Abstract
Background
Lactobacillus gasseri CP2305 (CP2305) is a strain of Lactobacillus isolated from a stool sample from a healthy adult that showed beneficial effects on health as a paraprobiotic. In a previous study, we demonstrated that CP2305-fermented heat-treated milk modified gut functions more than artificially acidified sour milk. Thus, the regulatory activity of the former beverage was attributed to the inactivated CP2305 cells.
Objective
The aim of this study was to elucidate the contribution of non-viable paraprobiotic CP2305 cells to regulating human gut functions. We thus conducted a randomized, placebo-controlled, double-blinded parallel group trial.
Design
The trial included 118 healthy participants with relatively low or high stool frequencies. The test beverage was prepared by adding 1×1010 washed, heat-treated, and dried CP2305 cells directly to the placebo beverage. The participants ingested a bottle of the assigned beverage daily for 3 weeks and answered daily questionnaires about defecation and quality of life. Fecal samples were collected and the fecal characteristics, microbial metabolite contents of the feces and composition of fecal microbiota were evaluated.
Results
The number of evacuations and the scores for fecal odors were significantly improved in the group that consumed the CP2305-containing beverage compared with those of the group that consumed the placebo (p=0.035 and p=0.040, respectively). Regarding the fecal contents of microbial metabolites, the level of fecal p-cresol was significantly decreased in the CP2305 group relative to that of the placebo group (p=0.013). The Bifidobacterium content of the intestinal microbiota was significantly increased in the CP2305 group relative to that of the placebo group (p<0.008), whereas the content of Clostridium cluster IV was significantly decreased (p<0.003). The parasympathetic nerve activity of the autonomic nervous system became dominant and the total power of autonomic activity was elevated in the CP2305 group (p=0.0401 and p=0.011, respectively).
Conclusions
The continuous ingestion of heat-treated CP2305 cells clearly affected intestinal functionality. This is the first report of sterilized Lactobacillus cells having a significant impact on the environment and functions of the intestinal tract. The observed effects might be due, at least in part, to the brain–gut interaction.
Keywords: abdominal symptoms, defecation habits, intraluminal environment, bacterial flora, heat-inactivated lactic acid-producing bacilli, parasympathetic activation
There are several hundred trillion enteric bacteria per gram of feces in the human intestinal tract, which together form a complicated gut microbiome (1, 2). Previous studies have revealed close relationships between the host and the gut microbiota in health and disease (3–5). Recently, obesity and mental health status have been reported to be affected by the composition of the gut microbiome (6–9).
Probiotics are defined as ‘live microorganisms that, when administered in adequate amounts, confer a health benefit on the host’ (10), and the administration of these organisms might change the composition of host gut microbiota. The health-promoting effects of various probiotics have been clarified and put to practical use worldwide. In addition, although less well studied than probiotics, a considerable amount of data has revealed ‘biogenic’ and ‘paraprobiotic’ functions, indicating that non-viable microbial cells, microbial fractions, or cell lysates can maintain human and animal health (11, 12). Non-viable materials of microbial origin have noticeable advantages over probiotics for the development of safer and more stable products (13). For example, a longer shelf life is an advantage in the use of paraprobiotics. This advantage can be achieved through delivery to widespread areas without refrigerating devices and facilities and reducing the risk of microbial translocation, infection or enhanced inflammatory responses in consumers with imbalanced or compromised immune systems.
Lactobacillus gasseri CP2305 (CP2305) is a lactic acid bacteria isolated from the stool of a healthy adult and studied for its beneficial effects on the maintenance and promotion of health (14). A series of studies on strains that relieve stress and ameliorate sleep quality and intestinal function have been conducted using non-viable and living cells (in submission). Regarding the beneficial effects of non-viable lactic acid bacteria on gut health, the administration of pasteurized Enterococcus faecalis EC-12 (200 mg; 1012/day) to eight healthy volunteers (22–26 years of age) for 14 days improved the fecal microbiota and reduced the level of fecal putrefactive products (12); however, to date, the beneficial effects of non-viable lactobacilli on gut functions have not been fully investigated. We previously demonstrated that ingesting pasteurized CP2305-fermented milk improved bowel habits and the intestinal environment compared with the effects of a placebo prepared using artificially acidified sour milk, consistent with the effects of fermented milk containing live CP2305 (14). Thus, we proposed that pasteurized CP2305 bacterial cells lead to these improvements. However, there was a possibility that rare extracellular metabolites of CP2305, likely produced during the production of fermented milk as the base product material, could serve as functional molecules.
To elucidate the effect of non-viable CP2305 cells on the intestinal function and the intestinal environment, we conducted a randomized, double-blind, placebo-controlled trial with participants of relatively low or comparatively high stool frequency through the consumption of test beverages containing washed, pasteurized, and dried CP2305 bacterial cells for 3 weeks. We demonstrate that these effects were observed through a relatively lower dose of pasteurized CP2305 powder (1×1010 counts per day), and this study was conducted with a larger number of participants (n=60) of a broader age range compared with the previous study.
Materials and methods
Supplementary beverages
The non-viable CP2305 cell preparation was produced as described below: CP2305 was cultured at 37°C for 18 h in an original food ingredients-based medium containing glucose, casein and fish protein peptone, yeast extract, pH adjuster, and emulsifier. The cultured CP2305 cells were concentrated using a ceramic filter, washed with excessive water, pasteurized at 90°C, and freeze-dried.
Test beverages containing CP2305 were prepared after blending high-fructose corn syrup, powdered skim milk, lactic acid, soybean polysaccharide, pectin, sodium citrate, flavors, sweeteners, and 1×1010 counts of powdered CP2305 in water, followed by pasteurization and packing into a 200-mL container. Placebo beverages were prepared using the same formula and procedure as the CP2305 beverage, except this product did not contain the pasteurized CP2305 powder. The nutritional content of the test and placebo beverages is described as follows. The CP2305 beverages provided a total of 214 kJ/d (51 kcal/d), 2.0 g of protein, 11 g of carbohydrates, 0 g of fat and 76 mg sodium in 200 mL, whereas the placebo beverage provided a total of 214 kJ/d (51 kcal/d), 2.0 g of protein, 11 g of carbohydrates, 0 g of fat and 76 mg sodium in 200 mL. These supplementary beverages were produced at Asahi Soft Drinks Co., Ltd. (Tokyo, Japan).
Subjects
The participants in the present study included healthy adult men and women who were recruited from a pool of volunteers at the Clinical Support Corporation and who had undergone a medical examination at the Medical Corporation Hokubukai Utsukushigaoka Hospital, affiliated with the Clinical Support Corporation, within a month prior to the onset of the trial. The selection criteria included individuals aged 20–70 years who consumed regular meals and had either relatively low frequencies of defecation (≤4 times per week) or comparatively high frequencies of bowel movements (≥10 times per week).
The exclusion criteria included individuals with a medical history of severe disorders, continuous medical treatment, a surgical history of the digestive system (except appendectomy), daily watery stool, pregnancy or the potential to become pregnant during the study, breast-feeding, constant usage of supplements and/or functional foods (including Food for Specified Health Uses [FOSHU]) affecting the test results, extremely irregular dietary habits, alternative work schedules or midnight shifts, high alcohol consumption, whole blood withdrawal of more than 400 mL within 12 weeks or more than 200 mL within 4 weeks, or blood component withdrawal prior to the study, and individuals considered unsuitable for the study according to the principal investigator for other reasons.
One hundred and twenty participants who fulfilled the eligibility criteria for the study agreed to participate. All the subjects were enrolled in the study prior to random allocation. Allocation into the CP2305 or placebo group was concealed from the investigator who enrolled the subjects and the nurses and the medical doctor in charge.
This study was approved by the Institutional Review Board of the Medical Corporation Hokubukai Utsukushigaoka Hospital according to an ethic principle and experimental plan based on the Helsinki Declaration. Prior to the trial, a medical doctor (K.T.) provided a full explanation concerning the purpose and methodology of the study to the subjects. All participants who agreed to participate in the study provided fully informed consent.
Trial design
This study was a randomized, double-blind, placebo-controlled design, and the experimental periods comprised 2 weeks of pre-observation, 3 weeks of intervention, and 2 weeks of post-observation. A concealed study coordinator (I.T.) randomly and blindly assigned the 120 participants into two groups matched for age, gender, and defecation frequency. One group (n=60) received the CP2305 beverage (CP2305 group), and the other group (n=60) received the placebo beverage (placebo group). The participants were instructed to consume one pack of the beverage daily. During the study period, the participants were also instructed to assess their health condition, maintain healthy living habits, such as diet and exercise, and answer a questionnaire concerning stool habits and the characteristic features of feces as described below.
The sample size was calculated as 60 individuals per group to detect changes in Bristol stool scale (BSS) scores using analysis of covariance (ANCOVA), with the initial score as the covariate in stool consistency. This sample size was considered suitable for detecting differences between the scores at the end of treatment and the initial scores at a p-value of 0.05 with 80% power, based on information gathered in a previous study (14). As a 5% attrition rate was expected, we employed 120 participants.
Study questionnaire
Each participant maintained a daily record of CP2305 or placebo beverage consumption, diet, exercise, and physical condition, including the presence of subjective symptoms during the trial. To assess changes in frequency of bowel movements, amount of output, feelings after evacuation, mundane abdominal symptoms, and changes in appearance and characteristic traits of feces, the participants were asked to record a daybook. During the 7 weeks of study, the subjects maintained a stool diary to record the frequency of bowel movements, abdominal symptoms, the feeling after defecation and the fecal characteristic traits (form, color tone, output, and intensity of odor). Fecal form and color were evaluated in accordance with the BSS and a fecal color chart. The recorded BSS scores for each subject were treated as a subjective BSS score. The BSS (15), which assesses the consistency and the shape of the feces, was defined using the following seven categories: 1, separate hard lumps, similar to nuts; 2, sausage-shaped but lumpy; 3, similar to a sausage but with cracks on the surface; 4, similar to a banana, smooth and soft; 5, soft blobs with clear-cut edges; 6, fluffy pieces with ragged edges, a mushy stool; and 7, watery, no solid pieces, entirely liquid. The stool scale was shown graphically on the questionnaire, and the participants used this scale to rate and record each event.
During the study, the participants also recorded the fecal color. The subjects determined the fecal color through comparison with the tones corresponding to five stages on a color card provided from the experimenters. The scores for the tones of the seven stages were 1, dark to 7, light brown. An example of the fecal color score was shown graphically on the questionnaire, and the participants used this information to rate and record each event.
Strain while evacuating, feelings of incomplete evacuation, pain while evacuating, and flatulence were categorized according to five classifications (1, none; 2, mild; 3, moderate; 4, severe; and 5, very severe), while fecal odor was scored according to three classifications (1, strong; 2, moderate; and 3, weak), and abdominal discomfort was scored according to four classifications (1, comfort; 2, a little comfort; 3, normal; and 4, discomfort). The participants selected the most likely number to suit the condition.
The fecal output was compared with a scale model, such as a circular cylinder, of which the diameter of the base is 2.5 cm and the length 5.0 cm. The fecal volume was reported as substantial quantities expressed as the number of the model equivalent.
Measurements and analytical values
At baseline (week 0) and at the end of the intervention (week 3), anthropometric measurements (body weight, body mass index (BMI), body fat percentage, systolic and diastolic blood pressure, pulse rate, and body temperature), blood sampling, and urinalysis were conducted at the hospital. Height was measured at screening only, and BMI was calculated based on this measurement. Investigators blinded to the study allocation assessed the BSS at weeks 0 and 3 and at the end of the post-observation period (week 5).
Blood examination and urinalysis were conducted at Sapporo Clinical Laboratory Inc. (Sapporo, Japan). The participants were required to fast for more than 12 h prior to visiting the hospital on the test dates. The following biochemical and hematological parameters were measured: total protein, albumin, total bilirubin, aspartate aminotransaminase (AST), alanine aminotransaminase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γ-GT), total cholesterol, HDL cholesterol, LDL cholesterol, triacylglycerol (TG), uric acid, blood urea nitrogen (BUN), creatine, sodium, potassium, chloride, glucose, white blood cells, red blood cells, hemoglobin, hematocrit, and platelets. The urinalysis, protein, glucose, urobilinogen, bilirubin, ketone bodies, occult blood, pH, and density were examined.
A medical doctor (K.T.) conducted medical examinations and inquiries, and subjective symptoms were assessed at each measurement. All of the analyses were performed with the supervision of a physician.
Analyses of fecal samples
Fecal samples were collected three times at the end of the observation, ingestion, and follow-up periods. These samples were objectively evaluated using the BSS (objective BSS), and the putrefactive products, organic acids, and composition of the fecal microbiota were analyzed.
Microbial metabolites, including decomposed putrefactive products and organic acids, were analyzed according to Ikeda et al. (16). Briefly, putrefactive products in the fecal samples, including phenol, 4-ethylphenol, p-cresol, indole, and skatole, were determined through the gas–liquid chromatography apparatus GC-2014 fitted with a flame ionization detector (Shimadzu Corporation, Kyoto, Japan). The fecal microbial metabolites were analyzed using an HPLC with a pH indicator (LaChrom Elite; Hitachi High-Technologies Corporation, Tokyo, Japan). Tartaric acid, malic acid, succinic acid, lactic acid, formic acid, acetic acid, propionic acid, isobutyric acid, butyric acid, isovaleric acid, and valeric acid were simultaneously separated.
The fecal microbiota composition was analyzed using terminal-restriction fragment length polymorphism (T-RFLP) at TechnoSuruga Laboratory Co., Ltd. (Shizuoka, Japan) as previously described (17). Briefly, the fecal samples collected from all participants were stored at −30°C, and fecal DNA samples were subsequently prepared. Using fluorescent-labeled primers, the 16S rRNA gene was amplified. The amplified products were digested with a restriction enzyme (Bsl I) and classified according to the distribution of operation taxonomic units. The length of the terminal-restriction fragments, corresponding to Bifidobacterium, Lactobacillus, Bacteroides, Prevotella, Clostridium cluster IV, Clostridium subcluster XIVa, Clostridium cluster XI, Clostridium cluster XVIII, and other bacteria, was determined. Quantitative PCR analyses targeted to the genus Bifidobacterium and the bacterial group Clostridium cluster IV were performed to confirm the changes in the populations of intestinal bacteria. The protocol of Rinttilä et al. (18) was used to determine the Bifidobacterium load, and our modified protocol, described below, was used to determine the load of Clostridium cluster IV. The forward primer and reverse primers used to analyze the Clostridium cluster IV were 5′-CAAGCACAAGCRGTGGAGT-3′ and 5′-AGAGTSCTCTTGCGTA-3′, respectively. The specificity of these primers was fully confirmed. The amplification parameters for Clostridium cluster IV were those of Rinttilä et al. (18) except that the primer concentrations used were 400 nM instead of 1 µM. The PCR program consisted of 35 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 20 s, and extension at 72°C for 30 s.
Autonomic function analysis
Autonomic nervous system activity was examined after measuring the variation in the fingertip heart rate before and after the intervention (19). TAS9 VIEW (YKC corporation, Tokyo, Japan), an acceleration pulse wave measurement apparatus, was used to analyze the heart rate variation. The autonomic nerve balance analysis conducted at a frequency level from 0.04 to 0.15 Hz was classified as low frequency (LF), while the analysis conducted at 0.15–0.40 Hz was classified as high frequency (HF). LF and HF were considered indicators for the activities of sympathetic and parasympathetic nerves, respectively. The ratio between LF and HF (LF/HF) revealed the overall balance of both nerves.
Statistical analysis
All statistical analyses were performed using JMP11 Pro (SAS Institute Japan Ltd., Tokyo, Japan) and IBM SPSS Statistics 20 (IBM Japan Ltd., Tokyo, Japan). The subjects were classified into the following three subgroups based on the initial objective BSS at baseline: hard type (BSS 1–3), normal type (BSS 3.5–4.5), and soft type (BSS 5–7) (15).
ANCOVA with repeated measures and ANCOVA were used to analyze the data, in which the initial values were employed as covariates. In addition, analysis of variance (ANOVA) for repeated measures was used. Provabilities less than 0.05 were considered significant.
Results
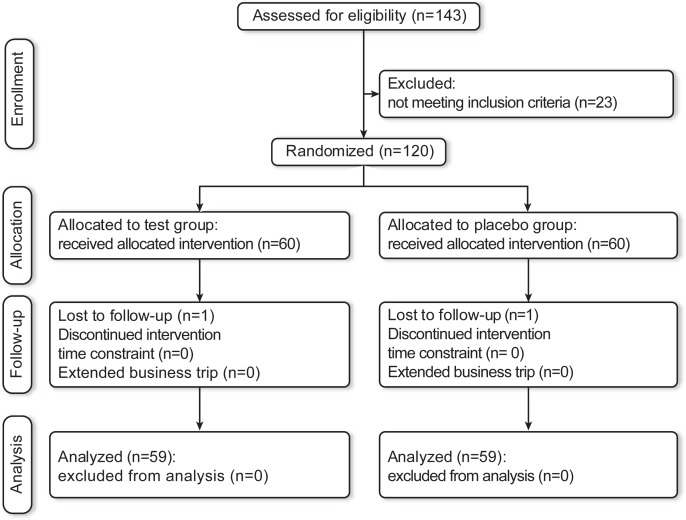
One hundred and forty-three participants were recruited, 23 of which were excluded, and randomly assigned to the pasteurized CP2305 or placebo treatment. A flow chart of subject recruitment, allocation, and analysis is shown in Fig. 1. One hundred and eighteen participants completed the study and were followed up during the study period. Two participants dropped out during the intervention. The baseline characteristics of the participants are presented in Table 1. No significant differences in gender, age, or BMI were observed between the CP2305 and placebo groups (p>0.250). High compliance was attained in the present study; the frequencies of CP2305 and placebo beverage consumption (%) were 99.92±0.081 for placebo and 99.92±0.081 for CP2305, respectively.
Fig. 1.
Flow chart showing the trial design.
Table 1.
Baseline characteristics of the study groups
| Placebo group | CP2305 group | |||
|---|---|---|---|---|
| Constipation | Frequency of bowel movements | Constipation | Frequency of bowel movements | |
| N (male/female) | 29 (10/19) | 30 (15/15) | 29 (10/19) | 30 (15/15) |
| Age (years) | 41.41±1.95 | 40.23±1.92 | 42.72±1.95 | 40.90±1.92 |
| Height (cm) | 162.35±1.44 | 165.21±1.42 | 161.07±1.44 | 163.25±1.42 |
| Weight (kg) | 58.82±2.34 | 64.75±2.30 | 56.81±2.34 | 63.55±2.30 |
| Body fat (%) | 27.47±1.39 | 27.53±1.37 | 26.57±1.39 | 28.54±1.37 |
| BMI (kg/m2) | 22.23±0.70 | 23.48±0.68 | 21.84±0.70 | 23.82±0.68 |
| Systolic blood pressure (mm Hg) | 118.81±3.39 | 121.32±3.33 | 118.53±3.39 | 120.40±3.33 |
| Diastolic blood pressure (mm Hg) | 79.88±5.17 | 74.58±5.08 | 70.91±5.17 | 72.05±5.08 |
| Pulse rate (beats/min) | 68.50±1.85 | 69.22±1.82 | 69.88±1.85 | 70.22±1.82 |
| Body temperature (°C) | 36.40±0.07 | 36.35±0.07 | 36.33±0.07 | 36.27±0.07 |
All values presented are the mean values±SEM (standard error of the mean).
BMI: body mass index.
BSS, bowel movements, characteristic features of feces, and abdominal symptoms
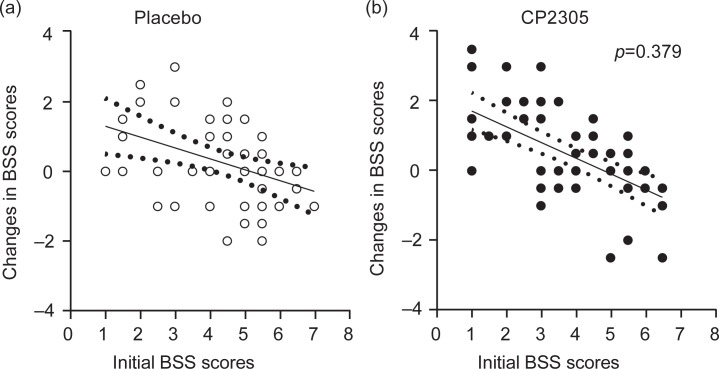
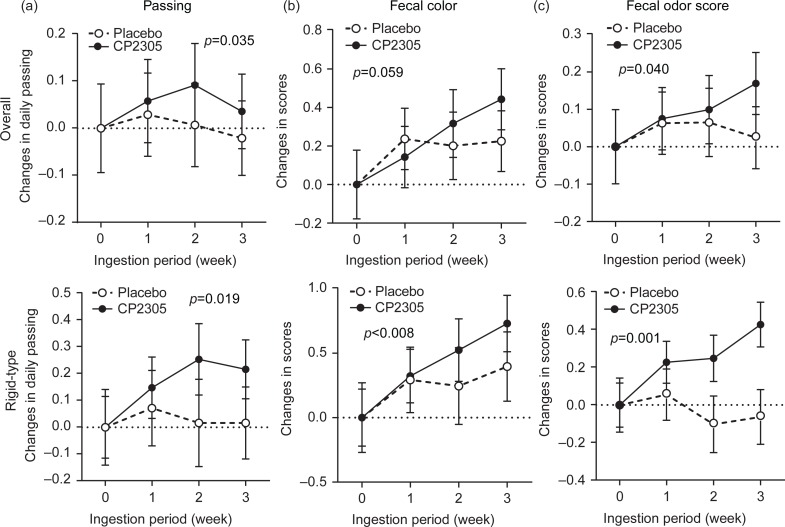
A tendency toward differences in the changes in the objective BSS scores of the two groups was observed; however, these differences were not significant (Fig. 2). Figure 3a shows the changes in the number of daily passing in overall groups and subgroups with rigid-type fecal characteristics. Bowel movement was affected through intervention with paraprobiotic CP2305 (1×1010 counts per day). In the subgroup with rigid-type fecal characteristics, a significant increase in the number of bowel movements was observed (p=0.019).
Fig. 2.
Changes in the objective BSS scores of the placebo and CP2305 groups. The solid line is the regression lines, and the dotted lines indicate the bounds of the 95% confidence intervals. ANCOVA was used to compare the adjustments in BSS scores in both beverages. A score of 4 shows the hardness of normal banana-shaped stool. Higher scores indicate softer stools and lower scores indicate harder stools.
Fig. 3.
Changes in the number of daily bowel movements and the fecal color tone and odor scores during the intervention period. (a) The number of daily bowel movements was summarized weekly and was distributed between 0 and infinity. The sum was divided by 7 to obtain the average number of daily bowel movements, and these data were analyzed parametrically (overall model and subgroup with rigid-type stool characteristics). (b) The fecal color tone of both groups was summarized in a diary every 7 days, with values ranging from 7 to 49, and the sum was divided by 7. The products were analyzed parametrically. Larger scores indicate brighter color tones. (c) Stool odor scores were summarized weekly and distributed from 7 to 21. The sum was divided by 7. The products were used for parametric analyses.
Among the fecal characteristics, slight lightening of fecal color tone was observed (Fig. 3b: p=0.059), particularly in participants in the subgroup with rigid-type stool characteristics (Fig. 3b: p<0.008). Figure 3c shows the changes in fecal odor scores. Almost no changes were observed in the placebo group, whereas the CP2305 group showed significant amendments in fecal odor (p=0.040).
The other parameters, such as abdominal refreshment after defecation, straining, incomplete evacuation, pain while evacuating, or flatulence, did not change between the CP2305 and placebo groups (data not shown).
Short chain fatty acids and putrefactive substances
Statistically significant changes in short chain fatty acids (SCFAs) (formate, acetate, propionate, butyrate, isobutyrate, isovalerate, and valerate) were not observed between the CP2305 and placebo groups, nor were there significant differences in other organic acids (tartrate, malate, succinate, and lactate) between the two groups (data not shown).
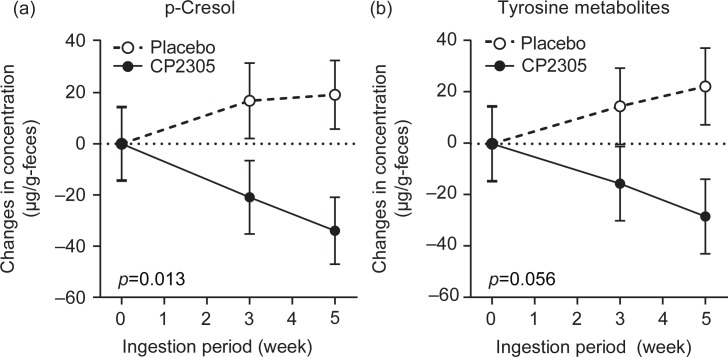
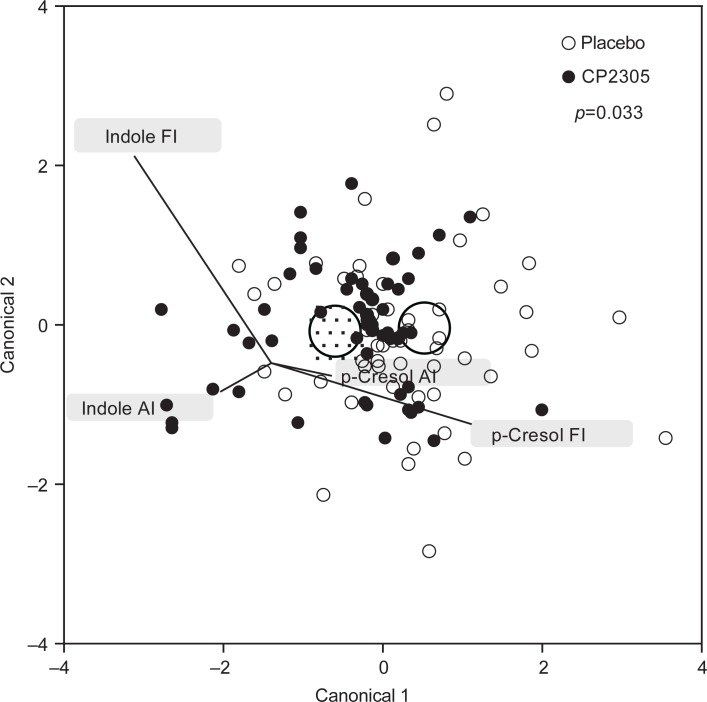
Among the putrefactive products, p-cresol showed a significant reduction (Fig. 4a: p=0.013), and tyrosine metabolites (phenol, p-cresol, and 4-ethylphenol) were reduced in the CP2305 group compared with the placebo group (Fig. 4b: p=0.056). The time-dependent changes in the levels of these products in the two groups were completely the opposite. Tryptophan metabolites (indole and skatole) did not show any significant differences (data not shown). The discrimination analysis using data for p-cresol and indole revealed that pasteurized CP2305 cells ameliorated the enteric environment (Fig. 5).
Fig. 4.
Changes in the concentrations of fecal p-cresol and tyrosine metabolites throughout the trial. (a) Changes in the concentrations of p-cresol in the stool samples. (b) Changes in the concentrations of fecal tyrosine metabolites, which reflect the sum of the concentrations of phenol, 4-ethylphenol and p-cresol.
Fig. 5.
Discrimination analysis of enteric environment showing changes in fecal p-cresol and indole. The circles in the figure show the 0.95 confidence level ellipses of both multivariate means. Both groups were differentiated through discrimination analysis using the changes in the concentrations of fecal p-cresol and indole. Abbreviations: AI, changes in the initial values and those after intervention; FI, changes in the initial values and those after post-observation.
Analyses of the fecal microbiota
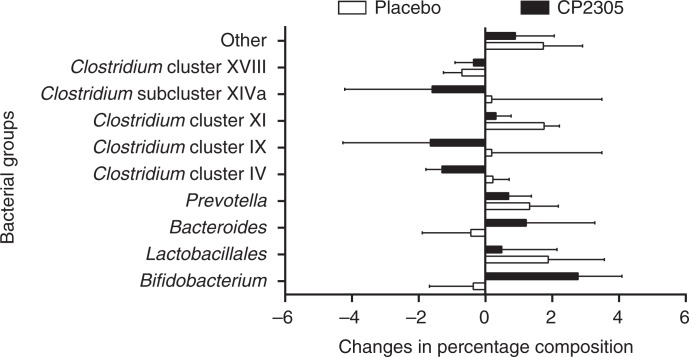
Changes in the percent composition of the fecal microbiota shown in Fig. 6 (nine selected bacterial groups) were analyzed using T-RFLP. Bifidobacterium was significantly increased during CP2305 ingestion (p<0.008), whereas Clostridium cluster IV was significantly decreased in the CP2305 group compared with the placebo (p<0.003). A significant increment in the Bifidobacterium population (p<0.01) and a significant reduction of the Clostridium cluster IV population were observed in the CP2305 group (p<0.05) but not in the placebo group. Both of these results were consistent with the quantitative PCR results (data not shown).
Fig. 6.
Changes in the composition of gut microbiota before and after intervention. Each participant consumed one pack (200 mL) of beverages containing pasteurized CP2305 cells or the same amount of placebo, daily for 3 weeks. Before and after intervention (week 0 and 3), the stool samples were collected, and terminal-restriction fragment length polymorphism (T-RFLP) profiling of the samples was conducted. ANCOVA was applied to analyze each bacterial compositional change.
Autonomous nervous activity
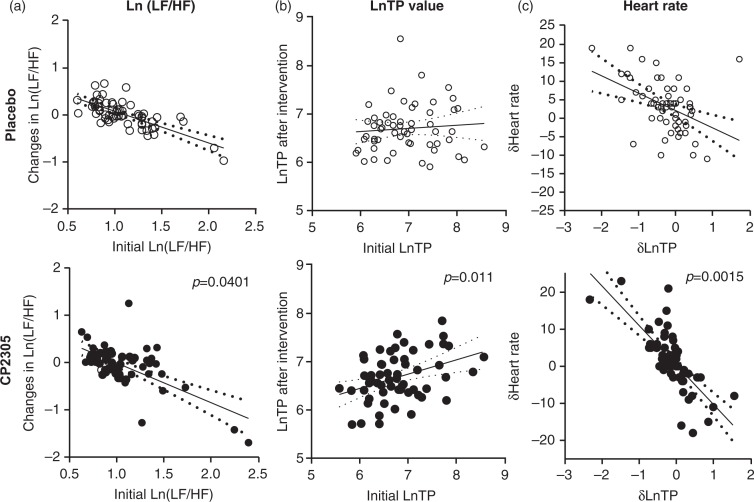
A significant reduction of the LF/HF ratio (Fig. 7a: p=0.0401) and a meaningful elevation of the total power of the autonomous nerve activity (Fig. 7b: p=0.011) were found in the CP2305-fed group. The heart rate of the participants in the CP2305 group was lowered after intervention (Fig. 7c: p=0.0015).
Fig. 7.
Changes in nerve activities after the intervention. (a) Changes in the ratio of sympathetic to parasympathetic nerve activities after the intervention, expressed as Ln (LF/HF) values. (b) Changes in total power (TP) of autonomous nerve activities after intervention. (c) Relationship between the changes in the TP of the autonomous nerve activity and the heartbeat rate. These values were analyzed using an ANCOVA. The solid line is the regression line, and the dotted lines indicate the bounds of the 95% confidence intervals.
Medical examinations and interviews
Measurements of serum biochemical and hematological parameters and urinalysis were conducted before and after the intervention period (weeks 0 and 3). There were no abnormal changes observed in the urinalysis and blood test for either the test or placebo groups (data not shown). Although two subjects were positive for uric protein and uric glucose prior to the intervention, no abnormal changes were observed before and after the intervention.
In the present study, transient diarrhea (four subjects in the placebo group and two subjects in the CP2305 group) and constipation (two subjects in the placebo group and one subject in the CP2305 group) were observed, but the medical doctor in charge (K.T.) determined that these symptoms were not attributed to the consumption of supplementary beverages. The symptoms were equally distributed between the CP2305 and placebo groups.
Discussion
In the present study, we demonstrate that the daily consumption of beverages containing non-viable CP2305 cells for 3 weeks ameliorated the intestinal environment and intestinal functions of healthy participants with a tendency toward constipation or frequent bowel movements. We also showed that these effects were achieved through relatively lower doses of pasteurized CP2305 powder. In addition, this study was conducted with a larger number of participants (n=120) and a broader age range (20–63 years of age) compared with a previous study of non-viable cocci, E. faecalis EC-12 (20).
In the T-RFLP profiling of fecal microbiota, Bifidobacterium was significantly increased in the CP2305 group compared with that of the placebo group (Fig. 6). The data suggest that Bifidobacterium growth in the intestine was promoted through the administration of non-viable CP2305, potentially reflecting improvements in the disturbance of defecation. Bifidobacterium is a dominant species in the intestinal microbiota, and these bacteria play important roles, such as suppressing the propagation of harmful bacteria; maintaining the healthy balance of the intestinal microbiota; regulating the immune system; and preventing infection, allergy, and cancer (21). Therefore, many efforts to increase the population of Bifidobacterium in the human intestine have been undertaken. One of these efforts involved the continuous ingestion of a specific strain of Bifidobacterium (21). Bifidobacterium bacteria in the intestinal tract produce succinate and lactate, as well as SCFAs, such as acetate and propionate. It has been suggested that these acids lower the pH of the intestine and inhibit the propagation of harmful bacteria and the production of putrefactive intestinal products. These SCFAs also act on the growth of intestinal epithelial cells, thereby regulating intestinal peristalsis and bowel habits. These findings have attracted much attention to probiotic products. In this trial, the increase in the Bifidobacterium content of the intestinal microbiota was fully reflected in the apparent increase in the levels of the endogenous strains. This result suggests that the gut microbiota was significantly changed by the continuous intake of paraprobiotic CP2305 cells.
However, a significant decrease in the Clostridium cluster IV population in the CP2305 group was also observed in this study (Fig. 6). This finding is quite different from the results of our previous clinical trial (14), in which a beverage produced using sterilized CP2305-fermented milk was used. In the previous trial, a significant increase in the level of Clostridium cluster IV bacteria was accompanied by a significant upregulation in the production of several SCFAs, including propionate, butyrate, and valerate. Thus, there may be contrasting mixed responses to ingested pasteurized CP2305 on a case-by-case basis. These results suggest that CP2305 cells have no specific agonistic and/or antagonistic effect on the investigated bacterial groups in the gastrointestinal tract. One explanation for these results is that the paraprobiotic CP2305 cells first affect gut functions, changing the environmental conditions of the intrinsic intestinal microbiota, and that thereafter the composition of the intestinal microbiota changes to adapt to the altered environmental conditions. Further studies are needed to verify this hypothesis.
Although in this discussion we mainly focused on the changes in the composition of the microbiota observed in the CP2305 group, as described above, the composition of the microbiota in the placebo group also changed. The reason for this change is unknown but it might be due to the limit of detection of the T-RFLP method and/or the effects of the constituents of the test beverage other than CP2305 cells. Figure 4a shows the suppression of fecal p-cresol production, likely resulting in peristalsis of the intestine and regulation of fecal habits. Evaluation of the peculiarities of feces and bowel habits revealed significantly improved fecal odor, number of evacuations, and feeling of incomplete evacuation in participants with a tendency toward constipation in the CP2305 group. However, significant changes in stool consistency, refreshment after defecation, straining, incomplete evacuation, abdominal pain, and abdominal fullness were not observed between the two groups. Questions regarding these topics are frequently asked in clinical trials of the effects of substances that regulate intestinal functions. However, care must be taken in deciphering the responses because they have the known limitations of subjective personal evaluations.
Whereas bifidogenic effects have been demonstrated after the administration of several probiotic Bifidobacterium strains, prebiotic oligosaccharides, and soluble dietary fibers, non-viable CP2305 cells are not bifidogenic materials. It is likely that bacterial cells or cell wall components elicit functions similar to soluble dietary fibers. Previous studies have reported that the pepsin hydrolysate of bovine lactoferrin possesses bifidogenic properties (22); thus, certain types of peptides derived from CP2305 cells might also increase the number of Bifidobacterium. In addition, cross-talk between bacterial cells and the mucosal surface of the intestine might also occur, leading to changes in intestinal functions. These include secretion of mucus, anti-bacterial peptides, and anti-bacterial antibodies, which act on enteric nerves, followed by changes in the peristaltic action and secretion and/or modifications of brain–gut interactions for regulating vital functions. Thus, the growth environment for the intestinal microbiota might be significantly affected. Indeed, we propose that these strains confer stress-relief and improve the quality of sleeping and abdominal symptoms, potentially through the brain–gut axis in animal and clinical studies (14). These effects have been observed using both non-viable CP2305 cells and living cells. In the present study, autonomic nervous activities were skewed toward the parasympathetic nerve-dominant condition after intervention. This effect might reflect the regulation of gut function through paraprobiotic CP2305. However, a divergent view has been reported using a fermented milk product produced from a strain in the same genus (7). The reason for this difference is not clear. One possibility is simply a difference among these strains. This difference might also reflect the conditions of the trials.
The group receiving CP2305 cells showed no changes in the concentrations of fecal SCFAs before and after intervention compared with the placebo group, consistent with the results of other studies using prebiotics (23, 24). Previous reports have suggested that this phenomenon might reflect the usage of SCFAs in enterocytes and enteric microbiota following the increased production of SCFAs (24, 25), reflecting the significant change in value detected in the feces. Bifidobacterium in the gut primarily produces acetate, propionate, and succinate, whereas it has been reported that SCFAs, such as acetate, propionate, and butyrate, stimulate the growth of Bifidobacterium. Thus, the increase in the population ratio of Bifidobacterium in the enteric microbiota might not be positively correlated with the concentration of SCFAs in the gut as observed in the present study.
The analysis of putrefactive products in feces indicated that p-cresol was significantly decreased in the CP2305 group (Fig. 4a). The reduction in p-cresol, a metabolite of the amino acid tyrosine produced in intestinal microbiomes, observed after the ingestion of CP2305 is also important for maintaining a healthy intestinal environment because potentially toxic protein degradation products, such as phenols and indoles, have also been implicated in increasing the incidence of colorectal cancer (26). The production of p-cresol in the large intestine is affected through the composition of intestinal microbiota, high tyrosine or high protein diets, and gut function, and the main contributing bacteria are anaerobes (26, 27). The reduction in the fecal p-cresol content suggests that CP2305-consumption alters the tyrosine metabolism of the intestinal microbiota and diminishes the toxicological risk of the tyrosine metabolites present in the gastrointestinal tract. In the present study, we observed a slight but not significant difference in changes in stool consistency, abdominal symptoms, SCFAs, putrefactive products in feces exclusive of p-cresol, and fecal microbiota exclusive of Bifidobacterium and Clostridium cluster IV between the two groups. One limitation concerning this trial is the deficiency of information about daily consumption of food intake. Although the intestinal microbial populations of the participants might have been affected by their diets, we did not impose strict dietary regulations on the participants or supply them with uniform diets, which might have affected the results. A previous study suggested that the handling of fecal samples from evacuation to analysis of the samples could affect the quantification of SCFAs (24). However, the data obtained in previous studies with the same study design and methods of appraisal showed ambiguous evidence for improving intestinal environments and defecation habits (14). The differences in the apparent efficacy of the test foods could be due to sampling bias, slight differences in the doses of CP2305 cells in each test food sample prepared, and differences in the pools of participants.
Conclusions
In conclusion, the daily consumption of beverages containing 1×1010 pasteurized CP2305 cells for 3 weeks in participants with low or frequent bowel movements improved the intestinal environment and function, although further investigations are needed to clarify the dose-response and establish evidence for mechanism of action. These results support the view that the consumption of beverages containing CP2305 can prevent the degeneration of quality of life and the occurrence of lifestyle-related diseases attributed to a decline in the intestinal environment. The advantages of non-viable cells contribute to its versatility compared with probiotics; they can be easily processed and incorporated into various accessible foods and beverages, and they exhibit a longer shelf life.
Acknowledgements
The authors thank the volunteers who participated in the study. The authors also thank Mr. Yusuke Shibata and Mr. Toshimasa Higuchi from the Products Development Center of Asahi Soft Drinks Co., Ltd. for the production of the placebo beverage and the beverage containing inactivated CP2305 cells, Ms. Hiromi Suzuki and Ms. Natsumi Koyama of Asahi Group Holdings, Ltd. for the analysis of intestinal microbial metabolites, and Dr. Izumi Yoshida and Dr. Yukiko Aoyama of the Tempstaff Co., Ltd. for excellent work in preparing this manuscript.
Conflict of interest and funding
This research received financial support from Asahi Group Holdings, Ltd. (Tokyo, Japan). TS, DS, YI, KA, YA, and SF are employees of Asahi Group Holdings, Ltd. The authors declare that there are no conflicts of interest.
References
- 1.Mitsuoka T, Ohno K, Benno Y, Suzuki K, Namba K. [The fecal flora of man. IV. Communication: comparison of the newly developed method with the old conventional method for the analysis of intestinal flora]. Die faekalflora bei menshen IV. Mitteilung: vergleich des neu entwickelten verfahrens mit den bisherigen tiblichen verfahren zur darmfloraanalyse. Zentralbl Bakteriol Orig A. 1976;A234:219–33. [In German] [PubMed] [Google Scholar]
- 2.Franks AH, Harmsen HJ, Raangs GC, Jansen GJ, Schut F, Welling GW. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol. 1998;64:3336–45. doi: 10.1128/aem.64.9.3336-3345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimizu K, Ogura H, Asahara T, Nomoto K, Morotomi M, Tasaki O, et al. Probiotic/synbiotic therapy for treating critically ill patients from a gut microbiota perspective. Dig Dis Sci. 2013;58:23–32. doi: 10.1007/s10620-012-2334-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ettinger G, MacDonald K, Reid G, Burton JP. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes. 2014;5:719–28. doi: 10.4161/19490976.2014.983775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. Gut microbiota and metabolic syndrome. World J Gastroenterol. 2014;20:16079–94. doi: 10.3748/wjg.v20.i43.16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minami J, Kondo S, Yanagisawa N, Odamaki T, Xiao JZ, Abe F, et al. Oral administration of Bifidobacterium breve B-3 modifies metabolic functions in adults with obese tendencies in a randomised controlled trial. J Nutr Sci. 2015;4:e17. doi: 10.1017/jns.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otomi K, Ymaguchi T, Watanabe S, Kobayashi A, Kobayashi H, Hashiguchi N. Effects of yogurt containing Lactobacillus gasseri OLL2716 on autonomic nerve activities and physiological functions. Health. 2015;7:397. [Google Scholar]
- 8.Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17:565–76. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–76. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Food and Agriculture Organization of the United Nations (FAO), World Health Organization (WHO) 2002. Guidelines for the evaluation of probiotics in food. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food, London, April 30 and May 1, 2002. [Google Scholar]
- 11.Taverniti V, Guglielmetti S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept) Genes Nutr. 2011;6:261–74. doi: 10.1007/s12263-011-0218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitsuoka T. Development of functional foods. Biosci Microbiota Food Health. 2014;33:117–28. doi: 10.12938/bmfh.33.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams CA. The probiotic paradox: live and dead cells are biological response modifiers. Nutr Res Rev. 2010;23:37–46. doi: 10.1017/S0954422410000090. [DOI] [PubMed] [Google Scholar]
- 14.Sawada D, Sugawara T, Ishida Y, Aihara K, Aoki Y, Takehara I, et al. Effect of continuous ingestion of a beverage prepared with Lactobacillus gasseri CP2305 inactivated by heat treatment on the regulation of intestinal function. Food Res Int. 2016;79:33–9. [Google Scholar]
- 15.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–4. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda N, Saito Y, Shimizu J, Ochi A, Mizutani J, Watabe J. Variations in concentrations of bacterial metabolites, enzyme activities, moisture, pH and bacterial composition between and within individuals in faeces of seven healthy adults. J Appl Bacteriol. 1994;77:185–94. doi: 10.1111/j.1365-2672.1994.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 17.Nagashima K, Hisada T, Sato M, Mochizuki J. Application of new primer-enzyme combinations to terminal restriction fragment length polymorphism profiling of bacterial populations in human feces. Appl Environ Microbiol. 2003;69:1251–62. doi: 10.1128/AEM.69.2.1251-1262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rinttilä T, Kassinen A, Malinen E, Krogius L, Palva A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol. 2004;97:1166–77. doi: 10.1111/j.1365-2672.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- 19.Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–81. [PubMed] [Google Scholar]
- 20.Terada A, Bukawa W, Kan T, Mitsuoka T. Effects of the consumption of heat-killed Enterococcus faecalis EC-12 preparation on microbiota and metabolic activity of the faeces in healthy adults. Microb Ecol Health Dis. 2004;16:188–94. [Google Scholar]
- 21.Tojo R, Suarez A, Clemente MG, de los Reyes-Gavilan CG, Margolles A, Gueimonde M, et al. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol. 2014;20:15163–76. doi: 10.3748/wjg.v20.i41.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oda H, Wakabayashi H, Yamauchi K, Sato T, Xiao JZ, Abe F, et al. Isolation of a bifidogenic peptide from the pepsin hydrolysate of bovine lactoferrin. Appl Environ Microbiol. 2013;79:1843–9. doi: 10.1128/AEM.03343-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleessen B, Sykura B, Zunft HJ, Blaut M. Effects of inulin and lactose on fecal microflora, microbial activity, and bowel habit in elderly constipated persons. Am J Clin Nutr. 1997;65:1397–402. doi: 10.1093/ajcn/65.5.1397. [DOI] [PubMed] [Google Scholar]
- 24.Linetzky Waitzberg D, Alves Pereira CC, Logullo L, Manzoni Jacintho T, Almeida D, Teixeira da Silva ML, et al. Microbiota benefits after inulin and partially hydrolyzed guar gum supplementation: a randomized clinical trial in constipated women. Nutr Hosp. 2012;27:123–9. doi: 10.1590/S0212-16112012000100014. [DOI] [PubMed] [Google Scholar]
- 25.Hengst C, Ptok S, Roessler A, Fechner A, Jahreis G. Effects of polydextrose supplementation on different faecal parameters in healthy volunteers. Int J Food Sci Nutr. 2009;60(Suppl 5):96–105. doi: 10.1080/09637480802526760. [DOI] [PubMed] [Google Scholar]
- 26.Bone E, Tamm A, Hill M. The production of urinary phenols by gut bacteria and their possible role in the causation of large bowel cancer. Am J Clin Nutr. 1976;29:1448–54. doi: 10.1093/ajcn/29.12.1448. [DOI] [PubMed] [Google Scholar]
- 27.Kawakami K, Kojima K, Makino I, Kato I, Onoue M. Fasting enhances p-Cresol production in the rat intestinal tract. Exp Anim. 2007;56:301–7. doi: 10.1538/expanim.56.301. [DOI] [PubMed] [Google Scholar]