Abstract
Recent synthetic efforts aimed at reconstructing the beginning of life on our planet point at the plausibility of scenarios fueled by extraterrestrial energy sources. In the current work we show that beyond nucleobases the sugar components of the first informational polymers can be synthesized in this way. We demonstrate that a laser-induced high-energy chemistry combined with TiO2 catalysis readily produces a mixture of pentoses, among them ribose, arabinose and xylose. This chemistry might be highly relevant to the Late Heavy Bombardment period of Earth’s history about 4–3.85 billion years ago. In addition, we present an in-depth theoretical analysis of the most challenging step of the reaction pathway, i.e., the TiO2-catalyzed dimerization of formaldehyde leading to glycolaldehyde.
Formation of simple sugars from one carbon feedstock molecules on early Earth has for long been considered as one of the most fundamental steps in the prebiotic syntheses leading to RNA. The oligomerization of formaldehyde, known as the formose reaction1,2, is the most widely recognized scenario in this context. Nevertheless, the formose reaction is plausible only if glycolaldehyde is present in the reaction mixture. Otherwise, the initial formaldehyde dimerization to glycolaldehyde is not feasible since it requires opposite polarity on the carbon atoms of the two reacting molecules. It has been demonstrated that if glycolaldehyde is present in the reaction mixture in trace amounts, simple sugars can be produced in autocatalytic cycles in the presence of borate minerals3. Since glycolaldehyde has been detected in our galaxy4, it was suggested that glycolaldehyde necessary for the synthesis of terrestrial sugars has been delivered from the space5. Another alternatives for glycolaldehyde production on the primitive Earth include UV–photolysis of formaldehyde6 and reaction of CH4 with CO27. The borate-chemistry proposed by Benner and coworkers3,8,9,10 offers not only an elegant way to the sequestration of pentoses from the prebiotic mix but also shows that the reaction can generate a sufficient amount of glycolaldehyde necessary to maintain production of aldopentoses. In this model pentoses are derived from glycolaldehyde and glyceraldehyde. The role of borates is to promote enolization of glycolaldehyde and slow down enolization of glyceraldehyde. In addition, as a side reaction branched pentoses are formed from the enol form of glyceraldehyde again in a borate-catalyzed reaction, and the retroaldol fragmentation thereof then produces sufficient amount of glycolaldehyde necessary to maintain the autocatalytic cycle. Zubay et al. have shown that Pb2+ cations can significantly increase the otherwise low yield of the formose reaction11. Reid and Orgel reported yields as high as 40% for the synthesis of sugars catalyzed by CaCO3 and apatite12. The peptide-catalyzed synthesis of sugars reported by Pizzarello and Weber proceeds with a relatively low yield but stereoselectively leads to D-sugars13.
Previously, we have successfully used Laser Induced Dielectric Breakdown (LIDB) plasma chemistry to simulate synthesis of nucleobases from small-molecular precursors in a high–density energy event, like impact of an extraterrestrial body14,15,16. It has been shown that such events are especially relevant to the Late Heavy Bombardment period ca. 4 billion years ago, which roughly coincides with the time when terrestrial life emerged.
The approach used in our previous papers14,15,16 is thus relevant to the impact of an icy, extraterrestrial body on an early Earth atmosphere. The great synthetic advantage of extraterrestrial impacts lies in their very broad energy spectrum: via a rovibrational excitation of the reaction components it facilitates a wide variety of chemical transformations. In the current work, we extend this approach towards the synthesis of sugars. We demonstrate that the crucial first step of the formose reaction yielding glycolaldehyde could be catalyzed by an anatase (TiO2) surface activated in such an impact.
Results and Discussion
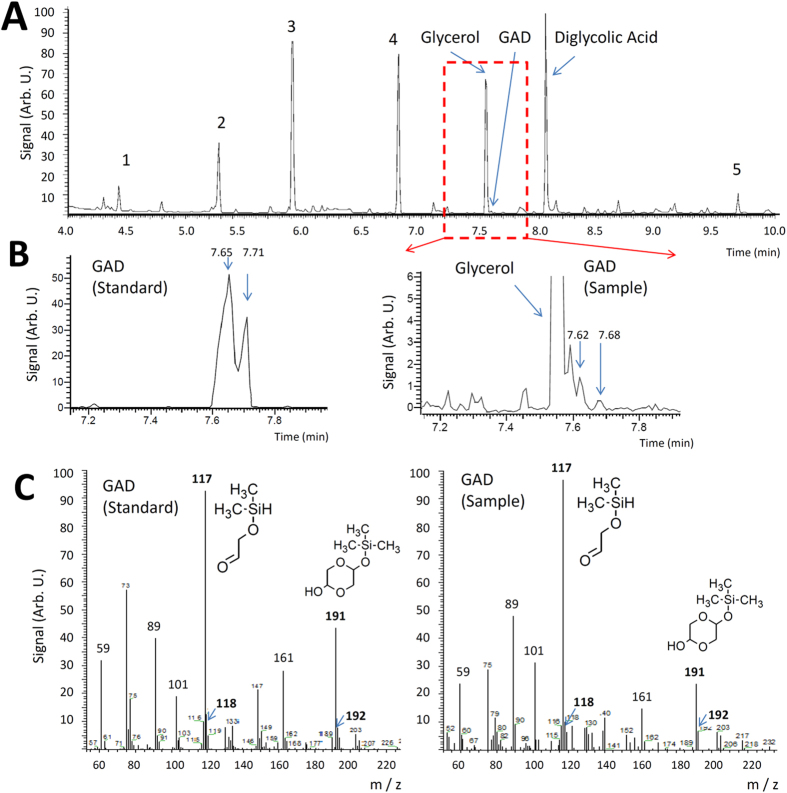
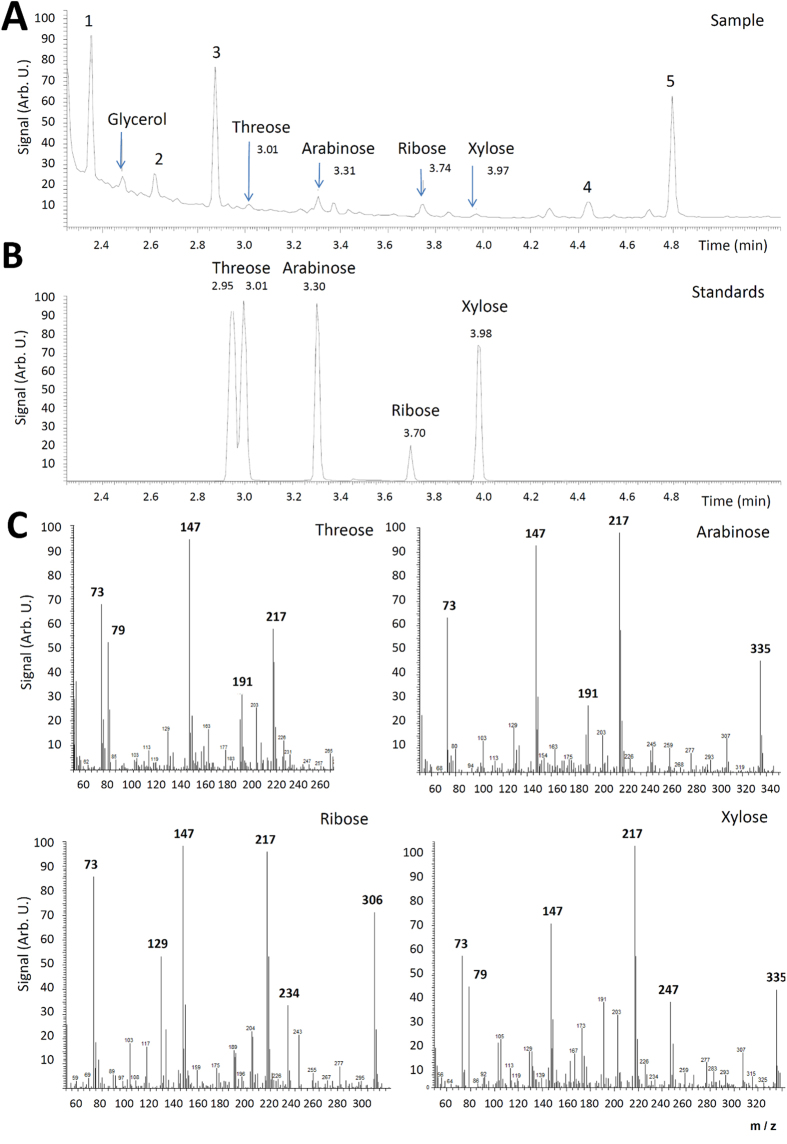
In our experiments, we mixed 1.0 g of paraformaldehyde sample with deionized water and a catalytic amount (0.1 g) of anatase form of titania TiO2. Then a frozen sample was prepared using liquid nitrogen, which was subsequently treated with 10 pulses of the Prague Asterix Laser System (PALS) (wavelength of 1.315 μm, energy of 150 J, mean output density of 1015 W/cm2) in a cell filled with 760 Torr of nitrogen inert gas. Formation of glycolaldehyde was hinted by an absorption at 853 cm−1 (assigned to a C-C stretching vibration)17 in the high resolution infrared spectrum of the vapor-phase products shown in Fig. S1 in the Supporting Information. An independent subsequent Gas Chromatography – Mass Spectroscopic (GC–MS) analysis after derivatization by silylation has confirmed presence of glycolaldehyde in an amount of 10 ppm in the evaporated liquid-phase product (see Fig. 1). In addition, we have identified four sugars, such as threose, arabinose, ribose and xylose, in a total amount of 110 ppm together with glycerol and diglycolic acid in the GC-MS spectra of the higher molecular weight products (see Fig. 2). In contrast, glycolaldehyde and the above mentioned sugars were either undetectable or formed in very low concentration (near the detection limit of >1 mg/kg) in the sample irradiated in the absence of TiO2. No sugars, glycerol, diglycolic acid or glycolaldehyde were detected in the non–irradiated blank sample prepared simultaneously (see Fig. S3 in the Supplementary Information). This shows that TiO2–catalysis and activation by high–energy pulses strongly cooperate in the studied scenario of sugar synthesis from formaldehyde.
Figure 1.
Panel (A) chromatogram of the derivatized sample of paraformaldehyde + TiO2 mixture treated with 10 laser pulses. Peaks of manifold products of mutual reactions among derivatization agents are marked 1–5. Glycerol and diglycolic acid have been identified using the NIST library43. Panel (B) chromatographic double peak of glycolaldehyde dimer (GAD) in a standard (100 ppm in deionized water) and the similar peak detected in the irradiated sample (Sample). Panel (C) Mass spectra of GAD in the irradiated sample as well as that of GAD standard along with two selected signatures of derivatized monomer and dimer.
Figure 2.
Panel (A) chromatogram of the derivatized sample of paraformaldehyde + TiO2 mixture treated with 10 laser pulses. Peaks of manifold products of mutual reactions among derivatization agents are marked 1–5. Panel (B) chromatogram of selected sugars measured for comparison. For the corresponding mass spectra detected in the sample see panel (C). For details see the Supplementary Information.
Our results indicate that in an impact-chemistry sugars can be synthesized from pure formaldehyde without even trace amounts of glycolaldehyde present in the reaction mixture. This implies that glycolaldehyde is produced in the very first step of the reaction pathway, via dimerization of formaldehyde promoted by the TiO2-anatase surface. (Let us note here, that glycolaldehyde detected in the product mixture could also be partly formed via retroaldolization of tetroses in further stages of the reaction, see in ref. 3). We suggest that high-energy pulses activate the TiO2-anatase surface by increasing the number of oxygen vacancies in the crystal structure18,19.
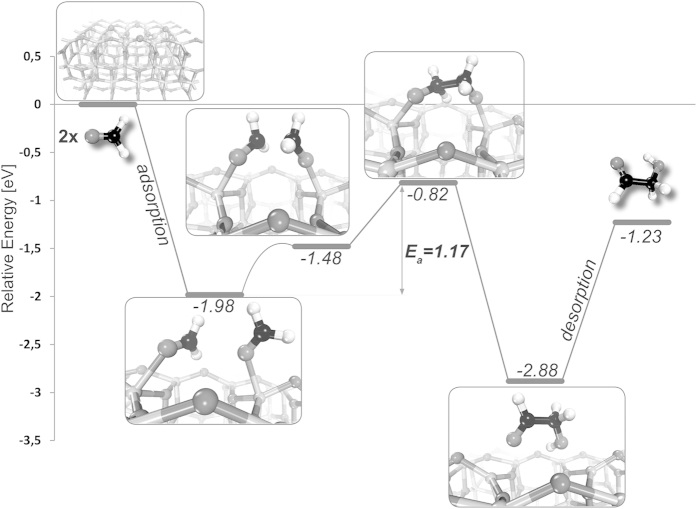
The feasibility of formaldehyde to glycolaldehyde dimerization is further supported by our quantum chemical calculations. We carried out density functional theory (DFT) computations of this reaction on the reactive (001) surface of TiO2-anatase with a surface O-vacancy placed in the middle of a 4 × 5 supercell. Since the O-vacancy forms a biradical state with each excess electron localized on one unsaturated Ti3+ cation20, we performed spin-polarized calculations with enforced triplet multiplicity. For this purpose we utilized the Quantum Espresso plane-wave DFT code21, and the PBE (Perdew-Burke-Ernzernhof) functional22 (see the Supplementary Information for more details). The results indicate that two formaldehyde molecules can be easily adsorbed on two neighboring unsaturated Ti3+ cations, with the total adsorption energy of −2.0 eV. These two formaldehydes can then effectively dimerize yielding glycolaldehyde with the energy barrier of approximately 1.2 eV (see Fig. 3 and Fig. S2 of the Supplementary Information for more detailed view of the reaction path). The biradical character of the defect enables the C-C bond formation and subsequent hydrogen atom transfer to occur within two reaction steps separated by a shallow plateau on the potential energy surface. Since the adsorption energy of the product is −1.7 eV, it is favorable for two further formaldehyde molecules to substitute glycolaldehyde in the reaction center. Therefore, the presence of O-vacancies enables a very effective catalytic cycle, without poisoning the active site of the TiO2 surface. Analogous computations conducted on the regular (001) surface showed that such catalyst poisoning occurs when no O-vacancies are present, i.e. the adsorption energy of the product is lower by 1.1 eV than the adsorption energy of two formaldehyde molecules. Thus, in agreement with the experimental findings, the creation of O-vacancies is essential to activate the catalyst and promote the formation of sugars. According to the theoretical results, the oxygen-deficient TiO2(001) surface also prevents the formation of C-O bond by anchoring oxygen atoms of the reacting formaldehyde molecules. Even though no polarity inversion is generated on any of the reacting species, glycolaldehyde formation is facilitated by oxygen-anchoring and localizing the unpaired electrons on the carbon atoms as the formaldehyde molecules approach each other.
Figure 3. Reaction path leading to glycolaldehyde on the modified TiO2-anatase (001) surface. Relative energies were derived from DFT computations.
It is reasonable to assume that the catalyst of the reaction, i.e. photoactive anatase TiO2, was available in a sufficiently high concentration on the early Earth. Anatase is known as a part of igneous metamorphic, weathered and hydrothermally altered rocks23 or meteoritic materials: see e.g. the Allan Hills meteorite A77307 (type: CO3.0 ordinary chondrite)24, the Martian meteorite EETA7900125, or the Chicxulub impact crater26. Our previous laboratory studies show that the catalytic activity of photoactive anatase TiO2 can be boosted by annealing at high temperature due to an increase of the number of vacancies inside the TiO2 structure18,19,27. Since vacancies are believed to be responsible for the catalytic activity, this suggests that the rapid rise of temperature during the extraterrestrial impact could activate TiO2 for catalysis. As our calculations suggest, if the TiO2-surface is activated, the dimerization proceeds with a relatively low activation energy in an exergonic manner.
As Benner et al. in ref. 9 formulate “Electrical discharge almost certainly generated HCHO continuously in the early terran atmosphere”28. This view is considered nowadays as a consensus supported by many other literature sources7,29,30. Moreover, formaldehyde is a rather common molecule in the space: it has been detected in extraterrestrial icy bodies, like comets31,32,33,34,35 and dark nebulas36. This suggests that formaldehyde must have been at least locally available on Earth during the Early and Late Heavy Bombardment eras either as a component of the primeval atmosphere or from extraterrestrial sources.
Thus, our experiments are compatible with a scenario, in which a high–energy impact is combined with terrestrial volcanic activity. Such an event could be plausible in a volcanic early Earth environment, when the impact of an extraterrestrial body could create the shock–wave necessary to push through the experimentally observed high–energy chemistry leading to glycolaldehyde and simple sugars.
The yield of the sugar synthesis presented above is relatively low (0.01%) as compared to other works reporting yields of 10–40%3,11,12. Nonetheless, one has to take into account that impact activities on the early Earth during the Late Heavy Bombardment were much higher than today, which is perhaps the best demonstrated by the fact that the amount of extraterrestrial material delivered to the Earth was astonishingly high and approached 109 tons/year16,37. In this light, even these relatively low yielding processes could produce an enormous amount of carbohydrates to establish a firm chemical background for the emergence of life.
Likewise, the current results, along with our previous related work16, may have an extraterrestrial implication as well: they may provide a clue for the origin of sugars and nucleobases in meteoritic materials38,39. In other words, they suggest that simple organic precursors present in impactors are transformed into more complex compounds during the fall of an icy extraterrestrial body into a planetary atmosphere.
Methods
Measurement of the high–resolution infrared spectrum of irradiated samples and vapor–phase standards
Glass irradiation cell equipped with a Pyrex window of 10 cm diameter has been filled with 1 g of paraformaldehyde (reagent grade, crystalline, CAS 30525–89–4, Sigma Aldrich), 1 ml of deionized water and 0.1 g of anatase TiO2 (99.8%, powder, CAS 1317–70–0, Sigma Aldrich), and 1 atm of inert nitrogen gas. The sample was then frozen using liquid nitrogen, transferred to the Prague Asterix Laser System facility (PALS) and irradiated with 10 laser pulses of 150 J in energy (time interval ≈350 ps, wavelength of 1.315 μm, output of 428 MW). The laser beam has been focused using CaF2 lens to achieve an output density approximately of 1014–1016 W/cm2. The experiment mimics the high–density energy plasma in an asteroid impact (plasma temperature of 4500 K, shock wave, emission of hard UV and XUV radiation).
Prior to spectroscopic measurements the frozen samples were melted in vacuum. High resolution Fourier transform infrared (HR–FTIR) spectra of the vapor phase were measured in a multipass White cell reaching an optical path of 35 m. The cell was interfaced to a sealable glass vacuum line used for the transfer of the vapors formed upon the evaporation of the icy reaction mixture from the irradiation cell.
The spectrometer Bruker IFS 125 HR was subsequently evacuated and operated in the measurement mode from 650–5500 cm−1 using a HgCdTe nitrogen cooled detector and a KBr beamsplitter. 100 scans were acquired with 40 kHz scanning mirror speed with a resolution of 0.02 cm−1. The measured interferograms were apodised with the Blackmann–Harris apodisation function.
For comparison, we have also recorded the spectrum of a sample, which was prepared in the same way as described above, but did not contain anatase TiO2. To identify the products formed upon the simulated high–density energy event, we have also recorded the vapor–phase spectra of paraformaldehyde (reagent grade, crystalline, CAS 30525–89–4, Sigma Aldrich), glycolaldehyde (crystalline dimer, mixture of stereoisomers, CAS 23147–58–2, Sigma Aldrich) and glyceraldehyde (DL mixture, assay ≥90%, CAS 56–82–6 Sigma Aldrich) – water mixtures. 1 g of powdered samples has been mixed with deionized water in a vessel, subsequently frozen by liquid nitrogen and evacuated. The frozen samples have been melted again and they have been evaporated under continuous stream of inert nitrogen gas (5 Torr) to the multipass cell in the temperature range from 50 °C up to 130 °C. 100 scans were recorded to acquire the spectra during the evaporation procedure.
GC–MS analysis of the non–volatile fraction of the products formed upon irradiation with a high–power laser
The irradiated and melted samples were evaporated under vacuum inside a vial vessel and analyzed for the presence of saccharides. The measurements were performed using a ITQ 1100 GC–Ion Trap MS system (ThermoScientific, USA), equipped with an Xcalibur MS Platform using a non–polar TG–SQC column (ThermoScientific, USA). 17 μL of hexamethyldisilazane (99% HMDS, CAS 999–97–3, Sigma Aldrich), 6 μL of chlorotrimethylsilane (99% TMCS, CAS 75–77–4, Sigma Aldrich), and 52 μL of pyridine (99.5% anhydrous, Scharlau) were added to the residue as derivatization agents and aprotic solvent, respectively. The vial was then heated at 70 °C for two hours. Subsequently, 0.5 μL of the sample was injected into the chromatograph, and the measurements were performed using a column temperature range of 180–280 °C with a temperature gradient of 30 °C min−1. The mass spectrum was compared with the GC chromatograms and MS spectra of D–forms of ribose, lyxose, xylose (99%), arabinose (98%), threose (60% syrup), ribulose (1 M solution) and xylulose and xylose (98% syrup) standards (all from Sigma Aldrich). Liquid–phase standards (i.e. threose, ribulose and xylulose) were evaporated under vacuum in the presence of phosphorus pentoxide prior to GC–MS analysis.
Quantum Chemical Calculations
Density Functional Theory calculations were performed using the Perdew-Burke-Ernzerhof22 exchange and correlation functional (abbreviated as PBE) within the generalized gradient approximation (GGA). The PBE functional was shown to yield generally very reliable results for anatase. In the calculations we considered a 4 × 5 (15.104 Å × 18.880 Å) surface supercell and a vacuum slab of 19.0 Å to separate the periodic images along the direction of the surface normal. We utilized the spin-restricted formalism when no surface defects were taken into consideration. In the case of surfaces containing O-vacancies we performed spin-polarized calculations with fixed triplet multiplicity, since it was shown that removal of a single oxygen atom from the anatase crystal results in the formation of a biradical in the electronic ground state20. The wave functions were expanded in plane waves applying the kinetic energy cutoff of 50 Ry, whereas a cutoff of 200 Ry was used for the augmented density. The minimum energy path leading to the surface-assisted glycolaldehyde formation was obtained with the Nudged Elastic Band method40. During this procedure 16 intermediate images were optimized on the path leading from the adsorbed substrates to the products. Additional single point computations with the HSE06 range-separated functional41 were performed on the PBE-optimized geometries to test the reliability of the generalized gradient approximation in estimating the relative energy differences. In the utilized HSE06 functional 25% of exact Hartree-Fock exchange was mixed with 75% of the PBE exchange in the short-range part, whereas the long-range part of the exchange potential was essentially described by PBE terms. The corresponding kinetic energy cutoff for the exact exchange operator calculations was set to 50 Ry. Electron-core interactions in both the PBE and HSE06 computations were described using the Troullier-Martins norm-conserving pseudopotentials42. The QUANTUM ESPRESSO package was used for all computations described above21.
Additional Information
How to cite this article: Civiš, S. et al. TiO2-catalyzed synthesis of sugars from formaldehyde in extraterrestrial impacts on the early Earth. Sci. Rep. 6, 23199; doi: 10.1038/srep23199 (2016).
Supplementary Material
Acknowledgments
We thank professor Rutger van Santen for helpful discussions. This research has been financially supported by the Ministry of Education, Youth and Sports of the Czech Republic under the project CEITEC 2020 (LQ1601). The authors would like also to thank the PALS facility for supporting the experiments, particularly Dr. Jiří Skála, Ing. Jiří Ullschmied, Pavel Prchal, and Jakub Mareš. We also thank the Ministry of Education, Youth and Sports of the Czech Republic for supporting the PALS infrastructure operation in the framework of the project LM 2010014. The calculations have been partially carried out on the Cartesius supercomputer at SURFsara, within the project SH-203-14. B.Sz and D.S. would like to acknowledge the support by a statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Chemistry and for the Faculty of Mechanical and Power Engineering of Wroclaw University of Technology for year 2016. B.Sz. and D.S. would like to acknowledge the Interdisciplinary Centre for Mathematical and Computational Modeling (ICM) and Wroclaw Center of Networking and Supercomputing (WCSS) for providing the access to supercomputer facilities (grant nr. G56-4).
Footnotes
Author Contributions J.E.Š. and M.F. invented research; S.C., R.S., B.M.S., D.S., O.I., A.K. and P.K. performed research; J.E.Š., M.F., R.S. and J.Š. wrote the paper.
References
- Breslow R. On the mechanism of the formose reaction. Tetrahedron Lett. 1, 22–26 (1959). [Google Scholar]
- Butlerow A. Bildung einer zuckerartigen Substanz durch Synthese. Justus Liebigs Ann. Chem. 120, 295–298 (1861). [Google Scholar]
- Kim H.-J. et al. Synthesis of carbohydrates in mineral-guided prebiotic cycles. J. Am. Chem. Soc. 133, 9457–9468 (2011). [DOI] [PubMed] [Google Scholar]
- Hollis J. M., Vogel S. N., Snyder L. E., Jewell P. R. & Lovas F. J. The spatial scale of glycolaldehyde in the galactic center. Astrophys. J. 554, L81–L85 (2001). [Google Scholar]
- Ricardo A., Carrigan M. A., Olcott A. N. & Benner S. A. Borate minerals stabilize ribose. Science 303, 196–196 (2004). [DOI] [PubMed] [Google Scholar]
- Pestunova O., Simonov A., Snytnikov V., Stoyanovsky V. & Parmon V. In Space Life Sciences: Astrobiology: Steps toward Origin of Life and Titan before Cassini Vol. 36 Advances in Space Research (eds Bernstein M., NavarroGonzalez R., & Raulin R.) 214–219 (Elsevier Science Ltd, 2005). [Google Scholar]
- Harman C. E., Kasting J. F. & Wolf E. T. Atmospheric production of glycolaldehyde under hazy prebiotic conditions. Orig. Life Evol. Biosph. 43, 77–98 (2013). [DOI] [PubMed] [Google Scholar]
- Ricardo A., Carrigan M. A., Olcott A. N. & Benner S. A. Borate minerals stabilize ribose. Science 303, 196 (2004). [DOI] [PubMed] [Google Scholar]
- Benner S. A., Kim H.-J. & Carrigan M. A. Asphalt, water, and the prebiotic synthesis of ribose, ribonucleosides, and RNA. Acc. Chem. Res. 45, 2025–2034 (2012). [DOI] [PubMed] [Google Scholar]
- Neveu M., Kim H.-J. & Benner S. A. The “strong” RNA world hypothesis: fifty years old. Astrobiology 13, 391–403 (2013). [DOI] [PubMed] [Google Scholar]
- Zubay G. Studies on the lead-catalyzed synthesis of aldopentoses. Orig. Life Evol. Biosph. 28, 13–26 (1998). [DOI] [PubMed] [Google Scholar]
- Reid C. & Orgel L. E. Model for origin of monosaccharides: synthesis of sugars in potentially prebiotic conditions. Nature 216, 455–455 (1967). [DOI] [PubMed] [Google Scholar]
- Weber A. L. & Pizzarello S. The peptide-catalyzed stereospecific synthesis of tetroses: a possible model for prebiotic molecular evolution. Proc. Natl. Acad. Sci., USA 103, 12713–12717 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferus M. et al. On the road from formamide ices to nucleobases: IR-spectroscopic observation of a direct reaction between cyano radicals and formamide in a high-energy impact event. J. Am. Chem. Soc. 134, 20788–20796 (2012). [DOI] [PubMed] [Google Scholar]
- Ferus M. et al. High-energy chemistry of formamide: a simpler way for nucleobase formation. J. Phys. Chem. A 118, 719–736 (2014). [DOI] [PubMed] [Google Scholar]
- Ferus M. et al. High-energy chemistry of formamide: A unified mechanism of nucleobase formation. Proc. Natl. Acad. Sci. USA 112, 657–662 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonniere P. & Pouchan C. Modelization of vibrational spectra beyond the harmonic approximation from an iterative variation–perturbation scheme: the four conformers of the glycolaldehyde. Theor Chem Acc 131, 1–8 (2012). [Google Scholar]
- Civis S. et al. Room temperature spontaneous conversion of OCS to CO2 on the anatase TiO2 surface. Chem. Commun. 50, 7712–7715 (2014). [DOI] [PubMed] [Google Scholar]
- Kavan L. et al. Oxygen-isotope labeled titania: (TiO2)-O18. Phys. Chem. Chem. Phys. 13, 11583–11586 (2011). [DOI] [PubMed] [Google Scholar]
- Aschauer U. et al. Influence of subsurface defects on the surface reactivity of TiO2: water on anatase (101). J. Phys. Chem. C 114, 1278–1284 (2010). [Google Scholar]
- Giannozzi P. et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys.: Condensed Mat. 21, 395502 (2009). [DOI] [PubMed] [Google Scholar]
- Perdew J. P., Burke K. & Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). [DOI] [PubMed] [Google Scholar]
- Bowles J. F. W., Howie R. A., Vaughan D. J. & Zussman J. Rock-Forming Minerals. Vol. 5A (The Geological Society of London, 2011). [Google Scholar]
- Han J. & Brearley A. J. Formation of TiO2 nanoparticles in a CAI-like object from an AOA in the Alpha 77307 CO3.0 carbonaceous chondrite. Meteorit. Planet. Sci. 46(S1), abstract No. 5190 (2011). [Google Scholar]
- Wang A., Kuebler K., Jolliff B. & Haskin L. A. Mineralogy of a Martian meteorite as determined by Raman spectroscopy. J. Raman Spectrosc. 35, 504–514 (2004). [Google Scholar]
- Zürcher L. & Kring D. A. Hydrothermal alteration in the core of the Yaxcopoil-1 borehole, Chicxulub impact structure, Mexico. Meteorit. Planet. Sci. 39, 1199–1221 (2004). [Google Scholar]
- Civiš S., Ferus M., Kubát P., Zukalová M. & Kavan L. Oxygen-isotope exchange between CO2 and solid Ti18O2. J. Phys. Chem. C 115, 11156–11162 (2011). [Google Scholar]
- Cleaves H. J. The prebiotic geochemistry of formaldehyde. Precambrian Res. 164, 111–118 (2008). [Google Scholar]
- Miller S. L. & Urey H. C. Organic compound synthesis on the primitive Earth. Science 130, 245–251 (1959). [DOI] [PubMed] [Google Scholar]
- Pinto J. P., Gladstone G. R. & Yung Y. L. Photochemical production of formaldehyde in Earth’s primitive atmosphere. Science 210, 183–184 (1980). [DOI] [PubMed] [Google Scholar]
- Schutte W. A., Allamandola L. J. & Sandford S. A. An experimental study of the organic molecules produced in cometary and interstellar ice analogs by thermal formaldehyde reactions. Icarus 104, 118–137 (1993). [DOI] [PubMed] [Google Scholar]
- Fomenkova M. N., Chang S. & Mukhin L. M. Carbonaceous components in the comet Halley dust. Geochim. Cosmochim. Acta 58, 4503–4512 (1994). [DOI] [PubMed] [Google Scholar]
- Biver N. et al. Chemical composition diversity among 24 comets observed at radio wavelengths. Earth Moon Planets 90, 323–333 (2002). [Google Scholar]
- Charnley S. B. & Rodgers S. D. Interstellar reservoirs of cometary matter. Space Sci. Rev. 138, 59–73 (2008). [Google Scholar]
- Mumma M. J. & Charnley S. B. The chemical composition of comets—emerging taxonomies and natal heritage. Annu. Rev. Astron. Astrophys. 49, 471–524 (2011). [Google Scholar]
- Palmer P., Zuckerman B., Buhl D. & Snyder L. E. Formaldehyde absorption in dark nebulae. Astrophys. J. 156, L147-& (1969). [Google Scholar]
- Koeberl C. Impact processes on the early Earth. Elements 2, 211–216 (2006). [Google Scholar]
- Callahan M. P. et al. Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc. Natl. Acad. Sci. USA 108, 13995–13998 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. et al. Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature 414, 879–883 (2001). [DOI] [PubMed] [Google Scholar]
- Henkelman G., Uberuaga B. P. & Jonsson H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000). [Google Scholar]
- Krukau A. V., Vydrov O. A., Izmaylov A. F. & Scuseria G. E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 125, (2006). [DOI] [PubMed] [Google Scholar]
- Troullier N. & Martins J. L. Efficient pseudopotentials for plane-wave calculations. Phys. Rev. B. 43, 1993–2006 (1991). [DOI] [PubMed] [Google Scholar]
- Stein E. NIST standard reference database 1A NIST/EPA/NIH mass spectral library (NIST 08) and NIST mass spectral search program (Version 2.0f), user’s guide. The NIST mass spectrometry data center. http://www.nist.gov/srd/nist1a.cfm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.