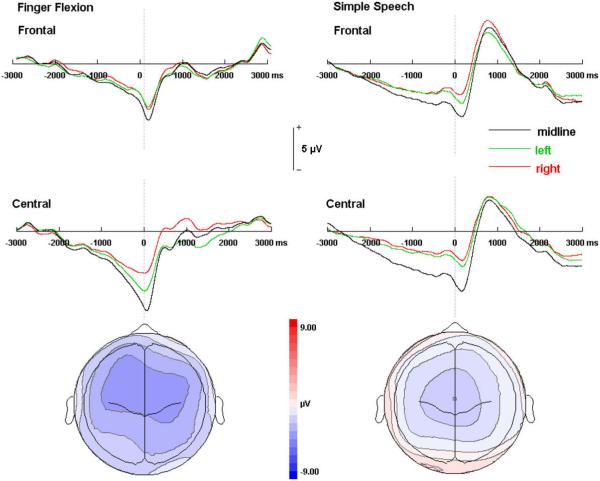
Experiment 2 was conducted in order to compare the topographic distributions of the BP prior to speech and simple distal limb movements. Fig. 2 illustrates the grand-average BP waveforms derived from finger flexions and the simple speech task (left) and topographic maps of the BP amplitude averaged over the last 2000 ms prior to movement onset. Both simple speech and finger movements produced symmetrical BP distributions over the frontal scalp region. However, right hand finger flexions produced a prominent left hemisphere asymmetry in the central BP distribution, most prominent within the final 500 ms prior to the onset of movement, while the BP derived from simple speech was symmetrical over the central region. BP onset latencies also differed between limb and speech movements. The BP preceded finger flexions by 2000 ms and for simple speech the BP began approximately 3000 ms prior to EMG onset.
Fig. 2.
Bereitschaftspotential waveforms from right hand finger flexions (top left) and simple speech (top right). Topographic maps of the mean amplitude from −2000 to 0 ms prespeech are shown on the bottom.
3.3. Experiment 3: impact of articulatory complexity and lexical access on the speech-related BP
Although the Bereitschaftspotential has been traditionally associated with motor planning and readiness prior to distal limb movement, it was proposed in the present study that lexical selection as well as articulatory complexity would contribute to variations in the topography of the BP preceding speech. Tasks were designed to dissociate the effects of these two aspects of spoken language production on the topographic distribution of the BP.
Specifically, it was hypothesized that increased articulatory complexity would produce a midline shift in maximum BP voltage to the anterior scalp midline. Thus, both the verbal fluency (VF) and word reading (WR) tasks, which required an identical degree of complex articulatory control (see Section 2), were expected to produce greater BP negativity at the frontocentral midline, while the simple speech (SS) task was expected to result in maximum BP amplitude over the vertex.
It was also expected that lexical selection would result in a further increase in BP amplitude at the anterior midline recording site (FCZ). Thus, it was hypothesized that the VF task, which, unlike the other two conditions, required a semantically based search of the mental lexicon and selection of a lexical target, would yield higher BP amplitude than both WR and SS at electrode FCZ.
Beyond this, lexical selection was expected to also produce lateralized asymmetries in the BP topography. It was therefore anticipated that the VF task would result in left lateralization of the BP waveform, while the WR and SS tasks would produce more symmetrical BP distributions.
3.4. Behavioral findings
As noted above, in each of the three tasks, participants were instructed to wait approximately 4 s, following textual cues, before making a spoken response. For the SS task, the mean response latency was 3.32 s and for WR and VF the means were 3.35 and 3.76 s, respectively (see Fig. 3). Participant’s mean response latencies by task were analyzed using a one-way repeated measure ANOVA. It was found that response latency did not significantly vary among tasks, F(230) = 2.58, p = .1114, ε = 0.7346.
Fig. 3.
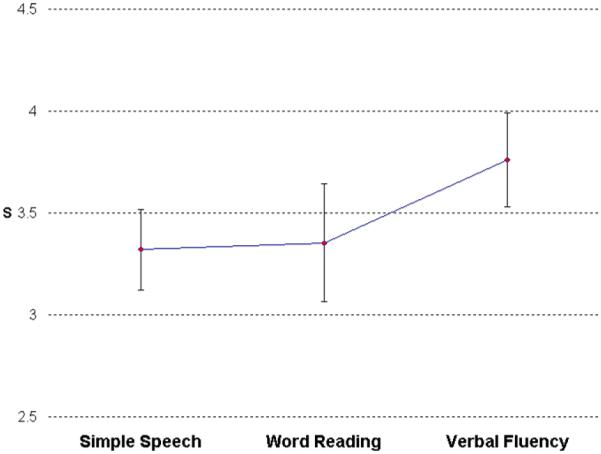
Mean articulator movement onset latencies from visual cues. No significant latency differences were found between conditions.
3.5. Electrophysiological findings
Separate 2 (electrode site) by 3 (task) repeated measures ANOVAs were performed in order to determine if task manipulations led to distinct topographic differences in the BP distribution.
3.6. Effects of articulatory complexity
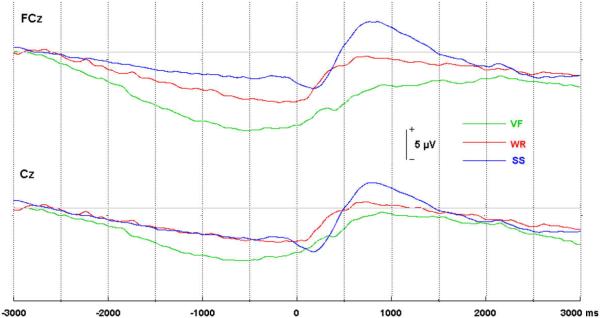
As hypothesized, the midline BP maximum amplitude was shifted to the anterior midline scalp for tasks involving complex articulatory movements (see Fig. 4). This was supported by a two-way repeated measures ANOVA, which revealed a significant interaction of electrode site and task F(268) = 7.35, p = .0046, ε = 0.6955. One tailed t-tests indicated that the BP mean amplitude was significantly greater at the anterior midline electrode (FCz) than the posterior midline electrode (Cz) for the VF, t(68) = 4.21, p = .0003, and WR, t(68) = 2.61, p = .0388, conditions. In contrast, the SS task produced maximum midline amplitude at the posterior site; amplitude differences between FCZ and CZ were not significant for the SS task, t(68) = −1.078, p = .9972 (see Table 2).
Fig. 4.
Comparison of averaged BP waveforms from anterior (FCz) and posterior (Cz) midline electrodes, in the verbal fluency (VF), word reading (WR), and simple speech (SS) conditions.
Table 2.
Midline anterior and posterior BP mean amplitudes by condition.
| Condition | Midline electrode site |
|
|---|---|---|
| Anterior (FCZ) | Posterior (CZ) | |
| Verbal fluency | −11.5349a,1 | −7.61528b,1 |
| Word reading | −7.5204a,2 | −5.08678b,2 |
| Simple speech | −4.03186a,3 | −5.03614a,2 |
Note. Means within the same row with differing alphabetic subscripts and means within the same column with differing numeric subscripts were significantly different at p < .02, when compared, using one-tailed Bonferroni corrected t-tests.
3.7. Effects of lexical access
Notable effects of lexical access were also found at the midline channels. A significant main effect of task was found for mean BP amplitude over the midline scalp region, F(268) = 30.15, p < .0001, ε = 0.6955. One tailed t-tests indicated that the midline BP mean amplitude was significantly greater for VF than the WR (t(68) = 4.97, p < .0001) and SS (t(68) = 7.65, p < .0001) conditions.
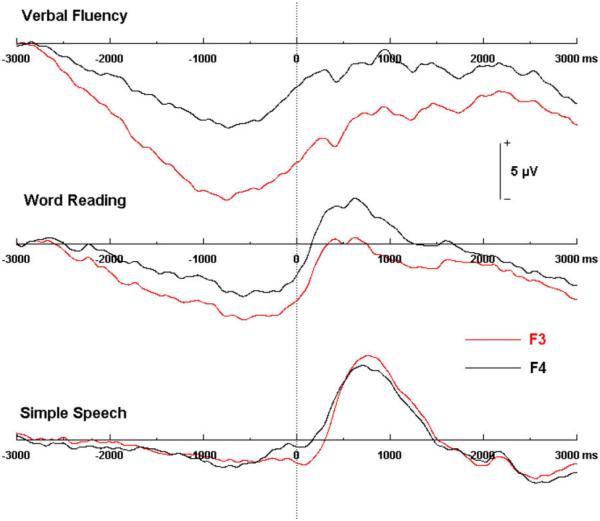
Support was also found for hypothesized task-specific hemispheric asymmetries in the BP topographic distribution (see Fig. 5). A repeated measures ANOVA of the mean BP amplitude recorded at frontal electrode sites F3 (left) and F4 (right) revealed a significant interaction of electrode site and task, F(268) = 4.27, p = .0198, ε = 0.9467. Left and right mean amplitudes within each of the tasks were compared with one tailed t-tests. VF produced a significant left-lateralized frontal distribution, t(68) = −4.34, p < .0003. Frontal hemispheric asymmetries were not found for tasks which did not require an online lexical search: comparisons of BP mean amplitudes between the left and right frontal sites were not significant for WR, t(68) = 1.45, p = .5257 and SS, t(68) = −0.33, p = 1 (see Table 3). Additionally, main effects of condition were found for means derived from recordings at the left frontal channel, F(268) = 30.07, p < .0001, ε = 0.9467 (see Fig. 5). One tailed t-tests indicated that amplitude differences between VF and WR (t(68) = 4.26, p = .0003) and WR and SS (t(68) = 3.23, p = .0067) were statistically significant. Amplitude differences at the right frontal channel were not significant (see Table 3).
Fig. 5.
Comparison of averaged BP waveforms from left (F3) and right (F4) frontal electrodes, in the verbal fluency, word reading, and simple speech conditions.
Table 3.
Frontal hemispheric asymmetries in BP mean amplitudes by condition.
| Condition | Frontal electrode site |
|
|---|---|---|
| Left (F3) | Right (F4) | |
| Verbal fluency | −11.591a,1 | −5.959b,1 |
| Word reading | −6.072a,2 | −4.185a,2 |
| Simple speech | −1.882a,3 | −1.445a,3 |
Note. Means within the same row with differing alphabetic subscripts and means within the same column with differing numeric subscripts were significantly different at p < .02, when compared using one-tailed Bonferroni corrected t-tests.
4. Discussion
The primary purpose of this study was to test the hypothesis that the features of the Bereitschaftspotential, an event related potential traditionally associated with motor planning, would be affected by task parameters specific to spoken language. It was anticipated that, consistent with what is known about relationships between movement complexity and the limb BP, the phonological complexity of an utterance would significantly modify the morphology and distribution of the speech BP. However, it was also expected that, when articulatory complexity was controlled for, tasks requiring lexical selection, retrieval of target words from the mental lexicon, would produce independent and dissociable effects on both the medial and lateral BP topography. This prediction is consistent with an overarching hypothesis – that motor systems play an integral role in lexical access, independent of articulation.
Before these hypotheses could be tested, pilot studies were conducted to demonstrate that the BP could be recorded prior to speech, independent of articulatory artifact.
4.1. Pilot studies: measuring an artifact-free speech BP
4.1.1. Comparison BP derived from speech and limb movements
We first sought to compare the topography and morphological features of the speech and distal limb BP collected in the same set of individuals under identical experimental conditions. The BP was recorded prior to right hand finger flexions and overt spoken repetitions of the word —Pool— in 6 participants. These movements were selected for comparison due to their relative simplicity from both a cognitive and motoric standpoint. Mean BP waveforms from the midline and left and right frontal and central scalp areas are presented with corresponding topographic maps in Fig. 2. Asymmetry in the BP amplitude was observed for finger but not simple speech movements, which were also associated with much earlier BP onset latencies. These observations coincide with the findings of studies of the BP associated with simple movements of the limb and simple and phonologically invariant speech utterances. The BP associated with the former typically develops a contralateral asymmetry approximately 500 ms prior to movement onset over the central scalp region (Deecke, 2000), whereas a relatively symmetrical distribution has been reported for the BP associated with simple speech movements (i.e. repetition of single, phonologically simplistic words) (Wohlert, 1993).
More complex utterances however, particularly in the unconstrained context employed in the present experiments, are more problematic due to the possibility of artifact contamination. This possibility arises because phonologically complex utterances involve the coordinated, but variable timing of multiple articulators.
4.1.2. Determining the onset of articulatory movements
A number of articulatory muscles have been implicated as potential sources of movement contamination in speech BP recordings due to their close spatial proximity to EEG electrode sites. Movement of the orbicularis oris often precedes the onset of audible speech sounds by 500 ms or more, and may have influenced the topography of the speech BP in the earliest of studies, which time-locked data averaging to the acoustic signal. Later studies utilized EMG measured from the orbicularis oris itself as a means of determining the onset of speech. However, it was still possible that movements of other articulatory muscles may have preceded the onset of EMG measured at the orbicularis oris and thereby contaminated the speech BP. Movements of the tongue and glottis for example have also been implicated as a potential source of contamination for the speech BP (Grabow and Elliott, 1974; Szirtes and Vaughan, 1977). Several authors attempted to reduce muscle artifacts from the tongue by tightly constraining the phonological patterns of the words spoken by participants. For example, in order to avoid preparatory tongue movements, Wohlert (1993) instructed participants to repeat the word —Pool— while deemphasizing the /l/ sound at the end of the word. This strategy had the consequence of limiting investigations of the speech BP to relatively simplistic and non-cognitively challenging tasks. Moreover, it is not entirely certain that limits on the types of articulatory gestures performed by participants to those believed to begin with lip movements would necessarily eliminate preparatory tongue movements. This point is highlighted by evidence from X-ray microbeam recordings of speech which has demonstrated that the order of articulatory movements varies considerably not only between utterances of varying phonological patterns, but also between repeated utterances of the same word (Westbury et al., 2000).
Several means of assessing the onset of speech related muscle movement were explored in the present study. While standard bipolar electrodes could be utilized for recording EMG from the orbicularis oris, overlying the lips, the motions of the tongue and glottis could not be accurately monitored through EMG surface recording. Two methods, novel to ERP research, were adopted in order to monitor tongue and glottal movements. First, Doppler imaging was examined as a means of determining the onset of tongue movement. As noted previously, direct measurements of tongue movement have been problematic, due to its enclosure in the oral cavity and pharynx. Doppler has been a popular means of imaging the tongue due to its noninvasive nature (Stone, 1989) and the temporal resolution it provides makes Doppler highly suitable as an index of the onset of tongue movement during articulation. Second, we used the electroglottogram (EGG) as a means of determining glottal adduction during speech. However, there were no observed instances of glottal movement preceding those of either the lips or tongue during the articulation of single words (see Table 1). Thus EGG measurements were discontinued during the primary experiment.
We found instead that, depending on the initial phoneme, the lips or tongue always were represented the source of first articulatory movement. Thus, by accurately measuring the activity of each of these articulators, it was possible to determine, on a word by word basis, the precise onset of the earliest reliable speech trigger and time-lock ERP averaging to these events in order to avoid movement-related contamination of the BP.
Confident that a reliable speech BP could be recorded, independent of muscle artifact, we were able to conduct the principal experiments, designed to examine the effects of articulatory complexity and lexical access on the features of the speechrelated BP.
4.2. Task-related modulation of the speech BP
4.2.1. Effects of articulatory complexity
Confirmatory evidence was found in support of the first hypothesis, that increased articulatory complexity would result in an anterior midline shift in the maximum BP voltage. It was anticipated that the verbal fluency (VF) and word reading (WR) tasks, in which the articulatory patterns of utterances were more complex, would produce greater BP negativity at the frontocentral midline, possibly due to the recruitment of more anterior portions of the SMA, while the simple speech (SS) task was expected to result in BP maximum over the vertex. A significant interaction between task and electrode site supported this hypothesis. Mean BP amplitudes in the VF and WR tasks were significantly greater at the anterior (FCZ) when compared to the posterior midline site (CZ). In contrast, the SS condition produced a greater BP amplitude at CZ than at FCZ, though the difference was non-significant (Fig. 4).
The notion that the anterior cortical midline regions subserve the production of relatively complex articulatory patterns is in agreement with findings from neuroimaging studies of both limb and speech movements. For example, Picard and Strick (1996) concluded from their review of PET movement studies that task complexity played a significant role in the pattern of medial premotor area activation. Movements characterized by more elementary temporal or spatial organization typically elicit increases in rCBF within the region of SMA proper. Tasks requiring more complex sequences of movement resulted in additional increases in the pre- SMA and rostral portions of the cingulate motor area (CMA).
A similar shift in topography of the BP has also been demonstrated for complex movements of the distal limbs (Niemann et al., 1991; Schreiber et al., 1983), and more recently, using both source localization techniques (Cui et al., 2000) and event related fMRI (Deecke, 2000), researchers have confirmed that this midline topographic shift in maximum BP amplitude is related to the recruitment of pre-SMA and CMA for tasks requiring more complex finger movements. The present study is the first to demonstrate a similar anterior progression for complex speech.
4.2.2. Effects of lexical access
In addition, our results also suggest that the anterior midline regions play a role in lexical selection underlying categorical word retrieval. The VF task, which required the participants to generate exemplars from several semantic categories, produced BP amplitudes at FCZ that were greater not only than the SS task, in which responses were stereotypic, but also greater than the WR task, in which the responses were identical with respect to articulatory complexity but were read rather than generated spontaneously.
These findings, along with those outlined above, suggest that the anterior midline cortical regions support two roles in the formulation of spoken language – the organization of complex articulatory movements as well as processes associated with early lexical access.
This interpretation is consistent with imaging studies that have demonstrated increased pre-SMA activation for speech tasks requiring the retrieval of semantic information, when compared to word reading (Petersen et al., 1988; Wise et al., 1991). Thus it is reasonable to infer that the anterior shift in BP amplitude demonstrated in the present study during the verbal fluency task may reflect involvement of the pre-SMA. Unlike the SMA proper, the pre-SMA has numerous reciprocal connections with the prefrontal cortex. It therefore seems plausible that the pre-SMA subserves functional roles higher order linguistic operations as well as articulation.
In addition to effects on the midline BP topography, a dissociation between the effects of articulatory complexity and lexical access was also found in the frontolateral scalp region. These results support the hypothesis that lexical search and selection would result in lateralized asymmetries in the BP topography. It was specifically predicted that the VF task would result in left lateralization of the BP waveform, while the WR and SS tasks, which require phonetic encoding and articulation but not selection of words, would produce a more symmetrical BP distribution. A significant interaction between condition and frontolateral electrode site provided confirmatory support for this: Mean BP amplitude differences between the left (F3) and right (F4) frontolateral electrode sites were found only for the VF condition, in which amplitude in the left hemisphere was significantly greater than the right. Furthermore, main effects of condition were found at the left frontal site (F3) between each of the three tasks. Left frontolateral mean BP amplitude was significantly greatest for the VF task, followed by WR and then SS. Amplitude differences were not significant between conditions at the right frontocentral site (F4) (Fig. 5).
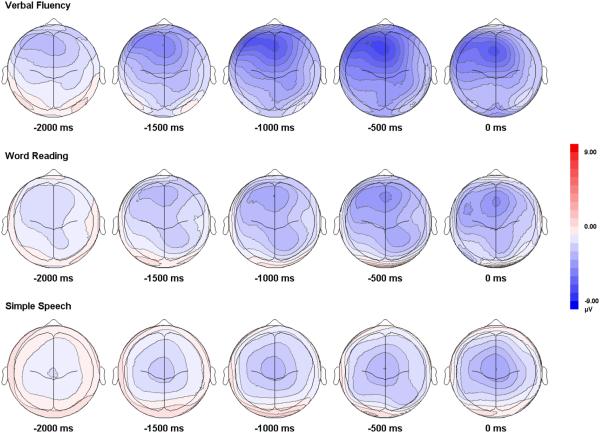
Considering these findings, it does appear that under certain conditions the speech-related BP is markedly left lateralized over the mid-frontal region (Fig. 6). As noted above, several previous studies found no evidence of hemispheric lateralization (Brooker and Donald, 1980; Grabow and Elliott, 1974; Morrell and Huntington, 1972; Wohlert, 1993). Possible reasons for this may have to do with differences in locations of the recording sites selected. Many of these studies recorded exclusively from electrodes over the central scalp region. Moreover, EMG contamination, resulting from movements of the tongue and other articulators, may possibly have masked any evidence of BP lateralization. Given the present results, however, a more likely explanation for these conflicting findings is that the paradigms used in earlier studies that found no evidence of lateralization in the speech BP employed simple speech tasks that did not require lexical search and selection.
Fig. 6.
Topographic voltage maps of BP amplitude from −2000 ms to speech onset from the verbal fluency, word reading, and simple speech conditions.
4.3. Language motor interactions
Left lateralization associated with language processing is not unexpected and ample evidence from imaging and electrophysiological studies lends support to this view. It may seem curious however that the topographic distribution of the BP, which is most commonly associated with readiness or preparation for volitional movements, should be modulated by tasks that differ purely in terms of cognitive–linguistic operations.
A growing body of literature suggesting a functional interaction between cortical motor and language networks may shed light on this finding. Converging evidence from a diverse range of disciplines suggests that the cortical system subserving language may have evolved from a pre-existing gestural system of communication (Corballis, 2003; Kimura, 1993; Liberman and Whalen, 2000; Lieberman, 1975, 1985, 1984). A link between the language and motor systems has also been demonstrated by several studies which found changes in hemodynamic activation and electrophysiological amplitudes within the motor and premotor cortices for spoken and written linguistic processes ranging from phonology to semantics (Hauk et al., 2004; Pulvermuller et al., 2001). Additionally, transcranial magnetic stimulation studies have demonstrated an interaction of language and motor systems during a lexical decision task (Pulvermuller et al., 2005).
Together, these findings suggest an active role of the cortical motor system in the processing of language. The results of the present experiment support this notion, specifically indicating that the mesial premotor structures and the prefrontal regions with which they interact may play an integral role in the process of searching for and retrieving words from the mental lexicon.
5. Conclusions
In summary, our findings demonstrate that accurate recordings of the BP prior to speech can be obtained when EEG data is timelocked to the earliest articulatory movement on a word by word basis. Due to the considerable variability present in the order of articulatory movements, it was necessary to monitor a number of muscles in order to identify the earliest movements associated with speech. This method improved the accuracy of responselocked averaging and eliminated preparatory muscle artifact contributions to the speech BP.
When measured accurately, the speech BP is similar to the BP preceding distal limb movement in that it appears as a slow negative deflection in the ERP and is maximal over the central and frontocentral scalp regions. Unlike the limb movement derived BP, the speech BP begins at much earlier latencies (approximately 2500 ms prior to the onset of speech).
Speech and language-related factors appear to significantly modulate the amplitude and topographic distribution of the speech BP. Not unexpectedly, the maximum midline amplitude of the speech BP shifts in an anterior direction for more articulatory complex speech utterances. More surprisingly though, it appears that cognitive processes associated with lexical search and selection result further increases in the anterior amplitude and left-lateralized frontal BP asymmetry. This novel finding supports the notion that the motor systems may play a significant role in the formulation of language.
Acknowledgements
The authors thank Dr. Ou Bai for his critical and insightful review of this manuscript. This work was supported by the Intramural Program of NIDCD.
References
- Brooker BH, Donald MW. The search for scalp-recordable speech potentials. Prog Brain Res. 1980;54:782–9. doi: 10.1016/S0079-6123(08)61703-3. [DOI] [PubMed] [Google Scholar]
- Corballis MC. From mouth to hand: gesture, speech, and the evolution of right-handedness. Behav Brain Sci. 2003;26:199–208. doi: 10.1017/s0140525x03000062. [DOI] [PubMed] [Google Scholar]
- Cui RQ, Huter D, Egkher A, Lang W, Lindinger G, Deecke L. High resolution DC-EEG mapping of the Bereitschaftspotential preceding simple or complex bimanual sequential finger movement. Exp Brain Res. 2000;134:49–57. doi: 10.1007/s002210000449. [DOI] [PubMed] [Google Scholar]
- Deecke L. The Bereitschaftspotential as an electrophysiological tool for studying the cortical organization of human voluntary action. Suppl Clin Neurophysiol. 2000;53:199–206. doi: 10.1016/s1567-424x(09)70158-8. [DOI] [PubMed] [Google Scholar]
- Deecke L, Engel M, Lang W, Kornhuber HH. Bereitschaftspotential preceding speech after holding breath. Exp Brain Res. 1986;65:219–23. doi: 10.1007/BF00243845. [DOI] [PubMed] [Google Scholar]
- Empson JA. Slow potentials preceding vocalisation. Biol Psychol. 1982;14:271–6. doi: 10.1016/0301-0511(82)90007-2. [DOI] [PubMed] [Google Scholar]
- Grabow JD, Elliott FW. The electrophysiologic assessment of hemispheric asymmetries during speech. J Speech Hear Res. 1974;17:64–72. doi: 10.1044/jshr.1701.64. [DOI] [PubMed] [Google Scholar]
- Grözinger B, Kornhuber HH, Kriebel J. Methodological problems in the investigation of cerebral potentials preceding speech: determining the onset and suppressing artefacts caused by speech. Neuropsychologia. 1975;13:263–70. doi: 10.1016/0028-3932(75)90002-0. [DOI] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermuller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–7. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Kimura D. Neuromotor mechanisms in human communication. Oxford University Press; New York: 1993. [Google Scholar]
- Kornhuber HH, Deecke L. Changes in the brain potential in voluntary movements and passive movements in man: readiness potential and reafferent potentials. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965;284:1–17. [PubMed] [Google Scholar]
- Kristeva R. Bereitschaftspotential of pianists. Ann NY Acad Sci. 1984;425:477–82. doi: 10.1111/j.1749-6632.1984.tb23570.x. [DOI] [PubMed] [Google Scholar]
- Liberman AM, Whalen DH. On the relation of speech to language. Trends Cogn Sci. 2000;4:187–96. doi: 10.1016/s1364-6613(00)01471-6. [DOI] [PubMed] [Google Scholar]
- Lieberman P. On the origins of language: an introduction to the evolution of speech. Macmillan; New York: 1975. [Google Scholar]
- Lieberman P. The biology and evolution of language. Harvard University Press; Cambridge, MA: 1984. [Google Scholar]
- Lieberman P. On the evolution of human syntactic ability: its pre-adaptive bases-motor control and speech. J Hum Evol. 1985;14:657–68. [Google Scholar]
- McAdam DW, Seales DM. Bereitschaftspotential enhancement with increased level of motivation. Electroencephalogr Clin Neurophysiol. 1969;27:73–5. doi: 10.1016/0013-4694(69)90111-4. [DOI] [PubMed] [Google Scholar]
- McAdam DW, Whitaker HA. Language production: electroencephalographic localization in the normal human brain. Science. 1971;172:499–502. doi: 10.1126/science.172.3982.499. [DOI] [PubMed] [Google Scholar]
- Morrell LK, Huntington DA. Electrocortical localization of language production. Science. 1972;174:1359–61. doi: 10.1126/science.174.4016.1359. [DOI] [PubMed] [Google Scholar]
- Niemann J, Winker T, Gerling J, Landwehrmeyer B, Jung R. Changes of slow cortical negative DC-potentials during the acquisition of a complex finger motor task. Exp Brain Res. 1991;85:417–22. doi: 10.1007/BF00229418. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–9. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–53. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F, Harle M, Hummel F. Walking or talking? Behavioral and neurophysiological correlates of action verb processing. Brain Lang. 2001;78:143–68. doi: 10.1006/brln.2000.2390. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F, Hauk O, Nikulin VV, Ilmoniemi RJ. Functional links between motor and language systems. Eur J Neurosci. 2005;21:793–7. doi: 10.1111/j.1460-9568.2005.03900.x. [DOI] [PubMed] [Google Scholar]
- Schreiber H, Lang M, Lang W, Kornhuber A, Heise B, Keidel M, et al. Frontal hemispheric differences in the Bereitschaftspotential associated with writing and drawing. Hum Neurobiol. 1983;2:197–202. [PubMed] [Google Scholar]
- Shibasaki H, Hallett M. What is the Bereitschaftspotential? Clin Neurophysiol. 2006;117:2341–56. doi: 10.1016/j.clinph.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Simonetta M, Clanet M, Rascol O. Bereitschaftspotential in a simple movement or in a motor sequence starting with the same simple movement. Electroencephalogr Clin Neurophysiol. 1991;81:129–34. doi: 10.1016/0168-5597(91)90006-j. [DOI] [PubMed] [Google Scholar]
- Stone M. A three-dimensional model of tongue movement based on ultrasound and x-ray microbeam data. J Acoust Soc Am. 1989;87:2207–17. doi: 10.1121/1.399188. [DOI] [PubMed] [Google Scholar]
- Szirtes J, Vaughan HG., Jr Characteristics of cranial and facial potentials associated with speech production. Electroencephalogr Clin Neurophysiol. 1977;43:386–96. doi: 10.1016/0013-4694(77)90261-9. [DOI] [PubMed] [Google Scholar]
- Westbury JR, Severson EJ, Lindstrom MJ. Kinematic event patterns in speech: special problems. Lang Speech. 2000;43:403–28. doi: 10.1177/00238309000430040401. [DOI] [PubMed] [Google Scholar]
- Wise R, Chollet F, Hadar U, Friston K, Hoffner E, Frackowiak R. Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain. 1991;114(Pt. 4):1803–17. doi: 10.1093/brain/114.4.1803. [DOI] [PubMed] [Google Scholar]
- Wohlert AB. Event-related brain potentials preceding speech and nonspeech oral movements of varying complexity. J Speech Hear Res. 1993;36:897–905. doi: 10.1044/jshr.3605.897. [DOI] [PubMed] [Google Scholar]