Abstract
Advanced or metastatic breast cancer is an incurable disease with high mortality rate worldwide and about 20% of breast cancers overexpress and amplify the human epidermal growth factor receptor 2 (HER2). Achievements in targeted therapy have benefited people during the past decades. Trastuzumab emtansine (T-DM1), a novel antibody-drug conjugate playing a powerful role in anti-tumor activity, not only blocks the HER2 signaling pathways, but also disturbs the microtubule dynamics. To access the efficacy and safety of T-DM1, we analyzed 9 clinical trials on T-DM1. Results showed that fatigue (0.604, 95% CI 0.551, 0.654), nausea (0.450, 95% CI 0.365, 0.537), increased transaminases (0.425, 95% CI 0.353, 0.500) and thrombocytopenia (0.383, 95% CI 0.322, 0.448) occurred more frequently in participants with single T-DM1. In controlled trials, increased transaminases (OR = 4.040, 95% CI 1.429, 11.427), thrombocytopenia (OR = 8.500, 95% CI 3.964, 18.226) and fatigue (OR = 1.288, 95% CI 1.041, 1.593) were statistically significant. Only thrombocytopenia appeared as severe adverse event (grade ≥ 3) in single-arm and control-arm studies. Meanwhile, T-DM1 stabilized cancer and prolonged life with notable improved progression-free survival (PFS) and overall survival (OS). In conclusion, it is a safe and effective agent in advanced or metastatic breast cancer, but should be carefully applied on patients with severe hepatic and neurological disease.
Breast cancer is one of the most common cancers among women worldwide1. It is the second leading cause of cancer death among women in the U.S, exceeded only by lung cancer2. With the advancements of chemoradiotherapy over the past two decades, the prognosis of breast cancer has improved and the 5-year overall survival rate is almost up to 90%3. However, metastatic breast cancer (MBC) remains a challenge with only about 22 month’s overall survival (OS)1. According to the expressions of estrogen, progesterone and human epidermal growth factor receptor 2 (HER2), breast cancers were traditionally divided into four types4. HER2 is overexpressed and amplified in about 20% of all breast cancers3,4 and functions as a poor prognostic factor5. Blockage of HER2 receptors has attracted much attention and in 1998 the US Food and Drug Administration (FDA) approved trastuzumab as an agent in HER2-positive breast cancer therapy6. However, some patients who were treated with trastuzumab still appeared disease progression and trastuzumab resistance limited the clinical application of trastuzumab. In 2013, the FDA approved the application of trastuzumab emtansine (T-DM1) on patients with trastuzumab resistance7,8.
T-DM1 is a novel antibody-drug conjugate composed of trastuzumab, derivative of maytansine 1 (DM1) and a non-reducible thioether linker9,10. Trastuzumab itself is a humanized monoclonal antibody targeting on HER2 receptors, thus stimulating antibody-dependent cell-mediated cytotoxicity (ADCC), inhibiting the PTEN-PI3K/AKT pathway and inducing apotosis3,11,12,13. Moreover, after T-DM1 binds to HER2, the HER2-T-DM1 complex enters into target cells through receptor-mediated endocytosis and releases DM1, causing cell cycle arrest and apoptosis via the inhibition of microtubule assembly11,12. To access the efficacy and safety of T-DM1, clinical trials were launched in different countries among patients with advanced or metastatic HER2-positive breast cancer. Studies have demonstrated that T-DM1 functioned well in patients. In a phase 1 trial, researchers14 have detected the maximum-tolerated dose and the optimal outcome was 3.6 mg/kg every three weeks. A large-scale trial15 comparing T-DM1 with lapatinib plus capecitabine among HER2-positive metastatic breast cancer patients indicated that T-DM1 significantly improved the OS and median progression-free survival (PFS). T-DM1 brought clinical benefits to patients, at the same time, the adverse events (AEs) were inevitable. The most common AEs caused by T-DM1 were fatigue, nausea, increased aminotransferases, thrombocytopenia, arthralgia, headache, anemia and pneumonia. Thus, we analyzed published clinical trials to evaluate the odd ratios (ORs) of adverse events and hazard ratios (HRs) for PFS and OS.
Results
Eligible articles
A total of 305 potentially relevant articles were searched in PubMed in June 2015. After reviewing the titles or abstracts, 282 unrelated articles were excluded. After reading the full texts of the remaining articles, 9 articles were finally included for meta-analysis. The basic process was showed in Fig. 1. Basic information of the 9 included articles was presented in Table 1. Eligible articles included 3 phase I trials and 3 phase II single-arm studies, and 1 phase II and 2 phase III studies were randomized controlled trials. For single-arm trials, we calculated the incidence of adverse events and analyzed 3 articles for PFS. In terms of controlled studies, Hurvitz et al. set two groups (T-DM1; trastuzumab plus docetaxel) to determine the contribution of T-DM1 in adverse events, Verma et al. made comparison between Lapatinib plus Capecitabine and T-DM1, Krop et al. conducted a multicenter trials comparing T-DM1 and physician’s choice of treatment, both adverse events and efficacy were analyzed.
Figure 1. Flow diagram of the literature search and trial selection process.
Table 1. Basic information of eligible articles.
| First author | Year of publication | phase | treatment | Number of patients |
|---|---|---|---|---|
| Krop.IE | 2010 | I | T-DM1 | 24 |
| Beeram.M | 2012 | I | T-DM1 | 28 |
| Yamamoto.H | 2015 | I | T-DM1 | 10 |
| Burris.HA 3rd | 2011 | II | T-DM1 | 112 |
| Krop.IE | 2012 | II | T-DM1 | 110 |
| Hurvitz.SA | 2013 | II | T-DM1 | 137(67,70) |
| Trastuzumab plus docetaxel | ||||
| Miller.KD | 2014 | Ib/IIa | T-DM1 and pertuzumab | 64 |
| Verma.S | 2012 | III | T-DM1 | 991(495; 496) |
| Lapatinib plus capecitabine | ||||
| Krop.IE | 2014 | III | T-DM1 | 602(404; 198) |
| Physician’s choice | ||||
Patients
A number of 2050 patients (T-DM1: 1308; control: 742) in 9 articles were included for analysis. For safety analysis, Hurvitz et al. excluded two patients from their study and included another two patients taking T-DM1 in control arm (T-DM1: 1310; Control: 738). Three trials (T-DM1: 962; Control: 738) compared T-DM1 with other agents. Miller et al. used T-DM1 and pertuzumab for single-arm study (n = 64). The others (n = 348) took single T-DM1 for therapy.
Safety analysis
All the included articles reported adverse events. After evaluating the all grade and grade ≥3 adverse events, we found that the most common events contained anemia, fatigue, increased transaminases, nausea, thrombocytopenia, arthralgia and headache. Single-arm studies were analyzed to calculate the adverse event rates and control trials were to determine the contribution of T-DM1 in adverse events. The results were presented in Table 2.
Table 2. The OR values and models of control-arm and the event rates of single-arm trials.
| Control-arm trials: | ||||||
|---|---|---|---|---|---|---|
| Adverse events | All grade |
Grade ≥3 |
||||
| Odds Ratio with 95% CI | Model | I2 | Odds Ratio with 95% CI | Model | I2 | |
| Anemia | 0.847(0.457,1.571) | Random Model | 67.184 | 1.220(0.643,2.316) | Fixed Model | 0.000 |
| Fatigue | 1.288(1.041,1.593) | Fixed Model | 0.000 | 0.774(0.427,1.402) | Fixed Model | 0.000 |
| Increased Transaminases | 4.040(1.429,11.427) | Random Model | 87.995 | 3.2007(0.828,10.912) | Random Model | 65.316 |
| Nausea | 0.843(0.664,1.069) | Fixed Model | 29.670 | 0.862(0.067,11.046) | Random Model | 62.427 |
| Thrombocytopenia | 8.500(3.964,18.226) | Random Model | 59.033 | 7.271(1.098,48.133) | Random Model | 75.811 |
| Single-arm trials: | ||||||
| Adverse events | Event rate with 95% CI | |||||
| Fixed model | ||||||
| Anemia | 0.216(0.171,0.269) | |||||
| Fatigue | 0.604(0.551,0.654) | |||||
| Increased Transaminases | 0.425(0.353,0.500) | |||||
| Thrombocytopenia | 0.383(0.322,0.448) | |||||
| Random model | ||||||
| Arthralgia | 0.201(0.107,0.348) | |||||
| Headache | 0.252(0.150,0.391) | |||||
| Nausea | 0.450(0.365,0.537) | |||||
| Pneumonia | 0.083(0.016,0.336) | |||||
With regard to single-arm trials (Fig. 2), the incidence of anemia ranged from 0.200 to 0.292 and the overall event rate accounted for 0.216 (95% CI 0.171, 0.269). Increased transaminases occurred more frequently in participants with the overall event rate being 0.425 (95% CI 0.353, 0.500). Thrombocytopenia showed a similar event rate of 0.383 (95% CI 0.322, 0.448). Nausea appeared differently in each trial, varying from 0.250 to 0.700 and the overall event rate was 0.450 (95% CI 0.365, 0.537). Fatigue was the most common adverse event with the highest rate of 0.604 (95% CI 0.551, 0.654). Arthralgia and headache occurred less frequently. Despite the high rates of adverse events, severe events (grade ≥ 3) were relatively rare. Only severe thrombocytopenia occurred in 10.7% (95% CI 0.073, 0.154) of participants, others seldom happened.
Figure 2. The adverse event rates and 95% CI in single-arm trials.
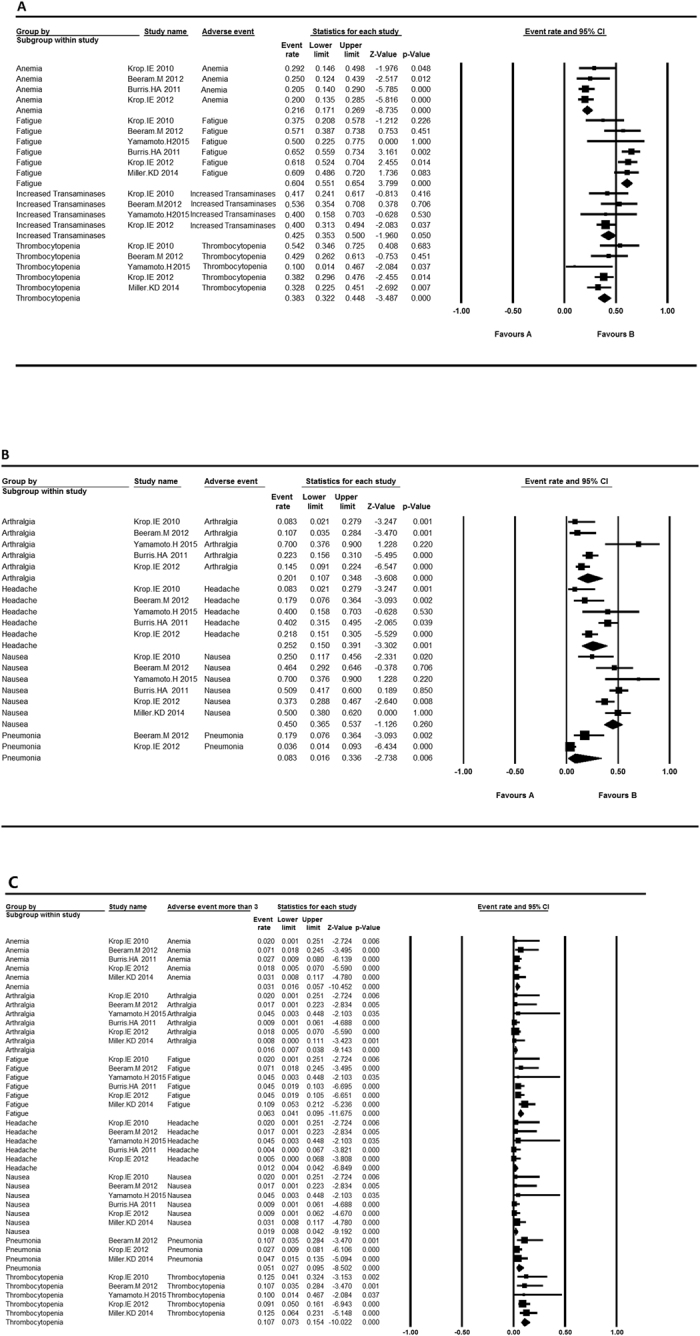
(A) The adverse event rates and 95% CI of fixed model in single-arm trials; (B) The adverse event rates and 95% CI of random model in single-arm trials; (C) The adverse event (grade more than 3) rates and 95% CI of fixed model in single-arm trials.
For three control-arm studies, we analyzed anemia, fatigue, increased transaminases, nausea and thrombocytopenia with OR values (Fig. 3). According to I2, fixed model was used in all grade fatigue and nausea, and grade ≥3 anemia and fatigue. Among all the adverse events, increased transaminases (OR = 4.040, 95% CI 1.429, 11.427), thrombocytopenia (OR = 8.500, 95% CI 3.964, 18.226) and fatigue (OR = 1.288, 95% CI 1.041, 1.593) were statistically significant. T-DM1 may play a dominant role in thrombocytopenia with the highest OR value. Similarly, only grade ≥3 thrombocytopenia (OR = 7.271, 95% CI 1.098, 48.133) appeared statistically significant.
Figure 3. The adverse event rates and 95% CI in control-arm trials.
(A) The adverse event rates and 95% CI of fixed model in control-arm trials; (B) The adverse event rates and 95% CI of random model in control-arm trials; (C) The adverse event (grade more than 3) rates and 95% CI of fixed model in control-arm trials; (D) The adverse event (grade more than 3) rates and 95% CI of random model in control-arm trials.
Efficacy analysis
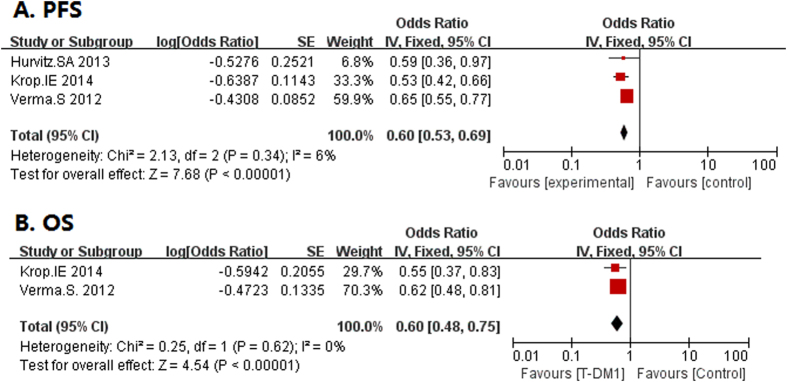
PFS data was presented in 3 single-arm studies and 3 controlled trials (Table 3). The median PFS varied from 4.6 to 6.9 months for single-arm studies. In controlled trials, the HRs for progression or death in three control trials ranged from 0.528 to 0.65 with a total OR of 0.64 (95% CI 0.55, 0.75) (Fig. 4), indicating a longer PFS in T-DM1 group. Only two controlled studies provided the OS data, with a total OR of 0.64 (95% CI 0.55, 0.75) (Fig. 4). Verma et al. showed both first and second interim analysis and Krop et al. only had second interim analysis, both indicating an improved survival than other groups.
Table 3. The PFS of control-arm trials and single-arm trials.
| Control-arm trials: | ||||
|---|---|---|---|---|
| study | PFS(median month) |
HR(95% CI) | P value | |
| T-DM1 | Control | |||
| Hurvitz.SA 2013 | 14.2 | 9.2 | 0.59(0.36,0.97) | 0.035 |
| Verma.S 2012 | 9.6 | 6.4 | 0.65(0.55,0.77) | <0.001 |
| Krop.IE 2014 | 6.2 | 3.3 | 0.528(0.422,0.661) | <0.0001 |
| Single-arm trials | ||||
| Study | Median PFS(95% CI)(month) | |||
| Burris.HA 3rd 2011 | 4.6(3.9,8.6) | |||
| Krop.IE 2012 | 6.9(4.2,8.4) | |||
| Miller.KD 2014 | 6.6(4.21,9.46) | |||
Figure 4. The HRs and 95% CI for PFS and OS in control-arm trials.
(A) PFS; (B) OS.
Discussion
Although the advanced or metastatic breast cancer remains incurable, the application of T-DM1 does benefit patients. According to our analysis, major common adverse events involved fatigue, nausea, increased transaminases and thrombocytopenia. The total event of fatigue reached more than 50% and nausea happened in nearly half of participants. The OR value of increased transaminases was more than 3, indicating the firm correlation with the toxicity of T-DM1. Compared to this, the event rate of anemia and thrombocytopenia seemed lower, but severe thrombocytopenia (grade ≥ 3) approached 10% and the OR valued more than 5, suggesting a prominent role of T-DM1 in hematologic toxicity. Other adverse events did not show such close correlations with T-DM1. Meanwhile, we summarized the PFS and OS in patients. As was shown in single-arm trials, T-DM1 stabilized the disease approximately for half a year. In controlled trials, T-DM1 was more effective than other therapies, even compared with the combination therapy with trastuzumab16. T-DM1 indeed brings hope and benefits to patients.
As described above, the components trastuzumab and DM1 both play a role in anti-tumor activity. Trastuzumab (Herceptin) is a humanized IgG antibody specific to HER2, which was approved for HER2-positive breast cancer therapy. It is reported to activate the tumor suppressor PTEN, down-regulate the ErbB2 and subsequently inhibit PTEN-PI3K/AKT signaling pathway, which is vital for diverse cell functions including cell growth, survival, proliferation and metabolism17,18,19,20. In T-DM1, trastuzumab not only functions as an antibody binding to the HER2-positive cells, inhibiting the HER2 signaling pathway and inducing antibody-mediated cellular cytotoxicity (ADCC), but also specifically conveys DM1 to target cells which disturbs the original cell function9,21. An experiment22 has reported that the major toxicities of T-DM1 were associated with DM1 rather than trastuzumab or thioether linker. DM1 is a tubulin-binding agent. Once separated from ADCs, free DM1 has a high affinity to the microtubules, thus suppressing microtubule dynamics and inhibiting mitosis at metaphase23,24. Combination of the two powerful anti-tumor agents with a thioether linker makes it possible to function effectively in advanced or metastatic breast cancer and minimize the exposure of DM1 to normal tissue owing to the stable linker25.
The DM1 part in T-DM1, a microtubule-inhibiting agent, plays a major role not only in anti-tumor activity, but also in adverse events. Fatigue is the most common adverse event mainly attributed to DM1. Fortunately, few patients experienced severe fatigue. Previous studies have demonstrated that microtubule-inhibiting chemotherapy agents are always accompanied by neurotoxicity and DM1 is no exception26, DM1 or T-DM1 shares the same mechanism that causes notable degeneration of axon in animal experiments and may be less reversible22,26. Patients with nerve neurological problems should be cautious when taking T-DM1.
T-DM1 was given intravenously at 3.6 mg/kg every three weeks. Krop et al.27 confirmed the maximum tolerated dose of T-DM1 mainly according to severe thrombocytopenia (grade ≥ 3). Thrombocytopenia might result from decreased production or accelerated destruction of platelet. Among T-DM1 treated patients, researchers found that T-DM1 inhibited the differentiation of megakaryocytes and the production of platelets was consequently reduced22,26. Moreover, Uppal et al.28 reported that T-DM1 entered the megakaryocytes (MKs) by binding to FcgRIIα independent of HER2, and affected the cytoskeleton of differentiating MKs without trastuzumab. Another trial found a significantly positive relationship between HER2 expression and increased platelets through vascular endothelial growth factor (VEGF)29, but whether thrombocytopenia was caused by decreased HER2-positive breast cancer cells still remained unknown. More subsequent researches are needed to explore the exhaustive mechanism.
A previous study26 has confirmed that increased transaminases was caused by maytansine, and FDA has taken hepatotoxicity into account which predicts liver damage in people based on monkey experiments. In addition, other researches have found that the clearance of T-DM1 mainly depend on hepatic-biliary and gastrointestinal route30,31,32, hence patients with hepatic diseases should be kept under surveillance.
The article analyzed the safety and efficacy of T-DM1 in available clinical trials. All eligible articles chose patients with advanced or metastatic breast cancer. Possible mechanisms of major adverse events were explained. The heterogeneity of included articles was analyzed based on different regions, different races, different therapies and previous treatments, even the Eastern Cooperative Oncology Group performance status (ECOG PS) was analyzed.
There are also some shortages in our analysis. Firstly, Brain-metastatic breast cancer markedly influences PFS and OS, but in all articles, analysis of subtypes and stages of breast cancer were ignored, so we did not consider these aspects. Secondly, some articles mentioned that T-DM1 had two black boxes, one for pregnant women, the other for cardiac toxicity32. For trastuzumab part, it has been reported to cause cardiac dysfunction33, but cardiac AEs did not appear to be such frequent or serious in T-DM1, thus we did not take it into account, and further trials are needed. Trastuzumab has been reported to cause severe AEs in both pregnant women and fetuses32, but we found no descriptions of such case in T-DM1. Lastly, Miller et al.34 prescribed T-DM1 and pertuzumab in patients, but there was no consideration of the AEs of pertuzumab and the interactions between the two drugs in the analysis. All of these might cause bias in our analysis.
To sum up, T-DM1 is a relatively safe and effective agent in the treatment of advanced or metastatic HER2-positive breast cancer, even among patients with asymptomatic or treated brain metastases and trastuzumab resistance14,15,16,25,27,34,35,36,37. Previous articles32,33,38,39 found the same common AEs in trials including increased transaminases, thrombocytopenia and fatigue, but they had no explanations about them and further researches are needed. Given the notable adverse events in platelet production and drug excretion pathway, patients should take regular laboratory examination and should be followed up. For patients with severe hepatic or neurological diseases, drugs should be taken under close surveillance or should not be prescribed.
Method
Article searching
Relevant articles were selected by searching databases through PubMed (until June 2015) without language or data limitations. Retrieval keywords included “T-DM1”, “TDM-1”, “trastuzumab emtansine/trastuzumab-emtansine”, “kadcyla”, “ado-trastuzumab emtansine” and “trastuzumab-DM1”. The search was focused on articles conducting clinical trials.
Inclusion and Exclusion criteria
The eligible criteria included: 1) any phase clinical trials evaluating the efficacy and safety of T-DM1 whether they had control groups or not; 2) patients in clinical trials were confirmed by pathology to have breast cancer, clinical evidence supported advanced or metastatic breast cancer; 3) efficacy and adverse events were available in the results; 4) full text could be downloaded. Articles were excluded if they were duplicate publications or without raw data.
Data extraction
Data extracted from all eligible articles included: 1) the basic information of studies: the first author name, year of publication, study design, number of participants, treatment and study phase. 2) the characteristics of major AEs (mentioned in at least 2 articles): T-DM1 groups or control groups, types of AEs and numbers of all grade and grade ≥3 AEs. 3) HRs for PFS or OS.
Statistical analysis
Data analysis was performed on Comprehensive Meta-Analysis (CMA) program 2 (Biostat, Englewood, NJ) and Review manager 5.2 (Copenhagen, Sweden). For single-arm studies, we calculated the proportion and derived 95% confidence interval (CI) of major AEs (both all grade and grade ≥3). For controlled trials, the OR was calculated to determine the role of T-DM1 in adverse events. Two-sided P values were considered significant when less than 0.10 and I2 ≥ 50% was used to decide on fixed-effects model or random-effects model in the analysis.
Additional Information
How to cite this article: Shen, K. et al. Safety and Efficacy of Trastuzumab Emtansine in Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: a Meta-analysis. Sci. Rep. 6, 23262; doi: 10.1038/srep23262 (2016).
Footnotes
Author Contributions K.S. collected, analyzed the data and wrote the article. X.M. and X.W. provided the idea. C.Z. modified the article, H.J. edited the pictures. All authors reviewed the manuscript.
References
- Sharp A. & Johnston S. R. Dose-reduced trastuzumab emtansine: active and safe in acute hepatic dysfunction. Case Rep Oncol. 8, 113–121 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breast Cancer Statistics. Available at: http://www.breastcancer.org/symptoms/understand_bc/statistics (Accessed May 11, 2015).
- Tinoco G., Warsch S., Gluck S., Avancha K. & Montero A. J. Treating breast cancer in the 21st century: emerging biological therapies. J Cancer. 4, 117–132 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp A. & Harper-Wynne C. Treatment of advanced breast cancer (ABC): the expanding landscape of targeted therapies. J Cancer Biol Res. 2, 1036 (2014). [Google Scholar]
- Eggemann H. et al. Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr Relat Cancer. 22, 725–733 (2015). [DOI] [PubMed] [Google Scholar]
- Kumar G. & Badve S. Milestones in the discovery of HER2 proto-oncogene and trastuzumab (herceptin). Connections. 13, 9–14 (2008). [Google Scholar]
- Murphy C. G. & Morris P. G. Recent advances in novel targeted therapies for HER2-positive breast cancer. Anticancer Drugs. 23, 765–776 (2012). [DOI] [PubMed] [Google Scholar]
- Oostra D. R. & Macrae E. R. Role of trastuzumab emtansine in the treatment of HER2-positive breast cancer. Breast Cancer (Dove Med Press). 6, 103–113 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila T. T., Li G., Parsons K., Phillips G. L. & Sliwkowski M. X. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat. 128, 347–356 (2011). [DOI] [PubMed] [Google Scholar]
- Lambert J. M. & Chari R. V. Ado-trastuzumab Emtansine (T-DM1): an antibody-drug conjugate (ADC) for HER2-positive breast cancer. J Med Chem. 57, 6949–6964 (2014). [DOI] [PubMed] [Google Scholar]
- Barok M., Joensuu H. & Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 16, 209 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K. C., Hageman K. & Cooper M. R. Ado-trastuzumab emtansine for the treatment of human epidermal growth factor receptor 2-positive metastatic breast cancer. Am J Health Syst Pharm. 71, 537–548 (2014). [DOI] [PubMed] [Google Scholar]
- Leyland-Jones B. Trastuzumab: hopes and realities. Lancet Oncol. 3, 137–144 (2002). [DOI] [PubMed] [Google Scholar]
- Krop I. E. et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 28, 2698–2704 (2010). [DOI] [PubMed] [Google Scholar]
- Verma S. et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 367, 1783–1791 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krop I. E. et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 15, 689–699 (2014). [DOI] [PubMed] [Google Scholar]
- Nagata Y. et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 6, 117–127 (2004). [DOI] [PubMed] [Google Scholar]
- Baselga J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist. 16 Suppl 1, 12–19 (2011). [DOI] [PubMed] [Google Scholar]
- Berns K. et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 12, 395–402 (2007). [DOI] [PubMed] [Google Scholar]
- Nahta R., Yu D., Hung M. C., Hortobagyi G. N. & Esteva F. J. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 3, 269–280 (2006). [DOI] [PubMed] [Google Scholar]
- Saini K. S. et al. Beyond trastuzumab: new treatment options for HER2-positive breast cancer. Breast. 20 Suppl 3, S20–27 (2011). [DOI] [PubMed] [Google Scholar]
- Poon K. A. et al. Preclinical safety profile of trastuzumab emtansine (T-DM1): mechanism of action of its cytotoxic component retained with improved tolerability. Toxicol Appl Pharmacol. 273, 298–313 (2013). [DOI] [PubMed] [Google Scholar]
- Lopus M. Antibody-DM1 conjugates as cancer therapeutics. Cancer Lett. 307, 113–118 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elster N. et al. HER2-family signalling mechanisms, clinical implications and targeting in breast cancer. Breast Cancer Res Treat. 149, 5–15 (2015). [DOI] [PubMed] [Google Scholar]
- Burris H. A. 3rd et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 29, 398–405 (2011). [DOI] [PubMed] [Google Scholar]
- Amiri-Kordestani L. et al. FDA approval: ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin Cancer Res. 20, 4436–4441 (2014). [DOI] [PubMed] [Google Scholar]
- Beeram M. et al. A phase 1 study of weekly dosing of trastuzumab emtansine (T-DM1) in patients with advanced human epidermal growth factor 2-positive breast cancer. Cancer. 118, 5733–5740 (2012). [DOI] [PubMed] [Google Scholar]
- Uppal H. et al. Potential mechanisms for thrombocytopenia development with trastuzumab emtansine (T-DM1). Clin Cancer Res. 21, 123–133 (2015). [DOI] [PubMed] [Google Scholar]
- Gu M. L. et al. Pre-treatment Elevated Platelet Count Associates with HER2 Overexpression and Prognosis in Patients with Breast Cancer. Asian Pac J Cancer Prev. 16, 5537–5540 (2015). [DOI] [PubMed] [Google Scholar]
- Shen B. Q. et al. Non-clinical Disposition and Metabolism of DM1, a Component of Trastuzumab Emtansine (T-DM1), in Sprague Dawley Rats. Drug Metab Lett (2015). [DOI] [PubMed] [Google Scholar]
- Girish S. et al. Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol. 69, 1229–1240 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peddi P. F. & Hurvitz S. A. Ado-trastuzumab emtansine (T-DM1) in human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer: latest evidence and clinical potential. Ther Adv Med Oncol. 6, 202–209 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelovac D. & Wolff A. C. The adjuvant treatment of HER2-positive breast cancer. Curr Treat Options Oncol. 13, 230–239 (2012). [DOI] [PubMed] [Google Scholar]
- Miller K. D. et al. Phase IIa trial of trastuzumab emtansine with pertuzumab for patients with human epidermal growth factor receptor 2-positive, locally advanced, or metastatic breast cancer. J Clin Oncol. 32, 1437–1444 (2014). [DOI] [PubMed] [Google Scholar]
- Yamamoto H. et al. Phase I and pharmacokinetic study of trastuzumab emtansine in Japanese patients with HER2-positive metastatic breast cancer. Jpn J Clin Oncol. 45, 12–18 (2015). [DOI] [PubMed] [Google Scholar]
- Krop I. E. et al. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J Clin Oncol. 30, 3234–3241 (2012). [DOI] [PubMed] [Google Scholar]
- Hurvitz S. A. et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 31, 1157–1163 (2013). [DOI] [PubMed] [Google Scholar]
- Baron J. M., Boster B. L. & Barnett C. M. Ado-trastuzumab emtansine (T-DM1): a novel antibody-drug conjugate for the treatment of HER2-positive metastatic breast cancer. J Oncol Pharm Pract. 21, 132–142 (2015). [DOI] [PubMed] [Google Scholar]
- Barroso-Sousa R., Santana I. A., Testa L., de Melo Gagliato D. & Mano M. S. Biological therapies in breast cancer: common toxicities and management strategies. Breast. 22, 1009–1018 (2013). [DOI] [PubMed] [Google Scholar]